Abstract
Previously, we showed that the Shiga toxin type 2 (Stx2)-expressing Escherichia coli O157:H7 strain 86-24 colonized mice better than did its isogenic stx2 negative mutant. Here, we confirmed that finding by demonstrating that Stx2 given orally to mice increased the levels of the 86-24 stx2 mutant shed in feces. Then we assessed the impact of Stx2-neutralizing antibodies, administered passively or generated by immunization with an Stx2 toxoid, on E. coli O157:H7 colonization of mice. We found that such antibodies reduced the E. coli O157:H7 burden in infected mice and, as anticipated, also protected them from weight loss and death.
Keywords: Shiga toxin, anti-Shiga toxin, E. coli O157:H7 colonization
1. Introduction
Escherichia coli O157:H7 is a food- and water-borne pathogen that can cause diarrhea, bloody diarrhea (known as hemorrhagic colitis), or in a fraction of cases, a life-threatening sequela called hemolytic uremic syndrome (HUS). In a 1999 publication, Mead et al. reported that in the United States E. coli O157:H7 was associated with an estimated 73,000 cases of intestinal disease each year and that about 3% of infected individuals required hospitalization. The rate of HUS that followed E. coli O157:H7 infection in that 1999 report was estimated as about 4%, and the number of individuals who died of HUS annually was listed as 61 [1]. Approximations of E. coli O157:H7-associated HUS and hospitalization rates from 2000–2006 were over 6% and nearly 42%, respectively [2]. That the severity of the most recent outbreaks of E. coli O157:H7 in the United States has increased even further is indicated by the fact that >50% of ill persons required hospitalization and >10% of infections led to the development of HUS [3–5].
Shiga toxins (Stxs, also called Vero toxins) made by E. coli O157:H7 and other serotypes of E. coli (collectively called Shiga toxin-producing E. coli or STEC) are considered to be responsible for the development of HUS [6]. Stxs are potent AB5 (one A polypeptide with enzymatic activity and 5 copies of a B or cell-binding polypeptide) cytotoxins. These toxins are N-glycosidases that inhibit protein synthesis by the depurination of a critical ribosomal residue important for protein elongation (reviewed in [7]). There are two serologically distinct groups of Stx: Stx1 and Stx2 (reviewed in [8]). The expression of both toxins is associated with human disease, but more recent outbreaks in the United States seem to be associated with STEC that produce Stx2 or a variant of Stx2 [9].
The Stxs are known to act systemically and therefore must transit from the site of STEC colonization in the gastrointestinal tract to the circulatory system (reviewed in [10]). That Stx may also act locally was suggested by an investigation from our laboratory in which we demonstrated that Stx2-expressing E. coli O157:H7 strain 86-24 adhered better to HEp-2 cells in culture and colonized to a greater extent in a mouse model of single organism infection than did its isogenic stx2 null mutant [11]. In that same report, we also demonstrated in vitro that Stx2 increases cell-surface expression of nucleolin, a eukaryotic receptor for the E. coli O157:H7 adhesin intimin [12, 13]. This latter result led to the speculation that Stx2 may augment E. coli O157:H7 adherence through its capacity to increase the number of receptors available for intimin-dependent adherence. Intimin is an outer membrane protein of E. coli O157:H7 and is the primary mediator of adherence for the bacterium [14, 15]. Although the E. coli O157:H7 type III secretion system (TTSS) product called Tir (for translocated intimin receptor), is the critical receptor for E. coli O157:H7 intimin after its injection into the eukaryotic cell, our previously published in vitro and in vivo data strongly suggest that nucleolin may play a role in the initial binding of the organism to the target cell surface before Tir is injected [13, 16].
In this study, we first sought to extend our observation that the wild-type E. coli O157:H7 strain 86-24 colonizes at higher levels in vivo than does its isogenic 86-24 stx2 mutant by feeding mice Stx2 and then assessing whether the 86-24 stx2 mutant colonized better than in animals not fed the toxin. We found that pre-treatment with toxin did enhance the capacity of E. coli O157:H7 to colonize mice with an intact commensal flora. We then tested the impact of anti-Stx2 neutralizing antibody administered passively or induced by active immunization on colonization. We found that anti-toxin not only, as expected, protected mice from the morbidity (as reflected by weight loss) and lethality of E. coli O157:H7 infection, but also reduced the level of colonization by the E. coli O157:H7 challenge strain.
2. Materials & Methods
2.1 Bacterial strains
Wild-type E. coli O157:H7 strain 86-24, first isolated in 1986 during an outbreak in Washington State believed to be linked with contaminated beef products [17], was used for all experiments. E. coli O157:H7 strain 86-24 produces Shiga toxin type 2 (Stx2) only. We made use of a toxin null mutant isogenic to strain 86-24, TUV 86-2. Both the wild-type and the isogenic mutant TUV 86-2 were generously provided by Dr. Arthur Donohue-Rolfe of Tufts University [18]. To aid in recovery and differentiation of the bacterium from normal flora in vivo, strains of both the wild-type and mutant resistant to nalidixic acid (NalR, 25 µg/mL) were used in this investigation. Bacteria were taken directly from a freezer stock and grown on Luria Bertani (LB) plates with nalidixic acid. Subsequently, a single colony was inoculated into LB broth supplemented with nalidixic acid for use as a starter culture. For infection of mice, bacterial starter cultures were diluted 1:100 into larger broth cultures and were grown overnight with aeration prior to concentration by centrifugation and resuspension to 40× in 20% glucose-phosphate buffered saline (PBS).
2.2 Anti-Stx2, normal rabbit serum, and immunoblot procedure
Polyclonal rabbit anti-Stx2 serum [19] was used in passive immunization studies and as a primary probe for the detection of Stx2 toxin or toxoid in immunoblot assays. Normal rabbit serum (NRS, purchased from Rockland Immunochemicals, Inc., Gilbertsville, PA) was used as a control in passive immunization experiments and immunoblot analyses.
Prior to use in animals, samples of the anti-Stx2 antiserum and NRS were pre-cleared against a laboratory strain of E. coli (to remove non-specific anti-E. coli antibodies) as follows. Two volumes of an overnight culture of DH5α were harvested by centrifugation (10 minutes at 5,000 rpm), washed three times in PBS, and resuspended in one volume of the serum sample. This mixture was allowed to incubate end-over end at 37°C for ~2 hours and then the bacteria were pelleted by centrifugation. The resulting supernatant that contained the pre-cleared serum was filtered through a 0.22 µm syringe filter. The filtrate was tested for sterility by spotting 100 µL of the material onto LB agar followed by overnight incubation of the plate at 37°C.
For immunoblot analyses, samples were either spotted directly onto nitrocellulose with the use of a dot blot manifold or electrophoretically mobilized into a sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE). Samples run by SDS-PAGE were transferred to nitrocellulose with a semi-dry transfer apparatus. Once samples were applied to nitrocellulose, the blots were rinsed in PBS-Tween (PBST) then blocked overnight with 5% dry milk in PBST. Blots were washed in PBST prior to incubation in primary antibody (anti-Stx2 or NRS at a dilution of 1:5,000) for ~2 hours. Blots were washed again and incubated for an additional hour in secondary antibody conjugated to horseradish peroxidase (goat anti-rabbit-HRP, Bio-Rad, Hercules, CA). After a final wash, blots were incubated with Amersham Bioscience’s enhanced chemiluminescence (ECL) Plus Western blotting detection reagents (Amersham Bioscience, GE Healthcare Bio-Sciences Corp., Piscataway, NJ). Blots were then developed onto Kodak XAR-5 film (purchased from VWR International, West Chester, PA) using a Series XXXV A Rapid Processor (Ti-Ba Enterprises, Inc., Rochester, NY).
2.3 Mouse infection model
For all experiments, we used an intact commensal flora (ICF) mouse model to study E. coli O157:H7 colonization and pathogenesis [20]. In this E. coli O157:H7 oral infection model, moderate levels of intestinal colonization can be established. Moreover, such manifestations of disease as weight loss and death are evident in at least 30% of E. coli O157:H7-challenged ICF animals. Lastly, renal tubular damage is evident in some infected animals as is an elevation in the level of blood urea nitrogen; these latter two findings are indicative of kidney damage.
The details of the model in brief are as follows. Six week-old female BALB/c mice were purchased from Charles River Labs (Wilmington, MA). The mice were housed in filter-top cages, in a temperature-, light-, and humidity- controlled room. Animals were provided access to food and water ad libitum. To ensure colonization with E. coli O157:H7 upon infection, animals were fasted overnight prior to infection; additionally, water access was restricted 2 hours before infection and returned thereafter. All animal experiments were approved by the Institutional Animal Care and Use Committee at the Uniformed Service University of the Health Sciences.
Mice were infected orally either by pipette feeding or intragastric administration (IG, gavage) with ~109 CFU of E. coli O157:H7 strain 86-24NalR (wild-type) or TUV 86-2 NalR (stx2 mutant) (at a feeding dose of 109 CFU, oral infection by pipette feeding or IG results in essentially equivalent colonization levels [20]). Animals were monitored for E. coli O157:H7 colonization by enumeration of the number of challenge organisms in their feces. Fecal pellets were suspended in PBS (1:10 dilution by weight), homogenized with a wooden stick, and debris pelleted by slow speed centrifugation. The resultant fecal supernatants were diluted and plated on sorbitol MacConkey Agar (SMAC) supplemented with nalidixic acid to determine the colony-forming-units (CFU) shed/g feces. In many experiments animals were housed individually after infection to prevent secondary infection from hyper-shedding animals.
2.4 Stx2 toxin and toxoid
Toxin feeding experiments were done with a histidine-tagged Stx2 protein (called Stx2-6H) that was generated and purified as previously described [11]. The resulting toxin was tested for activity on Vero cells and found to be about 10-fold less toxic than purified Stx2 without the tag. Nevertheless, the specific activity of Stx2-6H was still high [8 × 107 50% cytotoxic doses (CD50)/mg protein].
The clone for expression of a fully inactive Stx2 toxoid with a similar C-terminal 6-histidine tag (Stx2 Y77S E167D-6H) was created as follows. Splicing by overlap extension (SOE) PCR was used to introduce a mutation into the clone that expressed Stx2 E167D-6H [11] so as to ultimately generate a second amino acid change in that toxoid (Y77S). The DNA segments to be linked together with nucleotide changes were amplified by PCR from the Stx2 E167D-6H clone template [forward primer 2Y77S (TCAGTGGCCGGGTTCGTTAATACGG); reverse primer pTrcR (CCAGGCAAATTCTGTTTTATCAGACCGC); forward primer pTrcF (GACAATCTGTGTGGGCACTCGACCGG); reverse primer 2Y77SR (CCGTATTAACGAACCCGGCCACTGATAAATTATTTTGCTCAATAATCAGACGAAGATGGT)]. These upstream and downstream fragments were then connected by SOE PCR with pTrcF and pTrcR as primers. The resulting PCR product was subjected to a double restriction enzyme digest and ligated into pTrcHis2C. Transformants were screened by PCR for the presence of the mutated DNA. That the plasmid DNA inserts from those transformants that were positive by PCR were indeed those of the mutant genotype was confirmed by sequencing. The toxoid was expressed and purified in a similar manner to that of Stx2-6H protein described previously [11].
2.5 Toxin feeding and O157:H7 challenge studies
A crude preparation of Stx2 was made by sonically-disrupting a concentrated overnight culture of 86-24. The lysate was clarified by centrifugation and then filter sterilized. The concentration of Stx2 in the lysate was estimated by comparison with purified Stx2 on Western blot. The lysate was then adjusted to contain 0.1 µg, 1 µg, or 10 µg of Stx2 in 20% glucose-PBS. We selected those toxin amounts based on our knowledge of the load of bacteria present within the cecum of an infected, ICF mouse, 105–106 CFU of E. coli O157:H7 86-24NalR (data not shown); that level of bacteria produces approximately 0.1 µg of Stx2 in vitro. As we thought there might be loss of toxin during transit to the site of infection, we used 1-, 10-, and 100-fold doses (0.1 µg, 1 µg, or 10 µg) of Stx2 to maximize the chance that sufficient toxin was available to affect colonization. Prior to infection and daily thereafter, groups of three mice were fed 20% glucose-PBS alone or one of the lysates that contained Stx2. Mice were infected by pipette feeding with either the wild-type or the stx2 mutant bacteria. Colonization was monitored over the first 9 days post-infection. Colonization levels are reported as the geometric mean (GM) of CFU/g feces after normalization by inoculum load. Specifically, daily CFU counts were normalized to wild-type inocula by division of the CFU of the strain inoculum over the CFU of the inoculum of wild-type.
For the studies in which mice were given purified Stx2, five µg of pure Stx2-6H in Non-Fat Dry Milk (NFDM) or NFDM alone was administered orally, once daily by pipette to groups of 5 mice on days -1 through 7 and again on days 11 through 21. Mice were than challenged with a concentrated culture of E. coli O157:H7 strain 86-24NalR or TUV 86-2 NalR given orally by pipette feeding. Colonization was monitored by fecal shedding (see section 2.3.1) and reported as geometric mean (GM) of CFU/g feces, normalized by inoculum level (see above).
2.6 Assessment for presence of anti-Stx2 serum in the feces of mice
Animals were given a single intraperitoneally (IP)-administered dose of the polyclonal rabbit Stx2 antiserum (described above in section 2.2). Fecal pellets were collected from infected (n=8) and uninfected (n=8) animals at various times post-infection (1, 2, 3, 4, 5 days) and analyzed for the appearance of the Stx2 antibody by an anti-Stx2 ELISA (see section 2.10). Fecal material was also assessed for the presence of toxin by a commercially-available ELISA kit (Premier EHEC ELISA, Meridian diagnostics, Cincinnati, OH) as per the manufacturer’s protocol.
2.7 Passive immunization with anti-Stx2 antiserum or normal rabbit serum
Mice were injected IP with 200 µL of the polyclonal rabbit anti-Stx2 antiserum (described above in section 2.2) or 200 µL of NRS (pre-cleared and filter-sterilized as for the polyclonal anti-Stx2). Mice were then intragastrically infected with wild-type strain 86-24NalR. Animals received two doses of anti-serum (anti-Stx2 or NRS), 24 hours apart prior to infection (at either -24 and -1 hour or at -48 and -24 hours, depending on the study). Following infection, colonization was monitored by fecal shedding (see section 2.3).
2.8 Active immunization with an Stx2 toxoid
Prior to immunization, serum and fecal material were collected from the various groups to serve as a pre-immunization control. Groups of 11 animals were then immunized IP with either 5 µg of the toxoid Stx2 Y77S E167D-6H or PBS mixed 1:1 with the adjuvant TiterMax Gold (TiterMax USA, Inc., Norcross, GA). Animals received an initial injection followed by five boosts at three week intervals.
Three weeks after the fifth boost animals received a final dose of concentrated antigen (200 µg of toxoid in a total volume of ~100 µL) by intragastric (IG) administration. To generate this antigen, purified toxoid was further concentrated by use of a Centriplus centrifugal filtration device with a molecular weight cut-off of 10 kDa (Amicon Bioseperations, Millipore, Billerica, MA). The final toxoid concentration was determined by bicinchoninic acid (BCA) assay (Pierce, Thermo Fisher Scientific, Rockford, IL) in conjunction with an immunoblot analysis that included toxin standards.
After each IP boost and the single IG immunization, fecal pellets and blood from tail vein bleeds were collected from each animal to determine serum and fecal anti-Stx2 titers by a neutralization assay and an ELISA (see sections 2.9 and 2.10). Animals were then infected by gavage with greater than 109 CFU of E. coli O157:H7 strain 86-24NalR and colonization was monitored (see section 2.3).
2.9 Assessment of Stx2-neutralizing antibody capacity in fecal supernatants
Fecal samples were diluted 1:10 by weight into PBS, homogenized, and large debris pelleted. Fecal supernatants were removed, further diluted 1:10 into PBS, and filtered through a 0.80 µm or 0.45 µm syringe filter followed by a 0.22 µm syringe filter. The filtered fecal supernatant material was stored at −20°C prior to use.
The fecal supernatants were analyzed by a Vero cell neutralization of cytotoxicity assay for the presence of anti-toxin antibody. Each fecal sample was pre-incubated (at the 1:100 dilution) with 8 pg of Stx2 diluted into sample at a 1:1 ratio for a final concentration of 4 pg/100 µL for 2 hours at 37°C. Vero cells were then overlaid with 100 µL of the sample/toxin mixture and incubated 40–48 hours at 37°C 5% CO2 prior to fixation and staining in crystal violet. The wells in the Vero plate were read at 630 nm on a spectrophotometer.
2.10 Determination of fecal and serum anti-Stx2 ELISA titers
Serum was obtained from the blood collected prior to E. coli O157:H7 infection. Fecal samples were obtained and processed as described in section 2.9. Microtiter plates (96-well U-bottom) were coated with purified Stx2 (100 ng/well) in PBS overnight. Plates were blocked with 3% bovine serum albumin (BSA) for at least 16 hours prior to use. Before addition of samples, plates were washed in PBST. Separately, serum samples were diluted 1:50 in PBS then further serially diluted at 1:5 increments. Fecal samples were serially diluted 1:2 in intervals from a filtered, 1:100 stock dilution (see section 2.9) or from a fecal supernatant obtained from feces diluted 1:10 by weight (see section 2.6). The sample dilution series was incubated on the pre-blocked, washed plates at 37°C for 2 hours (serum) or at 4°C overnight (fecal). Next, plates were washed in PBST and a 1:3,000 dilution of secondary antibody, either goat anti-mouse IgG or goat anti-mouse IgA both conjugated to HRP, was added to each appropriate well. The plates were then incubated at room temperature for 1 hour and subsequently washed in PBST. Substrate solution [3,3’,5,5’-tetramethylbenzidine (TMB) peroxidase enzyme immunoassay (EIA) substrate kit, Bio-Rad Laboratories, Hercules, CA] was added to the wells of the washed plates and incubated for 15 minutes before the reaction was stopped with 1N sulfuric acid. The plates were then read on a spectrophotometric plate reader at a wavelength of 450 nm. For this assay, polyclonal mouse anti-Stx2 serum was used as a positive control.
2.11 Statistical analyses
Statistical analyses were calculated through application of the statistical software program SPSS v16 (SPSS Inc., Chicago, IL). Specific analyses were performed as described below.
For evaluation of the colonization levels of E. coli O157:H7 wild-type or toxin mutant in the lysate feeding experiment, all data were transformed to a log base 10 scale to meet the assumption for normality. A two-way analysis of variance (ANOVA) was done with the log base 10 CFU/g feces as the dependent variable and both group and day as fixed factors. Main effects of both group and day were investigated.
For most other statistical assessments of colonization levels, individual animals were monitored over time and repeated measures (RM) ANOVA was used to appraise differences in colonization levels among the groups. The RM ANOVA was estimated by a linear mixed model approach to incorporate all available data (to include results obtained from animals that died prior to the conclusion of the study). In these analyses, group was the between subjects factor and day was the within subjects factor. Differences on various days post-infection were then analyzed by means of a one-way ANOVA, with the data split by day post-infection. For the latter analysis, group became the independent variable.
In the evaluation of the systemic effects of passive immunization, the proportion of infected animals that shed toxin in their feces over time was compared by the Log Rank test.
For an assessment of the effect of vaccination on serum and fecal responses by ELISA, we first made use of a nonparametric statistical test on related samples (the Wilcoxon signed-rank test) as our samples failed to meet the assumption for normality even after transforming the data to log base 10. Additionally, we compared the IgG titers, IgA titers, and % neutralization values between the vaccinated and unvaccinated animals by the Mann-Whitney nonparametric statistical test.
3. Results
3.1 Role of Stx2 in E. coli O157:H7 colonization
In our previous study on the impact of Stx2 on colonization of mice by E. coli O157:H7, we found that when a mixture of wild-type 86-24NalR and the toxin null mutant TUV86-2NalR were fed to the animals, toxin produced by the wild-type bacteria complemented the defect in the colonizing capacity of the mutant [11]. Based on that observation, we hypothesized that Stx2 given orally to mice would increase intestinal colonization of a toxin null mutant (Note: US Recombinant DNA guidelines forbid the complementation of such a mutant by transformation with a plasmid that expresses Stx2 holotoxin [21]). To test this theory, we did a preliminary study to determine whether crude toxin preparations supplied exogenously would complement the colonization defect of the toxin null mutant. For that purpose, we fed groups of 3 mice varying dilutions of an Stx2-containing lysate of 86-24NalR such that animals received no toxin (20% glucose-PBS), 0.1 µg, 1 µg, or 10 µg of crude Stx2 prior to infection. The following day, we challenged lysate-treated or 20% glucose-PBS-treated mice orally with TUV86-2NalR and 20%glucose-PBS-treated mice with wild-type 86-24NalR as a control. We continued to administer the Stx2-containing lysates or 20% glucose-PBS alone to the animals daily thereafter while we monitored levels of O157:H7 shed in the feces as a surrogate for intestinal colonization (Fig. 1A). Although in this experiment colonization of either wild-type or the toxin null mutant at day 1 was not as high as expected, we were still able to confirm our previous finding that the mutant alone colonized to a lesser extent than did the wild-type alone (p=0.001). In addition, each lysate that contained toxin increased colonization by the mutant strain (p≤0.001).
Figure 1.
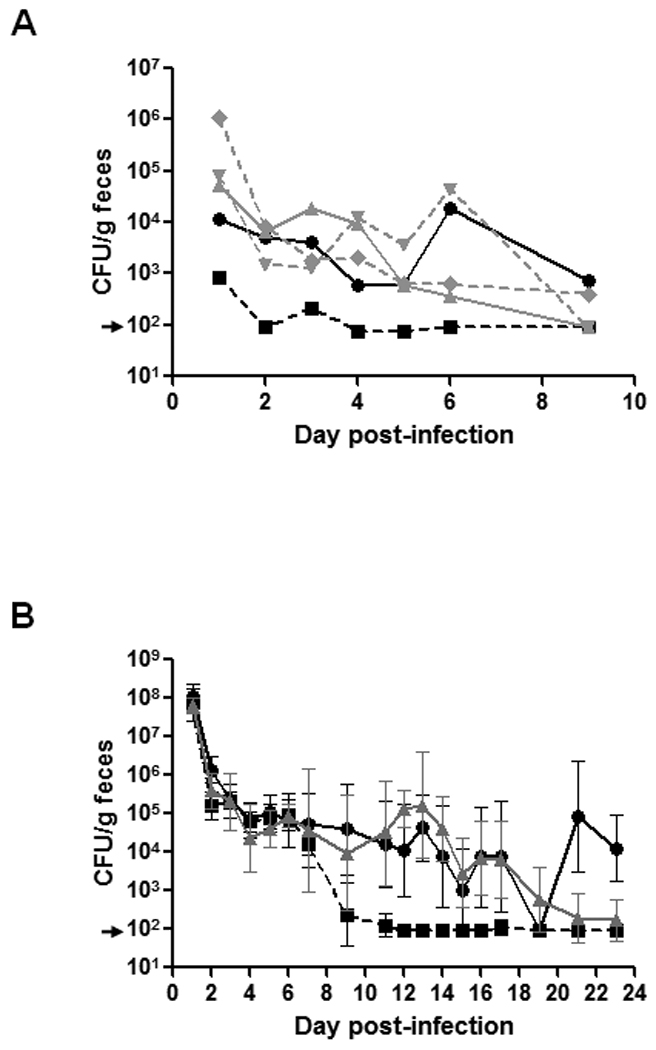
Colonization levels of E. coli O157:H7 stx2 mutant in mice following oral infection in the presence or absence of Stx2 provided in trans. (A) Mice infected with the stx2 mutant of strain 86-24 were orally administered sterile toxin-containing lysate of wild-type strain 86-24 adjusted to contain 0.1 µg (▲), 1.0 µg (♦), or 10 µg (▼) of Stx2. Lysate was given prior to and daily following infection. Control groups included mice that received 20% glucose-PBS alone and were infected with the wild-type (●) or the stx2 mutant (■). Colonization was monitored over a period of 9 days by the CFU shed/g feces. Analysis of the average CFU/g feces indicated that the group infected with wild-type or the groups infected with the stx2 mutant that received toxin-containing lysate had significantly higher levels of colonization (p≤0.001) than the group fed the stx2 mutant alone. (B) Mice orally infected with the stx2 mutant of strain 86-24 were fed 5 µg of purified toxin in NFDM (▲). Control groups of both wild-type (●) and stx2 mutant- (■) infected mice were given NFDM alone. Mice were provided toxin or NFDM alone prior to and following infection on days 0–7 and days 11–21. Colonization was monitored over a period of 23 days and is reported as the GM CFU shed/g feces. The error bars represent the 95% confidence interval. A mixed model RM ANOVA revealed that the stx2 mutant-infected group that received purified Stx2 had statistically higher levels of colonization on days 11–14 (p≤0.025). For all experiments the limit of detection was 102 CFU/g feces (marked by an arrow).
To ensure that Stx2 alone was responsible for the increased colonization of TUV86-2NalR when animals were given the 86-24 lysate, animals were fed 5 µg of purified Stx2-6H in NFDM (mutant treated group) or NFDM alone (mutant or wild-type control groups). As with the lysate-treatment experiments toxin was given 1 day before infection to prime the gastrointestinal tract. The mice were then orally infected by pipette with greater than 109 CFU of either wild-type 86-24NalR or the isogenic toxin null mutant, TUV 86-2NalR. In addition, animals continued to receive toxin in NFDM or NFDM alone (depending on the infection group).
One day after infection, the groups appeared comparably colonized with E. coli O157:H7 organisms, i.e., they shed between 107 – 108 CFU/g feces (Fig. 1 B). In fact, during the first week post-infection, colonization levels were similar [except at day 2 where the wild-type GM CFU/g feces was greater than the mutant (p=0.034)]. This was likely due to the high rate of initial colonization by both strains in this experiment. However, the bacterial load in the mutant-infected, NFDM-alone-treated group began dropping at day 9 and continued to decline, so that by day 12 post-infection this group had colonization levels below the detectable limit. As expected, the wild-type toxin-producing strain colonized to higher levels than did the toxin null mutant throughout the remainder of the experiment. Moreover, when toxin was supplied exogenously with the mutant, significantly higher levels of colonization, comparable to that of wild-type, were achieved than with the mutant alone from days 9–17 of infection. Specifically, on days 11–14 CFU levels were higher in the animals fed purified toxin when compared to the mutant alone by nearly 2 logs or greater (p≤0.025 for days 11–14). Thus, while there was not an obvious difference in colonization pattern early in infection (except at day 2; see above), at later time points toxin administration resulted in increased colonization levels for the stx2 mutant. We speculate that the large number of bacteria present during the first week of infection obscured the beneficial effects of toxin on colonization.
3.2 Impact of passively administered anti-Stx2 serum on O157:H7 colonization
Since exogenously supplied Stx2 increased colonization of TUV86-2, we next tested the impact of passively administered polyclonal rabbit anti-Stx2 serum on colonization by wild-type E. coli O157:H7 strain 86-24NalR. First we confirmed that this antiserum [19] both recognized Stx2 in a Western blot and neutralized the cytotoxic activity of Stx2 toward Vero cells (data not shown). Additionally, when animals received two doses of the antiserum, the anti-Stx2 could be detected in the feces from the majority of animals on day 1, but the number of mice with anti-Stx2 in the feces declined over time (data not shown). However, anti-Stx2 was still detectable in the feces up to 5 days post-immunization in 3/10 mice [day 5 was the latest time point assessed (data not shown)]. The detection of the anti-Stx2 antiserum in the feces of animals after IP administration indicated that at least a portion of antibody provided by this route transited to the gastrointestinal tract. Thus, we felt confident that we could assess the role of passively administered toxin-neutralizing antibody on colonization by wild-type E. coli O157:H7. In a first experiment to examine the effect on 86-24 colonization by the anti-Stx2 serum, groups of 5 animals were inoculated IP with two doses of either NRS or anti-Stx2 antibody and, later, infected by IG administration with about 2 × 109 CFU of E. coli O157:H7. Colonization levels of the animals were monitored by fecal shedding twice daily through day 4 and then once daily from days 5–7 (Fig. 2). The groups demonstrated a comparable level of initial colonization as reflected by the shedding of approximately 107 CFU/g feces on day 1 post-challenge. However, on days 3–5 post-infection, animals that received the anti-Stx2 antiserum had lower geometric mean colonization levels than did control mice (p<0.05). By day 7 post-infection, the colonization levels of both groups had dropped to just over 103 CFU/ g feces. We repeated these studies on two occasions and found a similar trend (data not shown).
Figure 2.
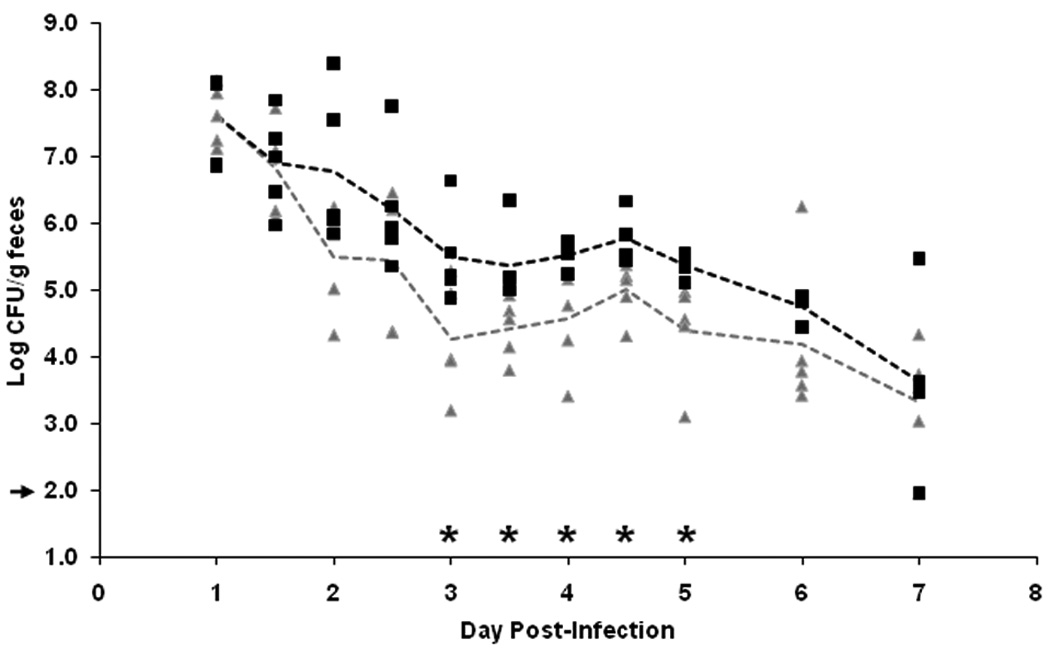
Impact of passively administered anti-Stx2 neutralizing antibodies on E. coli O157:H7 strain 86-24 colonization level. Mice received polyclonal rabbit anti-Stx2 serum (▲) or NRS (■) twice by IP administration prior to infection. Colonization was monitored following infection, twice daily for the first 4 days, and then once daily through day 7. Colonization was determined by enumerating the CFU shed/g feces and is reported for each animal (n=5). The dashed lines represent the GM for the anti-Stx2 (gray) and the NRS (black) treatment groups. Asterisks indicate specific days when statistically different colonization levels were observed. The limit of detection was 102 CFU/g feces (marked by an arrow).
3.3 Local and systemic impact of passively administered anti-Stx2 serum on toxicity of Stx2 delivered by E. coli O157
In the above study, a severely moribund infected animal within the NRS group was sacrificed to prevent undue suffering. We suspected that this mouse died due to the production of Stx2 by the infecting bacteria. Therefore, in a separate study we asked whether we could demonstrate neutralization of toxin made in vivo in the gut by the passively administered anti-Stx2 antibody. To address that question, groups of 8 mice were administered anti-Stx2 serum or NRS (as a control), challenged IG with 86-24NalR, and feces from the animals were collected for 5 days following infection. Supernatants of these fecal samples were evaluated by ELISA for rabbit anti-Stx2 antiserum or Stx2. Anti-Stx2 antibodies were detected in the feces of infected animals that received anti-Stx2 serum but, as expected, not in the feces of animals that received NRS (Fig. 3A). There was also a difference in detection of Stx2 in the feces of animals from both groups (Fig. 3B). Fewer mice given anti-Stx2 antibody had detectable Stx2 within their feces on day 1 post-infection than did mice given NRS. Moreover, by day 2 post-infection, mice given anti-Stx2 antibody had no detectable Stx2 in their feces while a portion of mice administered NRS still had measurable levels of Stx2 through day 3 of infection (Fig. 3B). Thus, a greater proportion of infected mice given NRS shed Stx2 in their feces over time in comparison to animals given anti-Stx2 (p=0.032). These findings suggest that the anti-Stx2 antibody at least partially bound the toxin produced at the site of infection within the gastrointestinal tract.
Figure 3.
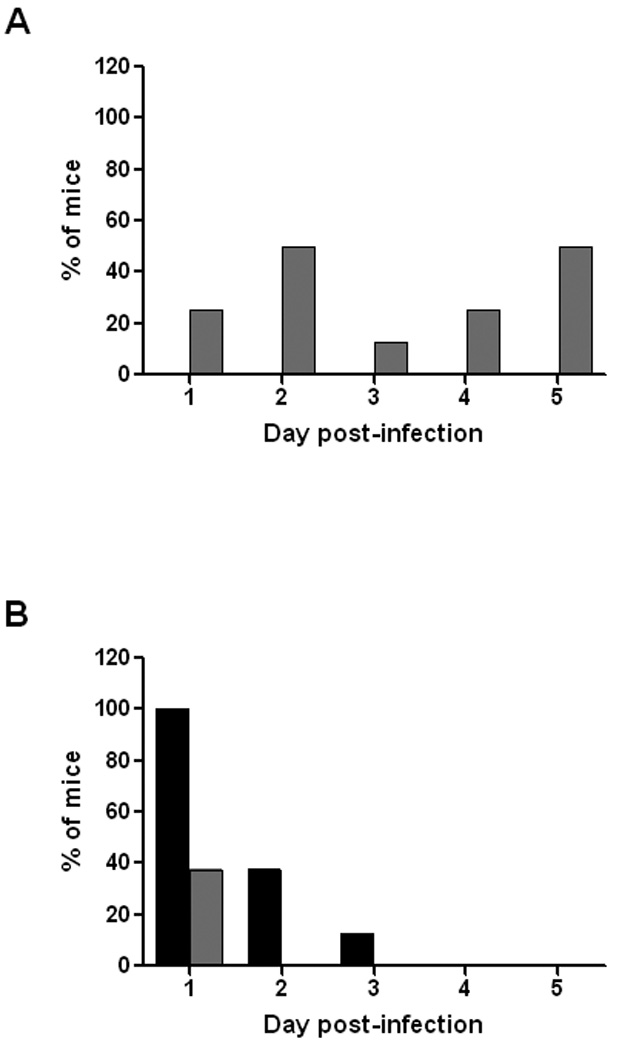
Post-infection detection of passively administered anti-Stx2 antibody or Stx2 produced by the infecting wild-type E. coli O157:H7 strain 86-24. The group of mice given NRS is depicted by black bars and the animals administered anti-Stx2 are represented by gray bars. (A) Percentage of mice (n=8) from the NRS and anti-Stx2 administration group with detectable anti-Stx2 antibody shed into the feces after E. coli O157:H7 infection. No detectable anti-Stx2 antibody was seen in the feces of mice administered NRS. (B) Percentage of mice (n=8) from the NRS and anti-Stx2 administration group that shed detectable levels of Stx2 into the feces post-infection.
In the experiment described above, animals that received NRS lost weight [in comparison to mice given anti-Stx2 serum (Fig. 4A)] and 7 of 8 mice succumbed to infection between days 4 and 5 (Fig. 4B). Overall, the cumulative lethality data from all passive transfer experiments were as follows: 12/38 mice given NRS and then infected with 86-24NalR died versus 0/43 mice treated with anti-Stx2 serum and then challenged with the bacterium. Therefore, passively administered anti-toxin antibody not only reduced the bacterial burden of E. coli O157:H7 in the gut but also protected the infected animals from Stx2-mediated death.
Figure 4.
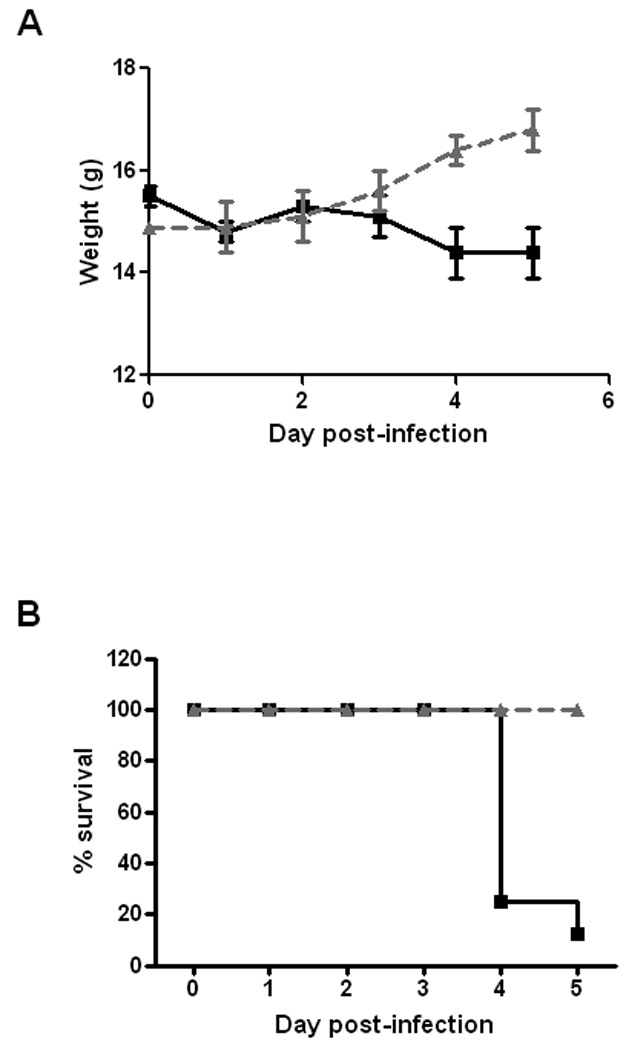
Effect of passive administration of rabbit anti-Stx2 serum or NRS on weight and survival of mice following E. coli O157:H7 infection. Groups of 8 animals received either two IP injections of anti-Stx2 antibody (▲) or NRS (■) prior to oral infection with wild-type E. coli O157:H7 strain 86-24. (A) Weight was monitored following infection and is displayed as the average of the group in grams. Animals that succumbed to infection were subsequently listed at their last recorded weight. Bars indicate the standard error of the mean. (B) Percentage of animals surviving by day post-E. coli O157:H7 infection.
3.3 Effect of active immunization with Stx2 toxoid on serum and fecal anti-Stx2 responses and on colonization
Next, we assessed the impact of active immunization with an Stx2 toxoid on E. coli O157:H7 colonization. From a pilot study, we knew that a lengthy immunization protocol would be required to detect an Stx2-neutralizing antibody response in the feces and that such a response was necessary to reduce colonization by the challenge strain (data not shown). Therefore, mice were given Stx2 Y77S E167D-6H over a series of inoculations (see Materials and Methods for details of procedure). Fecal and serum samples obtained after boosts number 5 (last IP immunization) and 6 (IG administration) were then analyzed for the presence of anti-Stx2 antibodies by ELISA and by the capacity to neutralize Stx2 on Vero cells (Fig. 5). High titers of anti-Stx2 IgG antibodies were present in the sera from all vaccinated mice by boost 5 with lower level titers seen in the unvaccinated animals (Fig. 5A, p<0.001). Much lower ELISA titers of anti-Stx2 IgG (Fig. 5B) and IgA (Fig. 5C) antibodies were detected in fecal homogenates, with no significant difference observed between the vaccinated and unvaccinated groups. In addition, antibodies were only detected from the feces of a proportion of the animals (Fig. 5B and C). Furthermore, IG administration of a concentrated amount of toxoid antigen (boost 6) did not result in significantly increased ELISA titers (Fig. 5B and C). Nevertheless, the fecal homogenates from some of the vaccinated animals after boost 5 and more of the mice after boost 6 had the capacity to ablate the cytotoxicity of Stx2 on Vero cells (Fig. 5D), an indication of Stx2-neutralizing antibody in those samples. In fact, the difference in % neutralization of fecal homogenates between the vaccinated and unvaccinated groups was near statistical significance following boost 6 (p=0.065).
Figure 5.
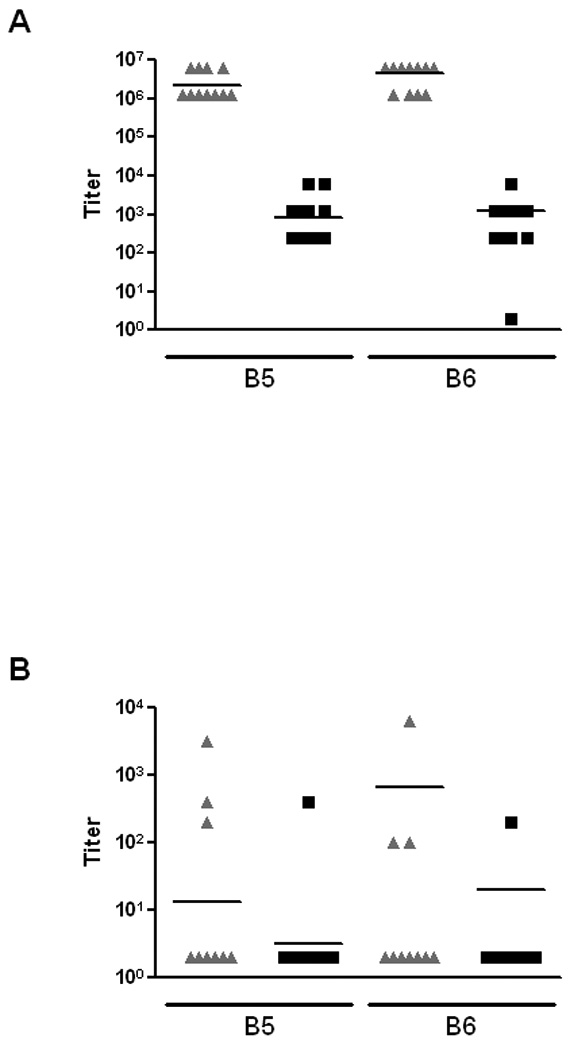
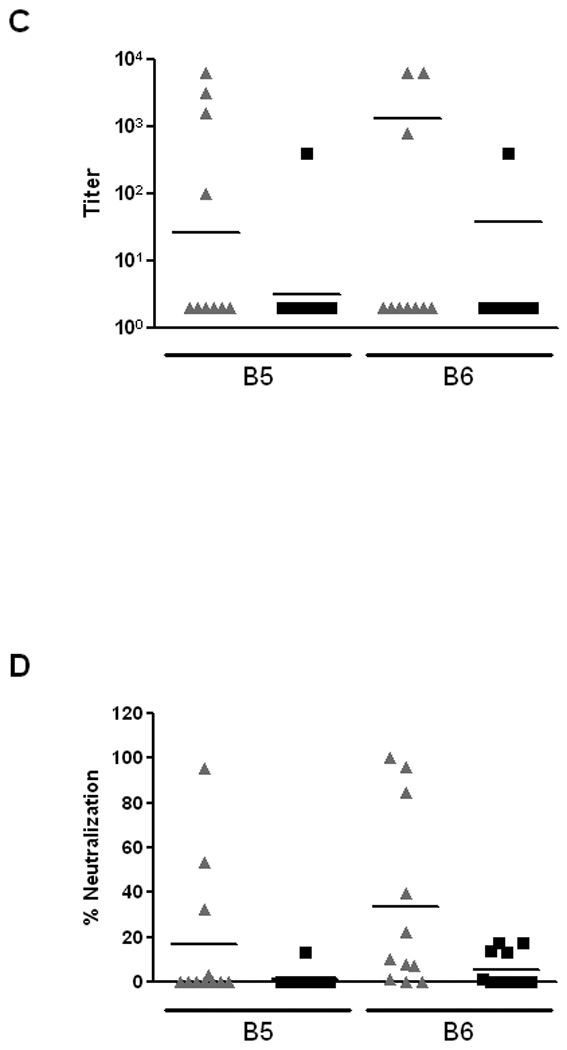
Evaluation of anti-Stx2 antibody response in serum or fecal filtrates by ELISA and in fecal filtrates by the capacity to neutralize Stx2 on Vero cells. Samples were obtained from mice following the last IP-immunization (boost 5, B5) and again after IG administration (boost 6, B6) of toxoid (▲) or PBS (■). (A) Serum IgG titers of Stx2 antibodies in vaccinated or mock-vaccinated mice. (B) Fecal IgG titers of Stx2 antibodies in vaccinated or mock-vaccinated mice. (C) Fecal IgA titers of Stx2 antibodies in vaccinated or mock-vaccinated mice. (D) Percent neutralization of 4 pg of Stx2 (~ 1–2 50% cytotoxic doses or CD50s) by fecal filtrates from vaccinated or mock-vaccinated mice.
Following the detection of toxin-neutralizing antibodies in the feces, mice were infected with 2.8 × 109 CFU of wild-type strain 86-24NalR and colonization was monitored by fecal shedding (Fig. 6). Fecal shedding of 86-24NalR on day 1 post-infection was comparable between groups. Thereafter, the vaccinated group showed a steady decline in colonization levels out as far as two weeks post-infection, and the bacterial burden of the group remained below 103 CFU/g feces for the remainder of the experiment. The unvaccinated group displayed an overall decline in colonization levels past day 3 post-infection but also seemed to undergo cyclical rebounds in colonization levels (seen on days 3, 7, 10, 16, and 17) before the GM CFU/g feces in that group finally dropped below 103 on day 22 and remained there for the duration of the experiment. Thus, as was seen with passive immunization, active immunization against Stx2 resulted in decreased colonization levels (p=0.048 by mixed model, RM ANOVA; one-way ANOVA demonstrated statistical differences from the control on days 3 and 10) and a shorter duration of colonization (p=0.047) as compared to the controls. However, unlike the passive experiments, no systemic disease (emaciation, fur ruffling, unsteady gait, or death) was apparent in any of the mice. This absence of an obvious toxic effect from Stx2 may reflect the fact that these mice were considerably older and, thus, heavier (presumably with a lower toxin to body weight ratio) than the animals used in the passive immunization experiments.
Figure 6.
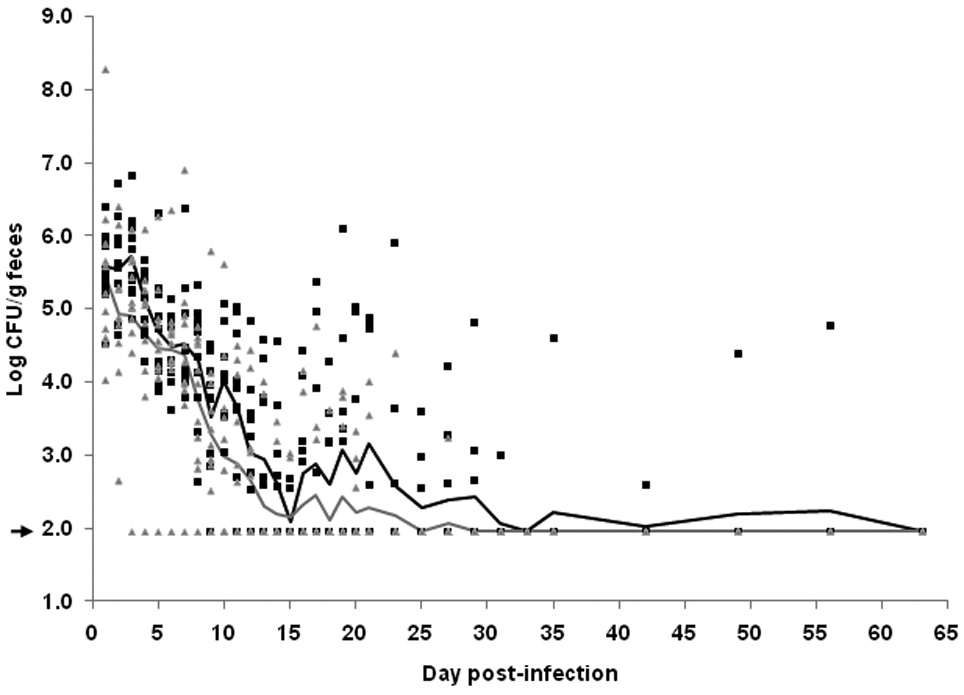
Effect of active immunization with Stx2 toxoid on E. coli O157:H7 colonization levels. Mice were vaccinated with Stx2 toxoid (▲) or mock-vaccinated with PBS (■). Colonization was monitored daily following infection for the first 21 days, then every other day through day 35, and then weekly through day 63 post-infection. Colonization was determined by enumeration of the CFU shed/g feces and is reported for each animal (n=10–11/group). The lines represent the GM for the vaccinated (gray) and the mock-vaccinated (black) group. The limit of detection was 102 CFU/g feces (marked by an arrow).
4. Discussion
Three major findings were derived from this investigation. First, we demonstrated that Stx2 provided exogenously to mice challenged with an E. coli O157:H7 stx2 mutant enhanced the intestinal colonizing capacity of that mutant. Second, we showed that Stx2-neutralizing antibodies administered passively to mice reduced the burden of wild-type, Stx2-expressing E. coli O157:H7 and protected animals from death. Third, we provided evidence that active immunization with an Stx2 toxoid resulted in toxin-neutralizing antibodies in the gut and reduced the level of wild-type, Stx2-expressing E. coli O157:H7 shed in the feces over time compared to unvaccinated, infected controls.
The notion that Stx plays a role in colonization came from work that suggested a rabbit diarrheagenic E. coli strain transfected with a phage that encodes Stx1 not only caused more serious disease than the control strain, but was also more enteroadherent [22]. In 2006, our laboratory presented evidence that Stx2 plays a role in E. coli O157:H7 adherence in vitro and colonization in vivo [11]. Subsequently, other investigators reported that Stxs can augment colonization of E. coli O157:H7 [23–28]. Conversely, several groups of researchers found no such role for toxin [29–33]. However, in those latter studies, a variety of factors may have affected the results. For example, introduction of the organism by a route that bypassed most of the gut [31] or the use of animals with no or reduced flora [33] could preclude detection of a role for toxin. Additionally, the conclusions in some of the reports are based on either a single time-point post-infection [29] or a limited temporal analysis [32, 33]. Finally, in one instance the authors excluded a role for Stx in gut adherence yet only measured colonization levels of an Stx-negative E. coli O157:H7 strain without inclusion of a wild-type toxigenic strain for comparison [30].
The findings described here and elsewhere suggest that Stxs can promote bacterial colonization by E. coli O157:H7 [11, 23–28]. The discovery that toxin plays a role in colonization by bacteria appears to be generalizable to a broader group of toxin-producing enteric microbes. In fact, there are reports of a role in gastrointestinal colonization for heat-labile toxin of enterotoxigenic E. coli (ETEC) [34, 35], the accessory toxins of Vibrio cholerae [36], VacA toxin of Helicobacter pylori [37, 38], and, most recently, Clostridium difficile transferase (CDT) toxin of C. difficile [39]. The mechanisms by which toxin directly or indirectly promote colonization in the gastrointestinal tract likely differ among these pathogens and are not fully understood. Some possible explanations include: a toxin-mediated increase in the level of a bacterial receptor on the surface of eukaryotic cells (as we have suggested for the effect of Stx2 on a eukaryotic intimin receptor, nucleolin), the capacity of toxin to directly function as a bacterial adhesion molecule, an indirect increase of/exposure to receptor as a result of toxin-mediated cell death, a toxin-promoted host-cell cytoskeletal change required for bacterial adherence, and/or a dampening of the host response in favor of initial colonization [35, 36]. Regardless of the mechanism, toxin production may either be required to augment colonization or simply provide a competitive advantage to the toxin-producing bacterium (see review by [38]).
Because Stx plays a role in both colonization and systemic disease, we propose the use of either toxoid vaccination or passive administration of anti-Stx antibodies to reduce or prevent STEC-mediated disease in people. The transfer of specific antibodies or anti-serum to patients is a well-established strategy to prevent or treat certain infectious disease agents or toxins (reviewed in [40, 41]). That anti-Stx2 antibody might be of use in the treatment of the serious consequences of E. coli O157:H7 infection was first demonstrated by Wadolkowski et al. in a mouse model [42]; in that and other early studies, such antibody was given before infection [42–44]. In subsequent evaluations of the efficacy of such passive therapy, anti-Stx antibodies were shown to provide protection against STEC-mediated illness and death even when given up to 4 days after bacterial challenge [44–47]. In this study, we found that anti-Stx2 antiserum administered IP prior to E. coli O157:H7 challenge prevented both weight loss and death of the infected mice. We also found that such treatment decreased the likelihood that mice would become highly colonized with E. coli O157:H7. To the best of our knowledge, the latter observation is a novel finding about the impact of passively administered anti-Stx antibodies in an animal model.
Several groups of investigators, including our own, have reported that active immunization with an Stx toxoid by parenteral [48–50] or oral routes [51] protects animals from the systemic manifestations of STEC disease. In a more recent paper by Zhu et al., StxB1 was used to immunize rabbits through a transcutaneous procedure. The animals were then challenged with an Stx1-producing rabbit diarrheagenic E. coli strain [52]. Immunized animals demonstrated systemic protection in the form of enhanced weight gain and a reduction in the histopathological effects of intoxication and also showed a statistically significant decrease in enteroadherent bacteria on the surface of their cecal tissues when compared to both naïve and adjuvant-alone-treated controls [52]. The later finding is consistent with our observation that active immunization against Stx2 resulted in decreased levels of E. coli O157:H7 colonization of mice after infection when compared to mock-vaccinated control.
In sum, our results suggest that a toxoid-based vaccine against STEC would be beneficial for two reasons. First, such a vaccine would prevent the systemic toxicity due to the Stxs that can lead to such serious consequences as HUS. Second, a toxoid-based vaccine would reduce the bacterial burden of E. coli O157:H7 in the gastrointestinal tract if infection should occur and would thus decrease the level of toxin made in vivo as well as the likelihood of person-to-person transmission of this serious food-borne pathogen.
Acknowledgments
The authors would like to thank Farhang Alem for his technical assistance with intragastric administration and Dr. Cara Olsen for her advice and assistance with the statistical analyses. This work was funded by the National Institutes of Health/National Institute for Allergy and Infectious Diseases grant number 5R37 AI20148 and the Uniformed Services University of the Health Sciences intramural student funding number T073MR-02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, et al. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5(5):607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gould LH, Demma L, Jones TF, Hurd S, Vugia DJ, Smith K, et al. Hemolytic uremic syndrome and death in persons with Escherichia coli O157:H7 infection, foodborne diseases active surveillance network sites, 2000–2006. Clin Infect Dis. 2009;49(10):1480–1485. doi: 10.1086/644621. [DOI] [PubMed] [Google Scholar]
- 3.Manning SD, Motiwala AS, Springman AC, Qi W, Lacher DW, Ouellette LM, et al. Variation in virulence among clades of Escherichia coli O157:H7 associated with disease outbreaks. Proc Natl Acad Sci U S A. 2008;105(12):4868–4873. doi: 10.1073/pnas.0710834105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC. Update on Multi-State Outbreak of E. coli O157:H7 Infections From Fresh Spinach, October 6, 2006. E coli O157:H7 Outbreak from Fresh Spinach. [October 6, 2006];2006 [cited 2009 October 28]; Available from: http://www.cdc.gov/foodborne/ecolispinach/100606.htm.
- 5.CDC. Multistate Outbreak of E. coli O157:H7 Infections Linked to Eating Raw Refrigerated Prepackaged Cookie Dough. [August 7, 2009];2009 doi: 10.1093/cid/cir831. [cited 2009; Available from: http://www.cdc.gov/ecoli/2009/0807.html. [DOI] [PubMed]
- 6.Karmali MA, Petric M, Lim C, Fleming PC, Arbus GS, Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985;151(5):775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 7.Obrig TG. Shiga toxin mode of action in E. coli O157:H7 disease. Front Biosci. 1997;2:d635–d642. doi: 10.2741/a219. [DOI] [PubMed] [Google Scholar]
- 8.Melton-Celsa AR, Smith MJ, O'Brien AD. Shiga Toxins: Potent Poisons, Pathogenicity Determinants, and Pharmacological Agents. In: Bock A, Curtiss R III, Kaper JB, Karp PD, Neidhardt FC, Nystrom T, et al., editors. EcoSal - Escherichia coli and Salmonella: Cellular and Molecular Biology. Washington, DC: ASM Press; 2005. Mar 29, posting date. [Google Scholar]
- 9.Gerner-Smidt P, Hyytia-Trees E, MacCannell D, Leeper MM, Gould LH, Lanier WA. Molecular Surveillence of Shiga toxin-producing Escherichia coli - the American experience. 3rd Pathogenic Escherichia coli Network (PEN) Conference, entitled Epidemiology and Transmission of VTEC and other Pathogenic Escherichia coli; 2008; pp. 78–82. [Google Scholar]
- 10.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11(1):142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson CM, Sinclair JF, Smith MJ, O'Brien AD. Shiga toxin of enterohemorrhagic Escherichia coli type O157:H7 promotes intestinal colonization. Proc Natl Acad Sci U S A. 2006;103(25):9667–9672. doi: 10.1073/pnas.0602359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinclair JF, O'Brien AD. Cell surface-localized nucleolin is a eukaryotic receptor for the adhesin intimin-gamma of enterohemorrhagic Escherichia coli O157:H7. J Biol Chem. 2002;277(4):2876–2885. doi: 10.1074/jbc.M110230200. [DOI] [PubMed] [Google Scholar]
- 13.Sinclair JF, Dean-Nystrom EA, O'Brien AD. The established intimin receptor Tir and the putative eucaryotic intimin receptors nucleolin and beta1 integrin localize at or near the site of enterohemorrhagic Escherichia coli O157:H7 adherence to enterocytes in vivo. Infect Immun. 2006;74(2):1255–1265. doi: 10.1128/IAI.74.2.1255-1265.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnenberg MS, Tzipori S, McKee ML, O'Brien AD, Alroy J, Kaper JB. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J Clin Invest. 1993;92(3):1418–1424. doi: 10.1172/JCI116718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKee ML, Melton-Celsa AR, Moxley RA, Francis DH, O'Brien AD. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect Immun. 1995;63(9):3739–3744. doi: 10.1128/iai.63.9.3739-3744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinclair JF, O'Brien AD. Intimin types alpha, beta, and gamma bind to nucleolin with equivalent affinity but lower avidity than to the translocated intimin receptor. J Biol Chem. 2004;279(32):33751–33758. doi: 10.1074/jbc.M401616200. [DOI] [PubMed] [Google Scholar]
- 17.Ostroff SM, Griffin PM, Tauxe RV, Shipman LD, Greene KD, Wells JG, et al. A statewide outbreak of Escherichia coli O157:H7 infections in Washington State. Am J Epidemiol. 1990;132(2):239–247. doi: 10.1093/oxfordjournals.aje.a115653. [DOI] [PubMed] [Google Scholar]
- 18.Gunzer F, Bohn U, Fuchs S, Muhldorfer I, Hacker J, Tzipori S, et al. Construction and characterization of an isogenic slt-ii deletion mutant of enterohemorrhagic Escherichia coli. Infect Immun. 1998;66(5):2337–2341. doi: 10.1128/iai.66.5.2337-2341.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokai-Kun JF, Melton-Celsa AR, O'Brien AD. Elastase in intestinal mucus enhances the cytotoxicity of Shiga toxin type 2d. J Biol Chem. 2000;275(5):3713–3721. doi: 10.1074/jbc.275.5.3713. [DOI] [PubMed] [Google Scholar]
- 20.Mohawk KL, Melton-Celsa AR, Zangari T, Carroll EE, O'Brien AD. Pathogenesis of Escherichia coli O157:H7 strain 86-24 following oral infection of BALB/c mice with an intact commensal flora. Microb Pathog. 2010;48(3–4):131–142. doi: 10.1016/j.micpath.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Services DoHaH., editor. NIH guidlines for research invovling recombinant DNA molecules. 2009. Sep, [Google Scholar]
- 22.Sjogren R, Neill R, Rachmilewitz D, Fritz D, Newland J, Sharpnack D, et al. Role of Shiga-like toxin I in bacterial enteritis: comparison between isogenic Escherichia coli strains induced in rabbits. Gastroenterology. 1994;106(2):306–317. doi: 10.1016/0016-5085(94)90587-8. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman MA, Menge C, Casey TA, Laegreid W, Bosworth BT, Dean-Nystrom EA. Bovine immune response to shiga-toxigenic Escherichia coli O157:H7. Clin Vaccine Immunol. 2006;13(12):1322–1327. doi: 10.1128/CVI.00205-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calderon Toledo C, Rogers TJ, Svensson M, Tati R, Fischer H, Svanborg C, et al. Shiga toxin-mediated disease in MyD88-deficient mice infected with Escherichia coli O157:H7. Am J Pathol. 2008;173(5):1428–1439. doi: 10.2353/ajpath.2008.071218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dean-Nystrom EA, Stoffregen WC, Bosworth BT, Moon HW, Pohlenz JF. Early attachment sites for Shiga-toxigenic Escherichia coli O157:H7 in experimentally inoculated weaned calves. Appl Environ Microbiol. 2008 Oct;74(20):6378–6384. doi: 10.1128/AEM.00636-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baines D, Erb S, McAllister T. Stx2 from enterohemorrhagic Escherichia coli O157:H7 promotes colonization in the intestine of cattle. Canadian Journal of Animal Science. 2008;88:581–584. [Google Scholar]
- 27.Yin X, Chambers JR, Wheatcroft R, Johnson RP, Zhu J, Liu B, et al. Adherence of Escherichia coli O157:H7 mutants in vitro and in ligated pig intestines. Appl Environ Microbiol. 2009;75(15):4975–4983. doi: 10.1128/AEM.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowe RM, Baines D, Selinger LB, Thomas JE, McAllister TA, Sharma R. Escherichia coli O157:H7 strain origin, lineage, and Shiga toxin 2 expression affect colonization of cattle. Appl Environ Microbiol. 2009;75(15):5074–5081. doi: 10.1128/AEM.00391-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritchie JM, Thorpe CM, Rogers AB, Waldor MK. Critical roles for stx2, eae, and tir in enterohemorrhagic Escherichia coli-induced diarrhea and intestinal inflammation in infant rabbits. Infect Immun. 2003;71(12):7129–7139. doi: 10.1128/IAI.71.12.7129-7139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Best A, La Ragione RM, Cooley WA, O'Connor CD, Velge P, Woodward MJ. Interaction with avian cells and colonisation of specific pathogen free chicks by Shiga-toxin negative Escherichia coli O157:H7 (NCTC 12900) Vet Microbiol. 2003;93(3):207–222. doi: 10.1016/s0378-1135(03)00031-2. [DOI] [PubMed] [Google Scholar]
- 31.Sheng H, Lim JY, Knecht HJ, Li J, Hovde CJ. Role of Escherichia coli O157:H7 virulence factors in colonization at the bovine terminal rectal mucosa. Infect Immun. 2006;74(8):4685–4693. doi: 10.1128/IAI.00406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornick NA, Helgerson AF, Sharma V. Shiga toxin and Shiga toxin-encoding phage do not facilitate Escherichia coli O157:H7 colonization in sheep. Appl Environ Microbiol. 2007;73(1):344–346. doi: 10.1128/AEM.01328-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eaton KA, Friedman DI, Francis GJ, Tyler JS, Young VB, Haeger J, et al. Pathogenesis of renal disease due to enterohemorrhagic Escherichia coli in germ-free mice. Infect Immun. 2008;76(7):3054–3063. doi: 10.1128/IAI.01626-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berberov EM, Zhou Y, Francis DH, Scott MA, Kachman SD, Moxley RA. Relative importance of heat-labile enterotoxin in the causation of severe diarrheal disease in the gnotobiotic piglet model by a strain of enterotoxigenic Escherichia coli that produces multiple enterotoxins. Infect Immun. 2004;72(7):3914–3924. doi: 10.1128/IAI.72.7.3914-3924.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen KP, Randolph MM, Fleckenstein JM. Importance of heat-labile enterotoxin in colonization of the adult mouse small intestine by human enterotoxigenic Escherichia coli strains. Infect Immun. 2006;74(2):869–875. doi: 10.1128/IAI.74.2.869-875.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olivier V, Salzman NH, Satchell KJ. Prolonged colonization of mice by Vibrio cholerae El Tor O1 depends on accessory toxins. Infect Immun. 2007;75(10):5043–5051. doi: 10.1128/IAI.00508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salama NR, Otto G, Tompkins L, Falkow S. Vacuolating cytotoxin of Helicobacter pylori plays a role during colonization in a mouse model of infection. Infect Immun. 2001;69(2):730–736. doi: 10.1128/IAI.69.2.730-736.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cover TL, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol. 2005;3(4):320–332. doi: 10.1038/nrmicro1095. [DOI] [PubMed] [Google Scholar]
- 39.Schwan C, Stecher B, Tzivelekidis T, van Ham M, Rohde M, Hardt WD, et al. Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog. 2009;5(10) doi: 10.1371/journal.ppat.1000626. e1000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keller MA, Stiehm ER. Passive immunity in prevention and treatment of infectious diseases. Clin Microbiol Rev. 2000;13(4):602–614. doi: 10.1128/cmr.13.4.602-614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casadevall A. Passive antibody administration (immediate immunity) as a specific defense against biological weapons. Emerg Infect Dis. 2002;8(8):833–841. doi: 10.3201/eid0808.010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wadolkowski EA, Sung LM, Burris JA, Samuel JE, O'Brien AD. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect Immun. 1990;58(12):3959–3965. doi: 10.1128/iai.58.12.3959-3965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karpman D, Connell H, Svensson M, Scheutz F, Alm P, Svanborg C. The role of lipopolysaccharide and Shiga-like toxin in a mouse model of Escherichia coli O157:H7 infection. J Infect Dis. 1997;175(3):611–620. doi: 10.1093/infdis/175.3.611. [DOI] [PubMed] [Google Scholar]
- 44.Donohue-Rolfe A, Kondova I, Mukherjee J, Chios K, Hutto D, Tzipori S. Antibody-based protection of gnotobiotic piglets infected with Escherichia coli O157:H7 against systemic complications associated with Shiga toxin 2. Infect Immun. 1999;67(7):3645–3648. doi: 10.1128/iai.67.7.3645-3648.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matise I, Cornick NA, Booher SL, Samuel JE, Bosworth BT, Moon HW. Intervention with Shiga toxin (Stx) antibody after infection by Stx-producing Escherichia coli. J Infect Dis. 2001;183(2):347–350. doi: 10.1086/317930. [DOI] [PubMed] [Google Scholar]
- 46.Sheoran AS, Chapman-Bonofiglio S, Harvey BR, Mukherjee J, Georgiou G, Donohue-Rolfe A, et al. Human antibody against shiga toxin 2 administered to piglets after the onset of diarrhea due to Escherichia coli O157:H7 prevents fatal systemic complications. Infect Immun. 2005;73(8):4607–4613. doi: 10.1128/IAI.73.8.4607-4613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamagami S, Motoki M, Kimura T, Izumi H, Takeda T, Katsuura Y, et al. Efficacy of postinfection treatment with anti-Shiga toxin (Stx) 2 humanized monoclonal antibody TMA-15 in mice lethally challenged with Stx-producing Escherichia coli. J Infect Dis. 2001;184(6):738–742. doi: 10.1086/323082. [DOI] [PubMed] [Google Scholar]
- 48.Gordon VM, Whipp SC, Moon HW, O'Brien AD, Samuel JE. An enzymatic mutant of Shiga-like toxin II variant is a vaccine candidate for edema disease of swine. Infect Immun. 1992;60(2):485–490. doi: 10.1128/iai.60.2.485-490.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bosworth BT, Samuel JE, Moon HW, O'Brien AD, Gordon VM, Whipp SC. Vaccination with genetically modified Shiga-like toxin IIe prevents edema disease in swine. Infect Immun. 1996;64(1):55–60. doi: 10.1128/iai.64.1.55-60.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacLeod DL, Gyles CL. Immunization of pigs with a purified Shiga-like toxin II variant toxoid. Vet Microbiol. 1991;29(3–4):309–318. doi: 10.1016/0378-1135(91)90138-6. [DOI] [PubMed] [Google Scholar]
- 51.Wen SX, Teel LD, Judge NA, O'Brien AD. A plant-based oral vaccine to protect against systemic intoxication by Shiga toxin type 2. Proc Natl Acad Sci U S A. 2006;103(18):7082–7087. doi: 10.1073/pnas.0510843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu C, Yu J, Yang Z, Davis K, Rios H, Wang B, et al. Protection against Shiga toxin-producing Escherichia coli infection by transcutaneous immunization with Shiga toxin subunit B. Clin Vaccine Immunol. 2008;15(2):359–366. doi: 10.1128/CVI.00399-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


