Abstract
Activation of trigeminal nerves and release of neuropeptides that promote inflammation are implicated in the underlying pathology of migraine and temporomandibular joint (TMJ) disorders. The overall response of trigeminal nerves to peripheral inflammatory stimuli involves a balance between enzymes that promote inflammation, kinases, and those that restore homeostasis, phosphatases. The goal of this study was to determine the effects of a cocoa-enriched diet on the expression of key inflammatory proteins in trigeminal ganglion neurons under basal and inflammatory conditions. Rats were fed a control diet or an isocaloric diet enriched in cocoa for 14 days prior to an injection of noxious stimuli to cause acute or chronic excitation of trigeminal neurons. In animals fed a cocoa-enriched diet, basal levels of the mitogen-activated kinase (MAP) phosphatases MKP-1 and MKP-3 were elevated in neurons. Importantly, the stimulatory effects of acute or chronic peripheral inflammation on neuronal expression of the MAPK p38 and extracellular signal-regulated kinases (ERK) were significantly repressed in response to cocoa. Similarly, dietary cocoa significantly suppressed basal neuronal expression of calcitonin gene-related peptide (CGRP) as well as stimulated levels of the inducible form of nitric oxide synthase (iNOS), proteins implicated in the underlying pathology of migraine and TMJ disorders. To our knowledge, this is first evidence that a dietary supplement can cause upregulation of MKP, and that cocoa can prevent inflammatory responses in trigeminal ganglion neurons. Furthermore, our data provide evidence that cocoa contains biologically active compounds that would be beneficial in the treatment of migraine and TMJ disorders.
Keywords: Cocoa, Trigeminal, Inflammation, Headache, temporomandibular joint disorders, MAPK, MKP
1. Introduction
Diseases involving inflammation and pain in the head and face such as migraine and temporomandibular joint (TMJ) disorders are reported to be some of the most debilitating human conditions (Carlsson, 1995; Lipton, 1993; Sessle, 1987). Migraine is a common neurovascular disease characterized by intense head pain that can persist for as long as 72 hours that afflicts 12% of adults in the United States (Ferrari, 1998; Lipton et al., 2001). Similarly, TMJ disorders involve complex symptoms of pain and dysfunction of the masticatory system, which includes the TMJ, muscles of mastication and the adjacent tissues (Herb et al., 2006), and affects >13 million people in the United States (Isong et al., 2008). Activation of the trigeminal nerve, which results in release of neuropeptides that mediate an inflammatory and nociceptive response, is implicated in the underlying pathology of both migraine and TMJ disorders (Buzzi, 2001; Williamson and Hargreaves, 2001). The trigeminal nerve is divided into three distinct regions: the ophthalmic (V1), maxillary (V2), and mandibular (V3) branches that provide sensory innervation to much of the head and face (Shankland, 2000). Cell bodies of the trigeminal nerve are located in the trigeminal ganglion (TG), which is comprised of both neurons and glial cells. Neurons in the adult TG are classified as pseudounipolar since a single axon leaves the cell body and splits to form two branches. The central branch exits the ganglion and enters the brainstem terminating at the principal sensory and spinal trigeminal nuclei, while the peripheral branch exits the ganglion to form the V1, V2, and V3 branches of the trigeminal nerve.
The mitogen-activated protein kinase (MAPK) signal transduction pathways are involved in the initiation and promotion of inflammatory and nociceptive responses (Ji, 2004a; Ji, 2004b). There are four distinct groups of MAPK present in mammalian cells: extracellular signal-regulated kinase (ERK1/2); Jun amino-terminal kinase (JNK 1/2/3); p38 proteins (p38α/β/γ/δ), and ERK5 (Chang and Karin, 2001). MAPK are activated by phosphorylation in response to a variety of inflammatory stimuli including cytokines, ceramides, nitric oxide, and transforming growth factor β (Lewis et al., 1998). Upon activation, these pathways are responsible for controlling many cellular functions, including inflammation, proliferation, differentiation, migration, and apoptosis (Turjanski et al., 2007). Furthermore, over-expression of MAPK pathways has been reported to facilitate inflammation and nociception, as well as tissue damage (Ji, 2004a).
The duration and magnitude of the MAPK response is controlled by MAP kinase phosphatases (MKP), which are enzymes involved in restoring elevated MAPK levels to normal via dephosphorylation of threonine or tyrosine residues on the MAPK (Dickinson and Keyse, 2006). There are 11 known MKP that are expressed in a variety of different cell types including neurons (Wu et al., 2006). Each MKP exhibits selectivity towards a particular MAPK. For example, while MKP-1 is reported to dephosphorylate the active forms of p38 and JNK, MKP-3 selectively dephosphorylates ERK (Keyse, 1998). Importantly, data from recent studies have provided evidence that mice lacking the MKP-1 gene have increased ERK, p38 and JNK activity (Wu et al., 2006) and increased inflammation (Wang and Liu, 2007).
We have recently found that an extract from cocoa beans can regulate MAPK pathways, as well as gene expression of the neuropeptide calcitonin gene-related peptide (CGRP) in cultured trigeminal ganglion neurons (Abbey et al., 2008). Theobroma cacao is a tree native to the tropical regions of the Americas that produces the cocoa seeds, which are used to make cocoa and chocolate. Historically, the Mayans and Aztecs harvested the pods containing seeds and used them in a variety of rituals and believed cocoa seeds had medicinal benefits (Dillinger et al., 2000). More recently, cocoa has shown promise in the treatment of a variety of ailments including insulin insensitivity, cardiovascular disease, and inflammation (Grassi et al., 2005; Selmi et al., 2006). Most research involving the beneficial effects of cocoa has been limited to in vitro studies and its antioxidant properties (Selmi et al., 2006). The purpose of this study was to determine if rats fed a diet enriched with cocoa would have increased basal MKP levels and repressed MAPK activity in trigeminal neurons in response to a noxious stimulus as well as chronic inflammation. In addition, the effect of a cocoa-enriched diet to repress expression of CGRP and the inducible form of nitric oxide synthase (iNOS), proteins implicated in inflammatory and nociceptive responses, was investigated.
2. Results
2.1 Expression of MKP in trigeminal ganglia in response to cocoa enriched diets
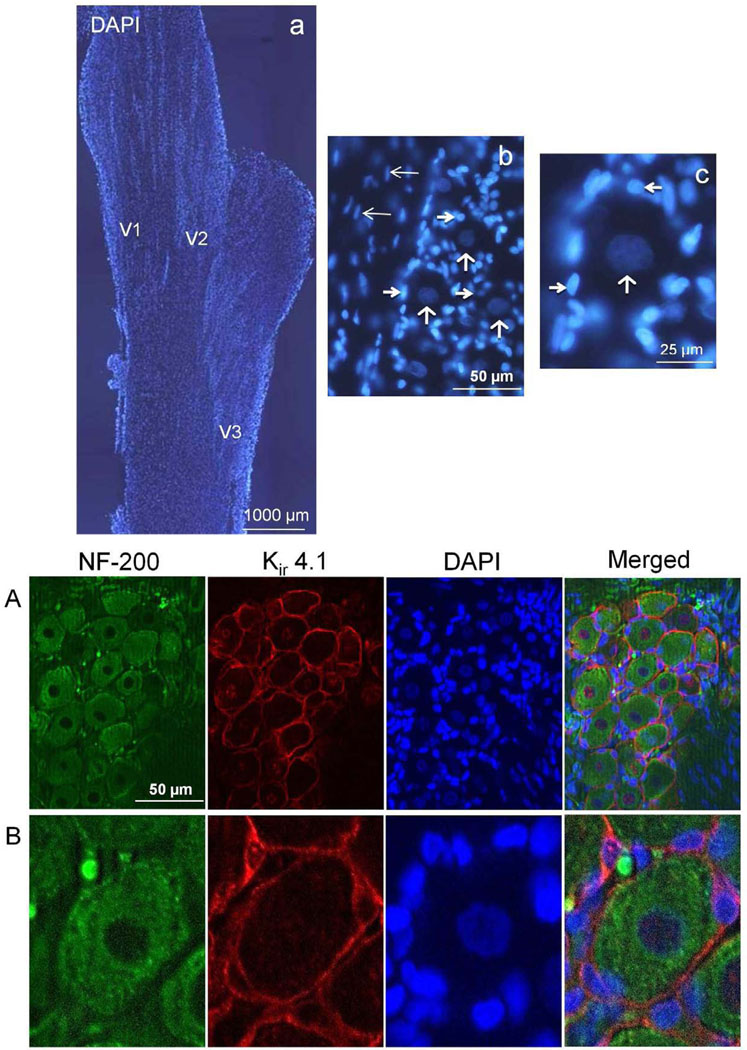
Initially, trigeminal ganglia from unstimulated control rats were isolated and longitudinal sections of the entire ganglion were stained with the fluorescent dye DAPI to identify all cellular nuclei (Fig. 1A). Three distinct regions of organized bands or clusters comprised of neurons and satellite glial cells were visible that correspond to the V1 (anteromedial), V2 (anterolateral), and V3 (posterolateral) regions of the ganglion. At higher magnification, functional units consisting of a single neuronal cell body and surrounding satellite glia cells were discernable. To aid in the identification of neurons and satellite glial cells, some sections were costained with antibodies directed against neurofilament protein (NF-200) and Kir 4.1. Neurofilament protein staining was observed in the cell bodies of trigeminal neurons as well as their processes, while Kir 4.1 expression was localized to satellite glial cells (Fig. 1B).
FIG. 1.
Organization and identification of neurons and glia in the trigeminal ganglion. The cellular organization of the trigeminal ganglion is shown in the top panels. (a) A longitudinal section of a trigeminal ganglion stained with the fluorescent dye DAPI to identify cell nuclei is shown at 40× magnification. The relative locations of the V1, V2, and V3 regions are indicated. (b) The cell bodies of neuronal cells are organized into bands or clusters within the ganglion. A band of neuronal (vertical arrows) and satellite glial cells (thick horizontal arrows) is shown at 400× magnification. Also visible are Schwann cells (thin horizontal arrows). (c) An enlarged image of a functional unit consisting of a single neuron (vertical arrow) surrounded by several satellite glia cells (horizontal arrows) is shown. Bottom panels: A 400× magnification of a neuron-satellite glia enriched region costained with antibodies against the neuronal cell marker NF-200 and the satellite glial cell marker Kir 4.1, as well as DAPI is shown (A). An enlarged image of a functional unit is shown in panel B. A merged image of the same sections stained for NF-200, Kir 4.1, and DAPI staining is shown in the bottom right panels.
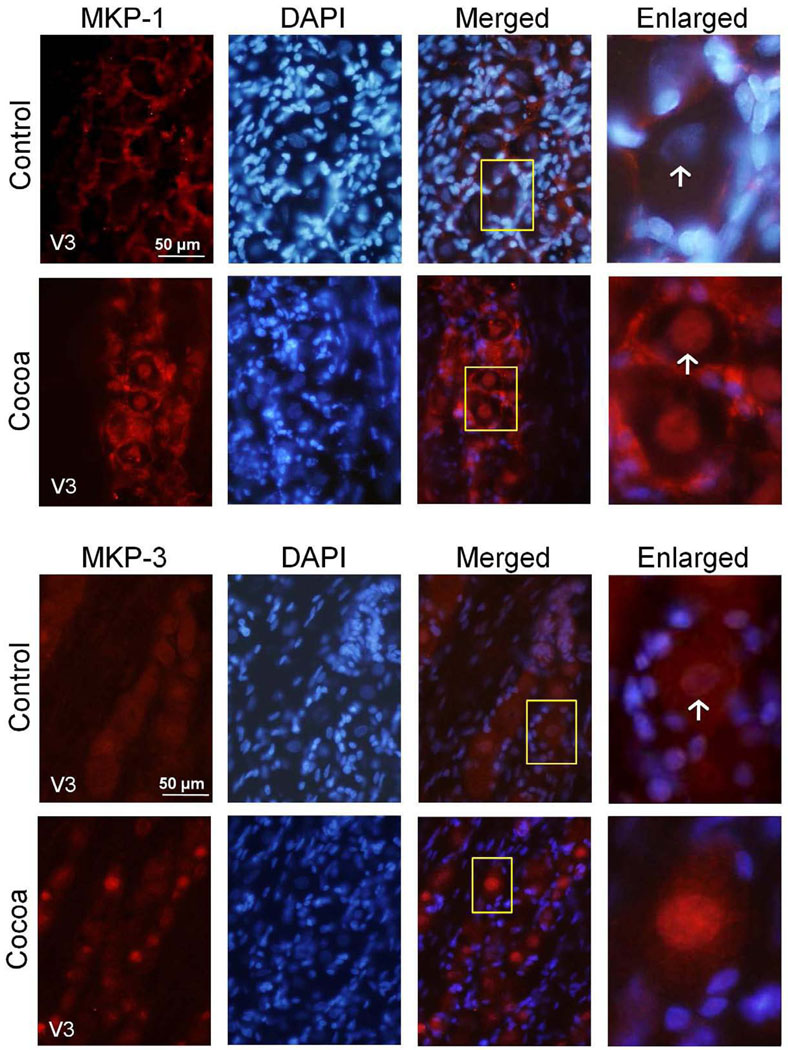
The expression of the MAPK phosphatases MKP-1 and MKP-3 in trigeminal ganglia obtained from animals fed a control diet or a cocoa enriched diet were investigated by immunohistochemistry. In animals fed a control diet, MKP-1 expression was observed primarily in the cytosol and nucleus of satellite glia in the V3 region of the ganglion (Fig. 2). In contrast, MKP-1 staining was barely detectable in neuronal cell bodies in the same animals. However, the intensity of MKP-1 staining was increased in the cytosol and nucleus in both neurons and satellite glia in rats fed a high cocoa (10% g/g) diet when compared to levels in control animals. In contrast to MKP-1 expression, MKP-3 staining was primarily observed in neuronal cells in ganglia obtained from control animals. The levels of nuclear MKP-3 staining in unstimulated control animals was minimal while rats consuming the high cocoa diets had an increase in nuclear neuronal MKP-3 expression as compared to control (Fig. 2). Importantly, there were no observable differences in weight due to changes in food or water consumption nor were any noticeable differences in grooming behaviors in rats on either low or high doses of cocoa when compared to rats fed control diets (data not shown).
FIG. 2.
MPK-1 and MKP-3 expression are increased in rats fed cocoa enriched diets. A section of the posterolateral portion of the ganglia (V3) was obtained from untreated animals (Control) or animals fed 10% cocoa (g/g) for 14 days. A 400× magnification of neuron-satellite glia enriched regions stained for MKP-1 (top panel) or MKP-3 (bottom panel) are shown in the left panels. The next three panels represent the same section stained with the nuclear dye DAPI, a merged image of MKP-1 or MKP-3 and DAPI staining, and an enlarged image from the tissue outlined by the yellow box on the merged image (right panel). Neurons are identified by vertical arrows.
2.2 Expression of MAPK proteins in the trigeminal ganglion in response to cocoa enriched diets during chronic and acute activation
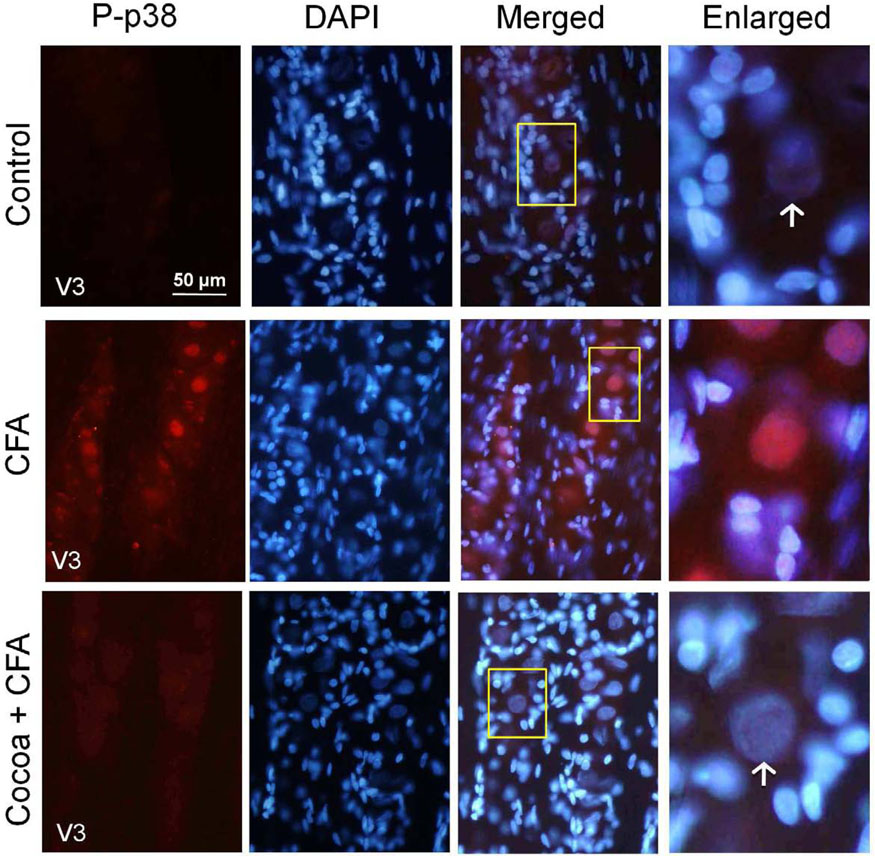
Expression of the active, phosphorylated form of p38 (P-p38) was barely detectable in the cytosol of neurons from ganglia obtained from rats fed control diets. In contrast, P-p38 staining was greatly increased both in the cytosol and nucleus of neurons, throughout the V3 region of the ganglion five days after injection of 50 µL of CFA (25 µg) into the joint capsule. However, rats on high cocoa diet and injected with CFA exhibited a marked decrease in both nuclear and cytosolic P-p38 expression in neurons when compared to CFA stimulation (Fig. 3). Rats on the high cocoa diet alone had no increase in P-p38 staining as compared to controls (data not shown). Similarly, rats injected with only the vehicle had no change in staining intensity compared to control uninjected rats (data not shown).
FIG. 3.
P-p38 expression in response to CFA is decreased in rats fed cocoa enriched diets. Sections from the posterolateral portion (V3) of the ganglion were obtained from untreated animals (Control), animals injected with CFA for five days, or animals fed 10% cocoa for 14 days and injected with CFA for 5 days prior to harvesting the ganglia (Cocoa + CFA). A 400× magnification of neuron-satellite glia enriched regions stained for P-p38 is shown in the left panels. The next three panels represent the same section stained with the nuclear dye DAPI, a merged image showing both P-p38 and DAPI staining, and an enlarged image taken from the tissue outlined by the yellow box on the merged image (right panel). Neurons are identified by vertical arrows.
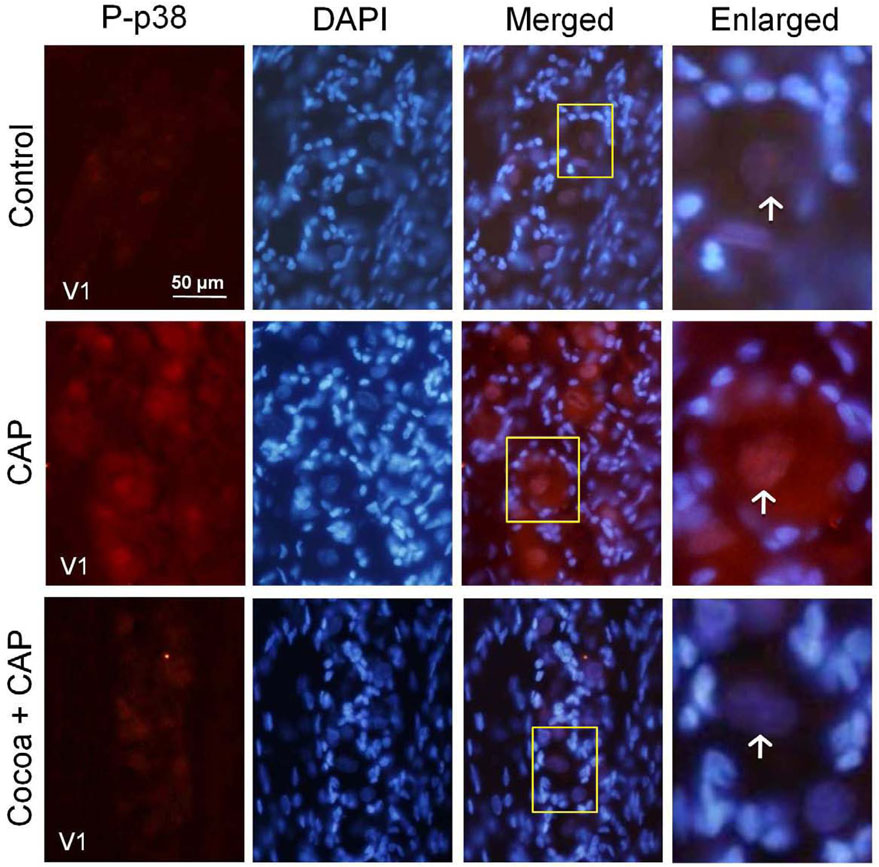
A similar staining pattern was seen in response to acute inflammation resulting from an injection of the noxious stimulus capsaicin into the eyebrow region to cause excitation of V1 trigeminal neurons (Fig. 4). Injection of 10 µM capsaicin caused an increase in cytosolic and nuclear neuronal staining of P-p38 in the V1 region after two hours. In contrast, rats injected with capsaicin after consuming a high cocoa diet did not have increased levels of P-p38 staining, but rather the level was more similar to that found in control tissue (Fig. 4).
FIG. 4.
Capsaicin stimulation of P-p38 expression is decreased in rats fed cocoa enriched diets. Sections of the anterolateral portion (V1) of the ganglia were obtained from untreated animals (Control), animals injected with capsaicin (CAP) for two hours, or animals fed 10% cocoa (g/g) diet for 14 days and injected with capsaicin for 2 hours prior to harvesting the ganglia (Cocoa + CAP). A 400× magnification of neuron-satellite glia enriched regions stained for P-p38 is shown in the left panels. The next three panels represent the same section stained with the nuclear dye DAPI, a merged image showing both P-p38 and DAPI staining, and an enlarged image taken from the tissue outlined by the yellow box on the merged image (right panel). Neurons are identified by vertical arrows.
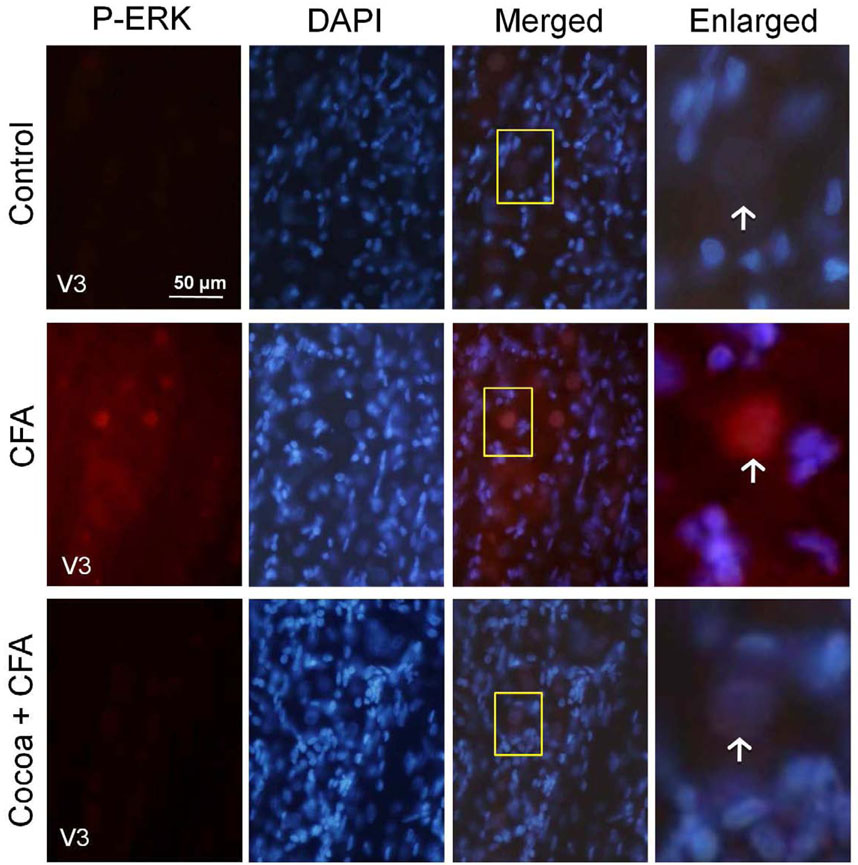
Active P-ERK expression was barely detectable in the neurons of rats on control diets. Rats on the high cocoa diet alone had no increase in P-ERK staining as compared to controls (data not shown). However, neuronal P-ERK was greatly increased throughout the V3 region of the ganglion in both the nucleus and cytosol of neurons in response to CFA injection into the joint capsule as compared to control levels. Rats consuming cocoa diets and injected with CFA showed a decrease in PERK expression both in the nucleus and cytosol of neurons when compared to CFA stimulation (Fig. 5).
FIG. 5.
The stimulatory effects of CFA on P-ERK expression are repressed in rats fed cocoa enriched diets. Sections from the posterolateral portion (V3) of the ganglion were obtained from untreated animals (Control), animals injected with CFA for five days, or animals fed 10% cocoa for 14 days and injected with CFA for 5 days prior to harvesting the ganglia (Cocoa + CFA). A 400× magnification of neuron-satellite glia enriched regions stained for P-ERK is shown in the left panels. The next three panels represent the same section stained with the nuclear dye DAPI, a merged image showing both P-ERK and DAPI staining, and an enlarged image taken from the tissue outlined by the yellow box on the merged image (right panel). Neurons are identified by vertical arrows.
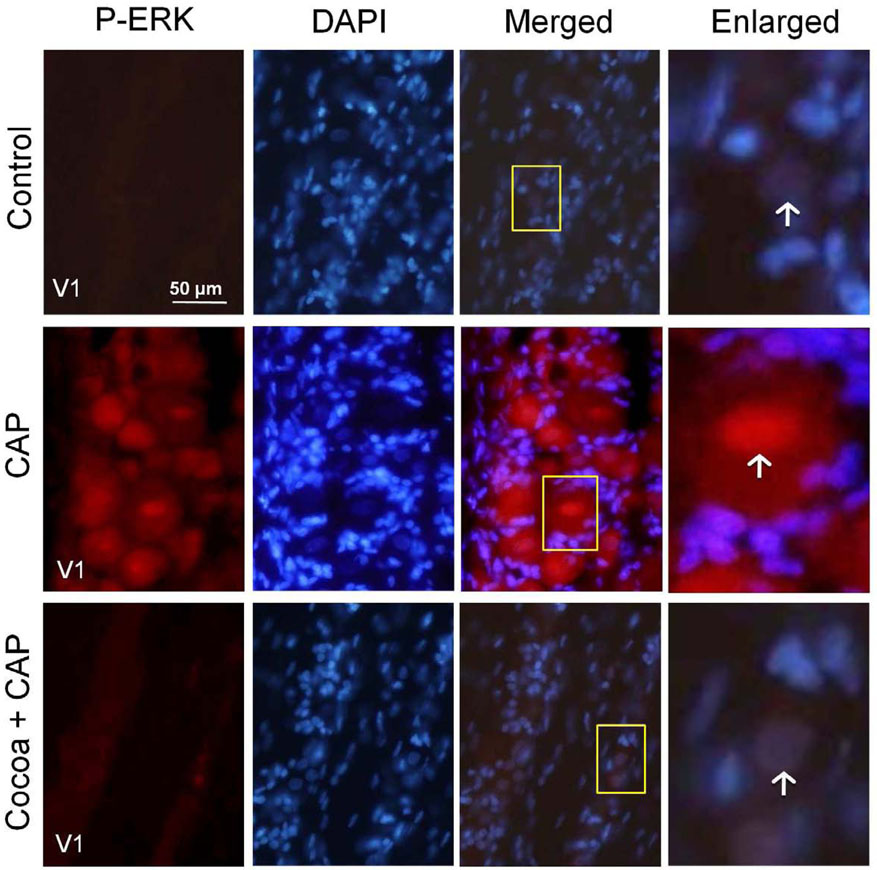
In response to acute stimulation caused by an injection of capsaicin into the eyebrow region, active P-ERK staining was increased in the cytosol of neurons. However, rats injected with capsaicin after consuming high cocoa diets had no change in P-ERK expression and levels were similar to those seen in control tissue (Fig. 6).
FIG. 6.
Increases in P-ERK expression in response to capsaicin (CAP) are repressed in rats fed cocoa enriched diets. Sections of the anterolateral portion (V1) of the ganglia were obtained from untreated animals (Control), animals injected with capsaicin (CAP) for two hours, or animals fed 10% cocoa (g/g) diet for 14 days and injected with capsaicin for 2 hours prior to harvesting the ganglia (Cocoa + CAP). A 400× magnification of neuron-satellite glia enriched regions stained for P-ERK is shown in the left panels. The next three panels represent the same section stained with the nuclear dye DAPI, a merged image showing both P-ERK and DAPI staining, and an enlarged image taken from the tissue outlined by the yellow box on the merged image (right panel). Neurons are identified by vertical arrows.
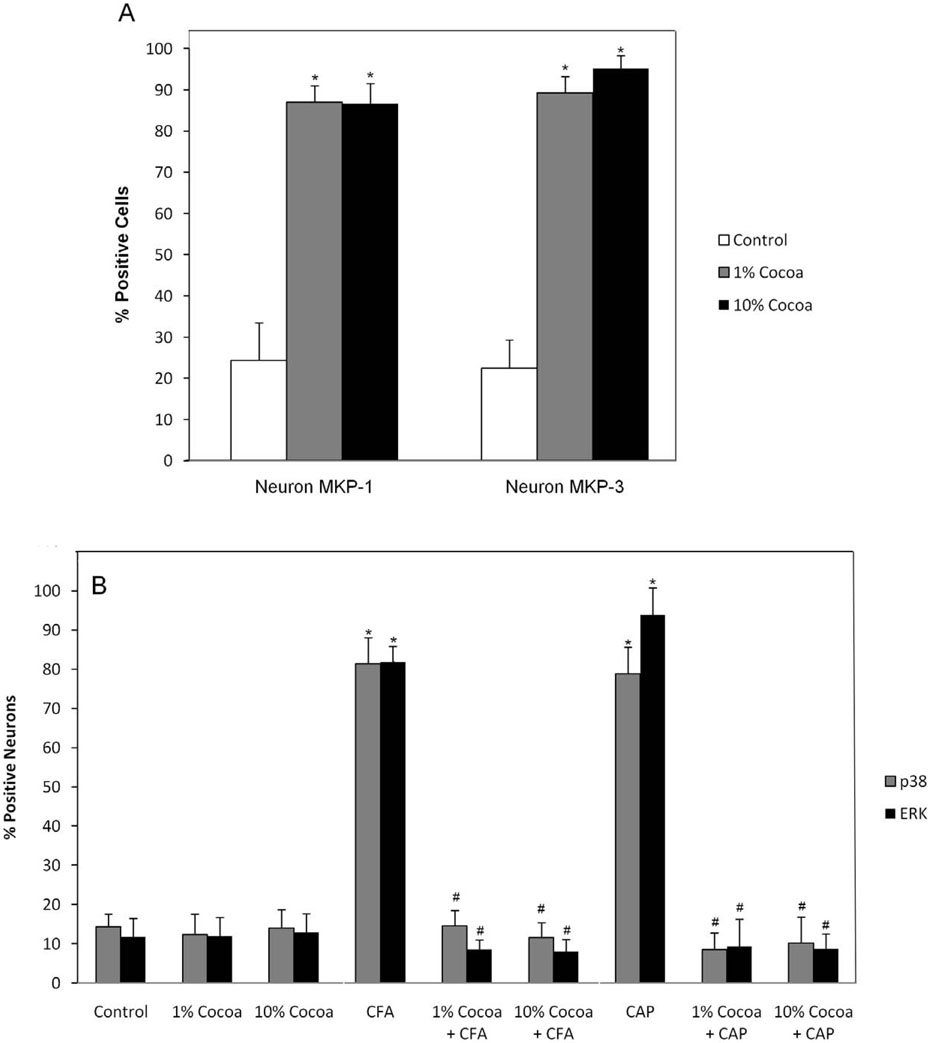
2.3 Effect of low and high cocoa-enriched diets on MKP and MAPK expression on trigeminal ganglion neurons
Based on cell count analysis, there were no major differences in the number of cells with readily detectable levels of MKP-1 or MKP-3 in ganglia obtained from animals fed low (1%) or high (10%) cocoa-enriched diets (Fig. 7A). For example, the percentage of MKP-1 and MKP-3 immunopositive neurons was almost identical to each other in the ganglion in response to a low or high cocoa diet. However, the staining intensities for MKP-1 and MKP-3 in response to the high cocoa diet was greater than that observed in ganglia obtained from animals fed a low cocoa diet (data not shown). In ganglion obtained from unstimulated control animals, the percentage of MKP-1 positive neuronal cells was 24.3 ± 9.2%, while the percentage significantly increased to 87 ± 4.0% (p < 0.01) and 86.6 ± 4.9% (p < 0.01) for the low and high cocoa diets, respectively. Similarly, while the percentage of MKP-3 positive neuronal cells was 22.4 ± 6.9% in control ganglion, the percentages were significantly increased to 89.3 ± 3.9% (p < 0.01) in response to a low cocoa diet and 95.2 ± 3.1% (p < 0.01) in ganglia from animals fed a high cocoa diet. Furthermore, the increases in the number of cells expressing MKP-1 and MKP-3 were seen in all regions of the ganglion (data not shown). Under unstimulated conditions, the percentages of P-p38 and P-ERK positive neuronal cells was not statistically different when comparing control levels to those seen in response to a low or high cocoa diet (Fig. 7B). For P-p38, the percentage was 14.3 ± 3.3% in control ganglia, 12.3 ± 5.3% for rats on a low cocoa diet, or 14.0 ± 4.7% for rats on a high cocoa diet. Similar results were obtained for P-ERK with values of 11.7 ± 4.8% in control tissue, 12.0 ± 4.7% in low cocoa diet, or 12.8 ± 4.9% for rats on a high cocoa diet. In contrast, animals fed a cocoa-enriched diet had a significantly lower percentage of P-p38 or P-ERK immunopositive cells when compared to animals injected with CFA or capsaicin. In response to CFA, P-p38 staining was detected in 81.4 ± 6.7% while P-ERK staining was seen in 81.9 ± 6.9% of neurons. The values for P-p38 were significantly reduced to 14.5 ±4.0% (p < 0.01) and 11.6 ± 3.8% (p < 0.01) in response to a low or high cocoa diet while P-ERK levels were significantly reduced to 8.6 ± 2.4% (p < 0.01) or 8.0 ± 3.1% (p < 0.01). A similar pattern of repression of P-p38 and P-ERK in response to dietary cocoa was observed to capsaicin injected animals. Capsaicin caused a significant increase in the percentage of neuronal cells expressing either P-p38 or P-ERK to 78.9 ± 6.6% (p < 0.01) and 93.9 ± 3.9% (p < 0.01), respectively. These percentages were significantly decreased in animals fed a cocoa-enriched diet to P-p38 levels of 8.5 ± 4.2% (p < 0.01) (low cocoa) and 10.5 ± 6.6%(p < 0.01) (high cocoa) while P-ERK levels were significantly decreased to 9.3 ± 6.9% (p < 0.01) (low cocoa) and 8.7 ± 3.8% (p < 0.01) (high cocoa). While the number of immunopositive cells was not significantly different between the diets, the 10% cocoa diet caused more marked changes in the intensity of MKP and MAPK staining when compared to both the low cocoa and control diets (data not shown).
FIG. 7.
Effect of cocoa-enriched diets on the expression of MKP and MAPK in trigeminal ganglion neurons and satellite glial cells. The data are reported as an average percentage ± SEM (n = 3 independent experiments) of the number of immunopositive neurons, which were identified by nuclear staining with DAPI. Percentage of trigeminal ganglion neurons staining positive for MKP-1 or MKP-3 (A) Percentage of trigeminal ganglion neurons staining positive for P-p38 and P-ERK under basal, unstimulated conditions or in response to cocoa, in response to CFA and cocoa, or in response to capsaicin (CAP) and cocoa (B). * p < 0.01 when compared to control while # p < 0.01 when compared to stimulation.
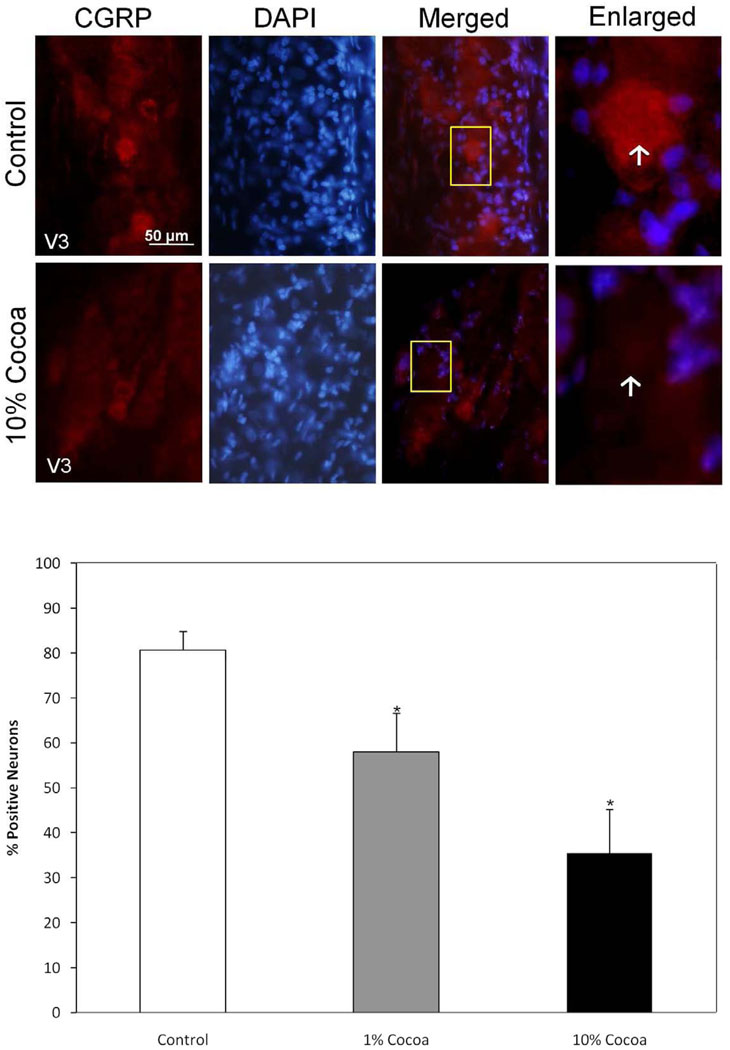
2.4 Expression of CGRP and iNOS in the trigeminal ganglion in response to cocoa enriched diets
In ganglia obtained from unstimulated control animals, CGRP staining was detected in many trigeminal neurons throughout the entire ganglia (Fig. 8). However, the intensity and number of CGRP-positively stained cells was significantly decreased in animals on both low and high cocoa diets. In control ganglia, the percentage of neuronal cells with levels of CGRP staining greater than background levels was 80.6 ± 4.2% but was significantly decreased to 58.0 ± 8.6% (p <0.01) and 35.5 ± 9.7% (p < 0.01) in ganglia from animals fed a low or high cocoa diet respectively. Similar results were obtained when counting only neuronal cells that express high levels of CGRP. In control tissue, 31.6 ± 4.5% of the neurons exhibited intense CGRP staining, while in animals consuming cocoa those values were significantly decreased to 25.6 ± 4.3% (p <0.01) in animals receiving the low cocoa diet and 17.3 ± 3.0% (p <0.01) in animals receiving the high cocoa diet.
FIG. 8.
Basal CGRP expression is decreased in rats fed cocoa enriched diets. A section of the posterolateral portion of the ganglia (V3) was obtained from untreated animals (Control) or animals fed 10% cocoa (g/g) for 14 days. A 400× magnification of neuron-satellite glia enriched regions stained for CGRP is shown in the left panels. The next three panels represent the same section stained with the nuclear dye DAPI, a merged image showing both CGRP and DAPI staining, and an enlarged image taken from the tissue outlined by the yellow box on the merged image (right panel). Neurons are identified by vertical arrows. Bottom panel: Effects of cocoa-enriched diets on the expression of CGRP in trigeminal ganglion neurons. The data are reported as an average percentage ± SEM (n = 3 independent experiments) of the number of immunopositive neurons compared to the total number of neurons as identified by nuclear staining with DAPI. * p < 0.01 when compared to control.
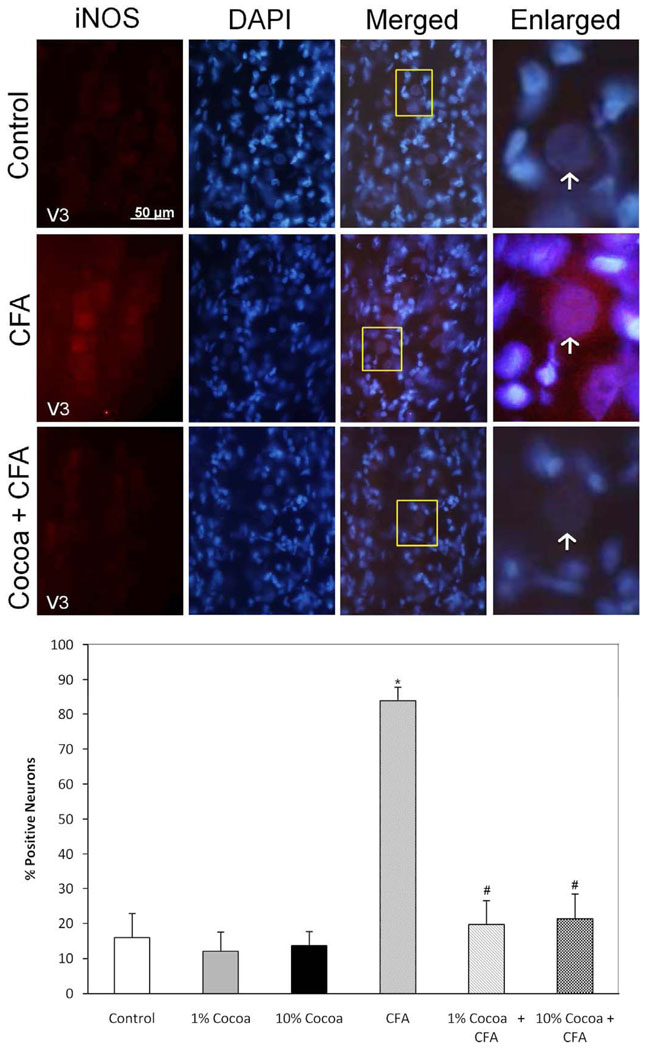
While expression of iNOS was minimally detectable in control ganglia, the level of staining was increased in neurons throughout the ganglion in response to CFA injection into the joint capsule (Fig. 9). The stimulatory effect of CFA on iNOS expression was greatly reduced to near basal levels in ganglia obtained from animals fed a low or high cocoa diet. No significant differences were observed in the percentage of immunopositive iNOS neurons in ganglia obtained from control (16.0 ± 6.9%) or from animals fed a low (12.1 ± 5.9%) or high (13.6 ± 4.4%) cocoa diet. In contrast, the increased percentage of iNOS positive neurons (83.8 ± 4.0%) was significantly reduced in response to a low (19.7 ± 6.8%) (p < 0.01) or high (21.4 ± 7.0%) (p < 0.01) cocoa diet.
FIG. 9.
Stimulated iNOS expression in response to CFA is repressed in rats fed cocoa enriched diets. A section of the posterolateral portion of the ganglia (V3) was obtained from untreated animals (Control), or animals injected with CFA for five days, or animals fed 10% cocoa for 14 days and injected with CFA for 5 days prior to harvesting the ganglia (Cocoa + CFA). A 400× magnification of neuron-satellite glia enriched regions stained for iNOS is shown in the left panels. The next three panels represent the same section stained with the nuclear dye DAPI, a merged image showing both iNOS and DAPI staining, and an enlarged image taken from the tissue outlined by the yellow box on the merged image (right panel). Neurons are identified by vertical arrows. Bottom panel: Effects of cocoa-enriched diets on the expression of iNOS in trigeminal ganglion neurons. The data are reported as an average percentage ± SEM (n = 3 independent experiments) of the number of immunopositive neurons compared to the total number of neurons as identified by nuclear staining with DAPI. * p < 0.01 when compared to control while # p < 0.01 compared to stimulation.
3. Discussion
In this study, acute as well as chronic in vivo models of inflammation and nociception were used to investigate cellular changes in trigeminal ganglion neurons and glia in response to diets enriched in cocoa. Overall, rats fed an isocaloric diet enriched with cocoa exhibited a repressed inflammatory response to acute as well as chronic stimulation of trigeminal ganglion neurons. For the acute model, which was used to mimic a migraine attack, the noxious stimulus capsaicin was injected into the eyebrow region of rats causing activation of the ophthalmic or V1 branch of the trigeminal nerve, an event implicated in the initiation of migraine pain. Capsaicin has been reported to bind to the transient receptor potential vanilloid subfamily member 1 (TRPV1) receptor (Caterina et al., 1997). TRPV1 receptors are abundantly expressed in sensory trigeminal ganglion neurons and upon activation mediate the release of CGRP and other molecules from neurons that promote peripheral inflammation and transmission of pain signals to the central nervous system (Thalakoti et al., 2007). The chronic inflammation model used in our study is a well-established model for TMJ pathology (Suzuki et al., 2007; Thut et al., 2007). CFA contains heat-killed mycobacteria that upon injection into the TMJ capsule results in a painful long-lasting inflammatory event in the joint that causes prolonged activation of the mandibular or V3 branch of the trigeminal nerve. In our study, we found that both the 1% and 10% (0.67 and 6.7% of total caloric intake) cocoa diets significantly repressed the number of trigeminal neurons with increased levels of P-p38 or P-ERK as well as increased the number of neurons with elevated levels of MKP-1 and MKP-3. The percent of cocoa used in our studies was less than that used in another study that reported changes in genes associated with fatty acid metabolism in animals fed a diet containing 12% cocoa (Matsui et al., 2005). Importantly, we found that animals on a cocoa-enriched diet exhibited normal behaviors as compared to the control animals. For example, no marked change in feeding, drinking, or grooming behaviors was seen in the animals on the cocoa diets. Thus, it appears that this type of diet is well-tolerated by the animals, at least for the 14 day time period that was required to see cellular changes. To our knowledge, this is the first evidence for the use of a dietary supplement to cause an upregulation of MKP, as well as the first evidence to show that cocoa might have suppressive effects on inflammatory and nociceptive responses in both acute and chronic models of trigeminal nerve activation.
In our study, rats fed a cocoa-enriched diet for two weeks showed a significant increase in basal MKP-1 and MKP-3 levels in neurons when compared to rats fed a normal diet. Typically, MKP are induced in response to inflammatory stimuli and thus function as an important feedback control mechanism to regulate the levels of active MAPK within cells, and ultimately, the magnitude of the inflammatory response. While inflammation is a normal protective response induced by tissue injury or infection, prolonged inflammation characteristic of a pathological state leads to release of mediators that cause sustained neuronal activation. Recent studies have provided evidence that MKP plays a crucial anti-inflammatory role in cells by modulating the levels of active MAPK and restoring homeostasis. For example, MKP-1 has been reported to play a critical role in the down regulation of the p38 MAPK pathway and inhibiting the response to inflammatory cytokines (Wang and Liu, 2007). Further evidence of an important anti-inflammatory role for MKP comes from recent studies in which rats deficient in MKP1 were shown to have increased production of iNOS as well as having a higher susceptible to inflammatory injuries (Wei et al., 2007; Zhao et al., 2006). Interestingly, the anti-inflammatory effects of glucocorticoids have been shown to be meditated, at least to some degree, by inducing a sustained expression of MKP-1 (Clark, 2003). Thus, it appears that daily consumption of cocoa may function to block inflammatory and nociceptive responses in the trigeminal ganglion by a similar mechanism as has been reported for glucocorticoids. While not a focus of this study, it should be noted that the beneficial effects of cocoa are likely to also involve inhibition of local peripheral inflammation mediated by pro-inflammatory cytokines (Ramiro-Puig and Castell, 2009; Ramiro et al., 2005). Although not directly shown in our study, but based on prior pharmacological studies (Keyse, 1998), it is likely that the cocoa mediated increase in MKP-1 expression would be involved in regulating levels of P-p38 in trigeminal neurons, while elevated MKP-3 levels would modulate P-ERK levels.
We showed that the elevated active levels of p38 and ERK seen in response to either acute or chronic stimulation of trigeminal neurons could be repressed by cocoa-enriched diets. This finding is in agreement with results from a previous study in immune cells that reported extracts from cocoa can suppress expression of the active forms of the MAPK, including ERK, JNK and p38 (Zhang et al., 2006). Importantly, activation of MAPK pathways in response to inflammatory mediators is thought to play a pivotal role in regulating acute inflammatory and nociceptive responses, as well as promoting progression to chronic pain. Specifically, increased levels of the active forms of p38 and ERK, which are involved in sustaining an inflammatory response, have also been reported to lead to development of chronic pain states while inhibition of these pathways blocks progression (Crown et al., 2006; Yang et al., 2005). Sustained elevated active levels of p38 and ERK in trigeminal neurons, as seen in our study, would likely lead to changes in synaptic plasticity, which is the underlying mechanism for peripheral sensitization (Garry et al., 2005). In addition, elevated levels of P-p38 and P-ERK in neurons seen in response to capsaicin and CFA would likely lead to increased neuron-satellite glia signaling that facilitates peripheral sensitization (Cheng and Ji, 2008) and intraganglion communication. Peripheral sensitization, which is defined as a decreased threshold of activation and increased rate of spontaneous firing of nociceptive neurons, is characteristic of both acute, as well as chronic pain conditions (Dodick and Silberstein, 2006). Importantly, we have shown that cocoa is able to cause an upregulation of the phosphatases that are known to inactivate p38 and ERK in sensory neurons and satellite glial cells within the trigeminal ganglion. Based on our findings, animals fed a cocoa-enriched diet had increased expression of MKP-1 in both trigeminal neurons as well as satellite glial cells. The elevated levels of MKP-1 would be expected to dephosphorylate p38 and ERK and thus, help to maintain homeostasis by decreasing neuronal excitability. Thus, based on our findings we propose that animals on a cocoa enriched diet would have an increased threshold for sensory nerve activation and be less likely to develop peripheral sensitization in response to inflammatory stimuli. We can only speculate that the ability of dietary cocoa to suppress increases in MAPK pathways caused by acute and chronic stimulation provides evidence that cocoa may be useful in the treatment of other inflammatory diseases such as arthritis and allergic rhinitis.
The ability of cocoa to decrease the expression of both CGRP and iNOS has important implications for the treatment of migraine and TMJ (Holmlund et al., 1991; Pietrobon and Striessnig, 2003; Takahashi et al., 2003). CGRP is a multifunctional neuropeptide that is abundantly expressed in trigeminal neurons, which when activated leads to peripheral release of CGRP that promotes an inflammatory response within the dura (migraine) or TMJ capsule and whose central release activates second order neurons resulting in pain (Hargreaves, 2007; Kopp, 2001). We found that animals on the high cocoa diet had significantly lower basal levels of CGRP in trigeminal ganglion neurons. This finding is in agreement with previously published results from our laboratory that provided evidence that CGRP secretion from stimulated trigeminal neurons can be directly inhibited by a methanol extract of Theobroma cacao beans (Abbey et al., 2008). In this study, we extend our findings to demonstrate that dietary cocoa can repress stimulated iNOS levels in trigeminal neurons. Activation of iNOS, which is a member of the family of nitric oxide synthase enzymes that also includes neuronal nitric oxide synthase (nNOS) and endothelial nitric oxide synthase (eNOS), leads to production of large amounts of nitric oxide (NO) (Liu et al., 2002). NO is an unstable free radical gas that functions as an important signaling molecule involved in the development and maintenance of inflammation and pain (Guzik et al., 2003; Yun et al., 1996). While iNOS is not expressed at high levels in a normal human TMJ, iNOS expression in the synovial lining of a diseased TMJ is greatly increased (Homma et al., 2001; Nagai et al., 2003; Takahashi et al., 2003). Furthermore, NO levels in synovial fluid obtained from patients with internal derangement and osteoarthritis of their TMJ were significantly increased when compared to control levels and correlated with disease stage and pain in the patients’ joint area (Suenaga et al., 2001; Takahashi et al., 1999). In addition, elevated levels of NO are also implicated in the underlying pathology of migraine and infusion of NO can cause migraine attacks (Iversen and Olesen, 1996; Olesen and Jansen-Olesen, 2000). Although the cellular mechanisms involved in cocoa regulation of CGRP and iNOS were not investigated in our study, it is probable that cocoa repression of CGRP and iNOS expression involves upregulation of MKP and inhibition of MAPK pathways since both CGRP and iNOS gene expression has been shown to be regulated through multiple pathways including the MAPK pathways (Bhat et al., 2002; Durham and Russo, 1998; Durham and Russo, 2000; Durham and Russo, 2003).
In summary, we have shown that rats fed diets enriched in cocoa have increased levels of MKP-1 and MKP-3, which likely leads to decreased levels of the MAPK proteins P-p38 and P-ERK, as well as decreased levels of CGRP and iNOS in trigeminal ganglia neurons. Our data demonstrate that consumption of cocoa would provide a natural therapeutic approach for minimizing trigeminal nerve activation in response to both acute and chronic noxious stimuli. Of particular clinical significance is our finding that a cocoa-enriched diet repressed chronic inflammation, which is characterized by persistent pain that is not easily treatable. Finally, results from our study support the dietary use of cocoa for the preventative treatment of migraine and TMJ disorders that involve trigeminal nerve activation.
4. Experimental Procedure
4.1 Animals
All animal studies were approved by the Institutional Animal Care and Use Committee at Missouri State University and were conducted in compliance with all established guidelines in the Animal Welfare Act and National Institutes of Health. A concerted effort was made to reduce the number of animals used and to minimize any suffering. Adult Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) (200–230 g) were housed in either plastic rat cages (VWR, West Chester, PA) or BioDaq unplugged feeding cages (Research Diets, Brunswick, NJ). All animals had unrestricted access to food and water and were kept on a 12-hour light/dark cycle. Food and water consumption as well as weight and behavioral changes were monitored and recorded daily to assess the overall health of the animals.
4.2 Diets
Raw cocoa powder (Theobroma cacao) was purchased from Nature’s First Law (San Diego, CA) and incorporated into two diets based on a modified AIN-76A rodent diet (Research Diets, New Brunswick, NJ). The low cocoa diet [1% (g/g) or 0.67% of total caloric intake] contained 9.545 g cocoa powder/kg pellet weight while the high cocoa diet [10% (g/g) or 6.7% of total caloric intake] contained 95.45 g cocoa powder/kg pellet weight (Table 1). All diets were iso-caloric and contained equal concentrations of carbohydrates, protein, and fat. Following a three-day acclimation period on the control diet (AIN-76A), rats were randomly placed into three groups receiving control, low cocoa, or high cocoa diet for two weeks.
Table 1.
Composition of diets.
| Diet | Control |
1% Cocoa |
10% Cocoa |
|||
|---|---|---|---|---|---|---|
| g/kg% | kcal% | g/kg% | kcal% | g/kg% | kcal% | |
| Protein | 20 | 21 | 20 | 21 | 20 | 21 |
| Carbohydrate | 66 | 68 | 66 | 68 | 65 | 68 |
| Fat | 5 | 12 | 5 | 12 | 5 | 12 |
| Fiber | 5 | 5 | 5 | |||
| Total | 100 | 100 | 100 | |||
| kcal/gm | 4 | 4 | 4 | |||
| Ingredient | ||||||
| g/kg | kcal | g/kg | kcal | g/kg | kcal | |
| Casein | 200 | 800 | 197 | 791 | 174 | 705 |
| DL-Methionine | 3 | 12 | 3 | 12 | 3 | 12 |
| Corn Starch | 150 | 600 | 150 | 600 | 148 | 60 |
| Sucrose | 500 | 2000 | 499 | 1999 | 490 | 1986 |
| Cellulose, BW200 | 50 | 0 | 46 0 |
0 | 12 | 0 |
| Com Oil | 50 | 450 | 48 | 435 | 33 | 297 |
| Mineral Mix S1001 |
35 | 0 | 35 | 0 | 35 | 0 |
| Vitamin Mix V10001 | 10 | 40 | 10 | 40 | 10 | 40 |
| Choline Bitartate |
2 | 0 | 2 | 0 | 2 | 0 |
| Cocoa Extract | 0 | 0 | 10 | 26 | 94 | 263 |
| Total | 3902 | 3902 | 3902 | |||
4.3 Acute and chronic models of trigeminal nerve activation
Rats were anesthetized by intraperitoneal injection of 0.3 mL ketamine and xylazine in solution (800 mg and 60 mg in 10 mL, respectively; Sigma, St. Louis, MO). Assessment of proper anesthesia was performed by monitoring the writhing reflex and tail flick reflex. After fourteen days of consuming control or cocoa diets, bilateral injections were performed with a 50 µL Hamilton syringe (Hamilton Company, Reno, NV) and a 26½ G needle (Becton Dickinson, Franklin Lakes, NJ). For the acute inflammation model, rats were injected bilaterally in the eyebrow regions (25 µL total) with 10 µM capsaicin (diluted in 100% dimethlysulfoxide (DMSO); Sigma-Aldrich, St. Louis, MO) two hours prior to being sacrificed. For the chronic inflammation model, rats received a bilateral injection of 50 µL complete Freund’s adjuvant (CFA, Sigma-Aldrich) in each TMJ capsule five days prior to being sacrificed. Prior to CFA injections, a 1:1 CFA/physiological saline emulsion was prepared by sonication of the mixture (3× each for 3 seconds). The time of treatment as well as amount of stimulatory agent, capsaicin or CFA, were based on previous studies (Garrett and Durham, 2009; Thalakoti et al., 2007). As controls, some rats did not receive any injections, while some animals were injected with only the vehicle.
4.4 Trigeminal tissue isolation and mounting
Trigeminal ganglia were removed from all rats following CO2 asphyxiation. Ganglia were mounted in OCT (Sakura Finetek, Torrance, CA) such that the ventral surface was in contact with the slide, quickly frozen, and stored at −20°C. Twenty-micron longitudinal sections of the entire trigeminal ganglion tissue were serially prepared using a cryostat (Microm HM 525, Thermo Scientific, Waltham, MA) and mounted on Superfrost Plus microscope slides (Fischer Scientific, Pittsburg, PA). Sections from the middle of the ganglion were then chosen for immunohistochemistry.
4.5 Immunohistochemistry
Slides containing sectioned ganglia were incubated in 4% paraformaldehyde for 30 minutes, followed by 0.1% Triton X-100 for 30 minutes to fix and permeabilize the cells. Sections were then incubated for 30 minutes in 5% donkey serum (Jackson ImmunoReasearch Laboratories, West Grove, PA) to reduce non-specific antibody binding. Sections were next incubated overnight at 4°C with rabbit polyclonal antibodies directed against the phosphorylated, active MAPK proteins ERK 1/2 (1:500 in PBS, Promega, Madison, WI) and p38 (1:200 Cell Signaling, Beverly, MA). Other sections were incubated with rabbit polyclonal antibodies directed against MKP-1 (1:500, Upstate Cell Signaling Solutions, Lake Placid, NY), MKP-3 (1:500, Santa Cruz Biotechnology), CGRP (1:1000, Sigma-Aldrich), or Kir 4.1 (1:1000, Alomone Labs, Jerusalem, Israel), or a mouse monoclonal antibody against iNOS (1:200, BD Biosciences, San Jose, CA) or Neurofilament 200 kDa (1:400, Millipore, Billerica, MA). Sections were incubated for one hour at room temperature in Rhodamine Red X-conjugated donkey anti-rabbit, Alexa Fluor 594 donkey anti-rabbit, donkey anti-mouse, or Alexa Fluor 488 donkey anti-mouse IgG antibodies, (diluted 1:100 in PBS, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) to detect immunofluorescent proteins by UV-fluorescence microscopy. Sections were mounted using Vectashield medium (H-1200) containing 4’,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) to identify cell nuclei. Images (40× and 400×) were collected using an Olympus DP70 camera mounted on an Olympus BX41 fluorescent microscope. Image acquisition was performed using Olympus MicroSuite Five image processing software (Center Valley, PA). In some cases, multiple image alignment was utilized to view the entire ganglia. Briefly, twelve 40× magnification images were collected and aligned to produce one large image of the entire ganglia.
4.6 Quantification of immunopositive cells
For cell count determination, three 400× images were used for each experimental condition repeated in three independent experiments. Images of V1 (capsaicin model) or V3 (CFA model) regions from tissue isolated from the middle portion of the ganglion were obtained. Only DAPI-stained images that contained a band of neurons (20–40 µm) and associated satellite glial cells as well as Schwann cells and nerve fibers were used for analysis. The total number of neurons in each image was determined by counting the number of neuronal DAPI-stained nuclei characterized by their large (> 10 µm) round appearance. Positively stained cells were identified as neurons or glia in enlarged merged images by colocalization of the DAPI signal and the rhodamine signal. Cells were considered positive if the intensity of staining was above that observed in Schwann cells and nerve fibers, which was considered background staining. The data are reported as the average percentage ± SEM of immunopositive neurons or glia compared to the total number of neuronal cells as determined from images costained with DAPI. The reported numbers are the average of the counts obtained by two laboratory staff members that were blinded to the experimental design. On average, the total number of neuronal cells per image was nine. Statistical analysis was performed using a Mann-Whitney U-Test. Results were considered significant when p < 0.05. All statistical tests were performed using Minitab Statistical Software (Version 15, Minitab, State College, PA).
Acknowledgments
This research project was supported by NIH grant DE017805.
Abbreviations used
- TMJ
temporomandibular joint
- MAPK
mitogen-activated protein kinase
- MAP
mitogen-activated kinase
- ERK
extracellular signal-regulated kinase
- JNK
Jun amino-terminal kinase
- MKP
MAP kinase phosphatase
- CGRP
calcitonin gene-related peptide
- iNOS
nitric oxide synthase
- TRPV1
transient receptor potential vanilloid subfamily member 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Abbey MJ, Patil VV, Vause CV, Durham PL. Repression of calcitonin gene-related peptide expression in trigeminal neurons by a Theobroma cacao extract. J Ethnopharmacol. 2008;115:238–248. doi: 10.1016/j.jep.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat N, Feinstein D, Shen Q, Bhat A. p38 MAPK-mediated transcriptional activation of inducible nitric-oxide synthase in glial cells. Roles of nuclear factors, nuclear factor kappa B, cAMP response element-binding protein, CCAAT/enhancerbinding protein-beta, and activating transcription factor-2. J Biol Chem. 2002;277:29584–29592. doi: 10.1074/jbc.M204994200. [DOI] [PubMed] [Google Scholar]
- Buzzi M. Trigeminal pain pathway: peripheral and central activation as experimental models of migraine. Funtional Neurology. 2001;16:77–81. [PubMed] [Google Scholar]
- Carlsson G, LeResche L. Epidemiology of temporomandibular disorders. In: Sessle PS, Dionne RA, editors. Temporomandibular disorders and related pain conditions. Progress in pain research and management. Vol., B. Seattle: IASP Press; 1995. pp. 211–226. [Google Scholar]
- Caterina M, Schumacher M, Tominaga M, Rosen T, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Cheng JK, Ji RR. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem Res. 2008;33:1970–1978. doi: 10.1007/s11064-008-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. MAP kinase phosphatase 1: a novel mediator of biological effects of glucocorticoids. J Endocrinol. 2003;178:5–12. doi: 10.1677/joe.0.1780005. [DOI] [PubMed] [Google Scholar]
- Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Hulsebosch CE. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp Neurol. 2006;199:397–407. doi: 10.1016/j.expneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Dickinson R, Keyse S. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Science. 2006;119:4607–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- Dillinger TL, Barriga P, Escarcega S, Jimenez M, Salazar Lowe D, Grivetti LE. Food of the gods: cure for humanity? A cultural history of the medicinal and ritual use of chocolate. J Nutr. 2000;130 doi: 10.1093/jn/130.8.2057S. 2057S–72S. [DOI] [PubMed] [Google Scholar]
- Dodick D, Silberstein S. Central sensitization theory of migraine: clinical implications. Headache. 2006;46:S182–S191. doi: 10.1111/j.1526-4610.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Durham P, Russo A. Serotonergic repression of mitogen-activated protein kinase control of the calcitonin gene-related peptide enhancer. Mol Endocrinol. 1998;12:1002–1009. doi: 10.1210/mend.12.7.0135. [DOI] [PubMed] [Google Scholar]
- Durham P, Russo A. Differential regulation of mitogen-activated protein kinase-responsive genes by the duration of a calcium signal. Mol Endocrinol. 2000;14:1570–1582. doi: 10.1210/mend.14.10.0529. [DOI] [PubMed] [Google Scholar]
- Durham P, Russo A. Stimulation of the calcitonin gene-related peptide enhancer by mitogen-activated protein kinases and repression by an antimigraine drug in trigeminal ganglia neurons. J Neurosci. 2003;23:807–815. doi: 10.1523/JNEUROSCI.23-03-00807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari MD. The economic burden of migraine to society. Pharmacoeconomics. 1998;13:667–676. doi: 10.2165/00019053-199813060-00003. [DOI] [PubMed] [Google Scholar]
- Garrett FG, Durham PL. Differential expression of connexins in trigeminal ganglion neurons and satellite glial cells in response to chronic or acute joint inflammation. Neuron Glia Biol. 2009:1–12. doi: 10.1017/S1740925X09990093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry E, Delaney A, Blackburn-Munro G, Dickinson T, Moss A, Nakalenbe I, Robertson D, Rosie R, Robberecht P, Mitchell R, Fleetwood-Walker S. Activation of p38 and p42/44 MAP kinase in neuropathic pain: Involvement of VPAC and NK receptors and mediation by spinal glia. Molecular and Cellular Neuroscience. 2005;30:523–537. doi: 10.1016/j.mcn.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611–614. doi: 10.1093/ajcn/81.3.611. [DOI] [PubMed] [Google Scholar]
- Guzik T, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- Hargreaves R. New migraine and pain research. Headache. 2007;47:S26–S43. doi: 10.1111/j.1526-4610.2006.00675.x. [DOI] [PubMed] [Google Scholar]
- Herb K, Cho S, Stiles M. Temporomandibular joint pain and dysfunctioning. Curr Pain and Headache Reports. 2006;10:408–414. doi: 10.1007/s11916-006-0070-7. [DOI] [PubMed] [Google Scholar]
- Holmlund A, Ekblom A, Hansson P, Lind J, Lundenberg T, Theodorsson E. Concentration of neuropeptide substance - P, Neurokinin A, calcitonin gene related peptide, neuropeptide Y and vasoactive intestinal polypeptide in the synovial fluid of the -human temporomandibular joint. Int. J. Oral Maxillofac Surg. 1991;20:228–231. doi: 10.1016/s0901-5027(05)80181-x. [DOI] [PubMed] [Google Scholar]
- Homma H, Takahashi T, Seki H, Ohtani M, Kondoh T, Fukuda M. Immunohistochemical localization of inducible nitric oxide synthase in synovial tissue of human temporomandibular joints with internal derangement. Arch Oral Biol. 2001;46:93–97. doi: 10.1016/s0003-9969(00)00086-8. [DOI] [PubMed] [Google Scholar]
- Isong U, Gangsky S, Plesh O. Temporomandibular joint and muscle disorder-type pain in U.S. adults: the National Health Interview Survey. J Orafac Pain. 2008;22:317–322. [PMC free article] [PubMed] [Google Scholar]
- Iversen H, Olesen J. Headache induced by a nitric oxide donor (nitroglycerin) responds to sumatriptan. A human model for development of migraine drugs. Cephalalgia. 1996;16:412–418. doi: 10.1046/j.1468-2982.1996.1606412.x. [DOI] [PubMed] [Google Scholar]
- Ji R. Mitogen-activated protein kinases as potential targets for pain killers. Curr Opin Investig Drugs. 2004a;5:71–75. [PubMed] [Google Scholar]
- Ji RR. Peripheral and central mechanisms of inflammatory pain, with emphasis on MAP kinases. Curr Drug Targets Inflamm Allergy. 2004b;3:299–303. doi: 10.2174/1568010043343804. [DOI] [PubMed] [Google Scholar]
- Keyse S. Protein phosphatases and the regulation of MAP kinase activity. Cell & Dev Bio. 1998;9:143–152. doi: 10.1006/scdb.1997.0219. [DOI] [PubMed] [Google Scholar]
- Kopp S. Neuroendocrine, immune, and local responses related to temporomandibular disorders. J Orofac Pain. 2001;15:9–28. [PubMed] [Google Scholar]
- Lewis T, Shapiro P, Ahn N. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- Lipton J, Ship JA, Larach-Robinson D. Estimated prevalence and distribution of reported orofacial pain in the United States. Journal of American Dental Association. 1993;124:115–121. doi: 10.14219/jada.archive.1993.0200. [DOI] [PubMed] [Google Scholar]
- Lipton R, Stewart W, Diamond S, Diamond M, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- Liu B, Gao H, Wang J, Jeohn G, Cooper C, Hong J. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann N Y Acad Sci. 2002;962:318–331. doi: 10.1111/j.1749-6632.2002.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Matsui N, Ito R, Nishimura E, Yoshikawa M, Kato M, Kamei M, Shibata H, Matsumoto I, Abe K, Hashizume S. Ingested cocoa can prevent high-fat diet-induced obesity by regulating the expression of genes for fatty acid metabolism. Nutrition. 2005;21:594–601. doi: 10.1016/j.nut.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Nagai H, Kumamoto H, Fukuda M, Takahashi T. Inducible nitric oxide synthase and apoptosis-related factors in the synovial tissues of temporomandibular joints with internal derangement and osteoarthritis. J Oral Maxillofac Surg. 2003;61:801–807. doi: 10.1016/s0278-2391(03)00155-1. [DOI] [PubMed] [Google Scholar]
- Olesen J, Jansen-Olesen I. Nitric oxide mechanisms in migraine. Pathol Biol (Paris) 2000;48:648–657. [PubMed] [Google Scholar]
- Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci. 2003;4:386–398. doi: 10.1038/nrn1102. [DOI] [PubMed] [Google Scholar]
- Ramiro-Puig E, Castell M. Cocoa: antioxidant and immunomodulator. Br J Nutr. 2009;101:931–940. doi: 10.1017/S0007114508169896. [DOI] [PubMed] [Google Scholar]
- Ramiro E, Franch A, Castellote C, Perez-Cano F, Permanyer J, Izquierdo-Pulido M, Castell M. Flavonoids from Theobroma cacao down-regulate inflammatory mediators. J Agric Food Chem. 2005;53:8506–8511. doi: 10.1021/jf0511042. [DOI] [PubMed] [Google Scholar]
- Selmi C, Mao T, Keen C, Schmitz H, Eric Gershwin M. The anti-inflammatory properties of cocoa flavanols. J Cardiovasc Pharmacol. 2006;47:S163–S176. doi: 10.1097/00005344-200606001-00010. [DOI] [PubMed] [Google Scholar]
- Sessle B. The neurobiology of facial and dental pain: present knowledge, future directions. Journal of Dental Research. 1987;66:962–981. doi: 10.1177/00220345870660052201. [DOI] [PubMed] [Google Scholar]
- Shankland W. The trigeminal nerve. Part I: An over-view. Cranio. 2000;18:238–248. doi: 10.1080/08869634.2000.11746137. [DOI] [PubMed] [Google Scholar]
- Suenaga S, Abeyama K, Hamasaki A, Mimura T, Noikura T. Temporomandibular disorders: relationship between joint pain and effusion and nitric oxide concentration in the joint fluid. Dentomaxillofac Radiol. 2001;30:214–218. doi: 10.1038/sj.dmfr.4600610. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Harada T, Asano M, Tsuboi Y, Kondo M, Gionhaku N, Kitagawa J, Kusama T, Iwata K. Phosphorylation of ERK in trigeminal spinal nucleus neurons following passive jaw movement in rats with chronic temporomandibular joint inflammation. J Orofac Pain. 2007;21:225–231. [PubMed] [Google Scholar]
- Takahashi T, Kondoh T, Ohtani M, Homma H, Fukuda M. Association between arthroscopic diagnosis of temporomandibular joint osteoarthritis and synovial fluid nitric oxide levels. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:129–136. doi: 10.1016/s1079-2104(99)70105-8. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Homma H, Nagai H, Seki H, Kondoh T, Yamazaki Y, Fukuda M. Specific expression of inducible nitric oxide synthase in the synovium of the diseased temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:174–181. doi: 10.1067/moe.2003.45. [DOI] [PubMed] [Google Scholar]
- Thalakoti S, Patil V, Damodaram S, Vause C, Langford L, Freeman S, Durham P. Neuron-Glia signaling in trigeminal ganglion: Implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut P, Hermanstyne T, Flake N, Gold M. An operant conditioning model to assess changes in feeding behavior associated with temporomandibular joint inflammation in the rat. J Orofacial Pain. 2007;21:7–18. [PubMed] [Google Scholar]
- Turjanski A, Vaque J, Gutkind J. MAP kinases and the control of nuclear events. Oncogene. 2007;26:3240–3253. doi: 10.1038/sj.onc.1210415. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu Y. Regulation of innate immune response by MAP kinase phosphatase-1. Cellular Signalling. 2007;19:1372–1382. doi: 10.1016/j.cellsig.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Zhao W, Wang Y, Pertovaara A. Pain-realated behavior following REM sleep deprivation in the rat: Influence of peripheral nerve injury, spinal glutamatergic receptors and nitric oxide. Brain Research. 2007;1148:105–112. doi: 10.1016/j.brainres.2007.02.040. [DOI] [PubMed] [Google Scholar]
- Williamson D, Hargreaves R. Neurogenic inflammation in the context of mirgraine. Microsc Res Tech. 2001;53:167–178. doi: 10.1002/jemt.1081. [DOI] [PubMed] [Google Scholar]
- Wu J, Roth R, Anderson E, Hong E, Lee M, Choi C, Neufer P, Shulman G, Kim J, Bennett A. Mice lacking MAP kinase phophatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab. 2006;4:61–73. doi: 10.1016/j.cmet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Yang CM, Hsieh HL, Lee CW. Intracellular signaling mechanisms underlying the expression of pro-inflammatory mediators in airway diseases. Chang Gung Med J. 2005;28:813–823. [PubMed] [Google Scholar]
- Yun H-Y, Dawson V, Dawson T. Neurobiology of nitric oxide. Crit Rev Neurobiol. 1996;10:291–316. doi: 10.1615/critrevneurobiol.v10.i3-4.20. [DOI] [PubMed] [Google Scholar]
- Zhang WY, Liu HQ, Xie KQ, Yin LL, Li Y, Kwik-Uribe CL, Zhu XZ. Procyanidin dimer B2 [epicatechin-(4beta-8)-epicatechin] suppresses the expression of cyclooxygenase-2 in endotoxin-treated monocytic cells. Biochem Biophys Res Commun. 2006;345:508–515. doi: 10.1016/j.bbrc.2006.04.085. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, Baliga RS, Meng X, Smith CV, Bauer JA, Chang CH, Liu Y. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med. 2006;203:131–140. doi: 10.1084/jem.20051794. [DOI] [PMC free article] [PubMed] [Google Scholar]