Abstract
The macrophage-tropic lentivirus, equine infectious anemia virus (EIAV), encodes the small auxiliary protein S2 from a short open reading frame that overlaps the amino terminus of env. EIAV S2 is dispensable for virus replication in cultured cells but is required for disease production. S2 is approximately 7kDa and has no overall amino acid sequence homology to other cellular or viral proteins. Therefore it is likely that S2 plays a role as an adaptor protein. To further investigate S2 function we performed a yeast-2-hybrid screen to identify cellular proteins that interact with EIAV S2. The screen identified two human cellular proteins, amplified in osteosarcoma (OS-9) and proteasome 26S ATPase subunit 3 (PSMC3) that interact with S2. The equine homologues of these proteins were cloned and their interactions with S2 confirmed using co-immunoprecipitation assays. We identified two OS9 isoforms that interact with S2 and a third splice variant that does not, indicating a region of OS9 apparently required for the S2 interaction. The roles of these cellular proteins during EIAV infection have not been determined.
Keywords: equine infectious anemia virus, EIAV, OS9, PSMC3, TBP-1, lentivirus
Equine infectious anemia virus (EIAV) is a macrophage-tropic retrovirus (genus Lentivirus) of equids. Natural transmission occurs mechanically as biting flies move between infected and naïve animals during feeding. Transmission can also occur via blood transfer through poor veterinary practices. Upon infection, acute disease may develop within days to weeks and is characterized by a high titer viremia accompanied by fever and pronounced thrombocytopenia. Clinical outcomes depend on the infecting virus strain, as well as host factors; horses frequently survive the acute disease episode to become life-long carriers. However some infected animals will develop a chronic form of the disease, manifested by recurring febrile episodes (Clements and Payne, 1994; Montelaro et al., 1993; Payne et al., 2006).
Organization of the EIAV genome is relatively simple when compared to primate lentivirus genomes (Payne et al., 2006). EIAV encodes gag, pol, env, tat and rev genes, but few additional auxiliary genes. In fact, EIAV appears to encode only two additional proteins, S2 and Ttm, neither of whose functions have been well characterized (Fagerness et al., 2006). The approximately 7 kDa (65–68 amino acid) S2 polypeptide is encoded by a short open reading frame (orf) that overlaps env. S2 translation presumably occurs by leaky scanning of a tricistronic mRNA encoding tat, S2 and env or from a bicitronic mRNA encoding S2 and env (Li et al., 1998; Schiltz et al., 1992).
The S2 orf is present in all EIAV strains characterized to date, suggesting functional significance (Fagerness et al., 2006; Li et al., 2000). Overall the S2 protein has no obvious sequence homology to other viral or host cell proteins. S2 function has been probed by generating S2 mutant proviruses and assessing viral replication in permissive cell lines or in primary monocyte-derived-macrophages (Covaleda et al., In press; Fagerness et al., 2006; Li et al., 2000; Li et al., 1998). Cell culture-based assays suggest that S2 does not play a major role in virion production or particle morphology, nor does it appear to be packaged into virions, although its presence may subtly enhance viral replication (Fagerness et al., 2006; Li et al., 2000; Li et al., 1998; Yoon et al., 2000). In contrast to effects observed in cultured cells, S2-deletion has a profound effect on replication and virulence when assayed in horses or Shetland ponies where S2 is required for high titer virus replication and the development of acute disease (Fagerness et al., 2006). To gain insight into possible mechanistic activities of the EIAV S2 protein, we performed a yeast 2-hybrid screen to identify host cell proteins that interact with S2. In this report we describe the interaction of EIAV S2 with two cellular proteins: amplified in osteosarcoma (OS-9) and proteasome 26S ATPase subunit 3 (PSMC3) a protein component of the 19S regulatory subunit of the 26S proteasome (Hoyle et al., 1997; Tanahashi et al., 1998).
The ProQest™ Two-Hybrid System with Gateway® Technology (Invitrogen) system was used to identify cellular proteins capable of interacting directly with the EIAV S2 protein. The S2 open reading frame, amplified by PCR, and was moved into pDEST™ 32 using recombination mediated cloning (Gateway® BP Clonase™). The forward primer attB1S2 (5′ GGGGACAAGTTTGTACAAAAAAGCAGCCTTGATGGGAGTATTTGGT 3′) contains an attB1 site (italicized); the S2 codons are underlined. The reverse primer sequence attB2S2 was (5′ GGGGACCACTTTGTACAAGAAAGCTGGGTTTTCTTGGTCTCTTGC 3′). Recombinants were isolated and subjected to DNA sequence analysis to confirm recovery of the desired constructs. The selected DB-S2 construct was tested for self-activation (in the presence of pEXP-AD502) as recommended by the manufacturer. DB-S2 was co-transformed into the yeast reporter strain MaV203 (Invitrogen) with a commercially available human spleen cDNA library (pPC86 vector, Invitrogen). Transformants were plated onto SC-Leu-Trp and the selective media SC-Leu-Trp-His +3AT. Patching and replica plating were performed exactly as described by the manufacturer to identify transformants displaying activation of the three reporter genes His3, Ura3 and LacZ. False positives were ruled out using the retransformation assays exactly as described by the manufacturer. After identification of possible S2-interactors, AD-Y plasmids were isolated from yeast and transformed into E. coli followed by selection on LB agar with ampicillin. Plasmid minipreps were performed followed by DNA sequence analysis. The proteins encoded by the cDNAs were identified using BLAST.
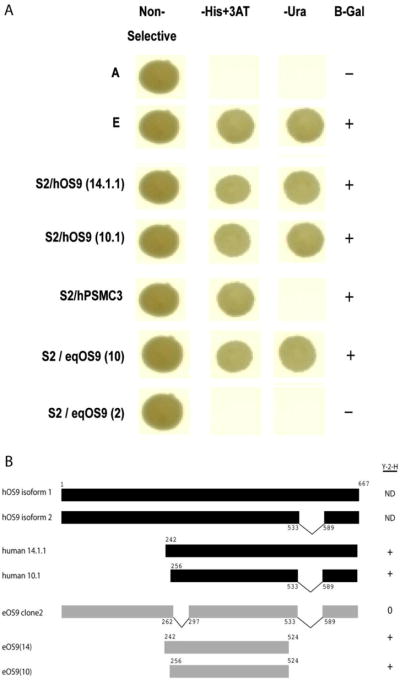
The screen identified three cDNAs, encoding two different cellular proteins. Figure 1 shows the pattern of reporter gene activation observed for each clone. Two cDNAs encoding human OS-9 (hOS-9) both displayed strong interaction phenotypes with S2, activating the three reporter genes in the system. One clone (designated LC14.1.1) encodes 430 amino acids of hOS-9; the second cDNA (LC10.1) encodes 419 amino acids. These partial cDNAs probably derive from the alternatively spliced isoforms 1 and 2 of hOS-9 as shown schematically in Figure 1B. A cDNA encoding 425 amino acids of the human PSMC3 (hPSMC3) was also recovered. Co-expression of this cDNA with EIAV S2 reproducibly activated two of three reporter genes in a pattern indicating a weak interaction (Fig. 1A).
Figure 1.
Summary of yeast two hybrid screen results. Panel A shows a strong interaction phenotype for two human OS9 cDNAs (S2/hOS9 14.1.1 and S2/hOS9 10.1) and the weak interaction of phenotype of human PMCS3 with EIAVS2 in the yeast two hybrid screen. Also shown is the strong interaction phenotype of eqOS9 clone 10 with S2 and the lack of interaction between eqOS9 clone 2 and S2. Row A is a negative control (pPC97 and pPC86, Invitrogen, with no inserts); row E is the strong positive control (pCL1Gal4, Invitrogen). Panel B. Schematic representation of known OS9 isoforms 1 and 2 for comparison with cDNAs recovered from the yeast two hybrid screen. Fragments of equine OS9 tested in the yeast two hybrid assay are also shown.
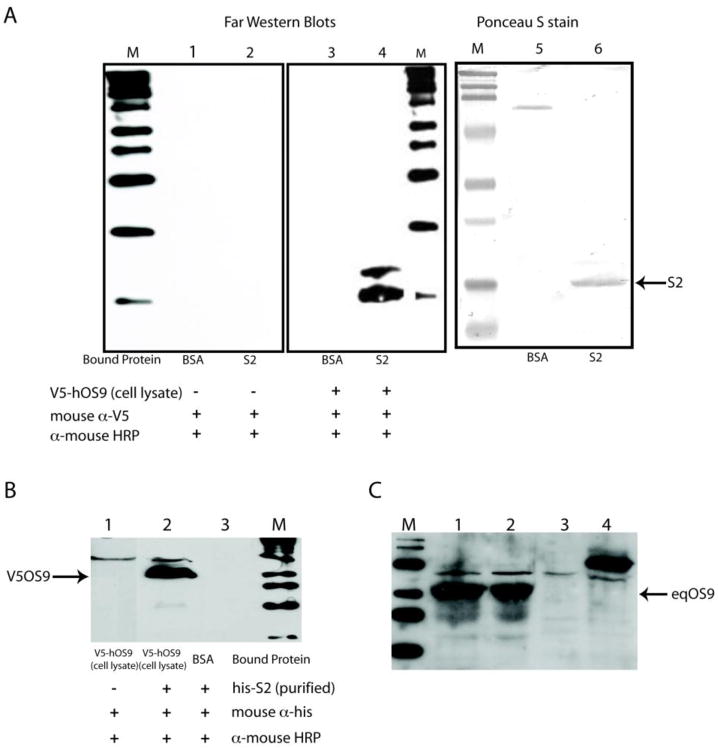
The interaction between hOS-9 and EIAV S2 was validated by performing far-western blot assays using his-tagged EIAV S2 and V5-tagged hOS-9. The EIAV S2 protein was cloned with a histidine tag into the bacterial expression vector pET32. The S2-his tagged protein was purified by Ni-agarose affinity chromatography. Human OS9 was expressed as a V5 fusion protein in Chinese hamster ovary (CHO) cells using pcDNA3.2/V5-DEST (Invitrogen). Primary antibodies used in these studies were mouse anti-his monoclonal antibody (Pierce), mouse anti-V5 monoclonal antibody (Invitrogen), mouse anti-human OS-9 (Center for Biomedical Invention, University of Texas Southwestern Medical School, Dallas TX) and goat anti-EIAV his-S2 (generated at Bethel Laboratories, Inc). Secondary antibodies were horseradish peroxidase conjugates (Sigma) and were detected using the ECL Western blotting detection system (Amersham). As shown in Figure 2, interactions between hOS-9 and S2 were observed between immobilized S2 and soluble hOS-9 (Fig. 2, panel A) and between soluble S2 and immobilized OS9 (Fig. 2, panel B). We also assayed equine macrophage for OS-9 expression and detected an abundantly expressed protein by western blot using antisera to human OS-9 (Fig. 2, panel C).
Figure 2.
Panel A. Far western blots showing the interaction of immobilized recombinant S2 with recombinant V5-tagged hOS9 in CHO cell lysates. Lanes 1–3 are reagent controls. Lane 4 shows the binding of V5-OS9 to the membrane at the position of bacterially expressed, histidine tagged S2 (his-S2). The larger product in lane 4 is not present in the controls and may result from V5-OS9 binding to a slower migrating form of his-S2 or alternatively, interaction of V5-OS9 with a contaminating bacterial protein in the lysate. V5-OS9 is detected using mouse anti-V5 antibody. Lanes 5 and 6 show the positions of S2 and the control protein, bovine serum albumin. Panel B. Far western blot showing the interaction of immobilized V5-OS9 with soluble his-S2. Panel C. Western blot using anti-human OS9 indicates that equine macrophages express OS9. Lanes 1 and 2 contain lysates from equine monocyte-derived macrophages. Lane 3 contains a CHO cell lysate. Lane 4 contains a cell lysate from V5-tagged human OS9 transfected CHO cells.
We next obtained cDNA clones for the equine homologues of OS-9 and PSMC3 (accession nos. GU055515, GU196834). Total RNA was prepared from equine monocyte-derived macrophages and cDNA was prepared using SuperScript® II Reverse Transcriptase (Invitrogen) and reverse PCR primers. Initial PCR products were recovered by TOPO TA cloning. As the equine genome sequence was unavailable at the time these studies were initiated, available sequences from other species were used to design degenerate primers for amplification of equine OS-9 and equine PSMC3 by RT-PCR. Primers used to amplify equine OS-9 were OS-9 Forward primer (5′ ATGGCGGCGGARDCGCTGCTGT) and Reverse primer (5′ GGGTCAGAAGTCAAAYTCRTCCAGGTCCCCTGT 3′). Primers for PSMC3 were forward primer 5′-TCCACGGAGGAGATCATC-3′ and reverse primer 5′-CTAGGCGTAGTATTGTAG-3′. The 5′ and 3′ ends of equine OS9 and PSMC3 transcripts were obtained using rapid amplification of cDNA ends (RACE) kits (Invitrogen).
Partial cDNAs of equine OS-9 (eqOS-9) similar to hOS-9 isoforms 1 and 2 were recovered; a cDNA indicating a novel splice variant was also recovered and is shown schematically in Figure. 1B. Three eqOS-9 fusions were tested in the yeast 2-hybrid assay. These included fragments starting at amino acids 242 and 256 and ending just upstream of the location of the alternatively spliced region of hOS-9 (Fig. 1B). Both of these fragments showed strong interacting phenotypes when tested with S2 (Fig. 1A). The novel eqOS-9 isoform was also tested, but this form failed to interact in the yeast 2-hybrid assay suggesting that a region of approximately 40 amino acids (262–297 of eqOS9) is necessary for the interaction of eOS9 and S2.
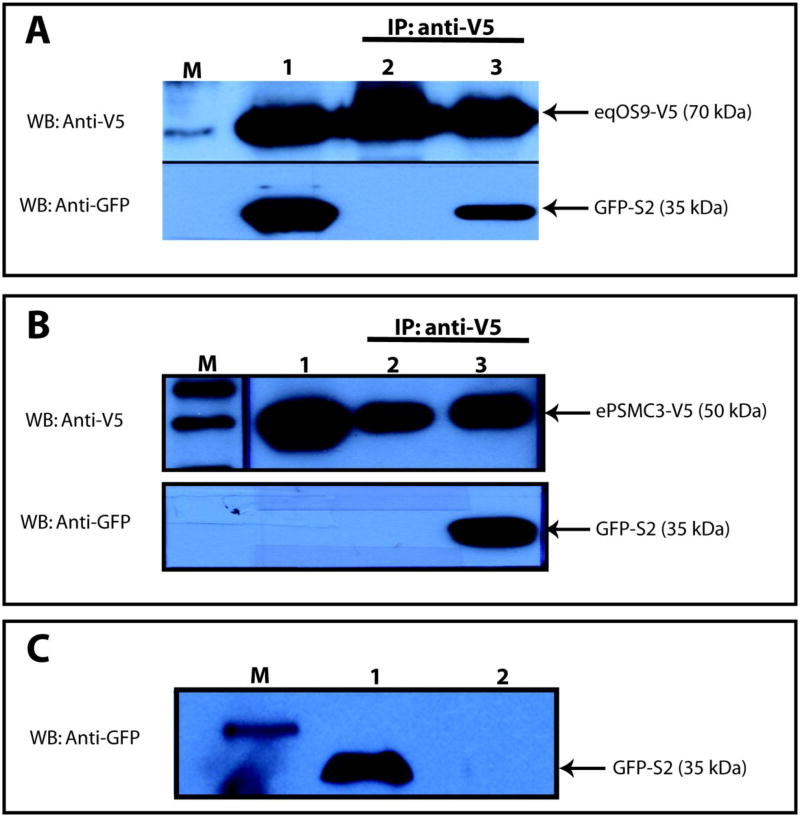
Interaction between EIAV S2 and eqOS-9 was also probed using a co-immunoprecipitation assay. Full length equine OS9 was expressed as a V5 fusion in pcDNA ™/V5–DEST (Invitrogen). EIAV S2 was expressed as an N-terminal GFP fusion. CHO cells were transfected using Lipofectamine ™ (Invitrogen) and cell lysates were harvested at 48 h post transfection. Protein expression was verified by western blot assay using mouse anti-V5 or mouse anti-GFP antibodies. Lysates were incubated with the appropriate antibody (anti-V5 or anti-GFP) and agarose G was used to collect the immunocomplexes. Samples were subjected to SDS-PAGE electrophoresis and proteins were transferred onto nitrocellulose membranes. As shown in Figure 3, when cell lysates were mixed, specific interactions between S2 and eqOS-9 were readily detected by co-immunoprecipitation.
Figure 3.
Co-immunoprecipitation of EIAV S2 with equine OS9 and PMSC3. Panel A. Co-IP of equine OS9-V5 and GFP-S2. Lane 1: Mixed lysates from equine OS9-V5 and S2-GFP transfected cells. Lane 2: Mixed lysates, equine OS9-V5 and GFP-control tranfected cells. Lane 3: Mixed lysates, equine OS9-V5 and GFP-S2 transfected cells. Lanes 2 and 3, immunoprecipitates with anti-V5. Western blots with anti-GFP or anti-V5 as indicated. Panel B. Co-IP of equine PSMC3-V5 and GFP-S2. Lane 1: Lysate from equine PSMC3-V5 transfected cells. Lane 2: ePSMC3-V5 transfected cell lysate mixed with GFP-control lysate; immunoprecipitated with anti-V5. Lane 3: ePSMC3-V5 transfected cell lysate mixed with GFP-S2 transfected cell lysate; immunoprecipitated with anti-V5. Panel C. GFP-S2 control. Lysate from GFP-S2 transfected cells was immunoprecipitated with anti-V5. Lane 1: Column flow-through. Lane 2. Column eluant. In all panels, M designates lanes with protein molecular weight markers.
A full-length cDNA encoding the equine homologue of PSMC3 (eqPSMC3) was also recovered. We determined that the sequence of the equine clone predicted a protein differing from hPSMC3 by only one amino acid. Because human PSMC3 gave only a weak interacting phenotype in the yeast two hybrid screen (Figure 1) we chose to directly test for the interaction of eqPSMC3 with S2 using the more specific co-immunoprecipitation assay. As shown in Figure 3 an interaction between S2 and eqPSMC3 was readily detected.
In summary, performing a yeast two-hybrid screen with a human spleen cDNA library revealed two S2 interacting proteins; their equine homologues were cloned and their interactions with S2 were confirmed. A rationale for using the commercially available human cDNA library was to avoid the need to prepare and extensively characterize an equine library. However we certainly recognize the likelihood of discovering additional S2 interacting proteins using an equine cDNA library.
The identification of OS-9 as an S2 interacting protein does not immediately provide a clear understanding of S2 function. OS9 is encoded on human chromosome 12q 13–15, a region frequently amplified in human cancers (Su et al., 1996). It is ubiquitously expressed and four isoforms have been identified (Bernasconi et al., 2008; Kimura et al., 1998). Both cytoplasmic and ER localization of OS-9 have been reported and multiple protein activities have been proposed. An early report describes a role for OS-9 in the regulation of proteosome-mediated degradation of the transcription factor HIF1-α in an oxygen dependent manner (Baek et al., 2005). OS-9 has also been reported to bind misfolded proteins in the ER lumen (Alcock and Swanton, 2009) or to play a role in protein trafficking by serving as a multi-target adaptor of proteins moving from the ER to the Golgi (Jansen et al., 2009; Litovchick et al., 2002; Wang et al., 2007). Various OS-9 isoforms appear to differ in their cellular location and/or function. For example, while transport of meprin-β by OS-9 requires isoform 1 it is inhibited by isoform 2 (Litovchick et al., 2002). In contrast, transport of dendritic cell-specific transmembrane protein (DC-STAMP) from the ER to the cis-golgi occurs in the presence of full-length OS9 isoforms 1 and 2, but is inhibited by a C-terminal deletion (Jansen et al., 2009). We observed an interaction of EIAV S2 with C-terminal deletions of both isoforms 1 and 2, but no interaction with a novel splice variant lacking amino acids 262 to 297. Thus the binding of S2 to OS9 may interrupt binding of some cellular OS9 partners, or may instead influence the trafficking or degradation of OS-9 containing complexes.
There is precedent for lentiviral accessory proteins in protein trafficking and degradation. HIV-1 Vpu, Vif and Vpr all induce the polyubiquitylation and proteasomal degradation of their cellular targets (reviewed in (Malim and Emerman, 2008)). HIV-1 Vpu is an accessory protein similar to EIAV S2 with regard to its position in the lentiviral genome and its expression strategy. Both Vpu and S2 are encoded by open reading frames that overlap env and both are expressed via leaky ribosome scanning (Krummheuer et al., 2007; Schiltz et al., 1992; Schwartz et al., 1992). HIV-1 Vpu activities include degradation of CD4 and interaction with proteasome members to inhibit IκB degradation (Malim and Emerman, 2008). Vpu is an integral membrane protein that contains a high proportion of charged residues in its cytoplasmic domain and has a pair of serine residues that are constitutively phosphorylated by casein kinase II (CKII). While S2 does not appear to have a transmembrane domain, it does have a myristilation signal such that myristilated S2 could direct the polypeptide to membranes. S2 also has a pair of conserved serine residues that could be substrates for CKII.
The interaction of EIAV S2 with PMSC3 is intriguing in that this cellular protein was first described as an HIV-1 Tat binding protein (thus designated Tat binding protein 1 or TBP1) (Nelbock et al., 1990). PMSC3/TBP1 (also referred to as 19S ATPase S6a) is one of 6 ATPases found in the base of the 19S regulatory subunit of the proteasome (Tanahashi et al., 1998) and is involved in diverse cellular processes. For example PMSC3/TBP1 promotes degradation of HIF1-α (Corn et al., 2003) but stabilizes cellular levels of the tumor suppressor p14ARF (Satoh et al., 2009). Other proteins that interact with PMSC3/TBP1 include the cellular tumor necrosis factor receptor associated factors (trafs) 4 and 6 (Rozan and El-Deiry, 2006) and the hepatitis B virus X protein (Barak et al. 2000). Proteasomal and nonproteasomal roles of the 19S regulatory subunit in transcription regulation of the HIV promoter have been described and the interaction between PSMC3/TBP1 and HIV Tat plays a role in the process by which 19S components facilitate transcriptional elongation (Lassot et al. 2007). Recently Traux et al. (2010) have demonstrated that this protein is crucial for regulating cytokine-inducible transcription of the transcription initiator of class II transactivator (CIITA), the master regulator of the major histocompatibility class II transcription complex (MHC-II).
As regards transcription regulation, there is no indication that S2 plays a role in viral gene expression. However our laboratory has recently demonstrated a role for S2 in enhancing pro-inflammatory cytokine and chemokine gene expression in infected macrophages (Covaleda et al. 2010). In these studies we observed that expression of interleukin (IL)-8, IL-34 and monocyte chemoattractant protein (MCP)-2 were enhanced by S2. As regards these genes, expression (as measured by quantitative PCR) was significantly increased by EIAV infection and there was also a significant difference in expression levels between the wild-type and S2-deleted viruses. In the case of IL-34, S2 appeared to be the dominant factor in expression levels as there was no difference in gene expression between the S2-deleted virus and mock-infected controls (Covaleda et al. 2010). Therefore it is intriguing to speculate that the interaction of S2 and PMSC3/TBP1 affects the role of the 19S regulatory subunit in transcription control, up-regulating a set of proinflammatory cytokines and chemokines. Up-regulation of immune modulators is certainly consistent with an S2 function that is not evident in cultured cells, but that has a pronounced effect in the animal; one such function could be the recruitment of susceptible cells to sites of virus replication.
Acknowledgments
We thank Dr. Rebecca Parr for expert advice and assistance with yeast 2-hybrid assays. We thank Melissa Harville for assistance with cloning and sequence analysis. This work was supported by Public Health Service grant number CA-59278 from the National Cancer Institute (to S.L.P and F.J.F).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcock F, Swanton E. Mammalian OS-9 is upregulated in response to endoplasmic reticulum stress and facilitates ubiquitination of misfolded glycoproteins. J Mol Biol. 2009;385(4):1032–1042. doi: 10.1016/j.jmb.2008.11.045. [DOI] [PubMed] [Google Scholar]
- Barak O, Aronheim A, Shaul Y. HBV X protein targets HIV Tat-binding protein 1. Virology. 2000;283 (1):110–120. doi: 10.1006/viro.2001.0883. [DOI] [PubMed] [Google Scholar]
- Baek JH, Mahon PC, Oh J, Kelly B, Krishnamachary B, Pearson M, Chan DA, Giaccia AJ, Semenza GL. OS-9 interacts with hypoxia-inducible factor 1alpha and prolyl hydroxylases to promote oxygen-dependent degradation of HIF-1alpha. Mol Cell. 2005;17 (4):503–512. doi: 10.1016/j.molcel.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Bernasconi R, Pertel T, Luban J, Molinari M. A dual task for the Xbp1-responsive OS-9 variants in the mammalian endoplasmic reticulum: inhibiting secretion of misfolded protein conformers and enhancing their disposal. J Biol Chem. 2008;283 (24):16446–16454. doi: 10.1074/jbc.M802272200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JE, Payne SL. Molecular basis of the pathobiology of lentiviruses. Virus Res. 1994;32 (2):97–109. doi: 10.1016/0168-1702(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Corn PG, McDonald ER, 3rd, Herman JG, El-Deiry WS. Tat-binding protein-1, a component of the 26S proteasome, contributes to the E3 ubiquitin ligase function of the von Hippel-Lindau protein. Nat Genet. 2003;35 (3):229–237. doi: 10.1038/ng1254. [DOI] [PubMed] [Google Scholar]
- Covaleda L, Fuller FJ, Payne SL. EIAV enhances pro-inflammatory cytokine and chemokine response in infected macrophages. Virology. 2010;397 (1):217–223. doi: 10.1016/j.virol.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerness AJ, Flaherty MT, Perry ST, Jia B, Payne SL, Fuller FJ. The S2 accessory gene of equine infectious anemia virus is essential for expression of disease in ponies. Virology. 2006;349 (1):22–30. doi: 10.1016/j.virol.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Hoyle J, Tan KH, Fisher EM. Localization of genes encoding two human one-domain members of the AAA family: PSMC5 (the thyroid hormone receptor-interacting protein, TRIP1) and PSMC3 (the Tat-binding protein, TBP1) Human Genet. 1997;99 (2):285–288. doi: 10.1007/s004390050356. [DOI] [PubMed] [Google Scholar]
- Jansen BJ, Eleveld-Trancikova D, Sanecka A, van Hout-Kuijer M, Hendriks IA, Looman MG, Leusen JH, Adema GJ. OS9 interacts with DC-STAMP and modulates its intracellular localization in response to TLR ligation. Mol, Immunol. 2009;46 (4):505–515. doi: 10.1016/j.molimm.2008.06.032. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Nakazawa M, Yamada M. Cloning and characterization of three isoforms of OS-9 cDNA and expression of the OS-9 gene in various human tumor cell lines. J Biochem. 1998;123 (5):876–882. doi: 10.1093/oxfordjournals.jbchem.a022019. [DOI] [PubMed] [Google Scholar]
- Krummheuer J, Johnson AT, Hauber I, Kammler S, Anderson JL, Hauber J, Purcell DF, Schaal H. A minimal uORF within the HIV-1 vpu leader allows efficient translation initiation at the downstream env AUG. Virology. 2007;363 (2):261–271. doi: 10.1016/j.virol.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Lassot I, Latreille D, Rousset E, Sourisseau M, Linares LK, Chable-Bessia C, Coux O, Benkirane M, Kiernan RE. The proteasome regulates HIV-1 transcription by both proteolytic and nonproteolytic mechanisms. Mol Cell. 2007;25 (3):369–383. doi: 10.1016/j.molcel.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Li F, Leroux C, Craigo JK, Cook SJ, Issel CJ, Montelaro RC. The S2 gene of equine infectious anemia virus is a highly conserved determinant of viral replication and virulence properties in experimentally infected ponies. J Virol. 2000;74 (1):573–579. doi: 10.1128/jvi.74.1.573-579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Puffer BA, Montelaro RC. The S2 gene of equine infectious anemia virus is dispensable for viral replication in vitro. J Virol. 1998;72 (10):8344–8348. doi: 10.1128/jvi.72.10.8344-8348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovchick L, Friedmann E, Shaltiel S. A selective interaction between OS-9 and the carboxyl-terminal tail of meprin beta. J Biol Chem. 2002;277 (37):34413–34423. doi: 10.1074/jbc.M203986200. [DOI] [PubMed] [Google Scholar]
- Malim MH, Emerman M. HIV-1 accessory proteins--ensuring viral survival in a hostile environment. Cell Host Microbe. 2008;3 (6):388–398. doi: 10.1016/j.chom.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Montelaro RC, Ball JM, Rushlow KE. Equine retroviruses. In: LJA, editor. The Retroviridae. Vol. 2. Plenum Press; New York and London: 1993. pp. 257–360. 4 vols. [Google Scholar]
- Nelbock P, Dillon PJ, Perkins A, Rosen CA. A cDNA for a protein that interacts with the human immunodeficiency virus Tat transactivator. Science (Washington, DC, US) 1990;248 (4963):1650–1653. doi: 10.1126/science.2194290. [DOI] [PubMed] [Google Scholar]
- Payne S, Lim WS, Fuller FJ, Ball JM. Equine infectious anemia virus as a model for lentiviral pathogenesis. In: Friedman H, Specter S, Bendinelli M, editors. Vivo Models of HIV Disease and Control/edited by Herman Friedman, Steven Specter, Mauro Bendinelli. Springer; New York: 2006. pp. 365–390. [Google Scholar]
- Rozan LM, El-Deiry WS. Identification and characterization of proteins interacting with Traf4, an enigmatic p53 target. Cancer Biology Therapy. 2006;5 (9):1228–1235. doi: 10.4161/cbt.5.9.3295. [DOI] [PubMed] [Google Scholar]
- Satoh T, Ishizuka T, Tomaru T, Yoshino S, Nakajima Y, Hashimoto K, Shibusawa N, Monden T, Yamada M, Mori M. Tat-binding protein-1 (TBP-1), an ATPase of 19S regulatory particles of the 26S proteasome, enhances androgen receptor function in cooperation with TBP-1-interacting protein/Hop2. Endocrinology. 2009;150 (7):3283–3290. doi: 10.1210/en.2008-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz RL, Shih DS, Rasty S, Montelaro RC, Rushlow KE. Equine infectious anemia virus gene expression: characterization of the RNA splicing pattern and the protein products encoded by open reading frames S1 and S2. J Virol. 1992;66 (6):3455–3465. doi: 10.1128/jvi.66.6.3455-3465.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Felber BK, Pavlakis GN. Mechanism of translation of monocistronic and multicistronic human immunodeficiency virus type 1 mRNAs. Mol Cell Biol. 1992;12 (1):207–219. doi: 10.1128/mcb.12.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YA, Hutter CM, Trent JM, Meltzer PS. Complete sequence analysis of a gene (OS-9) ubiquitously expressed in human tissues and amplified in sarcomas. Mol Carcinog. 1996;15 (4):270–275. doi: 10.1002/(SICI)1098-2744(199604)15:4<270::AID-MC4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Tanahashi N, Suzuki M, Fujiwara T, Takahashi E, Shimbara N, Chung CH, Tanaka K. Chromosomal localization and immunological analysis of a family of human 26S proteasomal ATPases. Bioch Biophys Res Commun. 1998;243 (1):229–232. doi: 10.1006/bbrc.1997.7892. [DOI] [PubMed] [Google Scholar]
- Truax AD, Koues OI, Mentel MK, Greer SF. The 19S ATPase S6a (S6′/TBP1) regulates the transcription initiation of class II transactivator. J Mol Biol. 2010;395 (2):254–269. doi: 10.1016/j.jmb.2009.10.035. [DOI] [PubMed] [Google Scholar]
- Wang Y, Fu X, Gaiser S, Kèottgen M, Kramer-Zucker A, Walz G, Wegierski T. OS-9 regulates the transit and polyubiquitination of TRPV4 in the endoplasmic reticulum. J Biol Chem. 2007;282 (50):36561–36570. doi: 10.1074/jbc.M703903200. [DOI] [PubMed] [Google Scholar]
- Yoon S, Kingsman SM, Kingsman AJ, Wilson SA, Mitrophanous KA. Characterization of the equine infectious anaemia virus S2 protein. J Gen Virol. 2000;81(Pt 9):2189–2194. doi: 10.1099/0022-1317-81-9-2189. [DOI] [PubMed] [Google Scholar]