Abstract
IL-17 mediates essential inflammatory responses in host defense and autoimmunity. The IL-17A/IL-17F signaling complex is composed of IL-17RA and IL-17RC, both of which are necessary for signal transduction. To date, the specific contribution of IL-17RC to downstream signaling remains poorly understood. To define the regions within the IL-17RC cytoplasmic tail required for signal transduction, we assayed signaling by a panel of IL-17RC deletion mutants. These findings reveal that IL-17RC inducibly associates with a specific glycosylated IL-17RA isoform, in a manner independent of the IL-17RC cytoplasmic tail. Using expression of the IL-17 target genes IL-6 and 24p3/lipocalin-2 as a readout, functional reconstitution of signaling in IL-17RC−/− fibroblasts required the SEFIR domain, a conserved motif common to IL-17R family members. Unexpectedly, the IL-17RC SEFIR alone was not sufficient to reconstitute IL-17-dependent signaling. Rather, an additional sequence downstream of the SEFIR was also necessary. We further found* that IL-17RC interacts directly with the adaptor/E3 ubiquitin ligase Act1, and that the functional IL-17RC isoforms containing the extended SEFIR region interact specifically with a phosphorylated isoform of Act1. Finally, we show that IL-17RC is required for in vivo IL-17-dependent responses during oral mucosal infections caused by the commensal fungus Candida albicans. These results indicate that IL-17RC is vital for IL-17-dependent signaling both in vitro and in vivo. Insight into the mechanisms by which IL-17RC signals helps shed light on IL-17-dependent inflammatory responses, and may ultimately provide an avenue for therapeutic intervention in IL-17-mediated diseases.
Introduction
Interleukin (IL)-17 (IL-17A) plays a major host protective function at mucosal surfaces by driving inflammatory responses in mesenchymal and epithelial cell types (1, 2). The inflammatory program initiated by IL-17A leads to induction of IL-6, CXC chemokines, matrix metalloproteinases, acute phase reactants and anti-microbial peptides such as β-defensins and lipocalin-2/24p3 (3, 4). The potent inflammatory properties of IL-17A establish its critical role in host defense against extracellular pathogens. Conversely, aberrations in the IL-17A signaling axis correlate with a number of autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus and psoriasis (5).
The fundamental mechanisms by which IL-17A transduces signals are still poorly understood (6). IL-17A and a closely related family member IL-17F mediate their inflammatory activities via the IL-17 receptor (IL-17R) complex, composed of IL-17RA and IL-17RC. IL-17RA was the first receptor to be indentified for this cytokine family (7). Bioinformatic studies identified a domain encoded within the IL-17RA intracellular tail that is found in all IL-17R family members, termed “similar expression to FGF/IL-17R (SEFIR)” (8). The SEFIR domain was discovered based on homology with a functional domain within Toll-/IL-1R family members (TIR). The SEFIR domain is also found in the signaling adaptor/E3 ubiquitin ligase Act1/CIKS (8), a downstream signaling intermediate in the IL-17R pathway. To recruit Act1 and promote downstream signaling, IL-17RA depends on homotypic interactions between its SEFIR domain and the Act1 SEFIR (9–11). Structure-function studies subsequently showed that the IL-17RA SEFIR motif is critical for activation of IL-17A-dependent downstream effector pathways including the MAPK, NF-κB, and C/EBP pathways (10, 12). Although the SEFIR domain is essential for IL-17RA-mediated signaling, it is not sufficient; rather, a region extending beyond the conserved SEFIR is also required for activation of NF-κB and downstream target gene expression (12).
While IL-17RA is required for IL-17 signaling, genetic evidence (13) as well as the recent crystal structure of the IL-17F-bound IL-17RA receptor subunit (14) indicates that the ligand-bound IL-17R complex is an IL-17RA and IL-17RC heterodimer (15). However, the precise stoichiometry and the relative biochemical contributions of the IL-17RA and IL-17RC subunits to downstream signaling are still not well defined (14). Notwithstanding the requirement of both IL-17RA and IL-17RC to transduce IL-17 signals, IL-17RA and IL-17RC have strikingly distinct tissue expression patterns. IL-17RA is expressed in most cell types, with high levels in hematopoietic cells relative to other cell types. In contrast, IL-17RC is expressed at high levels in glandular tissues such as the adrenal gland, prostate, liver, and thyroid, with little expression in hematopoietic tissues (16, 17). Recent reports, however, have identified IL-17-dependent responses in hematopoietic cell types (17–19). Therefore, it is not certain whether IL-17RC is indeed needed in all tissues to mediate IL-17-dependent signal transduction.
While the specific contributions of the IL-17RC subunit to IL-17 signaling remain unknown, previous studies indicated that the cytoplasmic tail of IL-17RC is essential for functional IL-17A-dependent responses in fibroblast cells (13, 20). Since IL-17RA uses sequences beyond the SEFIR domain to mediate signaling, we aimed to define regions within the IL-17RC cytoplasmic tail that are integral to IL-17A and IL-17F signaling and to determine IL-17RC function in IL-17-dependent immune responses in vivo. Here, we show that IL-17A and IL-17F promote the association of IL-17RC with a glycosylated IL-17RA isoform. This association is not dependent on the IL-17RC cytoplasmic tail. Moreover, the IL-17RC SEFIR domain is necessary but not sufficient to mediate IL-17 dependent signaling. Rather, an additional ~20–30 amino acid motif downstream of the IL-17RC SEFIR (“SEFEX”) is necessary for activation of NF-kB-dependent genes such as IL-6 and 24p3. We also found that this SEFEX region isis required for ligand-dependent interaction with a phosphorylated Act1 species. These data demonstrate for the first time that Act1 is phosphorylated and that the phospho-Act1 isoform preferentially associates with the IL-17R complex upon IL-17 stimulation. Finally, we show that both IL-17RC and IL-17RA are required in vivo for immunity to oral infection with the commensal yeast Candida albicans, and that IL-17RC functions indistinguishably from IL-17RA in this regard.
Materials and Methods
Cell Cultures and Luciferase Assays
Primary IL-17RC−/− fibroblasts were isolated from IL-17RC−/− adult tail-tips (20). IL-17RA−/− fibroblasts from IL-17RA−/− mice were immortalized with the SV40 T antigen as described (12). ST-2 murine stromal cells, HEK 293T cells and fibroblasts were cultured with α-MEM (Sigma, St. Louis, MO) with 10% FBS, L-Glutamine, and antibiotics (Invitrogen, Carlsbad, CA). HEK 293T cells were transfected using Fugene 6 per manufactuer’s instructions (Roche, Indianapolis, IN). IL-17RC−/− and IL-17RA−/− fibroblasts were transfected with the Amaxa MEF2 Nucleofector Kit per manufacturer’s instructions (Lonza, Germany), and luciferase assays were performed as previously described (21). Cytokines were from Peprotech (Rocky Hill, NJ).
Plasmids
IL-17RC cDNA (encoding full length, unspliced IL-17RC) was generated from ST-2 cells by RT-PCR. The IL-17RC truncations were generated by PCR with a C-terminal Myc tag, and confirmed by sequencing. The murine IL-17RA construct was previously described (12). Act1 cDNA was obtained by RT-PCR from ST2 cells, and fused to an N-terminal CFP tag in pcDNA3.1.Zeo (Invitrogen, Carlsbad CA). The 24p3 promoter luciferase reporter was previously described (21).
ELISA and Flow Cytometry
IL-6 ELISA kits were from eBioscience (San Diego, CA). Flow cytometry was performed on a FACSCalibur with Cell Quest Software (BD Biosciences, San Diego, CA). Cells were stained with an anti-IL-17RC Ab followed by a PE-anti-goat Ig Ab (R & D Systems, Minneapolis, MN).
Western Blotting, EMSA and Immunoprecipitation
Western blotting, EMSA and immunoprecipitation experiments were performed as described (12). Immunoprecipitates were incubated with shrimp alkaline phosphatase (Fermentas, Glen Burnie, MD) at 37°C for 1 h. Anti-mIL-17RA Abs were from R&D Systems. Anti-Act1 Abs were from Santa Cruz Technologies (Santa Cruz, CA) and anti-MYC Abs were from Cell Signaling (Cell Signaling, Beverly, MA). Mouse TrueBlo ULTRA Horseradish Peroxidase (HRP) anti-mouse IgG was from eBioscience (San Diego, CA). Densitometry was performed using Image J software on scanned gels.
Mouse model of OPC
IL-23p19−/− and IL-17RC−/− mice were generously provided by Genentech, produced in collaboration between Genentech and Lexicon Pharmaceuticals to analyze the function of 500 secreted and transmembrane proteins (20). IL-17RA−/− mice were provided by Amgen. Age- and sex-matched WT control mice were from The Jackson Laboratory (Bar Harbor, ME). Mice were infected under anesthesia by placing a 0.0025g cotton ball saturated with 2×107 CFU C. albicans (strain CAF2-1) sublingually for 75 min, as previously described (22, 23). If indicated, 225 mg/kg cortisone acetate (Sigma-Aldrich, St Louis MO) was injected days -1, 1 and 3 relative to infection. Tongue was homogenized and analyzed for CFU/g tissue, and paraffin-embedded tongue sections were stained with periodic-acid Schiff (PAS) by the University at Buffalo Histology Core Facility or the University of Pittsburgh Research Histology Services. Protocols were approved by the SUNY Buffalo and University of Pittsburgh IACUC.
Results
An experimental system for evaluating IL-17RC functional signaling domains
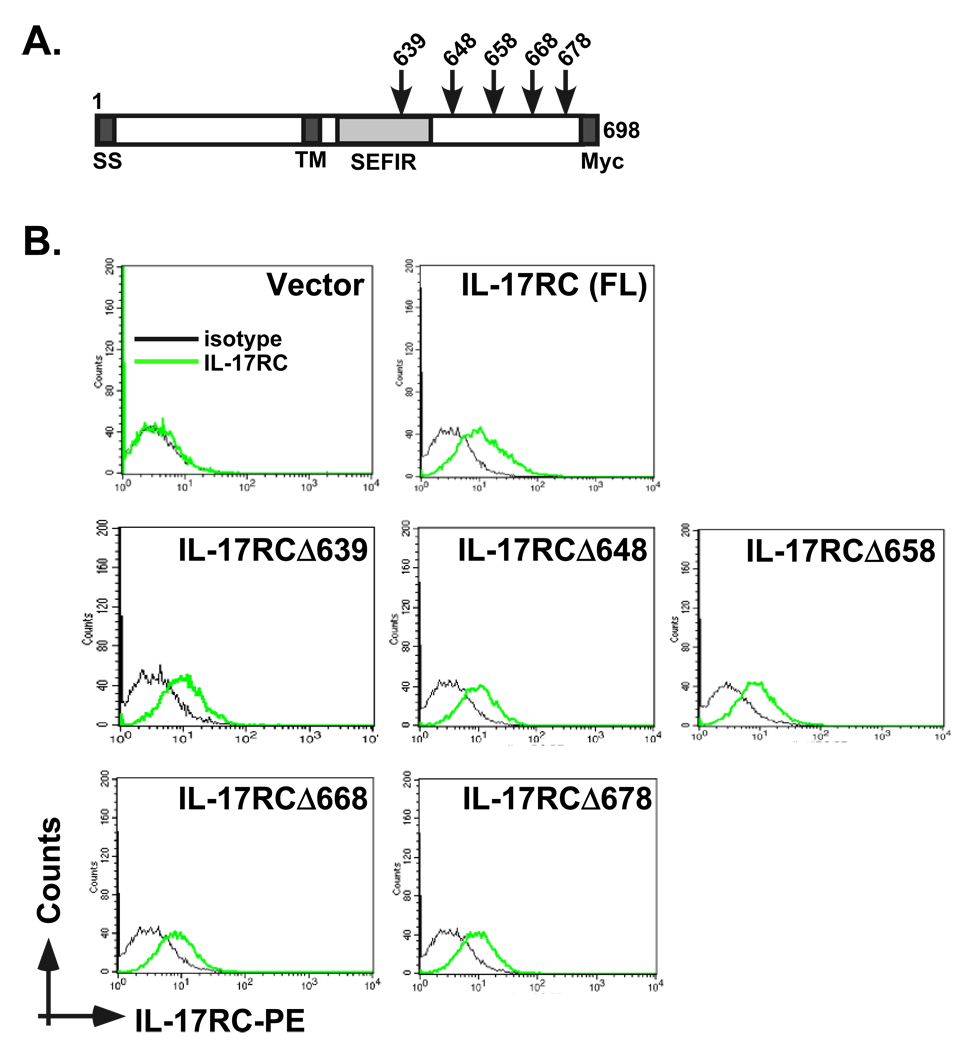
To delineate motifs within the IL-17RC intracellular domain required for functional signaling responses, we established a system to study IL-17 signaling analysis in murine IL-17RC−/− tail-tip fibroblasts and HEK293T cells. Due to the requirement of IL-17RC for IL-17 signaling and the failure of the human and murine receptor subunits to complement one another (13), IL-17RC−/− fibroblasts and HEK293T cells lacking mIL-17RC are deficient in IL-17-responses, and therefore provide us with a useful experimental platform to perform IL-17RC signaling analysis (13, 20). Accordingly, we created a series of murine carboxyl-terminally truncated IL-17RC mutants (Fig 1A). The IL-17RC truncations included deletions that lack the SEFIR signaling domain (amino acids 495–645), which is uniquely found on IL-17R family members and is critical for IL-17RA signaling (6, 8). Cell surface expression of these mutants was verified by flow cytometry (Fig 1B).
Figure 1. System for analyzing IL-17RC functional mutants.
A. Schematic diagram of murine IL-17RC and the constructs used in this report. The location of each deletion is indicated. The C-terminus is tagged with Myc. SS = signal sequence, TM = transmembrane domain, SEFIR = SEF/IL-17R conserved domain (amino acids 495–645). B. IL-17RC deletion mutants are equivalently expressed at the cell surface. HEK293T cells were transfected with the panel of IL-17RC deletions and stained for murine IL-17RC by flow cytometry. Black line = isotype control stain, grey line = IL-17RC stain.
IL-17RC association with IL-17RA does not require the IL-17RC intracellular domain
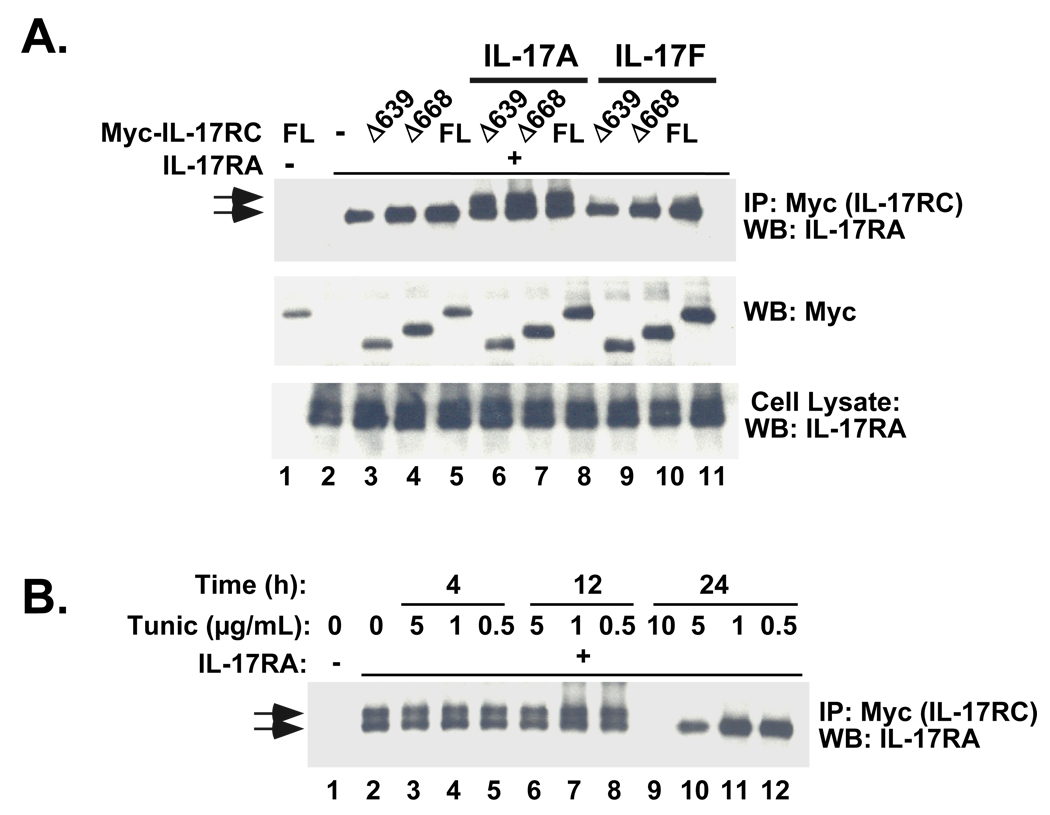
The ligand-bound IL-17R complex is reported to be composed of both IL-17RA and IL-17RC, and previous FRET studies suggested that IL-17RA forms homodimers, at least in the unliganded state (13, 14, 24). We thus questioned whether the association of IL-17RC and IL-17RA occurs in a ligand-dependent manner, and whether this interaction requires any portion of the IL-17RC intracellular domain. Accordingly, HEK293T cells were co-transfected with a plasmid encoding full-length murine IL-17RA together with various IL-17RC receptor truncations. There was baseline association of IL-17RA and IL-17RC, which was enhanced by treatment with IL-17A and and IL-17F (Fig 2A). Unlike the toll-like receptors (TLRs) (25), the association of IL-17RA with IL-17RC appeared to be independent of the IL-17RC cytoplasmic tail, as none of the IL-17RC cytoplasmic truncations were defective in association with IL-17RA (Fig 2A, Supplementary Fig. S2).
Figure 2. Stimulation of the IL-17R complex causes inducible association of IL-17RC with a specific glycosylated isoform of IL-17RA, independent of the cytoplasmic domain of IL-17RC.
A. HEK293T cells were transiently co-transfected with combinations of IL-17RC mutants and full length (FL) IL-17RA. Cells were treated with 200 ng/ml IL-17A or IL-17F for 20 minutes (lanes 6–8, 9–11, respectively) or with no cytokine (lanes 1–5), and lysates were immunoprecipitated with anti-Myc Abs. Immunoprecipitates (top and middle) were blotted with Abs to Myc and IL-17RA, as indicated and whole cell lysates (bottom) were immunoblotted with Abs to IL-17RC. B. HEK 293 cells were transfected with IL-17RA and IL-17RC. Cells were treated with the indicated concentrations of tunicamycin for 4, 12 or 24 hours prior to preparation of cell lysates. Lysates were immunoprecipiated with Abs to IL-17RC and immunoblotted with Abs to IL-17RA.
Interestingly, IL-17A and IL-17F treatment caused the association with a slower-migrating IL-17RA isoform, although IL-17A was more potent than IL-17F (Fig. 2A, lanes 6–11). Several differentially glycosylated forms of IL-17RA have been reported (7, 19, 26), but the biochemical nature of the specific IL-17RA molecule that is pulled down with IL-17RC was unclear. To assess whether glycosylation accounted for the larger IL-17RA isoform, we pretreated IL-17RA-transfected cells with tunicamycin to deglycosylate IL-17RA before immunoprecipitation. Upon tunicamycin treatment, the larger IL-17RA isoform resolved to a single band (Fig. 2B). These results indicate that IL-17A and IL-17F enhance formation of a multimeric receptor complex containing a specific glycosylated isoform of IL-17RA paired with IL-17RC.
An extended region beyond the SEFIR domain is required for functional IL-17RC signaling
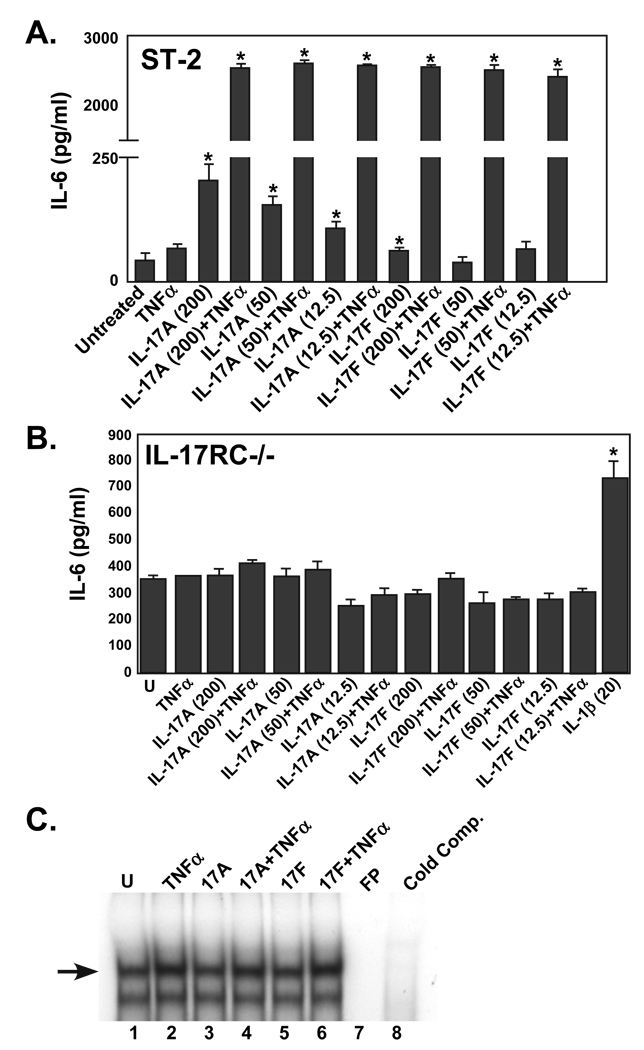
To delineate motifs within the IL-17RC cytoplasmic domain necessary for functional responses, primary fibroblasts from IL-17RC−/− mice (20) were prepared and assayed for IL-17-dependent signaling. Treatment of these cells with IL-17 or IL-17F alone or in combination with TNFα (with which IL-17 exhibits potent synergistic responses, (27, 28)) did not induce secretion of IL-6 or activation of NF-κB compared to ST-2 control cells or WT fibroblasts (Fig 3A–B and data not shown), whereas a TNFα control induced NF-κB (Fig. 3C) and an IL-1β control induced IL-6 secretion (Fig 3B). Induction of other target genes such as 24p3/lipocalin 2 and CXCL5 in response to IL-17A signaling was also impaired in these cells (data not shown). Therefore, IL-17RC is essential in fibroblasts for IL-17A-dependent signaling responses.
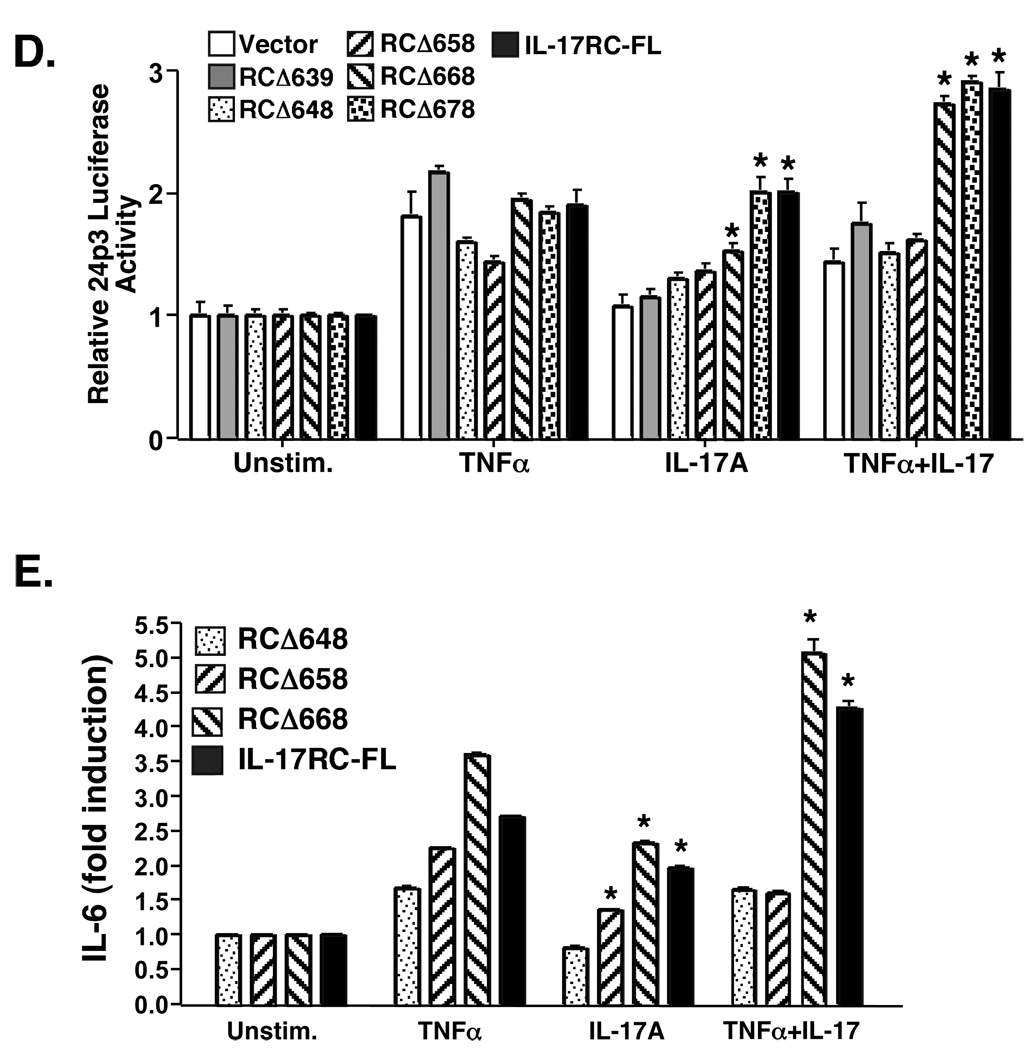
Figure 3. An extended region of the IL-17RC intracellular domain beyond the SEFIR is required for IL-17-dependent signal transduction.
A–B. ST-2 cells (A) or primary IL-17RC−/− fibroblasts (B) were stimulated with the indicated concentrations (ng/ml) of IL-17A, IL-17F or IL-1β in the presence or absence of TNFα (2 ng/ml) in triplicate. Supernatants were analyzed for IL-6 by ELISA. SD are shown. * p<0.01 C. IL-17RC−/− fibroblasts were stimulated with IL-17A, IL-17F and/or TNFα and nuclear extracts were subjected to EMSA with a 32P-labeled oligonucleotide NF-κB probe. Where indicated, a 50-fold excess of unlabeled probe was used as a control. FP = free probe alone. Arrow indicates NF-κB complex. D. IL-17RC−/− primary fibroblasts were transiently transfected with the indicated IL-17RC deletion mutants (FL= full length, unspliced IL-17RC) together with the 24p3-Luc reporter (21) and an internal Renilla luciferase control. Cells were stimulated for 5 hours with IL-17 (200 ng/ml), TNFα (2 ng/ml) or both cytokines. Cell lysates were subjected to standard luciferase assays in triplicate. Data were normalized to the R-luciferase control and to the untreated control samples. Standard deviations are shown, *p<0.01 by student’s t-Test relative to vector control. E. Supernatants from the cells transfected in panel C were analyzed in triplicate for IL-6 expression by ELISA and normalized to the unstimulated control. Standard deviations are shown, *p<0.01 relative to vector control.
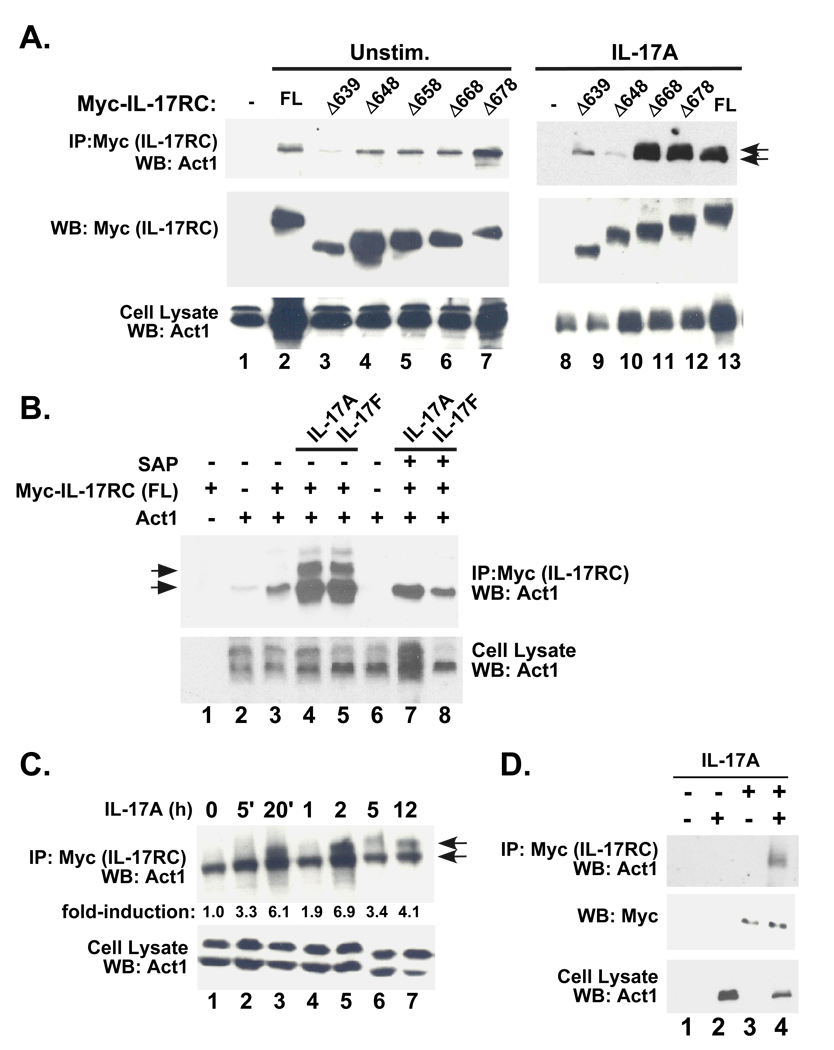
Since IL-17RC is required for signaling in these cells, we transfected IL-17RC−/− cells with the panel of IL-17RC receptor truncations in attempt to reconstitute signaling. Cells were then treated with IL-17 alone or in combination with TNFα and assessed for cytokine-dependent induction of prototypical IL-17 target genes, including 24p3/lipocalin 2 and IL-6 (12, 29). Cells expressing the IL-17RCΔ668, IL-17RCΔ678, and IL-17RC-FL receptors activated the 24p3 promoter reporter (Fig 3D) and produced IL-6 in response to IL-17A alone and in combination with TNFα (Fig 3E). There was a very small but statistically significant induction of IL-6 secretion with the IL-17RCΔ658 mutant following IL-17 treatment alone, but no activity in the synergistic condition or in IL-17-mediated activation of the 24p3 reporter. This result is likely due to the low signal-noise ratio of this assay, and that the IL-17RCΔ658 mutant does not recapitulate full-length IL-17RC function. Intriguingly, IL-17RC mutants such as IL-17RCΔ648 failed to respond to IL-17A, even though these deletions retain the entire conserved SEFIR domain (Fig 1A, 3D–E). This result suggests that, similar to IL-17RA, the IL-17RC SEFIR domain is not sufficient for functional IL-17-mediated signaling. Rather, an additional region downstream of the IL-17RC SEFIR is also necessary, the terminus of which extends to between amino acids 658 and 668. Hence, we term this the “SEFEX” domain, for “SEFIR-extension.”
The adaptor molecule Act1 is an E3 ubiquitin ligase required to link IL-17RA with downstream signaling pathways such as TRAF6 and NF-κB (30), although Act1 may not be fully necessary for ERK activation (10). Act1 has been shown to co-IP with IL-17RA (9, 10). To determine whether Act1 also associates with IL-17RC, HEK 293T cells were co-transfected with a CFP-tagged murine Act1 together with the panel of IL-17RC truncations. The association of Act1 with the IL-17RC mutants was evaluated by co-IP in the presence of IL-17A and IL-17F. A low baseline level of Act1 was pulled down with all IL-17RC deletion mutants tested (Fig 4A, lanes 1–7). Interestingly, this constitutively-associated Act1 represented the smaller of 2 isoforms of this protein (Fig 4A, bottom arrow). Notably, a larger Act1 isoform was inducibly associated with IL-17RC (Fig 4A, top arrow, lanes 11–13). Moreover, the larger isoform of Act1 associated only with IL-17RC variants that were shown to be capable of mediating signaling; namely, IL-17RCΔ668, IL-17RAΔ678 and IL-17RC-FL, perhaps suggesting that this Act1 isoform may be the version of this molecule competent for signaling in the IL-17 pathway.
Figure 4. IL-17RC inducibly associates with a phosphorylated isoform of Act1.
A. HEK293T cells were transiently co-transfected with Act1 together with various IL-17RC deletion mutants. Cells were treated with no cytokines or IL-17A or for 20 minutes. Lysates were immunoprecipitated with Abs to Myc and immunoblotted with α-Act1 Abs (top) or α-Myc Abs (middle). Whole cell lysates were immunoblotted with Abs to Act1 (bottom). Arrows indicate the 2 isoforms of Act1 that associate with IL-17RC-FL, IL-17RCΔ678 and IL-17RCΔ668. B. HEK293T cells were transiently co-transfected as described in panel A in the presence or absence of IL-17A or IL-17F. Lysates were then immunoprecipitated with anti-Myc Abs. Where indicated, immunoprecipitates were then treated with SAP for 1 hour at 37°C, and immunoblotted with Abs to Act1 (top). Whole cell lysates were immunoblotted with Abs to Act1 (bottom). C. HEK293T cells were transfected with Myc-IL-17RC (full length) together with Act and stimulated with IL-17A for the indicated times. Lysates were immunoprecipitated with anti-Myc Abs and immunoblotted for Act1 (top). Whole cell lysates were immunoblotted with Abs to Act1 (bottom). Intensity of the slower-migrating band was assessed by densitometry of replicate experiments. and values are indicated as fold-induction over the 0 time point. D. IL-17RA−/− fibroblasts were transfected with IL-17RC and Act1, stimulated with IL-17A and lysates immunoprecipitated with Abs to IL-17RC. IPs and lysates were blotted with Abs to Act1.
To better characterize the isoform of Act1 that correlates with functional IL-17RC receptor mutants, we examined whether this Act1 isoform was phosphorylated. To that end, HEK293T cells were co-transfected with Act1 and IL-17RC, stimulated with IL-17 for 20 minutes, and immunoprecipitates were treated with shrimp alkaline phosphatase (SAP) (Fig 4B). SAP treatment resulted in the complete disappearance of the larger Act1 isoform and also a diminution of both isoforms, confirming that the functional Act1 species represents a phosphorylated moiety. To characterize the kinetics of phospho-Act1 association with IL-17RC, a time course was performed. Phospho-Act1 co-association with IL-17RC showed a reproducibly bisphasic profile, rising to a ~6-fold induction 20 minutes after IL-17A stimulation, diminishing after 1hr, and reappearing to high levels at 2–12 hours (Fig 4C).
HEK293 cells express high levels of endogenous IL-17RA (although there is evidence that murine IL-17RC does not partner with human IL-17RA. Nonetheless, to determine whether IL-17RA is required to bridge Act1 and IL-17RC, co-transfection and pulldown experiments with IL-17RC and Act1 were performed in IL-17RA−/− cells stimulated with IL-17A (Fig. 4D). As shown, IL-17RC could still immunoprecipitate Act1, indicating.
IL-17RC−/− mice are susceptible to infection with Candida albicans
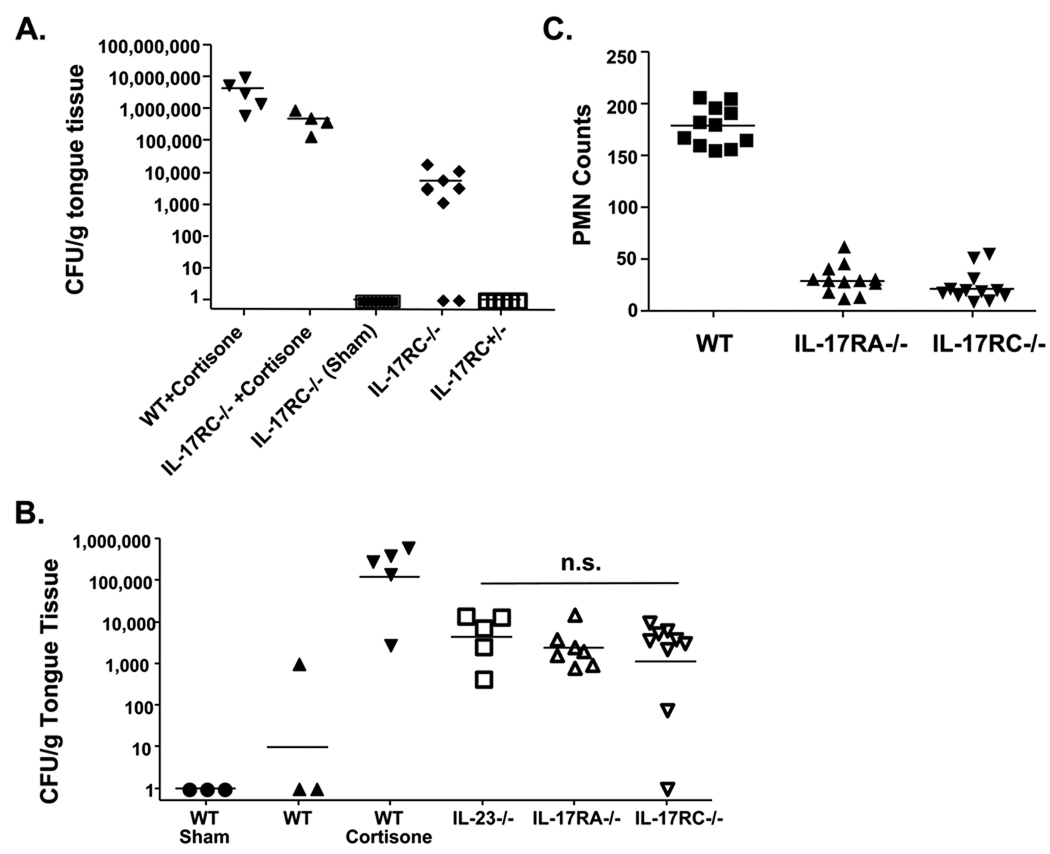
We have previously shown that IL-17RA−/− mice are highly prone to infection with Candida albicans, a commensal yeast of the human oral cavity that causes oropharyngeal candidiasis (OPC, thrush) in immunocompromised individuals (22). To determine whether IL-17RC−/− mice show a similar susceptibility and to establish whether IL-17RC is required for in vivo IL-17-dependent immune responses, mice were infected orally with C. albicans, and 5 days later the tongue was evaluated for fungal load and histological evidence of pathology. As a positive control, WT and IL-17RC−/− mice were immunosuppressed with cortisone. As shown, cortisone-treated mice had a high fungal load following infection (Fig 5A). There was no detectable colonization in Sham-infected IL-17RC−/− mice, which was expected since C. albicans is not a normal commensal in rodents. However, IL-17RC−/− mice showed a dramatic increase in fungal burden in the oral cavity, whereas heterozygous IL-17RC+/− mice fully cleared the infection with no residual fungal load by Day 5 (Fig 5A). Consistent with the fungal burden in the oral cavity, invasion of hyphal and psuedohyphal forms of C. albicans were visible on the tongue in cortisone-treated mice (data not shown) as well as infected IL-17RC−/− mice (Supplemental Fig. S1). The surface epithelium of the tongues in these mice were damaged by the invading yeast. In contrast, sham-infected mice and infected IL-17RC+/− mice showed no evidence of Candida, and the surface epithelium of the tongue was undamaged.
Figure 5. IL-17RC−/− mice are susceptible to oropharyngeal candidiasis equivalently to IL-17RA−/− or Th17−/− mice.
A. IL-17RC−/− mice are susceptible to OPC. IL-17RC−/−, IL-17RC+/− or age- and sex-matched WT mice were infected orally with Candida albicans, and 5 days post-inoculation mice were sacrificed and the oral fungal burden in the tongue was assessed, normalized to weight of tongue tissue. As positive controls for infection, WT and IL-17RC−/− mice were treated with cortisone acetate to cause susceptibility to infection. As a negative control, IL-17RC−/− mice were sham-infected with PBS. Bars indicate the mean fungal load per group. B. IL-17RC−/−, IL-17RA−/− and IL-23−/− mice show similar susceptibilities to OPC. IL-17RC−/−, IL-23p19−/− and IL-17RA−/− mice were subjected to OPC in the same experiment as outlined above. N.S.= not significant. C. PMN recruitment is defective in IL-17RA−/− and IL-17RC−/− mice. PAS-stained images of tongue tissue in infected mice were analyzed for PMN recruitment in a blinded analysis.
To determine whether there was any detectable difference in function between IL-17RC and IL-17RA in the context of OPC, we performed a side-by-side comparison of IL-17RC−/− and IL-17RA−/− mice. As an additional control, we included IL-23p19−/− mice, which are deficient in IL-17-producing Th17 cells (31). As shown, the IL-17RC−/−, IL-17RA−/−, and IL-23p19−/− mice were all susceptible to OPC, and there was no statistically significant difference between the fungal load between any of these cohorts (Fig. 5B). Furthermore, both the IL-17RA−/− and IL-17RC−/− mice exhibited defective neutrophil recruitment to sites of fungal infection (Fig. 5C). Therefore, these data support the concept that IL-17RC is an integral part of the IL-17 signaling complex, and its absence predisposes mice to similar infections as IL-17RA−/− mice (20).
Discussion
IL-17 has come into prominence with its identification as the signature cytokine of the Th17 lineage (32–34). However, to date the signaling mechanisms mediated by IL-17 are surprisingly poorly defined. The IL-17 receptor family is striking in that it bears little resemblance to better known cytokine families, and thus it has not been possible to infer how this receptor mediates signals based merely on homology with other systems (6). An important clue came with the discovery of the SEFIR domain and its homology to TIR domains (8), which was consistent with the similar panels of pro-inflammatory gene targets activated by IL-17 and TIR/IL-1R ligands (reviewed in (27)). Despite this homology, the SEFIR domain is functionally and structurally distinct from TIRs. First, unlike TIR-containing receptors, IL-17R does not engage the prototypical innate immune adaptors MyD88 or TRIF (9, 12). Second, SEFIR domains do not encode the “BB-loop” structure found in TIR domains that is required to mediate specificity of homotypic TIR-TIR interactions (25). Empirical structure-function studies of IL-17RA showed that, while the SEFIR is necessary for signaling, an extended region beyond the SEFIR is also required (12).
When IL-17RC was shown to be a key component of the IL-17R signaling cascade, this raised important questions about its mechanisms of action with respect to signal transduction. Given the genetic requirement for IL-17RC in transducing IL-17 signals (13) and the IL-17F-bound IL-17RA crystal structure suggesting a IL-17RA/IL-17RC heterodimeric receptor (14), we wished to determine the functional significance of the IL-17RC receptor in the IL-17 signaling axis. Using IL-17RC truncations, our data indicate that the IL-17RC cytoplasmic tail is not required for its association with IL-17RA (Fig 2). Furthermore, the IL-17RC SEFIR as well as an additional downstream 23 amino acids are required for IL-17-dependent IL-6 and 24p3 expression, and for a ligand-dependent association with a phosphorylated Act1 isoform (Figs 3–4). We have dubbed this a “SEFEX” domain, for SEFIR extension. We further show that IL-17RC is required for IL-17-dependent host defense against oropharyngeal candidiasis (Fig 5). Taken together, these results demonstrate that IL-17RC is indispensable for IL-17-dependent immune responses, and identifies key structural and biochemical requirements responsible for signaling downstream of the IL-17RC receptor.
The stoichiometry of how IL-17RC associates with IL-17RA is currently undefined. FRET studies of IL-17RA suggested that pre-assembled homodimers exist on the cell surface. However, the addition of IL-17 causes a conformational shift to occur, which could be explained by dissociation of IL-17RA homodimers or recruitment of additional subunits (24, 35). Subsequent genetic data, demonstrating that IL-17RC is required for the IL-17-dependent production of CXCL1, supported the idea that association with IL-17RC is involved in this process (13). Our present data indicate that a basal level of IL-17RA and IL-17RC may exist as pre-associated complexes, but that addition of IL-17A or IL-17F enhances this association (Fig. 2A). While our results must be interpreted in the context of an overexpression system, these findings are supported by recent biochemical studies of IL-17RA, which indicate that ligand-binding increases the affinity of the IL-17RA/IL-17RC interaction (14). Notably, we also find that IL-17 treatment induces IL-17RC to preferentially associate with a larger IL-17RA isoform. Tunicamycin treatment leads to the disappearance of the larger IL-17RA isoform, suggesting that this is a glycosolated IL-17RA isoform. Various N-linked glycosylated forms of IL-17RA have been reported, and the E3 ubiquitin ligases Act1 and TRAF6 have been shown to be associated with ubiquitinated forms of IL-17RA (7, 26, 30, 36). The relevance of these glycosylated species IL-17RA is unclear at present, but its ligand-dependent association argues that it may be critical for signaling.
IL-17RC has a higher affinity for IL-17F than IL-17A (16). However, our data and others (13) demonstrate that IL-17RC is nonetheless required for IL-17A-mediated signaling. IL-17RC also exists in multiple splice forms, some of which have differential recognition of IL-17-family ligands (16). In these studies we only evaluated the unspliced (full length) IL-17RC isoform, but it will be interesting to determine in the future whether there are differences in how other splice forms of IL-17RC participate in signaling.
Previous reports indicate that the IL-17RC cytoplasmic tail, which contains a SEFIR domain, is integral for functional IL-17 responses (8, 13, 37). We previously found that IL-17RA uses a SEFIR extension to mediate signaling (12). Analogously, we show here that IL-17RC uses both its SEFIR domain and an additional downstream sequence of 23 amino acids to activate signals such as IL-6 and 24p3 expression (Fig 3). Consistent with this result, Hu et al. find that the IL-17RC SEFIR is required for functional IL-17 signal transduction both in vitro and in vivo (37). Bioinformatic database searches and sequence alignments, however, indicate that the extended sequence downstream of the IL-17RC SEFIR lacks homology to the sequence downstream of the IL-17RA SEFIR, the Act1 SEFIR, or other known receptors or signaling intermediates (AWH, unpublished observations). Thus, the extended IL-17RC SEFEX domain may contribute a novel signaling function. Indeed, a chimeric receptor construct composed of the IL-17RA extracellular domain fused to the complete IL-17RC cytoplasmic tail cannot rescue IL-17-dependent signaling in IL-17RA−/− cells (R. Onishi and J. Park, unpublished data), implying that the role of IL-17RC is probably not simply to supply another SEFIR domain to more efficiently recruit additional Act1 molecules. Therefore, despite the fact that the IL-17RC cytoplasmic tail is much shorter than the IL-17RA intracellular domain (214 versus 521 amino acids), IL-17RC may possess novel signaling functions distinct from IL-17RA.
Act1 is an essential signaling component downstream of IL-17RA and is required for IL-17-dependent immune responses (9, 10). We now show that IL-17RC also associates with Act1 even in the absence of IL-17RA (Fig 5). Interestingly, IL-17 treatment leads to the association of IL-17RC with a larger phosphorylated form of Act1. This result indicates for the first time that Act1 is phosphorylated, and that phospho-Act1 associates with IL-17RC in a ligand-dependent manner. Even more striking, the only IL-17RC deletions that associate with this phospho-Act1 isoform are those that retain function, i.e. mutants that contain the IL-17RC SEFEX motif. It is possible that the SEFEX domain recruits other signaling intermediates needed to create a stable signaling scaffold to permit Act1 to efficiently transduce downstream signals, analogous to other systems such as the co-receptors of the T cell receptor (38).
Although good biochemical and genetic evidence supports a role for IL-17RC within the IL-17R complex, it is still not fully established whether IL-17RC is required for all aspects of IL-17 signal transduction in vivo. To evaluate the physiological function of IL-7RC, we used an oral fungal infection model (23) that we and others previously showed to be strongly IL-17/IL-17RA-dependent (22, 39). Here, we demonstrate that IL-17RC is required for host defense against infection with the yeast Candida albicans. (Fig 5). Moreover, susceptibility to OPC was identical between IL-17RC−/− and IL-17RA−/− mice (and also IL-23p19−/− mice (22)). This finding also suggests, albeit indirectly, that there is no additional role for IL-25 in this process, since IL-17RA but not IL-17RC participates in the IL-25 receptor complex (40). In line with these findings, IL-17RC was recently shown to participate in the pathogenesis of autoimmune inflammation of the central nervous system, an event highly dependent on the IL-17 signaling axis (37).
In conclusion, our results show that IL-17RC is critically important for IL-17-dependent signaling and immune responses. IL-17RC mediates signaling via an extended SEFIR domain, which is required for a ligand-dependent association with a phosphorylated Act1 isoform to promote downstream signaling. These studies provide the first report of a signaling intermediate directly downstream of the IL-17RC receptor, and are the first to define important structural sequence elements within this receptor. Lastly, like IL-17RA, IL-17RC is required for host defense against oral fungal infections caused by Candida albicans. It will be interesting in future studies to link IL-17RC signaling pathways directly to biological signals and ultimately exploit this knowledge to improve treatments for a host of human diseases.
Supplementary Material
Acknowledgements
We thank Genentech for kindly providing IL-17RC−/− and IL-23p19−/−mice, and Amgen for kindly providing IL-17RA−/− mice. We thank N. Patel for valuable technical assistance.
Abbreviations
- CIKS
cytokine inducer of NF-κB signaling
- SAP
shrimp alkaline phosphatase
- FRET
fluorescence resonance energy transfer
- OPC
oropharyngeal candidiasis
- PAS
periodic acid Schiff
- SEFIR
SEF/IL-17R signaling domain
- TIR
Toll-like receptor/IL-1 receptor signaling domain.
Footnotes
SLG was supported by NIH grant AR054389 and the Alliance for Lupus Research. LPK was supported by NIH grant AI067544, and JKK was supported by HL079142. AWH was supported by the Medical Scientist Training Program at the University at Buffalo, SUNY. HRC was supported by an NIH training grant to the Dept. of Oral Biology at the University at Buffalo (DE007034). NHS was supported by an NIH training grant to the University of Pittsburgh (CA082084). WO and FS are full time employees of Genentech, Inc.
References
- 1.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009 doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6:1133-141A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:133–141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol. 2005;77:388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 5.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–518. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 6.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 8.Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, Eisenhaber F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem Sci. 2003;28:226–229. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 9.Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281:35603–35607. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 10.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li X. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 11.Huang F, Kao CY, Wachi S, Thai P, Ryu J, Wu R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J Immunol. 2007;179:6504–6513. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 12.Maitra A, Shen F, Hanel W, Mossman K, Tocker J, Swart D, Gaffen SL. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc Natl Acad Sci U S A. 2007;104:7506–7511. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 14.Ely LK, Fischer S, Garcia KC. Structural basis of receptor sharing by interleukin 17 cytokines. Nature immunology. 2009 doi: 10.1038/ni.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho A, Gaffen S. IL-17RC: A partner in IL-17 signaling and beyond. Semin Immunopathol. 2010 doi: 10.1007/s00281-009-0185-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, Aujla SJ, Reardon B, Moore M, Shea P, Schreckhise R, Bukowski TR, Presnell S, Guerra-Lewis P, Parrish-Novak J, Ellsworth JL, Jaspers S, Lewis KE, Appleby M, Kolls JK, Rixon M, West JW, Gao Z, Levin SD. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, Le TV, Lorenz RG, Xu H, Kolls JK, Carter RH, Chaplin DD, Williams RW, Mountz JD. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cellmediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 21.Shen F, Hu Z, Goswami J, Gaffen SL. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138–24148. doi: 10.1074/jbc.M604597200. [DOI] [PubMed] [Google Scholar]
- 22.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamai Y, Kubota M, Kamai Y, Hosokawa T, Fukuoka T, Filler S. New model of oropharyngeal candidiasis in mice. Anti-microb. Agents Chemo. 2001;45:3195–3197. doi: 10.1128/AAC.45.11.3195-3197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer JM, Yi L, Shen F, Maitra A, Jiao X, Jin T, Gaffen SL. Evidence for ligand-independent multimerization of the IL-17 receptor. J Immunol. 2006;176:711–715. doi: 10.4049/jimmunol.176.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toshchakov V, Vogel S. Cell-penetrating TIR BB loop decoy peptides: A novel class of TLR signaling inhibitors and a tool to study topology of TIR-TIR interactions. Expt. Op. Biol. Ther. 2007;7:1035–1050. doi: 10.1517/14712598.7.7.1035. [DOI] [PubMed] [Google Scholar]
- 26.Yao Z, Spriggs MK, Derry JM, Strockbine L, Park LS, VandenBos T, Zappone JD, Painter SL, Armitage RJ. Molecular characterization of the human interleukin (IL)-17 receptor. Cytokine. 1997;9:794–800. doi: 10.1006/cyto.1997.0240. [DOI] [PubMed] [Google Scholar]
- 27.Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine. 2008;41:92–104. doi: 10.1016/j.cyto.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miossec P. Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 2003;48:594–601. doi: 10.1002/art.10816. [DOI] [PubMed] [Google Scholar]
- 29.Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, Qian W, Qian Y, Giltiay NV, Lu Y, Swaidani S, Misra S, Deng L, Chen ZJ, Li X. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci Signal. 2009;2:ra63. doi: 10.1126/scisignal.2000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Quinn DB, Palmer MT, Lee YK, Weaver CT. Emergence of the Th17 pathway and its role in host defense. Adv Immunol. 2008;99:115–163. doi: 10.1016/S0065-2776(08)00605-6. [DOI] [PubMed] [Google Scholar]
- 32.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annual review of immunology. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 33.Dong C. Regulation and pro-inflammatory function of interleukin-17 family cytokines. Immunol Rev. 2008;226:80–86. doi: 10.1111/j.1600-065X.2008.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Z, O'Shea JJ. Th17 cells: a new fate for differentiating helper T cells. Immunol Res. 2008;41:87–102. doi: 10.1007/s12026-007-8014-9. [DOI] [PubMed] [Google Scholar]
- 35.Kramer JM, Hanel W, Shen F, Isik N, Malone JP, Maitra A, Sigurdson W, Swart D, Tocker J, Jin T, Gaffen SL. Cutting edge: identification of a preligand assembly domain (PLAD) and ligand binding site in the IL-17 receptor. J Immunol. 2007;179:6379–6383. doi: 10.4049/jimmunol.179.10.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rong Z, Cheng L, Ren Y, Li Z, Li Y, Li X, Li H, Fu XY, Chang Z. Interleukin-17F signaling requires ubiquitination of interleukin-17 receptor via TRAF6. Cell Signal. 2007;19:1514–1520. doi: 10.1016/j.cellsig.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 37.Hu Y, Ota N, Peng I, Refino CJ, Danilenko DM, Caplazi P, Ouyang W. IL-17RC is required for IL-17A- and IL-17F-dependent signaling and the pathogenesis of experimental autoimmune encephalomyelitis. J Immunol. 2010;184:4307–4316. doi: 10.4049/jimmunol.0903614. [DOI] [PubMed] [Google Scholar]
- 38.Weiss A. TCR signal transduction: opening the black box. J Immunol. 2009;183:4821–4827. doi: 10.4049/jimmunol.0990083. [DOI] [PubMed] [Google Scholar]
- 39.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, Anders PM, Tocker JE, Comeau MR, Budelsky AL. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.