INTRODUCTION
For over a decade, clinical investigation of autologous hematopoietic stem cell transplantation (HCT) as therapy for multiple sclerosis (MS) has been ongoing. Several phase II studies have been completed or are in progress; however, no definitive prospective randomized studies comparing HCT versus alternative therapies for MS have been completed. The objectives of this 1.5 day workshop were to review ongoing studies of the Center for International Blood and Marrow Transplant Research (CIBMTR) and European Group for Blood and Marrow Transplantation (EBMT) in MS, including harmonization of the MS disease specific report forms, and to explore mechanisms by which the databases might serve as a resource to facilitate collaboration on the scale needed for pivotal studies. We sought to critically review progress to date in HCT for MS, identify challenges to the advancement of this therapeutic modality, and discuss opportunities for future collaborative clinical trials. Meeting participants included HCT physicians, neurologists, and imaging experts with particular interest in MS clinical research and immunologic mechanisms from North America and Europe.
AUTOLOGOUS HCT FOR MS – BACKGROUND AND CURRENT CLINICAL TRIALS
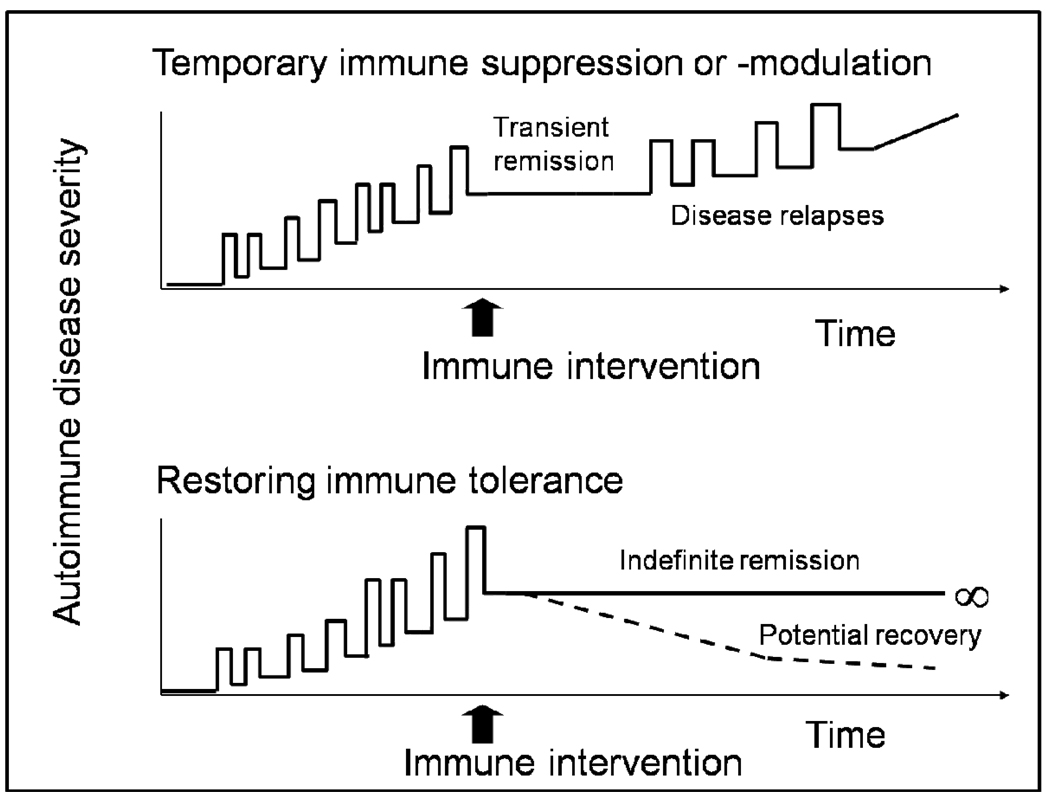
The purpose of high-dose immunosuppressive therapy (HDIT) with autologous HCT is to stop inflammation associated with MS,1,2 and thereby preserve neurologic function. The autologous graft serves to rescue hematopoietic activity after HDIT, or in the case of less immunosuppressive therapy to reduce the time to recovery of blood counts. Generation of a new and diverse T cell immune response may be one mechanism of action to explain remission of inflammatory disease activity which extends much longer than the duration of immune suppression (Figure 1).3 Currently, MS is the most common autoimmune disease indication for autologous HCT.4–6 Following the initial promising clinical experience7 over 350 consecutive cases have been reported by the EBMT during the last decade.8,9 Most patients who received autologous HCT for MS in the early studies had secondary progressive (SP) MS and relatively fewer had relapsing remitting (RR) disease, with Kurtzke Extended Disability Severity Scores (EDSS)10 at time of transplantation ranging from 3.0 to 9.5.4 Improvements in supportive care and better patient selection have contributed to improved outcomes with a significant reduction in transplant related mortality to 1.3% during 2001–2007.4,6 Recent studies have enrolled patients with earlier disease and are supportive of a role for intense immunosuppression with autologous HCT as treatment for rapidly evolving MS unresponsive to conventional therapies.11–14 Clinical studies performed to date have recently been comprehensively reviewed,4 and the retrospective report of the EBMT database in MS has been updated.8,9
Figure 1. Possible Outcomes of Autologous HCT for MS.
The objective of autologous HCT for MS is to reduce inflammation and progression of the disease for a prolonged period of time. Early MS, which has characteristic lesions that show active inflammation, has a chronic relapsing remitting course, and is followed by progressive disease in later years. There is growing evidence that the clinical effects of autologous HCT are not limited to transient immune suppression (top graph) but could be related to a “resetting” of the immune system.3 However, several years of long term follow up of patients transplanted for MS is needed to determine durability of remission from clinical disease activity. Immunologic mechanistic studies using patient samples and MRI studies to assess demyelination and re-myelination will also be needed to elucidate the mechanisms of HCT effects on MS.
At least one prospective randomized and other single arm phase II studies of autologous HCT for MS are currently active in the US, Canada and Europe (Table 1). Since it is now generally accepted that timing of HDIT relatively early in disease to reduce inflammation before irreversible neuronal damage occurs is important, these studies target MS patients with active disease and worsening disability as evidenced clinically by relapses, change in EDSS, and/or inflammatory MRI activity, and who have failed at least one approved first-line immunomodulatory MS therapy for enrollment. Three of the studies have either completed or closed to enrollment during 2009, and follow up of several years will be needed to evaluate outcomes, which will in turn be important for design of the next clinical trial(s).
Table 1.
Phase II Studies in Autologous HCT for MS
| Phase II Single Arm | Phase II Randomized | |||
|---|---|---|---|---|
| Study and/or Sponsor |
HALT MS ITN033AI |
MS BMT Canada |
ASTIMS EBMT |
Northwestern University |
| Transplant Arm | ||||
| Mobilization | GCSF + prednisone | Cy + GCSF | Cy + GCSF | Cy + GCSF |
| Graft | CD34 selected | CD34 selected | Unmanipulated | Unmanipulated |
| Conditioning | BEAM + ATG | Busulfan + Cy | BEAM + ATG | Cy + ATG |
| Alternative Arm | ||||
| NA | NA | Mitoxantrone | FDA-approved standard of care | |
| Inclusion / Exclusion | ||||
| Target MS Population | Relapsing remitting or progressive relapsing MS | Active MS with relapses or progression | Relapsing remitting or progressive relapsing or secondary progressive MS | Inflammatory MS failing interferon therapy |
| Age | 18–60 | 18–50 | 18–50 | 18–55 |
| EDSS | 3.0–5.5 | 3.0–6.0 | 3.5–6.5 | 2.0–6.0 |
| MS Criteria | Duration <15 years from diagnosis; T2 abnormalities on brain MRI consistent with MS; ≥ 2 relapses in <18 mo; worsening of EDSS; failure of standard drug therapy | MRI findings meet criteria of MS; evidence of current disease activity including worsening of EDSS in last 18 months or 2 relapses in last year or 3 relapses in last 3 years; failed at least one immunosuppressive drug | Relapsing remitting MS with at least 2 relapses per year and enhancing lesions on MRI; relapsing progressive MS with worsening EDSS during last year and enhancing lesions on MRI; secondary progressive MS with worsening EDSS during last year and enhancing lesions on MRI unless rapid deterioration | Inflammatory disease, based on both clinical and MRI activity, after ≥ 6 months of interferon or copaxone |
| Study Design | ||||
| Primary Outcome | Progression free survival at 5 years | Progression free survival at 3 years | New T2 lesions per year | Progression free survival at 5 years |
| Primary Outcome Measure | EDSS and/or clinical relapse and/or new MRI abnormalities consistent with MS | EDSS | MRI imaging (EDSS is a secondary outcome measure) | EDSS |
| Projected Accrual | 25 | 24 | 30 (21 accrued) | 110 |
| Date of Activation | July 2006; enrollment complete September 2009 | August 2001; enrollment complete July 2009 | January 2005; closed November 2009 due to lack of accrual | January 2006; number enrolled not available |
| References | ClinicalTrials.gov NCT00288626 http://www.halt-ms.org | Atkins A, Freedman M (2009);39 Chen JT et al (2006)40 | http://www.astims.org Mancardi G, Saccardi R (2008)4 | ClinicalTrials.gov NCT00273364 |
REGISTRY STUDIES IN HCT FOR MS
Both the CIBMTR and the EBMT collect information about patients with MS who have received HCT. The CIBMTR operates under a US Department of Health and Human Services (DHHS) mandate to collect information about all patients who receive either related or unrelated allogeneic HCT for any indication; reporting of autologous HCT is voluntary. Most European centers report both allogeneic and autologous HCT to the EBMT, although the requirement to report allogeneic HCT depends on the country, and reporting of autologous HCT is voluntary. As a part of this workshop, representatives of the Autoimmune Diseases Working Committee of the CIBMTR, and the Autoimmune Diseases Working Party of the EBMT, met to revise and harmonize the research forms in HCT for MS that will be used in the future by both registries. (See Table 2 and Figure 2). To complete registry forms at the patient care sites, close partnerships between the transplant and neurology services will be needed. Two registry-based studies of HCT for MS were discussed.
Table 2.
Multiple Sclerosis Forms Harmonization by CIBMTR and EBMT
| Assessments Timeline | |
|---|---|
| MS Diagnosis | Dates of onset of first symptoms and diagnosis of MS, family history of the disease, and imaging or laboratory evidence of MS are recorded. Previous MS specific treatments, not including symptomatic treatment during relapses, are documented. |
| Pre-HCT MS Disease Course | Disease manifestations within the 2-year interval which immediately precedes HCT are documented, including the number of MS relapses, extent of MRI abnormalities, and disease course. The EDSS is also collected for 1 and 2 years prior to presentation at the transplant center, if available. |
| Note: If EDSS has not been determined during the 1–2 year pre-transplant interval, reconstruction may be attempted by a study neurologist provided there is an objective detailed chart. Some centers have formalized this process. | |
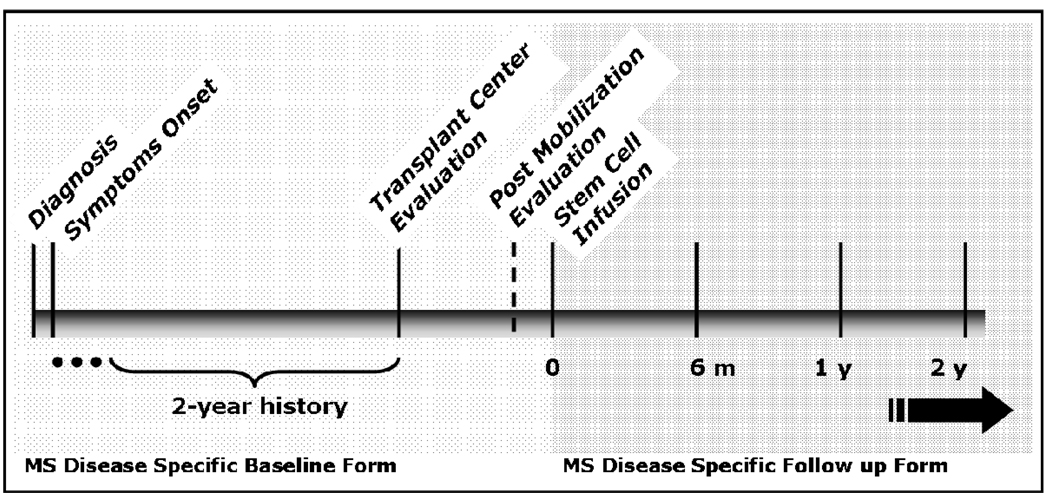
| Baseline: MS Assessment Immediately Preceding HCT | For autologous procedures, EDSS assessment and MRI findings are obtained within 2 weeks prior to the administration of stem cell mobilization agents (“Baseline Scan”) and again immediately prior to the administration of the conditioning regimen. Obtaining information about the mobilization and also the post-mobilization clinical assessment and MRI findings will allow evaluation of any effect of the mobilization procedure on neurologic status. For allogeneic procedures, EDSS is assessed and MRI findings are obtained within 2 weeks prior to administration of the conditioning regimen (“Baseline Scan”). |
| Post-HCT Follow Up of MS Disease Course | Follow up post-transplant is obtained at 6 months and 1 year, and yearly thereafter. Clinical relapses including date of each relapse after HCT are documented. EDSS assessment of disease severity and imaging studies of neurologic burden of disease are included. If needed, any MS specific treatments are recorded. |
| Challenges in Forms Design and Completion | |
| Forms Design | The clinical features and natural history of MS present certain challenges in design of disease specific registry forms. For example, disease activity in MS may manifest as clinical relapse or exacerbation (flare), findings revealed by neurologic imaging, and/or incremental worsening of the clinical scale used to assess severity of disease. |
| Forms Completion | We recommend here a minimum data set for patients who receive HCT for MS. For patients with MS who are enrolled in treatment clinical trials of any type, most of the assessments will have been performed as a part of those studies. For patients not enrolled in a clinical study, these suggestions may serve as guidance. To complete the neurology forms collaboration with a neurologist, preferably the treating neurologist, will be needed. To enhance accuracy, completion of forms in reasonable proximity to the events by staff familiar with MS is preferred. In addition, completion of MS-specific quality of life assessments is desirable. |
| MRI | MRI findings consistent with MS activity include: new gadolinium-enhancing and/or new T2 lesions, as compared to a “Baseline Scan” obtained before transplant. If available, an 8 weeks post-HCT MRI may also be used as a “Reference Scan” comparator for later MRI studies. |
Figure 2. Time Course of MS and Pre- and Post-HCT Events for MS Research Forms.
The “MS Disease Specific Baseline Form” will be used to capture information about MS disease activity pre-HCT. Diagnostic information, as well as assessment of MS activity including EDSS, clinical relapses, and MRI findings during the 2 years prior to HCT is of particular interest. The same form will be used to record baseline assessments both prior to mobilization and after collection of the graft for autologous HCT, and prior to receiving the preparative regimen, for allogeneic HCT. The “MS Disease Specific Follow Up Form” will be used to capture post-HCT information. (See also Table 2).
A. AUTOLOGOUS HCT FOR MS – LONG TERM FOLLOW UP
To assess long term follow up of patients who received autologous HCT for MS, CIBMTR and EBMT have approved a collaborative cross-sectional and retrospective study of patients transplanted between five and twelve years previously. Up to 250 patients from the combined registries may be eligible for further study. We will obtain information about disease status at baseline, and the transplant regimen used for each patient. Progression free survival will be the primary outcome. Secondary outcomes under consideration include interval change in MS-specific imaging (evolution of MRI MS-specific lesion load and presence of MS-specific gadolinium-enhancing lesions), time to progression, overall survival, causes of death, response to MS-specific treatment if needed post transplant and incidence of transplant-related late effects and secondary malignancies.
Feasibility issues for this type of long term follow up study of HCT for MS include: lack of comparable quality baseline data for the variety of patients and regimens that contribute to the CIBMTR and EBMT databases and differences in MS eligibility criteria for studies conducted a decade ago, as compared to today. To obtain a history of the course of disease retrospectively, participation of a study neurologist will be needed. Unambiguous milestones will need to be specified. Inclusion of only subjects who were enrolled in any clinical trial (not necessarily a transplant study) and from a center committed to clinical trials investigation may be preferable as such individuals will be more likely to have sufficient documentation of their MS disease course. MRI findings consistent with MS activity include: new gadolinium-enhancing and/or new T2 lesions. Comparison of imaging findings may be difficult, due either to outdated techniques or absence of such evaluations. Quantitative assessment of lesion load may not be possible for this type of study. The newly harmonized MS disease-specific research forms will be utilized to collect post transplant information for this long term follow up study, which will be a collaborative project of the CIBMTR and EBMT. For sites reporting to CIBMTR, limited reimbursement is available for completion of the MS-specific research forms.
B. ALLOGENEIC HCT IN MS – LONG TERM FOLLOW UP
To investigate the potential of allogeneic HCT to stabilize or cure MS, we have performed a cross sectional and retrospective clinical study to assess outcomes for eleven patients with coexistent MS who received allogeneic HCT for hematologic malignancy. The experience of allogeneic HCT for autoimmune diseases is limited as, due to the generally unfavorable risk-benefit ratio, this approach may be considered only for those having very advanced nonmalignant diseases.15 There are currently no clinical trials of allogeneic HCT as therapy for MS. However, for patients with coincident autoimmune disease who receive allogeneic HCT for treatment of malignancy, investigation of MS-related outcomes provides an opportunity to understand how transplant affects the patient’s autoimmune disease, and also the effects of the underlying immune dysregulation on transplant outcomes.16 Subjects were identified through research of the CIBMTR database or personal contact with transplant physicians worldwide. Ten patients alive at the time of initiation of this study, and pathologic samples from one patient who expired after allogeneic HCT were evaluated. Study participants received comprehensive follow up including clinical neurologic and MRI evaluations, after informed consent. Publication is pending. (Richard A. Nash, personal communication). In addition, Lu et al17 have reported on a single patient with mild MS who received a myeloablative preparative regimen and allogeneic HCT from a HLA matched unrelated donor for chronic myelogenous leukemia (CML). The patient developed GVHD and worsening, but not new, neurologic symptoms. At 140 days post-HCT, following demise due to adenovirus hepatitis, post mortem CNS examination revealed ongoing active and chronic active MS lesions. Most hematolymphatic cells in the brain were recipient cells, even though only donor cells were detected in peripheral blood. Further study of such cases will be needed to fully evaluate the potential of allogeneic HCT to affect the clinical course, and toxicities particular to this therapy, for patients with MS.
CLINICAL TRIALS IN AUTOLOGOUS HCT FOR MS – CHALLENGES IN STUDY DESIGN
During the next few years, as the phase II clinical trials of HCT for MS currently underway come to completion, it will be important to plan ahead for the next studies with special emphasis on randomized phase III trials. This will be especially critical in the event international multicenter collaboration is desired. Major issues in study design and implementation include the following.
A. FEASIBILITY
Patient accrual has been a challenge for clinical trials of autologous HCT for MS. Lack of wide acceptance by the neurology community due to the investigational status of the therapy, an insufficient number of transplant teams that include strong functional partnerships between transplant and disease specialists, absence of training programs in the field of HCT for autoimmune diseases, narrow eligibility criteria, referral patterns to transplant centers, and difficulty obtaining third party payer approval to cover costs of HCT in the US and some European countries are the most important impediments to accrual. The neurology community has been cautious to consider autologous HCT for MS due to concerns about safety, including toxicities and the risk of mortality in a disorder that, at least in the short term, is not life threatening. The relatively high toxicity of transplant versus existing and experimental new therapies has been a major disincentive for many neurologists to refer MS patients to transplant studies, in the absence of convincing evidence of efficacy. Furthermore, there is little enthusiasm to refer patients for additional phase II studies, given the general acceptance that a phase III randomized clinical trial is what is needed to evaluate efficacy. Given the concerns about risk-benefit of the procedure, entry criteria for early studies were highly selective for poor prognosis patients with aggressive MS that was too advanced, although, as outcomes have improved, it has become feasible to consider more broad entry criteria. As is entirely appropriate for a disease with a natural history that may be devastating in the long term, and no known cure, other potential novel therapies and competing drugs for MS are continually being developed; studies of this type may attract patients otherwise eligible for HCT. Sullivan et al18 have described the recent challenges of obtaining insurance coverage in the US for a NIH-sponsored clinical trial of autologous HCT for autoimmune disease. Finally, patients may be reluctant to enroll in randomized studies, due to individual treatment preferences of one study arm over another.
B. STUDY TEAM
A multidisciplinary team with expertise in neurology, imaging, immunology, hematology and transplantation is needed to design and manage studies of HCT for MS. Close partnership that includes real time consultation of neurology and HCT physicians is needed both during and in follow up of the transplant procedure, to provide the best patient care and to appropriately evaluate disease response.
C. OUTCOMES ASSESSMENT
The 30–40 year time course of evolution of MS,19 and heterogeneity of the disease, necessitate several years of clinical follow up and relatively large sample sizes for meaningful assessment of clinical trial endpoints. Clinical assessments include comparison of cumulative functional disability measured using the EDSS10 and / or number of clinical relapses and / or time to clinical relapse in one treatment arm, relative to the other. Success is then defined as less progression of EDSS and / or fewer relapses and / or a longer time to relapse and / or progression. The requirement or not for further immunomodulatory therapy may serve as an adjunct outcome. In contrast, to allow the opportunity of smaller sample size and shorter follow up, recent clinical studies to evaluate alternative drug therapies for MS have frequently used improvement of clinical status following treatment in one arm relative to the other as an outcome.20 However, it is unclear whether early improvements in disability indeed translate into longer progression free survival or other improvements in later MS outcomes after HCT. Additional clinical assessments, for example, the MS Functional Composite (MSFC) have been incorporated in recent clinical trials;21–24 the MS Impact Scale (MSIS-29)25 is commonly used to assess quality of life. Investigations of MRI surrogate markers26–29 and biomarkers30 of MS are ongoing.
CLINICAL TRIALS IN AUTOLOGOUS HCT FOR MS – FUTURE DIRECTIONS
Consideration of options for the next clinical study or group of studies is now timely. A future comparative prospective randomized clinical trial of transplant versus non-transplant treatment is clearly needed. Important questions in study design will include sample size, transplant regimen, non-transplant therapy or therapies, MS target population, and outcomes to be analyzed, including long term follow up. Issues of feasibility in study design due to the very large number of subjects required to demonstrate a significant difference between two treatment options, and/or the long period of several years of observation needed are likely to remain a challenge for future studies. Potential non-transplant comparators might include rituximab, alemtuzumab, daclizumab, cladribine, fingolimod, cyclophosphamide and mitoxantrone,31–36 and/or other new therapy(s) currently in development. A composite primary endpoint which includes MRI as well as clinical functional assessments could be used for phase II efficacy studies. For phase III or pivotal clinical trials of therapy for MS, the primary endpoint is a clinical functional assessment of disease, as required by the US Food and Drug Administration (FDA), 37 with the EDSS most commonly used for this purpose.
To continue discussion of both study design and the operational challenges for a prospective randomized study of autologous HCT for MS, representatives of this workshop met again recently as part of a larger ongoing international effort which is reported separately.38 International collaboration, including partnership with the CIBMTR and EBMT, may be desirable and may in fact be critical for successful completion of a definitive comparative study. The use of a single protocol required at all sites, vs. comparable protocols in North America and Europe, will be an important consideration. Use of a single protocol would ensure uniformity of subject entry/exclusion criteria and study design. If there are multiple protocols, comparable baseline as well as follow up assessments will be needed for all subjects. A decentralized plan would offer individual sites some degree of flexibility in site-specific protocol design and implementation and assume distributed responsibility for study costs. The regulatory challenges particular to each country will need to be addressed. Consideration of options for funding from multiple sources, for example the US National Institutes of Health, the national Multiple Sclerosis Societies, and other national and international resources will be needed.
Acknowledgment
We thank Steven Z. Pavletic, MD, and Maria Pia Sormani, MscStat, for comments on the manuscript.
APPENDIX 1: WORKSHOP PARTICIPANTS
Co-Chairs
Harold L. Atkins, MD, Bone Marrow Transplant Program, Ottawa General Hospital, Ottawa, Ontario, Canada
Linda M. Griffith, MD, PhD, Division of Allergy, Immunology and Transplantation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD
Paolo A. Muraro, MD, PhD, Department of Neurology, Imperial College, London, England, UK
Richard A. Nash, MD, Fred Hutchinson Cancer Research Center and University of Washington, Seattle, WA (who was unable to attend this workshop but whose participation was contributory)
Marcelo C. Pasquini, MD, MS, Center for International Blood and Marrow Transplant Research (CIBMTR) and Department of Medicine, Medical College of Wisconsin, Milwaukee, WI
Riccardo Saccardi, MD, Department of Hematology, Ospedale di Careggi, Florence, Italy
Speakers and Discussants
Manza Agovi, MPH, Center for International Blood and Marrow Transplant Research (CIBMTR) and Department of Medicine, Medical College of Wisconsin, Milwaukee, WI
Douglas L. Arnold, MD, Department of Neurology, McGill University – Montreal Neurological Institute, Montreal, Quebec, Canada
James D. Bowen, MD, Multiple Sclerosis Center, Swedish Neuroscience Institute, Seattle, WA
Jacqueline T. Chen, PhD, Department of Neurology, McGill University – Montreal Neurological Institute, Montreal, Quebec, Canada; and Department of Neurosciences, Cleveland Clinic, Cleveland, OH
Mark S. Freedman, MD, Department of Neurology, Ottawa General Hospital, Ottawa, Ontario, Canada
Kay Gardner, Center for International Blood and Marrow Transplant Research (CIBMTR) and National Marrow Donor Program (NMDP), Minneapolis, MN
Diane J. Knutson, Center for International Blood and Marrow Transplant Research (CIBMTR) and Department of Medicine, Medical College of Wisconsin, Milwaukee, WI
George Howard Kraft, MD, MS, Department of Rehabilitation Medicine, University of Washington, Seattle, WA
Gian Luigi Mancardi, MD, Department of Neuroscience, Opthalmology and Genetics, University of Genoa, Genoa, Italy (who was unable to attend this workshop but whose participation was contributory)
Roland Martin, MD, Institute for Neuroimmunology and Clinical Multiple Sclerosis Research, Center for Molecular Neurobiology, University Medical Center, Hamburg, Germany (who was unable to attend this workshop but whose participation was contributory)
Marie Matlack, MT (ASCP), Center for International Blood and Marrow Transplant Research (CIBMTR) and National Marrow Donor Program (NMDP), Minneapolis, MN
Michael K. Racke, MD, Department of Neurology, Ohio State University Medical Center, Columbus, OH
Jan Storek, MD, PhD, Departments of Medicine and Oncology, University of Calgary, Alberta, Canada
Paula A. Watry, RN, PA-C, Center for International Blood and Marrow Transplant Research (CIBMTR) and Department of Medicine, Medical College of Wisconsin, Milwaukee, WI
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Report of a workshop sponsored by the Center for International Blood and Marrow Transplant Research (CIBMTR), European Group for Blood and Marrow Transplantation (EBMT), and National Institute of Allergy and Infectious Diseases (NIAID); Minneapolis, MN, USA; November 9–10, 2008
The opinions expressed are those of the authors, and do not represent the position of the National Institute of Allergy and Infectious Diseases (NIAID), the National Institutes of Health (NIH), or the United States Government.
REFERENCES
- 1.Sospedra M, Martin R. Immunology of multiple sclerosis. Ann Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 2.Frohman EM, Racke MK, Raine CS. Multiple sclerosis - the plaque and its pathogenesis. New Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 3.Muraro PA, Douek DC, Packer A, et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J Exp Med. 2005;201:805–816. doi: 10.1084/jem.20041679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mancardi G, Saccardi R. Autologous haematopoietic stem-cell transplantation in multiple sclerosis. Lancet Neurol. 2008;7:626–636. doi: 10.1016/S1474-4422(08)70138-8. [DOI] [PubMed] [Google Scholar]
- 5.Saccardi R, Di Gioia M, Bosi A. Haematopoietic stem cell transplantation for autoimmune disorders. Curr Opin Hematol. 2008;15:594–600. doi: 10.1097/MOH.0b013e3283136700. [DOI] [PubMed] [Google Scholar]
- 6.Schippling S, Heesen C, Zander A, Martin R. Stem cell transplantation in multiple sclerosis. J Neurol. 2008;255 Suppl 6:43–47. doi: 10.1007/s00415-008-6008-8. [DOI] [PubMed] [Google Scholar]
- 7.Fassas A, Anagnostopoulos A, Kazis A, et al. Peripheral blood stem cell transplantation in the treatment of progressive multiple sclerosis: first results of a pilot study. Bone Marrow Transplant. 1997;20:631–638. doi: 10.1038/sj.bmt.1700944. [DOI] [PubMed] [Google Scholar]
- 8.Saccardi R, Kozak T, Bocelli-Tyndall C, et al. Autologous stem cell transplantation for progressive multiple sclerosis: update of the European Group for Blood and Marrow Transplantation Autoimmune Diseases Working Party database. Mult Scler. 2006;12:814–823. doi: 10.1177/1352458506071301. [DOI] [PubMed] [Google Scholar]
- 9.Farge D, Labopin M, Tyndall A, et al. Autologous hematopoietic stem cell transplantation (HSCT) for autoimmune diseases: an observational study on 12 years of experience from the European Group for Blood and Marrow Transplantation (EBMT) Working Party on Autoimmune Diseases. Haematol. 2009 doi: 10.3324/haematol.2009.013458. doi: 10.3324/haematol.2009.013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 11.Saccardi R, Mancardi GL, Solari A, et al. Autologous HSCT for severe progressive multiple sclerosis in a multicenter trial: impact on disease activity and quality of life. Blood. 2005;105:2601–2607. doi: 10.1182/blood-2004-08-3205. [DOI] [PubMed] [Google Scholar]
- 12.Capello E, Saccardi R, Murialdo A, et al. Intense immunosuppression followed by autologous stem cell transplantation in severe multiple sclerosis. Neurol Sci. 2005;26:S200–S203. doi: 10.1007/s10072-005-0514-6. [DOI] [PubMed] [Google Scholar]
- 13.Burt RK, Loh Y, Cohen B, et al. Autologous non-myeloablative haematopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. Lancet Neurol. 2009;8:244–253. doi: 10.1016/S1474-4422(09)70017-1. [DOI] [PubMed] [Google Scholar]
- 14.Stangel M. Hematopoietic stem cell transplantation: hope and hype. Nature Rev Neurol. 2009;5:300–302. doi: 10.1038/nrneurol.2009.63. [DOI] [PubMed] [Google Scholar]
- 15.Daikeler T, Hugle T, Farge D, et al. Allogeneic hematopoietic SCT for patients with autoimmune diseases. Bone Marrow Transplant. 2009;44:27–33. doi: 10.1038/bmt.2008.424. [DOI] [PubMed] [Google Scholar]
- 16.Griffith LM, Pavletic SZ, Tyndall A, et al. Feasibility of allogeneic hematopoietic stem cell transplantation for autoimmune disease: position statement from a National Institute of Allergy and Infectious Diseases and National Cancer Institute - sponsored international workshop, Bethesda, MD, March 12 and 13, 2005. Biol Blood Marrow Transplant. 2005;11:862–870. doi: 10.1016/j.bbmt.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Lu J-Q, Storek J, Metz L, et al. Continued disease activity in a patient with multiple sclerosis after allogeneic hematopoietic cell transplantation. Arch Neurol. 2009;66:116–120. doi: 10.1001/archneurol.2008.522. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan KM, Siebold JR, Mineishi S, et al. Denials of treatment coverage by health insurance carriers restrict patient recruitment on a randomized clinical trial: experience of 95 patients with systemic sclerosis (SSc) enrolled in the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial. Biol Blood Marrow Transplant. 2010;16(Issue 2) Supplement 1:S164. [Google Scholar]
- 19.Weinshenker BG, Bass B, Rice GPA, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989;112:133–146. doi: 10.1093/brain/112.1.133. [DOI] [PubMed] [Google Scholar]
- 20.Ebers GC, Heigenhauser L, Daumer M, Lederer C, Noseworthy JH. Disability as an outcome in MS clinical trials. Neurology. 2008;71:624–631. doi: 10.1212/01.wnl.0000313034.46883.16. [DOI] [PubMed] [Google Scholar]
- 21.Cutter GR, Baier ML, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999;122:871–882. doi: 10.1093/brain/122.5.871. [DOI] [PubMed] [Google Scholar]
- 22.Cohen JA, Cutter JR, Fischer JS, et al. Use of the multiple sclerosis functional composite as an outcome measure in a phase 3 clinical trial. Arch Neurol. 2001;58:961–967. doi: 10.1001/archneur.58.6.961. [DOI] [PubMed] [Google Scholar]
- 23.Petzold A, Eikelenboom MJ, Keir G, et al. The new global multiple sclerosis severity score (MSSS) correlates with axonal but not glial biomarkers. Mult Scler. 2006;12:325–328. doi: 10.1191/135248505ms1277oa. [DOI] [PubMed] [Google Scholar]
- 24.Liu C, Po ALW, Blumhardt LD. "Summary measure" statistic for assessing the outcome of treatment trials in relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psych. 1998;64:726–729. doi: 10.1136/jnnp.64.6.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The multiple sclerosis impact scale (MSIS-29): a new patient-based outcome measure. Brain. 2001;124:962–973. doi: 10.1093/brain/124.5.962. [DOI] [PubMed] [Google Scholar]
- 26.Bar-Zohar D, Agosta F, Goldstaub D, Filippi M. Magnetic resonance imaging metrics and their correlation with clinical outcomes in multiple sclerosis: a review of the literature and future perspectives. Mult Scler. 2008;14:719–727. doi: 10.1177/1352458507088102. [DOI] [PubMed] [Google Scholar]
- 27.Chen JT, Collins DL, Atkins HL, Freedman MS, Arnold DL Canadian MS/BMT Study Group. Magnetization transfer ratio evolution with demyelination and remyelination in multiple sclerosis lesions. Ann Neurol. 2008;63:254–262. doi: 10.1002/ana.21302. [DOI] [PubMed] [Google Scholar]
- 28.Sormani MP, Bonzano L, Roccatagliata L, Cutter GR, Mancardi GL, Bruzzi P. Magnetic resonance imaging as a potential surrogate for relapses in multiple sclerosis: a meta-analytic approach. Ann Neurol. 2009;65:268–275. doi: 10.1002/ana.21606. [DOI] [PubMed] [Google Scholar]
- 29.Rudick RA, Fisher E, Lee JC, Duda JT, Simon J. Brain atrophy in relapsing multiple sclerosis: relationship to relapses, EDSS, and treatment with interferon beta-1a. Mult Scler. 2000;6:365–372. doi: 10.1177/135245850000600601. [DOI] [PubMed] [Google Scholar]
- 30.Bielekova B, Martin R. Development of biomarkers in multiple sclerosis. Brain. 2004;127:1463–1478. doi: 10.1093/brain/awh176. [DOI] [PubMed] [Google Scholar]
- 31.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 32.Coles AJ, Compston DAS, et al. CAMMS223 Trial Investigators. Alemtuzumab vs interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359:1786–1801. doi: 10.1056/NEJMoa0802670. [DOI] [PubMed] [Google Scholar]
- 33.Bielekova B, Richert N, Howard T, et al. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon beta. Proc Natl Acad Sci U S A. 2004;101:8705–8708. doi: 10.1073/pnas.0402653101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice GPA, Filippi M, Comi G. Cladribine and progressive MS: clinical and MRI outcomes of a multicenter controlled trial. Neurology. 2000;54:1145–1155. doi: 10.1212/wnl.54.5.1145. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan C, Kaplin AI, Brodsky RA, et al. Reduction of disease activity and disability with high-dose cyclophosphamide in patients with aggressive multiple sclerosis. Arch Neurol. 2008;65:1044–1051. doi: 10.1001/archneurol.65.8.noc80042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartung HP, Gonsette R, Konig N, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360:2018–2025. doi: 10.1016/S0140-6736(02)12023-X. [DOI] [PubMed] [Google Scholar]
- 37.Providing clinical evidence of effectiveness for human drug and biological products. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER); Guidance for Industry. 1998 May; ( http://www.fda.gov/cder/guidance/index.htm) or ( http://www.fda.gov/cber/guidelines.htm)
- 38.Freedman M, Amato MP, Atkins H, et al. Haematopoietic stem cell transplantation for severe autoimmune diseases; panel session report: multiple sclerosis. Bone Marrow Transplant. 2010;45 Supplement 1s:S8–S10. [Google Scholar]
- 39.Atkins H, Freedman M. Immune ablation followed by autologous hematopoietic stem cell transplantation for the treatment of poor prognosis multiple sclerosis. Meth Mol Biol Neural Cell Transplant. 2009;549:231–246. doi: 10.1007/978-1-60327-931-4_16. [DOI] [PubMed] [Google Scholar]
- 40.Chen JT, Collins DL, Atkins HL, et al. Brain atrophy after immunoablation and stem cell transplantation in multiple sclerosis. Neurol. 2006;66:1935–1937. doi: 10.1212/01.wnl.0000219816.44094.f8. [DOI] [PubMed] [Google Scholar]