Abstract
The use of Drug Delivery Systems as nanocarriers for chemotherapeutic agents can improve the pharmacological properties of drugs by altering drug pharmacokinetics and biodistribution. Among the many drug delivery systems available, both micelles and liposomes have gained the most attention in recent years due to their clinical success. There are several formulations of these nanocarrier systems in various stages of clinical trials, as well as currently clinically approved liposomal-based drugs. In this review, we discuss these drug carrier systems, as well as current efforts that are being made in order to further improve their delivery efficacy through the incorporation of targeting ligands. In addition, this review discusses aspects of drug resistance attributed to the remodeling of the extracellular matrix that occurs during tumor development and progression, as well as to the acidic, hypoxic, and glucose deprived tumor microenvironment. Finally, we address future prospective approaches to overcoming drug resistance by further modifications made to these drug delivery systems, as well as the possibility of coencapsulation/coadministration of various drugs aimed to surmount some of these microenvironmental-influenced obstacles for efficacious drug delivery in chemotherapy.
Keywords: Liposomes, Micelles, Extracellular Matrix, Drug Delivery, Chemotherapy, Tumor Microenvironment, Nanocarriers
Introduction
One of the many challenges in chemotherapy is the delivery of an effective dose of a given cytotoxic agent to the tumor site, while at the same time minimizing unintended harmful side effects. The use of Drug Delivery Systems (DDS) can improve the pharmacological properties of traditional chemotherapeutics by altering drug pharmacokinetics and biodistribution [1, 2]. DDS can include liposomes, micelles, dendrimers, as well as various polymeric-based systems [3–5]. Among the many DDS available, both micelles and liposomes have gained popularity in recent years due to their relative clinical success (details below). For example, several micellar-based drugs are currently in various stages of clinical trials for the delivery of both doxorubicin (a topoisomerase II inhibitor) as well as paclitaxel (a drug that interferes with the normal function of microtubule breakdown) in order to treat gastric and pancreatic cancers [6, 7]. On the other hand, DaunoXome and Doxil are examples of clinically-approved liposomal-based drugs that are currently used to treat either Kaposi’s Sarcoma [8], or both ovarian and recurrent breast cancer [1, 8].
The clinical success of chemotherapeutics encapsulated within lipid-based vesicle-like DDS such as micelles or liposomes can be explained using a number of arguments. For example, due to their small size (~100nm or less), these DDS readily extravasate from circulation through vascular gaps or defects attributed to ongoing angiogenesis that is typical of tumor sites [9] (Figure 1), which have been reported to be ~200 nm or greater [10]. DDS retention within these sites is generally high due to the poor lymphatic drainage observed within tumors [11, 12]. Furthermore, their lower size limit of ~ 20 nm in diameter ensures that these vehicles do not randomly penetrate normal vessel walls. In addition, DDS also serves to minimize the undesirable side effects which can occur using conventional (nonencapsulated) drugs. This includes peripheral neurotoxicity commonly associated with the use of both cisplatin (which crosslinks DNA, thereby interfering with cell division) and vincristine (inhibits assembly of microtubules) [13, 14], or cardiotoxicity that generally results with the use of anthracyclines (DNA intercalators) such as doxorubicin and daunorubicin [15, 16].
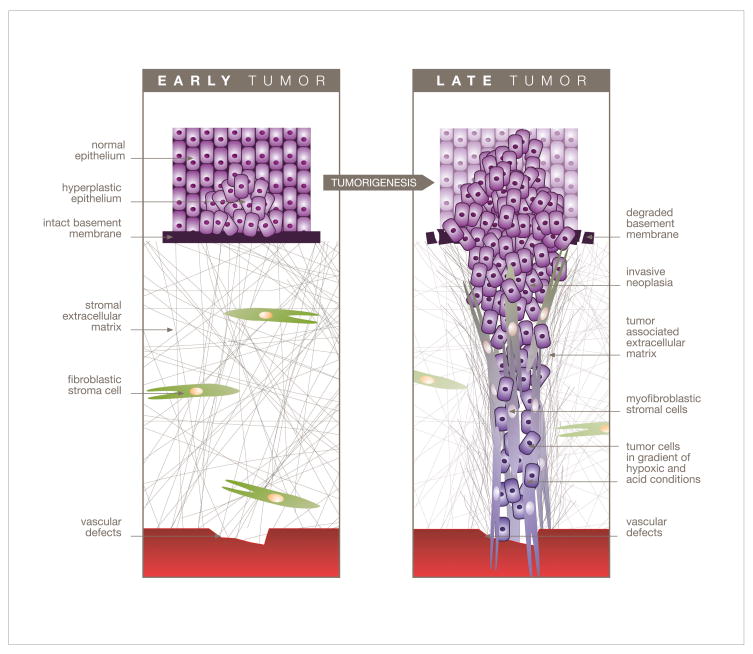
Figure 1.
Diagrammatic representation depicting tumor and mesenchyme progressive stages during tumor development. With respect to the blood vessel and proximal tumor cells, distal cells reside in acidic and hypoxic conditions. The invasive tumor cells will travel through a stromal reaction that contains (among others) myofibroblastic stromal cells localized within the signature parallel-organized tumor-associated extracellular matrix, just prior to their intravasation towards hematogenous metastasis.
The use of DDS however, does present some obstacles to efficacious drug delivery. For instance, low bioavailability resulting from minimal drug accumulation within the tumor site can occur as DDS are particularly susceptible to opsonization and therefore subjected to undesired reticuloendothelial system (RES) uptake, resulting in low drug efficacy. However, surface coating DDS with polyethylene glycol (PEG) has been shown to dramatically improve their circulation times in vivo by substantially reducing protein adsorption and opsonization [11, 17, 18], thereby allowing for increased accumulation of the encapsulated drug within tumor tissues. The use of “PEG-lipids” is ideal as they are water soluble, biocompatible, and confer weak immunogenicity to these systems [19]. In fact, the clinically approved liposomal-based drug Doxil (liposomal-based doxorubicin) is pegylated (Mr 2000) [20, 21], thereby allowing it to preferentially accumulate within tumors via enhanced circulation times. This coupled with the fact that that there is generally poor lymphatic drainage at tumor sites results in a phenomenon commonly referred to as the enhanced permeability and retention (EPR) effect [22–24]. In addition, longer circulation times associated with many micellar-based drugs can also be attributed to PEG-lipids used as hydrophilic corona-forming blocks [6, 25]. However, while the presence of the PEG moiety is useful for controlling the pharmacokinetics of the drug, it can also dramatically reduce tumor cellular uptake because it presents a steric barrier between the DDS and the tumor cells [21, 26]. Unfortunately, this form of “passive” delivery of encapsulated drugs today is still therefore mostly based on leakage in the tumor microenvironment, followed by the possibility of neoplastic cellular uptake of the free drug. As a result, many research groups are currently working on a more “active” form of delivery. Unlike passive delivery, active targeting seeks to further improve the colocalization between the drug and cancer cells, and in some cases it also attempts to improve cellular internalization via receptor-mediated endocytosis, through the addition of surface ligands to DDS [6, 27]. These ligands specifically recognize and preferentially bind receptors present on the cells of interest, thereby allowing for a more precise method of delivery [28]. Patients could therefore receive much higher doses of the chemotherapeutic agent with possibly less non-specific effects, and thus more frequent treatments.
In this review, we discuss some of the recent work involving surface modifications made to both micelles and liposomes in order to actively target tumor cells. While these modifications may improve the delivery of chemotherapeutics to tumor tissue, overall drug efficacy also depends on both the specific tumor cell responsiveness to the given drug and the altered (host’s) microenvironmental settings, which are typical of tumor-associated stromal reactions. Therefore, we also discuss drug resistance attributed to tumor-associated extracellular matrix (ECM) remodeling and the stressful conditions that exist within the tumor microenvironment. Finally, future prospective possibilities to overcoming drug resistance utilizing a combinatorial approach of various DDS modifications, and/or coencapsulation/coadministration of various drugs are also discussed.
Micelles
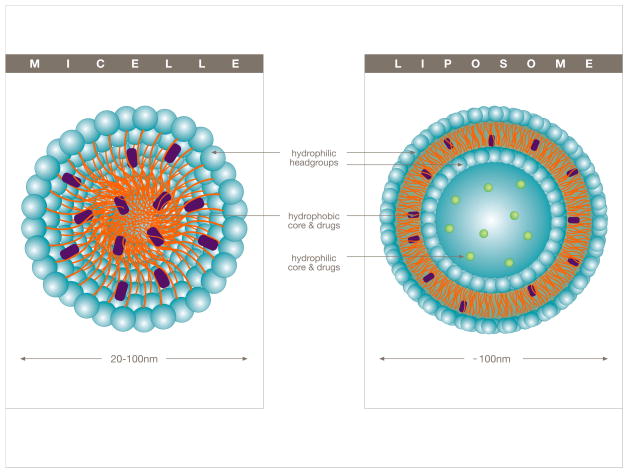
Micelles are small (5–100 nm in diameter) colloidal dispersions that are constructed from amphiphilic molecules (possessing both hydrophilic and hydrophobic properties) such as lipids, which contain a hydrophobic core (figure 2A) and a hydrophilic head (micellar corona) oriented outwardly. Micelles are therefore large enough to escape renal excretion, yet small enough to extravasate from circulation into the tumor tissue [25, 29] through the imperfect tumor vasculature. Their hydrophobic core allows for the delivery of chemotherapeutics, which are often sparingly/poorly soluble in water. The solubilization of hydrophobic drugs also reduces the common risk of potential drug aggregation following intravenous administration, which can lead to severe adverse side effects arising from complications such as the formation of an embolism [2, 30]. As with all DDS, their ability to evade the RES of the immune system is necessary in order to achieve a high bioavailability of the drug. To this end, the hydrophilic micellar corona allows for increased circulation times by reducing opsonization [25, 31], and the low critical micelle concentration (CMC), which is defined as the concentration threshold at which micelles are formed, provide for an ideally stable construct not easily disassociated in vivo [2, 6].
Figure 2.
Schematic depicting the structure of micelles (left) and their ability to incorporate hydrophobic drugs within their internal hydrophobic core, or liposomes (right) which can accommodate both hydrophilic as well as hydrophobic drugs either in the internal aqueous (hydrophilic core) compartment or within the liposomal bilayer (hydrophobic core).
Due to the fact that there are several micellar-based formulations currently in various stages of clinical trials, micelles have received a lot of attention as an optimal DDS. For example, NK105, NC-6004, and SP1049C are paclitaxel, cisplatin, and doxorubicin based drugs currently in Phase-II, Phase-I/II, and Phase-III stages of clinical trials respectively [7, 32]. While proving to be very promising, all of these drugs are based on a passive form of delivery, and great efforts are currently being made in order to further improve overall drug efficacy by actively delivering drugs such as these to the tumor site. For example, previous ligands used in order to confer targeting capabilities to micelles based on highly expressed cancer cell surface receptors include various proteins (including antibodies and specific ligand peptides), carbohydrates, as well as vitamins [28, 33–36]. More recently, active targeting via folate receptor-mediated endocytosis using micelles containing a folate moiety has been shown to be more than four-times as cytotoxic to ovarian carcinoma cells (A2780) than their non-targeted micelle counterparts [37]. Additional ligands that have recently been reported to create targeted micelles include both receptor-specific peptides as well as antibodies (immunomicelles). For example, GRGDS-modified micelles (the peptide GRGDS is a specific ligand for the αVβ3-integrin, which is known to be overexpressed in various metastatic cancer cells) have demonstrated enhanced cytotoxicity against metastatic melanoma B16F10 cells compared to non-targeted micelles [38]. In addition, promising in vivo results have recently been obtained using encapsulated meso-tetraphenylporphorine as a photosensitizing agent in photodynamic therapy within immunomicelles containing the tumor-specific monoclonal antibody (mAb 2C5) used as a targeting ligand against murine lewis lung carcinoma [22]. In fact, female C57BL/6 mice treated with the non-targeted micellar formulation in this latter study resulted in ~50% tumor inhibition compared to untreated control, whereas treatment with mAb 2C5-immunomicelles resulted in almost complete tumor inhibition during the 35 day experiment.
While micelles prove to be a very promising DDS, their smaller size when compared to larger carriers such as liposomes limit their ability to carry a substantial dose of the chemotherapeutic agent to the tumor. Furthermore, the use of micelles increases the risk of premature release of the drug prior to reaching the intended target when compared to larger DDS, as smaller sized carriers experience faster release rates when compared to larger vesicles [39, 40].
Liposomes
Liposomes are composed of a phospholipid bilayer which entirely surrounds an internal aqueous core used for drug encapsulation (Figure 2B). While these DDS can vary in size quite dramatically with diameters ranging from a few nanometers to several microns, liposomes of ~100 nm in diameter have been shown to be optimal for the delivery of chemotherapeutics to tumors [41, 42]. As such, they tend to be larger than micelles and therefore have the ability to deliver greater amounts of the chemotherapeutic agent to the tumor site while minimizing the risk associated with premature leakage. In addition, liposomes also have the ability to accommodate both hydrophilic as well as hydrophobic drugs, either in the internal aqueous core or in the lipid bilayer respectively [43, 44] (Figure 2B). When compared to conventional (unencapsulated) drugs, liposomal treatment has been shown to dramatically reduce some of the traditional side effects associated with chemotherapy, such as nausea and vomiting [45].
As with micelles, the clinical success of liposomes has also made them very popular drug carriers for various chemotherapeutics. For example, the clinically approved drugs DaunoXome and Doxil are liposomal formulations that encapsulate the commonly used chemotherapeutic agents daunorubicin and doxorubicin respectively. CPX-1 is a new irinotecan (topoisomerase-I inhibitor) HCI/floxuridine liposomal-based drug (molar ratio 1:1) which is currently in Phase-II clinical trials for the treatment of Colorectal cancer [7]. However, all of these formulations are once again based on a passive form of delivery, and therefore current work is aimed at actively targeting systems like these to tumor cells. In fact, MCC465 and MBP-426 are both targeted liposomal-based drugs currently in Phase-I clinical trials [7, 23], and there are many other systems that may prove to be very promising in the near future. For example, the cancer cell surface receptor CD44, which recognizes the extracellular tumor microenvironment rich hyaluronic acid, is found at elevated levels amongst various tumorigenic cells, as is the case in melanoma [46, 47]. This cell surface receptor has been successfully targeted using liposomes coated with not only hexameric fragments of hyaluronic acid [48], but also with a CD44-binding triple-helical peptide-amphiphile ligand (α1(IV)1263–1277 PA) [5]. Moreover, cisplatin-loaded liposomes containing a new cationic lipid known as TRX-20 that is specific for overexpressed chondroitin sulfate, which is associated with many types of tumor cells, exhibit far greater tumor growth inhibition than non-targeted liposomes [49]. As many cancer cells have been shown to overexpress the tyrosine kinase receptor HER2, anti-HER2 immunoliposomes have previously been constructed and have been shown to exhibit superior anticancer activity when compared to their non-targeted counterpart [50]. Various liposomal formulations containing peptide sequences specific for certain upregulated integrins (major receptors for cell-matrix interactions) in both primary and metastatic melanoma have also been suggested [51]. More recently, transferrin-coated liposomes, which specifically recognize the commonly cancer-overexpressed transferrin receptor have been shown to exhibit far greater tumor site-specificity in tumor-bearing mice than non-targeted liposomes [52].
Tumor Microenvironment
While the use of DDS such as micelles and liposomes have resulted in very encouraging results, many obstacles still remain which limit their overall efficacy. Nevertheless, they have proved to dramatically enhance the pharmacokinetics and biodistribution of encapsulated drugs. However, following extravasation from circulation, there remain considerable challenges to overcome attributed to drug resistance within the harsh conditions of the tumor microenvironment.
Tumor associated ECM
The tumor microenvironment [53–55], collectively known as stroma, is complex and is composed of connective tissue that contains an altered ECM as well as several different cell types. The various cells found within the tumor-stroma include endothelial cells which form new vasculature, immune and inflammatory cells such as macrophages, bone marrow-derived mesenchymal stem cells that differentiate into various components of the stroma, lipocytes, smooth muscle cells and fibroblastic cells which become myofibroblastic or activated as the tumor progresses and are responsible for secreting the mesenchymal ECM [53–56]. During tumor development, fibroblastic stroma progression, also known as stromagenesis, results in significant changes to the surrounding mesenchyme in response to tumor growth, which are believed to promote tumorigenesis [56, 57]. Importantly to this review, one of the results of these stromal-modifications is the remodeling of the ECM, which can alter tumor cell responsiveness to various chemotherapeutics [58–62]. Further contributing to overall drug resistance is the low pH, as well as the hypoxic and glucose-deprived conditions that exist in the tumor microenvironment [63–65].
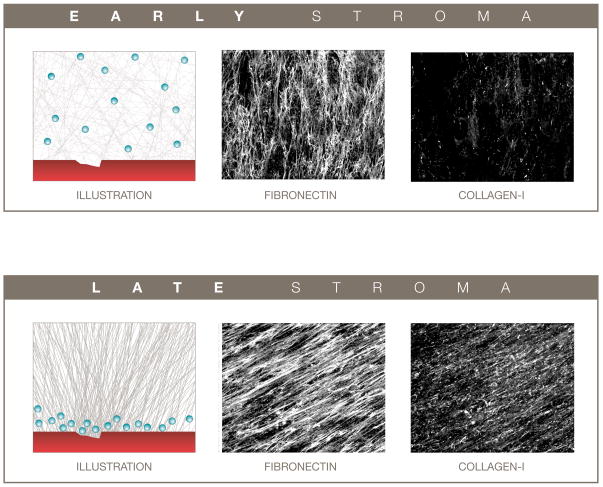
During tumor-induced mesenchymal stroma progression [66, 67], the rapidly proliferating stromal fibroblasts (as well as other cells recruited to the tumor-associated site) undergo morphological changes and begin to express myofibroblastic markers such as α-smooth muscle actin (α-SMA) [68–70]. Among some of the results of this myofibroblastic differentiation, is the increased deposition of ECM proteins such as collagen I and differential spliced forms of fibronectin [68], resulting in a significantly dense tumor-microenvironment which shares many characteristics of wound healing [71]. Further contributing to this phenotype is the parallel orientated fiber organization of ECM components such as collagen I and fibronectin [69, 72] (Figure 3), resulting in a tightly packed and greatly organized ECM. This reorganization of the ECM provides what is believed to be a less penetrable than normal or than non-tumorigenic extracellular environment for chemotherapeutics following drug extravasation into the tumor-microenvironment near the tumor cells [73]. In fact, due to the recent development of fibroblast-derived three-dimensional (3-D) matrices [66, 74, 75], this level of organization can be mimicked in vitro using normal (Figure 3A) vs. carcinoma- or tumor-associated (Figure 3B) fibroblasts [69, 76]. Consequentially, one can understand how smaller DDS (e.g. micelles) could be able to penetrate deeper into this densely organized tumor-microenvironment than larger carriers (e.g. liposomes) [2, 6]. Furthermore, following DDS escape (when drugs are released from their delivery systems), some commonly used cytotoxic agents, such as doxorubicin, have limited tumor tissue penetrability due to a high affinity between this drug and various components of the extracellular environment [77, 78]. This can further contribute to observed drug resistance as the uniform distribution of the drug within the tumor microenvironment can be limited.
Figure 3.
The penetration of extravasated chemotherapeutic agents from circulation into the tumor microenvironment can be influenced by the tumor-associated stromal compartment. A normal-like less organized and loosely packed early tumor microenvironment (top) can provide for greater drug penetration, while a more organized and tighter packed late tumor microenvironment (bottom) may result in decreased drug penetration. Left panels are representative illustrations of damaged blood vessels (red) and putative extravasated drugs (e.g., DDS, blue spheres) penetrating through the assorted ECMs. Middle and right panels depict reconstructed confocal images of indirect immunofluorescence showing in vivo-like ECM fibronectin (middle) and collagen I (right) fibers derived from assorted fibroblasts. Confocal images are reproductions from Amatangelo, Bassi et al. 2005 [69] presented with permission from the American Journal of Pathology.
Integrin-mediated drug resistance can also occur as a direct result of stromal ECM remodeling during tumorigenesis [60]. Integrins are composed of two noncovalently associated heterodimeric transmembrane subunits, designated alpha (α) and beta (β) [79, 80]. They facilitate bidirectional signal transduction between the ECM and cells. Therefore, changes in the extracellular environment can influence not only cellular adhesions, but also cell behavior via differential integrin engagement. For example, great differences in integrin content and signaling have been observed between “3-D matrix adhesions” formed in physiological three-dimensional environments compared to focal and fibrillar adhesions observed in cells cultured in traditional two-dimensional culture dishes [75, 81, 82]. The differential integrin engagement between distinctly different substrates or extracellular environments can further alter cellular behavior, including responsiveness to chemotherapeutics. For example, ligation of β1-integrin by various extracellular matrix ligands in metastatic breast cancer cells has been shown to result in resistance to both paclitaxel and vincristine [83]. Fibroblast-derived 3-D matrix-induced resistance to taxol treatment in pancreatic cancer cells (PANC-1) been shown to be in a β1-integrin-dependent manner [58]. More recently, we have compared metastatic breast cancer cell behavior induced by ECMs representative of early and late-stage stromagenesis, which resulted in clear differences in β1-integrin blockage responses, suggesting possible changes in chemosensitivity based on biochemical/structural differences of the surrounding environment [76].
Low Stromal pH
Drug resistance can also occur due to the relatively low pH observed within the typical tumor microenvironment (Figure 1) [63]. Most chemotherapeutics are membrane permeable in their neutral form, and relatively membrane impermeable in their low pH induced charged form. This lowering of the pH occurs in part because tumor cells often use glycolysis rather than oxidative metabolism, and high interstitial pressure causing poor perfusion results in reduced clearance of the resulting acidic products [63, 84]. Indeed, the pH in the tumor microenvironment has been reported to be anywhere from ~6.5 to about 7.2 [37, 84]. The lower pH in the tumor microenvironment with respect to both tumor intracellular pH and normal tissue causes drug protonation of weakly basic drugs such as vincristine and doxorubicin [85–87], resulting in decreased cellular uptake, which therefore contributes to drug resistance. Although many solutions have been proposed to overcome this form of resistance to include alkalinization of the tumor pH to enhance the activity of these mildly basic chemotherapeutics [85], the encapsulation of these drugs within DDS serves to effectively overcome this obstacle. The DDS shields cytotoxic agents from the microenvironmental settings, and therefore allows tumor cells to internalize the drug. However, following cellular internalization, the pH gradient between the neutral/alkaline cytoplasm and acidic intracellular organelles (e.g. endosomes and lysosomes) can also lead to drug resistance [88]. Due to the fact that these DDS are often sequestered in acidic endosomes following cellular internalization [77, 89], endosomal pH triggered release mechanisms have been developed and incorporated within these systems. For example, pH sensitive doxorubicin loaded micelles composed of poly(histidine (His)-co-phenylalanine (Phe))-b-poly(ethylene glycol) (PEG) and poly(L-lactic acid) (PLLA)-b-PEG have been shown to be almost four-times as cytotoxic towards ovarian carcinoma cells (A2780) at pH 6.0 when compared to neutral pH conditions [37]. In fact, more recently, the Bae group has shown that a modified version of this micelle formulation (second generation optimized for pH 6.0 rather than 6.8) effectively suppressed the growth of existing Multidrug-resistant ovarian tumors in mice [90]. Hydrophobic acetal groups have also been incorporated within micellar formulations to generate acid-sensitive micelles [91, 92]. Similarly, liposomes containing various pH sensitive molecules have been constructed and proven to be quite effective. For example, vinyl ethers, acetals, and ketals have all been used for the pH triggered release of liposomal contents [93–95]. More recently, acid-sensitive liposomes containing ortho ester phosphocholine have proven to be particularly sensitive to changes in pH [96]. Rather than incorporating pH triggered release mechanisms within drug carriers, the additional use of lysosomotropic agents such as chloroquine designed to buffer pre-lysosomal vesicles can be used to facilitate DDS escape [88, 97].
Stromal matrix metalloproteinases
The use of pH triggered release mechanisms may prove to be a very effective modification in targeted-based DDS intended for receptor-mediated endocytosis. However, the introduction of such molecules within pegylated DDS-based drugs intended for passive delivery (e.g. Doxil), would not be beneficial as these pegylated systems seldomly gain access to the intracellular space [21, 26]. Nevertheless, the elevated levels of proteases such as matrix metalloproteinases (MMPs) within the tumor microenvironment provide an opportunity for the shedding of the PEG moiety following extravasation of these systems into the microenvironment and thus facilitate the subsequent cellular internalization of these DDS-based drugs [26]. In fact, tumor site-specific activity is anticipated as premature MMP-mediated PEG removal in circulation is greatly reduced due to inhibition of the these proteases by serum proteins such as α2-macroglobulin [98]. This concept has previously been applied to the development of pro-drugs, which have been successfully developed using both doxorubicin [99] as well as methotrexate [100]. Both of these systems have a relatively bulky moiety conjugated to the cytotoxic agent via a MMP-susceptible peptide sequence rendering them inactive. They are designed to remain in this form while in circulation and exposure to MMPs in the tumor microenvironment following extravasation liberates an active from of the drug. More recently, a similar concept has been applied to improving the overall drug efficacy of DDS-based chemotherapeutics. Liposomes containing a MMP-2-cleavable PEG-peptide lipid have recently been developed and have shown to exhibit significant tumor cellular uptake following exposure to MMP-2 [101]. In addition, Hatakeyama and colleagues have obtained similar results using a comparable system [26]. Liposomes have also been designed to include a MMP-specific (MMP-9) triggered release mechanism intended to facilitate drug release in the interstitial space [102, 103].
Hypoxic and glucose-deprived microenvironment
During tumor development, rapidly growing tumors quickly exhaust available resources and, because of high necrosis, limited vasculature, and increased interstitial pressure, the tumor microenvironment is characteristically hypoxic and glucose deprived (Figure 1). These harsh conditions can further contribute to chemotherapeutic resistance. For example, topoisomerase II inhibitors such as doxorubicin and etoposide can be particularly ineffective against hypoxic/glucose-starved tumor cells [64, 65]. This in part, is attributed to the fact that these stressful microenvironmental conditions also result in protein folding disruption within the endoplasmic reticulum, including that of topoisomerase II [104, 105]. As the cytotoxic effect of both doxorubicin and etoposide is directly related to the number of active topoisomerase II molecules, it is believed that drug resistance ensues due to a reduction in the numbers of these enzymes. In addition, hypoxic conditions can cause dramatic changes in gene expression mediated by hypoxia-inducible factor 1 (HIF-1), resulting in reduced apoptotic potential of tumor cells, and therefore decreased sensitivity to chemotherapeutics [65, 106].
To overcome the stress induced resistance attributed to the hypoxic and glucose deprived conditions found in the tumor microenvironment, several solutions have been proposed to include drugs that selectively target hypoxic cells such as tirapazamine, which is selectively activated by reductases that reside in these harsh environments by releasing oxygen radicals believed to induce damage to cancerous DNA [107]. Previous work has shown that resistance attributed to hypoxia and glucose deprivation can be overcome using the proteasome inhibitor lactacystin, which has shown to significantly enhance the antitumor activity of etoposide in a solid tumor model [64]. The possibility of using this type of combinatorial approach along with DDS-based drugs is discussed below.
Conclusions and Future Perspectives
The use of DDS such as micelles and liposomes can facilitate the delivery of chemotherapeutic agents to tumor sites, while at the same time minimizing unintended harmful side effects associated with the use of these drugs. In addition, DDS allow for the administration of an increased cumulative dose of the drug. For example, in mice the maximum tolerated dose of liposomal-encapsulated doxorubicin (55 mg/kg) is significantly higher than that of free doxorubicin (6 mg/kg) [24, 108]. Although there are many DDS-based drugs currently in various stages of clinical trials or already clinically approved, future work aims to improve drug efficacy by the incorporation of targeted ligands within these formulations. However, while this may improve how these drug formulations reach their desired destinations within the tumor site, drug resistance attributed to tumor-associated stromal ECM and ECM remodeling, as well as to the stressful tumor microenvironmental conditions remain challenges in effective chemotherapeutic treatments.
The increased deposition of Collagen I during tumorigenesis, and the relative fiber organization of modified ECMs, can result in a less penetrable tumor microenvironment for chemotherapeutics following extravasation. The coencapsulation/coadministration of DDS-based drugs and tumor-associated stromal-depleting drugs may in fact serve to overcome this form of resistance. Recently, Olive and coworkers have shown a dramatic improvement in the delivery and drug efficacy of gemcitabine (a commonly used antimetabolite drug) in mice for the treatment of pancreatic cancer when coadministered with a drug known as IPI-926 [109]. IPI-926 has been shown to be involved in the depletion of tumor-associated stromal-tissue via inhibition of the Hedgehog cellular signaling pathway [109, 110]. Future work involving the coencapsulation/coadministration of tumor-associated stromal-depleting drugs such as IPI-926 and liposomal-based drugs would not only allow for increased penetration and therefore uniform drug biodistribution within the tumor microenvironment, but also for a more effective dose of the drug to be delivered to the tumor cells without causing undesired non-specific secondary effects. However, it should also be mentioned that it remains unclear how tumor-associated stromal-depleting drugs would influence the metastatic potential and/or additional tumorigenic behaviors (normally affected by the tumor microenvironment), of cancer cells.
Integrin-mediated chemoresistance is an additional significant challenge in the administration of effective chemotherapeutic treatments, which is also directly affected by the ECM. The development of in vitro assays that effectively mimic in vivo-like conditions, such as tumor-associated fibroblast-derived 3-D matrices, could prove to be an invaluable tool to predicting overall drug efficacy. Although both Matrigel and polymerized collagen have been shown to influence tumor cell responsiveness to various chemotherapeutic agents [111] these systems may not accurately represent the true nature of a mesenchymal stroma. For example, Matrigel essentially mimics the basement membrane [112], which is often degraded as tumors progress, while polymerized collagen consists of a pure preparation of collagen I [113]. Therefore, these systems lack the numerous fibrous proteins and additional biological active molecules associated with the mesenchymal environment. Furthermore, the use of fibroblast-derived 3-D matrices could also account for resistance attributed to DDS penetrability of the tumor-associated environment [58]. In addition, following DDS escape, these in vitro assays could prove to be valuable in determining the overall distribution of commonly used DDS-based drugs within the ECM, as many are known to have a high affinity for various components of the extracellular environment (e.g. doxorubicin).
Many modifications to DDS-based drugs have been attempted with varying degrees of success. It may very well be that a combinatorial approach using several of these modifications results in the development of improved DDS-based drugs when compared to those currently used in the clinic. For example, liposomes that contain both a MMP-2-cleavable PEG-peptide and a pH sensitive release mechanism would potentially allow for not only enhance cellular uptake following the shedding of the PEG moiety, but would also allow for endosomal escape following internalization. The additional modification of a targeting ligand to such a system may prove to further enhance its overall efficacy. Furthermore, the coencapsulation of some of the drugs mentioned in this review within these modified DDS, and/or the coadministration of these modified systems along with conventional (unencapsulated) drugs such as stromal-depleting drugs may also result in very efficacious chemotherapeutic treatments. Additionally, successful combinatorial approaches aimed at overcoming drug resistance attributed to the extracellular environment in various cancer cells may be quickly identified with improved in vitro assays that effectively mimic the tumor microenvironment such as tumor-associated fibroblast derived matrices, thereby allowing for dramatically improved chemotherapeutic cocktails in order to more effectively treat cancer.
Acknowledgments
We would like to thank Perla Ovseiovich from “Paralelo 19” (Mexico City) for the art work, Anne Carson for accurate proofreading, as well as Matthew K. Robinson and Daniel Bassi for informative discussions. This work was supported by the following: Ovarian Cancer Research Fund, NIH/NCI CA06927, RO1-CA113451 (EC), Fox Chase Cancer Center’s internal director’s fund and an appropriation from the Commonwealth of Pennsylvania. Additional funds were provided by Fox Chase Cancer Center via institutional support of the Kidney Keystone Program and the Ewing Trust for Pancreatic Cancer research, as well as from the Killgore Research Center at West Texas A&M University. The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the foundations and institutes mentioned above.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen TM, Cullis PR. Drug delivery systems: Entering the mainstream. Science. 2004;303:1818–22. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 2.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007b;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 3.Sahoo SK, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug Discov Today. 2003;8:1112–20. doi: 10.1016/s1359-6446(03)02903-9. [DOI] [PubMed] [Google Scholar]
- 4.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–60. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 5.Rezler EM, Khan DR, Lauer-Fields J, Cudic M, Baronas-Lowell D, Fields GB. Targeted drug delivery utilizing protein-like molecular architecture. Journal of the American Chemical Society. 2007b;129:4961–72. doi: 10.1021/ja066929m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco E, Kessinger CW, Sumer BD, Gao J. Multifunctional micellar nanomedicine for cancer therapy. Exp Biol Med (Maywood) 2009;234:123–31. doi: 10.3181/0808-MR-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumura Y, Kataoka K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 2009;100:572–9. doi: 10.1111/j.1349-7006.2009.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torchilin VP. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007a;9:E128–47. doi: 10.1208/aapsj0902015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–84. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 10.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4607–12. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabizon AA. Stealth liposomes and tumor targeting: one step further in the quest for the magic bullet. Clin Cancer Res. 2001a;7:223–5. [PubMed] [Google Scholar]
- 12.Roux E, Lafleur M, Lataste E, Moreau P, Leroux JC. On the characterization of pH-sensitive liposome/polymer complexes. Biomacromolecules. 2003;4:240–8. doi: 10.1021/bm025651x. [DOI] [PubMed] [Google Scholar]
- 13.Bianchi R, Brines M, Lauria G, Savino C, Gilardini A, Nicolini G, et al. Protective effect of erythropoietin and its carbamylated derivative in experimental Cisplatin peripheral neurotoxicity. Clin Cancer Res. 2006;12:2607–12. doi: 10.1158/1078-0432.CCR-05-2177. [DOI] [PubMed] [Google Scholar]
- 14.Wang WS, Chiou TJ, Liu JH, Fan FS, Yen CC, Chen PM. Vincristine-induced dysphagia suggesting esophageal motor dysfunction: a case report. Japanese journal of clinical oncology. 2000;30:515–8. doi: 10.1093/jjco/hyd132. [DOI] [PubMed] [Google Scholar]
- 15.Rivera E. Liposomal anthracyclines in metastatic breast cancer: clinical update. The oncologist. 2003;8 (Suppl 2):3–9. doi: 10.1634/theoncologist.8-suppl_2-3. [DOI] [PubMed] [Google Scholar]
- 16.Swarbrick J, Boylan J. Liposomes as Pharmaceutical Dosage Forms. Encyclopedia of Pharmaceutical Dosage Forms. 1994:1–39. [Google Scholar]
- 17.Bedu-Addo FK, Tang P, Xu Y, Huang L. Effects of polyethyleneglycol chain length and phospholipid acyl chain composition on the interaction of polyethyleneglycol-phospholipid conjugates with phospholipid: implications in liposomal drug delivery. Pharm Res. 1996;13:710–7. doi: 10.1023/a:1016091314940. [DOI] [PubMed] [Google Scholar]
- 18.Photos PJ, Bacakova L, Discher B, Bates FS, Discher DE. Polymer vesicles in vivo: correlations with PEG molecular weight. J Control Release. 2003;90:323–34. doi: 10.1016/s0168-3659(03)00201-3. [DOI] [PubMed] [Google Scholar]
- 19.Bouvier M, Wiley DC. Antigenic peptides containing large PEG loops designed to extend out of the HLA-A2 binding site form stable complexes with class I major histocompatibility complex molecules. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:4583–8. doi: 10.1073/pnas.93.10.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabanes A, Even-Chen S, Zimberoff J, Barenholz Y, Kedar E, Gabizon A. Enhancement of antitumor activity of polyethylene glycol-coated liposomal doxorubicin with soluble and liposomal interleukin 2. Clin Cancer Res. 1999;5:687–93. [PubMed] [Google Scholar]
- 21.Gabizon AA. Pegylated liposomal doxorubicin: Metamorphosis of an old drug into a new form of chemotherapy. Cancer Invest. 2001b;19:424–36. doi: 10.1081/cnv-100103136. [DOI] [PubMed] [Google Scholar]
- 22.Roby A, Erdogan S, Torchilin VP. Enhanced in vivo antitumor efficacy of poorly soluble PDT agent, meso-tetraphenylporphine, in PEG-PE-based tumor-targeted immunomicelles. Cancer Biol Ther. 2007;6:1136–42. doi: 10.4161/cbt.6.7.4345. [DOI] [PubMed] [Google Scholar]
- 23.Matsumura Y, Gotoh M, Muro K, Yamada Y, Shirao K, Shimada Y, et al. Phase I and pharmacokinetic study of MCC-465, a doxorubicin (DXR) encapsulated in PEG immunoliposome, in patients with metastatic stomach cancer. Ann Oncol. 2004;15:517–25. doi: 10.1093/annonc/mdh092. [DOI] [PubMed] [Google Scholar]
- 24.Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Frechet JM, Dy EE, et al. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16649–54. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Husseini GA, Pitt WG. Micelles and nanoparticles for ultrasonic drug and gene delivery. Adv Drug Deliv Rev. 2008;60:1137–52. doi: 10.1016/j.addr.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatakeyama H, Akita H, Kogure K, Oishi M, Nagasaki Y, Kihira Y, et al. Development of a novel systemic gene delivery system for cancer therapy with a tumor-specific cleavable PEG-lipid. Gene Ther. 2007;14:68–77. doi: 10.1038/sj.gt.3302843. [DOI] [PubMed] [Google Scholar]
- 27.Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev. 1999;51:691–744. [PubMed] [Google Scholar]
- 28.Wang YG, Wang X, Zhang YF, Yang SJ, Wang JC, Zhang X, et al. RGD-modified polymeric micelles as potential carriers for targeted delivery to integrin-overexpressing tumor vasculature and tumor cells. J Drug Target. 2009;17:459–67. doi: 10.1080/10611860902974085. [DOI] [PubMed] [Google Scholar]
- 29.Dennis MS, Zhang M, Meng YG, Kadkhodayan M, Kirchhofer D, Combs D, et al. Albumin binding as a general strategy for improving the pharmacokinetics of proteins. J Biol Chem. 2002;277:35035–43. doi: 10.1074/jbc.M205854200. [DOI] [PubMed] [Google Scholar]
- 30.Degim IT, Celebi N. Controlled delivery of peptides and proteins. Curr Pharm Des. 2007;13:99–117. doi: 10.2174/138161207779313795. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Mongayt D, Torchilin VP. Polymeric micelles for delivery of poorly soluble drugs: preparation and anticancer activity in vitro of paclitaxel incorporated into mixed micelles based on poly(ethylene glycol)-lipid conjugate and positively charged lipids. Journal of drug targeting. 2005;13:73–80. doi: 10.1080/10611860400011935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kabanov AV. Polymer genomics: an insight into pharmacology and toxicology of nanomedicines. Adv Drug Deliv Rev. 2006;58:1597–621. doi: 10.1016/j.addr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagasaki Y, Yasugi K, Yamamoto Y, Harada A, Kataoka K. Sugar-installed block copolymer micelles: their preparation and specific interaction with lectin molecules. Biomacromolecules. 2001;2:1067–70. doi: 10.1021/bm015574q. [DOI] [PubMed] [Google Scholar]
- 34.Vinogradov S, Batrakova E, Li S, Kabanov A. Polyion complex micelles with protein-modified corona for receptor-mediated delivery of oligonucleotides into cells. Bioconjug Chem. 1999;10:851–60. doi: 10.1021/bc990037c. [DOI] [PubMed] [Google Scholar]
- 35.Leamon CP, Weigl D, Hendren RW. Folate copolymer-mediated transfection of cultured cells. Bioconjug Chem. 1999;10:947–57. doi: 10.1021/bc990066n. [DOI] [PubMed] [Google Scholar]
- 36.Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. Immunomicelles: Targeted pharmaceutical carriers for poorly soluble drugs. Proc Natl Acad Sci USA. 2003;100:6039–44. doi: 10.1073/pnas.0931428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D, Lee ES, Oh KT, Gao ZG, Bae YH. Doxorubicin-loaded polymeric micelle overcomes multidrug resistance of cancer by double-targeting folate receptor and early endosomal pH. Small. 2008;4:2043–50. doi: 10.1002/smll.200701275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong XB, Mahmud A, Uludag H, Lavasanifar A. Multifunctional polymeric micelles for enhanced intracellular delivery of doxorubicin to metastatic cancer cells. Pharm Res. 2008;25:2555–66. doi: 10.1007/s11095-008-9673-5. [DOI] [PubMed] [Google Scholar]
- 39.Kim SY, Shin IG, Lee YM. Preparation and characterization of biodegradable nanospheres composed of methoxy poly(ethylene glycol) and DL-lactide block copolymer as novel drug carriers. J Control Release. 1998;56:197–208. doi: 10.1016/s0168-3659(98)00083-2. [DOI] [PubMed] [Google Scholar]
- 40.Evans DF, Wennerstrom H. The Colloidal Domain: Where Physics, Chemistry, Biology, and Technology Meet. New York: VCH Publishers, Inc; 1994. [Google Scholar]
- 41.Siwak DR, Tari AM, Lopez-Berestein G. The potential of drug-carrying immunoliposomes as anticancer agents. Commentary re: J. W. Park et al., Anti-HER2 immunoliposomes: enhanced efficacy due to targeted delivery. Clin. Cancer Res. 8: 1172–1181, 2002. Clin Cancer Res. 2002;8:955–6. [PubMed] [Google Scholar]
- 42.Torchilin VP, Weissig V. Liposomes: A Practical Approach. 2. Oxford University Press; 2003. [Google Scholar]
- 43.New RRC. Liposomes: A Practical Approach. 1. Oxford University Press; 1990. [Google Scholar]
- 44.Khan DR, Rezler EM, Lauer-Fields J, Fields GB. Effects of drug hydrophobicity on liposomal stability. Chem Biol Drug Des. 2008;71:3–7. doi: 10.1111/j.1747-0285.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- 45.Lasic DD. Novel applications of liposomes. Trends in Biotechnology. 1998;16:307–21. doi: 10.1016/s0167-7799(98)01220-7. [DOI] [PubMed] [Google Scholar]
- 46.Faassen AE, Mooradian DL, Tranquillo RT, Dickinson RB, Letourneau PC, Oegema TR, et al. Cell surface CD44-related chondroitin sulfate proteoglycan is required for transforming growth factor-b-stimulated mouse melanoma cell motility and invasive behavior on type I collagen. J Cell Sci. 1993;105:501–11. doi: 10.1242/jcs.105.2.501. [DOI] [PubMed] [Google Scholar]
- 47.Faassen AE, Schrager JA, Klein DJ, Oegema TR, Couchman JR, McCarthy JB. A cell surface chondroitin sulfate proteoglycan, immunologically related to CD44, is involved in type I collagen-mediated melanoma cell motility and invasion. J Cell Biol. 1992;116:521–31. doi: 10.1083/jcb.116.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eliaz RE, Szoka J, FC Liposome-encapsulated doxorubicin targeted to CD44: A strategy to kill CD44-overexpressing tumor cells. Cancer Res. 2001;61:2592–601. [PubMed] [Google Scholar]
- 49.Lee CM, Tanaka T, Murai T, Kondo M, Kimura J, Su W, et al. Novel chondroitin sulfate-binding cationic liposomes loaded with cisplatin efficiently suppress the local growth and liver metastasis of tumor cells in vivo. Cancer Res. 2002;62:4282–8. [PubMed] [Google Scholar]
- 50.Park JW, Kirpotin DB, Hong K, Shalaby R, Shao Y, Nielsen UB, et al. Tumor targeting using anti-her2 immunoliposomes. J Control Release. 2001;74:95–113. doi: 10.1016/s0168-3659(01)00315-7. [DOI] [PubMed] [Google Scholar]
- 51.Rezler EM, Khan DR, Tu R, Tirrell M, Fields GB. Peptide-Mediated Targeting of Liposomes to Tumor Cells. Methods Mol Biol. 2007a:269–98. doi: 10.1007/978-1-59745-430-8_10. [DOI] [PubMed] [Google Scholar]
- 52.Li X, Ding L, Xu Y, Wang Y, Ping Q. Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int J Pharm. 2009;373:116–23. doi: 10.1016/j.ijpharm.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 53.Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem. 2007;101:805–15. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
- 54.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–50. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 55.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–9. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 56.Beacham DA, Cukierman E. Stromagenesis: the changing face of fibroblastic microenvironments during tumor progression. Semin Cancer Biol. 2005;15:329–41. doi: 10.1016/j.semcancer.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Ghajar CM, Bissell MJ. Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging. Histochem Cell Biol. 2008;130:1105–18. doi: 10.1007/s00418-008-0537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serebriiskii I, Castello-Cros R, Lamb A, Golemis EA, Cukierman E. Fibroblast-derived 3D matrix differentially regulates the growth and drug-responsiveness of human cancer cells. Matrix Biol. 2008 doi: 10.1016/j.matbio.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rintoul RC, Sethi T. The role of extracellular matrix in small-cell lung cancer. Lancet Oncol. 2001;2:437–42. doi: 10.1016/S1470-2045(00)00421-6. [DOI] [PubMed] [Google Scholar]
- 60.Zutter MM. Integrin-mediated adhesion: tipping the balance between chemosensitivity and chemoresistance. Adv Exp Med Biol. 2007;608:87–100. doi: 10.1007/978-0-387-74039-3_6. [DOI] [PubMed] [Google Scholar]
- 61.Hodkinson PS, Mackinnon AC, Sethi T. Extracellular matrix regulation of drug resistance in small-cell lung cancer. Int J Radiat Biol. 2007;83:733–41. doi: 10.1080/09553000701570204. [DOI] [PubMed] [Google Scholar]
- 62.Li ZW, Dalton WS. Tumor microenvironment and drug resistance in hematologic malignancies. Blood Rev. 2006;20:333–42. doi: 10.1016/j.blre.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373–84. [PubMed] [Google Scholar]
- 64.Ogiso Y, Tomida A, Tsuruo T. Nuclear localization of proteasomes participates in stress-inducible resistance of solid tumor cells to topoisomerase II-directed drugs. Cancer Res. 2002;62:5008–12. [PubMed] [Google Scholar]
- 65.Cosse JP, Sermeus A, Vannuvel K, Ninane N, Raes M, Michiels C. Differential effects of hypoxia on etoposide-induced apoptosis according to the cancer cell lines. Mol Cancer. 2007;6:61. doi: 10.1186/1476-4598-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Castello-Cros R, Cukierman E. Stromagenesis during tumorigenesis: characterization of tumor-associated fibroblasts and stroma-derived 3D matrices. Methods Mol Biol. 2009;522:275–305. doi: 10.1007/978-1-59745-413-1_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pavlakis K, Messini I, Vrekoussis T, Yiannou P, Keramopoullos D, Louvrou N, et al. The assessment of angiogenesis and fibroblastic stromagenesis in hyperplastic and pre-invasive breast lesions. BMC Cancer. 2008;8:88. doi: 10.1186/1471-2407-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–17. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 69.Amatangelo MD, Bassi DE, Klein-Szanto AJ, Cukierman E. Stroma-derived three-dimensional matrices are necessary and sufficient to promote desmoplastic differentiation of normal fibroblasts. Am J Pathol. 2005;167:475–88. doi: 10.1016/S0002-9440(10)62991-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–38. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 72.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–503. [PubMed] [Google Scholar]
- 74.Beacham DA, Amatangelo MD, Cukierman E. Preparation of extracellular matrices produced by cultured and primary fibroblasts. Curr Protoc Cell Biol. 2007;Chapter 10(Unit 10):9. doi: 10.1002/0471143030.cb1009s33. [DOI] [PubMed] [Google Scholar]
- 75.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–12. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 76.Castello-Cros R, Khan DR, Simons J, Valianou M, Cukierman E. Staged stromal extracellular 3D matrices differentially regulate breast cancer cell responses through PI3K and beta1-integrins. BMC Cancer. 2009;9:94. doi: 10.1186/1471-2407-9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tunggal JK, Cowan DS, Shaikh H, Tannock IF. Penetration of anticancer drugs through solid tissue: a factor that limits the effectiveness of chemotherapy for solid tumors. Clin Cancer Res. 1999;5:1583–6. [PubMed] [Google Scholar]
- 78.El-Kareh AW, Secomb TW. Two-mechanism peak concentration model for cellular pharmacodynamics of Doxorubicin. Neoplasia. 2005;7:705–13. doi: 10.1593/neo.05118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 80.DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol. 2003;15:572–82. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 81.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–10. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 82.Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002;14:633–9. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- 83.Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20:4995–5004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- 84.Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69:2260–8. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raghunand N, Gillies RJ. pH and drug resistance in tumors. Drug Resist Updat. 2000;3:39–47. doi: 10.1054/drup.2000.0119. [DOI] [PubMed] [Google Scholar]
- 86.Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther. 2006;5:1275–9. doi: 10.1158/1535-7163.MCT-06-0024. [DOI] [PubMed] [Google Scholar]
- 87.Raghunand N, He X, van Sluis R, Mahoney B, Baggett B, Taylor CW, et al. Enhancement of chemotherapy by manipulation of tumour pH. Br J Cancer. 1999;80:1005–11. doi: 10.1038/sj.bjc.6690455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luciani F, Spada M, De Milito A, Molinari A, Rivoltini L, Montinaro A, et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst. 2004;96:1702–13. doi: 10.1093/jnci/djh305. [DOI] [PubMed] [Google Scholar]
- 89.Lee CM, Tannock IF. Inhibition of endosomal sequestration of basic anticancer drugs: influence on cytotoxicity and tissue penetration. Br J Cancer. 2006;94:863–9. doi: 10.1038/sj.bjc.6603010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim D, Gao ZG, Lee ES, Bae YH. In Vivo Evaluation of Doxorubicin-Loaded Polymeric Micelles Targeting Folate Receptors and Early Endosomal pH in Drug-Resistant Ovarian Cancer. Molecular pharmaceutics. 2009 doi: 10.1021/mp900021q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gillies ER, Frechet JM. pH-Responsive copolymer assemblies for controlled release of doxorubicin. Bioconjug Chem. 2005;16:361–8. doi: 10.1021/bc049851c. [DOI] [PubMed] [Google Scholar]
- 92.Gillies ER, Jonsson TB, Frechet JM. Stimuli-responsive supramolecular assemblies of linear-dendritic copolymers. Journal of the American Chemical Society. 2004;126:11936–43. doi: 10.1021/ja0463738. [DOI] [PubMed] [Google Scholar]
- 93.Boomer JA, Thompson DH. Synthesis of acid-labile diplasmenyl lipids for drug and gene delivery applications. Chem Phys Lipids. 1999;99:145–53. doi: 10.1016/s0009-3084(99)00033-x. [DOI] [PubMed] [Google Scholar]
- 94.Qualls MM, Thompson DH. Chloroaluminum phthalocyanine tetrasulfonate delivered via acid-labile diplasmenylcholine-folate liposomes: intracellular localization and synergistic phototoxicity. Int J Cancer. 2001;93:384–92. doi: 10.1002/ijc.1339. [DOI] [PubMed] [Google Scholar]
- 95.Guo X, Szoka FC., Jr Chemical approaches to triggerable lipid vesicles for drug and gene delivery. Acc Chem Res. 2003;36:335–41. doi: 10.1021/ar9703241. [DOI] [PubMed] [Google Scholar]
- 96.Huang Z, Guo X, Li W, MacKay JA, Szoka FC., Jr Acid-triggered transformation of diortho ester phosphocholine liposome. Journal of the American Chemical Society. 2006;128:60–1. doi: 10.1021/ja057024w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen QR, Zhang L, Stass SA, Mixson AJ. Co-polymer of histidine and lysine markedly enhances transfection efficiency of liposomes. Gene Ther. 2000;7:1698–705. doi: 10.1038/sj.gt.3301294. [DOI] [PubMed] [Google Scholar]
- 98.Woessner JF, Nagase H. Matrix Metalloproteinases and TIMPs. Oxford: Oxford University Press; 2000. [Google Scholar]
- 99.Kline T, Torgov MY, Mendelsohn BA, Cerveny CG, Senter PD. Novel antitumor prodrugs designed for activation by matrix metalloproteinases-2 and -9. Molecular pharmaceutics. 2004;1:9–22. doi: 10.1021/mp0340183. [DOI] [PubMed] [Google Scholar]
- 100.Chau Y, Tan FE, Langer R. Synthesis and characterization of dextran-peptide-methotrexate conjugates for tumor targeting via mediation by matrix metalloproteinase II and matrix metalloproteinase IX. Bioconjugate Chem. 2004;15:931–41. doi: 10.1021/bc0499174. [DOI] [PubMed] [Google Scholar]
- 101.Terada T, Iwai M, Kawakami S, Yamashita F, Hashida M. Novel PEG-matrix metalloproteinase-2 cleavable peptide-lipid containing galactosylated liposomes for hepatocellular carcinoma-selective targeting. J Control Release. 2006;111:333–42. doi: 10.1016/j.jconrel.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 102.Sarkar NR, Rosendahl T, Krueger AB, Banerjee AL, Benton K, Mallik S, et al. Uncorking of liposomes by matrix metalloproteinase-9. Chem Commun. 2005:999–1001. doi: 10.1039/b416827e. [DOI] [PubMed] [Google Scholar]
- 103.Elegbede AI, Banerjee J, Hanson AJ, Tobwala S, Ganguli B, Wang R, et al. Mechanistic studies of the triggered release of liposomal contents by matrix metalloproteinase-9. Journal of the American Chemical Society. 2008;130:10633–42. doi: 10.1021/ja801548g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gray MD, Mann M, Nitiss JL, Hendershot LM. Activation of the unfolded protein response is necessary and sufficient for reducing topoisomerase IIalpha protein levels and decreasing sensitivity to topoisomerase-targeted drugs. Mol Pharmacol. 2005;68:1699–707. doi: 10.1124/mol.105.014753. [DOI] [PubMed] [Google Scholar]
- 105.Tomida A, Tsuruo T. Drug resistance mediated by cellular stress response to the microenvironment of solid tumors. Anticancer Drug Des. 1999;14:169–77. [PubMed] [Google Scholar]
- 106.Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57:1009–14. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gandara DR, Lara PN, Jr, Goldberg Z, Le QT, Mack PC, Lau DH, et al. Tirapazamine: prototype for a novel class of therapeutic agents targeting tumor hypoxia. Semin Oncol. 2002;29:102–9. doi: 10.1053/sonc.2002.31531. [DOI] [PubMed] [Google Scholar]
- 108.Hong R-L, Huang C-J, Tseng Y-L, Pang VF, Chen S-T, Liu J-J, et al. Direct comparison of liposomal doxorubicin with or without polyethylene glycol coating in C-26 tumor bearing mice: Is surface coating with polyethylene glycol beneficial? Clin Cancer Res. 1999;5:3645–52. [PubMed] [Google Scholar]
- 109.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tremblay MR, Lescarbeau A, Grogan MJ, Tan E, Lin G, Austad BC, et al. Discovery of a potent and orally active hedgehog pathway antagonist (IPI-926) J Med Chem. 2009;52:4400–18. doi: 10.1021/jm900305z. [DOI] [PubMed] [Google Scholar]
- 111.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–24. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 112.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–86. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 113.Grinnell F, Rocha LB, Iucu C, Rhee S, Jiang H. Nested collagen matrices: a new model to study migration of human fibroblast populations in three dimensions. Exp Cell Res. 2006;312:86–94. doi: 10.1016/j.yexcr.2005.10.001. [DOI] [PubMed] [Google Scholar]