Abstract
Wnt signaling can be divided into three pathways, namely the canonical Wnt/β-catenin pathway, and the non-canonical (or heretical) Wnt/Ca2+ and planar cell polarity (PCP) pathways, the Wnt/Ca2+ pathway was shown to play a major role in cancer. Moreover, increasing data points to the importance of the non-canonical and PCP pathways in several aspects of tumor progression. The recent advances in understanding the players and mechanisms by which the Wnt pathways contribute to cancer progression have led to the identification of numerous targets that are already or could be considered for therapeutical purposes.
1. Introduction
Wnt signaling is a complex process and has been implicated in a large number of diseases, most notably cancer. The variety of receptors and ligands involved in Wnt signaling lead to a multitude of diverse signal transduction cascades. The Wnt family of proteins consists of 19 known human members. These secreted proteins share 20–85 % amino acid identity and have a conserved pattern of 23–24 cysteine residues. Following their synthesis, these secreted Wnt proteins are modified by glycosylation and can bind to the Frizzled (Fzd) family of receptors. To date, ten members of this family of receptors have been identified, all of which are seven-pass transmembrane proteins characterized by an extracellular N-terminal conserved cystein-rich domain (CRD) that interacts with both Wnts and other Wnt co-receptors [1, 2]. The Fzd co-receptors, low-density lipoprotein receptor-related proteins, LRP5 and -6 are single pass transmembrane proteins and in the presence of the Fzd receptor form a co-receptor complex to which Wnts bind resulting in activation of downstream signaling [3]. The CRD domain that binds Wnt ligands was also shown to be present on the single-pass tyrosine kinase ROR2, which has also been involved in Wnt signaling [4], and other receptors for Wnts are discussed in the last section of this review. Fzd receptors are G-protein coupled receptors and downstream signaling upon Wnt binding requires heterotrimeric G proteins [5]. The activation of specific G protein subunits are dependent on the Wnt ligand subtype binding to different Fzd family members [6, 7]
Interaction of Wnts with their receptors and co-receptors are associated with three signaling pathways, namely the canonical Wnt/β-catenin pathway, and the non-canonical (or heretical) Wnt/Ca2+ and planar cell polarity (PCP) pathways. The Fzd receptors have the ability to discriminate between different Wnt ligands, and as such, activation of one of these three pathways is dictated by the nature of the ligand/receptor interaction. The proteins encoded by the WNT genes play a role in normal development but also in tumorigenesis [1], [8] and the inappropriate activation of the Wnt pathway results in the onset of several types of cancer [9]. In this review, we will go over the main Wnt pathways, how these pathways are modified in different types of cancers and discuss potential targets in these pathways.
2.1. The Canonical Wnt Pathway
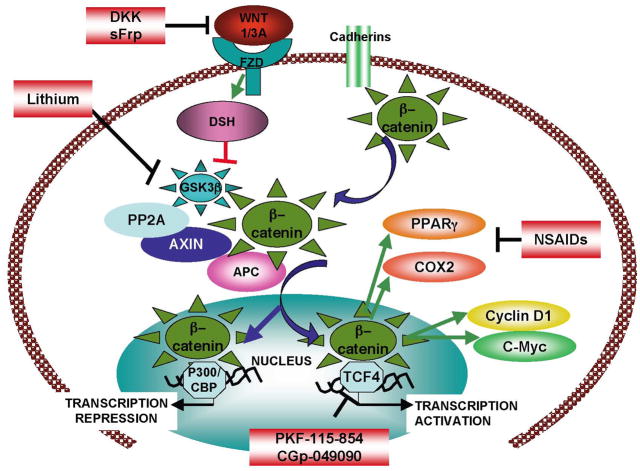
The canonical Wnt signaling pathway involves a key mediator, β-catenin. In the absence of Wnt,β-catenin is phosphorylated and targeted for degradation. In a first step,β-catenin is phosphorylated by CKIα and/or CKIε at residue ser45 [10]. This then allows GSK3β to phosphorylate β-catenin on residues 41, 37, and 33 [11]. The phosphorylation of β-catenin by CKI and GSK3β, occurs within a complex of several proteins including the scaffolding protein Axin and the tumor suppressor gene product APC (Adenomatous Polyposis coli) [12]. This complex, often referred to as the destruction complex, also contains a protein closely related to Axin, Conductin (Axin2) [13]. Within this complex, Axin binds directly to GSK3β and its substrate β-catenin [14]. Phosphorylation by GSK3β leads to the ubiquitinylation of β-catenin by βTrCP, a component of the E3 ubiquitin ligase, and ultimately to its degradation via the proteasome [15–17]. In the absence of Wnt ligand, this Axin-containing destruction complex is constitutively active, resulting in the phosphorylation and degradation of β-catenin [18]. Under these conditions, and in the absence of β-catenin to stimulate transcription, Tcf (T-cell specific transcription Factor) represses transcription of Wnt target genes by interacting with co-repressors HDAC and Groucho [19–21].
Activation of the canonical Wnt pathway requires the binding of Wnt to one of the Fzd receptors in the presence of the co-receptor, LRP 5/6 [3, 22]. Formation of this complex leads to the phosphorylation of LRP by CKI, allowing the recruitment of Axin to the complex at the membrane, thereby preventing the activation of the destruction complex responsible for β–catenin phosphorylation by GSK3β [23–27]. GSK3β is prevented from binding to Axin by the phosphoprotein Dishevelled (Dvl/Dsh) and its recruitment of the GSK3β binding protein (GBP), which prevents binding of GSK3β to Axin (Figure 1). Dishevelled therefore inhibits the β-catenin degradation machinery. Axin has distinct binding sites for GSK3 and β-catenin [12, 23, 28, 29]
Figure 1. The Wnt/β-Catenin Signaling Pathway and Points of Intervention.
Receptor activation upon binding of Wnt 1 or Wnt3A leads to stabilization of β-catenin which localizes to the nucleus where it associates with transcription factors and activates transcription of genes involved in proliferation and tumor progression. In the absence of Wnt, transcription of these genes is prevented by phosphorylation of β-catenin by GSK3β within a destruction complex and its subsequent degradation via the proteasome.
Once stabilized, β-catenin can enter the cell nucleus and associate with the transcription factors Lef (Lymphoid enhanced transcription factor) and Tcf (T-cell factor), leading to the transcription of Wnt target genes (Figure 1). The conversion of the Tcf repressor complex mentioned above into a transcriptional activator complex is thought to involve the displacement of Groucho from Tcf/Lef and recruitment of the histone acetylase CBP/p300 that acts as a co-activator by binding to the β-catenin-Tcf complex [30, 31]. Other factors that contribute to the activation of transcription include Brg1, a component of the SWI/SNF chromatin remodeling complex [32], as well as Bcl9 bound to Pygopus which mediates the interaction between the complex and chromatin [33–37]. It is important to note that several members of the pathway can be regulated independently of Wnt signaling. In particular, GSK3β can be inhibited by ILK, (Integrin Linked Kinase) [38], and is at the intersection of numerous pathways that might regulate its expression [39]. Regulation of β-catenin, and in particular its degradation can also be induced by p53 activation [40].
2.2. The Canonical Wnt pathway in Cancer
The stabilization of β-catenin, lack of degradation and ultimately nuclear accumulation is used as evidence of an activated Wnt/β-catenin pathway. Indeed, such accumulation has been detected by immunohistochemical staining in a number of human tumors including colorectal, lung, breast, cervical, skin, and liver. In hepatocellular carcinoma, β-catenin accumulation has been linked to poorly differentiated morphology [41], high proliferative activity [42], and poor prognosis [41–43]. The fate of β-catenin, namely, its accumulation or degradation, is regulated by numerous proteins, which, if not regulated or expressed appropriately would account for increased β-catenin expression in cancer. This dysregulation may occur due to mutations in the various members of the signaling pathway, or to epigenetic events. Mutations in Wnt themselves are rare and although Wnt-1 was identified as a mammary oncogene in mouse transgenic studies [44–47], no mutations in this gene have been linked to cancers in humans. Mutations affecting downstream targets however, are quite frequent in cancer [48] [49].
Amongst these are mutations in β-catenin itself. Indeed, activating β-catenin mutations at one of the sites that are phosphorylated by GSK3β have been identified in 50% of colon cancers that have wild type APC. These mutations prevent the β-catenin degradation via the proteasome [50–52]. These activating point mutations in β-catenin were found in the rare cases of colorectal cancers with wild type APC, thereby preventingβ-catenin degradation and leading to the inappropriate formation of the Tcf/β-catenin transcription complex [50]. Mutations in β-catenin have also been identified in a number of other tumors such as brain tumors, ovarian cancers, prostate cancer, and hepatocellular carcinomas [53–55]. In addition to these mutations in β-catenin, mutations affecting other components of the canonical Wnt pathway have been detected in different types of tumors. In particular, mutations affecting the interaction of β-catenin with the destruction complex, resulting in an increase in cytoplasmic β-catenin, play a major role in the development of a large number of tumors [9, 48].
The Adenomatous Polyposis Coli gene (APC), one of the components of the destruction complex, was identified as a tumor suppressor protein. Germline mutations in the gene coding for this protein is linked to an inherited form of colorectal cancer known as Familial Adenomatous Polyposis (FAP), which is characterized by a large number of colorectal polyps in early adulthood [56, 57]. In addition, somatic mutations of the APC gene are found in more than 80% of sporadic colorectal cancers [58–61]. Mutations in the APC protein results in the inability to downregulate β-catenin, which ultimately results in the activation of Wnt target gene transcription mediated by Lef/Tcf. Loss of APC occurs at the very initial steps of colorectal cancer and has been shown to accelerate the process of tumor initiation [62]. Whether it is the loss of APC or mutations in the Phosphorylation sites of β-catenin, the consequence remains a defect in the downregulation of β-catenin and transcription of genes that include cyclin D1 and c-myc [63, 64], both of which have been implicated in cell cycle progression and its deregulation. This defect in β-catenin degradation is found in numerous types of tumors, and makes the destruction complex an attractive target in terms of finding mechanism to increase its activation in these types of tumors.
One of the major constituents of the destruction complex is Axin, whose concentration was shown to be the rate-limiting factor in regulating the efficiency of the destruction complex [25, 65]. Interestingly, the overexpression of Axin induces β-catenin degradation in cell lines with mutated APC [12, 66, 67]. Therefore stabilization of Axin would provide a mechanism by which to stimulate β-catenin degradation. Recently, stabilization of Axin was reported in the presence of a small inhibitor of the poly(ADP-ribose) polymerase tankyrase [68]. Furthermore, in this same study, this inhibitor could inhibit the growth of β-catenin-dependent (loss of APC) colorectal cells. Other small molecules acting on Axin protein stability have since been described[69].
At the center of the Wnt/β-catenin pathway and a member of this destruction complex is, as mentioned above, the protein GSK3β. To date, no inactivating mutations have been detected in human cancers [70]. Although, from its role in phosphorylating β-catenin, GSK3β would be predicted to act as a tumor suppressor, its activity was deregulated in colorectal cancer and this was suggested to play a major role in colon cancer cell proliferation and survival [71]. Several studies have described an important role for GSK3β in nuclear factor-κB (NF-κB)-mediated cell survival [72], [73], and GSK3β has been shown to destabilize p53 [74, 75] and PTEN [76], altogether favoring a role for GSK3β in promoting cancer. GSK3β protein expression was also detected in human ovarian carcinoma [77]. However, studies done on breast, lung and non-melanoma skin cancers have shown inactivation of GSK3β in these cancer tissues and have further demonstrated that its activation induced apoptosis of cancer cells [78–81]. Despite this, studies done recently in prostate, pancreatic, and colorectal cancer cell lines reveal that the use of GSK3β inhibitors lead to significant decreases in cell growth and proliferation [82, 83]. It was also shown that in APC mutant mice, treatment with the GSK3β inhibitor lithium did not result in a significant increase in the number of tumors in these mice [84]. Therefore, although GSK3β appears to play a controversial role in cancer, the use of GSK3β inhibitors seem to correlate with an overall positive outcome, although the mechanism by which the inhibitory effect of inhibitors such as lithium affect GSK3β in the context of the Wnt pathway has not been well studied.
Unlike colorectal cancer, breast cancers do not display genetic alterations in any of the genes coding for β-catenin (CTNNB1), or components of the destruction complex such as APC or AXIN1 [85, 86], [87–89]. However, primary breast tumors overexpress the Wnt target gene cyclin D1 [90]. Accumulation of β-catenin and induction of cyclin D1 has been correlated with a poor prognosis in breast cancer [90]. This induction of cyclin D1 was shown to be due to the overexpression of Wnt ligands resulting in autocrine activation of Wnt signaling in breast cancer cells [91]. The use of monoclonal antibodies directed against these Wnts and Fzd receptors have shown promising results in vitro [92], [93] [94–98], but targeting the secreted ligands might be challenging due to the ubiquitous expression of the Fzd receptors and the possible activation of both canonical and non-canonical pathways.
2.3. Targeting the Canonical Wnt Pathway In Cancer
Ultimately, the Wnt/β-catenin pathway is linked to cancer via the activation of Tcf/β-catenin activated genes. Targeting the Tcf/β-catenin complex has so far proven to be a promising approach. Indeed, compounds acting on specifically disrupting the Tcf/β-catenin interaction (small molecule antagonists) have been identified and were shown to inhibit proliferation of colorectal cancer cells (HCC) [99]. These same molecules were also shown to exhibit anti-tumor activity against multiple myeloma [100]. In a recent study by Wei et al [101], these antagonists were reported to inhibit human hepatocellular carcinoma cell growth with limited cytotoxicity to normal hepatocytes. This effect was shown to involve the disruption of the interaction between Tcf4 and β-catenin and result in the downregulation of the Tcf4/β-catenin downstream oncogene c-myc, the expression of which alone was previously shown to induce HCC [102]. This same study showed that these antagonists could slow the growth of xenografts in nude mice in preliminary studies. This approach of targeting the pathway at the level of transcription shows promise and would also have the advantage of acting downstream of mutations contributing to the stabilization and increased presence of β-catenin in the transcription complex.
Amongst the Wnt target genes are PPAR-γ and COX-2, both of which have been implicated in the development of colorectal carcinomas. Moreover, both of these are inhibited by non-steroidal anti-inflammatory drugs (NSAIDs) which have been shown to inhibit colon tumorigenesis. COX-2 produces eicosanoids from arachidonic acid. These eicosanoids are themselves PPAR-γ ligands which, in collaboration with retinoic acid receptors, stimulate transcription. PPAR-γ is upregulated in early carcinogenesis by the Tcf-β-catenin complex. Treatment of mutant mice with a selective COX-2 inhibitor was shown to reduce polyp number [103] and the treatment of FAP patients with the COX-2 inhibitor Celecoxib showed significant decrease in the number of colorectal polyps [104, 105]. Also, levels of nuclear β-catenin in FAP patients was reduced substantially in polyps of FAP patients treated with the NSAID sulindac sulphide for 6 months [106]. The use of NSAIDs has been associated with intestinal bleeding and kidney damage and development of more selective, less toxic NSAIDs are still under development.
3. Endogenous Wnt Antagonists
Activation of the Wnt pathway is regulated by secreted Wnt inhibitors (Reviewed in [49]). These inhibitors affect the binding of the Wnt ligands to the receptors or co-receptors. These Wnt antagonists include the members of the secreted frizzled related proteins (sFRPs) that bind to Wnt proteins directly, and the members of the Dikkopf (Dkk) family that bind to the Wnt co-receptors LRPs. Whereas the sFRPs block all three Wnt pathways, the Dkks only inhibit the canonical Wnt/β-catenin pathway [107]. These secreted Wnt inhibitors function to keep Wnt signaling below a certain level of activation. Because these secreted proteins maintain levels of Wnt signaling below a certain threshold, any mechanism leading to their reduced function would lead to increased β-catenin-mediated transcription, such as that observed in numerous types of cancers. A number of studies have indeed described the silencing via promoter hypermethylation of the genes coding for some of the secreted Wnt antagonists in colorectal cancer [108–111]. The transcriptional inactivation of sFRPs has been detected in a number of cancers including colorectal cancer [108], non-small-cell lung cancer [112], breast cancer [113], ovarian cancer [113, 114]. It should be noted that overexpression of sFRPs has also been reported in some types of cancers [115–117], although the mechanisms for this overexpression or its consequence is not fully understood. The ability of sFRPs to inhibit Wnt signaling makes them an attractive target for therapeutic use. It was shown that restoring sFRP expression in colon cancer results in a reduction in Wnt signaling, even in the case of otherwise constitutive pathway activation due to mutations in proteins downstream of receptor activation [109]. Manipulating the expression of these proteins therefore appears to be a promising approach to downregulating Wnt signaling, especially in tumors that are characterized by mutations in the pathway, such as colorectal cancer.
Members of the Dkk family have also been shown to have an inhibitory effect on Wnt signaling, although activation of Wnt signaling, in particular by Dkk2 has been reported [118]. The Dkks inhibit Wnt signaling by binding and modulating the Wnt co-receptors LRP-5 and -6. Dkks bind to their receptor Kremen and to LRP5/6, inducing LRP endocytosis and preventing downstream signaling [119–121]. As is the case for sFRPs, epigenetic inactivation has also been reported for DKKs [111, 122]. It is clear that either potentiating the activity of endogenous Wnt antagonists, or designing efficacious drugs against targets of the canonical Wnt pathway could have a beneficial effect on many cancers. However, some cancers, such as melanoma, may not fall into this category. It has been demonstrated that β-catenin, while critical for the initial transformation of melanocytes [123], may actually have protective effects in the later stages. Chien et al have shown that activation of β-catenin in melanoma led to increased differentiation, and decreased tumor growth. On the other hand, non-canonical Wnt signaling can increase the metastatic potential of melanoma cells [124]. Thus, therapies targeted towards the β-catenin pathway may have no effect, or detrimental effects in melanoma patients, and instead therapies targeted toward the non-canonical Wnt pathway may be more beneficial.
4. Heretical Wnt Pathways
The non-canonical, or heretical, Wnt pathways are β-catenin-independent Wnt activated pathways. Unlike the canonical Wnts, heretical Wnts are unable to transform mammary epithelial cells [125] and are thought to be involved primarily in cell movement and polarity [126, 127]. There are two major heretical Wnt pathways, the planar cell polarity (PCP) pathway, and the Wnt/Ca2+ pathway.
4.1. The Wnt/PCP Pathway
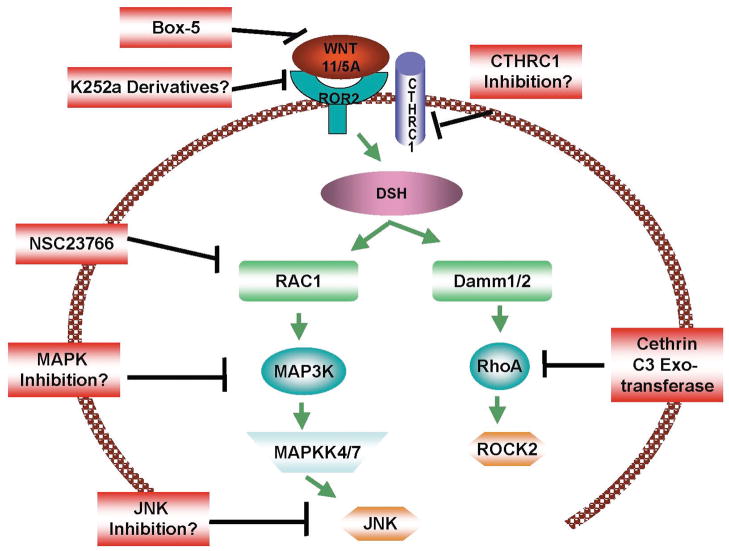
The Wnt/PCP pathway has been best described in development, where it coordinates the polarization of cells along embryonic axes. This involves the activation of STAT3, and JAK/STAT signaling [128]. Wnts that play a role in Wnt/PCP signaling include Wnt5A, Wnt11, and Wnt7a [129]. During Wnt/PCP signaling, Wnt/Fz/ROR2 interactions recruit disheveled to the membrane, which triggers the recruitment of vang and prickle to the membrane of adjacent cells, and the balance between these regulates polarity [130–132]. Disheveled-dependent Wnt/PCP signaling then transduces signals via Jun, Daam, RhoA, Rac, Cdc42 and Profilin, and these have cytoskeletal effects that ultimately control both polarity and motility (Figure 2). Since these features are critical for tumor progression, the role of Wnt/PCP has been implicated in cancer.
Figure 2. The Wnt/PCP Signaling Pathway and Points of Intervention.
Receptor activation by Wnt leads to the recruitment of Dsv, resulting in the activation of Rac1 and RhoA, both of which are involved in cytoskeletton remodeling and establishment of an EMT phenotype.
4.2. Wnt/PCP pathway in cancer and potential targets
Discerning the Wnt/PCP and Wnt/Ca2+ pathways in human cancer is quite a difficult task, since both involve key molecules such as Wnt5A and ROR2. Here we will attempt to make a somewhat shaky distinction between the two by assigning the downstream effectors of Jnk and Rac and Rho to the Wnt/PCP pathway, and those of calcium and PKC to the Wnt/Ca2+ pathway. It is important to point out however that in elements of both pathways can be found in each, depending on the context of the tumor. Not much is known about Wnt/PCP signaling in cancer, but two excellent reviews have pulled together the information available to give us a comprehensive insight into this, and we direct the interested reader to these reviews [129, 133]. In Wnt/PCP signaling during development, Wnt5A activates Dsh and Jnk and Rho [134]. LRP6 [135] and Fzd7 [136] seem to be critical receptors in mediating this process. Fzd 7 has been shown to promote hepatocellular cancer and colon carcinoma, and targeting Fzd 7 can inhibit the invasion of these cells [137, 138]. Dsh has been shown to be critical for β-catenin induced tumorigenesis as mentioned above, but may also play a role in β-catenin independent metastasis. Rac and Rho are also well known promoters of the metastatic phenotype in many cancer types, resulting in effects on the cytoskeleton [139]. Inhibitors of Rac and Rho therefore, would be useful in cancer therapy, and indeed this is an active field of study. Many of the inhibitors designed to target Rac and Rho actually target Ras, since this is one of the major pathways that activate these molecules. However, small molecule inhibitors of Rac and Rho are under also development. NSC23766 is a small molecule inhibitor of Rac that can suppress the growth and invasion of prostate cancer cells [140]. It has been shown that in a chimeric in vivo leukemia model that is clinically similar to human disease, this inhibitor can also suppress the growth and proliferation of leukemia [141]. This inhibitor does not affect either Cdc42 or Rho, and since both of these are also implicated in Wnt/PCP signaling, finding inhibitors to these molecules is also critical. C3 exotransferase has been used in vitro to inhibit both Cdc42 and Rho, but its use as a clinical agent is unknown [142]. Cethrin, a Rho inhibitor that has been used in the clinic to treat spinal cord injuries [143], may be of use in cancers that have Rho as an important intermediate.
As with the targeting of many intermediate proteins that are necessary for many cellular processes and in many cell types, the identification and use of a specific mediator expressed in cancer, but not normal cells, could significantly decrease toxicity. For example, the activation of Rho and Rac during Wnt/PCP signaling is mediated by the collagen triple helix repeat containing protein 1 (Cthrc1). Cthrc1 mediates the binding of Wnt5A to both Fzd and Ror2, and is thought to specifically activate Wnt/PCP signaling [144]. Knockout of CTHRC1 synergizes with mutations in Vangl2 to affect PCP signaling [144], and Vangl itself is implicated in the metastasis of tumors [145]. CTHRC1 is upregulated in the invasive stages of many cancers including melanoma, lung, breast, gastric, pancreatic, cervical, ovarian and thyroid cancers [146] making it an attractive target. It is possible that the in vivo use of CTHRC1 siRNA may have therapeutic effects. Failing the development of such specific inhibitors, adjuvant therapy combining a few of the above mentioned inhibitors may have the desired effects on tumor ablation.
4.3. The Wnt Calcium Pathway
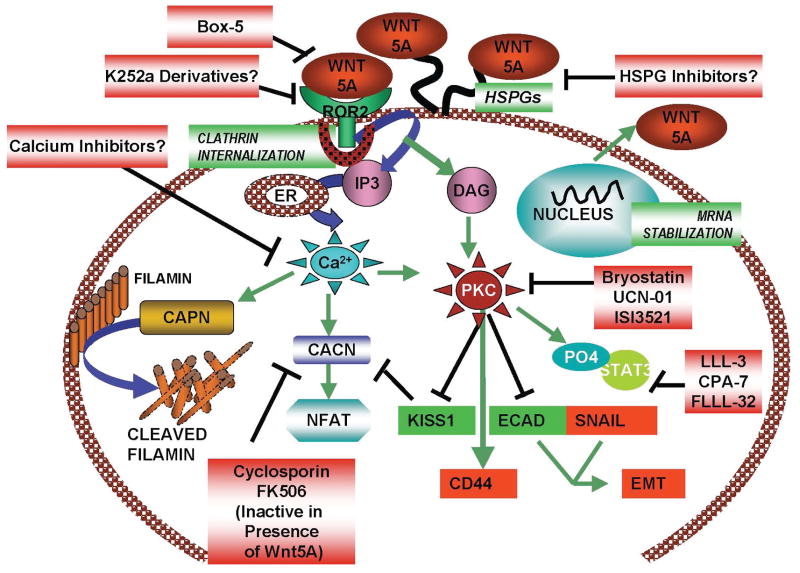
The other well-described heretical Wnt pathway involves the release of intracellular calcium downstream of Wnt signaling (Figure 3). Members of the Wnt family involved in the Wnt/Ca2+ signaling pathway include Wnt5a, Wnt11, and Wnt4, and activation of the Fzd receptors by these Wnts was shown to result in the activation of heterotrimeric G proteins [7]. This results in phospho-inositol turnover in the membrane and the release of calcium from its intracellular stores. The increase in intracellular calcium results in the activation of calcium-dependent signaling molecules, such as calmodulin-dependent protein kinase II (CAMKII) and protein kinase C (PKC) [147, 148]. These molecules can have a cornucopia of effects on downstream signaling, that is often dependent on the cellular context.
Figure 3. The Wnt/Ca2+ Signaling Pathway and Points of Intervention.
Binding of Wnt5A to ROR2 leads to the activation of Ca2+ and PKC, which in turn stabilizes Wnt5A mRNA and increases its secretion. Secreted Wnt5A is presented to the receptor by HSPGs, resulting in a positive feedback loop. The Wnt5A-mediated increase in Ca2+ resluts in an increase in calpain-dependent filamin cleavage as well as activation of calcineurin and NFAT. Increases in PKC are associated with changes in the expression of proteins involved in the promotion of a metastatic phenotype including the upregulation of CD44 and SNAIL, loss of KISS1 and ECAD and establishment of an EMT. Increases in PKC are also associated with an increase in phospho-STAT3, which in melanoma leads to a decrease in melanosomal antigens.
Calcium initiated effects include the activation of CAMKII. Wnt5A was shown to inhibit the activation of the canonical Wnt pathway via a number of different mechanisms including activation of CamKII [149], and through induction of Siah, a member of the E3 ubiquitin ligase complex [150]. Wnt5A, via CAMKII, has also been involved in the recruitment of macrophages during the inflammatory response to lipopolysaccahride [151], and its effect on macrophage activation may also play a role in breast cancer metastasis as discussed below [152]. Wnt5A activation of CAMKII is also critical for the proliferation of HUVEC cells, which may also have implications for angiogenesis and cancer [153]. We have also shown that Wnt5A regulates CAMKII in melanoma cells [154].
In addition to CAMKII, other calcium related molecules such as the calcium activate protease calpain (which will be discussed further in the next section) and calcineurin are activated by Wnt/Ca2+ signaling and these have downstream effects. For example, calcineurin is known to de-phosphorylate NFAT, causing its nuclear translocation [155]. This process is tightly regulated by GSK3β which phosphorylates NFAT to keep it in the cytoplasm. Wnt5A causes NFAT nuclear translocation in endothelial cells, even in the presence of cyclosporin A and FK506, calcineurin inhibitors, and it is thought that this may be due to the inhibition of GSK3β [156]. Data from our laboratory also show that Wnt5A inhibits the metastasis suppressor Kiss-1[154]. Kiss-1 is known to inhibit calcineurin, thus Wnt5A may also increase calcineurin expression via the inhibition of Kiss-1, resulting in NFAT translocation. Once in the nucleus, NFAT is capable of activating transcription programs that can contribute to cancer metastasis. It has been shown in mammary epithelial cells that Wnt5A can activate calcineurin and NFAT via a complex with CKIα [157]. This is a strong and durable interaction, but is regulated by Wnt5a signaling via Yes and Cdc42 [157]. This is a prime example of how complex Wnt signaling can be, even when discussing the same Wnt within the same cell type!
PKC is the other major intermediate activated by Wnt/Ca2+ signaling. PKC is important in many cellular processes and is a critical element for Wnt5A signaling. We and others have shown repeatedly that many of the effects of Wnt5A can be mimicked by phorbol esters, and cannot occur in the presence of PKC inhibitors [154, 158, 159]. PKC is even important in the expression of Wnt5A, as studies have shown that PKC activation can cause the stabilization of Wnt5A mRNA, thus leading to an increase in Wnt5A levels [160]. In neuronal cells, nerve growth factor requires Wnt5A for axonal branching and growth of neuronal cells, in a PKC-dependent manner [161]. In T-cells, Wnt5A mediates the interaction between the chemokine CXCL12 and its receptor CXCR4, and PKC is critical in the transduction of this signal cascade, that leads to the migration of T-cells in response to CXCL12 [162]. In melanoma cells a similar activation of CXCR4 by Wnt5A involves the activation of PKC, and the formation of a Rac-Actin Myosin polarity complex [163], again blurring the distinction between Wnt/Ca2+ and Wnt/PCP signaling in human cancer.
4.4. Wnt/Ca2+ pathway in cancer and potential targets
Wnt5A, in accordance with its different effects in the presence of different receptors, has been shown to have either a tumor suppressive or an oncogenic function, depending on the type of cancer. Its expression is downregulated in colorectal cancer [164, 165], neuroblastoma [166], ductal breast cancer [167, 168], and leukemias [169–171], and this downregulation was shown to be associated with higher tumor grade [172]. Conversely, Wnt5A was shown to be overexpressed in gastric cancer [173], pancreatic cancer [174], non-small cell lung cancer [175], and prostate cancer [176]. Wnt5A gene expression was found to be increased in more metastatic melanoma cells [177]and increased expression led to increased motility [178].
In melanoma, in which Wnt5A signaling has been well studied, expression of Wnt5A is correlated with a poor prognosis [179]. We have recently shown that the Wnt5A-mediated activation of the calcium-activated protease, calpain, results in the cleavage of the cytoskeletal protein filamin [180]. It has been shown that Wnt5A, via ROR2, can mediate the motility of various cell types [181, 182] by regulating the formation of lamellopodia and that ROR2 binding to filamin is essential for this process [183]. Our data indicate that cleavage of filamin by Wnt5A could be inhibited by chelation of calcium by BAPTA-AM, underscoring the importance of calcium in this process.
Over the last decade or so we have slowly begun to unravel the intricacy of Wnt5A signaling in melanoma, and recently summarized these findings in a review [184]. Briefly, our data indicate that Wnt5A binds to its receptor ROR2, and this binding is supported by heparan sulphate proteoglycans such as syndecan 1 and syndecan 4 [185]. Upon ROR2 binding, Wnt5A and ROR are internalized via clathrin, and this process both activates and is mediated by PKC [186]. The result of this is the activation of CD44, suppression of Kiss-1, activation of Snail and Vimentin, and an epithelial to mesenchymal transition [154]. In addition, in melanoma cells, PKC activation leads to the activation of STAT3, which in turn inhibits the expression of melanoma differentiation antigens such as MART-1 and GP100. The effect of this is the decreased immunogenicity of melanoma cells, as these antigens often act as “red flags” to the immune cells [187]. In fact, because of this, MART1 and GP100 are often used as targets of immunotherapy [188], and one can speculate that first down-regulating Wnt5A signaling might increase the efficacy of such drugs.
Increases in PKC activation have been shown to increase the migration of melanoma cells, while its inhibition was able to decrease melanoma metastasis [189–192] and melanoma cell motility [154], making PKC an attractive target, at least for melanoma metastasis. PKC inhibitors have been investigated in the context of cancer therapy (reviewed in [193]. Although several compounds have shown initial activity in melanoma (bryostatin and UCN-01), non-Hodgkin’s lymphoma (ISIS 3521, bryostatin, and UCN-01), and ovarian carcinoma (ISIS 3521 and bryostatin) in phase I studies, the activity of these drugs used as single agent in the phase II studies that were reported have been limited [194]. Again, because PKC is such an ubiquitous enzyme, finding specific, potent drugs with limited toxicity pose a problem, and targeting the source of aberrant signaling may hold the answer for future therapy.
Since Wnt5A is the best-described heretical Wnt member in human cancer, it is an obvious and highly specific target. Functional antibodies against Wnt5A have not been made available, however a study a few years ago laid the groundwork for a Wnt5A antagonist that could be used in the clinic. Foxy-5, a hexapeptide that acts as an agonist of Wnt5A was developed for use in breast cancer [195]. In breast, cancer, the expression of Wnt5A is a marker of better prognosis, since it acts as a tumor suppressor. Foxy-5 indeed was very effective in eradicating breast cancer in in vivo models, by mimicking Wnt5A expression [196]. Very recently the same group demonstrated that the N-butyloxycarbonyl hexapeptide, Box-5, could inhibit the growth and invasion of melanoma cells, and Box-5 may prove to be an exciting new molecule for cancer therapy [197].
5. Tyrosine Kinase Receptors: A Wnt-Wnt Situation for Cancer Therapy?
When possible, targeting a receptor rather than a secreted ligand is often more desirable. Recently several tyrosine kinase receptors, which are often good targets for cancer therapy, have been associated with the Wnt pathway. Wnt5A was shown to signal via ROR2, a Wnt co-receptor [4]. Wnt5A is the only known ligand for ROR2, which makes this receptor tyrosine kinase a target of particular interest. It was shown that while Wnt5A knockdown reduced ROR2 levels, the inhibition of ROR2 did not affect Wnt5A expression levels, but did inhibit Wnt5A downstream signaling and resulting increase in metastasis [186]. Therefore, inhibiting specifically ROR2 would result in the specific inhibition of Wnt5A-mediated effects. ROR2 bears homology to neurotrophic tyrosine kinases, most closely, NTRK2. Neurotrophic tyrosine kinase receptors demonstrate increased expression in many cancers. In melanoma cells, for example, the neurotrophic Trks p75NTR [198] and TrkC [199] are overexpressed during metastatic progression. Neurotrophic Trks are of great interest as targets for cancer therapy, because, outside of the brain, they are found quite specifically on malignant cells [200], and inhibitors of nTrks have been shown to have efficacy in vivo [201]. NTRK2, the neurotrophic Trk closest in homology to ROR2, can be inhibited by molecules such as K252a and it is entirely possible that these may have some efficacy in the treatment of melanoma. It has been shown that K252a prevents the proliferation of melanoma cells [202], and a derivative of K252a, KT6124, has been shown to inhibit B16 melanomas in vivo [203]. It is possible that a similar derivative designed to target ROR2 may also have beneficial effects for melanoma patients.
Another tyrosine kinase receptor of importance in neural development that is involved in Wnt signaling is the tyrosine kinase Ryk, or Derailed (in Drosophila). Ryk is essential for transducing Wnt signals during synaptogenesis and axon guidance [204]. Unlike ROR2, Ryk can bind both canonical and heretical Wnts. When complexed with fz8, Ryk can bind Wnt1 and activate TCF/LEF dependent transcription [205]. However, when complexed with Fz7, Ryk binds Wnt11 and activates a beta-arrestin2 mediated pathway [206]. Finally, when complexed with Wnt5A, Ryk can signal via the release of intracellular calcium [204]. Because of its ability to transduce both canonical and heretical Wnt signals effectively, Ryk is a less attractive target than ROR2.
Although Ryk itself may not be a good target for cancer therapy, the partners to which it binds may be. Ryk is known to complex with Ephrin receptors, which can also activate and bind Disheveled [207]. It is unknown whether this association requires Wnt signaling, but certainly Ephrin receptors are associated with the increased malignancy of tumors, specifically colo-rectal and gastro-intestinal tumors, and have been implicated in angiogenesis [208–210]. Ryk complexes with EphB, and inhibitors to EphB do not seem to be available, although inhibitors such as Dasitinib will target EphA [211]. Dasitinib also targets src, which is activated by Wnt5A/Ryk signaling [212]. Several inhibitors for src exist, including PP2, SU6656 and Dasitinib, which has been used in clinical trials for chronic myeloid leukemia (CML) [213]. It was recently reported that in a 2 year follow-up of 2000 CML patients who received Dasitinib after failing Imatinib, 94% were still surviving, and 80% showed no signs of progressive disease [214].
Finally, Bruton’s tyrosine kinase (BTK) is a novel receptor that has been identified as a regulator of Wnt signaling [215]. This tyrosine kinase regulates canonical Wnt signaling, and was identified by a combinatorial small molecule screen, as well as an siRNA screen. Loss of BTK by either pharmacological or genetic intervention resulted in an elevation of canonical Wnt signaling. BTK appears to inhibit β-catenin signaling via a direct interaction with a molecule known as CDC73, which is a member of the transcriptional elongation complex [36]. Increases in CDC73 repress β-catenin activity, and CDC73 has been reported to be a tumor suppressor gene [216]. Enhancing the expression of BTK or CDC73 in cancers where canonical Wnt signaling is important for progression may be of benefit to the patient.
6. Conclusion
The Wnt signaling pathway is ripe with molecular targets for cancer therapy. In addition to key intermediates downstream of Wnt signaling, the discovery of new tyrosine kinase receptors provides a variety of potential targets. In this review we have not touched upon additional molecules such as norrin, r-spondin, CCN2, etc, but these may also prove to be valuable targets, and we refer the interested reader to two excellent reviews that discuss these proteins [205, 217]. The identification and efficacy of small molecule inhibitors of Wnt, such as Box-5 are extremely promising for cancer therapy. The immense amount of work in the Wnt signaling field is opening up a whole new avenue for molecular therapy, and one can only hope that among the targets identified, we will find one to which we will be able to develop the “next Gleevec”.
Acknowledgments
TCC and ATW are supported by the Intramural Research Program of the National Institute on Aging. We regret that we were unable to cite all of the work of many great scientists in the Wnt signaling field due to space constraints.
References
- 1.Nusse R, Varmus HE. Wnt genes. Cell. 1992;69(7):1073–87. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- 2.Miller JR. The Wnts. Genome Biol. 2002;3(1):REVIEWS3001. doi: 10.1186/gb-2001-3-1-reviews3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Bu G. LRP5/6 in Wnt signaling and tumorigenesis. Future Oncol. 2005;1(5):673–81. doi: 10.2217/14796694.1.5.673. [DOI] [PubMed] [Google Scholar]
- 4.Saldanha J, Singh J, Mahadevan D. Identification of a Frizzled-like cysteine rich domain in the extracellular region of developmental receptor tyrosine kinases. Protein Sci. 1998;7(8):1632–5. [PMC free article] [PubMed] [Google Scholar]
- 5.Slusarski DC, V, Corces G, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390(6658):410–3. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- 6.Liu T, Liu X, Wang H, Moon RT, Malbon CC. Activation of rat frizzled-1 promotes Wnt signaling and differentiation of mouse F9 teratocarcinoma cells via pathways that require Galpha(q) and Galpha(o) function. J Biol Chem. 1999;274(47):33539–44. doi: 10.1074/jbc.274.47.33539. [DOI] [PubMed] [Google Scholar]
- 7.Malbon CC, Wang H, Moon RT. Wnt signaling and heterotrimeric G-proteins: strange bedfellows or a classic romance? Biochem Biophys Res Commun. 2001;287(3):589–93. doi: 10.1006/bbrc.2001.5630. [DOI] [PubMed] [Google Scholar]
- 8.McMahon AP, Gavin BJ, Parr B, Bradley A, McMahon JA. The Wnt family of cell signalling molecules in postimplantation development of the mouse. Ciba Found Symp. 1992;165:199–212. doi: 10.1002/9780470514221.ch12. discussion 212–8. [DOI] [PubMed] [Google Scholar]
- 9.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17(1):45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, et al. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16(9):1066–76. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10(12):1443–54. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 12.Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol. 1998;8(10):573–81. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- 13.Korswagen HC, Coudreuse DY, Betist MC, van de Water S, Zivkovic D, Clevers HC. The Axin-like protein PRY-1 is a negative regulator of a canonical Wnt pathway in C. elegans. Genes Dev. 2002;16(10):1291–302. doi: 10.1101/gad.981802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farr GH, 3rd, Ferkey DM, Yost C, Pierce SB, Weaver C, Kimelman D. Interaction among GSK-3, GBP, axin, and APC in Xenopus axis specification. J Cell Biol. 2000;148(4):691–702. doi: 10.1083/jcb.148.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16(13):3797–804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latres E, Chiaur DS, Pagano M. The human F box protein beta-Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta-catenin. Oncogene. 1999;18(4):849–54. doi: 10.1038/sj.onc.1202653. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, He X. beta-Trcp couples beta-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci U S A. 1999;96(11):6273–8. doi: 10.1073/pnas.96.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimelman D, Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25(57):7482–91. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- 19.Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, et al. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature. 1998;395(6702):604–8. doi: 10.1038/26982. [DOI] [PubMed] [Google Scholar]
- 20.Stadeli R, Hoffmans R, Basler K. Transcription under the control of nuclear Arm/beta-catenin. Curr Biol. 2006;16(10):R378–85. doi: 10.1016/j.cub.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Chen G, Fernandez J, Mische S, Courey AJ. A functional interaction between the histone deacetylase Rpd3 and the corepressor groucho in Drosophila development. Genes Dev. 1999;13(17):2218–30. doi: 10.1101/gad.13.17.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407(6803):530–5. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 23.Itoh K, V, Krupnik E, Sokol SY. Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and beta-catenin. Curr Biol. 1998;8(10):591–4. doi: 10.1016/s0960-9822(98)70229-5. [DOI] [PubMed] [Google Scholar]
- 24.Kishida S, Yamamoto H, Ikeda S, Kishida M, Sakamoto I, Koyama S, et al. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J Biol Chem. 1998;273(18):10823–6. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- 25.Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1(1):E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters JM, McKay RM, McKay JP, Graff JM. Casein kinase I transduces Wnt signals. Nature. 1999;401(6751):345–50. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- 27.Smalley MJ, Dale TC. Wnt signalling in mammalian development and cancer. Cancer Metastasis Rev. 1999;18(2):215–30. doi: 10.1023/a:1006369223282. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17(5):1371–84. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakanaka C, Weiss JB, Williams LT. Bridging of beta-catenin and glycogen synthase kinase-3beta by axin and inhibition of beta-catenin-mediated transcription. Proc Natl Acad Sci U S A. 1998;95(6):3020–3. doi: 10.1073/pnas.95.6.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hecht A, Vleminckx K, Stemmler MP, van Roy F, Kemler R. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J. 2000;19(8):1839–50. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takemaru KI, Moon RT. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J Cell Biol. 2000;149(2):249–54. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker N, Hurlstone A, Musisi H, Miles A, Bienz M, Clevers H. The chromatin remodelling factor Brg-1 interacts with beta-catenin to promote target gene activation. EMBO J. 2001;20(17):4935–43. doi: 10.1093/emboj/20.17.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, et al. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell. 2002;109(1):47–60. doi: 10.1016/s0092-8674(02)00679-7. [DOI] [PubMed] [Google Scholar]
- 34.Thompson B, Townsley F, Rosin-Arbesfeld R, Musisi H, Bienz M. A new nuclear component of the Wnt signalling pathway. Nat Cell Biol. 2002;4(5):367–73. doi: 10.1038/ncb786. [DOI] [PubMed] [Google Scholar]
- 35.Parker DS, Jemison J, Cadigan KM. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development. 2002;129(11):2565–76. doi: 10.1242/dev.129.11.2565. [DOI] [PubMed] [Google Scholar]
- 36.Mosimann C, Hausmann G, Basler K. Parafibromin/Hyrax activates Wnt/Wg target gene transcription by direct association with beta-catenin/Armadillo. Cell. 2006;125(2):327–41. doi: 10.1016/j.cell.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 37.Sierra J, Yoshida T, Joazeiro CA, Jones KA. The APC tumor suppressor counteracts beta-catenin activation and H3K4 methylation at Wnt target genes. Genes Dev. 2006;20(5):586–600. doi: 10.1101/gad.1385806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan C, Costello P, Sanghera J, Dominguez D, Baulida J, de Herreros AG, et al. Inhibition of integrin linked kinase (ILK) suppresses beta-catenin-Lef/Tcf-dependent transcription and expression of the E-cadherin repressor, snail, in APC−/− human colon carcinoma cells. Oncogene. 2001;20(1):133–40. doi: 10.1038/sj.onc.1204052. [DOI] [PubMed] [Google Scholar]
- 39.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116(Pt 7):1175–86. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levina E, Oren M, Ben-Ze’ev A. Downregulation of beta-catenin by p53 involves changes in the rate of beta-catenin phosphorylation and Axin dynamics. Oncogene. 2004;23(25):4444–53. doi: 10.1038/sj.onc.1207587. [DOI] [PubMed] [Google Scholar]
- 41.Endo K, Ueda T, Ueyama J, Ohta T, Terada T. Immunoreactive E-cadherin, alpha-catenin, beta-catenin, and gamma-catenin proteins in hepatocellular carcinoma: relationships with tumor grade, clinicopathologic parameters, and patients’ survival. Hum Pathol. 2000;31(5):558–65. doi: 10.1053/hp.2000.6683. [DOI] [PubMed] [Google Scholar]
- 42.Inagawa S, Itabashi M, Adachi S, Kawamoto T, Hori M, Shimazaki J, et al. Expression and prognostic roles of beta-catenin in hepatocellular carcinoma: correlation with tumor progression and postoperative survival. Clin Cancer Res. 2002;8(2):450–6. [PubMed] [Google Scholar]
- 43.Wong CM, Fan ST, Ng IO. beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 2001;92(1):136–45. doi: 10.1002/1097-0142(20010701)92:1<136::aid-cncr1301>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 44.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 45.Brown AM, Wildin RS, Prendergast TJ, Varmus HE. A retrovirus vector expressing the putative mammary oncogene int-1 causes partial transformation of a mammary epithelial cell line. Cell. 1986;46(7):1001–9. doi: 10.1016/0092-8674(86)90699-9. [DOI] [PubMed] [Google Scholar]
- 46.Rijsewijk F, van Deemter L, Wagenaar E, Sonnenberg A, Nusse R. Transfection of the int-1 mammary oncogene in cuboidal RAC mammary cell line results in morphological transformation and tumorigenicity. EMBO J. 1987;6(1):127–31. doi: 10.1002/j.1460-2075.1987.tb04729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edwards PA, Hiby SE, Papkoff J, Bradbury JM. Hyperplasia of mouse mammary epithelium induced by expression of the Wnt-1 (int-1) oncogene in reconstituted mammary gland. Oncogene. 1992;7(10):2041–51. [PubMed] [Google Scholar]
- 48.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14(15):1837–51. [PubMed] [Google Scholar]
- 49.Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene. 1999;18(55):7860–72. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- 50.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275(5307):1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 51.Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58(6):1130–4. [PubMed] [Google Scholar]
- 52.Ilyas M, I, Tomlinson P, Rowan A, Pignatelli M, Bodmer WF. Beta-catenin mutations in cell lines established from human colorectal cancers. Proc Natl Acad Sci U S A. 1997;94(19):10330–4. doi: 10.1073/pnas.94.19.10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zurawel RH, Chiappa SA, Allen C, Raffel C. Sporadic medulloblastomas contain oncogenic beta-catenin mutations. Cancer Res. 1998;58(5):896–9. [PubMed] [Google Scholar]
- 54.Palacios J, Gamallo C. Mutations in the beta-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res. 1998;58(7):1344–7. [PubMed] [Google Scholar]
- 55.Voeller HJ, Truica CI, Gelmann EP. Beta-catenin mutations in human prostate cancer. Cancer Res. 1998;58(12):2520–3. [PubMed] [Google Scholar]
- 56.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66(3):589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 57.Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253(5020):665–9. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 58.Levy DB, Smith KJ, Beazer-Barclay Y, Hamilton SR, Vogelstein B, Kinzler KW. Inactivation of both APC alleles in human and mouse tumors. Cancer Res. 1994;54(22):5953–8. [PubMed] [Google Scholar]
- 59.Smith KJ, Johnson KA, Bryan TM, Hill DE, Markowitz S, Willson JK, et al. The APC gene product in normal and tumor cells. Proc Natl Acad Sci U S A. 1993;90(7):2846–50. doi: 10.1073/pnas.90.7.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, et al. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992;1(4):229–33. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- 61.Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359(6392):235–7. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 62.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87(2):159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 63.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 64.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398(6726):422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 65.Salic A, Lee E, Mayer L, Kirschner MW. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol Cell. 2000;5(3):523–32. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- 66.Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280(5363):596–9. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 67.Kishida M, Koyama S, Kishida S, Matsubara K, Nakashima S, Higano K, et al. Axin prevents Wnt-3a-induced accumulation of beta-catenin. Oncogene. 1999;18(4):979–85. doi: 10.1038/sj.onc.1202388. [DOI] [PubMed] [Google Scholar]
- 68.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–20. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 69.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5(2):100–7. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Behrens J, Lustig B. The Wnt connection to tumorigenesis. Int J Dev Biol. 2004;48(5–6):477–87. doi: 10.1387/ijdb.041815jb. [DOI] [PubMed] [Google Scholar]
- 71.Shakoori A, Ougolkov A, Yu ZW, Zhang B, Modarressi MH, Billadeau DD, et al. Deregulated GSK3beta activity in colorectal cancer: its association with tumor cell survival and proliferation. Biochem Biophys Res Commun. 2005;334(4):1365–73. doi: 10.1016/j.bbrc.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 72.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406(6791):86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 73.Schwabe RF, Brenner DA. Role of glycogen synthase kinase-3 in TNF-alpha-induced NF-kappaB activation and apoptosis in hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2002;283(1):G204–11. doi: 10.1152/ajpgi.00016.2002. [DOI] [PubMed] [Google Scholar]
- 74.Kulikov R, Boehme KA, Blattner C. Glycogen synthase kinase 3-dependent phosphorylation of Mdm2 regulates p53 abundance. Mol Cell Biol. 2005;25(16):7170–80. doi: 10.1128/MCB.25.16.7170-7180.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qu L, Huang S, Baltzis D, Rivas-Estilla AM, Pluquet O, Hatzoglou M, et al. Endoplasmic reticulum stress induces p53 cytoplasmic localization and prevents p53-dependent apoptosis by a pathway involving glycogen synthase kinase-3beta. Genes Dev. 2004;18(3):261–77. doi: 10.1101/gad.1165804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maccario H, Perera NM, Davidson L, Downes CP, Leslie NR. PTEN is destabilized by phosphorylation on Thr366. Biochem J. 2007;405(3):439–44. doi: 10.1042/BJ20061837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rask K, Nilsson A, Brannstrom M, Carlsson P, Hellberg P, Janson PO, et al. Wnt-signalling pathway in ovarian epithelial tumours: increased expression of beta-catenin and GSK3beta. Br J Cancer. 2003;89(7):1298–304. doi: 10.1038/sj.bjc.6601265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Farago M, Dominguez I, Landesman-Bollag E, Xu X, Rosner A, Cardiff RD, et al. Kinase-inactive glycogen synthase kinase 3beta promotes Wnt signaling and mammary tumorigenesis. Cancer Res. 2005;65(13):5792–801. doi: 10.1158/0008-5472.CAN-05-1021. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo RL, et al. Adiponectin modulates the glycogen synthase kinase-3beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 2006;66(23):11462–70. doi: 10.1158/0008-5472.CAN-06-1969. [DOI] [PubMed] [Google Scholar]
- 80.Ding Q, He X, Xia W, Hsu JM, Chen CT, Li LY, et al. Myeloid cell leukemia-1 inversely correlates with glycogen synthase kinase-3beta activity and associates with poor prognosis in human breast cancer. Cancer Res. 2007;67(10):4564–71. doi: 10.1158/0008-5472.CAN-06-1788. [DOI] [PubMed] [Google Scholar]
- 81.Li Z, Tan F, Thiele CJ. Inactivation of glycogen synthase kinase-3beta contributes to brain-derived neutrophic factor/TrkB-induced resistance to chemotherapy in neuroblastoma cells. Mol Cancer Ther. 2007;6(12 Pt 1):3113–21. doi: 10.1158/1535-7163.MCT-07-0133. [DOI] [PubMed] [Google Scholar]
- 82.Martinez A. Preclinical efficacy on GSK-3 inhibitors: towards a future generation of powerful drugs. Med Res Rev. 2008;28(5):773–96. doi: 10.1002/med.20119. [DOI] [PubMed] [Google Scholar]
- 83.Ougolkov AV, Billadeau DD. Targeting GSK-3: a promising approach for cancer therapy? Future Oncol. 2006;2(1):91–100. doi: 10.2217/14796694.2.1.91. [DOI] [PubMed] [Google Scholar]
- 84.Gould TD, Gray NA, Manji HK. Effects of a glycogen synthase kinase-3 inhibitor, lithium, in adenomatous polyposis coli mutant mice. Pharmacol Res. 2003;48(1):49–53. [PubMed] [Google Scholar]
- 85.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653(1):1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 86.Brown AM. Wnt signaling in breast cancer: have we come full circle? Breast Cancer Res. 2001;3(6):351–5. doi: 10.1186/bcr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ueda M, Gemmill RM, West J, Winn R, Sugita M, Tanaka N, et al. Mutations of the beta- and gamma-catenin genes are uncommon in human lung, breast, kidney, cervical and ovarian carcinomas. Br J Cancer. 2001;85(1):64–8. doi: 10.1054/bjoc.2001.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Howe LR, Brown AM. Wnt signaling and breast cancer. Cancer Biol Ther. 2004;3(1):36–41. doi: 10.4161/cbt.3.1.561. [DOI] [PubMed] [Google Scholar]
- 89.Brennan KR, Brown AM. Wnt proteins in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2004;9(2):119–31. doi: 10.1023/B:JOMG.0000037157.94207.33. [DOI] [PubMed] [Google Scholar]
- 90.Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, et al. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci U S A. 2000;97(8):4262–6. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Benhaj K, Akcali KC, Ozturk M. Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncol Rep. 2006;15(3):701–7. [PubMed] [Google Scholar]
- 92.Mikami I, You L, He B, Xu Z, Batra S, Lee AY, et al. Efficacy of Wnt-1 monoclonal antibody in sarcoma cells. BMC Cancer. 2005;5:53. doi: 10.1186/1471-2407-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, et al. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene. 2005;24(18):3054–8. doi: 10.1038/sj.onc.1208511. [DOI] [PubMed] [Google Scholar]
- 94.Rhee CS, Sen M, Lu D, Wu C, Leoni L, Rubin J, et al. Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene. 2002;21(43):6598–605. doi: 10.1038/sj.onc.1205920. [DOI] [PubMed] [Google Scholar]
- 95.He B, You L, Uematsu K, Xu Z, Lee AY, Matsangou M, et al. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia. 2004;6(1):7–14. doi: 10.1016/s1476-5586(04)80048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mazieres J, You L, He B, Xu Z, Lee AY, Mikami I, et al. Inhibition of Wnt16 in human acute lymphoblastoid leukemia cells containing the t(1;19) translocation induces apoptosis. Oncogene. 2005;24(34):5396–400. doi: 10.1038/sj.onc.1208568. [DOI] [PubMed] [Google Scholar]
- 97.You L, He B, Xu Z, Uematsu K, Mazieres J, Mikami I, et al. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene. 2004;23(36):6170–4. doi: 10.1038/sj.onc.1207844. [DOI] [PubMed] [Google Scholar]
- 98.You L, He B, Xu Z, Uematsu K, Mazieres J, Fujii N, et al. An anti-Wnt-2 monoclonal antibody induces apoptosis in malignant melanoma cells and inhibits tumor growth. Cancer Res. 2004;64(15):5385–9. doi: 10.1158/0008-5472.CAN-04-1227. [DOI] [PubMed] [Google Scholar]
- 99.Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, et al. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell. 2004;5(1):91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 100.Sukhdeo K, Mani M, Zhang Y, Dutta J, Yasui H, Rooney MD, et al. Targeting the beta-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc Natl Acad Sci U S A. 2007;104(18):7516–21. doi: 10.1073/pnas.0610299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wei W, Chua MS, Grepper S, So S. Small molecule antagonists of Tcf4/beta-catenin complex inhibit the growth of HCC cells in vitro and in vivo. Int J Cancer. 2009 doi: 10.1002/ijc.24810. [DOI] [PubMed] [Google Scholar]
- 102.Sandgren EP, Quaife CJ, Pinkert CA, Palmiter RD, Brinster RL. Oncogene-induced liver neoplasia in transgenic mice. Oncogene. 1989;4(6):715–24. [PubMed] [Google Scholar]
- 103.Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87(5):803–9. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 104.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342(26):1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 105.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1(1):11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 106.Boon EM, Keller JJ, Wormhoudt TA, Giardiello FM, Offerhaus GJ, van der Neut R, et al. Sulindac targets nuclear beta-catenin accumulation and Wnt signalling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. Br J Cancer. 2004;90(1):224–9. doi: 10.1038/sj.bjc.6601505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116(Pt 13):2627–34. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 108.Suzuki H, Gabrielson E, Chen W, Anbazhagan R, van Engeland M, Weijenberg MP, et al. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat Genet. 2002;31(2):141–9. doi: 10.1038/ng892. [DOI] [PubMed] [Google Scholar]
- 109.Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36(4):417–22. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 110.Caldwell GM, Jones C, Gensberg K, Jan S, Hardy RG, Byrd P, et al. The Wnt antagonist sFRP1 in colorectal tumorigenesis. Cancer Res. 2004;64(3):883–8. doi: 10.1158/0008-5472.can-03-1346. [DOI] [PubMed] [Google Scholar]
- 111.Aguilera O, Fraga MF, Ballestar E, Paz MF, Herranz M, Espada J, et al. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene. 2006;25(29):4116–21. doi: 10.1038/sj.onc.1209439. [DOI] [PubMed] [Google Scholar]
- 112.Fukui T, Kondo M, Ito G, Maeda O, Sato N, Yoshioka H, et al. Transcriptional silencing of secreted frizzled related protein 1 (SFRP 1) by promoter hypermethylation in non-small-cell lung cancer. Oncogene. 2005;24(41):6323–7. doi: 10.1038/sj.onc.1208777. [DOI] [PubMed] [Google Scholar]
- 113.Zhou Z, Wang J, Han X, Zhou J, Linder S. Up-regulation of human secreted frizzled homolog in apoptosis and its down-regulation in breast tumors. Int J Cancer. 1998;78(1):95–9. doi: 10.1002/(sici)1097-0215(19980925)78:1<95::aid-ijc15>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 114.Takada T, Yagi Y, Maekita T, Imura M, Nakagawa S, Tsao SW, et al. Methylation-associated silencing of the Wnt antagonist SFRP1 gene in human ovarian cancers. Cancer Sci. 2004;95(9):741–4. doi: 10.1111/j.1349-7006.2004.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hrzenjak A, Tippl M, Kremser ML, Strohmeier B, Guelly C, Neumeister D, et al. Inverse correlation of secreted frizzled-related protein 4 and beta-catenin expression in endometrial stromal sarcomas. J Pathol. 2004;204(1):19–27. doi: 10.1002/path.1616. [DOI] [PubMed] [Google Scholar]
- 116.Abu-Jawdeh G, Comella N, Tomita Y, Brown LF, Tognazzi K, Sokol SY, et al. Differential expression of frpHE: a novel human stromal protein of the secreted frizzled gene family, during the endometrial cycle and malignancy. Lab Invest. 1999;79(4):439–47. [PubMed] [Google Scholar]
- 117.Wissmann C, Wild PJ, Kaiser S, Roepcke S, Stoehr R, Woenckhaus M, et al. WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J Pathol. 2003;201(2):204–12. doi: 10.1002/path.1449. [DOI] [PubMed] [Google Scholar]
- 118.Wu W, Glinka A, Delius H, Niehrs C. Mutual antagonism between dickkopf1 and dickkopf2 regulates Wnt/beta-catenin signalling. Curr Biol. 2000;10(24):1611–4. doi: 10.1016/s0960-9822(00)00868-x. [DOI] [PubMed] [Google Scholar]
- 119.Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3(7):683–6. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 120.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411(6835):321–5. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 121.Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11(12):951–61. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 122.Sato H, Suzuki H, Toyota M, Nojima M, Maruyama R, Sasaki S, et al. Frequent epigenetic inactivation of DICKKOPF family genes in human gastrointestinal tumors. Carcinogenesis. 2007;28(12):2459–66. doi: 10.1093/carcin/bgm178. [DOI] [PubMed] [Google Scholar]
- 123.Larue L, Luciani F, Kumasaka M, Champeval D, Demirkan N, Bonaventure J, et al. Bypassing melanocyte senescence by beta-catenin: a novel way to promote melanoma. Pathol Biol (Paris) 2009;57(7–8):543–7. doi: 10.1016/j.patbio.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 124.Chien AJ, Moore EC, Lonsdorf AS, Kulikauskas RM, Rothberg BG, Berger AJ, et al. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci U S A. 2009;106(4):1193–8. doi: 10.1073/pnas.0811902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shimizu H, Julius MA, Giarre M, Zheng Z, Brown AM, Kitajewski J. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 1997;8(12):1349–58. [PubMed] [Google Scholar]
- 126.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13(8):680–5. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 127.Kikuchi A, Yamamoto H. Tumor formation due to abnormalities in the beta-catenin-independent pathway of Wnt signaling. Cancer Sci. 2008;99(2):202–8. doi: 10.1111/j.1349-7006.2007.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miyagi C, Yamashita S, Ohba Y, Yoshizaki H, Matsuda M, Hirano T. STAT3 noncell-autonomously controls planar cell polarity during zebrafish convergence and extension. J Cell Biol. 2004;166(7):975–81. doi: 10.1083/jcb.200403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Y. Wnt/Planar cell polarity signaling: a new paradigm for cancer therapy. Mol Cancer Ther. 2009;8(8):2103–9. doi: 10.1158/1535-7163.MCT-09-0282. [DOI] [PubMed] [Google Scholar]
- 130.Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, Wilson SW, Tada M. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130(17):4037–46. doi: 10.1242/dev.00567. [DOI] [PubMed] [Google Scholar]
- 131.Takeuchi M, Nakabayashi J, Sakaguchi T, Yamamoto TS, Takahashi H, Takeda H, et al. The prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr Biol. 2003;13(8):674–9. doi: 10.1016/s0960-9822(03)00245-8. [DOI] [PubMed] [Google Scholar]
- 132.Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, et al. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306(1):121–33. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Katoh M. WNT/PCP signaling pathway and human cancer (review) Oncol Rep. 2005;14(6):1583–8. [PubMed] [Google Scholar]
- 134.Kim GH, Han JK. JNK and ROKalpha function in the noncanonical Wnt/RhoA signaling pathway to regulate Xenopus convergent extension movements. Dev Dyn. 2005;232(4):958–68. doi: 10.1002/dvdy.20262. [DOI] [PubMed] [Google Scholar]
- 135.Tahinci E, Thorne CA, Franklin JL, Salic A, Christian KM, Lee LA, et al. Lrp6 is required for convergent extension during Xenopus gastrulation. Development. 2007;134(22):4095–106. doi: 10.1242/dev.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Medina A, Reintsch W, Steinbeisser H. Xenopus frizzled 7 can act in canonical and non-canonical Wnt signaling pathways: implications on early patterning and morphogenesis. Mech Dev. 2000;92(2):227–37. doi: 10.1016/s0925-4773(00)00240-9. [DOI] [PubMed] [Google Scholar]
- 137.Merle P, Kim M, Herrmann M, Gupte A, Lefrancois L, Califano S, et al. Oncogenic role of the frizzled-7/beta-catenin pathway in hepatocellular carcinoma. J Hepatol. 2005;43(5):854–62. doi: 10.1016/j.jhep.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 138.Ueno K, Hazama S, Mitomori S, Nishioka M, Suehiro Y, Hirata H, et al. Down-regulation of frizzled-7 expression decreases survival, invasion and metastatic capabilities of colon cancer cells. Br J Cancer. 2009;101(8):1374–81. doi: 10.1038/sj.bjc.6605307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Soon LL, Yie TA, Shvarts A, Levine AJ, Su F, Tchou-Wong KM. Overexpression of WISP-1 down-regulated motility and invasion of lung cancer cells through inhibition of Rac activation. J Biol Chem. 2003;278(13):11465–70. doi: 10.1074/jbc.M210945200. [DOI] [PubMed] [Google Scholar]
- 140.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101(20):7618–23. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wei J, Wunderlich M, Fox C, Alvarez S, Cigudosa JC, Wilhelm JS, et al. Microenvironment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell. 2008;13(6):483–95. doi: 10.1016/j.ccr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yao H, Dashner EJ, van Golen CM, van Golen KL. RhoC GTPase is required for PC-3 prostate cancer cell invasion but not motility. Oncogene. 2006;25(16):2285–96. doi: 10.1038/sj.onc.1209260. [DOI] [PubMed] [Google Scholar]
- 143.Baptiste DC, Fehlings MG. Pharmacological approaches to repair the injured spinal cord. J Neurotrauma. 2006;23(3–4):318–34. doi: 10.1089/neu.2006.23.318. [DOI] [PubMed] [Google Scholar]
- 144.Yamamoto S, Nishimura O, Misaki K, Nishita M, Minami Y, Yonemura S, et al. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008;15(1):23–36. doi: 10.1016/j.devcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 145.Cantrell VA, Jessen JR. The planar cell polarity protein Van Gogh-Like 2 regulates tumor cell migration and matrix metalloproteinase-dependent invasion. Cancer Lett. 2009 doi: 10.1016/j.canlet.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 146.Tang L, Dai DL, Su M, Martinka M, Li G, Zhou Y. Aberrant expression of collagen triple helix repeat containing 1 in human solid cancers. Clin Cancer Res. 2006;12(12):3716–22. doi: 10.1158/1078-0432.CCR-06-0030. [DOI] [PubMed] [Google Scholar]
- 147.Kuhl M, Sheldahl LC, Malbon CC, Moon RT. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem. 2000;275(17):12701–11. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- 148.Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16(7):279–83. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- 149.Kuhl M, Geis K, Sheldahl LC, Pukrop T, Moon RT, Wedlich D. Antagonistic regulation of convergent extension movements in Xenopus by Wnt/beta-catenin and Wnt/Ca2+ signaling. Mech Dev. 2001;106(1–2):61–76. doi: 10.1016/s0925-4773(01)00416-6. [DOI] [PubMed] [Google Scholar]
- 150.Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162(5):899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 2008;28(3):504–10. doi: 10.1161/ATVBAHA.107.157438. [DOI] [PubMed] [Google Scholar]
- 152.Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, et al. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci U S A. 2006;103(14):5454–9. doi: 10.1073/pnas.0509703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cheng CW, Yeh JC, Fan TP, Smith SK, Charnock-Jones DS. Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem Biophys Res Commun. 2008;365(2):285–90. doi: 10.1016/j.bbrc.2007.10.166. [DOI] [PubMed] [Google Scholar]
- 154.Dissanayake SK, Wade M, Johnson CE, O’Connell MP, Leotlela PD, French AD, et al. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem. 2007;282(23):17259–71. doi: 10.1074/jbc.M700075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Medyouf H, Ghysdael J. The calcineurin/NFAT signaling pathway: a novel therapeutic target in leukemia and solid tumors. Cell Cycle. 2008;7(3):297–303. doi: 10.4161/cc.7.3.5357. [DOI] [PubMed] [Google Scholar]
- 156.Murphy LL, Hughes CC. Endothelial cells stimulate T cell NFAT nuclear translocation in the presence of cyclosporin A: involvement of the wnt/glycogen synthase kinase-3 beta pathway. J Immunol. 2002;169(7):3717–25. doi: 10.4049/jimmunol.169.7.3717. [DOI] [PubMed] [Google Scholar]
- 157.Dejmek J, Safholm A, Kamp Nielsen C, Andersson T, Leandersson K. Wnt-5a/Ca2+-induced NFAT activity is counteracted by Wnt-5a/Yes-Cdc42-casein kinase 1alpha signaling in human mammary epithelial cells. Mol Cell Biol. 2006;26(16):6024–36. doi: 10.1128/MCB.02354-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yu JM, Kim JH, Song GS, Jung JS. Increase in proliferation and differentiation of neural progenitor cells isolated from postnatal and adult mice brain by Wnt-3a and Wnt-5a. Mol Cell Biochem. 2006;288(1–2):17–28. doi: 10.1007/s11010-005-9113-3. [DOI] [PubMed] [Google Scholar]
- 159.Koyanagi M, Haendeler J, Badorff C, Brandes RP, Hoffmann J, Pandur P, et al. Non-canonical Wnt signaling enhances differentiation of human circulating progenitor cells to cardiomyogenic cells. J Biol Chem. 2005;280(17):16838–42. doi: 10.1074/jbc.M500323200. [DOI] [PubMed] [Google Scholar]
- 160.Jonsson M, Smith K, Harris AL. Regulation of Wnt5a expression in human mammary cells by protein kinase C activity and the cytoskeleton. Br J Cancer. 1998;78(4):430–8. doi: 10.1038/bjc.1998.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bodmer D, Levine-Wilkinson S, Richmond A, Hirsh S, Kuruvilla R. Wnt5a mediates nerve growth factor-dependent axonal branching and growth in developing sympathetic neurons. J Neurosci. 2009;29(23):7569–81. doi: 10.1523/JNEUROSCI.1445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ghosh MC, Collins GD, Vandanmagsar B, Patel K, Brill M, Carter A, et al. Activation of Wnt5A signaling is required for CXC chemokine ligand 12-mediated T-cell migration. Blood. 2009;114(7):1366–73. doi: 10.1182/blood-2008-08-175869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science. 2008;320(5874):365–9. doi: 10.1126/science.1151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dejmek J, Dejmek A, Safholm A, Sjolander A, Andersson T. Wnt-5a protein expression in primary dukes B colon cancers identifies a subgroup of patients with good prognosis. Cancer Res. 2005;65(20):9142–6. doi: 10.1158/0008-5472.CAN-05-1710. [DOI] [PubMed] [Google Scholar]
- 165.Ying J, Li H, Yu J, Ng KM, Poon FF, Wong SC, et al. WNT5A exhibits tumor-suppressive activity through antagonizing the Wnt/beta-catenin signaling, and is frequently methylated in colorectal cancer. Clin Cancer Res. 2008;14(1):55–61. doi: 10.1158/1078-0432.CCR-07-1644. [DOI] [PubMed] [Google Scholar]
- 166.Blanc E, Roux GL, Benard J, Raguenez G. Low expression of Wnt-5a gene is associated with high-risk neuroblastoma. Oncogene. 2005;24(7):1277–83. doi: 10.1038/sj.onc.1208255. [DOI] [PubMed] [Google Scholar]
- 167.Jonsson M, Dejmek J, Bendahl PO, Andersson T. Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res. 2002;62(2):409–16. [PubMed] [Google Scholar]
- 168.Dejmek J, Leandersson K, Manjer J, Bjartell A, Emdin SO, Vogel WF, et al. Expression and signaling activity of Wnt-5a/discoidin domain receptor-1 and Syk plays distinct but decisive roles in breast cancer patient survival. Clin Cancer Res. 2005;11(2 Pt 1):520–8. [PubMed] [Google Scholar]
- 169.Liang H, Chen Q, Coles AH, Anderson SJ, Pihan G, Bradley A, et al. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell. 2003;4(5):349–60. doi: 10.1016/s1535-6108(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 170.Roman-Gomez J, Jimenez-Velasco A, Cordeu L, Vilas-Zornoza A, San Jose-Eneriz E, Garate L, et al. WNT5A, a putative tumour suppressor of lymphoid malignancies, is inactivated by aberrant methylation in acute lymphoblastic leukaemia. Eur J Cancer. 2007;43(18):2736–46. doi: 10.1016/j.ejca.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 171.Ying J, Li H, Chen YW, Srivastava G, Gao Z, Tao Q. WNT5A is epigenetically silenced in hematologic malignancies and inhibits leukemia cell growth as a tumor suppressor. Blood. 2007;110(12):4130–2. doi: 10.1182/blood-2007-06-094870. [DOI] [PubMed] [Google Scholar]
- 172.Kremenevskaja N, von Wasielewski R, Rao AS, Schofl C, Andersson T, Brabant G. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene. 2005;24(13):2144–54. doi: 10.1038/sj.onc.1208370. [DOI] [PubMed] [Google Scholar]
- 173.Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, et al. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66(21):10439–48. doi: 10.1158/0008-5472.CAN-06-2359. [DOI] [PubMed] [Google Scholar]
- 174.Ripka S, Konig A, Buchholz M, Wagner M, Sipos B, Kloppel G, et al. WNT5A--target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis. 2007;28(6):1178–87. doi: 10.1093/carcin/bgl255. [DOI] [PubMed] [Google Scholar]
- 175.Huang CL, Liu D, Nakano J, Ishikawa S, Kontani K, Yokomise H, et al. Wnt5a expression is associated with the tumor proliferation and the stromal vascular endothelial growth factor--an expression in non-small-cell lung cancer. J Clin Oncol. 2005;23(34):8765–73. doi: 10.1200/JCO.2005.02.2871. [DOI] [PubMed] [Google Scholar]
- 176.Wang Q, Williamson M, Bott S, Brookman-Amissah N, Freeman A, Nariculam J, et al. Hypomethylation of WNT5A, CRIP1 and S100P in prostate cancer. Oncogene. 2007;26(45):6560–5. doi: 10.1038/sj.onc.1210472. [DOI] [PubMed] [Google Scholar]
- 177.Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406(6795):536–40. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 178.Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1(3):279–88. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 179.Da Forno PD, Pringle JH, Hutchinson P, Osborn J, Huang Q, Potter L, et al. WNT5A expression increases during melanoma progression and correlates with outcome. Clin Cancer Res. 2008;14(18):5825–32. doi: 10.1158/1078-0432.CCR-07-5104. [DOI] [PubMed] [Google Scholar]
- 180.O’Connell MP, Fiori JL, Baugher KM, Indig FE, French AD, Camilli TC, et al. Wnt5A activates the calpain-mediated cleavage of filamin A. J Invest Dermatol. 2009;129(7):1782–9. doi: 10.1038/jid.2008.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8(7):645–54. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 182.Billiard J, Way DS, Seestaller-Wehr LM, Moran RA, Mangine A, Bodine PV. The orphan receptor tyrosine kinase Ror2 modulates canonical Wnt signaling in osteoblastic cells. Mol Endocrinol. 2005;19(1):90–101. doi: 10.1210/me.2004-0153. [DOI] [PubMed] [Google Scholar]
- 183.Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, Ohta Y, et al. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol. 2006;175(4):555–62. doi: 10.1083/jcb.200607127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.O’Connell MP, Weeraratna AT. Hear the Wnt Ror: how melanoma cells adjust to changes in Wnt. Pigment Cell Melanoma Res. 2009 doi: 10.1111/j.1755-148X.2009.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.O’Connell MP, Fiori JL, Kershner EK, Frank BP, Indig FE, Taub DD, et al. HSPG modulation of WNT5A signal transduction in metastatic melanoma cells. J Biol Chem. 2009 doi: 10.1074/jbc.M109.028498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.O’Connell MP, Fiori JL, Xu M, Carter AD, Frank BP, Camilli TC, et al. The Orphan Tyrosine Kinase Receptor, ROR2, Mediates Wnt5A Signaling in Metastatic Melanoma. Oncogene. 2009 doi: 10.1038/onc.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dissanayake SK, Olkhanud PB, O’Connell MP, Carter A, French AD, Camilli TC, et al. Wnt5A regulates expression of tumor-associated antigens in melanoma via changes in signal transducers and activators of transcription 3 phosphorylation. Cancer Res. 2008;68(24):10205–14. doi: 10.1158/0008-5472.CAN-08-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer Regression in Patients After Transfer of Genetically Engineered Lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nakamura K, Yoshikawa N, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Effect of PKC412, an inhibitor of protein kinase C, on spontaneous metastatic model mice. Anticancer Res. 2003;23(2B):1395–9. [PubMed] [Google Scholar]
- 190.Mapelli E, Banfi P, Sala E, Sensi M, Supino R, Zunino F, et al. Effect of protein kinase C inhibitors on invasiveness of human melanoma clones expressing different levels of protein kinase C isoenzymes. Int J Cancer. 1994;57(2):281–6. doi: 10.1002/ijc.2910570225. [DOI] [PubMed] [Google Scholar]
- 191.Legg JW, Lewis CA, Parsons M, Ng T, Isacke CM. A novel PKC-regulated mechanism controls CD44 ezrin association and directional cell motility. Nat Cell Biol. 2002;4(6):399–407. doi: 10.1038/ncb797. [DOI] [PubMed] [Google Scholar]
- 192.Dennis JU, Dean NM, Bennett CF, Griffith JW, Lang CM, Welch DR. Human melanoma metastasis is inhibited following ex vivo treatment with an antisense oligonucleotide to protein kinase C-alpha. Cancer Lett. 1998;128(1):65–70. doi: 10.1016/s0304-3835(98)00052-4. [DOI] [PubMed] [Google Scholar]
- 193.Goekjian PG, Jirousek MR. Protein kinase C inhibitors as novel anticancer drugs. Expert Opin Investig Drugs. 2001;10(12):2117–40. doi: 10.1517/13543784.10.12.2117. [DOI] [PubMed] [Google Scholar]
- 194.Swannie HC, Kaye SB. Protein kinase C inhibitors. Curr Oncol Rep. 2002;4(1):37–46. doi: 10.1007/s11912-002-0046-7. [DOI] [PubMed] [Google Scholar]
- 195.Safholm A, Tuomela J, Rosenkvist J, Dejmek J, Harkonen P, Andersson T. The Wnt-5a-derived hexapeptide Foxy-5 inhibits breast cancer metastasis in vivo by targeting cell motility. Clin Cancer Res. 2008;14(20):6556–63. doi: 10.1158/1078-0432.CCR-08-0711. [DOI] [PubMed] [Google Scholar]
- 196.Safholm A, Leandersson K, Dejmek J, Nielsen CK, Villoutreix BO, Andersson T. A formylated hexapeptide ligand mimics the ability of Wnt-5a to impair migration of human breast epithelial cells. J Biol Chem. 2006;281(5):2740–9. doi: 10.1074/jbc.M508386200. [DOI] [PubMed] [Google Scholar]
- 197.Jenei V, Sherwood V, Howlin J, Linnskog R, Safholm A, Axelsson L, et al. A t-butyloxycarbonyl-modified Wnt5a-derived hexapeptide functions as a potent antagonist of Wnt5a-dependent melanoma cell invasion. Proc Natl Acad Sci U S A. 2009;106(46):19473–8. doi: 10.1073/pnas.0909409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Innominato PF, Libbrecht L, van den Oord JJ. Expression of neurotrophins and their receptors in pigment cell lesions of the skin. J Pathol. 2001;194(1):95–100. doi: 10.1002/path.861. [DOI] [PubMed] [Google Scholar]
- 199.Xu X, Tahan SR, Pasha TL, Zhang PJ. Expression of neurotrophin receptor Trk-C in nevi and melanomas. J Cutan Pathol. 2003;30(5):318–22. doi: 10.1034/j.1600-0560.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- 200.Weeraratna AT, Arnold JT, George DJ, DeMarzo A, Isaacs JT. Rational basis for Trk inhibition therapy for prostate cancer. Prostate. 2000;45(2):140–8. doi: 10.1002/1097-0045(20001001)45:2<140::aid-pros8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 201.Weeraratna AT, Dalrymple SL, Lamb JC, Denmeade SR, Miknyoczki S, Dionne CA, et al. Pan-trk inhibition decreases metastasis and enhances host survival in experimental models as a result of its selective induction of apoptosis of prostate cancer cells. Clin Cancer Res. 2001;7(8):2237–45. [PubMed] [Google Scholar]
- 202.Truzzi F, Marconi A, Lotti R, Dallaglio K, French LE, Hempstead BL, et al. Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. J Invest Dermatol. 2008;128(8):2031–40. doi: 10.1038/jid.2008.21. [DOI] [PubMed] [Google Scholar]
- 203.Akinaga S, Ashizawa T, Gomi K, Ohno H, Morimoto M, Murakata C, et al. Antitumor effect of KT6124, a novel derivative of protein kinase inhibitor K-252a, and its mechanism of action. Cancer Chemother Pharmacol. 1992;29(4):266–72. doi: 10.1007/BF00685943. [DOI] [PubMed] [Google Scholar]
- 204.Keeble TR, Halford MM, Seaman C, Kee N, Macheda M, Anderson RB, et al. The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J Neurosci. 2006;26(21):5840–8. doi: 10.1523/JNEUROSCI.1175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hendrickx M, Leyns L. Non-conventional Frizzled ligands and Wnt receptors. Dev Growth Differ. 2008;50(4):229–43. doi: 10.1111/j.1440-169X.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 206.Kim GH, Her JH, Han JK. Ryk cooperates with Frizzled 7 to promote Wnt11-mediated endocytosis and is essential for Xenopus laevis convergent extension movements. J Cell Biol. 2008;182(6):1073–82. doi: 10.1083/jcb.200710188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Trivier E, Ganesan TS. RYK, a catalytically inactive receptor tyrosine kinase, associates with EphB2 and EphB3 but does not interact with AF-6. J Biol Chem. 2002;277(25):23037–43. doi: 10.1074/jbc.M202486200. [DOI] [PubMed] [Google Scholar]
- 208.Liu W, Ahmad SA, Jung YD, Reinmuth N, Fan F, Bucana CD, et al. Coexpression of ephrin-Bs and their receptors in colon carcinoma. Cancer. 2002;94(4):934–9. doi: 10.1002/cncr.10122. [DOI] [PubMed] [Google Scholar]
- 209.Martiny-Baron G, Korff T, Schaffner F, Esser N, Eggstein S, Marme D, et al. Inhibition of tumor growth and angiogenesis by soluble EphB4. Neoplasia. 2004;6(3):248–57. doi: 10.1593/neo.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Brantley-Sieders D, Schmidt S, Parker M, Chen J. Eph receptor tyrosine kinases in tumor and tumor microenvironment. Curr Pharm Des. 2004;10(27):3431–42. doi: 10.2174/1381612043383160. [DOI] [PubMed] [Google Scholar]
- 211.Piccaluga PP, Paolini S, Martinelli G. Tyrosine kinase inhibitors for the treatment of Philadelphia chromosome-positive adult acute lymphoblastic leukemia. Cancer. 2007;110(6):1178–86. doi: 10.1002/cncr.22881. [DOI] [PubMed] [Google Scholar]
- 212.Wouda RR, Bansraj MR, de Jong AW, Noordermeer JN, Fradkin LG. Src family kinases are required for WNT5 signaling through the Derailed/RYK receptor in the Drosophila embryonic central nervous system. Development. 2008;135(13):2277–87. doi: 10.1242/dev.017319. [DOI] [PubMed] [Google Scholar]
- 213.Keam SJ. Dasatinib: in chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. BioDrugs. 2008;22(1):59–69. doi: 10.2165/00063030-200822010-00007. [DOI] [PubMed] [Google Scholar]
- 214.Pavlu J, Marin D. Dasatinib and chronic myeloid leukemia: two-year follow-up in eight clinical trials. Clin Lymphoma Myeloma. 2009;9(6):417–24. doi: 10.3816/CLM.2009.n.083. [DOI] [PubMed] [Google Scholar]
- 215.James RG, Biechele TL, Conrad WH, Camp ND, Fass DM, Major MB, et al. Bruton’s tyrosine kinase revealed as a negative regulator of Wnt-beta-catenin signaling. Sci Signal. 2009;2(72):ra25. doi: 10.1126/scisignal.2000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yip L, Seethala RR, Nikiforova MN, Nikiforov YE, Ogilvie JB, Carty SE, et al. Loss of heterozygosity of selected tumor suppressor genes in parathyroid carcinoma. Surgery. 2008;144(6):949–55. doi: 10.1016/j.surg.2008.08.030. discussion 954–5. [DOI] [PubMed] [Google Scholar]
- 217.Rey JP, Ellies DL. Wnt modulators in the biotech pipeline. Dev Dyn. 239(1):102–14. doi: 10.1002/dvdy.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]