Abstract
We studied orchiectomy specimens from 130 patients immuhistochemically with testicular germ cell tumor (TGCT) using anti-core 2 N-acetylglucosaminyltransferase-1 (C2GnT-1) antibody. The incidence of C2GnT-1 positivity in stage I disease (29.5%, 21/71) was significantly lower than that in higher stages (84.7%, 50/59) (P < 0.001, χ2 test). This significant difference was also found when the cases were divided into seminoma and NSGCT according to histopathological classification. Kaplan-Meier plots and the log rank test showed that in the patients with stage I seminoma, C2GnT-1-positive cases had a higher risk for recurrence (P < 0.001). This was also the case with the patients with stage I NSGCT (P < 0.001). To determine whether C2GnT-1 promotes aggressive behavior of cancer cells, a C2GnT-1-negative human TGCT cell line, JKT-1, was stably transfected with a mammalian expression vector containing C2GnT-1 cDNA. In vitro assays revealed that JKT-1-C2 cells are more invasive than mock transfectants, although there are no differences in proliferation activity. When orthotopically inoculated into athymic nude mice, JKT-1-C2 cells produced larger testicular tumors extending to the retroperitoneum with mesenteric metastasis, while mock transfectants produced small tumors without metastasis (P < 0.01, Mann-Whitney’s U-test). When injected via the tail vein, JKT-1-C2 cells produced a number of metastatic lung foci. In contrast, mock transfectants produced a small number of nodules (p < 0.01, Mann-Whitney’s U-test). These results strongly suggest that C2GnT-1 enhances the metastatic potential of TGCT and may be a reliable biomarker for aggressive potential of TGCT.
Keywords: core 2 N-acetylglucosaminyltransferase-1, testicular germ cell tumor, metastasis
Testicular germ cell tumor (TGCT) is the most common malignancy in young males, and the frequency has risen over the recent decades.1,2 Studies of the cell surface antigens of germ cell tumors in the 1980s revealed that majority of them are carbohydrate molecules such as the stage-specific embryonic antigens.3,4 They can be detected by specific monoclonal antibodies, and their localization and essential roles in differentiation have been elucidated. However, their roles in tumor progression and metastasis remain unclear.
Aberrant glycosylation of tumors defines the stage, direction and fate of tumor progression, and the expression of specific carbohydrate epitopes in certain tumors affects invasive and metastatic potential. The correlation between glycosylation and malignancy and/or metastatic potential varies largely between tumor types.5–7 We have studied the roles of glycolipid in TGCT.8–10 We found that Gb3 glycolipid expression was upregulated in TGCT,8 and that galactosylgloboside might be an indicator of recurrence in seminoma10; however, we were unable to demonstrate the role of glycolipids in the metastatic potential of TGCT. We then focused on finding a relationship between O-glycans and clinical features in patients with TGCT.
Core 2 β1,6-N-acetylglucosaminyltransferase-1 (C2GnT-1) is a key enzyme in the formation of core 2 branched O-glycans (Galβ1 → 3GlcNAcβ1 → 6GalNAcα → Ser/Thr) by catalyzing the transfer of GlcNAc from UDP-GlcNAc with a β1,6-linkage to the α-GalNAc of core 1 O-glycan (Galβ1 → 3GalNAcα → Ser/Thr). Sialyl Lewis A as well as sialyl Lewis X present in the nonreducing terminals of O-glycans are formed via this particular branch structure,11 and C2GnT-1 cDNA was isolated from human promyelocytic leukemia HL-60 cells by expression cloning.12 Previously, we showed that expression of core 2 branched O-glycans is closely correlated with the malignant potential of colorectal cancer cells13 and pulmonary adenocarcinoma.14
In this study, we first examined the expression of C2GnT-1 immunohistochemically using paraffin-embedded orchiectomy specimens to determine the relationship between C2GnT-1 expression and aggressive potential of human TGCT. We then tested whether gene transfer of C2GnT-1 promotes the aggressive potential of human TGCT cell line.
Material and Methods
Human patients
Consecutive 130 patients with TGCT treated at the Department of Urology, Tohoku University Hospital, Sendai, Japan, were enrolled in this study. Sixty-five patients had seminoma and 65 had non-seminomatous germ cell tumor (NSGCT). Formalin-fixed paraffin-embedded tumor specimens obtained by routine orchiectomy were subjected to immunohistochemistry using anti-C2GnT-1 antibody.15 C2GnT-1 expression was compared to the clinical outcome. Informed consent was obtained from each patient. The Ethical Committee of Hirosaki University School of Medicine approved the protocol of this study. Clinical stage was determined according to the American Joint Committee on Cancer staging system.16
Patient follow-up
Patients with stage I disease were followed according to routine surveillance policy (i.e., observation alone after radical orchiectomy)17 until relapse was detected. Serum chemistry and tumor marker (hCG, AFP) were examined every 3 months, computed tomography was performed every 3 months for first 2 year after orchiectomy. Quarterly follow-up of biochemical exam and radiological studies continued at least 5 years. Recurrences were diagnosed when tumor marker rose and/or tumor detection of computed tomography. The median follow-up period was 52 months (range, 13–144). Primary chemotherapy with 3–4 cycles’ bleomycin and etopside plus cisplatin according to the IGCCCG risk classification is the treatment of choice in advanced disease. Postchemotherapy retroperitoneal lymph node dissection (RPLND) was performed in cases of residual disease and salvage therapy should be performed for standard therapy resistance disease.
Immunohistochemistry
Deparaffinized specimens were incubated with in-house produced rabbit anti-human C2GnT-1 polyclonal antibody15 as the primary antibody. Anti-rabbit immunoglobulin antibody conjugated with horseradish peroxidase (Nichirei, Tokyo, Japan) was used as the secondary antibody, and peroxidase activity was visualized with aminoethylcarbazol (AEC) solution (Nichirei, Tokyo, Japan). A control experiment was done by omitting the primary antibody from the staining procedure. Noncancerous germ cells served as the internal negative control. Based on the staining status of the Golgi apparatus, specimens with 10% or more positive cancer cells were judged as C2GnT-1 positive by single experienced pathologist who was blind to the clinical outcome.
Cell line
JKT-1 is a human TGCT cell line provided by Dr. Keigo Kinugawa (Department of Urology, Kawasaki Medical School, Kurashiki, Japan).18 This cell line was maintained in α-minimal essential medium (α-MEM) containing 10% fetal bovine serum (FBS) in a humidified 5% CO2 atmosphere at 37° C.
Plasmid preparation
The plasmid pcDNA3-C2GnT-1 was constructed by first isolating a 2.0 kb BamHI-XhoI human C2GnT-1 fragment containing the entire coding region.12 This fragment cDNA was then cloned into pcDNA3 (neo) plasmid vector.
Establishment of stable transfectants
JKT-1 cells, which do not express C2GnT-1, were transfected with pcDNA3-C2GnT-1 using LipofectAMINE (Invitrogen, Carlsbad, CA) as described in the protocol provided by the supplier. After 2 weeks in G418 selection (400 µg/ml; Gibco-BRL, Grand Island, NY), 20 single colonies were used for immunochemical detection of C2GnT-1 using anti-C2GnT-1 polyclonal antibody. Five stable transfectants expressing C2GnT-1 were established (JKT-1-C2). Among the stable transfectants, 2 clones (JKT-1-C2-1 and -2) were subjected to tumor assays. Mock transfectant (vector only) JKT-1-pcDNA3 cells were used as the control cell line.
Immunohistochemistry of JKT-1 cells
JKT-1 cells were cultured on glass coverslips and fixed with 1% paraformaldehyde in phosphate-buffered saline. The cells were subjected to immunocytochemistry using by anti-C2GnT-1 antibody. The staining procedure was same as immunohistochemistry described earlier.
Flow cytometric analysis
JKT-1-C2 cells and mock transfectant cells underwent fluorescence-activated cell sorting (FACS) analysis after incubation with anti-C2GnT antibody, anti-sialyl Lewis X monoclonal antibody (CSLEX-1) and anti-sialyl Lewis A monoclonal antibody (CSLEA) followed by incubation with FITC-conjugated secondary antibodies. Without FITC-conjugated secondary antibody was assumed to be negative control. Analyses were carried out by FACSCalibur flow cytometry using the Cell Quest program (Becton-Dickinson, San Jose, CA).
In vitro growth kinetics
JKT-1-C2 cells and mock transfectant cells were seeded in 96-well plates at 105 cells/ml in α-MEM containing 10% FBS and 400 µg/ml of G418 and cultured several times. The number of living cells was measured each day using a Cell Counting Kit (Wako Pure Chemical Industries, Tokyo, Japan). Triplicate cultures were used for each analysis.
Invasion assay
A transwell cell culture chamber (Costar, Cambridge, MA) was used for in vitro invasion assays with modifications.19 Briefly, the bottom of the upper chamber was sealed with a polyvinylpyrrolidone-free polycarbonate filter with a pore size of 8 µm. The upper face of the filter was covered with 100 µg/ml of Matrigel (Collaborative Research, Bedford, MA). The lower face was covered with 50 µg/ml of fibronectin (Wako Pure Chemical Industries, Tokyo, Japan) in α-MEM medium. Cells (1 × 105) were plated in the upper chamber and incubated in a humidified CO2 incubator at 37°C for 4 hr. The lower chamber was filled with serum-free α-MEM medium. Cells that did not migrate through the membrane were removed, and the cells that migrated to the lower face of the membrane were fixed with methanol followed by Giemsa staining. The number of cells on the lower face was counted under a microscope. The mean number of 10 different fields was plotted. These assays were carried out in triplicate. The standard deviation of the values was always within 5%.
Tumor challenge
JKT-1-C2-1 and mock transfectants were injected into the testis or via the tail vein. Balb/c nude (nu/nu) mice, 6- to 8-week-old males obtained from CLEA JAPAN (Tokyo, Japan), were used for tumor cell injection. The mice were anesthetized with avertin, and 2 × 106 JKT-1-C2-1 cells and mock-transfected JKT-1 cells were suspended in 100 µl of serumfree α-MEM and inoculated bilaterally into the testis or into the tail vein using a 30G fine needle. Four weeks later, the mice were sacrificed, and the testis and visceral organs were removed. In the case of tail vein injection, the lungs were removed and fixed with Bouin’s solution, and the metastatic lung foci number was counted under a dissecting microscope. Metastatic nodule larger than 1 mm was detected as valuable focus.
Statistics
The χ2 test was used to assess the association between C2GnT-1 expression and clinical stage. Recurrence-free survival in patients with stage I disease was estimated by Kaplan-Meier curves. Differences between groups were evaluated using the log rank test. Other statistical analyses for in vitro and in vivo experiments were done using Mann-Whitney’s U-test.
Results
Positive correlation of C2GnT-1 expression with clinical stage
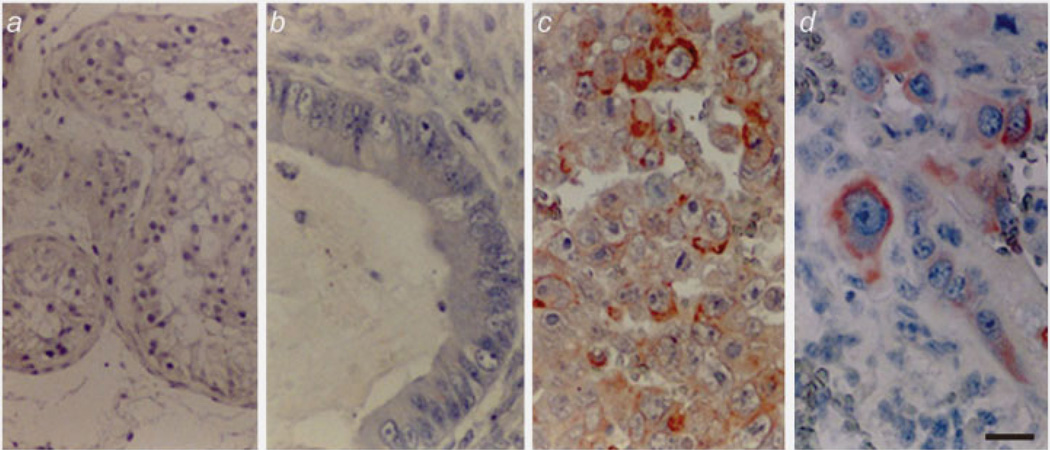
We examined 130 tumor specimens immunohistochemically from patients with TGCT (Table 1). C2GnT-1 was found to be positive in 12/43 (28%), 8/13 (62%), 8/9 (89%) in stage I, II, III seminoma, respectively. In NSGCT, C2GnT-1 was found to be positive in 9/28 (28%), 13/13 (100%), 21/24 (88%) in stage I, II, III, respectively. C2GnT-1 was more often positive in embryonal carcinoma and choriocarcinoma. Reflecting these findings, C2GnT-1 was much positive in 50/59 patients (84.7%) in higher stages (II + III) than 21/71 (29.5%) in stage I (p < 0.001). This significant difference was also found when the cases were divided into seminoma and NSGCT according to histopathological classification (Table 1). Representative photographs of immunohistochemistry are shown in Figure 1.
Table 1.
C2GnT-1 expression and clinical stage
| Clinical stage3 | ||||||
|---|---|---|---|---|---|---|
| N | Age | I | II | III | p value | |
| All | 130 | 31.8 ± 10.1 | 71 | 26 | 33 | |
| Semnoma | 65 | 34.3 ± 8.23 | 43 | 13 | 9 | |
| Relapsed/died | 7/0 | 0/0 | 4/2 | |||
| C2GnT-1 positive/all (%) | 12/43 (28) | 8/13 (62) | 8/9 (89) | 0.0012 | ||
| NSGCT1 | 65 | 29.4 ± 11.1 | 28 | 13 | 24 | |
| Embryonal carcinoma | 3 | 2 | 7 | |||
| Yolk sac carcinoma | 2 | 1 | 0 | |||
| Choriocarcinoma | 0 | 0 | 1 | |||
| Mixed | 18 | 8 | 15 | |||
| Relapsed/died | 9/0 | 2/1 | 10/7 | |||
| C2GnT-1 positive/all (%) | 9/28 (28) | 13/13 (100) | 21/24 (88) | 0.0012 | ||
Non-seminomatous germ cell tumor.
Statistical significance between stage I and II + III.
AJCC 6, American Joint Committee on Cancer, 6th edition, 2002.
Figure 1.
Immunohistochemistry of human TGCT using anti-C2GnT-1. Normal testis (a) and teratoma of low-grade malignancy (b) were negative for anti-C2GnT-1. Embryonal carcinoma (c) and choriocarcinoma (d), which have high malignant potential, were strongly positive for anti-C2GnT-1. Scale bar: 10 µm.
C2GnT-1- positive cases had higher risk for recurrence in patients with stage I disease
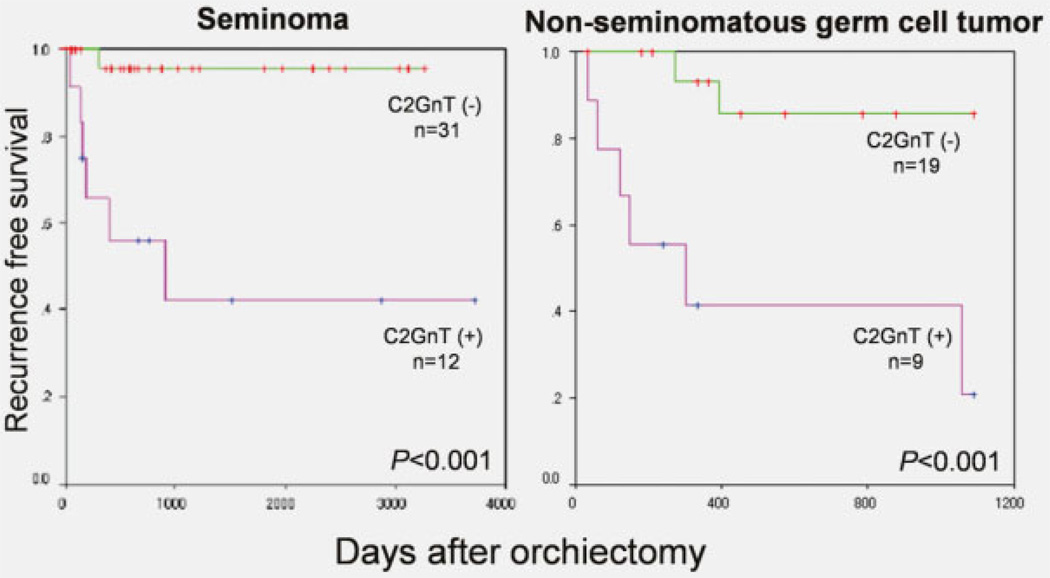
Kaplan-Meier plots and the log rank test showed that in the patients with stage I seminoma and NSGCT, C2GnT-1-positive cases had a higher risk for recurrence (p < 0.001) (Fig. 2). Because almost of all the cases of stage II and III were positive in G2GnT-1, there was no prognostic significance in stage II and III disease.
Figure 2.
Recurrence-free survival and C2GnT-1 expression in stage I disease. In patients with stage I seminoma and NSGCT, C2GnT-1-positive cases had a significantly higher risk for recurrence.
Immunocytochemical and flowcytometric analysis of JKT-1-C2 cells
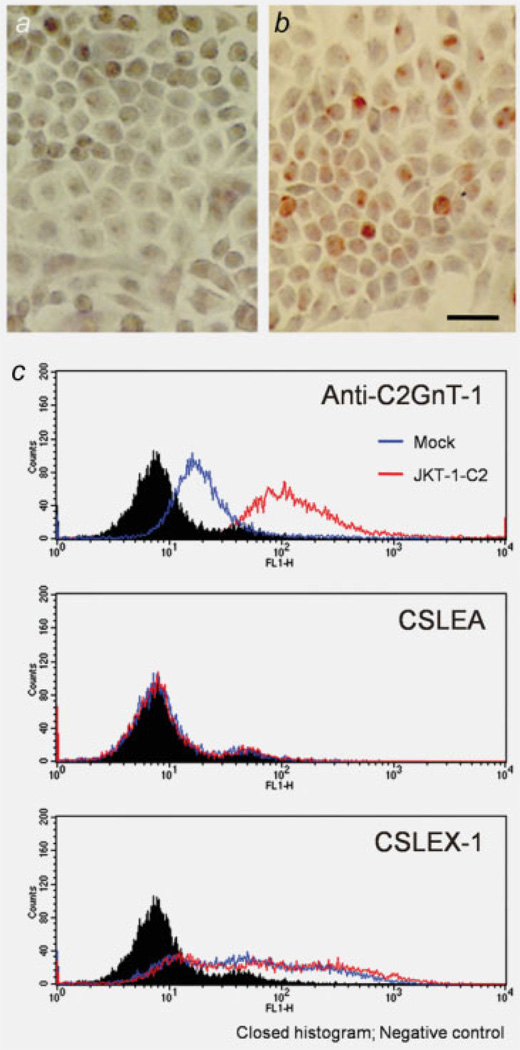
Closed histograms were negative control, open histograms of blue and red were JKT-1-mock and JKT-1-C2 cells, respectively. As shown in Figures 3a and 3c, top, open histogram of blue, the parent JKT-1 cells did not express C2GnT-1. After transfection of mammalian expression vector harboring C2GnT-1 cDNA, these transfected cells expressed C2GnT-1 and were designated JKT-1-C2 (Figs. 3b and 3c, top). These findings indicating the transfection of C2GnT-1 did not influence to the expression pattern of sialyl Lewis A and X (Fig. 3c, middle and bottom). We used anti-C2GnT-1 antibodies to screen stable transfectants, and 5 independent stably transfected JKT-1-C2 cell lines were isolated.
Figure 3.
Immunocytochemical and flow cytometric analyses of JKT-1 cells. On immunocytochemistry using anti-C2GnT-1, the parent JKT-1 cells did not express C2GnT-1 (a), CSLEA (c, middle) but positive in CSLEX-1 (c, bottom). After transfection of mammalian expression vector harboring C2GnT-1 cDNA, these transfected cells became positive for C2GnT-1 (b). C2GnT-1 was also detected in JKT-1-C2 by flowcytometric analysis. Transfection of C2GnT-1 did not influence to the expression pattern of sialyl Lewis A and X (c, middle, bottom). Closed histograms were negative control, open histograms of blue and red were JKT-1-mock and JKT-1-C2 cells, respectively. Scale bar: 10 µm.
In vitro growth kinetics
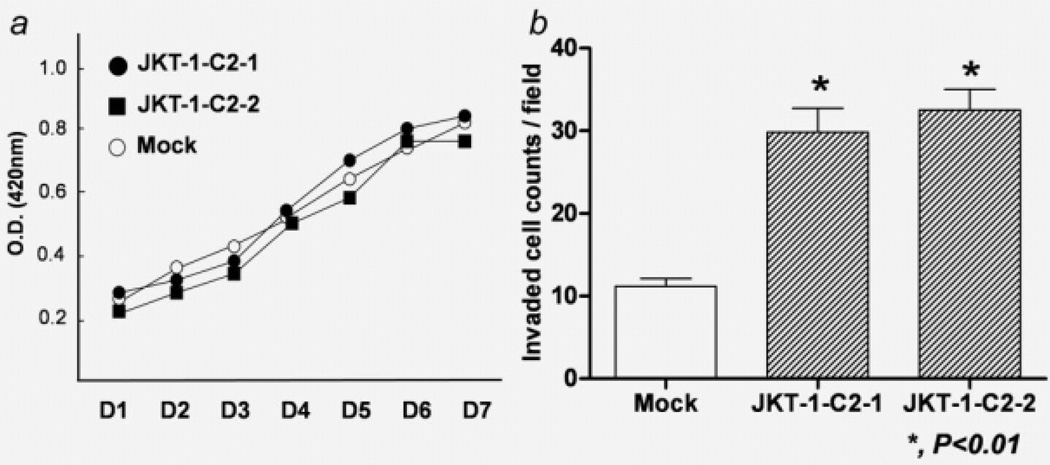
Because malignancy is closely associated with cell proliferation activity, we checked the in vitro growth kinetics of the cells. The results showed no significant difference between the viable cell number of JKT-1-C2 cells and mock transfectants during in vitro culture (Fig. 4a).
Figure 4.
In vitro growth curve and invasion assay. There were no significant differences in proliferation potential between mock transfectants and JKT-1-C2 cells (a). JKT-1-C2-1 and -2 cells had higher invasion potential than mock transfectants (b).
Enhancement of invasion potential in JKT-1-C2 cells
JKT-1-C2 cells and mock transfectants were subjected to invasion assays using a transwell cell culture system. These assays revealed that JKT-1-C2 cells are more invasive than JKT-1-mock cells (Fig. 4b). The number of invasive cells with a mean ± SD/fields were significantly larger in JKT-1-C2-1 (31.6 ± 6.18) and JKT-1-C2-2 (32.5 ± 3.53) than mock transfectant cells (10.8 ± 3.54) (p < 0.01). The possibility of clonal deviation in these results is excluded, as the differences between JKT-1-C2 clones were not significant.
JKT-1-C2 cells produced larger tumors with mesenteric metastasis through orthotopic inoculation and large number of metastatic lung foci through tail vein injection
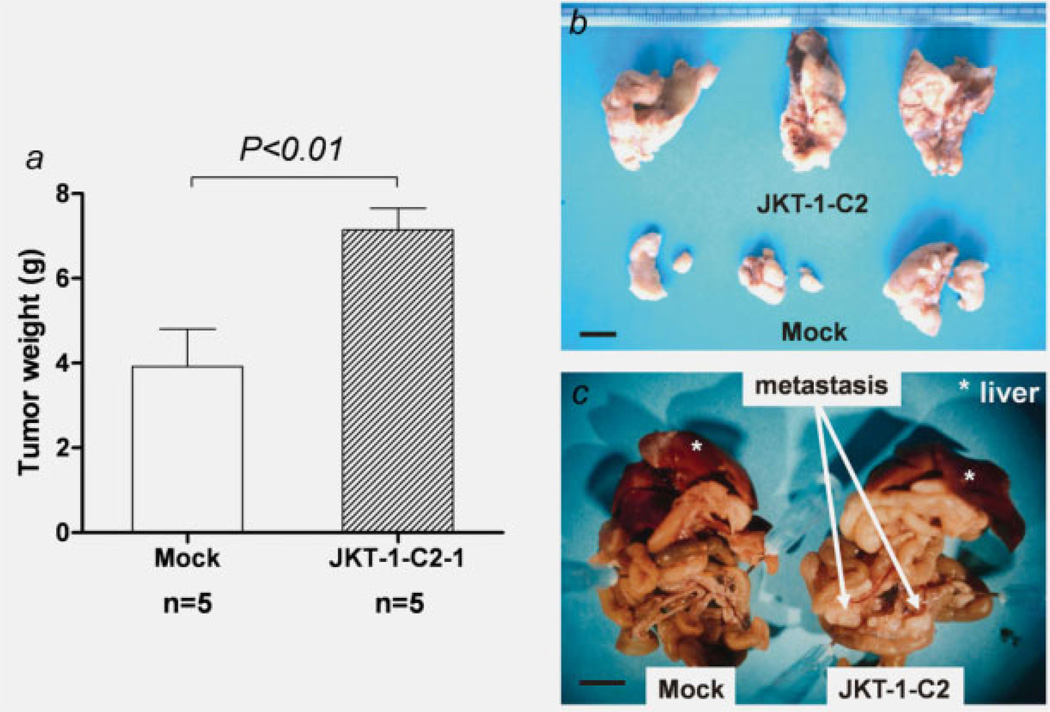
As the clinical data (Table 1, Fig. 2) indicated a strong correlation between C2GnT-1 positivity and malignant potential, we investigated whether JKT-1-C2 cells have aggressive potentials in athymic nude mice. The weights of tumor with a mean ± SD gram were significant larger in JKT-1-C2 cells (7.14 ± 1.15 g, n = 5) extending to the peritoneum in all mice, while mock transfectants produced small tumors (3.92 ± 1.97 g, n = 5; p < 0.01; Figs. 5a and 5b). Mesenteric metastasis was noted in all mice orthotopically inoculated with JKT-1-C2 cells, while there were no such findings in mice inoculated with JKT-1-mock cells (Fig. 5c).
Figure 5.
Tumor formation by orthotopic inoculation. Mice were given bilateral intra-testicular injections of 2 × 106 JKT-1-C2-1 cells or mock-transfected JKT-1. JKT-1-C2-1 cells produced huge tumors extending to the retroperitoneal space, while mock transfectants produced small tumors (a, b). JKT-1-C2-1 cells also produced extensive mesenteric metastasis (arrows), while there were no such findings in mice inoculated with mock transfectants (c). Scale bar: 1 cm.
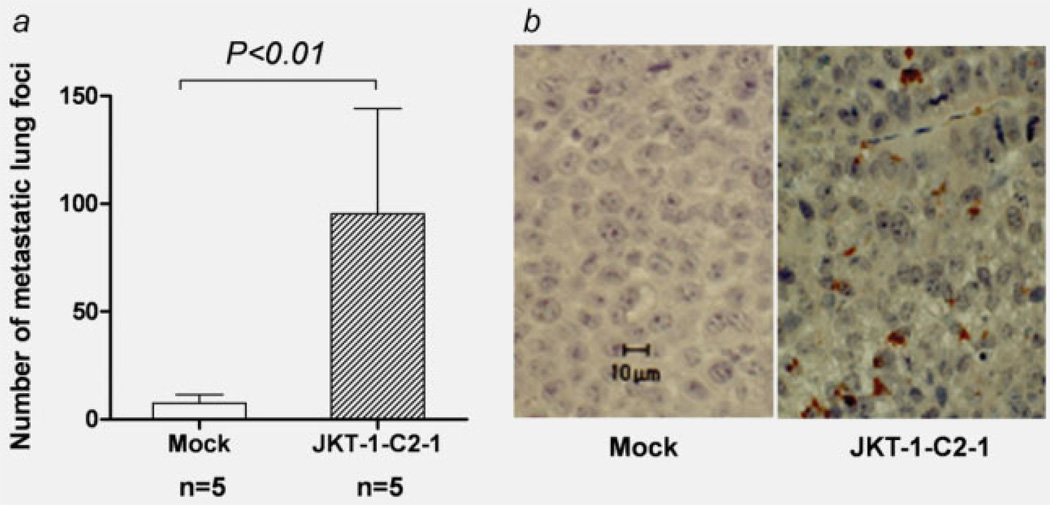
When injected via the tail vein, the number of metastatic lung foci with a mean ± SD were significant increased in JKT-1-C2-1 (95.3± 84.5, n = 5) compared to JKT-1-mock cells (7.6 ± 7.79, n = 5; p < 0.01; Fig. 6a). Immunohistochemistry using anti-C2GnT-1 antibody revealed that metastatic tumors in the mice injected with JKT-1-C2 were positive for C2GnT-1 even after 4 weeks of the injection (Fig. 6b).
Figure 6.
Metastatic lung foci with tail vein injection. When injected via the tail vein, JKT-1-C2-1 cells produced a number of metastatic lung foci compared to the mock transfectants (a). Immunohistochemistry using anti-C2GnT-1 antibody revealed that the metastatic tumors in mice injected with JKT-1-C2-1 were positive for C2GnT-1 (b). Scale bar: 10 µm.
Discussion
TGCTs are broadly divided into 2 groups: seminomas and NSGCT.20 Despite its aggressive potential, recent advances in the treatment of TGCT have achieved a remarkable cure rate of more than 95%.2 However, there are still major controversies concerning its management, especially in stage I disease.16,21,22 This controversy is at least partly caused by the lack of a useful biomarker to enable precise prediction of recurrence and risk stratification. Until date, several clinico-pathologic parameters have been advocated as predictors of recurrence in seminoma23 and NSGCT.24 However, their clinical usefulness is still limited. If there were a potential biomarker, management of stage I TGCT would be dramatically improved.
Cancer cells often express surface carbohydrates different from normal cell and expression of O-linked core structure defines tumor cell malignancy. Overexpression of core 2 branched O-glycans is seen in breast and prostate cancer cells.27,28 Previously, we demonstrated that expression of core 2 branched O-glycans in colorectal cancer is closely correlated with malignant potential13 and that expression of C2GnT mRNA was significantly enhanced in association with malignant transformation of lung cancer.14 In the present study, we tested whether this is also the case with TGCT. By contrast, core 3 branched O-glycans is synthesized in normal cells but apparently downregulated in gastric, fibrosarcoma, colorectal and prostate cancer cells.29–32 Lee et al. mentioned upregulation of core 3 syntheses decreased core 1 syntheses.32 These findings suggest that core 2 and core 3 syntheses may conflict in biosynthetic pathways of mucintype O-glycan but not all mechanisms are revealed, and future studies should define the mechanisms.
The present results of immunohistochemistry revealed that C2GnT-1 expression is closely related to clinical tumor stage in human TGCT. This finding was noted in both seminoma and NSGCT, which suggests a possible clinical application of C2GnT-1 as a novel biomarker for malignant potential. Furthermore, C2GnT-1 expression showed a strong correlation with tumor recurrence in patients with stage I disease. C2GnT-1 expression may be a promising biomarker for TGCT in clinical settings.
JKT-1-C2 was characterized by its highly aggressive potential in vitro and in vivo. Interestingly, in vitro cell kinetics was not influenced by C2GnT-1 gene transfer. Thus, it was suggested that the mechanism of enhanced aggressive potentials may be caused by interaction between the alteration of cell surface carbohydrates and their ligands.
It is widely accepted that selectin-dependent cell adhesion is of vital importance in blood-borne metastasis.6 Cell surface glycoproteins decorated with sialylated, fucosylated epitopes such as sialyl Lewis X are ligands for selectins. Not only sialyl Lewis X terminal moieties but also proximal core 2 structures generated by C2GnT-1 contribute to the formation of binding epitopes for selectins.25 In the present FACS analysis, however, neither sialyl Lewis X nor sialyl Lewis A was changed following gene transfer of C2GnT-1. These findings suggest that the as yet unidentified structures or the core 2 branched structure itself may serve as physiologically relevant ligands for selectins.26 It is necessary to determine possible roles of core 2 branched structure in cancer progression.
In the present invasion assay, overexpression of C2GnT-1 enhanced invasion potential of human TGCT cell line. Little is known about the interesting relationship between cell surface oligosaccharide alteration and invasion potential. This mechanism should also be addressed in the next study.
Although precise mechanism of the induced aggressive features of human TGCT by C2GnT-1 overexpression is unclear, C2GnT-1 has considerable use as a biomarker representing the malignant potential of human TGCT.
Acknowledgments
Grant sponsor: Japan Society for the Promotion of Science; Grant number: 21791483; Grant sponsor: CREST, Japan Science and Technology Agency; Grant number: 2310080004
Abbreviations
- AEC
aminoethylcarbazol
- C2GnT-1
core 2 N-acetylglucosaminyltransferase-1
- CSLEA
anti-sialyl Lewis A monoclonal antibody
- CSLEX-1
anti-sialyl Lewis X monoclonal antibody
- FBS
fetal bovine serum
- Gal
β-d-galactose
- GalNAc
β -d-N-acetylgalactosamine
- GlcNAc
β-d-N-Acetylglucosamine
- JKT-1
human TGCT cell line
- NSGCT
non-seminomatous germ cell tumor
- Ser
serine
- TGCT
testicular germ cell tumor
- Thr
threonine
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun M. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242–253. doi: 10.1056/NEJM199707243370406. [DOI] [PubMed] [Google Scholar]
- 3.Shevinsky LH, Knowles BB, Damjanov I, Solter D. Monoclonal antibody to murine embryos defines a stage-specific embryonic antigen expressed on mouse embryos and human teratocarcinoma cells. Cell. 1982;30:697–705. doi: 10.1016/0092-8674(82)90274-4. [DOI] [PubMed] [Google Scholar]
- 4.Kannagi R, Cochran NA, Ishigami F, Hakomori S, Andrews PW, Knowles BB, Solter D. Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2:2355–2361. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 6.Fukuda M. Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 1996;56:2237–2244. [PubMed] [Google Scholar]
- 7.Dennis JW, Granovsky M, Warren CE. Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta. 1999;1473:21–34. doi: 10.1016/s0304-4165(99)00167-1. [DOI] [PubMed] [Google Scholar]
- 8.Ohyama C, Fukushi Y, Satoh M, Saitoh S, Orikasa S, Nudelman E, Straud M, Hakomori S. Changes in glycolipid expression in human testicular tumor. Int J Cancer. 1990;45:1040–1044. doi: 10.1002/ijc.2910450610. [DOI] [PubMed] [Google Scholar]
- 9.Ohyama C, Orikasa S, Satoh M, Saito S, Ohtani H, Fukushi Y. Globotriaosylceramide glycolipid in seminoma: its clinicopathological importance in differentiation from testicular malignant lymphoma. J Urol. 1992;148:72–75. doi: 10.1016/s0022-5347(17)36513-8. [DOI] [PubMed] [Google Scholar]
- 10.Ohyama C, Orikasa S, Kawamura S, Satoh M, Saito S, Fukushi Y, Levery SB, Hakomori S. Galactosylgloboside expression in seminoma. Inverse correlation with metastatic potential. Cancer. 1995;76:1043–1050. doi: 10.1002/1097-0142(19950915)76:6<1043::aid-cncr2820760619>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.Schachter H, Brockhausen I. The biosynthesis of serine (threonine)-N-acetylgalactosamine-linked carbohydrate moieties. In: Allen HJ, Kisailus EC, editors. Glycoconjugates: composition, structure and function. New York: Marcel Dekker; 1992. pp. 263–332. [Google Scholar]
- 12.Bierhuizen MF, Fukuda M. Expression cloning of a cDNA encoding UDP-GlcNAc:Gal beta 1–3-GalNAc-R (GlcNAc to GalNAc) beta 1–6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc Natl Acad Sci USA. 1992;89:9326–9330. doi: 10.1073/pnas.89.19.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimodaira K, Nakayama J, Nakamura N, Hasebe O, Katsuyama T, Fukuda M. Carcinoma-associated expression of core 2 beta-1,6-N-acetylglucosaminyltransferase gene in human colorectal cancer: role of O-glycans in tumor progression. Cancer Res. 1997;57:5201–5206. [PubMed] [Google Scholar]
- 14.Machida E, Nakayama J, Amano J, Fukuda M. Clinicopathological significance of core 2 beta1,6-N-acetylglucosaminyltransferase messenger RNA expressed in the pulmonary adenocarcinoma determined by in situ hybridization. Cancer Res. 2001;61:2226–2231. [PubMed] [Google Scholar]
- 15.Skrincosky D, Kain R, El-Battari A, Exner M, Kerjaschki D, Fukuda M. Altered Golgi localization of core 2 beta-1,6-N-acetylglucosaminyl-transferase leads to decreased synthesis of branched O-glycans. J Biol Chem. 1997;272:22695–22702. doi: 10.1074/jbc.272.36.22695. [DOI] [PubMed] [Google Scholar]
- 16.Fleming ID, Greene FL, Balch CM, Fritz A, Haller DG, Morrow M, Page DL. AJCC cancer staging manual. 6th edn. New York: Springer-Verlag; 2002. p. 317. [Google Scholar]
- 17.Raghavan D. Active surveillance for stage I testis cancer: attaining maturity at 21 years. Eur J Cancer. 2000;36:1891–1494. doi: 10.1016/s0959-8049(00)00163-5. [DOI] [PubMed] [Google Scholar]
- 18.Kinugawa K, Hyodo F, Matsuki T, Jo Y, Furukawa Y, Ueki A, Tanaka H. Establishment and characterization of a new human testicular seminoma cell line, JKT-1. Int J Urol. 1998;5:282–287. doi: 10.1111/j.1442-2042.1998.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 20.Ulbright TM. Germ cell neoplasms of the testis. Am J Pathol. 1993;17:1075–1091. doi: 10.1097/00000478-199311000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Einhorn LH. Testicular cancer: an oncological success story. Clin Cancer Res. 1997;3:2630–2632. [PubMed] [Google Scholar]
- 22.Pont J, Albrecht W, Postner G, Sellner F, Angel K, Holtl W. Adjuvant chemotherapy for high-risk clinical stage I nonseminomatous testicular germ cell cancer: long-term results of a prospective trial. J Clin Oncol. 1996;14:441–448. doi: 10.1200/JCO.1996.14.2.441. [DOI] [PubMed] [Google Scholar]
- 23.Warde P, Specht L, Horwich A, Oliver T, Panzarella T, Gospodarowicz M, von der Maase H. Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J Clin Oncol. 2002;20:4448–4452. doi: 10.1200/JCO.2002.01.038. [DOI] [PubMed] [Google Scholar]
- 24.Vergouwe Y, Steyerberg EW, Eijkeman MJ, Albers P, Habbema JD. Predictors of occult metastasis in clinical stage I nonseminoma: a systematic review. J Clin Oncol. 2003;21:4092–4099. doi: 10.1200/JCO.2003.01.094. [DOI] [PubMed] [Google Scholar]
- 25.Renkonen J, Rabina J, Mattila P, Grenman R, Renkonen R. Core 2 beta1,6-N-acetylglucosaminyltransferases and alpha1,3-fucosyl-transferases regulate the synthesis of O-glycans on selectin ligands on oral cavity carcinoma cells. APMIS. 2001;109:500–506. doi: 10.1111/j.1600-0463.2001.apm090703.x. [DOI] [PubMed] [Google Scholar]
- 26.Wagers AJ, Stoolman LM, Craig R, Knibbs RN, Kansas GS. An sLex-deficient variant of HL60 cells exhibits high levels of adhesion to vascular selectins: further evidence that HECA-452 and CSLEX1 monoclonal antibody epitopes are not essential for high avidity binding to vascular selectins. J Immunol. 1998;160:5122–5129. [PubMed] [Google Scholar]
- 27.Dalziel M, Whitehouse C, McFarlane I, Brockhausen I, Gschmeissner S, Schwientek T, Clausen H, Burchell JM, Taylor-Papadimitriou J. The relative activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine O-glycan structure and expression of a tumorassociated epitope on MUC1. J Biol Chem. 2001;276:11007–11015. doi: 10.1074/jbc.M006523200. [DOI] [PubMed] [Google Scholar]
- 28.Hagisawa S, Ohyama C, Takahashi T, Endoh M, Moriya T, Nakayama J, Arai Y, Fukuda M. Expression of core 2 beta1,6-N-acetylglucosaminyltransferase facilitates prostate cancer progression. Glycobiology. 2005;15:1016–1024. doi: 10.1093/glycob/cwi086. [DOI] [PubMed] [Google Scholar]
- 29.Vavasseur F, Dole K, Yang J, Matta KL, Myerscough N, Corfield A, Paraskeva C, Brockhausen I. O-glycan biosynthesis in human colorectal adenoma cells during progression to cancer. Eur J Biochem. 1994;222:415–424. doi: 10.1111/j.1432-1033.1994.tb18880.x. [DOI] [PubMed] [Google Scholar]
- 30.Iwai T, Kudo T, Kawamoto R, Kubota T, Togayachi A, Hiruma T, Okada T, Kawamoto T, Morozumi K, Narimatsu H. Core 3 synthase is down-regulated in colon carcinoma and profoundly suppresses the metastatic potential of carcinoma cells. Proc Natl Acad Sci USA. 2005;102:4572–4577. doi: 10.1073/pnas.0407983102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vavasseur F, Yang JM, Dole K, Paulsen H, Brockhausen I. Synthesis of O-glycan core 3: characterization of UDP-GlcNAc: GalNAc-R beta 3-N-acetylglucosaminyltransferase activity from colonic mucosal tissues and lack of the activity in human cancer cell lines. Glycobiology. 1995;5:351–357. doi: 10.1093/glycob/5.3.351. [DOI] [PubMed] [Google Scholar]
- 32.Lee SH, Hatakeyama S, Yu SY, Bao X, Ohyama C, Khoo KH, Fukuda MN, Fukuda M. Core3 O-glycan synthase suppresses tumor formation and metastasis of prostate carcinoma PC3 and LNCaP cells through down-regulation of alpha2beta1 integrin complex. J Biol Chem. 2009;284:17157–17169. doi: 10.1074/jbc.M109.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]