Summary
Neurodegenerative diseases are a horrendous burden for their victims, their families, and society as a whole. For half a century scientists have pursued the hypothesis that these diseases involve a chronic viral infection in the brain. However, efforts to consistently detect a specific virus in brains of patients with such diseases as Alzheimer’s or multiple sclerosis have generally failed. Neuropathologists have become increasingly aware that most patients with neurodegenerative diseases demonstrate marked deterioration of the brain olfactory bulb in addition to brain targets that define the specific disease. In fact, the loss of the sense of smell may precede overt neurological symptoms by many years.
This realization that the olfactory bulb is a common target in neurodegenerative diseases suggests the possibility that microbes and/or toxins in inhaled air may play a role in their pathogenesis. With regard to inhaled viruses, neuropathologists have focused on those viruses that infect and kill neurons. However, a recent study shows that a respiratory virus with no neurotropic properties can rapidly invade the mouse olfactory bulb from the nasal cavity. Available data suggest that this strain of influenza is passively transported to the bulb via the olfactory nerves (mechanism unknown), and is taken up by glial cells in the outer layers of the bulb. The infected glial cells appear to be activated by the virus, secrete proinflammatory cytokines, and block further spread of virus within the brain. At the time that influenza symptoms become apparent (15 h post-infection), but not prior to symptom onset (10 h post-infection), proinflammatory cytokine-expressing neurons are increased in olfactory cortical pathways and hypothalamus as well as in the olfactory bulb. The mice go on to die of pneumonitis with severe acute phase and respiratory disease symptoms but no classical neurological symptoms. While much remains to be learned about this intranasal influenza-brain invasion model, it suggests the hypothesis that common viruses encountered in our daily life may initiate neuroinflammation via olfactory neural networks. The numerous viruses that we inhale during a lifetime might cause the death of only a few neurons per infection, but this minor damage would accumulate over time and contribute to age-related brain shrinkage and/or neurodegenerative diseases. Elderly individuals with a strong innate inflammatory system, or ongoing systemic inflammation (or both), might be most susceptible to these outcomes. The evidence for the hypothesis that common respiratory viruses may contribute to neurodegenerative processes is developed in the accompanying article.
Hypothesis
Classical neurodegenerative disorders (NDs), such as Alzheimer’s and Parkinson’s, are most commonly seen in the elderly and are increasing in incidence in the developed world as the population lives longer [1]. Millions of people are affected by NDs [2], which are generally fatal. Some NDs have been associated with viral infections [1], [3], [4] and are commonly assumed to involve a chronic viral infection of the brain. However, repeated efforts to consistently detect specific virions, viral proteins, or viral nucleic acid in the brains of ND patients, even when highly sensitive molecular techniques are used, have generally failed [5], [6], [7], [8].
Extensive clinical evidence indicates that olfactory bulb (OB) degeneration is associated with all NDs investigated so far, and that impairment of odorant detection may precede clinically-apparent NDs by as much as 10 years [9], [10]. As the OB is anatomically linked to the nasal passages via the olfactory nerves (see “Tutorial on olfactory system anatomy”), respiratory viruses could play a role in OB degeneration if they were able to traverse those nerves. Nerve transport has been demonstrated for some neurovirulent viruses (i.e., viruses that replicate in neurons, and spread from neuron to neuron [11], [12], [13]), but is generally assumed not to occur with non-neurovirulent viruses. However, highly sensitive molecular techniques have recently demonstrated that a mouse-adapted human influenza strain that is neither neurotropic nor neurovirulent can rapidly enter the mouse OB following intranasal instillation ([14], “Experimental evidence supporting the hypothesis”). In the OB the virus appears to be ‘trapped’ by glial cells ([14], “Major mechanisms of neuroinflammation” and “An influenza animal model demonstrating a gliotropic infection”) and initiate wide-spread neuroinflammation. 1 Therefore, an alternative hypothesis to ND causation by a persistent neurovirulent viral infection is that over a person’s lifetime, multiple ‘common cold’ and ‘flu-like’ viruses invade the brain from the nasal passages via the olfactory nerves. Such viruses are then captured and killed by glial cells in the OB, resulting in glial production of inflammatory mediators. These mediators, in turn, induce a cascade of proinflammatory cytokines in neurons and glia within olfactory cortical pathways and beyond with the potential to damage the neurons. The resulting brain damage is too limited to provoke classical neurological symptoms, but it accumulates over the individual’s lifetime and can potentially contribute to the development of late-life NDs in genetically- or age-susceptible individuals. The key elements of this hypothesis are summarized in Fig. 1 , and supporting evidence will be presented in “Experimental evidence supporting the hypothesis” and “Supporting evidence from the literature”.
Fig. 1.
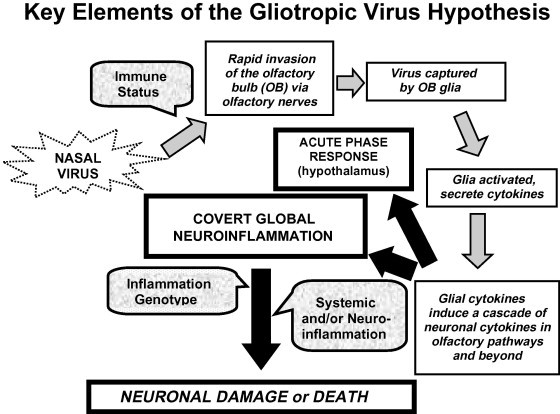
This schematic outlines the hypothetical sequence of events involved in induction of neuroinflammation by viruses associated with common colds, flu-like infections, or other routine viral infections replicating in the upper respiratory tract. Gray arrows track the key local and global processes over time. Black arrows pointing to boxes with heavy black frames indicate the ultimate outcomes of gliotropic viral invasion. Text in rounded stippled boxes identifies important host factors affecting outcomes, such as immune status (which may determine the success of the viral invasion process), inflammation genotype (which may influence the severity and persistence of neuroinflammation), or inflammation status (such as ongoing systemic or neuroinflammation from the infection itself or chronic inflammatory disorders). Underlying the hypothesis is the widely accepted concept that sufficiently severe neuroinflammation can lead to neuronal degeneration.
For the purposes of this discussion the term ND will apply both to ‘normal’ age-related brain shrinkage and senile dementia, as well as classical NDs ranging from essential tremor [18] to Alzheimer’s, recognizing that the pathogenesis of these conditions may be dissimilar [19] but that neuroinflammation contributes to all [20]. A wide variety of brain insults, both extrinsic [21] and intrinsic, can induce acute neuroinflammation (see Table 1 ); many of these insults occur through inhalation ([21], [22], Table 1) and can potentially cause OB pathology (see “Tutorial on olfactory system anatomy”). While this article will focus on commonly-encountered viruses, it is likely that any systemic inflammatory event [23], inhaled toxic chemicals ([22], [24], [25], Table 1), or head trauma [26], [27], [28] will amplify neuroinflammation by inducing additive or synergistic inflammatory events [22], [24]. Gene polymorphisms affecting inflammation severity and persistence are likely to be important players in ND development [24], [29], [30], [31], regardless of the cause. The underlying assumption of the hypothesis is that neuroinflammation, if sufficiently severe, will lead to some level of neurodegeneration [32].
Table 1.
Some common sources of neuroinflammatory stimuli.a
| Extrinsic stimuli (air-borne) | Intrinsic stimuli |
|---|---|
| Gaseous and particulate pollutants | Systemic inflammation |
| Toxic industrial chemicals, heavy metals | Dead brain cells, amyloid, other detritus |
| Pesticides and herbicides | Head trauma |
| Non-neurovirulent (gliotropic) viruses | Focal ischemia |
| Other microbes | Global ischemia |
| Neurovirulent (neurotropic) viruses | Excitatory amino acids, seizures |
The order of the listed stimuli reflects the relative frequency with which they occur in urban life.
The realization that olfactory system degeneration accompanies, or precedes, the onset of clinically-apparent NDs has led to the development of the olfactory vector hypothesis, discussed in Refs. [10], [13], [33]. However, the olfactory vector hypothesis has focused exclusively on viruses capable of replicating in, and killing, neurons [13]. The hypothesis articulated in this article instead emphasizes a central role for much more commonly experienced non-neurovirulent viruses, such as human influenza, that infect glia (i.e., are gliotropic) but not neurons.
The material presented below encompasses several massive research areas. Space limitations require that only representative examples be included – reviews are referenced when available. The discussion will start with tutorials on relevant olfactory system anatomy in “Tutorial on olfactory system anatomy” and major players in neuroinflammation in “Major mechanisms of neuroinflammation”. The limitations of traditional viral brain invasion models (“Traditional viral brain invasion models”), together with the influenza work that has led to my hypothesis, is summarized in “An influenza animal model demonstrating a gliotropic infection”. Some of the broader implications of the hypothesis are touched on in “Implications of the influenza-OB model”. Clinical evidence that common respiratory viruses affect olfactory function is summarized in “Clinical evidence of olfactory pathology caused by common respiratory viruses”. Influenza-associated pediatric brain pathologies are discussed in “Clinical evidence of brain damage in pediatric influenza infections”. Some viruses traditionally associated with human NDs are briefly discussed in “Association of viruses with human NDs”, together with recent studies of viruses interacting with glia rather than neurons. Host factors that are likely to influence susceptibility to viral brain invasion [34] or characteristics of the neuroinflammatory state [32] are described in “Host factors influencing outcomes from OB invasion by common viruses”. Limitations of the available data that support this hypothesis are summarized in “Limitations of available experimental data” together with important parameters for testing the hypothesis with other viruses (“Important parameters for investigating non-neurotropic viruses in the brain”). Then some prophylactic and therapeutic approaches to minimizing neuroinflammation caused by common viruses, and perhaps other neurological insults listed in Table 1, are briefly discussed in “Some approaches to prevention or treatment of respiratory virus-associated neuroinflammation”. The conclusions and major goals of this article are summarized in “Conclusions and goals of this article”.
Tutorial on olfactory system anatomy
This overview of the relevant spatial and organizational relationships of the olfactory system is intended to assist those readers with a limited background in olfactory neuroanatomy. Anatomical and functional details for the neuroanatomist are provided elsewhere [13], [35].
The olfactory system is comprised of (1) olfactory neurons located in the upper region of both nasal passages that, together with their associated glial and support cells, make up the olfactory nerves (ONs) [33], [35]; (2) the right and left lobes of the OB comprising the front-most region of the brain; and (3) neurons projecting from the OB to the olfactory cortex and beyond [36]. The OB that will be referenced in this article is the main OB, so-called to distinguish it from the accessory OB that processes signals from the vomeronasal organs [37].
The olfactory system senses air-borne odorants via the dendrites of the olfactory neurons lining the neuroepithelium in the uppermost nasal cavity region [35]. As odorant sensing requires a distinct dendritic receptor and olfactory network for each odorant, the olfactory receptor genes comprise the largest single gene family in the mammalian genome [35] and the ONs contain millions of axons. These axons coalesce into 15–20 large axon fascicles, or filia, that comprise the olfactory nerves [13]. The filia penetrate a perforated bony structure in the skull above the nose termed the cribriform plate. At the point where the ONs enter the cranial cavity they form the outermost layer of the OB, i.e., the ON layer. The olfactory neurons synapse in the adjacent glomerular layer with mitral neurons that form the third layer of the bulb. After odorant signals from the glomeruli are further processed within the succeeding cell layers of the multi-laminate OB, the signals are then transmitted to the rest of the brain via neurons projecting primarily to the olfactory cortex and anatomically related structures adjacent to the OB [35].
The olfactory neurons are unique in that they are the only neurons in the body with their dendritic processes directly exposed to the environment; only a thin layer of mucus protects the dendrites from substances in inhaled air [38]. Therefore the olfactory neuron dendrites (and potentially the OB) are exposed to air-borne chemical toxins and particulates as well as any infectious agents that enter the body via the nose, mouth, or eyes and replicate at some point in the nasal passages. The ONs themselves are also exposed to blood-borne pathogens because they lack a blood–nerve barrier [39].
An additional feature of the ONs is a structure not yet described in other nerves, i.e., channels derived from glia-like olfactory ensheathing cells (OECs) and the adjacent connective tissue [40]. These channels serve as support systems for olfactory neuron regeneration and have been demonstrated to transport mineral nanoparticles about 100 nm in diameter to the OB glomerular layer within 24 h [41]. Though no viruses have been shown to be transported through OEC channels, most are smaller in size than the active nanoparticles. More conventional nerve transport mechanisms employed by neurotropic viruses are discussed in Ref. [42].
Major mechanisms of neuroinflammation
Central role of glia in neuroinflammation
Glia, particularly microglia and astrocytes, form the intrinsic innate immune system of the brain. In vivo techniques now make it clear that microglia play a prominent role in neuroinflammation and serve as the convergence point for many NDs [20]. Microglia make up about 12% of the general brain mass [24] and are particularly densely distributed in the OB glomerular layer [43] and in the substantia nigra [24] targeted in Parkinson’s. Microglia express neurotransmitter receptors [44], are regulated in part by neurons [44], and produce trophic as well as toxic factors for neurons [24]. When activated by pathogens, toxins, cellular debris or excitatory amino acids microglia release a broad range of inflammatory products such as eicosenoids, metalloproteinases, proteases [45], and oxygen and nitrogen free radicals [46] capable of damaging nearby cells. Activated microglia also produce the key proinflammatory cytokines interleukin (IL)1β [47] and tumor necrosis factor (TNF)α [48]. These cytokines, particularly IL1β, are toxic for neurons in vitro and in vivo and can induce neurodegeneration [49], [50]; when combined the toxicity of the two cytokines is synergistic [51], [52]. Activated microglial products also open the blood–brain barrier to circulating inflammatory cells and blood-borne soluble mediators [53].
Recently studies have shown that another category of phagocytic glial cells is prominent in the OB glomerular layer, i.e., dendritic cells [54]. Dendritic cells and microglia are both derived from macrophages, are morphologically similar, express many of the same antigens, and cannot be distinguished from one another without uncommonly used selective stains. The role of dendritic cells in neuroinflammation is not known.
Prominent role of viruses in brain infections
The epidemiological association of viruses (rather than other infectious agents) with NDs may be related to their vigorous induction of type I interferons (IFNs). While these IFNs are known for their anti-viral action, they also have a complex role in immune regulation [55], [56]. IFNαs induce numerous genes that could be relevant to NDs, including the chemokine MIP-1α [57], [58] and IL-16, a marker for activated microglia [59]. Sustained expression of IFNα in the brain leads to neurodegeneration [60]. IFN-induced gene polymorphisms appear to determine susceptibility to multiple sclerosis [61] and chronic hepatitis C infections [31]. IFNα is not induced by bacteria or other microbes in substantial quantities [62], and therefore viruses are more likely to initiate neuroinflammatory processes driven by these mediators than are other infectious agents.
Of course, another feature of viruses is their small size relative to other pathogens, which may facilitate their entry into the OB and perhaps other brain regions as well.
Experimental evidence supporting the hypothesis
Traditional viral brain invasion models
Standard viral neuropathology models have employed large doses of viruses isolated from infected brains that are both neurotropic and neurovirulent. These animal models were originally developed to detect viruses, but over the years have morphed into neuropathology models. These models frequently employ intracerebral inoculation, or OB inoculation [63], to assure brain infection [39]. In addition, the models also commonly employ immunologically immature neonates and unnatural host species as well as the unnatural infection routes mentioned above that bypass host defenses. A comparison of herpes infection by the intranasal route (no symptoms) and the intracerebral route (encephalitis and death) [64] reveals the importance of the route used to infection outcomes.
The OB is sometimes examined in models employing neurovirulent viruses inoculated intranasally and it is generally positive for virus [12], [65], [66], [67]. It is currently customary to wait 2–3 days before examining the brain for virus in experimental models, though acute infection may occur within hours [14]. Modern molecular techniques [such as polymerase chain reaction (PCR), particularly the nested technique] increase the sensitivity of virus detection by orders of magnitude and allow detection of the viral replication products in the brain within hours of infection when gliotropic properties are most likely to be perceptible (see “Important parameters for investigating non-neurotropic viruses in the brain”).
An influenza animal model demonstrating a gliotropic infection
By using nested PCR and looking at tissue hours instead of days post-infection (PI), we have shown that immunocompetent mice demonstrate rapid (within 4 h) influenza viral invasion of the OB following intranasal inoculation, with or without anesthesia. The strain employed was a mouse-adapted human strain of a H1N1 influenza A virus, PR8 [14]. PR8 and other human influenza strains 2 have no recognized neurovirulent properties in immunocompetent animals [39].
Influenza is a minus-stranded RNA virus, and the presence of plus strand indicates that the virus is undergoing at least partial replication. Using nested PCR for both of these viral RNA strands, we see the presence of PR8 replication intermediates at 4, 7 and 15 h PI [14]. These intermediates are seen up to 96 h in the OB but are not detected in the somatosensory cortex (which lacks projections from the OB) at any time period examined [14], [71]. They are also seen at 15 h in OBs of mice bearing a functional IFN-induced Mx1 gene that inhibits viral replication and attenuates viral symptoms (Sawatski, et al., unpublished findings). At 15 h PI (the time at which the mice become clinically ill with the dose of virus used) we also see a significantly increased expression of proinflammatory cytokine mRNAs [14]. These cytokines include IL1β and TNFα, both potent and synergistic mediators of neuroinflammation (see “Major mechanisms of neuroinflammation”). We also detect mRNAs for IFN-induced enzymes [14] that serve as sensitive markers of IFN expression in the infected OB. Expression of these IFN-induced enzymes is significantly increased at 7 h PI and very strongly up-regulated at 15 h PI [14].
Immunohistochemical evidence shows that the influenza viral antigen is associated with glial cells that have an activated morphology in the OB glomerular layer [14], [36], but not in neurons or in subsequent OB laminae or cortical pathways [36]. Viral antigen is also seen in fusiform cells (possibly OECs – see “Tutorial on olfactory system anatomy”) within the olfactory nerves [36]. These immunohistochemical studies also show that neuroinflammation (in the form of an increased number of cytokine-expressing neurons) is activated in the OB, olfactory cortex (piriform and olfactory tubercle), the amygdala and the hypothalamus at the time of illness onset, but not at 10 h PI, prior to illness onset [36]. Increased neuronal expression of proinflammatory cytokines associated with the onset of the APR has not, to my knowledge, been described previously. We have not yet examined cytokine expression in the brain beyond 15 h PI, nor have glial cells been quantified or characterized beyond the OB outer layers [36].
Because viral replication is seen in the OB at 4 h PI, prior to completion of influenza replication in nasal tissues (7–8 h), the viral RNA detected in the OB is likely to represent input virus. The fact that the virus enters the OB glomerular layer suggests that it is being transported via either active axonal transport [42] or passively via OEC channels, though the minimum time required for either transport route in mice is not known. The persistence of viral replication intermediates to at least 96 h PI in the OB suggests the possibility that virus continues to enter the brain via its olfactory nerve route throughout the infection, or is replicating in a susceptible OB cell type. It is not known if complete infectious virions are formed in the OB, but (as stated above) viral antigen is detected only in the ON and OB glomerular layers [36].
Implications of the influenza-OB model
It is probable that rapid viral invasion of the OB is not unique to influenza virus, and that other respiratory agents that reach adequate concentrations in the upper nasal cavity can also enter the OB by the same mechanism. Suggestive evidence that this may occur in routine human respiratory infections is presented in “Clinical evidence of olfactory pathology caused by common respiratory viruses”. Whether the wide-spread neuroinflammation seen at the time of PR8 illness onset leads to neurodegeneration in infection-affected brain regions [72] is yet to be determined. But global neuroinflammation and neurodegeneration following a localized brain injury have been seen in a cortical impact head trauma model [73], suggesting that possibility. The up-regulation of neuronal cytokines by glial cytokines that seems to occur in our intranasal influenza model [36] suggests a mechanism by which local neuroinflammation can become global.
Over a normal lifespan an individual may experience dozens of viral infections that infect the respiratory tract, including infections with classically neurovirulent viruses that are manifested primarily as rashes (measles, mumps, rubella, varicella, etc.) or stomatis (herpes simplex). Each of these infections could potentially cause low-level neuroinflammation and neuronal loss, which would accumulate over time. While these low-level brain infections may not cause classical neurological symptoms (such as seizures), the headaches, fevers, anorexia and somnolence [or the acute phase response referenced in (Fig. 1)] commonly experienced in ‘flu-like’ infections could be related to brain inflammation. Indeed it might be appropriate to consider these common ‘flu’ symptoms as ‘neurological,’ regardless of their origin, since they clearly involve the brain.
Supporting evidence from the literature
Clinical evidence of olfactory pathology caused by common respiratory viruses
As stated in “Hypothesis”, there is a growing awareness that degeneration of the OB occurs early in many ND patients prior to onset of overt neurological symptoms [9]. Anosmia (loss of the sense of smell) or other olfaction disorders (ODs) are functional markers of OB degeneration that are seen relatively early in many ND patients [74]. Clinical ODs have been associated with recently-experienced respiratory viral infections [75], [76], including pandemic influenza [77], rhinoviruses, parainfluenza 3 virus [76], and coronaviruses [78]. Head trauma, toxin exposure, cardiac bypass and certain drugs are the other identifiable causes of ODs [75], [79], [80]. The pathogenesis of post-viral ODs is thought to result from destruction of olfactory receptor neurons within the nose, but central pathology within the OB cannot be eliminated [75].
Functional magnetic resonance imaging can map OD changes within the brain [81] but this technique has not been applied to ODs specifically associated with respiratory viral infections. Because the insipient OD is not apparent during the active infection, conducting imaging studies of the acute process is very difficult. However, imaging the OB following infection recovery may provide insights into the pathogenesis of infection-associated ODs. Clinical studies are also needed to determine if ODs early in life are commonly linked to NDs later in life.
Clinical evidence of brain damage in pediatric influenza infections
Recently an association of influenza virus with encephalopathy or encephalitis in children, particularly Japanese children, has been recognized [82]. Very recent studies suggest that a mitochondrial mutation may underlie acute influenza encephalopathy, which can be considered a form of Reye’s syndrome [83]. Similar encephalopathies have been reported in infants severely ill with respiratory syncytial virus [84] and recently. Post-influenza encephalitis occurring 3–4 weeks after influenzal disease is also seen relatively frequently in Japanese children, and is thought to be an autoimmune disorder similar to Guillain–Barré [85]. These disorders are distinct from acute influenza encephalitis/encephalopathy (manifested during the clinical respiratory infection in small children [86] and adults [3], including pandemic H1N1 2009 [87]), though all of these diseases suggest brain damage by influenza virus. Olfactory disorders have not been reported in these pediatric neurological diseases associated with influenza.
Association of viruses with human NDs
To date, no specific virus or viral family has been consistently associated with a specific human ND (with the notable exceptions of HIV dementia [88], progressive multi-focal leukoencephalopathy [89] and possibly amyotrophic lateral sclerosis [90]). This failure to detect definitive viral associations in more common NDs may simply reflect the possibility that the contributing viruses cause a “hit-and-run” neuropathology [8] via glial activation and do not persist in the brains of ND patients. This concept would also account for the wide variety of common viruses occasionally detected in both the brains of ND patients [91], [92], [93], [94] and in normal control brains as well [4], [95]. Further, the concept would account for the wide variety of viral antibodies in serum and spinal fluid of ND patients [96], [97].
Extensive epidemiologic evidence implicates influenza and other common viruses, as well as certain neurotropic parasites, in the pathogenesis of selected classical NDs (such as multiple sclerosis [98], Parkinson’s [99] 3 and Alzheimer’s [1]) and NDs better defined by behavioral changes, such as schizophrenia and bipolar disorder [94]. The most compelling evidence for an infection-ND relationship is found in schizophrenia [5], [105], [106], a disease in which progressive deterioration of neural connectivity occurs in the presence of neuroinflammation [107]. A large Danish study in a cohort of 1746,366 persons (including 2669 subjects with schizophrenia) reveals that schizophrenia incidence is increased in subjects with a high risk of childhood infections [108]. Recently a large prospective study in Finland demonstrated a striking relationship between clinically-apparent CNS infections in children (mostly males, who are more susceptible than females to severe infections from birth to 5 years of age [109]) and the occurrence of schizophrenia or other psychoses in adulthood [105]. Extensive epidemiological evidence suggests that schizophrenia in the offspring is associated with influenza infections in the mother during pregnancy [1], [106], though animal models suggest that any severe systemic inflammatory stimulus in the pregnant dam can result in schizophrenia-like lesions in the offspring [110].
A few recent experimental studies have observed that gliotropic viruses can contribute to neurodegeneration. Studies of the neurotropic Borna disease virus indicate that glial activation by the virus precedes neuronal destruction [111], suggesting that gliotropism rather than neurotropism may initiate the neuropathology associated with this virus. Further evidence of this phenomenon has been seen with an endogenous mouse Moloney leukemia retrovirus model [112], in which glial activation by the virus is responsible for motor neuron degeneration, not neuronal infection per se. A growing interest in the role of glia in virus-associated NDs, combined with early analysis (24 h or less post-infection), natural routes of infection, and ultrasensitive detection techniques (see “Important parameters for investigating non-neurotropic viruses in the brain”) may reveal that many viruses that cause neuropathology are initially gliotropic, and that acute glia-based neuroinflammation initiates neurodegeneration whether or not the neurons themselves become infected.
Host factors influencing outcomes from OB invasion by common viruses
The severity and persistence of neuroinflammation following a brain insult is likely to be influenced by several genes. Limited evidence points to cytokine polymorphisms as determinants of the amount of brain damage occurring after respiratory infections [24], [29], [30], [31]. In addition, the individual’s genetic propensity to mount a vigorous systemic inflammatory response may also prove relevant to ND development. There is experimental [113], [114] and clinical [115] evidence showing that systemic inflammation intensifies brain inflammation, even if the systemic inflammation is asymptomatic [116]. A recent study has shown that cognitive decline progresses more rapidly in Alzheimer’s patients experiencing frequent infections and other causes of systemic inflammation [117].
Another host factor of critical importance is age. Invasion of gliotropic viruses may increase in young children as a consequence of an immature innate immune system [118], [119] and limited acquired immunity. Viral invasion of the brain may also increase in older individuals due to immune senescence and other changes in the aged [1], [115]. Aging changes also may amplify the impact of systemic inflammation on neuroinflammation [120].
Experiences with animal models using neurotropic viruses confirm the importance of genetic background to the development of neuropathology. Dramatic mouse strain differences in susceptibility are seen in mouse hepatitis virus and Theiler’s virus models [121], [122]. Commonly employed mouse strains with very different immune responses, the BALB/c and C57BL/6 strains, respond very differently to disseminated viruses [123], [124] and intranasal viruses [125]. In general, C57BL/6 mice are more resistant to viruses than BALB/c mice, but when they do succumb it is largely due to T cell-driven inflammation [124], not viral damage per se.
Gender differences also affect neuropathology outcomes. Female mice recover better than males in a vesicular stomatitis virus encephalitis model, and appear to produce more anti-viral nitric oxide [126]. Women seem to fare better than men following head trauma [127] but not olfactory damage [75]. Schizophrenia [105] and Parkinson’s [128] occur more frequently in males, while Alzheimer’s [129] and multiple sclerosis occur more frequently in females [130]. The role of sex hormones in neuroinflammation appears to be very complex, as would be expected.
Acquired immune status may also be an important determinant of viral neuroinvasion. The C57BL/6J mice used in our influenza-OB model are immunologically naïve with respect to influenza virus. Studies of pandemic influenza patients (by definition, a non-immune population) indicate that influenza encephalitis is more common than during inter-pandemic periods [131] where partial immunity prevails. It is likely that pre-existing immunity will impair OB invasion, as previous intranasal exposure to an adenovirus vector prevents the invasion of the OB commonly seen with the vector [132]. Maternal antibody can prevent or reduce brain invasion with a respiratory virus that is a natural pathogen for swine [133]. The role of pre-existing immunity in the influenza-OB model described in “An influenza animal model demonstrating a gliotropic” infection is under investigation.
A less studied host factor is termed brain or cognitive reserve [134], which is estimated by various measures of intelligence. This factor may determine an individual’s ‘incubation period’ for a clinically-apparent ND. Time of onset of both HIV-associated dementia [135] and clinical Alzheimer’s appears to involve brain reserve [136]. Studies have demonstrated that over 70% of the neurons in the substantia nigra or the ventral horns of the spinal cord must degenerate before clinical disease is apparent [12]. Individuals with more extensive and better-developed neural networks may bypass brain damage more effectively and remain functional longer.
Research issues
Limitations of available experimental data
Our data from the influenza-OB model are meaningful only if the phenomenon is a general one and occurs in humans and/or farm animals. To date we have focused on characterizing influenza invasion of the OB using high doses of PR8 in mature male C57BL/6 (influenza-resistant) mice. The effects on OB invasion by PR8 of the following experimental variables remain to be investigated: (1) mouse gender; (2) pre-immunization with a related viral strain; (3) virus doses that infect but fail to make the animals ill; (4) viral strains that are more sensitive to IFN or other innate immune responses; (5) mice with chemically ablated olfactory nerve dendrites or transected olfactory nerves. 4 There is also a need for ultrastructural studies of infected olfactory nerves to characterize the location of the virus in the nerve. General research issues include analysis of OB viral invasion: (1) by different flu-like respiratory viruses (respiratory syncytial virus, non-neurovirulent mouse hepatitis virus, etc.) in intranasal infections using the procedural guidelines listed in “Important parameters for investigating non-neurotropic viruses in the brain”; (2) in different animal species. Finally, all viral models, including the influenza-OB model, need to be examined for neurodegeneration as well as neuroinflammation.
Confirming the relevance of animal models, such as the influenza-OB model, to human brain diseases will require the use of non-invasive techniques that can be employed to follow subjects prospectively over many years. As a baseline the EEG changes previously reported in ongoing respiratory infections [137], [138] need to be confirmed and extended using sensitive imaging methods in patients hospitalized with severe respiratory infections but lacking neurological symptoms. If the EEG changes correlate with neuroinflammation, less expensive EEG analyses could then be used to select subjects for prospective analysis of brain damage using such imaging techniques as those that detect gross neurodegeneration in schizophrenics [15], [139], HIV dementia and Alzheimer’s patients [15], [140]. Subjects with olfactory dysfunction following a respiratory infection would also be excellent candidates for prospective studies using the functional MRI imaging techniques that were mentioned earlier [81]. A focus on the OB in imaging studies is probably appropriate until other brain regions are clearly implicated in gliotropic virus pathology.
Important parameters for investigating non-neurotropic viruses in the brain
Several parameters of our PR8 model have allowed us to identify the virus as gliotropic. Most of these parameters are described in reference [14] and include using: (1) light anesthesia during inoculation to minimize swallowing of the inoculum; (2) relatively large volumes of viral inoculum that flood the nasal cavity (25 μl/nostril–10 μl was less efficient); (3) early brain tissue harvesting to assure that the virus has not yet been cleared by resident glia or invading leukocytes; (4) highly sensitive nested PCR techniques for virus detection; (5) real-time PCR to quantify cytokine induction and virus levels (when detectable); (6) histochemistry studies to identify glial cell infection; (7) highly purified virus to prevent cytokine induction artifacts [141]; and (8) thoroughly inactivated virus as a control to provide both a similar antigenic stimulus and a control for cytokine-inducing viral contaminants [141]. Finally, the use of nested PCR primers that detect both viral genomic and replication intermediates provides useful information on viral replication status in the tissue of interest.
Some approaches to prevention or treatment of respiratory virus-associated neuroinflammation
Prevention of viral invasion of the brain
If common viruses do contribute to NDs, and immunity prevents this, more aggressive public health measures aimed at preventing a majority of viral infections in children and adults are warranted. While vaccines are the obvious approach, the possible role of respiratory viruses in ND etiology argues for caution when developing live virus intranasal vaccines.
Prophylactic and therapeutic approaches to preventing neuroinflammation
In addition to preventing respiratory infections, safe pharmacological agents that dampen neuroinflammation [142], [143], [144] are needed, regardless of the etiology of NDs. Selected statins that cross the blood–brain barrier inhibit neuroinflammation [145], and a large study has revealed a reduction in dementias and Parkinson’s in patients receiving this class of statins, but not other statins [146]. Dietary fish oil, a major source of omega-3 fatty acids, also appears to protect against dementia and Alzheimer’s pathology, perhaps as effectively as statins [145] and with less risk. Mercury-free fish oil is inexpensive, widely available, free of known side effects, and probably cardio-protective [147]. Promising data on a neuroprotective role of vitamin D3 [148] suggest that this vitamin could also be useful as a prophylactic, though current data are not totally convincing [149].
Some therapeutic approaches are suggested by investigations in neuroinflammation models. Products of cyclooxygenase 2 (COX-2), an enzyme induced in the brain by proinflammatory cytokines, have a prominent role in neuroinflammation [150], [151], and COX-2 inhibitors have been proposed as potentially neuroprotective agents [152]. Acetaminophen may also have promise [153]. Another possible acute treatment is minocycline, which can suppress microglial activation [144]. Several drugs targeted at blocking neuroinflammation are under development [143], [154], [155]. Using such drugs in acute viral infections could prove beneficial in the long-term.
Conclusions and goals of this article
The key points of this article are sketched in Fig. 1. While there is a consensus that activated glia play a major role in ND development, the concept that commonly-encountered gliotropic viruses could initiate or contribute to age-related brain shrinkage, senile dementia or classical NDs (such as Alzheimer’s) via glial activation has not been considered previously. The language of neurovirology employs the terms “neuroinvasive”, “neurotropic” and “neurovirulent” in the context of viruses infecting the brain, reflecting the focus on viruses that can directly infect and kill neurons and cause neurological symptoms as currently defined. But in recent decades, the glia (particularly the microglia) have received more attention, and this article attempts to broaden the framework of neurovirology to include “hit-and-run” gliotropic viruses as possible players in the etiology of ND development.
Some novel ideas introduced in this article include (1) the concept that cumulative damage from numerous mild viral infections with both gliotropic and neurotropic viruses over a lifetime can contribute to ND development; (2) olfactory ensheathing cell (OEC) channels may serve as conduits for rapid viral invasion of the OB; (3) EEG abnormalities during acute viral infections may predict neurologically important viruses; and (4) acute viral symptoms may include cytokine cascades in the brain invoked by gliotropic viruses taken up by the OB (“Implications of the influenza-OB model”) as well as cytokines from the periphery acting upon the hypothalamus through conventional pathways [156]. The article also identifies a number of host factors that may contribute to neurological outcomes of covert viral invasions of the OB.
Direct evidence for many aspects of this complex hypothesis is limited (particularly its relevance to human disease and applicability to viruses other than virulent influenza), but all aspects are testable in animal models and some can be investigated in clinical settings with the imaging techniques described in “Limitations of available experimental data” as well as long-term prospective studies of possible associations between NDs and such conditions as encephalopathies or olfactory deficiencies.
In summary, this article presents a novel hypothesis of ND etiology and is intended to stimulate the extensive research needed to confirm (or deny) the assumptions underlying the hypothesis and to broaden the research to other respiratory virus models in animals and humans.
Conflicts of interest statement
The author declares that there are no financial or personal relationships influencing the conduct of this work or its conclusions.
Acknowledgements
The author is extremely grateful to the individuals who have conducted the influenza laboratory research at Washington State University that underlies the hypothesis presented: Dr. Victor Leyva-Grado, Dr. Lynn Churchill and Mr. Stewart Bohnet. The author is also especially grateful for the guidance and support provided by Dr. James M. Krueger, in whose laboratory the research was conducted.
Funding provided by the Child Health and Development Institute, National Institutes of Health, Bethesda, MD, USA (Grant No. HD36520).
Footnotes
Neuroinflammation is often defined as a state of glial activation that results in the secretion of cytokines and other inflammatory mediators within the brain in the absence of inflammatory exudates [15] – this definition generally applies to acute conditions and is employed in the discussion to follow. However, the term is also used to describe persistent brain inflammation that may involve activated leukocytes that have crossed the blood–brain barrier. This form of neuroinflammation is found in more chronic neurological disorders such as HIV dementia [16] and multiple sclerosis [17], and will be referred to in this discussion as chronic neuroinflammation.
The exceptions are mouse brain-adapted neurotropic strains of human influenza viruses [39]. These viral strains (NWS, NSW) grow in and kill neurons and can cause selective neurodegeneration [68] or a lethal encephalitis when inoculated by the intracerebral route [39]. However, these strains of influenza are highly atypical and should not be compared to epidemic or other human strains of influenza, which can be taken up by glia but not neurons [69]. Avian influenza strains, such as the H5N1 strains of current concern, tend to be neuroinvasive and neurovirulent [70], unlike human strains.
The association of Parkinson’s with influenza virus is based on the high incidence of post-encephalitic Parkinson’s (PEP) seen in young adults between 1920 and 1924 following the 1918 influenza pandemic. PEP, however, was associated with von Economo’s encephalitis (or encephalitis lethargica, EL), not influenza. EL was first diagnosed during a streptococcal disease epidemic in 1915–1916 [100] that preceded the influenza pandemic [3]. EL is still seen, and is thought to be an autoimmune complication of streptococcal infections affecting the basal ganglia [3], [101]. There is no direct association of influenza virus with Parkinson’s disease [102], though a role for respiratory viruses in sporadic Parkinson’s may yet be demonstrated [103] –perhaps as an amplifier of inhaled chemical injury [104].
A recent publication [157] has employed olfactory nerve transection in the mouse influenza model described here and shown some alteration in responses. However, the completeness of the nerve transection was not documented in this study.
References
- 1.Mattson M.P. Infectious agents and age-related neurodegenerative disorders. Ageing Res Rev. 2004;3(1):105–120. doi: 10.1016/j.arr.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirtz D., Thurman D.J., Gwinn-Hardy K., Mohamed M., Chaudhuri A.R., Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68(5):326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 3.Kristensson K. Avian influenza and the brain – comments on the occasion of resurrection of the Spanish flu virus. Brain Res Bull. 2006;68:406–413. doi: 10.1016/j.brainresbull.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 4.Vahlenkamp T.W., Enbergs H.K., Muller H. Experimental and natural Borna disease virus infections: presence of viral RNA in cells of the peripheral blood. Vet Microbiol. 2000;76(3):229–244. doi: 10.1016/s0378-1135(00)00242-x. [DOI] [PubMed] [Google Scholar]
- 5.Yolken R.H. Viruses and schizophrenia: a focus on herpes simplex virus. Herpes. 2007;11(Suppl. 2):83A–88A. [PubMed] [Google Scholar]
- 6.Sierra-Honigmann A.M., Carbone K.M., Yolken R.H. Polymerase chain reaction (PCR) search for viral nucleic acid sequences in schizophrenia. Br J Psychiatry. 1995;166(1):55–60. doi: 10.1192/bjp.166.1.55. [DOI] [PubMed] [Google Scholar]
- 7.McCall S., Henry J.M., Reid A.H., Taubenberger J.K. Influenza RNA not detected in archival brain tissues from acute encephalitis lethargica cases or in postencephalitic Parkinson cases. J Neuropathol Exp Neurol. 2001;60:696–704. doi: 10.1093/jnen/60.7.696. [DOI] [PubMed] [Google Scholar]
- 8.Atkins G.J., McQuaid S., Morris-Downes M.M., Galbraith S.E., Amor S., Cosby S.L. Transient virus infection and multiple sclerosis. Rev Med Virol. 2000;10(5):291–303. doi: 10.1002/1099-1654(200009/10)10:5<291::AID-RMV278>3.0.CO;2-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strous R.D., Shoenfeld Y. To smell the immune system: olfaction, autoimmunity and brain involvement. Autoimm Rev. 2006;6(1):54–60. doi: 10.1016/j.autrev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Doty R.L. Studies of human olfaction from the University of Pennsylvania Smell and Taste Center. Chem Senses. 1997;22(5):565–586. doi: 10.1093/chemse/22.5.565. [DOI] [PubMed] [Google Scholar]
- 11.Johnson R.T., Mims C.A. Pathogenesis of viral infections of the nervous system. N Engl J Med. 1968;278(1):23–30. doi: 10.1056/NEJM196801042780106. [DOI] [PubMed] [Google Scholar]
- 12.Mohammed A.H., Norrby E., Kristensson K. Viruses and behavioural changes: a review of clinical and experimental findings. Rev Neurosci. 1993;4(3):267–286. doi: 10.1515/revneuro.1993.4.3.267. [DOI] [PubMed] [Google Scholar]
- 13.Doty R.L. The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann Neurol. 2008;63(1):7–15. doi: 10.1002/ana.21327. [DOI] [PubMed] [Google Scholar]
- 14.Majde J.A., Bohnet S.G., Ellis G.A., Churchill L., Leyva-Grado V., Wu M. Detection of mouse-adapted human influenza virus in the olfactory bulb of mice within hours after intranasal infection. J Neurovirol. 2007;13(5):399–409. doi: 10.1080/13550280701427069. [DOI] [PubMed] [Google Scholar]
- 15.Cagnin A., Kassiou M., Meikle S.R., Banati R.B. In vivo evidence for microglial activation in neurodegenerative dementia. Acta Neurol Scand. 2006;114(s185):107–114. doi: 10.1111/j.1600-0404.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- 16.Ghafouri M., Amini S., Khalili K., Sawaya B.E. HIV-1 associated dementia: symptoms and causes. Retrovirol. 2006;3(May 19):28–39. doi: 10.1186/1742-4690-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lassmann H., Bruck W., Lucchinetti C.F. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17(2):210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thanvi B., Lo N., Robinson T. Essential tremor – the most common movement disorder in older people. Age Ageing. 2006;35(4):344–349. doi: 10.1093/ageing/afj072. [DOI] [PubMed] [Google Scholar]
- 19.Terry R.D. Alzheimer’s disease and the aging brain. J Geriatr Psychiatry Neurol. 2006;19(3):125–128. doi: 10.1177/0891988706291079. [DOI] [PubMed] [Google Scholar]
- 20.Frank-Cannon T, Alto L, McAlpine F, Tansey M. Does neuroinflammation fan the flame in neurodegenerative diseases? Available from: http://www.molecularneurodegeneration.com/content/4/1/47; 2009. [DOI] [PMC free article] [PubMed]
- 21.Brown R.C., Lockwood A.H., Sonawane B.R. Neurodegenerative diseases: an overview of environmental risk factors. Environ Health Perspect. 2005;113(9):1250–1256. doi: 10.1289/ehp.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangano E.N., Hayley S. Inflammatory priming of the substantia nigra influences the impact of later paraquat exposure: neuroimmune sensitization of neurodegeneration. Neurobiol Aging. 2009;30:1361–1378. doi: 10.1016/j.neurobiolaging.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Perry V.H., Cunningham C., Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7(2):161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 24.Block M.L., Hong J.S. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76(2):77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Prediger R.D., Rial D., Medeiros R., Figueiredo C.P., Doty R.L., Takahashi R.N. Risk is in the air: an intranasal MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) rat model of Parkinson’s disease. Ann NY Acad Sci. 2009;1170:629–636. doi: 10.1111/j.1749-6632.2009.03885.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen J.K., Johnston K.M., Petrides M., Ptito A. Neural substrates of symptoms of depression following concussion in male athletes with persisting postconcussion symptoms. Arch Gen Psychiatry. 2008;65(1):81–89. doi: 10.1001/archgenpsychiatry.2007.8. [DOI] [PubMed] [Google Scholar]
- 27.Sayer N.A., Chiros C.E., Sigford B., Scott S., Clothier B., Pickett T. Characteristics and rehabilitation outcomes among patients with blast and other injuries sustained during the global war on terror. Arch Phys Med Rehab. 2008;89(1):163–170. doi: 10.1016/j.apmr.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 28.Martin L.J., Al-Abdulla N.A., Brambrink A.M., Kirsch J.R., Sieber F.E., Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: a perspective on the contributions of apoptosis and necrosis. Brain Res Bull. 1998;46(4):281–309. doi: 10.1016/s0361-9230(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 29.Gentile D.A., Doyle W.J., Zeevi A., Piltcher O., Skoner D.P. Cytokine gene polymorphisms moderate responses to respiratory syncytial virus in adults. Hum Immunol. 2003;64:93–98. doi: 10.1016/s0198-8859(02)00705-x. [DOI] [PubMed] [Google Scholar]
- 30.Waters R.J., Nicoll J.A. Genetic influences on outcome following acute neurological insults. Curr Opinion Crit Care. 2005;11(2):105–110. doi: 10.1097/01.ccx.0000155354.78617.91. [DOI] [PubMed] [Google Scholar]
- 31.Fortunato G., Calcagno G., Bresciamorra V., Salvatore E., Filla A., Capone S. Multiple sclerosis and hepatitis C virus infection are associated with single nucleotide polymorphisms in interferon pathway genes. J Interferon Cytokine Res. 2008;28(3):141–152. doi: 10.1089/jir.2007.0049. [DOI] [PubMed] [Google Scholar]
- 32.Giunta B, Fernandez F, Nikolic WV, Obregon D, Rrapo E, Town T, et al. Inflammaging as a prodrome to Alzheimer’s disease. Available from: http://www.jneuroinflammation.com/content/5/1/51; 2008. [DOI] [PMC free article] [PubMed]
- 33.Loseva E., Yuan T.F., Karnup S. Neurogliogenesis in the mature olfactory system: a possible protective role against infection and toxic dust. Brain Res Rev. 2009;59(2):374–387. doi: 10.1016/j.brainresrev.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beutler B., Eidenschenk C., Crozat K., Imler J.L., Takeuchi O., Hoffmann J.A. Genetic analysis of resistance to viral infection. Nat Rev Immunol. 2007;7(10):753–766. doi: 10.1038/nri2174. [DOI] [PubMed] [Google Scholar]
- 35.Shipley, MT, Ennis M, Puche AC. Olfactory system. In: Paxinos G, editor. The rat nervous system. 3rd ed. San Diego; 2004. p. 923–64.
- 36.Leyva-Grado V., Churchill L., Wu M., Williams T.J., Majde J.A., Taishi P. Influenza virus- and cytokine-immunoreactive cells in the murine olfactory and central autonomic nervous systems before and after illness onset. J Neuroimmunol. 2009;211:73–83. doi: 10.1016/j.jneuroim.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori I., Goshima F., Ito H., Koide N., Yoshida T., Yokochi T. The vomeronasal chemosensory system as a route of neuroinvasion by herpes simplex virus. Virology. 2005;334(1):51–58. doi: 10.1016/j.virol.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 38.Mori I., Nishiyama Y., Yokochi T., Kimura Y. Olfactory transmission of neurotropic viruses. J Neurovirol. 2005;11(2):129–137. doi: 10.1080/13550280590922793. [DOI] [PubMed] [Google Scholar]
- 39.Schlesinger R.W., Husak P.J., Bradshaw G.L., Panayotov P.P. Mechanisms involved in natural and experimental neuropathogenicity of influenza viruses: evidence and speculation. Adv Virus Res. 1998;50:289–379. doi: 10.1016/s0065-3527(08)60811-8. [DOI] [PubMed] [Google Scholar]
- 40.Li Y., Field P.M., Raisman G. Olfactory ensheathing cells and olfactory nerve fibroblasts maintain continuous open channels for regrowth of olfactory nerve fibres. Glia. 2005;52(3):245–251. doi: 10.1002/glia.20241. [DOI] [PubMed] [Google Scholar]
- 41.Elder A., Gelein R., Silva V., Feikert T., Opanashuk L., Carter J. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114(8):1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharmaceut Sci. 2000;11(1):1–18. doi: 10.1016/s0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 43.Fiske B.K., Brunjes P.C. Microglial activation in the developing rat olfactory bulb. Neuroscience. 2000;96(4):807–815. doi: 10.1016/s0306-4522(99)00601-6. [DOI] [PubMed] [Google Scholar]
- 44.Biber K., Neumann H., Inoue K., Boddeke H.W.G.M. Neuronal ‘on’ and ‘off’ signals control microglia. Trends Neurosci. 2007;30(11):596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg G.A. Matrix metalloproteinases and neuroinflammation in multiple sclerosis. Neuroscientist. 2002;8(6):586–595. doi: 10.1177/1073858402238517. [DOI] [PubMed] [Google Scholar]
- 46.Deng Y., Thompson B.M., Gao X., Hall E.D. Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Exp Neurol. 2007;205(1):154–165. doi: 10.1016/j.expneurol.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simi A., Tsakiri N., Wang P., Rothwell N.J. Interleukin-1 and inflammatory neurodegeneration. Biochem Soc Transact. 2007;035(5):1122–1126. doi: 10.1042/BST0351122. [DOI] [PubMed] [Google Scholar]
- 48.Sriram U., Biswas C., Behrens E.M., Dinnall J.A., Shivers D.K., Monestier M. IL-4 suppresses dendritic cell response to type I interferons. J Immunol. 2007;179(10):6446–6455. doi: 10.4049/jimmunol.179.10.6446. [DOI] [PubMed] [Google Scholar]
- 49.Cacquevel M., Lebeurrier N., Cheenne D., Vivien D. Cytokines in neuroinflammation and Alzheimers disease. Curr Drug Targets. 2004;5:529–534. doi: 10.2174/1389450043345308. [DOI] [PubMed] [Google Scholar]
- 50.Rothwell N.J., Relton J.K. Involvement of cytokines in acute neurodegeneration in the CNS. Neurosci Biobehav Rev. 1993;17(2):217–227. doi: 10.1016/s0149-7634(05)80152-6. [DOI] [PubMed] [Google Scholar]
- 51.Chao C.C., Hu S.X., Ehrlich L., Peterson P.K. Interleukin-1 and tumor necrosis factor-α synergistically mediate neurotoxicity: Involvement of nitric oxide and of N-methyl-d-aspartate receptors. Brain Behav Immun. 1995;9(4):355–365. doi: 10.1006/brbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- 52.Brabers N.A., Nottet H.S. Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. Eur J Clin Invest. 2006;36(7):447–458. doi: 10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 53.Esiri M.M. The interplay between inflammation and neurodegeneration in CNS disease. J Neuroimmunol. 2007;184(1–2):4–16. doi: 10.1016/j.jneuroim.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 54.Bulloch K., Miller M.M., Gal-Toth J., Milner T.A., Gottfried A.B., Waters E.M. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult and injured mouse brain. J Comp Neurol. 2008;508:687–710. doi: 10.1002/cne.21668. [DOI] [PubMed] [Google Scholar]
- 55.Traynor T.R., Majde J.A., Bohnet S.G., Krueger J.M. Interferon type I receptor-deficient mice have altered disease symptoms in response to influenza virus. Brain Behav Immun. 2007;21:311–322. doi: 10.1016/j.bbi.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amadori M. The role of IFN-alpha as homeostatic agent in the inflammatory response: a balance between danger and response? J Interferon Cytokine Res. 2007;27(3):181–190. doi: 10.1089/jir.2006.0110. [DOI] [PubMed] [Google Scholar]
- 57.Taylor J.L., Grossberg S.E. The effects of interferon-α on the production and action of other cytokines. Semin Oncol. 1998;25(Suppl. 1):23–29. [PubMed] [Google Scholar]
- 58.Li X., Hanson C., Cmarik J.L., Ruscetti S. Neurodegeneration induced by PVC-211 murine leukemia virus is associated with increased levels of vascular endothelial growth factor and macrophage inflammatory protein 1α and is inhibited by blocking activation of microglia. J Virol. 2009;83(10):4912–4922. doi: 10.1128/JVI.02343-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ludwiczek O., Kaser A., Koch R.O., Vogel W., Cruikshank W.W., Tilg H. Activation of caspase-3 by interferon alpha causes interleukin-16 secretion but fails to modulate activation induced cell death. Eur Cytokine Netw. 2001;12(3):478–486. [PubMed] [Google Scholar]
- 60.Akwa Y., Hassett D.E., Eloranta M.L., Sandberg K., Masliah E., Powell H. Transgenic expression of IFN-α in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J Immunol. 1998;161(9):5016–5026. [PubMed] [Google Scholar]
- 61.Enevold C., Oturai A.B., Sorensen P.S., Ryder L.P., Koch-Henriksen N., Bendtzen K. Multiple sclerosis and polymorphisms of innate pattern recognition receptors TLR1-10, NOD1-2, DDX58, and IFIH1. J Neuroimmunol. 2009;212:125–131. doi: 10.1016/j.jneuroim.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Scott K., Manunta M., Germain C., Smith P., Jones M., Mitchell P. Qualitatively distinct patterns of cytokines are released by human dendritic cells in response to different pathogens. Immunology. 2005;116(2):245–254. doi: 10.1111/j.1365-2567.2005.02218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mori I., Goshima F., Koshizuka T., Koide N., Sugiyama T., Yoshida T. Differential activation of the c-Jun N-terminal kinase/stress-activated protein kinase and p38 mitogen-activated protein kinase signal transduction pathways in the mouse brain upon infection with neurovirulent influenza A virus. J Gen Virol. 2003;84(Pt 9):2401–2408. doi: 10.1099/vir.0.19188-0. [DOI] [PubMed] [Google Scholar]
- 64.Boggian I., Buzzacaro E., Calistri A., Calvi P., Cavaggioni A., Mucignat-Caretta C. Asymptomatic herpes simplex type 1 virus infection of the mouse brain. J Neurovirol. 2000;6(4):303–313. doi: 10.3109/13550280009030756. [DOI] [PubMed] [Google Scholar]
- 65.Perlman S., Sun N., Barnett E.M. Spread of MHV-JHM from nasal cavity to white matter of spinal cord. Transneuronal movement and involvement of astrocytes. Adv Exp Med Biol. 1995;380:73–78. doi: 10.1007/978-1-4615-1899-0_10. [DOI] [PubMed] [Google Scholar]
- 66.Rudd P.A., Cattaneo R., von Messling V. Canine distemper virus uses both the anterograde and the hematogenous pathway for neuroinvasion. J Virol. 2006;80(19):9361–9370. doi: 10.1128/JVI.01034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoon H.A., Eo S.K., Aleyas A.G., Cha S.Y., Lee J.H., Chae J.S. Investigation of pseudorabies virus latency in nervous tissues of seropositive pigs exposed to field strain. J Vet Med Sci. 2006;68(2):143–148. doi: 10.1292/jvms.68.143. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi M., Yamada T., Nakajima S., Nakajima K., Yamamoto T., Okada H. The substantia nigra is a major target for neurovirulent influenza A virus. J Exp Med. 1995;181(6):2161–2169. doi: 10.1084/jem.181.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bradshaw G.L., Schlesinger R.W., Schwartz C.D. Effects of cell differentiation on replication of A/WS/33, WSN, and A/PR/8/34 influenza viruses in mouse brain cell cultures: biological and immunological characterization of products. J Virol. 1989;63:1704–1714. doi: 10.1128/jvi.63.4.1704-1714.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bright R.A., Cho D.S., Rowe T., Katz J.M. Mechanisms of pathogenicity of influenza A (H5N1) viruses in mice. Avian Dis. 2003;47(3 Suppl.):1131–1134. doi: 10.1637/0005-2086-47.s3.1131. [DOI] [PubMed] [Google Scholar]
- 71.Bohnet S.G., Sawatzki N., Majde J.A., Churchill L., Krueger J.M. Persistent brain infection of PR8 influenza at 48 and 96 hours post-infection. Sleep. 2009;32(Abstract Suppl.):A402. [Google Scholar]
- 72.Chao C.C., Hu S., Peterson P.K. Glia: the not so innocent bystanders. J Neurovirol. 1996;2(4):234–239. doi: 10.3109/13550289609146886. [DOI] [PubMed] [Google Scholar]
- 73.Hall E.D., Sullivan P.G., Gibson T.R., Pavel K.M., Thompson B.M., Scheff S.W. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice. more than a focal brain injury. J Neurotrauma. 2005;22(2):252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- 74.Hawkes C. Olfaction in neurodegenerative disorder. Adv Otorhinolaryngol. 2006;63:133–151. doi: 10.1159/000093759. [DOI] [PubMed] [Google Scholar]
- 75.Welge-Lussen A., Wolfensberger M. Olfactory disorders following upper respiratory tract infections. Adv Otorhinolaryngol. 2006;63:125–132. doi: 10.1159/000093758. [DOI] [PubMed] [Google Scholar]
- 76.Sugiura M., Aiba T., Mori J., Nakai Y. An epidemiological study of postviral olfactory disorder. Acta Oto-laryngol. 1998;538(6 Suppl. 538):191–196. doi: 10.1080/00016489850182918. [DOI] [PubMed] [Google Scholar]
- 77.Henkin R.I., Larson A.L., Powell R.D. Hypogeusia, dysgeusia, hyposmia, and dysosmia following influenza-like infection. Ann Oto Rhino Laryngol. 1975;84(5 Pt 1):672–682. doi: 10.1177/000348947508400519. [DOI] [PubMed] [Google Scholar]
- 78.Suzuki M., Saito K., Min W.P., Vladau C., Toida K., Itoh H. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harris R., Davidson T.M., Murphy C., Gilbert P.E., Chen M. Clinical evaluation and symptoms of chemosensory impairment: one thousand consecutive cases from the nasal dysfunction Clinic in San Diego. Am J Rhinol. 2006;20(1):101–108. [PubMed] [Google Scholar]
- 80.Schiffman S.S. Critical illness and changes in sensory perception. Proc Nutr Soc. 2007;66(03):331–345. doi: 10.1017/S0029665107005599. [DOI] [PubMed] [Google Scholar]
- 81.Levy L.M., Henkin R.I., Lin C.S., Finley A. Rapid imaging of olfaction by functional MRI (fMRI): identification of presence and type of hyposmia. J Comput Assisted Tomography. 1999;23(5):767–775. doi: 10.1097/00004728-199909000-00026. [DOI] [PubMed] [Google Scholar]
- 82.Mizuguchi M., Yamanouchi H., Ichiyama T., Shiomi M. Acute encephalopathy associated with influenza and other viral infections. Acta Neurol Scand. 2007;115(s186):45–56. doi: 10.1111/j.1600-0404.2007.00809.x. [DOI] [PubMed] [Google Scholar]
- 83.Yao D., Kuwajima M., Chen Y., Shiota M., Okumura Y., Yamada H. Impaired long-chain fatty acid metabolism in mitochondria causes brain vascular invasion by a non-neurotropic epidemic influenza A virus in the newborn/suckling period: implications for influenza-associated encephalopathy. Mol Cell Biochem. 2007;299(1):85–92. doi: 10.1007/s11010-005-9046-x. [DOI] [PubMed] [Google Scholar]
- 84.Antonucci R., Fanos V. Acute encephalopathy associated with respiratory syncytial virus infections in childhood. A literature review. Minerva Pediatr. 2005;57(3):137–142. [PubMed] [Google Scholar]
- 85.Hayase Y., Tobita K. Influenza virus and neurological diseases. Psychiatry Clin Neurosci. 1997;51(4):181–184. doi: 10.1111/j.1440-1819.1997.tb02580.x. [DOI] [PubMed] [Google Scholar]
- 86.Yokota S. Influenza-associated encephalopathy–pathophysiology and disease mechanisms. Nip Rinsho. 2003;61(11):1953–1958. [PubMed] [Google Scholar]
- 87.Centers for Disease Control and Prevention (CDC) Neurologic complications associated with novel influenza A (H1N1) virus infection in children – Dallas, Texas, May 2009. MMWR: Morbidity and Mortality Weekly Report. 2009;58(28):773–778. [PubMed] [Google Scholar]
- 88.Sasseville V.G., Lackner A.A. Neuropathogenesis of simian immunodeficiency virus infection in macaque monkeys. J Neurovirol. 1997;3(1):1–9. doi: 10.3109/13550289709015787. [DOI] [PubMed] [Google Scholar]
- 89.Khalili K., White M.K. Human demyelinating disease and the polyomavirus JCV. Mult Scler. 2006;12(2):133–142. doi: 10.1191/135248506ms1264oa. [DOI] [PubMed] [Google Scholar]
- 90.Ravits J. Sporadic amyotrophic lateral sclerosis: a hypothesis of persistent (non-cytolytic) enteroviral infection. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6(2):77–87. doi: 10.1080/14660820510027026. [DOI] [PubMed] [Google Scholar]
- 91.Frank O., Giehl M., Zheng C., Hehlmann R., Leib-Mosch C., Seifarth W. Human endogenous retrovirus expression profiles in samples from brains of patients with schizophrenia and bipolar disorders. J Virol. 2005;79(17):10890–10901. doi: 10.1128/JVI.79.17.10890-10901.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hobbs J.A. Detection of adeno-associated virus 2 and parvovirus B19 in the human dorsolateral prefrontal cortex. J Neurovirol. 2006;12(3):190–199. doi: 10.1080/13550280600827351. [DOI] [PubMed] [Google Scholar]
- 93.Gordon L., McQuaid S., Cosby S.L. Detection of herpes simplex virus (types 1 and 2) and human herpesvirus 6 DNA in human brain tissue by polymerase chain reaction. Clin Diagn Virol. 1996;6(1):33–40. doi: 10.1016/0928-0197(95)00203-0. [DOI] [PubMed] [Google Scholar]
- 94.Yolken R.H., Torrey E.F. Viruses, schizophrenia, and bipolar disorder. Clin Microbiol Rev. 1995;8:131–145. doi: 10.1128/cmr.8.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haga S., Yoshimura M., Motoi Y., Arima K., Aizawa T., Ikuta K. Detection of Borna disease virus genome in normal human brain tissue. Brain Res. 1997;770(1–2):307–309. doi: 10.1016/s0006-8993(97)00903-7. [DOI] [PubMed] [Google Scholar]
- 96.Niebuhr D.W., Millikan A.M., Cowan D.N., Yolken R., Li Y., Weber N.S. Selected infectious agents and risk of schizophrenia among US military personnel. Am J Psychiatry. 2008;165(1):99–106. doi: 10.1176/appi.ajp.2007.06081254. [DOI] [PubMed] [Google Scholar]
- 97.Leweke F.M., Gerth C., Koethe D., Klosterklotter J., Ruslanova I., Krivogorsky B. Antibodies to infectious agents in individuals with recent onset schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2004;254(1):4–8. doi: 10.1007/s00406-004-0481-6. [DOI] [PubMed] [Google Scholar]
- 98.Steiner I., Nisipianu P., Wirguin I. Infection and the etiology and pathogenesis of multiple sclerosis. Curr Neurol Neurosci Rep. 2001;1(3):271–276. doi: 10.1007/s11910-001-0030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takahashi M., Yamada T. Viral etiology for Parkinson’s disease – a possible role of influenza A virus infection. Jpn J Infect Dis. 1999;52(3):89–98. [PubMed] [Google Scholar]
- 100.Ottolini M.G., Burnett M.W. History of US military contributions to the study of respiratory infections. Mil Med. 2005;170(4 Suppl.):66–70. doi: 10.7205/milmed.170.4s.66. [DOI] [PubMed] [Google Scholar]
- 101.Dale R.C., Church A.J., Surtees R.A.H., Lees A.J., Adcock J.E., Harding B. Encephalitis lethargica syndrome: 20 new cases and evidence of basal ganglia autoimmunity. Brain. 2004;127(Pt1):21–33. doi: 10.1093/brain/awh008. [DOI] [PubMed] [Google Scholar]
- 102.Casals J., Elizan T.S., Yahr M.D. Postencephalitic parkinsonism – a review. J Neural Transm. 1998;105(6–7):645–676. doi: 10.1007/s007020050086. [DOI] [PubMed] [Google Scholar]
- 103.Hawkes C.H., Del T.K., Braak H. Parkinson’s disease: the dual hit theory revisited. Ann NY Acad Sci. 2009;1170:615–622. doi: 10.1111/j.1749-6632.2009.04365.x. [DOI] [PubMed] [Google Scholar]
- 104.Franco J., Prediger R.D.S., Pandolfo P., Takahashi R.N., Farina M., Dafre A.L. Antioxidant responses and lipid peroxidation following intranasal 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration in rats: increased susceptibility of olfactory bulb. Life Sci. 2007;80(20):1906–1914. doi: 10.1016/j.lfs.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 105.Koponen H., Rantakallio P., Veijola J., Jones P., Jokelainen J., Isohanni M. Childhood central nervous system infections and risk for schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2004;254(1):9–13. doi: 10.1007/s00406-004-0485-2. [DOI] [PubMed] [Google Scholar]
- 106.Brown A.S., Begg M.D., Gravenstein S., Schaefer C.A., Wyatt R.J., Bresnahan M. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61(8):774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 107.Bernstein H.G., Steiner J., Bogerts B. Glial cells in schizophrenia: pathophysiological significance and possible consequences for therapy. Expert Rev Neurotherap. 2009;9(7):1059–1071. doi: 10.1586/ern.09.59. [DOI] [PubMed] [Google Scholar]
- 108.Westergaard T., Mortensen P.B., Pedersen C.B., Wohlfahrt J., Melbye M. Exposure to prenatal and childhood infections and the risk of schizophrenia: suggestions from a study of sibship characteristics and influenza prevalence. Arch Gen Psychiatry. 1999;56(11):993–998. doi: 10.1001/archpsyc.56.11.993. [DOI] [PubMed] [Google Scholar]
- 109.Jensen-Fangel S., Mohey R., Johnsen S.P., Andersen P.L., Sorensen H.T., Ostergaard L. Gender differences in hospitalization rates for respiratory tract infections in Danish youth. Scand J Infect Dis. 2004;36(1):31–36. doi: 10.1080/00365540310017618. [DOI] [PubMed] [Google Scholar]
- 110.Gilmore J.H., Jarskog L.F., Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNFα, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol. 2005;159(1–2):106–112. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 111.Ovanesov MV, Moldovan K, Smith K, Vogel MW, Pletnikov MV. Persistent Borna disease virus (BDV) infection activates microglia prior to a detectable loss of granule cells in the hippocampus. Available from: http://www.jneuroinflammation.com/content/5/1/16; 2008. [DOI] [PMC free article] [PubMed]
- 112.Zachary J.F., Baszler T.V., French R.A., Kelley K.W. Mouse Moloney leukemia virus infects microglia but not neurons even though it induces motor neuron disease. Mol Psychiatry. 1997;2(2):104–106. doi: 10.1038/sj.mp.4000219. [DOI] [PubMed] [Google Scholar]
- 113.Perry V.H., Newman T.A., Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4(2):103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- 114.Mori K., Kaneko Y.S., Nakashima A., Nagatsu I., Takahashi H., Ota A. Peripheral lipopolysaccharide induces apoptosis in the murine olfactory bulb. Brain Res. 2005;1039(1–2):116–129. doi: 10.1016/j.brainres.2005.01.078. [DOI] [PubMed] [Google Scholar]
- 115.Dunn N., Mullee M., Perry V.H., Holmes C. Association between dementia and infectious disease: evidence from a case–control study. Alzheimer Dis Assoc Disord. 2005;19(2):91–94. doi: 10.1097/01.wad.0000165511.52746.1f. [DOI] [PubMed] [Google Scholar]
- 116.Teeling J.L., Felton L.M., Deacon R.M.J., Cunningham C., Rawlins J.N.P., Perry V.H. Sub-pyrogenic systemic inflammation impacts on brain and behavior, independent of cytokines. Brain Behav Immun. 2007;21(6):836–850. doi: 10.1016/j.bbi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 117.Holmes C., Cunningham C., Zotova E., Woolford J., Dean C., Kerr S. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73(10):768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martyn C.N. Infection in childhood and neurological diseases in adult life. Br Med Bull. 1997;53(1):24–39. doi: 10.1093/oxfordjournals.bmb.a011603. [DOI] [PubMed] [Google Scholar]
- 119.Sharma A., Valadi N., Miller A.H., Pearce B.D. Neonatal viral infection decreases neuronal progenitors and impairs adult neurogenesis in the hippocampus. Neurobiol Dis. 2002;11(2):246–256. doi: 10.1006/nbdi.2002.0531. [DOI] [PubMed] [Google Scholar]
- 120.Chen J., Buchanan J.B., Sparkman N.L., Godbout J.P., Freund G.G., Johnson R.W. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22(3):301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sieve A.N., Steelman A.J., Young C.R., Storts R., Welsh T.H., Welsh C.J. Chronic restraint stress during early Theiler’s virus infection exacerbates the subsequent demyelinating disease in SJL mice. J Neuroimmunol. 2004;155(1–2):103–118. doi: 10.1016/j.jneuroim.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 122.Olson J.K., Girvin A.M., Miller S.D. Direct activation of innate and antigen-presenting functions of microglia following infection with Theiler’s virus. J Virol. 2001;75(20):9780–9789. doi: 10.1128/JVI.75.20.9780-9789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guida J.D., Fejer G., Pirofski L.A., Brosnan C.F., Horwitz M.S. Mouse adenovirus type 1 causes a fatal hemorrhagic encephalomyelitis in adult C57BL/6 but not BALB/c mice. J Virol. 1995;69(12):7674–7681. doi: 10.1128/jvi.69.12.7674-7681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karupiah G. Type 1 and type 2 cytokines in antiviral defense. Vet Immunol Immunopathol. 1998;63(1–2):105–109. doi: 10.1016/s0165-2427(98)00086-5. [DOI] [PubMed] [Google Scholar]
- 125.Christian A.Y., Barna M., Bi Z., Reiss C.S. Host immune response to vesicular stomatitis virus infection of the central nervous system in C57BL/6 mice. Viral Immunol. 1996;9(3):195–205. doi: 10.1089/vim.1996.9.195. [DOI] [PubMed] [Google Scholar]
- 126.Barna M., Komatsu T., Bi Z., Reiss C.S. Sex differences in susceptibility to viral infection of the central nervous system. J Neuroimmunol. 1996;67(1):31–39. doi: 10.1016/0165-5728(96)00022-7. [DOI] [PubMed] [Google Scholar]
- 127.Rogers E., Wagner A.K. Gender, sex steroids, and neuroprotection following traumatic brain injury. J Head Trauma Rehab. 2006;21(3):279–281. doi: 10.1097/00001199-200605000-00008. [DOI] [PubMed] [Google Scholar]
- 128.Marchetti B., Serra P.A., L’Episcopo F., Tirolo C., Caniglia S., Testa N. Hormones are key actors in gene x environment interactions programming the vulnerability to Parkinson’s disease: glia as a common final pathway. Ann NY Acad Sci. 2005;1057(Dec):296–318. doi: 10.1196/annals.1356.023. [DOI] [PubMed] [Google Scholar]
- 129.Webber K.M., Casadesus G., Perry G., Atwood C.S., Bowen R., Smith M.A. Gender differences in Alzheimer disease: the role of luteinizing hormone in disease pathogenesis. Alzheimer Dis Assoc Disord. 2005;19(2):95–99. doi: 10.1097/01.wad.0000165512.90864.3f. [DOI] [PubMed] [Google Scholar]
- 130.Schwendimann RN, Alekseeva N. Gender issues in multiple sclerosis. In: Alireza M, editor. International review of neurobiology – the neurobiology of multiple sclerosis, vol. 79. San Diego; 2007. p. 377–92. [DOI] [PubMed]
- 131.Koskiniemi M., Rantalaiho T., Piiparinen H., Von Bonsdorff C.H., Farkkila M., Jarvinen A. Infections of the central nervous system of suspected viral origin: a collaborative study from Finland. J Neurovirol. 2001;7(5):400–408. doi: 10.1080/135502801753170255. [DOI] [PubMed] [Google Scholar]
- 132.Lemiale F., Kong W.p., Akyurek L.M., Ling X., Huang Y., Chakrabarti B.K. Enhanced mucosal immunoglobulin A response of intranasal adenoviral vector human immunodeficiency virus vaccine and localization in the central nervous system. J Virol. 2003;77(18):10078–10087. doi: 10.1128/JVI.77.18.10078-10087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kritas S.K., Nauwynck H.J., Pensaert M.B., Kyriakis S.C. Effect of the concentration of maternal antibodies on the neural invasion of Aujeszky’s disease virus in neonatal pigs. Vet Microbiol. 1997;55(1–4):29–36. doi: 10.1016/s0378-1135(96)01303-x. [DOI] [PubMed] [Google Scholar]
- 134.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
- 135.Stern R.A., Silva S.G., Chaisson N., Evans D.L. Influence of cognitive reserve on neuropsychological functioning in asymptomatic human immunodeficiency virus-1 infection. Arch Neurol. 1996;53(2):148–153. doi: 10.1001/archneur.1996.00550020052015. [DOI] [PubMed] [Google Scholar]
- 136.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(3 Suppl. 2):S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- 137.Gibbs E.L., Gibbs F.A., Grossman H. Electroencephalographic evidence of encephalitis in children with supposedly “uncomplicated” childhood diseases. Trans Am Neurol Assoc. 1956:159–160. 81st Meeting. [PubMed] [Google Scholar]
- 138.Grossman H.J., Gibbs E.L., Spies H.W. Electroencephalographic studies on children having measles with no clinical evidence of involvement of the central nervous system. Pediatrics. 1956;18(4):556–560. [PubMed] [Google Scholar]
- 139.Csernansky J.G. Neurodegeneration in schizophrenia: evidence from in vivo neuroimaging studies. Sci World J. 2007;7(2):135–143. doi: 10.1100/tsw.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Apostolova L.G., Thompson P.M. Brain mapping as a tool to study neurodegeneration. Neurotherapeutics. 2007;4(3):387–400. doi: 10.1016/j.nurt.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Majde J.A. Microbial cell-wall contaminants in peptides: a potential source of physiological artifacts. Peptides. 1993;14:629–632. doi: 10.1016/0196-9781(93)90155-a. [DOI] [PubMed] [Google Scholar]
- 142.Ma Y.P., Ma M.M., Ge S., Guo R.B., Zhang H.J., Frey W.H., II Intranasally delivered TGF-β1 enters brain and regulates gene expressions of its receptors in rats. Brain Res Bull. 2007;74(4):271–277. doi: 10.1016/j.brainresbull.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 143.Minghetti L., Greco A., Potenza R.L., Pezzola A., Blum D., Bantubungi K. Effects of the adenosine A2A receptor antagonist SCH 58621 on cyclooxygenase-2 expression, glial activation, and brain-derived neurotrophic factor availability in a rat model of striatal neurodegeneration. J Neuropathol Exp Neurol. 2007;66(5):363–371. doi: 10.1097/nen.0b013e3180517477. [DOI] [PubMed] [Google Scholar]
- 144.Wu D.C., Jackson-Lewis V., Vila M., Tieu K., Teismann P., Vadseth C. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22(5):1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Farooqui A.A., Ong W.Y., Horrocks L.A., Chen P., Farooqui T. Comparison of biochemical effects of statins and fish oil in brain: the battle of the titans. Brain Res Rev. 2007;56(2):443–471. doi: 10.1016/j.brainresrev.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 146.Wolozin B., Wang S.W., Li N.-C., Lee A., Lee T.A., Kazis L.E. Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med. 2007;5(July):20–31. doi: 10.1186/1741-7015-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kakar P., Watson T., Lip G.Y. New approaches to therapy with omega-3 fatty acids. Curr Atheroscler Rep. 2008;10(1):79–87. doi: 10.1007/s11883-008-0012-4. [DOI] [PubMed] [Google Scholar]
- 148.Moore M.E., Piazza A., McCartney Y., Lynch M.A. Evidence that vitamin D3 reverses age-related inflammatory changes in the rat hippocampus. Biochem Soc Transact. 2005;33(Pt 4):573–577. doi: 10.1042/BST0330573. [DOI] [PubMed] [Google Scholar]
- 149.McCann J.C., Ames B.N. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22(4):982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 150.Laflamme N., Lacroix S., Rivest S. An essential role of interleukin-1beta in mediating NF-kappaB activity and COX-2 transcription in cells of the blood–brain barrier in response to a systemic and localized inflammation but not during endotoxemia. J Neurosci. 1999;19(24):10923–10930. doi: 10.1523/JNEUROSCI.19-24-10923.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Garcia-Bueno B., Madrigal J.L., Lizasoain I., Moro M.A., Lorenzo P., Leza J.C. Peroxisome proliferator-activated receptor gamma activation decreases neuroinflammation in brain after stress in rats. Biol Psychiatry. 2005;57(8):885–894. doi: 10.1016/j.biopsych.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 152.Strauss K.I. Antiinflammatory and neuroprotective actions of COX2 inhibitors in the injured brain. Brain Behav Immun. 2008;22(3):285–298. doi: 10.1016/j.bbi.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tripathy D, Grammas P. Acetaminophen inhibits neuronal inflammation and protects neurons from oxidative stress. Available from: http://www.jneuroinflammation.com/content/6/1/10; 2009. [DOI] [PMC free article] [PubMed]
- 154.Skaper S.D. The brain as a target for inflammatory processes and neuroprotective strategies. Ann NY Acad Sci. 2007;1122(1):23–34. doi: 10.1196/annals.1403.002. [DOI] [PubMed] [Google Scholar]
- 155.Vink R., Nimmo A.J. Multifunctional drugs for head injury. Neurotherapeutics. 2009;6(1):28–42. doi: 10.1016/j.nurt.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dantzer R., Kelley K.W. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21(2):153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Leyva-Grado V., Churchill L., Harding J., Krueger J.M. The olfactory nerve has a role in the body temperature and brain cytokine responses to influenza virus. Brain Behav Imm. 2010;24:281–288. doi: 10.1016/j.bbi.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


