Abstract
In many mammals, the availability of familiar conspecifics in the home environment can affect immune function and morbidity. Numerous sex differences exist in immune responses, but whether the social environment impacts the immune system differently in males and females is not fully understood. This study examined behavioral and physiological responses to simulated bacterial infection in adult male and female Wistar rats housed either with 3 same-sex non-siblings (Group) or alone (Isolate). Rats were injected with bacterial lipopolysaccharide (E. coli LPS; 150 µg/kg, i.p.), and behavioral (orectic, locomotor, and social) and physiological (thermoregulatory, cytokine, and corticosterone) inflammatory responses were measured. Among males, LPS-induced fever, suppressed locomotor activity, and inhibited feeding behavior and the magnitude of these responses were greater in Isolate relative to Group housed individuals. In contrast, among females group housing exacerbated behavioral and physiological symptoms of simulated infection. LPS treatments elicited IL-1β production in all groups, but plasma IL-1β concentrations were higher and peaked earlier in Isolate relative to Group males, and in Group relative to Isolate females. Furthermore, plasma concentrations of TNFα and IL-2 were higher in Group relative to Isolate males. Plasma corticosterone concentrations did not vary as a function of social housing conditions. Together, the data indicate that the social environment markedly influences innate immune responses. Group housing exacerbates inflammatory responses and sickness behaviors in females, but attenuates these responses in males. These sex differences are mediated in part by differential effects of the social environment on pro- and anti-inflammatory cytokine production.
Keywords: innate immune responses, IL-1β, corticosterone, sex-differences, social behavior
Introduction
Social isolation is a powerful risk factor for increased morbidity and mortality (Cacioppo et al., 2000; House et al., 2001; House et al., 1988). The stable presence or absence of familiar conspecifics has long been known to alter immune responsiveness in rodent models, with social isolation in particular generally associated with impaired measures of immune function (Edwards et al., 1980; Plaut et al., 1969; Rabin et al., 1987a; Vessey, 1964). Social isolation impacts diverse aspects of the immune system: individual- relative to group-housing results in decreased mitogen-stimulated lymphocyte proliferation (Bartolomucci et al., 2003; Jessop et al., 1988), decreased antigen-specific IgG production (Demas et al., 2004; Klein et al., 1997; Shanks et al., 1994), delayed wound healing (Detillion et al., 2004), increased parasitic load (Schuster & Schaub, 2001), and increased tumor growth following tumor cell transplantation (Kerr et al., 1997; Kerr et al., 2001; Strange et al., 2000) across different animal models. The role of social organization (i.e., solitary vs. group living species) on isolation-induced changes in immunity has received limited empirical study (but see, Klein et al., 1997).
Independent of social organization, male mammals are at greater risk than females for broad-based morbidity and mortality. This is especially true for the symptoms of bacterial infection, which are exacerbated in males relative to females (Brabin & Brabin, 1992; Grossman, 1985; Schuster & Schaub, 2001). This sexual diphenism is regulated in part by a higher expression of the LPS-binding Toll-like receptor-4 (TLR4) and CD14 in macrophages of males relative to females (Marriott et al., 2006). In addition, endogenous male sex steroids suppress some aspects of cell-mediated immune function (Gaillard & Spinedi, 1998).
Although rats are a canonical model organism for the study of psychoneuroimmunological mechanisms, interactions between sex and social organization are seldom considered in studies of immune function. We hypothesized that the social environment would alter immunological and behavioral responses to a simulated bacterial infection in a sex-specific manner. To test this hypothesis, male and female Wistar rats that had been raised in social isolation or in social groups were treated with LPS; over the days that followed, body temperature, locomotion, food intake, and social behavior were quantified. To identify immunological and neuroendocrine mechanisms that mediate social and sex differences in LPS-elicited innate immune responses, blood cytokine and corticosterone concentrations were determined during the hours before and after LPS treatment.
Materials and Methods
Animals and Photoperiod Treatments
Male and female Wistar rats (HsdRccHan:WIST) (n = 75) were bred at the University of Chicago from stock purchased from Harlan (Indianapolis, IN, USA). Pups were weaned at 21–25 days of age and housed with same-sex non-siblings in groups of 3/cage (Group) or 1/cage (Isolate). Final sample sizes are indicated in Fig. 1. In the Group condition, only one (focal) animal received the injection (LPS or saline) on any given experimental run. Otherwise, Group and Isolate animals were treated and handled in an identical manner. At all times, rats were housed in in polypropylene cages (25.9 × 47.6 × 20.9 cm) at 22±1°C and 50±5% humidity and had ad libitum access to food (Teklad 8640; Harlan, Indianapolis, IN, USA) and filtered tap water. Rats were housed under a light-dark cycle that provided 14 h of light and 10 h of darkness per day (onset of darkness: 12:00h C.S.T.). A dim (<0.1 lux) red light remained on at all times to facilitate behavioral observations during the scotophase. Experiments began when rats were 90 – 150 days of age. All procedures conformed to guidelines of “Principles of Laboratory Animal Care” (NIH publication No. 86-23, revised 1985) and the USDA Guidelines for the Care and Use of Laboratory Animals, and were pre-approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Chicago.
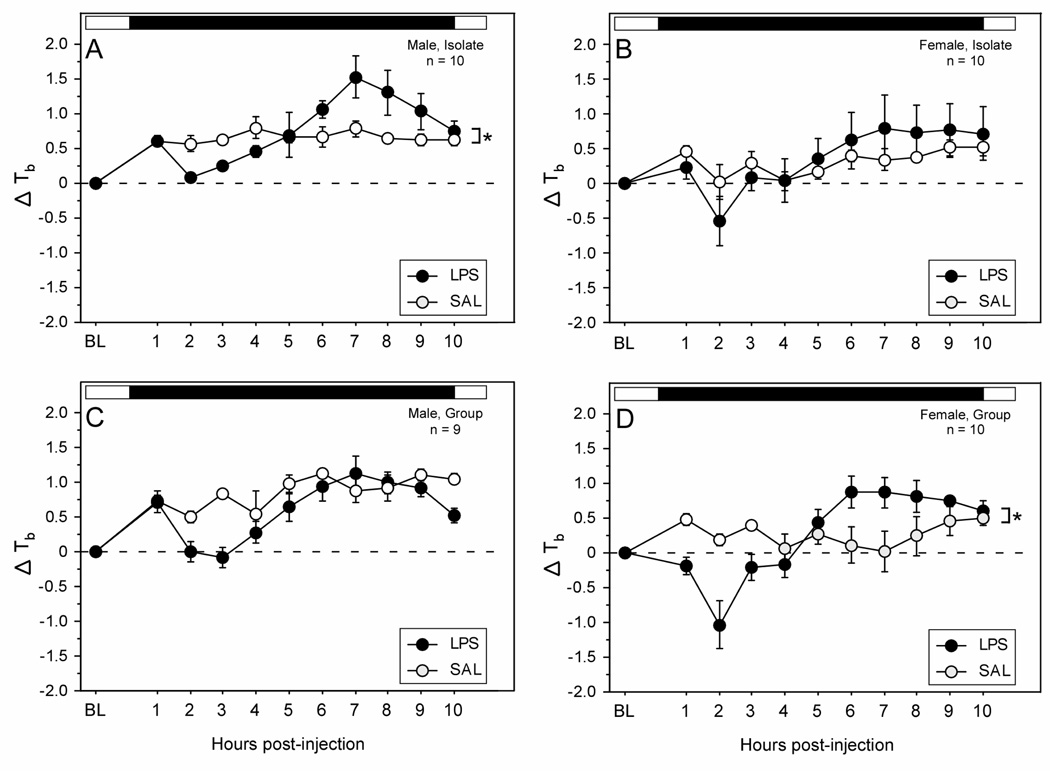
Figure 1.
Mean (±SEM) hourly change in body temperature of male (A, C) and female (B, D) Wistar rats housed 1/cage (Isolate; panels A and B) or 3/cage (Group; panels C and D) prior to and following treatment with lipopolysaccharide (150 µg/kg i.p.; LPS) or saline (at time 0). Open:filled bars above each plot indicate the daily light:dark cycle. Within each panel: * p < 0.05 vs. saline.
Body temperature and activity telemetry
Body temperature (Tb) and locomotor activity were recorded telemetrically from rats (n = 39) bearing temperature-sensitive radiotransmitters (G2 Emitter; Respironics Inc.; Bend, OR) which were implanted i.p. under deep surgical anesthesia (sodium pentobarbital, 100 mg/kg, i.p.) according to methods described in detail elsewhere (Prendergast et al., 2002). After recovery from surgery, rats were returned to their home cages and received buprenorphine (0.5 mg/kg, s.c.) as an analgesic at 12 h intervals for 48 h. One week later, Isolate and Group cages, each containing one radiotransmitter-bearing animal (i.e. the focal animal), were placed on receiver boards (ER-4000; Respironics Inc.); Tb data were collected every 5 min and transmitted to a PC using VitalView software (Respironics Inc.).
Injection treatments
After ≥ 2 days of baseline data collection, rats received an i.p. injection of either 150 µg/kg of E. coli lipopolysaccharide (LPS; serotype 0127:B8; Sigma) or 0.9% sterile physiological saline (SAL) 10–30 minutes before the onset of darkness in a counter-balanced, blocked design, with all focal animals receiving both LPS and SAL treatments in random order. In the Group condition, only one animal received the injection on any given experimental run. Injection volumes ranged from 0.1–0.4 ml. Successive injections were separated by 7 days. LPS is the biologically-active fragment of endotoxin from gram-negative bacteria; it is nonreplicating. This dose and strain of LPS was used because it reliably elicits physiological and behavioral symptoms of infection in Wistar rats (Bluthe et al., 2001; Dogan et al., 2000; Dogan et al., 2002). During the 2 days prior to, and for 3 days after, injection treatments, physiological (Tb) and behavioral (locomotor activity, food intake, social interactions) measures were obtained. Estrous cycles were not monitored in female rats. Ovarian cyclicity affects tolerance to repeated LPS injections (Engeland et al., 2006), and responsiveness to IL-1β treatments (Avitsur et al., 1995), however, naive behavioral and physiological responses to LPS do not change across the estrous cycle (Engeland et al., 2006).
Food intake
The uneaten portion of food (both food in the cage hopper and crumbs on the cage floor) was weighed (± 0.1 g) each day before the onset of darkness (11:30 – 12:00h) and again immediately after lights on (22:00 – 22:30h) to determine nightly food intake for each cage. Food weights were used to identify the magnitude of anorexia and to validate behavioral measures of feeding in Isolate rats (see Results); food weights obtained from Group cages were not further analyzed.
Behavioral data collection and analysis
Behavior in each cage was recorded individually with infrared video cameras (Q-See, Model #QSOCWC, DPSI, Anaheim, CA, USA) mounted on tripods and positioned approximately 50 cm from the side of each cage. Video output was recorded digitally on a programmable recorder (Lorex, L174V-081; Lorex Inc., Markham, Ontario, Canada). Video coding of feeding behavior and social behavior was conducted using the Etholog behavioral scoring program (Ottoni, 2000).
In some rodents, subordinate status attenuates behavioral responsiveness to LPS treatment (Cohn and de Sa-Rocha, 2006); however, social hierarchies within male and female rat groups were not characterized in the current study, thus it was not possible to determine relations between dominance/subordinance status and immune/behavioral responses to LPS.
Night time feeding behavior was quantified using a discontinuous one-zero time-sampling procedure (Tyler, 1979), in which 10 minutes of video from each of the 10 hours of the dark phase was scored by a trained observer who was blind both to the injection treatment condition and the sex of the rat. Behavioral acts scored were: (1) the number of visits to the food hopper in which the focal animal was observed eating food, and (2) the amount of time spent eating at the food hopper (in a rearing position, with head directed toward the hopper performing chewing motions). The focal animal was identified on video by the presence of stainless steel ear tags (National Band & Tag Company; Newport, KY).
Social behaviors were quantified by scoring the number of (1) social contacts initiated and (2) social contacts received by the focal (injected) rat. Social contacts between all familiar cagemates were exclusively affiliative (side-by-side contact, anogenital investigation, nose-to-nose contact, contact with forepaws); none of the observed interactions resulted in injury. In addition, the total amount of time spent huddling was also scored. Huddling was operationally defined as the duration of time the focal rat spent in direct physical contact with one or more cagemates while all participants were sedentary.
Blood collection
A separate cohort of rats (n = 36) that did not receive radiotransmitter implants provided data on cytokine and glucocorticoid responses to LPS challenge. With the exception of radiotransmitter implantation surgery, all social (Group, Isolate), sex (male, female), and injection (LPS, SAL) methods were identical to those described above. One week prior to injection treatments, rats were anesthetized using a mixture of 3% isoflurane and medical oxygen, and 1 ml of whole blood was obtained via the right retro-orbital sinus using a sterilized Pasteur pipette coated with sodium heparin. Rats were subsequently injected with 150 µg/kg LPS or SAL at the time of lights-off (12:00h C.S.T.), and additional blood samples of 500–1000 µl were obtained 2 and 6 h and 10 h after LPS treatment, as described above. Blood collection at these times were performed in the dark, assisted by a dim (<0.1 lux) overhead red light. Immediately after each blood collection event, rats were administered 1 ml of warm sterile 0.9% physiological saline s.c. All bleeding was performed in a separate room that was acoustically isolated from the main housing chamber. Final sample sizes for each treatment group are indicated in Fig. 5 and 6.
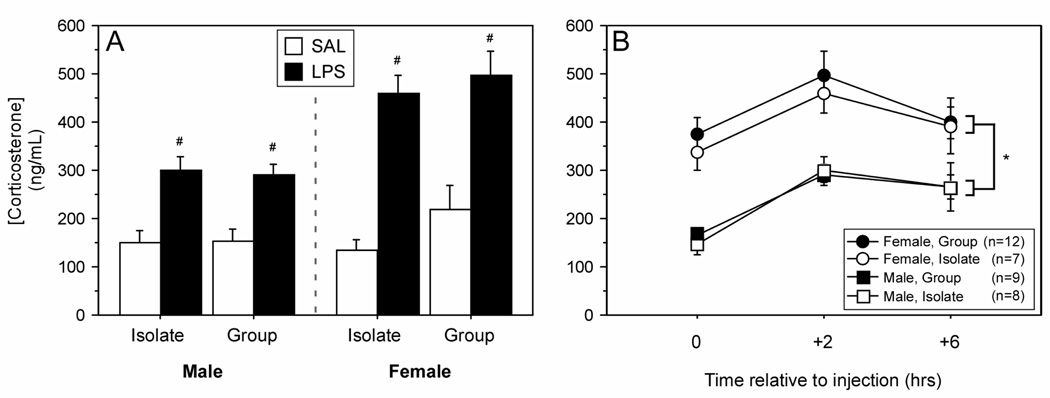
Figure 5.
Mean (±SEM) plasma concentrations of corticosterone measured by specific EIA. Male and female Wistar rats were housed 1/cage (Isolate) or 3/cage (Group) prior to (A) and following injections (B) (150 µg/kg i.p.). # p < 0.05 vs. SAL value within housing condition and sex. * p < 0.05, male vs. female.
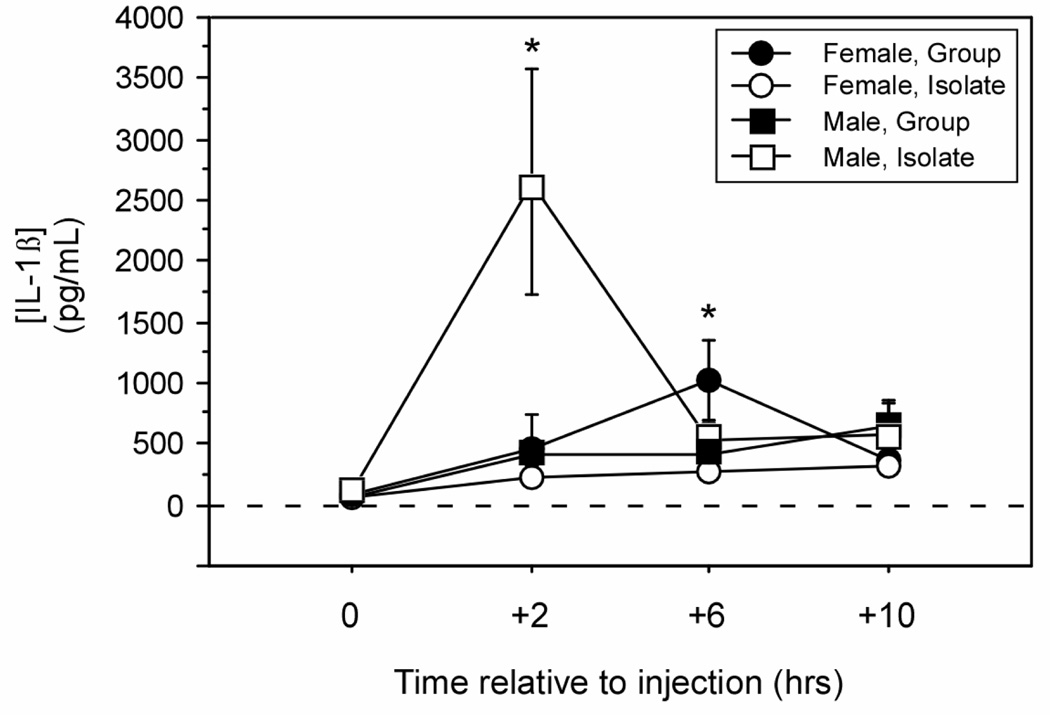
Figure 6.
Mean (±SEM) plasma concentrations of IL-1β measured by specific EIA. Male and female Wistar rats were housed 1/cage (Isolate) or 3/cage (Group) prior to and following treatment with LPS (150 µg/kg i.p.). * p < 0.05 vs. Group-male value; # p < 0.05 vs. Isolate-female value.
Following collection, blood samples were gently mixed with 100 units of sodium heparin, kept on ice for 60 – 90 min, and centrifuged at ∼300 × g for 30 min at 4°C. Plasma was stored at −80° C until assayed for IL-1β and corticosterone concentrations by enzyme-linked immunosorbent assays (EIAs; see below).
Corticosterone assay
Corticosterone was measured using an EIA (Correlate-EIA Kit, Assay Designs) according to the manufacturer’s instructions. The EIA uses a polyclonal antibody to corticosterone to competitively bind plasma corticosterone and alkaline-phosphatase conjugated corticosterone. Briefly, plasma samples were thawed at room temperature, diluted 1:30 in assay buffer, and applied to a microplate. Following incubation and automated washing (SkanWasher 400, Molecular Devices, Sunnyvale, CA), plates were read on a microplate reader at 405 nm, and values were determined by extrapolation from a standard curve using SoftMaxPro 5.0 software (Molecular Devices). The corticosterone EIA had a sensitivity of <27.0 pg/ml, an intra-assay CV of 7.7% and an inter-assay CV of 9.7%. All samples, standards, and replicates were assayed in duplicate.
IL-1β EIA assay
IL-1β was measured by EIA (Quantikine, Rat IL-1β Kit; R&D Systems) according to the manufacturer’s instructions. Briefly, plasma samples were thawed and centrifuged at room temperature, and diluted 1:3 in assay buffer. Diluted samples and standards were added in duplicate to a microplate coated with an affinity-purified polyclonal antibody specific for rat IL-1β. After binding to the immobilized antibody, wells were washed, HRP-conjugated secondary antibodies were added, wells were washed again, and a colorimetric reaction was performed to permit visualization of a reaction product. Finally, the optical density of each microplate well was read at 450 nm using a microplate reader (Emax, Molecular Devices) with correction at 570 nm. Sample values were determined by extrapolation from a standard curve. The IL-1β EIA had a sensitivity of <5.0 pg/ml, an intra-assay CV of 5.5%, and an inter-assay CV of 4.6%. All samples, standards, and replicates were assayed in duplicate.
Multiplex cytokine assay
In a subset of samples which corresponded to the peak of IL-1β production (+2 h and +6 h post-LPS treatment; as determined in the time-course above), protein concentrations of IL-1β and 8 additional cytokines (TNFα, IFNγ, GM-CSF, IL-2, IL-4, IL-6, IL-10, and IL-1α) were determined using the Bioplex Protein Array system (Bio-Rad, Hercules, CA), according to methods described in de Jager et al. (2003). Briefly, plasma samples were thawed at 20°C and assayed for cytokine levels using a multiplex bead-based immunoassay kit (Bio-Rad). Cytokine assays were performed according to the manufacturer’s protocol.
Statistical Analyses
All statistical analyses were performed using StatView 5.0.1 (SAS Institute, Cary, NC). Dependent variables were compared between sex, social conditions, and injection treatments using factorial ANOVA. Dependent variables that were sampled repeatedly were first compared with repeated-measures ANOVA with time as a within-subject factor, and separately, by timepoint. Post-hoc pairwise comparisons were conducted using Fisher’s protected least significant difference to limit experiment-wise error when the overall ANOVA was significant. Reliability of behavioral recordings for the assessment of food intake was determined by Pearson rank correlation (r). Values of r ≥ 0.70 were considered reliable; this value was chosen because, for example, with a Pearson correlation coefficient of 0.70 between food intake and food hopper visits, approximately half of the variance in food weight would be accounted for by the number of visits to the food hopper (i.e., R2 = 0.49) (Martin & Bateson, 2002). Differences were considered statistically significant if P≤0.05.
Results
Fever
Overall, males experienced significantly greater body temperature changes in response to LPS treatment than females (sex × treatment × time: F (24, 648) = 1.6, p < 0.05). In several experimental groups, LPS treatments induced a dual change in Tb, characterized by hypothermia followed by fever (Fig. 1; cf. Dogan et al., 2000). Housing condition (Isolate, Group), sex (male, female), and treatment (LPS, SAL) interacted to affect the pattern of change in Tb on the day of injection (housing × sex × injection × time : F (24,648) = 1.6, p < 0.05). Isolate males exhibited a dual change in Tb characterized by hypothermia beginning ∼2 h after LPS treatment, and followed by a fever which began ∼6 h post-treatment (Fig. 1A; p < 0.05); Group males exhibited a hypothermic response to LPS, but did not exhibit fever (Fig. 1C). In contrast to males, Isolate female rats injected with LPS did not exhibit significant changes in Tb relative to saline-injected controls (Fig. 1B). Group females, however, exhibited a dual change in Tb following LPS treatment, comparable to that observed in Isolate males (Fig. 1D; p < 0.05): hypothermia was evident beginning ∼2 h after LPS treatment, and was followed by fever occurring ∼6 h after treatment.
Locomotor activity
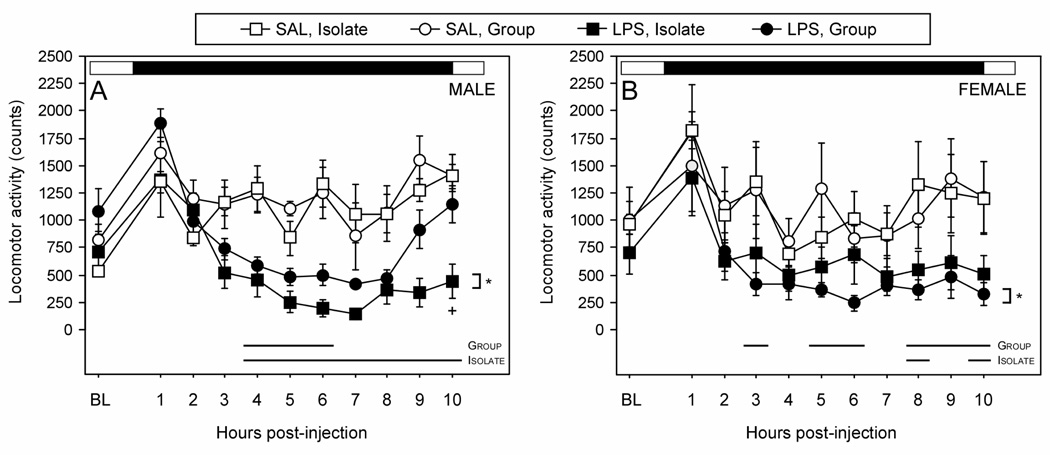
During the scotophase immediately following injection treatments, rats treated with LPS were less active in the home cage (Fig. 2; treatment × time: F (10, 310) = 9.4, p < 0.05). Overall, males were significantly more affected by LPS treatment than females (sex × treatment × time: F (10, 310) = 2.6, p < 0.05). In males, Isolates injected with LPS exhibited decreased locomotor activity throughout the scotophase in relation to SAL animals (p < 0.05), while Group males injected with LPS did not decrease activity in comparison to SAL. Group females exhibited decreased locomotor activity throughout the scotophase following LPS treatment (p < 0.05), similar to Isolate males; however, LPS treatment did not significantly decrease locomotor activity over the same timecourse in Isolate females.
Figure 2.
Mean (±SEM) hourly locomotor activity counts of male (A) and female (B) Wistar rats housed 1/cage (Isolate) or 3/cage (Group) prior to and following treatment with LPS (150 µg/kg i.p.) or saline (at time 0). The daily light:dark cycle is depicted by a black and white bar above each graph. Within each panel, * p < 0.05 vs. SAL within Housing condition. Time interval over which LPS values differed pairwise from SAL values within sex (p < 0.05) are indicated along the abscissa with dark bars.
Post-hoc pairwise comparisons at each timepoint also indicated a differential effect of housing condition across the sexes. Locomotor activity was reduced in LPS-treated Isolate males (relative to SAL) in 7 of 10 total one-hour blocks (hours 4–10) following injection (p < 0.05, all comparisons), but only in 3 of 10 total one-hour blocks (hours 4–6) following injection in Group males. In contrast to males, activity levels of Isolate females injected with LPS were diminished in only 2 of 10 total one-hour blocks (at hours 8 and 10), while Group females injected with LPS exhibited a decrease in activity in 6 of 10 total one-hour blocks (at hours 3, 5, 6, 8, 9, and 10)(p < 0.05, all comparisons).
Behavioral anorexia
Among Isolate rats, the amount of time spent at the food hopper was significantly correlated with the amount of food consumed (Pearson’s r = 0.77), validating the use of the 10-minute time sampled technique for estimating food intake (Martin and Bateson, 2002).
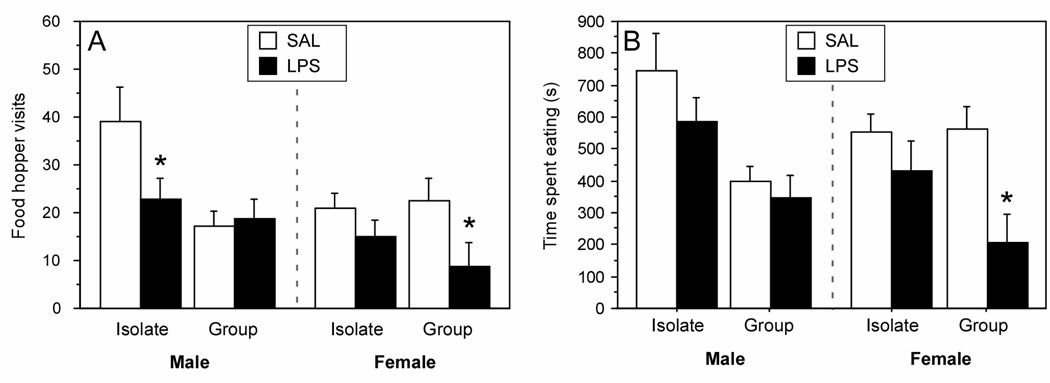
Housing condition (Isolate, Group), sex (male, female), and treatment (LPS, SAL) interacted to affect the number of visits to the food hopper (housing × sex × injection: F (1, 70) = 3.9, p < 0.05). Isolate males decreased the number of visits to the food hopper after LPS treatment relative to saline-treated controls (p < 0.05), but Group males did not differ in the number of food hopper visits after LPS treatment (p > 0.05). In contrast to males, LPS inhibited visits to the food hopper in Group females (p < 0.05), but not in Isolate females (p > 0.05).
Analyses of the total duration of time spent eating yielded a similar pattern of results (Fig. 3B). Significant main effects for housing and sex conditions revealed that males spent more time eating than females (F (1, 70) = 12.4, p < 0.05), and Isolate rats spent more time eating than Group rats (F (1, 70) = 9.2, p < 0.05). In males injected with LPS, neither Isolate nor Group rats significantly differed from saline-treated controls (p > 0.05, both comparisons). Isolate females injected with LPS did not spend less time eating relative to SAL, but Group females injected . with LPS spent significantly less time eating (p < 0.05).
Figure 3.
The total number of visits to the food hopper (A) and total duration of time spent eating (B) of male and female Wistar rats housed 1/cage (Isolate) or 3/cage (Group) and treated treatment with LPS (150 µg/kg i.p.) or SAL are presented as the mean (±SEM). Feeding behavior was sampled in 10-min bins, once per h, for the first 10 h (scotophase) following injections. * p < 0.05 vs. SAL value within sex and social condition.
Body weight
Animals treated with LPS experienced greater body weight declines the day after the injection in relation to animals treated with saline (F (1,70) = 39.5, p < 0.05). Post-hoc analysis revealed that each sex × housing condition (i.e. Isolate-male, Group-male, Isolate-female, Group-female) experienced a decline in body weight following LPS treatment compared to saline-injected controls (p < 0.05, all comparisons). There was no sex-specific effect of housing in the response to LPS (sex × housing × treatment: F (1, 70) = 0.53, p > 0.05).
Social behavior
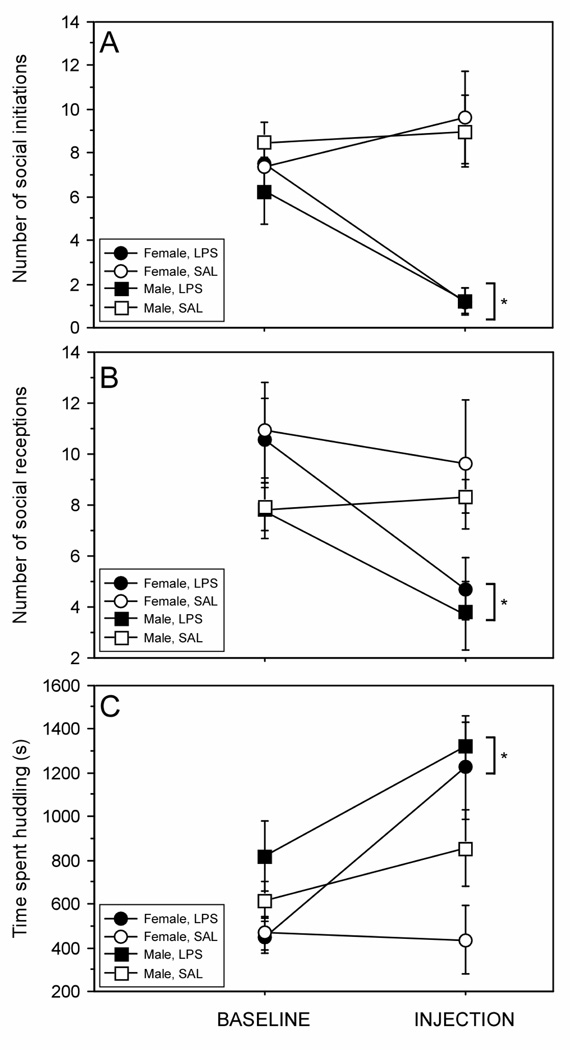
In Group male and female rats, treatment with LPS decreased the number of affiliative interaction initiations (Fig. 4A; treatment × time: F (1, 34) = 15.0, p < 0.05). However, no sex difference was evident in the effect of LPS on this measure (treatment × sex × time: F (1, 34) = 0.65, p > 0.05). LPS treatment also decreased the number of times an individual received an affiliative interaction (Fig. 4B; treatment × time: F (1, 34) = 6.07, p < 0.05). Males and females likewise did not differ in the effect of LPS on this measure of social behavior (treatment × sex × time: F (1, 34) < 0.1, p > 0.05). In contrast, huddling behavior was markedly increased following LPS treatment (Fig. 4C; treatment × time: F (1, 34) = 8.17, p < 0.05); this increase was comparable in males and females (treatment × sex × time: F (1, 34) = 2.12, p > 0.05).
Figure 4.
The total number of initiated (A) and received (B) social interactions, and the total duration of time spent in side-by-side social contact (C) of male and female Wistar rats housed 3/cage (Group) and treated with LPS (150 µg/kg i.p.) or saline are presented as the mean (±SEM). Social behavior was sampled in 10-min bins, once per h, for the first 10 h (scotophase) following LPS and saline treatment. Values represent the total frequency or duration over 10 hourly 10-minute bins at baseline (24 hours prior to treatment) and following injection. See Methods for description of behavioral criteria. * p < 0.05 vs. saline value.
Corticosterone
LPS elicited significant increases in plasma corticosterone concentrations in all groups, with peak concentrations achieved within 2 h of LPS injection (Fig. 5A; treatment × housing × sex: F (1, 60) = 74.17, p < 0.05). Corticosterone concentrations were significantly higher in females relative to males throughout the scotophase following injection treatments (Fig. 5B; sex: F (1, 32) = 54.87, p < 0.05). Corticosterone concentrations following LPS treatment were not affected by housing condition in either sex (Fig. 5B; sex × housing × time: F (1, 32) = 0.47, p > 0.05).
Cytokines
Plasma IL-1β concentrations were measured by EIA at prior to (0 h), and 2 h, 6 h, and 10 h after treatment with LPS. Both housing condition (Isolate, Group) and sex (male, female) interacted to affect both overall plasma IL-1β concentrations (sex × housing: F (1, 32) = 5.9, p < 0.05) and its pattern of change throughout the scotophase following LPS injections (Fig. 6; sex × housing × time: F (3, 96) = 5.7, p < 0.05). Peak IL-1β occurred earlier in males (at +2 h) relative to females (at +6 h). Post-hoc comparison by timepoint revealed that amongst LPS-injected males, Isolate animals displayed higher IL-1β concentrations than Group animals at 2 h post-treatment (p < 0.05); in contrast, amongst LPS-injected females, Group animals displayed higher IL-1β concentrations than Isolate animals at 6 h post-treatment (p < 0.05).
Plasma concentrations of 9 cytokines (including IL-1β) were run by multiplex at times (+2 and +6 hours, post-LPS) that corresponded to the peaks in IL-1β concentration obtained by EIA. Multiplex determination of plasma IL-1β concentrations replicated findings obtained by EIA. Housing condition and sex interacted produce a trend in overall IL-1β concentrations (sex × housing: F (1, 32) = 3.4, p = 0.07), but interacted to significantly alter IL-1β concentrations over time (sex × housing × time: F (1, 32) = 4.1, p < 0.05). Also as obtained by EIA, Isolate males exhibited higher concentrations of IL-1β production relative to Group males at 2 h post-LPS treatment (F (1,15) = 6.9, p < 0.05). However, IL-2 concentrations were elevated at 6 h (F (1, 15) = 5.6, p < 0.05) and TNFα concentrations were elevated at 2 h (F (1, 15) = 8.0, p < 0.05) in Group relative to Isolate males. Concentrations of all other cytokines were comparable between Isolate and Group animals injected with LPS (see Table 1).
Table 1.
Mean (±SEM) plasma cytokine concentrations (pg/mL) as measured 2 h and 6 h after LPS treatment (150 µg/kg i.p.) in male and female Wistar rats that were housed 1/cage (Isolate) or 3/cage (Group).
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 h | 6 h | 2 h | 6 h | |||||
| Isolate | Group | Isolate | Group | Isolate | Group | Isolate | Group | |
| IL-1β | 2291 ± 580 |
887 ± 126* |
648 ± 125 | 865 ± 144.1 |
403 ± 76 | 729 ± 374 | 814 ± 193 | 1487 ± 487 |
| IL-1α | 21 ± 4 | 23± 4 | 14 ± 6 | 21 ± 7.2 | 88 ± 69 | 14 ± 2 | 27 ± 5 | 33 ± 8 |
| IL-2 | 2619 ± 592 |
3907 ± 507 |
1976 ± 627 |
3647 ± 383.3* |
2470 ± 1093 |
3228 ± 432 |
3372 ± 1155 |
3199 ± 507 |
| IL-4 | 147 ± 30 | 235 ± 28 | 120 ± 48 | 202 ± 33.7 | 94 ± 32 | 160 ± 15 | 179 ± 51 | 187 ± 27 |
| IL-6 | 7357 ± 1080 |
9203 ± 1031 |
5310 ± 1357 |
6639 ± 672.7 |
8845 ± 1196 |
8500 ± 861 |
8693 ± 2113 |
6583 ± 815 |
| IL-10 | 464 ± 67 | 917 ± 230 | 697 ± 426 | 1117 ± 491.8 |
371 ± 59 | 400 ± 30 | 821 ± 146 | 656 ± 96 |
| TNFα | 714 ± 198 | 1741 ± 283* |
542± 300 | 880 ± 334.8 |
1384 ± 420 |
900 ± 164 | 498 ± 111 | 352 ± 35 |
| IFNγ | 579 ± 104 | 2063 ± 683 |
686 ± 431 | 1504 ± 716.9 |
494 ± 236 | 430 ± 54 | 1221 ± 556 |
918 ± 129 |
| GM- CSF |
38 ± 6 | 76 ± 19 | 64 ± 39 | 99 ± 43.2 | 31 ± 6 | 38 ± 3 | 82 ± 26 | 60 ± 11 |
p < 0.05 vs Isolate value, within sex.
Discussion
The presence of same-sex conspecifics altered behavioral and physiological responses to simulated bacterial infection differently in male relative to female rats. Male rats housed in social isolation exhibited larger changes in body temperature, greater and more enduring suppression of locomotor activity, and greater anorexia relative to males housed in social groups with familiar conspecifics. In marked contrast, among females, these symptoms of infection were greater when rats were housed in social groups, relative to in isolation. Social control of LPS-induced IL-1β production may be partially responsible for this pattern of outcomes, as IL-1β concentrations were significantly higher in Isolate males and Group females (i.e. groups that exhibited exacerbated sickness responses). No clear effect of the social environment was evident in corticosterone responses to LPS. The concordance between IL-1β production and behavioral and febrile responses to LPS obtained in the present study is consistent with an extensive literature documenting a primary role for IL-1β in the expression of sickness behaviors (Bluthe et al., 1991; Bluthe et al., 1992; Kelley et al., 1997; Kent et al., 1992). The present work suggests that the social environment exerts markedly dissimilar effects on early innate immune responses to pathogens, which culminate in sex differences in symptom severity.
Fever, anorexia, and suppression of locomotor behavior are classic sickness behaviors (Bluthe et al., 2000; Dantzer, 2001; Hart, 1988; Kelley et al., 2003) and are mediated in large part by the proinflammatory cytokines IL-1β and TNFα (Dantzer, 2001; Kent et al., 1992; Konsman et al., 2002). The present results further suggest that differences in IL-1β production may account for the observed sex differences in thermoregulation and behavior. These findings, obtained initially by EIA, were subsequently confirmed by multiplex measurements of IL-1β. Taken together, these data strongly suggest that sex and the social environment interact to affect an individual’s capacity for IL-1β production. How long or how frequently an animal must be exposed to conspecifics for the social modulation of innate immune function to manifest remains unanswered by the present results. Data on this issue, however, may have implications for how the social environment affects the immune system.
The majority of other measured cytokines did not differ systematically between Group and Isolate animals, in either sex (see Table 1). However, TNFα and IL-2 concentrations were elevated in Group relative to Isolate males at 2 h and 6 h (respectively) following LPS treatments. Increased IL-2 production accompanied by more robust behavioral and thermoregulatory responses to LPS in Group males is consistent with an anti-inflammatory role for this cytokine. IL-2 is critical for the development of (CD4+/CD25+) regulatory T cells (Tregs) (Sakaguchi et al., 2009; Setoguchi et al., 2005; Thornton et al., 2004a; Thornton et al., 2004b). Tregs can suppress proliferation of naive and memory T cells and inhibit production and release of proinflammatory cytokines through several mechanisms (Fontenot et al., 2005; Piccirillo et al., 2004; Ziegler et al., 2006). However, the degree to which Treg-mediated effects of IL-2 are relevant to the observed effects of the social environment on behavioral and physiological responses to LPS are not presently known.
LPS-induced TNFα concentrations were also elevated in Group relative to Isolate males. These data are difficult to reconcile with the established role of TNFα in mediating acute phase inflammatory responses. Paradoxically, TNFα concentrations were lowest in the experimental condition (i.e. Isolate males) that responded with the most robust behavioral, thermoregulatory, and IL-1β responses to LPS treatment. TNFα responses to LPS typically peak early (within 1 h) whereas IL-1β responses lag in time, peaking 2 to 6 hours later (Chensue et al., 1991). Perhaps lower TNFα at +2 h in Group males reflected a robust peak in TNFα in this group that was rapidly terminated. This conjecture cannot be addressed within the present dataset, as earlier (+1 h) blood samples were not obtained.
The mechanisms by which sex and the social environment interact to alter LPS-mediated IL-1β production are not known. Corticosterone, which suppresses the production of and signaling by proinflammatory cytokines (Barnes, 1998; Beishuizen & Thijs, 2003; Besedovsky et al., 1986; Del Rey et al., 1987), did not explain the observed pattern of IL-1β secretion. Consistent with prior reports (Handa et al., 1994; Kitay, 1961), females produced greater plasma corticosterone concentrations than did males. However, sex differences in corticosterone concentrations were unaffected by the social environment. This observation is consistent with previous work in mice, which demonstrated that differences in antibody production between isolated and group-housed animals were not explained by serum corticosterone concentrations (Rabin et al., 1987b).
Thermoregulatory changes were stimulated by systemic LPS administration and, in a majority of groups, resulted in a dual Tb change in which hypothermia preceded fever. Such a biphasic course of thermoregulatory responses to LPS has been reported previously, and depends on several factors, including environmental conditions, dose, host strain, and bacterial serotype (Avistur et al., 1995; Dogan et al., 2002; Kluger et al., 1998; Long et al., 1991; Moltz, 1993; Romanovsky et al., 1997). Higher doses of LPS and subthermoneutral ambient temperatures (Ta) both exacerbate the magnitude of hypo- and hyperthermia (Dogan et al., 2000; Dogan et al., 2002; Leon, 2004). We interpret greater magnitude changes in Tb (relative to saline-injected controls) to indicate a more robust acute phase response (cf. Dogan et al., 2000; 2002). Accordingly, in males isolation resulted in the greatest deviations in thermoregulation relative to saline, whereas in females group-housing produced the most profound changes in thermoregulation. Group males injected with LPS exhibited an initial decline in Tb, but no fever Isolate females injected with LPS did not differ from saline controls at any timepoint. The pattern of enhanced thermoregulatory responses to LPS in Isolate males and Group females was consistent with the pattern of IL-1β responses observed in this study.
Juvenile rats are commonly used as social stimuli in behavioral investigations of endotoxin and/or cytokines. This methodology is motivated in part by the relative absence of sexual or aggressive interactions that occur between juvenile and adult rats (Bluthe et al., 1992). In the present study the use of familiar adults rather than novel juveniles revealed an unexpected and an ecologically adaptive pattern of results. Consistent with the existing literature using juvenile stimulus animals, the initiation and reception of affiliative social behaviors with familiar adult cagemates was decreased following LPS treatment; however, the duration of time spent in side-by-side contact (huddling) with cagemates was markedly increased in LPS-treated rats. Thus, the use of age-matched familiar conspecifics has allowed identification of a social behavior, huddling, that increases in frequency during the expression of sickness behaviors. One possible function of the increased affiliative drive to huddle may be to facilitate thermoregulation during the energetically costly interval of anorexia and fever of the acute phase response (Bartolomucci, 2007). This observation underscores the concept that, rather than being omnibus in nature, social behaviors are highly specific; indeed, categorically different responses to infection may be observed when ecologically-appropriate stimuli are implemented and behavior is examined over long time intervals. The ambivalent social state that occurs during illness may provide a useful model for further investigations of how cytokines affect distinct aspects of social behavior.
Thermoregulatory, locomotor, and ingestive responses to LPS were exacerbated in Isolate males and Group females but body mass responses did not exhibit a similar pattern. Rats in all groups exhibited a comparable loss of body weight. This outcome is consistent with existing reports that observed sex differences in food intake, but that did not observe sex differences in the more integrative response of body weight (Pitychoutis et al., 2009).
A number of prior reports document sex differences, the absence thereof, and the social effects thereupon in multiple aspects of the response to LPS treatment (Ashdown et al., 2007; Engeland et al., 2003; Franklin et al., 2003; Gayle et al., 2006). It is impossible to reconcile every outcome in the present report with all prior reports on sex differences in LPS-induced sickness responses, as idiosyncratic methodological differences confound such direct comparisons. For example, in the present report we observed no significant febrile response to LPS in Isolate females, whereas Ashdown et al. (2007) reported a clear elevation in Tb among single-housed females injected with LPS. However, in Ashdown et al. LPS was administered at the dark-to-light phase transition, whereas in the present report LPS was injected at the onset of the dark phase. The nocturnal circadian rise in Tb among control-treated rats likely renders modest febrile responses statistically unresolvable. The timing of LPS treatment likewise influences subsequent changes in locomotor activity levels (Franklin et al., 2007). Additionally, in contrast to the present report, Engeland et al. (2003) reported enhanced suppression of locomotor activity by LPS in males relative to females; but differences in rat strain, LPS strain, LPS dosage and social group size preclude direct reconciliation of these data. Moreover, some reports do not specify whether group-housed LPS-treated rats are injected simultaneously or discretely. In this study, only one animal per cage was treated with LPS. It is conceivable, that the expression of sickness behaviors may be altered by the presence of sick animals in the immediate environment. Ultimately, many methodological differences - including species, host strain, strain of LPS, timing of injection, dosage, and dependent measures – preclude direct reconciliation of every outcome in the present report with prior published data. However, the present data identify sex differences in sickness behaviors that are parallelled by changes in peripheral proinflammatory cytokine production; this model may be useful for future dissections of the neuroimmune mechanisms mediating sex differences in sickness responses.
The adaptive significance of robust IL-1β and sickness response in Isolate males and Group females may be interpretable within an ecological context. Rats in nature typically live in large groups in which exposure to the opposite sex is available (Barnett, 1955; Calhoun, 1961; Calhoun, 1962; Lore & Flannelly, 1977). However, environmental conditions in laboratory studies reflect a highly contrived scenario, in which segregation from the opposite sex is the norm. According to life-history trade-off theory, animals divert finite energy resources toward physiological functions that increase reproductive success, and in polygynous mammals, maximization of reproductive success is presumed to occur by prioritization of mate quantity in males and mate quality in females (Bateman, 1948; Kelly & Jennions, 2009; Rolff, 2002). Under conditions of social isolation, male rats are devoid of sexual partners, but also of potential competitors. Therefore, when contending with an acute infection, a socially-isolated male might maximize its fitness by prioritizing robust immune resonses at the cost of maintaining characteristics that would make it attractive to potential mates. Female rats, on the other hand, typically live communally and share the same nest and burrow with other females and their young; close living quarters offer an increased opportunity for the social transmission of infectious disease. In this ecological context, female rats may have evolved to respond to group-living with enhanced innate immune responses. In general, normal (i.e. robust) proinflammatory cytokine responses may only be elicited under conditions which match a species-specific, ecologically normal (i.e., species-appropriate) scenario: solitary males and communal-living females.
A sizable literature on the clinical outcomes of human sepsis points toward a sexual diphenism characterized by increased male susceptibility (Adrie et al., 2007; Coyle et al., 2006; Schroder et al., 2000; Suffredini, 2007; Wichmann et al., 2000). Indeed, investigations into the immunological effects of gonadal steroids have demonstrated an immunoenhancing effect of estrogens and immunosuppressive effect of androgens (Jarrar et al., 2000; Knoferl et al., 2002a; Knoferl et al., 2002b; Mouihate et al., 2003; Wichman et al., 1996). However, some large-scale studies have produced inconsistent findings in which women may experience greater risk for negative outcomes than men (Crabtree et al., 1999; Eachempati et al., 1999), or intragender developmental fluctuations in circulating gonadal steroids (for example, menopause in women) fail to yield the predicted change in risk (Sperry et al., 2008). Taken together, these studies suggest that factors in addition to gonadal steroids participate in the sex differences in symptoms of infection. The results of the present experiment suggest that gender-based differences in how individuals respond to the presence or absence of social partners may play an important role in determining the innate immune response to a simulated bacterial infection.
In summary, social isolation of male rats resulted in greater LPS-induced changes in body temperature, suppression of locomotor activity, and anorexia compared to male rats living in social groups. In contrast, female rats housed in social groups exhibited greater symptoms of infection compared to those that were housed in social isolation. Taken together, the data indicate that the presence of same-sex conspecifics attenuates innate immune inflammatory responses and sickness behaviors in males, but potentiates these symptoms in females. Further investigations that specify the effect of the environmental context on host immunity may help to clarify the role of sex in immune function.
Acknowledgements
This work was supported by NIH Grant AI-67406 from the National Institute of Allergy and Infectious Diseases, NIH Grant F31-CA130267 from the National Cancer Institute, and a Social Sciences Divisional Research grant from the University of Chicago. We thank Jerome Galang, Mina Kang, and Curtis Wilkerson for expert technical assistance. This work was submitted in partial fulfillment of the requirements for the Ph.D degree in Comparative Human Development at the University of Chicago.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: All authors declare that there are no conflicts of interest.
REFERENCES
- Adrie C, Azoulay E, Francais A, Clec'h C, Darques L, Schwebel C, Nakache D, Jamali S, Goldgran-Toledano D, Garrouste-Orgeas M, Timsit JF. Influence of gender on the outcome of severe sepsis - A reappraisal. Chest. 2007;132:1786–1793. doi: 10.1378/chest.07-0420. [DOI] [PubMed] [Google Scholar]
- Ashdown H, Poole S, Boksa P, Luheshi GN. Interleukin-1 receptor antagonist as a modulator of gender differences in the febrile response to lipopolysaccharide in rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1667–R1674. doi: 10.1152/ajpregu.00274.2006. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Donchin O, Barak O, Cohen E, Yirmiya R. Behavioral effects of interleukin-1 beta: modulation by gender, estrus cycle, and progesterone. Brain Behav Immun. 1995;9:234–241. doi: 10.1006/brbi.1995.1022. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- Barnett SA. Competition among wild rats. Nature. 1955;175:126–127. doi: 10.1038/175126b0. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A. Social stress., immune functions and disease in rodents. Frontiers in Neuroendocrinology. 2007;28:28–49. doi: 10.1016/j.yfrne.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Palanza P, Sacerdote P, Ceresini G, Chirieleison A, Panerai AE, Parmigiani S. Individual housing induces altered immunoendocrine responses to psychological stress in male mice. Psychoneuroendocrinology. 2003;28:540–558. doi: 10.1016/s0306-4530(02)00039-2. [DOI] [PubMed] [Google Scholar]
- Bateman AJ. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Beishuizen A, Thijs LG. Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J Endotoxin Res. 2003;9:3–24. doi: 10.1179/096805103125001298. [DOI] [PubMed] [Google Scholar]
- Besedovsky H, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233:652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Bristow A, Lestage J, Imbs C, Dantzer R. Central injection of interleukin-13 potentiates LPS-induced sickness behavior in rats. Neuroreport. 2001;12:3979–3983. doi: 10.1097/00001756-200112210-00025. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R, Kelley KW. Interleukin-1 mediates behavioural but not metabolic effects of tumor necrosis factor alpha in mice. Eur J Pharmacol. 1991;209:281–283. doi: 10.1016/0014-2999(91)90184-r. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R, Kelley KW. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 1992;573:318–320. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Laye S, Michaud B, Combe C, Dantzer R, Parnet P. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. 2000;12:4447–4456. [PubMed] [Google Scholar]
- Brabin L, Brabin BJ. Parasitic infections in women and their consequences. Adv Parasitol. 1992;31:1–81. doi: 10.1016/s0065-308x(08)60020-2. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Ernst JM, Burleson MH, McClintock MK, Malarkey WB, Hawkley LC, Kowalewski RB, Paulsen A, Hobson JA, Hugdahl K, Spiegel D, Berntson GG. Lonely traits and concomitant physiological processes: the MacArthur social neuroscience studies. Int J Psychophysiol. 2000;35:143–154. doi: 10.1016/s0167-8760(99)00049-5. [DOI] [PubMed] [Google Scholar]
- Calhoun JB. Determinants of social organization exemplified in a single population of domesticated rats. Trans. N.Y. Acad. Sci. 1961;23:437–442. doi: 10.1111/j.2164-0947.1961.tb01371.x. [DOI] [PubMed] [Google Scholar]
- Calhoun JB. In: The ecology and sociology of the Norway rat. U.S. Dept. of Health, E., and Welfare, editor. Bethesda, MD: Public Health Service Publication; 1962. [Google Scholar]
- Chensue SW, Terebuh PD, Remick DG, Scales WE, Kunkel SL. In vivo biologic and immunohistochemical analysis of interleukin-1 alpha, beta and tumor necrosis factor during experimental endotoxemia. Kinetics, Kupffer cell expression, and glucocorticoid effects. Am J Pathol. 1991;138:395–402. [PMC free article] [PubMed] [Google Scholar]
- Cohn DW, de Sa-Rocha LC. Differential effects of lipopolysaccharide in the social behavior of dominant and submissive mice. Physiology and Behavior. 2006;87:932–937. doi: 10.1016/j.physbeh.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Coyle SM, Calvano SE, Lowry SF. Gender influences in vivo human responses to endotoxin. Shock. 2006;26:538–543. doi: 10.1097/01.shk.0000232589.39001.4d. [DOI] [PubMed] [Google Scholar]
- Crabtree TD, Pelletier SJ, Gleason TG, Pruett TL, Sawyer RG. Gender-dependent differences in outcome after the treatment of infection in hospitalized patients. Jama-Journal of the American Medical Association. 1999;282:2143–2148. doi: 10.1001/jama.282.22.2143. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Del Rey A, Besedovsky H, Sorkin E, Dinarello CA. Interleukin-1 and glucocorticoid hormones integrate an immunoregulatory feedback circuit. Ann N Y Acad Sci. 1987;496:85–90. doi: 10.1111/j.1749-6632.1987.tb35749.x. [DOI] [PubMed] [Google Scholar]
- Demas GE, Johnson C, Polacek KM. Social interactions differentially affect reproductive and immune responses of Siberian hamsters. Physiol Behav. 2004;83:73–79. doi: 10.1016/j.physbeh.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Detillion CE, Craft TK, Glasper ER, Prendergast BJ, DeVries AC. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29:1004–1011. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Dogan MD, Ataoglu H, Akarsu ES. Effects of different serotypes of Escherichia coli lipopolysaccharides on body temperature in rats. Life Sci. 2000;67:2319–2329. doi: 10.1016/s0024-3205(00)00821-3. [DOI] [PubMed] [Google Scholar]
- Dogan MD, Ataoglu H, Akarsu ES. Characterization of the hypothermic component of LPS-induced dual thermoregulatory response in rats. Pharmacol Biochem Behav. 2002;72:143–150. doi: 10.1016/s0091-3057(01)00736-5. [DOI] [PubMed] [Google Scholar]
- Eachempati SR, Hydo L, Barie PS. Gender-based differences in outcome in patients with sepsis. Archives of Surgery. 1999;134:1342–1347. doi: 10.1001/archsurg.134.12.1342. [DOI] [PubMed] [Google Scholar]
- Edwards EA, Rahe RH, Stephens PM, Henry JP. Antibody response to bovine serum albumin in mice: the efffects of psychosocial environmental change. Proc Soc Exp Biol Med. 1980;164:478–481. doi: 10.3181/00379727-164-40899. [DOI] [PubMed] [Google Scholar]
- Engeland CG, Kavaliers M, Ossenkopp KP. Sex differences in the effects of muramyl dipeptide and lipopolysaccharide on locomotor activity and the development of behavioral tolerance in rats. Pharmacol Biochem Behav. 2003;74:433–447. doi: 10.1016/s0091-3057(02)01024-9. [DOI] [PubMed] [Google Scholar]
- Engeland CG, Kavaliers M, Ossenkopp KP. Influence of the estrous cycle on tolerance development to LPS-induced sickness behaviors in rats. Psychoneuroendocrinology. 2006;31:510–525. doi: 10.1016/j.psyneuen.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Franklin AE, Engeland CG, Kavaliers M, Ossenkopp KP. Lipopolysaccharide-induced hypoactivity and behavioral tolerance development are modulated by the light-dark cycle in male and female rats. Psychopharmacology (Berl) 2003;170:399–408. doi: 10.1007/s00213-003-1554-3. [DOI] [PubMed] [Google Scholar]
- Franklin AE, Engeland CG, Kavaliers M, Ossenkopp KP. The rate of behavioral tolerance development to repeated lipopolysaccharide treatments depends upon the time of injection during the light-dark cycle: a multivariable examination of locomotor activity. Behav Brain Res. 2007;180:161–173. doi: 10.1016/j.bbr.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Gaillard RC, Spinedi E. Sex- and stress-steroids interations and the immune system: evidence for a neuroendocrine-immunological sexual dimorphism. Domestic Animal Endocrinology. 1998;15:345–352. doi: 10.1016/s0739-7240(98)00028-9. [DOI] [PubMed] [Google Scholar]
- Gayle DA, Desai M, Casillas E, Beloosesky R, Ross MG. Gender-specific orexigenic and anorexigenic mechanisms in rats. Life Sci. 2006;79:1531–1536. doi: 10.1016/j.lfs.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Grossman CJ. Interactions between the gonadal steroids and the immune system. Science. 1985;227:257–261. doi: 10.1126/science.3871252. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- House JS. Social isolation kills, but how and why? Psychosomatic Medicine. 2001;63:273–274. doi: 10.1097/00006842-200103000-00011. [DOI] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Jarrar D, Wang P, Knoferl MW, Kuebler JF, Cioffi WG, Bland KI, Chaudry IH. Insight into the mechanism by which estradiol improves organ functions after trauma-hemorrhage. Surgery. 2000;128:246–252. doi: 10.1067/msy.2000.107376. [DOI] [PubMed] [Google Scholar]
- Jessop JJ, Gale K, Bayer BM. Time-dependent enhancement of lymphocyte activation by mitogens after exposure to isolation or water scheduling. Life Sci. 1988;43:1133–1140. doi: 10.1016/0024-3205(88)90472-9. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17 Suppl 1:S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Hutchison K, French R, Bluthe RM, Parnet P, Johnson RW, Dantzer R. Central interleukin-1 receptors as mediators of sickness. Ann N Y Acad Sci. 1997;823:234–246. doi: 10.1111/j.1749-6632.1997.tb48395.x. [DOI] [PubMed] [Google Scholar]
- Kelly CD, Jennions MD. Sexually dimorphic immune response in the harem polygynous Wellington tree weta Hemideina crassidens. Physiological Entomology. 2009;34:174–179. [Google Scholar]
- Kent S, Bluthe RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, Vannice JL. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci U S A. 1992;89:9117–9120. doi: 10.1073/pnas.89.19.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr LR, Grimm MS, Silva WA, Weinberg J, Emerman JT. Effects of social housing condition on the response of the Shionogi mouse mammary carcinoma (SC115) to chemotherapy. Cancer Res. 1997;57:1124–1128. [PubMed] [Google Scholar]
- Kerr LR, Hundal R, Silva WA, Emerman JT, Weinberg J. Effects of social housing condition on chemotherapeutic efficacy in a Shionogi carcinoma (SC115) mouse tumor model: influences of temporal factors, tumor size, and tumor growth rate. Psychosom Med. 2001;63:973–984. doi: 10.1097/00006842-200111000-00017. [DOI] [PubMed] [Google Scholar]
- Kitay JI. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- Klein SL, Hairston JE, DeVries AC, Nelson RJ. Social environment and steroid hormones affect species and sex differences in immune function among voles. Hormones and Behavior. 1997;32:30–39. doi: 10.1006/hbeh.1997.1402. [DOI] [PubMed] [Google Scholar]
- Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D. Role of fever in disease. Ann N Y Acad Sci. 1998;856:224–233. doi: 10.1111/j.1749-6632.1998.tb08329.x. [DOI] [PubMed] [Google Scholar]
- Knoferl MW, Angele MK, Diodato MD, Schwacha MG, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002a;235:105–112. doi: 10.1097/00000658-200201000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoferl MW, Angele MK, Schwacha MG, Bland KI, Chaudry IH. Preservation of splenic immune functions by female sex hormones after trauma-hemorrhage. Crit Care Med. 2002b;30:888–893. doi: 10.1097/00003246-200204000-00029. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Leon LR. Hypothermia in systemic inflammation: role of cytokines. Front Biosci. 2004;9:1877–1888. doi: 10.2741/1381. [DOI] [PubMed] [Google Scholar]
- Long NC, Morimoto A, Nakamori T, Murakami N. The effect of physical restraint on IL-1 beta- and LPS-induced fever. Physiol Behav. 1991;50:625–628. doi: 10.1016/0031-9384(91)90556-4. [DOI] [PubMed] [Google Scholar]
- Lore R, Flannelly K. Rat Societies. Scientific American. 1977;236:106–116. doi: 10.1038/scientificamerican0577-106. [DOI] [PubMed] [Google Scholar]
- Marriott I, Bost KL, Huet-Hudson YM. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: A possible mechanism for gender-based differences in endotoxic shock susceptibility. Journal of Reproductive Immunology. 2006;71:12–27. doi: 10.1016/j.jri.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Martin P, Bateson P. Measuring behaviour: An introductory guide. Cambridge, UK: Cambridge University Press; 2002. [Google Scholar]
- Moltz H. Fever: causes and consequences. Neurosci Biobehav Rev. 1993;17:237–269. doi: 10.1016/s0149-7634(05)80009-0. [DOI] [PubMed] [Google Scholar]
- Mouihate A, Pittman QJ. Neuroimmune response to endogenous and exogenous pyrogens is differently modulated by sex steroids. Endocrinology. 2003;144:2454–2460. doi: 10.1210/en.2002-0093. [DOI] [PubMed] [Google Scholar]
- Ottoni EB. EthoLog 2.2: a tool for the transcription and timing of behavior observation sessions. Behav Res Methods Instrum Comput. 2000;32:446–449. doi: 10.3758/bf03200814. [DOI] [PubMed] [Google Scholar]
- Piccirillo CA, Thornton AM. Cornerstone of peripheral tolerance: naturally occurring CD4+CD25+ regulatory T cells. Trends Immunol. 2004;25:374–380. doi: 10.1016/j.it.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Pitychoutis PM, Nakamura K, Tsonis PA, Papadopoulou-Daifoti Z. Neurochemical and behavioral alterations in an inflammatory model of depression: sex differences exposed. Neuroscience. 2009;159:1216–1232. doi: 10.1016/j.neuroscience.2009.01.072. [DOI] [PubMed] [Google Scholar]
- Plaut SM, Ader R, Friedman SB, Ritterson AL. Social factors and resistance to malaria in the mouse: effects of group Vs individual housing on resistance to Plasmodium berghei infection. Psychosom Med. 1969;31:536–552. doi: 10.1097/00006842-196911000-00007. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Freeman DA, Zucker I, Nelson RJ. Periodic arousal from hibernation is necessary for initiation of immune responses in ground squirrels. Am J Physiol Regulatory Integrative Comp Physiol. 2002;282:R1054–R1062. doi: 10.1152/ajpregu.00562.2001. [DOI] [PubMed] [Google Scholar]
- Rabin BS, Lyte M, Hamill E. The influence of mouse strain and housing on the immune response. Journal of Neuroendocrinology. 1987a;17:11–16. doi: 10.1016/0165-5728(87)90027-0. [DOI] [PubMed] [Google Scholar]
- Rabin BS, Lyte M, Hamill E. The influence of mouse strain and housing on the immune response. J Neuroimmunol. 1987b;17:11–16. doi: 10.1016/0165-5728(87)90027-0. [DOI] [PubMed] [Google Scholar]
- Rolff J. Bateman's principle and immunity. Proc Biol Sci. 2002;269:867–872. doi: 10.1098/rspb.2002.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanovsky AA, Kulchitsky VA, Akulich NV, Koulchitsky SV, Simons CT, Sessler DI, Gourine VN. The two phases of biphasic fever--two different strategies for fighting infection? Ann N Y Acad Sci. 1997;813:485–490. doi: 10.1111/j.1749-6632.1997.tb51737.x. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Wing K, Yamaguchi T. Dynamics of peripheral tolerance and immune regulation mediated by Treg. Eur J Immunol. 2009;39:2331–2336. doi: 10.1002/eji.200939688. [DOI] [PubMed] [Google Scholar]
- Schroder J, Kahlke V, Book M, Stuber F. Gender differences in sepsis: Genetically determined? Shock. 2000;14:307–310. [PubMed] [Google Scholar]
- Schuster JP, Schaub GA. Experimental Chagas disease: the influence of sex and psychoneuroimmunological factors. Parasitol Res. 2001;87:994–1000. doi: 10.1007/s004360100474. [DOI] [PubMed] [Google Scholar]
- Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Renton C, Zalcman S, Anisman H. Influence of change from grouped to individual housing on a T-cell-dependent immune response in mice: antagonism by diazepam. Pharmacol Biochem Behav. 1994;47:497–502. doi: 10.1016/0091-3057(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Sperry JL, Nathens AB, Frankel HL, Vanek SL, Moore EE, Maier RV, Minei JP. Characterization of the gender dimorphism after injury and hemorrhagic shock: Are hormonal differences responsible? Critical Care Medicine. 2008;36:1838–1845. doi: 10.1097/CCM.0b013e3181760c14. [DOI] [PubMed] [Google Scholar]
- Strange KS, Kerr LR, Andrews HN, Emerman JT, Weinberg J. Psychosocial stressors and mammary tumor growth: an animal model. Neurotoxicol Teratol. 2000;22:89–102. doi: 10.1016/s0892-0362(99)00049-5. [DOI] [PubMed] [Google Scholar]
- Suffredini AF. Systemic inflammation and sexual dimorphism: more than meets the eye. Crit Care Med. 2007;35:1610–1612. doi: 10.1097/01.CCM.0000266793.09378.22. [DOI] [PubMed] [Google Scholar]
- Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004a;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- Thornton AM, Piccirillo CA, Shevach EM. Activation requirements for the induction of CD4+CD25+ T cell suppressor function. Eur J Immunol. 2004b;34:366–376. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- Tyler S. Time-sampling: A matter of convention. Animal Behaviour. 1979;27:801–810. [Google Scholar]
- Vessey SH. Effects of Grouping on Levels of Circulating Antibodies in Mice. Proc Soc Exp Biol Med. 1964;115:252–255. doi: 10.3181/00379727-115-28883. [DOI] [PubMed] [Google Scholar]
- Wichmann MW, Inthorn D, Andress HJ, Schildberg FW. Incidence and mortality of severe sepsis in surgical intensive care patients: the influence of patient gender on disease process and outcome. Intens Care Med. 2000;26:167–172. doi: 10.1007/s001340050041. [DOI] [PubMed] [Google Scholar]
- Wichmann MW, Zellweger R, DeMaso CM, Ayala A, Chaudry IH. Mechanism of immunosuppression in males following trauma-hemorrhage. Archives of Surgery. 1996;131:1186–1192. doi: 10.1001/archsurg.1996.01430230068012. [DOI] [PubMed] [Google Scholar]
- Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]