Abstract
Background
Age prevalence curves from areas endemic for schistosomiasis suggest that humans develop partial immunity to reinfection beginning in early adolescence. We conducted a 2-year longitudinal study to determine if children infected with S. mansoni develop protection-related immune responses upon treatment with PZQ and if the development of these immune responses is accelerated by frequent treatment upon reinfection.
Methods
8-10 year old children were tested for S. mansoni every four months and treated with praziquantel (PZQ) when positive (Arm A, N=68) or tested and treated at the end of the 2-year follow-up (Arm B, N=49).
Results
Children in Arm A who remained free of infection during follow-up had significantly higher baseline levels of schistosome-specific IgE than children with ≥2 repeat S. mansoni diagnoses. Children with ≥2 repeat S. mansoni diagnoses significantly increased their levels of anti-schistosome IgE and CD23+ B cells after receiving ≥3 PZQ treatments over the course of follow-up. No increase in either parameter was seen in children who received only the baseline PZQ treatment.
Conclusions
B cell activation and anti-schistosomal IgE are associated with resistance to S. mansoni in children, and these immunological parameters can be increased by multiple rounds of infections and PZQ-induced cures.
Keywords: schistosomiasis, children, praziquantel, IgE, B lymphocytes
Introduction
Schistosoma mansoni age prevalence curves from endemic areas suggest that intensity and prevalence of infection peak in the early teen years. Prevalence then plateaus while infection intensity sharply declines as individuals enter the third decade of life. Immunologic studies suggest that the decline in intensity is in part attributable to development of immunity to new infections [1-3]. As the lifespan of S. mansoni worms is approximately 5-10 years [4, 5], this resistance to reinfection coincides with the time at which worms from the initial infection begin to die. These findings have lead to the hypothesis that worm death, rather than worm maintenance, is responsible for inducing resistance to reinfection [6]. We have previously shown that adult males occupationally exposed to S. mansoni developed increased resistance to reinfection upon repeated cycles of treatment, reinfection, and retreatment [7].
The most consistent immune parameter associated with resistance to reinfection is increased levels of schistosome-specific IgE [8-11]. B lymphocytes are the producers of all immunoglobulins, including IgE, and recently we have reported an association between the CD23+ B cell subset and increased resistance to reinfection in our cohort of adult males [12]. CD23 is the low affinity IgE receptor (FceRII) and its expression on B cells is in part considered an indication of their maturity [13]. CD23 binds to a variety of membrane and soluble molecules, such as CD21, CD11b, CD11c and IgE and in its soluble form can act as a B cell proliferation factor [14]. The functional roles of the “a” and “b” isoforms of membrane-bound and soluble CD23 include B cell development, IgE binding, cell adhesion, antigen presentation to T cells and the regulation of IgE synthesis [15-19].
It has been postulated that resistance to reinfection can develop sooner than in the early adolescence in areas of high endemnicity or where there are programs leading to early treatment of infections in children [20-22]. World Health Assembly Resolution 54.19 recommends periodic mass treatment of children with the drug praziquantel (PZQ) in areas endemic for schistosomiasis. Although intended to control morbidity, the periodic killing of adult worms might have the additional benefit of hastening the development of resistance to reinfection by inducing premature worm death. However, the appropriate interval at which treatment should be given to control morbidity or enhance resistance to reinfection has not been extensively evaluated in different epidemiologic settings. The purpose of the current study was to determine if 8-10 year old children infected with S. mansoni develop protective immune responses upon treatment with PZQ and if the development of these anti-schistosome immune responses is accelerated by more frequent treatment over a two-year time period.
Materials and Methods
Study population
All subjects began the study as 8-10 year old children recruited from eight primary schools located within three kilometers of Lake Victoria in the Asembo Bay area of the Nyanza Province in western Kenya. The area is highly endemic for S. mansoni. A 2001 survey of schools in this area found S. mansoni prevalence ranging from 35-80% [23].
After an initial screening of 485 children, 155 of the 179 children diagnosed positive for S. mansoni were enrolled in a 2-year longitudinal study. Children were assigned into treatment Arm A (N=88) or Arm B (N=67). Assignments were made by school except in the case of one school with the largest number of students and the highest S. mansoni prevalence. Students in this school were randomized to Arm A or Arm B. The final study population consisted of 68 children from Arm A (77.3%) and 49 children from Arm B (73.1%) who completed the 2-year follow-up.
Study procedures
In the baseline survey, all consenting children aged 8-10 years attending the study schools were tested for the presence of S. mansoni eggs by the Kato-Katz method using 2 slides from a single stool sample. Children positive for S. mansoni were then asked to enroll in a 2-year longitudinal study and assigned to arms A or B as described above. At baseline and each follow-up encounter, children provided a stool sample for testing for S. mansoni eggs as well as Ascaris lumbricoides, hookworm, and Trichuris trichiura by the Kato-Katz method, again by 2 slides from a single stool sample. Children were also bled by venipuncture for immunological testing as well as testing for malaria parasitemia via Giemsa-stained blood smears. Children infected with S. mansoni, soil-transmitted helminths (STHs), or malaria were treated with PZQ (40 mg/kg), albendazole (400 mg), or Co-artem (6 doses administered over 3 days), respectively.
Children in Arm A were tested and treated every 4 months, for a total of 6 follow-up encounters following baseline testing. Children in Arm B were tested and treated only at baseline and again at the end of the 2-year follow-up.
At study entry, a short questionnaire was administered to students in both study arms in which they were asked to characterize how often they went to the lake to do such activities as bathing, swimming, or fishing as: almost every day; sometimes (once a week); rarely (once a month); or never. A similar questionnaire was administered to the parents regarding the water contact behavior of their child. All questionnaires were verbally administered in the local language.
Written informed consent was obtained from the parent/guardian of each child prior to enrollment, and the children gave verbal assent for participation in the study. Study procedures were approved by the institutional review boards of the University of Georgia and the Centers for Disease Control and Prevention, the Scientific Steering Committee of the Kenya Medical Research Institute (KEMRI), and the KEMRI/National Ethics Review Committee of Kenya.
Immunological testing
Antibody evaluation
Assays for soluble worm antigen preparation (SWAP)-specific IgE were performed on plasma from the venous blood using a standardized ELISA as previously described [24]. An external positive control (PC) comprised of a pooled sample of high-responding adults and a negative control of normal human serum (NHS) from non-endemic volunteers was run on each plate. The optical density of the anti-SWAP IgE value for each sample was standardized according to the following formula:
(Sample − NHS) / (PC − NHS)
Whole blood phenotyping
EDTA-anticoagulated whole blood was analyzed by flow cytometry. Data acquisition and analysis were done on a dual laser FACS Calibur flow cytometer using FlowJo software. B cells were defined as cells that were CD19 positive. Gating to separate CD23-negative B cells from CD23-positive B cells was based on florescence minus one (FMO) stained samples as previously reported [12].
Statistical analyses
The Wilcoxon rank sum test or the Kruskal-Wallis test was used for two-sample or multiple-sample group comparisons, respectively. The Wilcoxon signed rank test was used for paired comparisons of the same children at different time points. All analyses were performed with GraphPad Prism 5 or SAS version 9.1.
Results
Baseline demographics
Baseline demographic characteristics of the study population are shown in Table 1. There were no significant differences between the two study arms in intensity of S. mansoni infection, gender, or coinfection with malaria or soil-transmitted helminths. Both groups had a similarly high frequency of self-reported water contact, with approximately 95% of children reporting contact with the lake at least once per month, and >85% reporting weekly contact with the lake.
Table 1.
Baseline demographic characteristics of study arms A and B
| Arm A N=68 |
Arm B N=49 |
p-value | |
|---|---|---|---|
| Gemetric mean S. mansoni eggs per gram feces (95% CI)) | 94 (66, 133) | 79 (56, 110) | 0.5599 |
| Gender (% male) | 55.9 | 57.1 | 0.8926 |
| Plasmodium falciparum prevalence (%) | 28.1 | 21.4 | 0.4545 |
| Soil-transmitted helminth prevalence (%) | 57.4 | 40.4 | 0.0756 |
| Any water contacta (%) | 94.9 | 95.7 | 0.8421 |
| Frequent water contactb (%) | 84.8 | 89.4 | 0.4877 |
Contact with lake at least once a month
Contact with lake at least once a week
In addition, there were no significant differences in any of these demographic factors between those children that did and did not complete the entire two years of follow-up.
S. mansoni infection over time
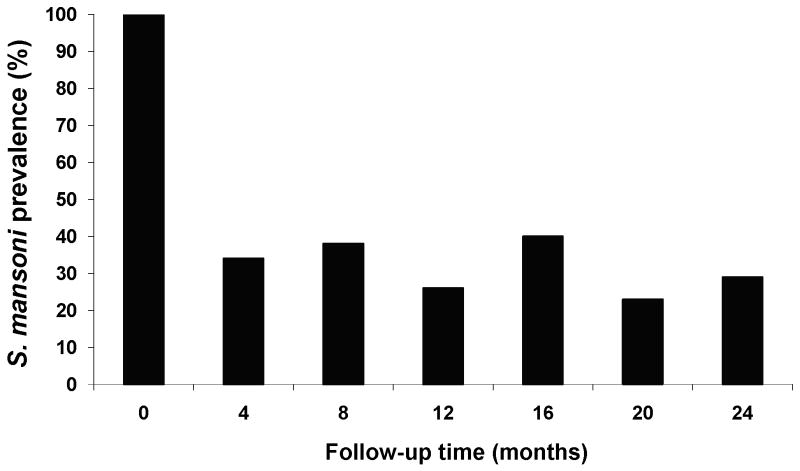
The prevalence of S. mansoni infection at each follow-up timepoint in Arm A is shown in Figure 1. Prevalence was reduced to 34% four months after the initial PZQ treatment. However, children became rapidly reinfected in this endemic area. Even with infected children being diagnosed and treated at four-month intervals, prevalence of S. mansoni never fell below 23% during the two years of follow-up.
Figure 1.
Prevalence of S. mansoni infection at each follow-up timepoint in Arm A
Thirty-eight children (56%) in Arm A had two or more positive S. mansoni diagnoses following treatment for the S. mansoni infection present at baseline and prior to the two-year follow-up. Nineteen children (28%) had one repeat S. mansoni diagnosis during the course of follow-up, and 11 children (16%) went at least two years without becoming reinfected with S. mansoni, although 2 of these children were infected at the 24 month follow-up.
Geometric mean baseline egg counts (95% confidence interval) in children with 0, 1, and ≥2 repeat S. mansoni diagnoses were 54 (18, 159) 64 (32, 127), and 135 (87, 209) eggs per gram feces, respectively. Although there was a trend towards higher initial intensity of infection in children with a greater number of repeat S. mansoni diagnoses, the difference between the groups only approached statistical significance (p=0.0591). There were no differences in self-reported or parent-reported water contact between these groups.
Forty-three percent of children in Arm B were infected with S. mansoni at the 2-year follow-up, which approximates the overall baseline prevalence of 37% found in the initial survey of these schools. Mean intensity of infection among stool-positive children at the 2-year follow-up was 99 (±172) epg in Arm A and 172 (±219) epg in Arm B (difference between arms not significant, p=0.2975).
Changes in anti-SWAP IgE
Standardized anti-SWAP IgE levels at baseline and 2-year follow-up in Arms A and B are shown in Figure 2. Although anti-SWAP IgE slightly increased over the 2-year period, there were no statistically significant differences between baseline and 2-year follow-up anti-SWAP IgE in either arm. There were also no statistically significant differences in baseline anti-SWAP IgE between Arms A and B nor final anti-SWAP IgE between arms A and B.
Figure 2.
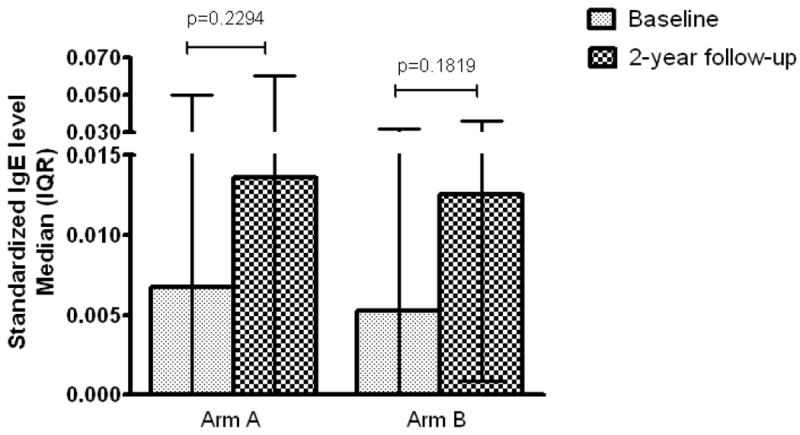
Anti-SWAP IgE at baseline and 2-year follow-up in Arms A and B
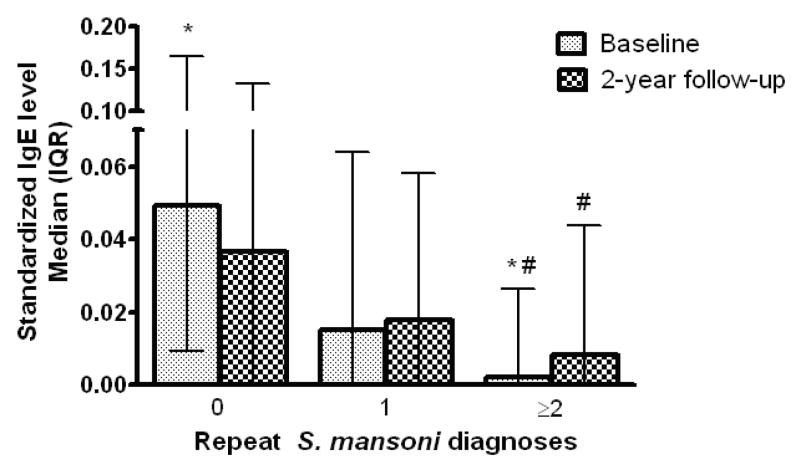
Figure 3 depicts standardized anti-SWAP IgE levels at baseline and final follow-up for children in Arm A stratified by the number of repeat S. mansoni diagnoses during the two years of follow-up. The baseline anti-SWAP IgE level in children with ≥2 repeat diagnoses was significantly lower that the baseline anti-SWAP IgE in the children who went at least 2 years without becoming reinfected. There was also a significant trend for decreasing baseline anti-SWAP IgE among children with 0, 1, and ≥2 repeat S. mansoni diagnoses (p=0.0019). Children who had ≥2 repeat diagnoses, and thus received a total of 3 or more treatments with PZQ over the two year follow-up, demonstrated significant increases in their anti-SWAP IgE between baseline and the final 2-year follow-up. There were no significant changes in anti-SWAP IgE over time in those children without a repeat diagnosis and thus who received only the initial PZQ treatment at baseline, nor in those children with one repeat diagnosis (total of 2 PZQ treatments, Figure 3).
Figure 3.
Anti-SWAP IgE at baseline and final follow-up in Arm A, stratified by the number of PZQ treatments received during the 2-year follow-up. *Difference in baseline anti-SWAP between 0 and ≥2 diagnoses groups p<0.05; # Difference between baseline and final bleed p<0.05 by Wilcoxon paired test.
Changes in CD23+ B cells
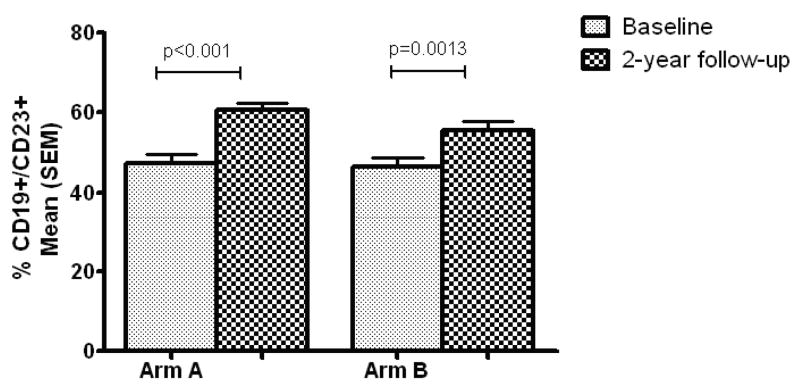
The mean percentage of CD23+ B cells in children in both study arms at baseline and final follow-up is shown in Figure 4. The percentage of CD23+ B cells significantly increased between baseline and the two-year follow-up in both arm A and arm B, but the difference in % CD23+ B cells between the two arms at the two-year time point was not statistically significant (p=0.0761).
Figure 4.
Percent CD19+/CD23+ cells at baseline and 2-year follow-up in Arms A and B
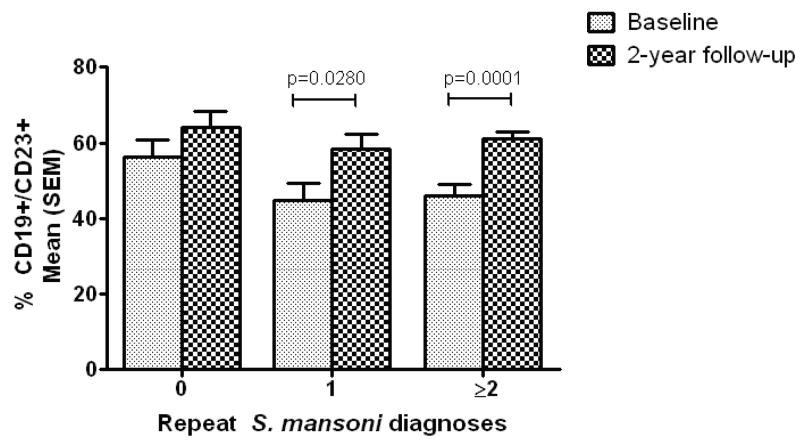
When Arm A was stratified by the number of repeat S. mansoni diagnoses during follow-up (Figure 5), changes in CD23+ B cells showed a pattern similar to that seen with anti-SWAP IgE levels. Although the difference did not reach statistical significance (p=0.0979), children that were not reinfected before the 2-year follow-up had a higher percentage of CD23+ B cells at baseline that those children who had 2 or more repeat S. mansoni diagnoses over the course of follow-up. The percentage of CD23+ B cells increased between baseline and follow-up in children who had two or more repeat S. mansoni diagnoses over the course of follow-up, and therefore received ≥3 PZQ treatments, as well as in those children who had one repeat diagnosis and received 2 PZQ treatments. However, the percent of CD23+ B cells did not change significantly in those children who did not get reinfected before the final follow-up and therefore had received only the initial PZQ treatment.
Figure 5.
Percent CD19+/CD23+ cells at baseline and final follow-up, stratified by the number of PZQ treatments received during the 2-year follow-up
Co-infection with malaria and soil-transmitted helminths
There was no difference in baseline anti-SWAP IgE level between children co-infected with S. mansoni and P. falciparum and children without P. falciparum (standardized ODs: 0.058 versus 0.055, p=0.2819) or between children co-infected with S. mansoni and STHs and children without STHs (0.062 versus 0.046, p=0.9426). There was also no difference in CD23+ B cells between children co-infected with S. mansoni and P. falciparum and children without P. falciparum (49.3% versus 46.9%, p=0.5327). There was a higher percentage of CD23+ B cells in children co-infected with S. mansoni and STHs than in children without STHs (57.8% versus 41.5%, p=0.0184). However, the difference in CD23+ B cells in children infected with STHs and those uninfected with STHs did not persist at the first follow-up 4 months after treatment with ALB and/or PZQ (50.8% in STH infected children and 51.6% in uninfected children). This was true regardless of S. mansoni infection status at 4 months (data not shown). At no other time throughout the course of follow-up did children infected with STHs have a higher percentage of CD23+ B cells than children not infected with STHs.
Discussion
Our data suggest a high force of S. mansoni transmission in this area along Lake Victoria in western Kenya. After two years of diagnosing and treating infected children every four months, the prevalence of S. mansoni in Arm A of our cohort was still approximately 30%. This is close to the overall prevalence of 37% found in our initial survey of 8-10 year old children in the study area. More than half (56%) of the children in Arm A had 2 or more positive S. mansoni diagnoses during the course of two years after treatment of the initial infection. Since we did not assess cure after each treatment, we cannot be certain that each positive diagnosis was a reinfection rather than a cure failure. Since the cure rate of PZQ is >70% after one dose [25, 26], the majority of these positive diagnoses likely represented true cures and reinfections.
The original design of the study was to examine differences in immune parameters between children kept as free from S. mansoni infection as possible for 2 years (Arm A) and children treated only once 2 years prior (Arm B). However, when Arm A was analyzed as a group, we did not find a difference in levels of schistosome-specific IgE between arms A and B at the end of the study. What we did not anticipate was that some children in this area had developed an apparent level of resistance to reinfection prior to age 8-10 when they were enrolled in this study, as they did not become reinfected for two years following one PZQ treatment. When Arm A was stratified according to the number of S. mansoni diagnoses each child had during follow-up, which is a close approximation to how many times the child was reinfected, those children that did not get reinfected for at least 2 years after treatment of their baseline infection had significantly higher levels of anti-SWAP IgE and borderline significantly higher levels of CD23+ B cells than the more susceptible children with at least 2 repeat S. mansoni diagnoses (and likely ≥2 reinfections) within 2 years. After 3 or more PZQ treatments, both anti-SWAP IgE and the percentage of CD23+ B cells had significantly increased in the more susceptible children, indicating that frequent treatment of infected children can drive immune responses to more closely resemble those of the children who remained uninfected over the course of follow-up.
Numerous studies have reported an association between higher levels of schistosome-specific IgE and resistance to reinfection with S. mansoni [8-11]. We recently reported increases in anti-SWAP IgE that paralleled the change from a state of susceptibility to S. mansoni infection to a state of increased resistance to reinfection in our cohort of adult males undergoing repeated cycles of treatment and reinfection [20]. We also recently reported a correlation between the percentage of CD23+ B cells and the level of resistance to S. mansoni reinfection in our cohort of adult males, suggesting that B cells expressing CD23 may play a role in the development of protective immunity [12]. CD23 is the low affinity receptor for IgE and has shown to be essential for IgE-mediated enhancement of specific immune responses in murine models [28].
We did not see an increase in anti-SWAP IgE in children who received 2 PZQ treatments during the course of follow-up. However, this group was likely a combination of children who failed to cure after the initial PZQ treatment, children who had already developed an intermediate level of resistance, and children who had more than one reinfection but were not diagnosed as repeat positives due to the insensitivity of the Kato-Katz test on a single stool. While there was an overall increase in CD23+ B cells over the two-year period in both study arms, this was not unexpected as studies have shown that the proportion of B lymphocytes expressing CD23 slowly increase until age 12 in normal pediatric populations [29, 30]. However, within Arm A, the increase was more dramatic in the more susceptible children who received 3 or more PZQ treatments than in the more resistant children who only required 1 initial PZQ treatment.
A high level of co-infection between S. mansoni and malaria and STHs was present in this population. We found no association between levels of schistosome-specific IgE and co-infection with either malaria or STHs. Children co-infected with STHs and S. mansoni at baseline had a statistically significantly higher percentage of CD23+ B cells than children without STH infection. A higher percentage of CD23+ B cells in co-infected children was only seen at baseline when they likely had long term S. mansoni and STH infections. The difference did not persist after baseline treatment of their infections.
In conclusion, we found that some children in this highly endemic area exhibited a phenotype indicative of resistance to S. mansoni reinfection by 8-10 years of age. Infections in lakeside communities have been shown to begin very early in life [31], and some of our 8-10 year old subjects could have been infected for 7-9 years at the time of their enrollment, providing an opportunity for them to have already experienced dying worms. The resistant phenotype was characterized by high schistosome-specific IgE and elevated levels of CD23+ B cells. Frequent PZQ treatment of more susceptible children increased these immune responses towards protective levels. The effect of PZQ treatment on the production of anti-SWAP IgE and CD23+ B cells was obscured when data were analyzed without accounting for differences in prior development of apparent resistance to S. mansoni.
Acknowledgments
This work is published with the permission of the Director, Kenya Medical Research Institute. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Financial support: This work was supported by PHS grants R01 AI053695 (DGC) and T32 AI060546 (CLB) from the NIAID and D43 TW007123 (PNMM) from the FIC of the National Institutes of Health; Centers for Disease Control and Prevention (WES); and the Kenya Medical Research Institute (EMOM, PNMM, DMSK)
Footnotes
Conflicts of interest: None reported
Presentations at meetings: Portions of this work were presented by Carla L. Black at the American Society for Tropical Medicine and Hygiene 58th Annual Meeting, November 18-22, Washington, D.C. Abstract # 381.
References
- 1.Kabatereine NB, Vennervald BJ, Ouma JH, Kemijumbi J, Butterworth AE, Dunne DW, Fulford AJC. Adult resistance to schistosomiasis mansoni: age-dependence of reinfection remains constant in communities with diverse exposure patterns. Parasitology. 1999;118:101–5. doi: 10.1017/s0031182098003576. [DOI] [PubMed] [Google Scholar]
- 2.King CH, Keating CE, Muruka JF, Ouma JM, Houser H, Siongok TKA, Mahmoud AAF. Urinary tract morbidity in schistosomiasis haematobia: associations with age and intensity of infection in an endemic area of Coast Province, Kenya. Am J Trop Med Hyg. 1988;39:361–8. doi: 10.4269/ajtmh.1988.39.361. [DOI] [PubMed] [Google Scholar]
- 3.Wilkins HA, Goll PH, Marshall TFD, Moore PJ. Dynamics of Schistosoma haematobium infection in a Gambian community. III. Acquisition and loss of infection. Trans R Soc Trop Med Hyg. 1984;78:227–32. doi: 10.1016/0035-9203(84)90283-9. [DOI] [PubMed] [Google Scholar]
- 4.Fulford AJ, Butterworth AE, Ouma JH, Sturrock RF. A statistical approach to schistosome population dynamics and estimation of the life span of Schistosoma mansoni in man. Parasitology. 1995;110(Pt 3):301–16. doi: 10.1017/s0031182000080896. [DOI] [PubMed] [Google Scholar]
- 5.Warren KS, Mahmoud AAF, Cumming P, Murphy DJ, Houser HB. Schistosomiasis mansoni in Yemeni in California: duration of infection, presence of disease, therapeutic management. Am J Trop Med Hyg. 1974;23:902–9. doi: 10.4269/ajtmh.1974.23.902. [DOI] [PubMed] [Google Scholar]
- 6.Woolhouse MEJ, Hagan P. Seeking the ghost of worms past. Nature Med. 1999;5:1225–27. doi: 10.1038/15169. [DOI] [PubMed] [Google Scholar]
- 7.Karanja DMS, Hightower AW, Colley DG, et al. Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and effect of HIV-1 co-infection on susceptibility to schistosomiasis: a longitudinal study. Lancet. 2002;360:592–6. doi: 10.1016/S0140-6736(02)09781-7. [DOI] [PubMed] [Google Scholar]
- 8.Dunne DW, Butterworth AE, Fulford AJC, et al. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992;22:1483–94. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- 9.Caldas IR, Correa-Oliveira R, Colosimo E, Carvalho OS, Massara CL, Colley DG, Gazzinelli G. Susceptibility and resistance to Schistosoma mansoni reinfection: parallel cellular and isotypic immunologic assessment. Am J Trop Med Hyg. 2000;62:57–64. doi: 10.4269/ajtmh.2000.62.57. [DOI] [PubMed] [Google Scholar]
- 10.Webster M, Fallon PG, Fulford AJC, Butterworth AE, Ouma JH, KImani G, Dunne DW. Effect of praziquantel and oxamniquine treatment on human isotype responses to Schistosoma mansoni: elevated IgE to adult worm. Parasite Immunol. 1997;19:333–5. doi: 10.1046/j.1365-3024.1997.d01-211.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhaosong Z, Haiwei W, Suchen C, et al. Association between IgE antibody against soluble egg antigen and resistance to reinfection with Schistosoma japonicum. Trans R Soc Trop Med Hyg. 1997;91:606–8. doi: 10.1016/s0035-9203(97)90047-x. [DOI] [PubMed] [Google Scholar]
- 12.Mwinzi PNM, Ganley-Leal L, Black CL, Secor WE, Karanja DMS, Colley DG. Circulating CD23+ B cell subset correlates with the development of resistance to Schistosoma mansoni reinfection in occupationally exposed adults who have undergone multiple treatments. J Infect Dis. 2009;199:272–9. doi: 10.1086/595792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suryani S, Fulcher DA, Santner-Nanan B, et al. Differential expression of CD21 identifies developmentally and functionally distinct subsets of human transitional B cells. Blood. 2010;115:519–529. doi: 10.1182/blood-2009-07-234799. [DOI] [PubMed] [Google Scholar]
- 14.McClosky N, Hunt J, Beavil RL, et al. Soluble CD23 monomers inhibit and oligomers stimulate IgE synthesis in human B cells. J Biol Chem. 2007;282:24083–24091. doi: 10.1074/jbc.M703195200. [DOI] [PubMed] [Google Scholar]
- 15.Aubry JP, Pochon S, Graber P, Jansen KU, Bonnefoy JY. CD21 is a ligand for CD23 and regulates IgE production. Nature. 1992;358:505–507. doi: 10.1038/358505a0. [DOI] [PubMed] [Google Scholar]
- 16.Su TT, Guo B, Wei B, Braun J, Rawlings DJ. Signaling in transitional type 2 B cells is critical for peripheral B-cell development. Immunol Rev. 2004;197:161–178. doi: 10.1111/j.0105-2896.2004.0102.x. [DOI] [PubMed] [Google Scholar]
- 17.Hjelm F, Karlsson MC, Heyman B. A novel B cell-mediated transport of IgE-immune complexes to the follicle of the spleen. J Immunol. 2008;180:6604–6610. doi: 10.4049/jimmunol.180.10.6604. [DOI] [PubMed] [Google Scholar]
- 18.Rambert J, Mamani-Matsuda M, Moynet D, et al. Molecular blocking of CD23 supports its role in the pathogenesis of arthritis. PLoS One. 2009;4:e4834. doi: 10.1371/journal.pone.0004834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stief A, Texido G, Sansig G, Eibel H, Le Gros G, van der Putten H. Mice deficient in CD23 reveal its modulatory role in IgE production but no role in T and B cell development. J Immunol. 1994;152:3378–3390. [PubMed] [Google Scholar]
- 20.Mutapi F, Ndhlovu PD, Hagan P, Woolhouse MEJ. A comparison of humoral responses to Schistosoma haematobium in areas with low and high levels of infection. Parasite Immunol. 1997;19:255–63. doi: 10.1046/j.1365-3024.1997.d01-206.x. [DOI] [PubMed] [Google Scholar]
- 21.Mutapi F, Ndhlovu PD, Hagan P, et al. Chemotherapy accelerates the development of acquired immune responses to Schistosoma haematobium infection. J Infect Dis. 1998;178:289–93. doi: 10.1086/517456. [DOI] [PubMed] [Google Scholar]
- 22.Mutapi F, Hagan P, Woolhouse MEJ, Mduluza T, Ndhlovu PD. Chemotherapy-induced, age-related changes in antischistosome antibody responses. Parasite Immunol. 2003;25:87–97. doi: 10.1046/j.1365-3024.2003.00610.x. [DOI] [PubMed] [Google Scholar]
- 23.Handzel T, Karanja DMS, Addiss DG, et al. Geographic distribution of schistosomiasis and soil-transmitted helminthes in western Kenya: implications for antihelminthic treatment. Am J Trop Med Hyg. 2003;69:318–23. [PubMed] [Google Scholar]
- 24.Ganley-Leal LM, Mwinzi PN, Cetre-Sossah CB, et al. Correlation between eosinophils and protection against reinfection with Schistosoma mansoni and the effect of human immunodeficiency virus type 1 coinfection in humans. Infect Immun. 2006;74:2169–76. doi: 10.1128/IAI.74.4.2169-2176.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gryseels B, Nkulikyinka L, Coosemans MH. Field trials of praziquantel an oxamniquine for the treatment of schistosomiasis mansoni in Burundi. Trans R Soc Trop Med Hyg. 1987;81:641–44. doi: 10.1016/0035-9203(87)90439-1. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. The Control of Schistsomiasis. WHO Technical Report Series. 1993:1–86. [PubMed] [Google Scholar]
- 27.Black CL, Mwinzi PNM, Muok EMO, et al. Influence of exposure history on the immunology and development of resistance to human schistosomiasis mansoni. PLoS Negl Trop Dis. 2009 doi: 10.1371/journal.pntd.0000637. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujiwara H, Kikutani H, Suematsu S, et al. The absence of IgE antibody-mediated augmentation of immune responses in CD23-deficient mice. Proc Natl Acad Sci USA. 1994;91:6835–9. doi: 10.1073/pnas.91.15.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veneri D, Ortolani R, Franchini M, Tridente G, Pizzolo G, Vella A. Expression of CD27 and CD23 on peripheral blood B lymphocytes in humans of different ages. Blood Transfus. 2009;7:29–34. doi: 10.2450/2008.0007-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erkeller-Yuksel FM, Deneys V, Yuksel B, et al. Age-related changes in human blood lymphocyte populations. J Pediatr. 1992;120:216–22. doi: 10.1016/s0022-3476(05)80430-5. [DOI] [PubMed] [Google Scholar]
- 31.Odogwu SE, Ramamurthy NK, Kabatereine NB, et al. Schistosoma mansoni in infants (aged <3 years) along the Ugandan shoreline of Lake Victoria. Ann Trop Med Parasitol. 2006;100:315–26. doi: 10.1179/136485906X105552. [DOI] [PubMed] [Google Scholar]