Abstract
Astrocytes communicate with neurons, endothelial and other glial cells through transmission of intercellular calcium signals. Satellite glial cells (SGCs) in sensory ganglia share several properties with astrocytes, but whether this type of communication occurs between SGCs and sensory neurons has not been explored. In the present work we used cultured neurons and SGCs from mouse trigeminal ganglia to address this question. Focal electrical or mechanical stimulation of single neurons in trigeminal ganglion cultures increased intracellular calcium concentration in these cells and triggered calcium elevations in adjacent glial cells. Similar to neurons, SGCs responded to mechanical stimulation with increase in cytosolic calcium that spread to the adjacent neuron and neighboring glial cells. Calcium signaling from SGCs to neurons and among SGCs was diminished in the presence of the broad-spectrum P2 receptor antagonist suramin (50 µM) or in the presence of the gap junction blocker carbenoxolone (100 µM), whereas signaling from neurons to SGCs was reduced by suramin, but not by carbenoxolone. Following induction of submandibular inflammation by Complete Freund’s Adjuvant injection, the amplitude of signaling among SGCs and from SGCs to neuron was increased, whereas the amplitude from neuron to SGCs was reduced. These results indicate for the first time the presence of bidirectional calcium signaling between neurons and SGCs in sensory ganglia cultures, which is mediated by the activation of purinergic P2 receptors, and to some extent by gap junctions. Furthermore, the results indicate that not only sensory neurons, but also SGCs release ATP. This form of intercellular calcium signaling likely plays key roles in the modulation of neuronal activity within sensory ganglia in normal and pathological states.
Keywords: Gap junctions, neuron–glial communication, orofacial hypersensitivity, purinergic receptors
INTRODUCTION
Intercellular calcium waves (ICWs) provide a mechanism for long-range signaling between astrocytes (see Scemes and Giaume, 2006; Scemes and Spray, 2008 for reviews). Transmission of ICWs can involve both gap junction-mediated direct cytosol-to-cytosol diffusion of second messengers such as IP3 and Ca2+, and release of molecules such as ATP that diffuse through extracellular space, interacting with receptors on neighboring cells to mobilize calcium from the intracellular and extracellular compartments (Scemes and Giaume, 2006).
The main type of glial cell in sensory ganglia is the satellite glial cell (SGC). These cells share many properties with astrocytes of the central nervous system (CNS), including expression of glutamine synthetase and various neurotransmitter transporters (for a review see Hanani, 2005). Moreover, SGCs presumably fulfill similar roles in clearance of extracellular potassium from active neurons and also metabolite delivery and removal from neurons. However, SGCs differ in some respect from astrocytes, particularly by their tight enwrapment of neuronal cell bodies (Pannese, 1981), which enables them to be highly responsive to neuronal somatic ATP release, as recently shown in dorsal root ganglia (Zhang et al., 2007). SGCs in both dorsal root and trigeminal ganglia are coupled by gap junctions (Hanani et al., 2002; Cherkas et al., 2004; Huang et al., 2005) and express purinergic receptors (Weick et al., 2003; Zhang et al., 2007; Ceruti et al., 2008). The presence of these receptors is noteworthy as they have been shown to be involved in the extracellular mediation of ICW spread in astrocytes (Scemes and Giaume, 2006; Scemes and Spray, 2008). Also, SGCs possess mechanisms for release of cytokines (Takeda et al., 2007; Zhang et al., 2007) and possibly other chemical messengers.
It is likely that SGCs communicate with neurons chemically, but currently little is known about this topic (Hanani, 2005; Takeda et al., 2007). Moreover, whether intercellular communication through transmission of ICWs can occur in peripheral ganglia has not been previously investigated, with the exception of myenteric ganglia (Zhang et al., 2003; Gulbransen and Sharkey, 2009). The findings reported in this study provide evidence that sensory neurons and SGCs can communicate through transmission of Ca2+ signals and that this form of signaling depends on both activation of purinergic receptors and gap junction coupling.
OBJECTIVE
We tested the hypothesis that neurons and SGCs in mouse trigeminal ganglia communicate via the transmission of ICWs. We used short term co-cultures of neurons and SGCs and measured changes in intracellular Ca2+ with the Ca2+ indicator Fura-2.
METHODS
All animal handling and experimental protocols were approved by the Animal Institute and Committee of the Albert Einstein College of Medicine.
Animals and trigeminal ganglion culture preparation
C57BL/6 mice of either sex (2–3 months old; Charles River Laboratories, Boston, MA, USA) were anesthetized with isoflurane (Abbott Laboratories, IL, USA) and euthanized by decapitation. The skull was then opened and the brain removed, exposing both trigeminal ganglia. The ganglia were transferred into cold Dulbecco’s phosphate-buffered saline (DPBS, pH 7.4; Mediatech Cellgro, Herdon, VA, USA). Next, the ganglia were cleaned from connective tissue and transferred to a 1.5 ml vial containing DPBS and collagenase (1 mg/ml, Type 1A; Sigma, St. Louis, MO, USA). The vial was then placed on a tilting stage (with a rate of 50 min−1) for 45 min at 37°C. After this digestion period, the collagenase dissociation solution was substituted by fresh DPBS, the ganglia were then triturated by repeated pipetting until complete tissue homogenization, spun down (1000 RPM for 5 min), resuspended and placed in glass-bottomed MatTek dishes (MatTek, Ashland, MA, USA) in Dulbecco’s modified Eagle medium (GIBCO, Invitrogen, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (GIBCO) and 1% penicillin–streptomycin (Mediatech Cellgro) and transferred to a humidified 5% CO2 incubator at 37°C for 1 – 3 days.
Orofacial tactile hypersensitivity model
Submandibular inflammation was induced by injecting 20 µl of 1:1 Complete Freund’s Adjuvant (CFA) solution (Sigma, St. Louis, MO, USA) in saline under the submandibular skin of mice anesthetized with xylazine (3 mg/kg) and ketamine (135 mg/kg). Controls were injected with 20 µl of saline. Seven days after treatment the animals were euthanized and the trigeminal ganglia were removed and cultured as described above.
Imaging of intracellular calcium transients and induction of intercellular Ca2+ signaling in trigeminal ganglion cultures
Cultures grown in MatTek dishes were loaded with the ratio-metric intracellular Ca2+ indicator Fura-2AM (10 µM; Molecular Probes, Eugene, OR, USA) for 45 min at 37°C in a CO2 humidified incubator. Following this, the cells were rinsed and bathed in Tyrode solution [(in mM) 137.0 NaCl, 2.7 KCl, 0.5 MgCl2, 1.8 CaCl2, 12.0 NaHCO3, 0.5 NaH2PO4, 5.5 glucose, and 5 HEPES; pH 7.4] throughout the experimental procedure. Changes in Fura-2 fluorescence intensities emitted at two excitation wavelengths (340 and 380 nm) were acquired at 1.0 Hz after background and shading correction using filters and shutter (Lambda DG-4, Sutter Instruments, Novato, CA, USA) driven by a computer through Metafluor software (Molecular Devices, Downingtown, PA, USA) as previously described (Suadicani et al., 2004, 2006). Intracellular Ca2+ levels determined from regions of interest placed on cells were obtained from Fura-2 ratio images using an in vitro calibration curve as previously described (Suadicani et al., 2004). Briefly, fluorescence ratio values obtained online from regions of interest placed on cells were translated into intracellular Ca2+ concentrations following the in vitro calibration curve , where [Ca2+]i is the calculated intracellular calcium concentration, KD is the dissociation constant of free Ca2+ for Fura-2 (KD = 224 nM) (Grynkiewicz et al., 1985), R is the ratio intensity, Rmin is the ratio of the intensity obtained at zero calcium, Rmax is the ratio of the intensity at saturated calcium, is the fluorescence intensity measured at zero calcium at 380 nm and is the fluorescence intensity measured with saturated calcium at 380 nm. Cells were visualized using a Nikon TE2000-U inverted microscope equipped with 40× S Fluor objective (Nikon, Japan) and a CoolSnap HQ2 CCD camera (Photometrics, Tucson, AZ, USA). Changes in intracellular Ca2+ concentration ≥100 nM were considered as a response. Intercellular Ca2+ signaling was induced by either focal electrical or mechanical stimulation. Single neurons were stimulated electrically using patch-type pipettes with resistance ~4 MΩ when filled with extracellular solution. The pipettes were placed rather far (~5–10 µm) above the neuron somata, so as to avoid possible mechanical stimulation due to tip movement. Under this condition, increases in intracellular Ca2+ level were observed at stimulus duration from 0.5 to 10 ms and 40–60 V. For this study, pulse duration was set at 10 ms, when we obtained consistent and reversible responses from stimulated neurons. Single SGCs and also neurons were stimulated mechanically using glass micropipettes driven by a micromanipulator. For each experimental condition, at least six fields from at least three independent cultures were analyzed and averaged. ‘N’ in text and figure legends refers either to total number of fields analyzed or number of responding cells. Transmission of Ca2+ signals in the absence or presence of suramin (50 µM; Sigma-Aldrich St. Louis, MO, USA) or carbenoxolone (100 µM; Sigma-Aldrich) was compared in relation to the Amplitude of the Ca2+ signal generated in the stimulated and in the responding cells, and transmission Efficacy (the number of responding/total number of cells in each field of view) (Suadicani et al., 2004). In experiments where ICWs were induced, the velocity of ICW spread was also quantified (Suadicani et al., 2004). In the pharmacological experiments the antagonists were added to the bath solution at least 15 min before the measurements were done.
Statistics
Values of amplitude and efficacy determined in the presence of the pharmacological blockers were compared to control values using unpaired t-test and considered significantly different at the level of P < 0.05.
RESULTS
Trigeminal ganglion cultures
Cultures were used 2–3 days after their preparation. At this time after plating the cultures were typically constituted by neurons closely apposed by glial cells (see Fig 1 and Fig 2), whose identity could be confirmed by their positive immunoreactivity for the marker tubulin β III isoform and glutamine synthetase, respectively (our unpublished results and Ceruti et al., 2008). Viability of neurons in the cultures was confirmed by their ability to fire action potentials (data not shown). At days 2–3 after culturing many SGCs migrate a short distance from the parent neurons and are distinctly visible. These cells were selected for the mechanical stimulation, to avoid direct stimulation of the neurons.
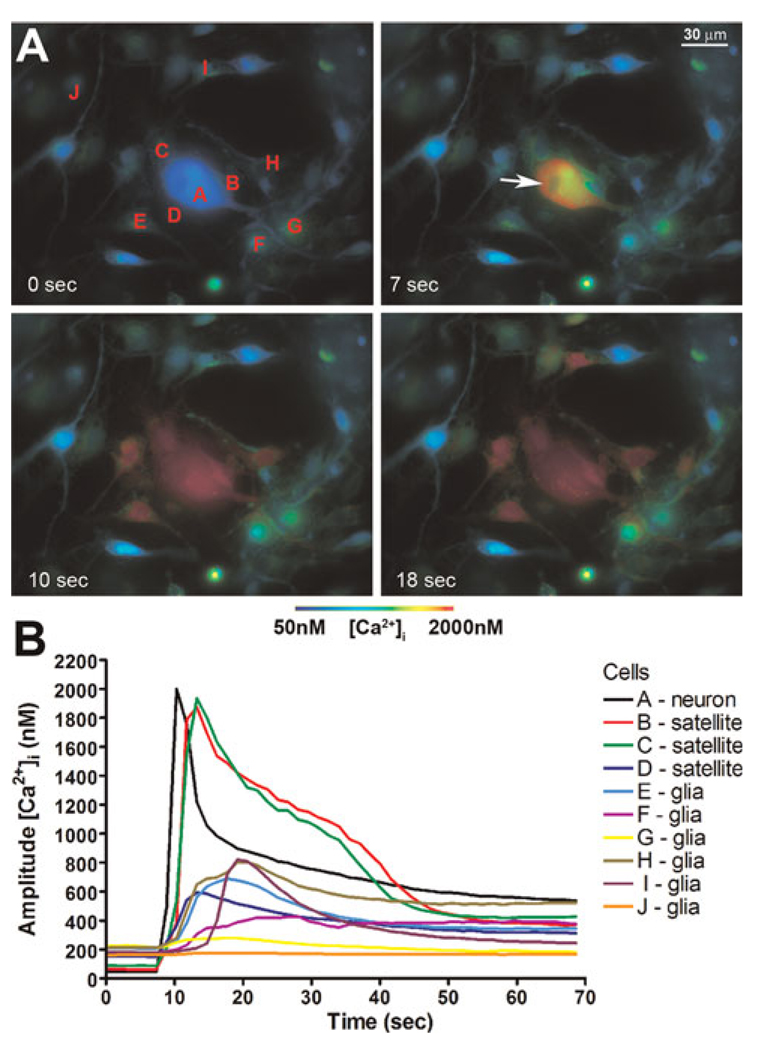
Fig. 1. Focal electrical stimulation of single sensory neurons triggers intercellular calcium wave (ICW) spread in trigeminal ganglion cultures.
Cultures were loaded with the intracellular Ca2+ indicator Fura-2 AM and changes in intracellular Ca2+ levels were monitored by epifluorescence. (A) Pseudo-colored time-lapse display of ICW spread induced by focal electrical stimulation of a single neuron. Note the prompt increase in intracellular Ca2+ level of the stimulated neuron (cell A, Panel A) that was transmitted not only to adjacent satellite glial cells (SGCs, cells B–D in Panel A) but also to other glial cells (i.e. cells E–I, Panel A) located farther from the stimulated neuron. (B) Graphic representation of the ICW spread triggered by electrical stimulation of the neuron ‘A’ depicted in Panel A.
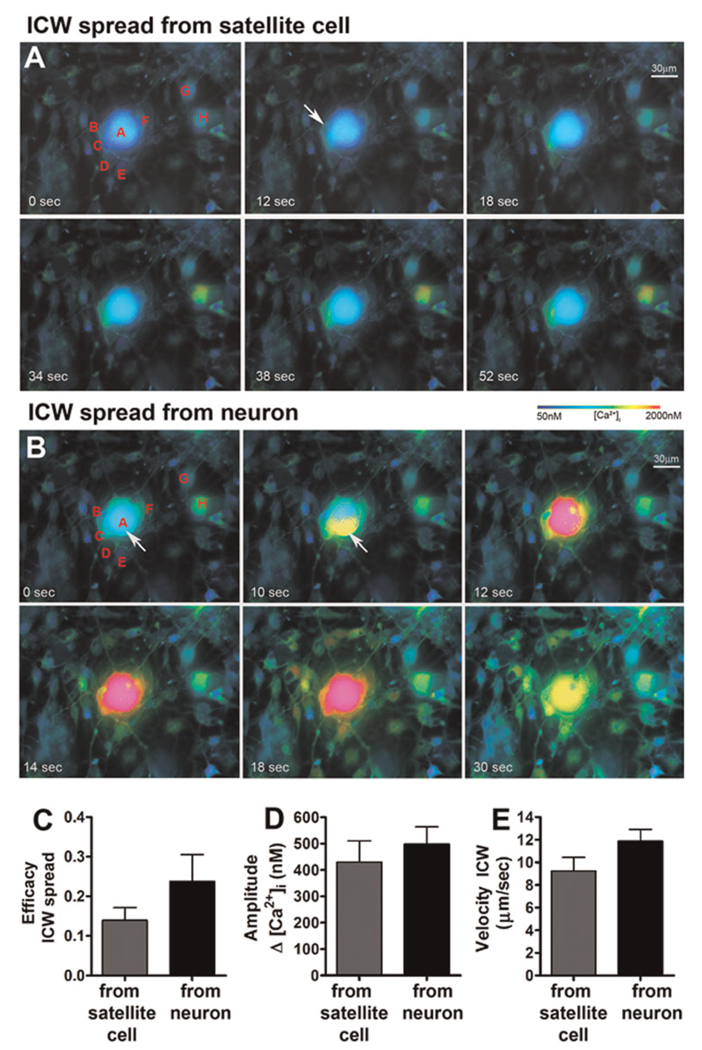
Fig. 2. Calcium signals generated in satellite glial cells (SGCs) and sensory neurons by mechanical stimulation can be communicated to neighboring cells in trigeminal ganglion cultures.
Cultured cells were loaded with the intracellular Ca2+ indicator Fura-2 AM and changes in intracellular Ca2+ levels were monitored by epifluorescence. (A) Pseudo-colored time lapse display of intercellular Ca2+ signaling in a trigeminal ganglion culture induced first by focal mechanical stimulation of one of the SGCs (cell B) and then (B) by stimulation of a sensory neuron (cell A). Stimulation of SGC ‘B’ (Panel A, white arrow) induced responses in the adjacent SGCs (cells C and D), in the neuron ‘A’ and in a few other glial cells (i.e. cells G–H) located more distantly from the stimulated SGC. Stimulation of neuron ‘A’ (Panel B, white arrow) induced increase in the intracellular Ca2+ of not only the previously stimulated SGC ‘B’, demonstrating the bidirectionality of the Ca2+ signaling between neuron–SGC, but also in the other SGCs (cells C–F) and in the majority of glial cells located more remotely in the field of the stimulated neuron. Comparison of the (C) efficacy (number of responding cells per total number of cells in the field), (D) amplitude of the Ca2+ signal generated in responding cells and (E) velocity of the Ca2+ signal spread generated in SGCs and neurons. Note the similar amplitude and velocity but higher, although not significantly different, efficacy of the Ca2+ signal spread generated in neurons compared to that generated in SGCs. Bars correspond to mean ± SEM of 12 and 16 fields for neuron and SGC stimulation.
ICW spread in trigeminal ganglion cultures
Electrical stimulation of a single neuron in the cultures evoked a sharp and transient rise in the Ca2+ level of the stimulated neuron that was followed by a fast Ca2+ response in adjacent SGCs that was also communicated to neighboring glial cells, spreading as a typical ICW (Fig. 1). Given the technical impossibility of electrically stimulating a single SGC without also stimulating the adjacent neurons, we used focal mechanical stimulation to investigate whether activation of single SGCs would also evoke the transmission of Ca2+ signals. As shown in Fig. 2A, focal mechanical stimulation of a single SGC evoked a steep and transient increase in intracellular Ca2+ of the stimulated cell that was promptly followed by rise in Ca2+ of not only the adjacent neuron and SGCs, but also in other glial cells located more distantly from the stimulated cell. Mechanical stimulation of single neurons also triggered transmission of intercellular Ca2+ signals. Figure 2B illustrates the transmission of a typical ICW generated by mechanical stimulation of a neuron that was previously engaged in SGC signaling (see Fig. 2A). The overall properties of the ICWs initiated by SGC or neuron stimulation were not significantly different (Fig. 2C–E), but the efficacy (number of responding cells/total number of cells in each field) of the Ca2+ signaling triggered by neuron stimulation was higher than that observed upon SGC stimulation (Fig. 2C and compare Fig. 2A,B). Although in this study we focused on the intercellular Ca2+ signaling between neurons and glial cells, we have also observed that focal mechanical stimulation of single neurons could also trigger responses in neurons located at closer range of the stimulated cell. However, because of the low density of neurons in our cultures, we did not quantify the Ca2+ signals generated in neighboring neurons.
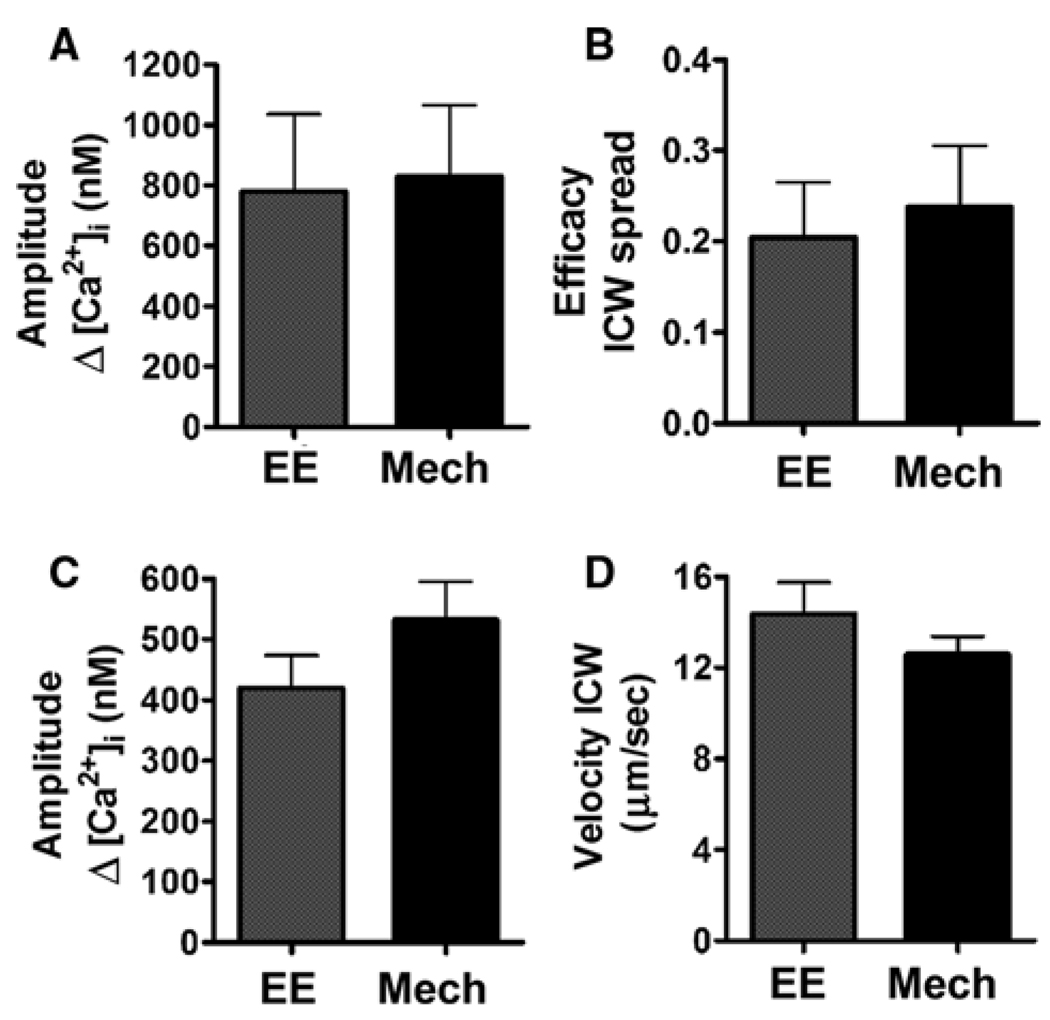
We recognize that mechanical stimulation is not a physiological stimulus for either glial cells or neurons (except perhaps during traumatic injury). However, it allows selective and controlled stimulation of a single cell or cell regions, which is an essential requisite to study bidirectional neuron–SGC signaling, given their close proximity and the small size of SGCs. Moreover, our data demonstrating that the pattern (compare Fig 1A and Fig 2B) and properties of ICWs (Fig. 3) generated by electrical and by mechanical stimulation of single neurons are not significantly different, corroborate the use of this stimulation paradigm in this study.
Fig. 3. Comparison of the intercellular Ca2+ signaling induced by focal electrical (EE) and mechanical (Mech) stimulation of single sensory neurons in trigeminal ganglion cultures.
(A) Amplitude of the Ca2+ response induced in the stimulated neurons, (B) efficacy of the intercellular Ca2+ wave (ICW) spread (number of responding cells per total number of cells in the field), (C) amplitude of the Ca2+ signal evoked in glial cells and (D) velocity of the ICW spread. Note the similar amplitude of the Ca2+ response of neurons to EE and Mech stimulation, and the similar properties of the neuron-generated ICWs that spread among glial cells in response to these two stimuli. Bars correspond to mean ± SEM of 12 and 10 fields each for EE and Mech stimulation, respectively.
Ca2+ signaling between neurons and SGCs
As shown in Fig 1 and Fig 2, neurons and SGCs can communicate through transmission of Ca2+ signals. Focal stimulation of single neurons in cultured trigeminal ganglia triggered increase in Ca2+ levels in surrounding SGCs and, reciprocally, focal stimulation of a single SGC induced a rise in the cytosolic Ca2+ levels of the adjacent neuron as well as those of other surrounding SGCs. In this bidirectional neuron–SGC signaling an average of 71% of the SGCs responded to neuronal stimulation (Efficacy = 0.71 ± 0.05; N = 54 fields), whereas the incidence of neuronal response to SGC stimulation was 37% (Efficacy = 0.37 ± 0.08; N = 34 fields) and the efficacy of SGC–SGC communication was 41% (Efficacy = 0.41 ± 0.07; N = 30 fields). The amplitude of the response of mechanically stimulated neurons (Δ [Ca2+]i=625.4 ± 80.7 nM; N = 49) was significantly lower (P < 0.05) than that observed in mechanically stimulated SGCs (Δ [Ca2+]i=940.1 ± 128.4 nM; N = 36). Despite this lower Ca2+ mobilization in stimulated neurons, the response of SGCs to neuronal activation (Δ [Ca2+]i=694.1 ± 55.6; N = 120) was significantly higher than the responses observed in neurons (Δ [Ca2+]i = 172.1 ± 15.4; N = 13; P < 0.01) or SGCs (Δ [Ca2+]i = 246.6 ± 27.5; N = 31; P < 0.0001) evoked by SGC stimulation.
Pharmacological dissection of the pathways involved in the Ca2+ signaling between neurons and SGCs
Intercellular calcium signaling is mediated by both extracellular diffusion of Ca2+-mobilizing transmitter molecules, of which ATP is most prominent, and by gap junction-mediated cytosol-to-cytosol diffusion of Ca2+-mobilizing intracellular second messengers (Scemes and Giaume, 2006). We have previously shown that SGCs surrounding a given neuron in the trigeminal ganglion are coupled by gap junctions (Cherkas et al., 2004) and it has also been shown that SGCs express purinergic receptors (Weick et al., 2003; Ceruti et al., 2008). Moreover, recent findings demonstrating release of ATP from the soma of electrically stimulated dorsal root ganglia neurons in culture (Zhang et al., 2007) suggest that not only gap junctions but also ATP and activation of purinergic receptors are involved in the transmission of Ca2+ signals between neurons and SGCs.
To investigate the participation and relative contributions of these signaling pathways we used a broad-spectrum purinergic receptor antagonist, suramin (50 µM), a gap junction channel blocker, carbenoxolone (100 µM), and measured their impact on the intercellular Ca2+ signaling between neurons and SGCs in trigeminal ganglion cultures. We found that neither blocker had significant effects on the amplitude of the Ca2+ response recorded in the mechanically stimulated neurons (in suramin: 974.3 ± 315.0 nM, N = 6; in carbenoxolone: 969.9 ± 223.3 nM, N = 12) or in mechanically stimulated SGCs (see Table 1). However, these treatments had profound effect on the Ca2+ signaling between these cells, as detailed below.
Table 1.
Effect of P2 receptor and gap junction blockade on Ca2+ signal transmission from mechanically stimulated satellite glial cell (SGC) to adjacent SGCs and neurons.
| Direction of signaling | Experimental condition | Amplitude Ca2+ response in stimulated SGC (nM) |
Amplitude Ca2+ response in neighboring SGCs or neuron (nM) |
|---|---|---|---|
| SGC to SGCs | Control | 940.1 ± 128.4 (36) | 246.6 ± 27.5 (31/85) |
| Suramin (50 µM) | 781.8 ± 324.8 (7) | 239 (2/15) | |
| Carbenoxolone (100 µM) | 1180.0 ± 203.7 (14) | 115 (1/24) | |
| SGC to neuron | Control | 940.1 ± 128.4 (36) | 172.1 ± 15.4 (13/36) |
| Suramin (50 µM) | 781.8 ± 324.8 (7) | No response (0/7) | |
| Carbenoxolone (100 µM) | 1180.0 ± 203.7 (14) | No response (0/14) |
Data correspond to mean ± SEM (number of stimulated SGCs and number of responding SGCs or neurons/total number of cells analyzed)
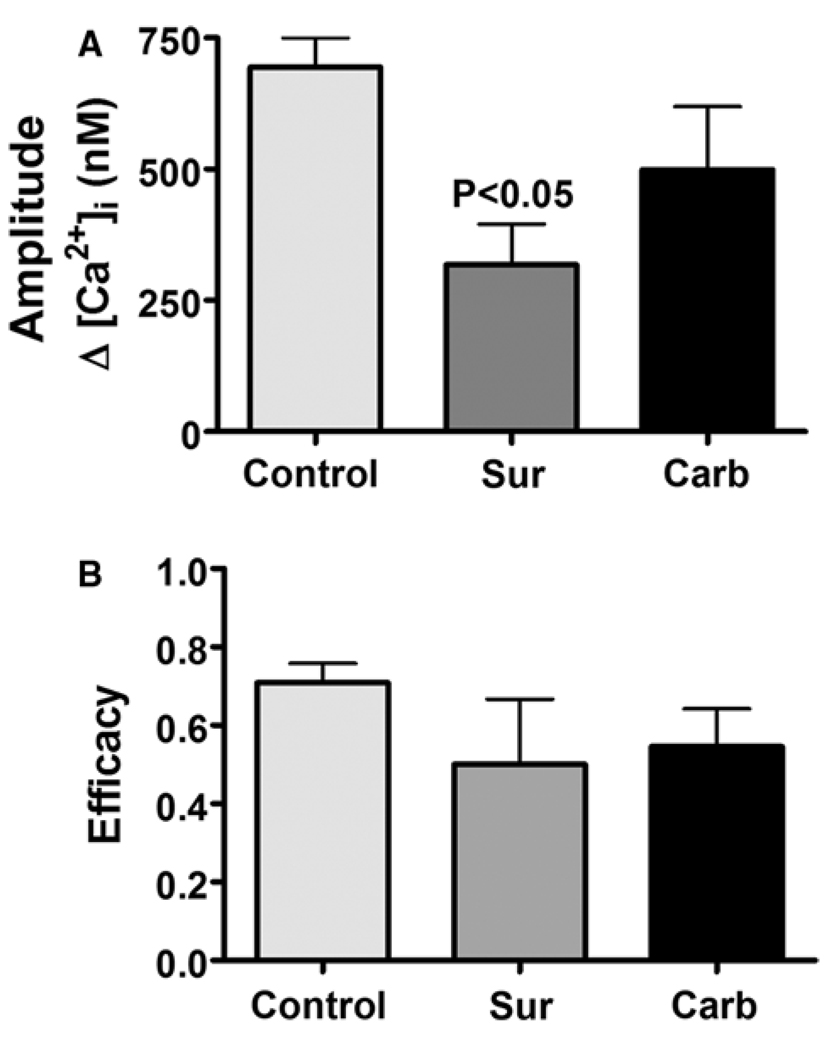
Treatment of cultures with 50 µM suramin significantly (P < 0.05) reduced by 60% the amplitude of the Ca2+ signal generated in SGCs in response to neuronal stimulation (Fig. 4A), whereas in the presence of 100 µM carbenoxolone the response was only minimally altered (Fig. 4A). The efficacy of the neuron-to-SGC signaling was not significantly altered in the presence of either suramin or carbenoxolone (Fig. 4B).
Fig. 4. Comparison of the effects of P2 receptor and gap junction blockade on the amplitude and efficacy of the Ca2+ signaling from neurons to satellite glial cells (SGCs) in trigeminal ganglion cultures.
Ca2+ signals were initiated by focal mechanical stimulation of a single neuron in Fura-2 AM loaded cultures and increases in intracellular Ca2+ levels in surrounding SGCs were recorded by epifluorescence. (A) Amplitude of the Ca2+ signal induced in SGCs in response to neuron stimulation and (B) efficacy of the neuron to SGC signaling (number of responding SGCs per total number of SGCs surrounding the stimulated neuron) determined in the absence (Control) and in the presence of either the broad-spectrum P2 receptor blocker suramin (Sur, 50 µM) or the gap junction blocker carbenoxolone (Carb, 100 µM). Note in suramin-treated cultures the significant reduction in the amplitude of SGC response to neuron stimulation, whereas carbenoxolone treatment had lesser effect. The efficacy of neuron to SGC signaling in suramin and in carbenoxolone-treated cultures was not significantly different from that observed in control conditions. Bars correspond to mean ± SEM; number of fields analyzed, 54 in control conditions, 9 in suramin- and 13 in carbenoxolone-treated cultures.
In contrast to neuron-to-SGC signaling, treatment of cultures with suramin or carbenoxolone abolished the Ca2+ signaling from SGCs to neurons and among SGCs (see Table 1). This asymmetry of the effects of carbenoxolone in the neuron–SGC bidirectional signaling is likely due to coupling primarily among SGCs, where Ca2+ rises in the population may summate to activate the neuron, either through gap junction-mediated spread of Ca2+ mobilizing second messenger or by ATP release from the glia. Although direct neuron–glial coupling has been found in CNS cultures (Froes et al., 1999; Rozental et al., 2001), such coupling has not been reported in sensory ganglia. By contrast, SGC dye coupling is strong and gap junctions between these cells are abundant (Pannese, 1981; Hanani et al., 2002). These observations indicate the functional importance of gap junction-mediated SGC coupling, which enables them to function as a unit. SGCs are small compared with sensory neurons, and a single cell may not be able to alter neuronal function; however, several mutually connected SGCs can act in concert and exert strong influence on an adjacent neuron.
Ca2+ signaling between neurons and SGCs in trigeminal ganglion cultures is modulated in an orofacial tactile hypersensitivity model
Peripheral injury or inflammation induces profound effects on both SGCs and neurons in sensory ganglia (Hanani, 2005; Devor, 2006). To investigate whether such stimuli alter the ability of neurons and SGCs to communicate through transmission of Ca2+ signals, we induced peripheral submandibular inflammation by CFA injection, and the trigeminal ganglia were removed and cultured seven days later. As shown in Table 2, this treatment significantly altered the bidirectional Ca2+ signaling between neurons and SGCs, and also the signaling among SGCs. In cultures obtained from CFA-treated mice, the amplitude of the Ca2+ signal generated in SGCs in response to neuronal activation was significantly reduced by 30% (P < 0.05; Table 2), whereas the response of neurons to SGC activation was increased two-fold (P < 0.001; Table 2). The amplitude of the Ca2+ signal generated in SGCs in response to activation of an adjacent SGC was also increased by almost two-fold in cultures from CFA-treated mice (P < 0.05; Table 2). CFA treatment did not alter either the number of SGCs recruited into the Ca2+ signaling initiated by neuronal or SGC stimulation or the likelihood that a neuron would response to SGC stimulation.
Table 2.
Effect of Complete Freund’s Adjuvant (CFA)-induced submandibular inflammation on intercellular Ca2+ signaling between neurons and satellite glial cells (SGCs) in trigeminal ganglion cultures.
| Direction of signaling | Experimental condition | Amplitude Ca2+ response in stimulated neuron or SGC (nM) |
Amplitude Ca2+ response in neighboring SCGs or neuron (nM) |
CFA treated/control |
|---|---|---|---|---|
| Neuron to SGCs | Control | 625.4 ± 80.7 (49) | 694.1 ± 55.6 (120/187) | 0.7 |
| CFA treated | 605.6 ± 122.3 (30) | 488.1 ± 65.4* (52/82) | ||
| SGC to SCGs | Control | 940.1 ± 128.4 (36) | 246.6 ± 27.5 (31/85) | 1.9 |
| CFA treated | 886.0 ± 131.5 (29) | 464.1 ± 98.7* (19/68) | ||
| SGC to neuron | Control | 940.1 ± 128.4 (36) | 172.1 ± 15.4 (13/36) | 2.1 |
| CFA treated | 886.0 ± 131.5 (29) | 363.3 ± 47.9** (11/29) |
Data correspond to mean ± SEM (number of stimulated neurons or SGCs, and number of responding SGCs or neurons/total number of cells analyzed).
P < 0.05
P < 0.001 compared to control. Significant differences were determined by unpaired t-test.
CONCLUSIONS
Direct stimulation of neurons or SGCs resulted in an increase in Ca2+ level in the stimulated cell, followed by Ca2+ increase in neighboring cells.
The P2 receptor blocker suramin had a strong inhibitory action on the spread of Ca2+ signals from both neurons and SGCs, whereas the gap junction blocker carbenoxolone had a weaker inhibitory effect, attenuating only the Ca2+ signal spreading from SGCs.
DISCUSSION
The ability of glial cells to communicate through transmission of Ca2+ signals was first described in cultured astrocytes (Cornell-Bell et al., 1990; Charles et al., 1991). Since then, a growing body of evidence suggests that through this form of signaling, astrocytes can interact with other glial cells, neurons and endothelial cells in the CNS, thereby modulating synaptic transmission and mediating neurovascular coupling (Nedergaard et al., 2003; Haydon and Carmignoto, 2006; Scemes and Giaume, 2006; Scemes and Spray, 2008).
Despite the numerous studies and growing understanding of the mechanisms and functional relevance of this homo- and hetero-cellular Ca2+ signaling in the CNS, there is still very little information about this topic in the peripheral nervous system, with only one study describing the presence of intercellular Ca2+ signaling in enteric glial cells (Zhang et al., 2003). The main type of glial cell in peripheral ganglia (except in the enteric nervous system) is the SGC. These cells are believed to play roles similar to those of astrocytes, such as regulating the neuronal environment (Hanani, 2005). However, unlike astrocytes, SGCs form a tight envelope around individual neurons, and the space between the two cell types is very narrow (about 20 nm; Pannese, 1981). Such restriction in extracellular space would not only permit SGCs to tightly control the neuronal extracellular space (for a review see Hanani, 2005), but would also make these cells highly susceptible to factors released from neuronal somata. Indeed, there is direct evidence that ATP released from dorsal root ganglion neurons increases intracellular Ca2+ levels in SGCs (Zhang et al., 2007). This report and previous studies by us and others, showing that SGCs express various purinergic receptor (P2R) subtypes and respond to ATP with increases in intracellular Ca2+ concentration (Weick et al., 2003; Zhang et al., 2005; Ceruti et al., 2008), suggest that like astrocytes, SGCs can respond to ATP released during neuronal activity and in turn modulate neuronal activity in the sensory ganglia.
In this study we show for the first time that SGCs and neurons from mouse trigeminal ganglia can communicate bidrectionally through transmission of Ca2+ signals. The mechanisms underlying this phenomenon appear to be quite similar to those observed in the CNS, in agreement with previous reports that SGCs share many features with astrocytes (Hanani, 2005). Transmission of astrocytic Ca2+ signals is mediated by two pathways, one involving release of ATP from stimulated astrocytes followed by activation of P2Rs, and another involving gap junction-mediated cytosol-to-cytosol diffusion of generated Ca2+-mobilizing intracellular second messengers (Scemes and Giaume, 2006). The participation of these two pathways in the transmission of Ca2+ signals between neurons and SGCs, as well as among SGCs, was demonstrated by our findings that communication between these cells was significantly impaired by suramin, a broad-spectrum P2R antagonist, and by carbenoxolone, a gap junction blocker.
On the basis of previous work, communication between SGCs via gap junctions is expected, but the observation that the P2R blocker suramin inhibited communication between these cells and with neurons is novel as it indicates that SGCs are able to release ATP. It has been shown that SGCs release cytokines (Takeda et al., 2007; Zhang et al., 2007), and our study provides evidence for the release of the transmitter ATP from these cells. It will be of great interest to explore whether besides ATP, SGCs would also release other neurotransmitters, such as glutamate, as observed in astrocytes (Parpura et al., 2004; Montana et al., 2006). Neurotransmitter release from SGCs is likely to have important implications for the function of sensory ganglia under various conditions.
The data presented in this work were obtained from cells in culture, and one question that might arise is of their relevance to the intact ganglion. It has to be mentioned that the trigeminal ganglion cultures used in this work were short term (2–3 days) and are likely to retain many of the properties of the original tissue, as described by Ceruti et al. (2008). We showed that homo- and hetero-cellular Ca2+ signaling in trigeminal ganglion cultures depend on the presence of P2Rs and gap junctions, and that both these prerequisites exist in intact ganglia (Hanani et al., 2002; Weick et al., 2003). Therefore it is very likely that SGCs and neurons can communicate through transmission of Ca2+ signals in the intact tissue. We have attempted to evoke transmission of Ca2+ signals in the intact ganglia, but this proved not to be feasible, mainly because of major technical difficulties encountered while trying to stimulate single cells in the ganglion. Cultures, because of their two-dimensional organization and the easy accessibility of cells in them, are ideal for this type of work, and indeed are widely used to study intercellular Ca2+ signaling.
There is strong evidence that nerve injury enhances SGC coupling via gap junctions (Hanani et al., 2002; Cherkas et al., 2004; Huang et al., 2005), and we have proposed that this may contribute to chronic pain in mice. Recently we tested this idea on mice in which hind paw inflammation was induced to cause pain (Dublin and Hanani, 2007). We found that coupling among SGCs and among neurons was increased and that treatment of these mice with carbenoxolone significantly reduced pain (as demonstrated by diminished paw retraction behavior), which supports a role for gap junctions in the mechanisms involved in pain signaling (see also Spataro et al., 2004). As the results of the present work indicate that SGCs are capable of communication with neurons and neighboring SGCs through transmission of intercellular Ca2+ signals, which depend in part on gap junctional coupling, it can be suggested that Ca2+ signaling is enhanced after injury and may contribute to the functional abnormalities associated with chronic pain. In this study we tested this idea using an orofacial pain/inflammation model in mice. We found that in trigeminal ganglion cultures obtained from CFA-treated mice the amplitude of Ca2+ signal observed in SGCs and neurons in response to SGC activation increased two-fold compared to controls, whereas the response of SGCs to neuronal stimulation was significantly decreased (by 30%). The mechanism by which inflammation increased SGC signaling is not known, but it can be partly explained by our observation that in the same inflammation model used here, the sensitivity of SGCs to ATP is greatly augmented (Hanani et al., 2007). Another contributing factor may be increased gap junctional coupling after injury (Hanani et al., 2002; Cherkas et al., 2004), which would be in agreement with the apparent analgesic action of carbenoxolone (Dublin and Hanani, 2007; Huang et al., 2009). However, why transmission from neurons to SGCs was diminished after inflammation is not clear, and it can be proposed that somatic neuronal ATP release is reduced by inflammation.
The number of sensory neurons that innervate the submandibular region is a small fraction of the total number of neurons in the trigeminal ganglia. Although we sampled the cells randomly, a large fraction of the cells were affected by CFA injections. This indicates that the cellular changes induced in the cells innervating the submandibular region have spread to uninvolved neurons and their attendant SGCs, probably via chemical signals. Such a spread after peripheral injury to the trigeminal nerves was reported previously (Stephenson and Byers, 1995; Thalakoti et al., 2007).
Overall, based on our findings of enhanced SGC signaling in cultures from CFA-treated mice, it is likely that homo- and hetero-cellular Ca2+ signaling in sensory ganglia would be augmented in chronic pain states, thereby increasing neuronal activity and amplifying transmission of pain signals reaching the CNS. Greater sensory neuronal activity would conform with the current view that hyperexcitability and spontaneous (ectopic) electrical activity of sensory neurons contribute to neuropathic pain (Devor, 2006).
In summary, in this study we demonstrate that neurons and SGCs from sensory ganglia can communicate through transmission of Ca2+ signals and that this form of intercellular signaling involves the activation of purinergic receptors, and to a lesser extent, gap junctions. In addition, we found that not only the bidirectional neuron–SGC, but also the signaling among SGCs is altered in cultures obtained from a mouse model of inflammation, suggesting that Ca2+ signaling between these cells would play major roles in the modulation of sensory ganglia function under both normal and pathological states.
ACKNOWLEDGEMENTS
This work was supported by the US–Israel Binational Science Foundation (BSF 2003262, 2007311), the Israel Science Foundation (212/08), NIH grants NS41282 and DK060037, and AHA grant 0735377N.
Footnotes
Statement of interest
None.
REFERENCES
- Ceruti S, Fumagalli M, Villa G, Verderio C, Abbracchio MP. Purinoceptor-mediated calcium signaling in primary neuron–glia trigeminal cultures. Cell Calcium. 2008;43:576–590. doi: 10.1016/j.ceca.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6:983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- Cherkas PS, Huang TY, Pannicke T, Tal M, Reichenbach A, Hanani M. The effects of axotomy on neurons and satellite glial cells in mouse trigeminal ganglion. Pain. 2004;110:290–298. doi: 10.1016/j.pain.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Devor M. Responses of nerves to injury in relations to neuropathic pain. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. 5th edition. Dover: Elsevier Churchill Livingstone; 2006. pp. 905–928. [Google Scholar]
- Dublin P, Hanani M. Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain. Brain Behavior and Immunity. 2007;21:592–598. doi: 10.1016/j.bbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Froes MM, Correia AH, Garcia-Abreu J, Spray DC, Campos de Carvalho AC, Neto MV. Gap-junctional coupling between neurons and astrocytes in primary central nervous system cultures. Proceedings of the National Academy of Sciences of the U.S.A. 1999;96:7541–7546. doi: 10.1073/pnas.96.13.7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology. 2009;136:1349–1358. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Research Brain Research Reviews. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Hanani M, Huang TY, Cherkas PS, Ledda M, Pannese E. Glial cell plasticity in sensory ganglia induced by nerve damage. Neuroscience. 2002;114:279–283. doi: 10.1016/s0306-4522(02)00279-8. [DOI] [PubMed] [Google Scholar]
- Hanani M, Kushnir R, Cherkas PS. Nerve damage or inflammation augment purinergic signaling in satellite glial cells in mouse sensory ganglia. Neuron Glia Biology. 2007;3:S108. [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiological Reviews. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Huang TY, Belzer V, Hanani M. Gap junctions in dorsal root ganglia: possible contribution to visceral pain. European Journal of Pain. 2009 doi: 10.1016/j.ejpain.2009.02.005. (in press) [DOI] [PubMed] [Google Scholar]
- Huang TY, Cherkas PS, Rosenthal DW, Hanani M. Dye coupling among satellite glial cells in mammalian dorsal root ganglia. Brain Research. 2005;1036:42–49. doi: 10.1016/j.brainres.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia. 2006;54:700–715. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends in Neuroscience. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Pannese E. The satellite cells of the sensory ganglia. Advances in Anatomy Embryology and Cell Biology. 1981;65:1–111. doi: 10.1007/978-3-642-67750-2. [DOI] [PubMed] [Google Scholar]
- Parpura V, Scemes E, Spray DC. Mechanisms of glutamate release from astrocytes: gap junction “hemichannels”, purinergic receptors and exocytotic release. Neurochemistry International. 2004;45:259–264. doi: 10.1016/j.neuint.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Rozental R, Andrade-Rozental AF, Zheng X, Urban M, Spray DC, Chiu FC. Gap junction-mediated bidirectional signaling between human fetal hippocampal neurons and astrocytes. Develomental Neuroscience. 2001;23:420–431. doi: 10.1159/000048729. [DOI] [PubMed] [Google Scholar]
- Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54:716–725. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scemes E, Spray DC. Connexin expression (gap junctions and hemichannels) in astrocytes. In: Parpura V, Haydon PG, editors. Astrocytes in Pathophysiology of the Nervous System. Berlin: Springer; 2008. pp. 107–150. [Google Scholar]
- Spataro LE, Sloane EM, Milligan ED, Wieseler-Frank J, Schoeniger D, Jekich BM, et al. Spinal gap junctions: potential involvement in pain facilitation. Journal of Pain. 2004;5:392–405. doi: 10.1016/j.jpain.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Stephenson JL, Byers MR. GFAP immunoreactivity in trigeminal ganglion satellite cells after tooth injury in rats. Experimental Neurology. 1995;131:11–22. doi: 10.1016/0014-4886(95)90003-9. [DOI] [PubMed] [Google Scholar]
- Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. Journal of Neuroscience. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suadicani SO, Flores CE, Urban-Maldonado M, Beelitz M, Scemes E. Gap junction channels coordinate the propagation of intercellular Ca2+ signals generated by P2Y receptor activation. Glia. 2004;48:217–229. doi: 10.1002/glia.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Kadoi J, Nasu M, Takahashi M, Kitagawa J, et al. Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain. 2007;129:155–166. doi: 10.1016/j.pain.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, et al. Neuron–glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weick M, Cherkas PS, Hartig W, Pannicke T, Uckermann O, Bringmann A, et al. P2 receptors in satellite glial cells in trigeminal ganglia of mice. Neuroscience. 2003;120:969–977. doi: 10.1016/s0306-4522(03)00388-9. [DOI] [PubMed] [Google Scholar]
- Zhang W, Segura BJ, Lin TR, Hu Y, Mulholland MW. Intercellular calcium waves in cultured enteric glia from neonatal guinea pig. Glia. 2003;42:252–262. doi: 10.1002/glia.10215. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron–satellite glial cell communication in dorsal root ganglia. Proceedings of the National Academy of Sciences of the U.S.A. 2007;104:9864–9869. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Han P, Faltynek CR, Jarvis MF, Shieh CC. Functional expression of P2X7 receptors in non-neuronal cells of rat dorsal root ganglia. Brain Research. 2005;1052:63–70. doi: 10.1016/j.brainres.2005.06.022. [DOI] [PubMed] [Google Scholar]