Abstract
Purkinje neurons fire spontaneous action potentials at ~50 spikes/sec and generate more than 100 spikes/sec during cerebellum-mediated behaviors. Many voltage-gated channels, including Ca channels, can inactivate and/or facilitate with repeated stimulation, raising the question of how these channels respond to regular, rapid trains of depolarizations. To test whether Ca currents are modulated during firing, we recorded voltage-clamped Ca currents, predominantly carried by P-type Ca channels, from acutely dissociated mouse Purkinje neurons at 30–33°C (1 mM Ca). With 0.5 mM intracellular EGTA, 1-second trains of either spontaneous action potential waveforms or brief depolarizing steps at 50 Hz evoked Ca tail currents that were stable, remaining within 5% of the first tail current throughout the train. Higher frequency trains (100 and 200 Hz) elicited a maximal inactivation of <10%. To test whether this stability of Ca currents resulted from a lack of modulation or from an equilibrium between facilitation and inactivation, we manipulated the permeant ion (Ca vs. Ba) and Ca buffering (0.5 vs. 10 mM EGTA). With low buffering, Ba accelerated the initial inactivation evoked by 1-second trains, but reduced its extent at 200 hz, consistent with an early calcium-dependent facilitation (CDF) and late calcium-dependent inactivation (CDI) at high frequencies. Increasing the Ca buffer favored CDF. These data suggest that stable Ca current amplitudes result from a balance of CDF, CDI, and voltage-dependent inactivation. This modest net Ca-dependent modulation may contribute to the ability of Purkinje neurons to sustain long periods of regular firing and synaptic transmission.
Keywords: P-type, CaV2.1, calcium channel, Ca dependent inactivation, spontaneous firing, cerebellum
Introduction
Cerebellar Purkinje neurons generate regular trains of high-frequency action potentials, both in vivo and in vitro.1-3 In cerebellar slices and isolated Purkinje cells, spontaneous action potentials persist at rates near 50 spikes/sec and spike shapes remain constant. This regularity of firing suggests that the ion channels of Purkinje cells are not subject to strong cumulative inactivation or facilitation with repetitive depolarizations.4 Many types of voltage-gated Ca channels, however, are susceptible to both these forms of modulation.5 Whether Ca channels of Purkinje cells are modulated during spiking is of interest because Ca activates K channels, stimulates biochemical cascades, and triggers neurotransmitter release. Thus, changes in Ca current amplitudes during rapid firing are likely to alter coupling to these processes.
Several studies have demonstrated that high-voltage-activated (HVA) Ca channels are modulated by both Ca-independent and Ca-dependent mechanisms. For instance, in L-type, N-type and P/Q-type channels, the amplitudes of Ba currents decay with sustained or repeated depolarizations, indicative of voltage-dependent inactivation.6,7 These channels are also regulated by the influx of Ca, which binds to Ca-sensing proteins such as calmodulin or neuronal Ca binding proteins.8-11 In general, increases in local Ca concentration augment Ca currents (Ca-dependent facilitation, or CDF), whereas more widespread or global increases in Ca concentration decrease current amplitudes (Ca-dependent inactivation, or CDI).5,12 While such modulation may constitute a meaningful signal in response to synaptically driven activity,13 fluctuating Ca current amplitudes during spontaneous firing seem more likely to generate noise than signal.
Interestingly, Purkinje cells have a narrow palette of HVA Ca channels: Most Ca current is carried by P-type (CaV2.1) channels.7,14,15 Although expressed CaV2.1 channels can also respond to high local Ca with CDF and elevated global Ca with CDI,9,10,12 in Purkinje neurons from young mice (P10), depolarizations at rates of ~100 Hz elicit only moderate CDF.18 Moreover, P-type channels show considerably less voltage-dependent inactivation than N-type currents.6,7,16,17 Together, these data raise the possibility that that the near exclusive expression of P-type channels plays an adaptive role in Purkinje cells by limiting their susceptibility to modulation.
To test this hypothesis, we recorded Ca currents in Purkinje neurons isolated from P16-P21 mice, at 30–33°C, with 1 mM Ca as the charge carrier. We find that action potential waveforms that mimic spontaneous firing, or trains of brief depolarizations at comparable rates, evoke Ca tail currents that are remarkably stable over periods as long as 1 second. This stability, however, does not result from a resistance to modulation, as manipulations of the permeant ion, intracellular Ca buffering, and stimulation rate reveal a capacity for both CDF and CDI. Nevertheless, during spontaneous firing, CDF, CDI and voltage-dependent inactivation are balanced such that the Ca influx associated with each action potential remains nearly constant.
Results
Stability of Ca tail currents evoked by action potential waveform trains
To assess the extent of modulation of somatic Ca currents during spontaneous firing, we made whole-cell recordings from acutely isolated Purkinje neurons. To maximize relevance of the currents to physiological conditions, recordings were made from neurons from P16-P21 mice, whose electrophysiological properties are indistinguishable from adult Purkinje neurons,19 at 30–33°C, and with divalent cation concentrations close to those found in the rodent brain (1 mM Ca and 1 mM Mg).20 Cells were held at −60 mV to inactivate most of the low-voltage activated (T-type) Ca current,7,21 and nimodipine was added to the external solution in all experiments to block the small amount of L-type Ca current present in Purkinje neurons.4 Na and K currents were blocked with TTX and TEA.
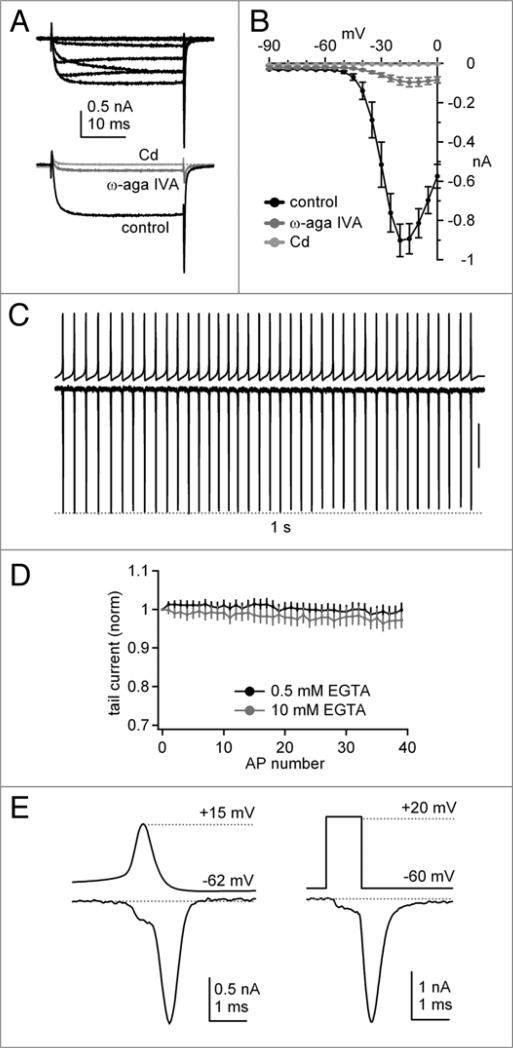
Consistent with previous reports, raw Ca currents evoked by step depolarizations were large (−901 ± 82 pA at −20 mV, n = 7; Fig. 1A and B) and were blocked by 90% by ω-agatoxin IVA (200 nM; residual current −90 ± 20 pA, n = 7) (Fig. 1A and B), confirming that P-type channels carry most of the current in Purkinje cells.7,14 The remaining current was fully blocked by 300 μM Cd (Fig. 1A). Alternatively, in the absence of ω-agatoxin IVA, the total Ca current could be completely blocked by 300 μM Cd. Subtraction of records obtained in Cd from control records therefore isolated P-type current along with any small N- or R-type currents that may have been present; L-type currents were again blocked in all records by nimodipine. In subsequent experiments, we isolated Ca currents by Cd subtraction, which permitted a rapid blockade of current and removal of capacitative currents, while avoiding the potential relief of ω-agatoxin IVA block by depolarization.14 With this method, Ca currents were evoked by a voltage-clamp command waveform composed of a 1-sec train of 40 action potentials previously recorded from an intact Purkinje cell in a cerebellar slice.22 During the train, the peak amplitude of Ca currents evoked by each action potential waveform remained virtually unchanged (Fig. 1C), remaining within 5% of the first current, with intracellular EGTA at either 0.5 mM (n = 7) or 10 mM (n = 5) (Fig. 1D). Comparing the Ca current in response to a single action potential waveform with the current evoked by a 1-ms step from −60 mV to +20 mV indicated similar kinetics of the evoked currents (Fig. 1E). In both cases, the Ca current activated during the initial phase of depolarization, but primarily flowed as a tail current upon repolarization.23 In response to the action potential waveform, the tail current reached a peak of −1.46 ± 0.21 nA (n = 7) and decayed fully during the interspike interval. Together, the data illustrate that action potential-evoked somatic Ca currents maintain a nearly constant amplitude during spontaneous firing in Purkinje neurons.
Figure 1.
P-type Ca currents evoked by step depolarizations and action potential waveforms in Purkinje neurons at 30–33°C. (a) Top, Representative raw Ca currents elicited by depolarizing steps from −60 to 0 mV in 10 mV increments. holding potential in all experiments, −60 mV. Bottom, Blockade of raw Ca current at −20 mV by 200 nM ω-agatoxin IVa, and blockade of residual current by 300 μM Cd. same cell as above. (B) Mean current-voltage relation for raw currents in control (black circles), after addition of ω-agatoxin IVa (dark gray circles), and after addition of 300 μM Cd (light gray circles, n = 7, same cells throughout, 10 mM BAPTA). (C) Command voltage consisting of a pre-recorded 1-sec train of 40 Purkinje neuron action potentials (top) and corresponding 300 μM Cd-sensitive Ca currents (bottom). Vertical scale bar, 0.5 nA. (D) Average Ca tail current amplitudes normalized to the first tail current evoked by the action potential (AP) train, for 0.5 mM EGTA (black, n = 7) and 10 mM EGTA (gray, n = 5). Note y-scale, which is repeated on all related plots. (e) Left, Cd-sensitive Ca current evoked by a single action potential waveform command, shown on an expanded time base. Right, Cd-sensitive Ca current evoked by a single 1-ms step depolarization from −60 to +20 mV, on the same time base. Data from the same cell as at left.
Ca-dependent modulation in low Ca buffer
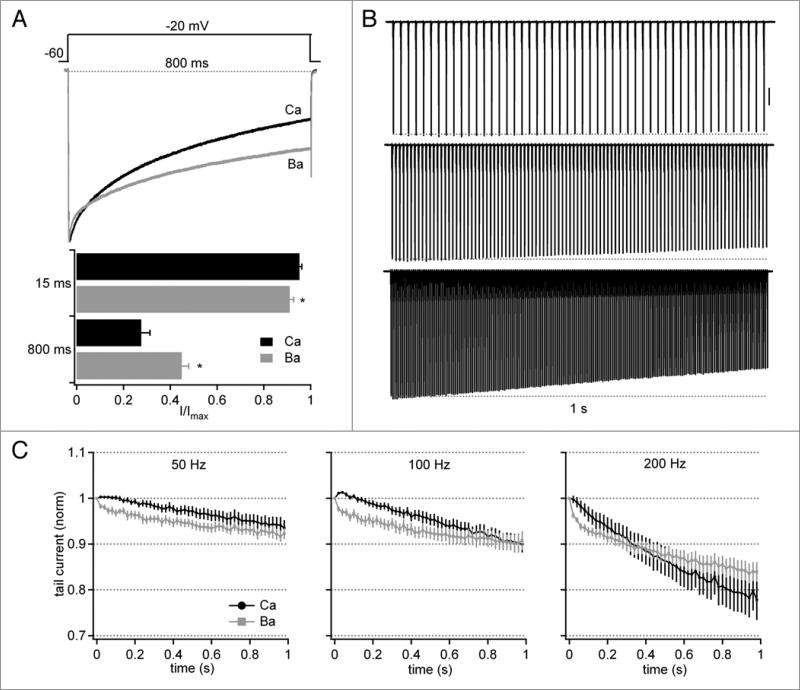
The stability of these currents is notable because CaV2.1 (P-type) channels, including those in P10 Purkinje cells, have been shown to be subject to both CDF and CDI.5,10,18,24 We therefore tested whether the absence of overt modulation of Ca currents in response to action potential waveforms resulted because Purkinje cells in our preparation lacked the capacity for modulation, or because CDF and CDI indeed took place, but the net effects were negligible. We began by comparing currents evoked by a long (800 ms) step depolarization to −20 mV, with either Ca or Ba as the charge carrier and low (0.5 mM) Ca buffering by EGTA (Fig. 2A, top). The initial phase of inactivation was consistently more rapid in Ba, such that the normalized current after 15 ms dropped by 8.9 ± 1.6% in Ba but only 4.8 ± 0.1% in Ca (n = 8, p < 0.05). The current carried by Ca, however, decreased to a greater extent during the step, such that the final amplitude of the normalized current in Ca was 27.6 ± 3.7%, whereas it remained at 44.8 ± 2.9% in Ba (same cells, p < 0.05, Fig. 2A, bottom). The long steps therefore provided evidence that Ca-dependent modulation of the currents indeed can occur, with Ca flux counteracting voltage-dependent inactivation early in the record, but intensifying inactivation later in the record.
Figure 2.
Facilitation and inactivation of tail currents evoked by stimulus trains with 0.5 mM intracellular EGTA. (A) Top, Normalized Ca and Ba currents from a single Purkinje cell during an 800-ms depolarizing step from −60 to −20 mV. Bottom, Fraction of current (I/Imax) remaining 15 ms and 800 ms after the onset of depolarization (n = 8). (B) Representative records of Cd-sensitive Ca current elicited by 50-Hz (top), 100-Hz (middle) or 200-Hz (bottom) trains of 1 ms pulses to +20 mV for 1 s. Vertical scale bar, 1 nA. (C) Average tail current amplitudes normalized to the first tail current evoked by the train (n = 7). For 100 and 200 hz records, every second or fourth point is plotted, respectively.
To test how these forms of modulation are expressed during stimuli that mimic physiological rates of firing, we recorded Ca tail currents evoked by 1-sec trains of 1-ms step depolarizations from −60 to +20 mV (as in Fig. 1E) applied at 50, 100 or 200 Hz (Fig. 2B). These brief steps were preferable to pre-recorded action potential trains because they permitted identical depolarizations and facilitated experimental control of the rate of stimulation. With these stimuli, the net changes in Ca current were again quite small. Currents showed no overt CDF, as their amplitudes did not increase by more than 1% of the initial tail current at any frequency. Consistent with the currents evoked by long steps, however, currents measured in Ba initially decreased more rapidly, indicating that CDF did occur, and that it reduced the net effect of voltage-dependent inactivation (Fig. 2C). For instance, examining the fifth tail current indicated that Ca currents slightly but significantly exceeded Ba currents in all cases, yielding normalized currents of 100 ± 0.5% vs. 97 ± 0.8% at 50 Hz, 100 ± 0.5% vs. 97 ± 1% at 100 Hz, and 100 ± 1% vs. 96 ± 0.7% at 200 Hz, (n = 14, p < 0.05 all comparisons). By the end of the 50- and 100-Hz trains, currents in Ca were indistinguishable from those in Ba at 50 and 100 Hz (94 ± 2% vs. 92 ± 1% at 50 Hz, 90 ± 2% vs. 90 ± 2% at 100 Hz). At 200 Hz, however, tail currents decreased more in Ca than in Ba (78 ± 2% vs. 84 ± 2%, p < 0.05), suggesting that Purkinje Ca channels show significant CDI only with prolonged stimulation at very high frequencies. Together the data suggest that CDF and voltage-dependent inactivation balance each other at spontaneous and elevated firing rates, yielding only slight net changes in Ca tail currents.
Ca-dependent modulation in high Ca buffer
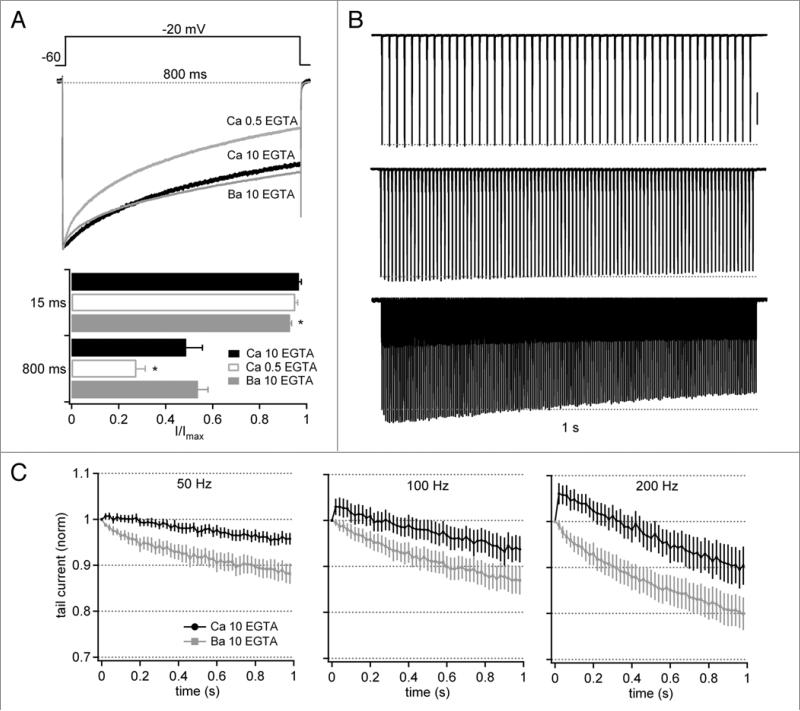
Despite the observation that the normalized Ca current was larger than the Ba current except at the end of sustained trains at 200 Hz, the buffering condition of 0.5 EGTA is predicted to be permissive of CDI, which is induced by global increases in Ca concentration.9,24 This idea raises the possibility that CDI also contributed to the profile of Ca tail currents even with 50- and 100-Hz trains. To test this idea, we repeated the experiments in 10 mM EGTA, a concentration of buffer that is expected to eliminate CDI while allowing CDF to proceed. During the long step, inactivation of currents carried by Ca was significantly reduced with 10 mM relative to 0.5 mM EGTA (Fig. 3A). Moreover, the amount of current remaining at the end of the step with Ca (54 ± 4%, n = 5) was indistinguishable from that recorded with Ba in the same cells (49 ± 7%), consistent with the loss of CDI. The small, significant augmentation of the early current in Ca relative to Ba was still evident, however, again suggestive of a facilitation that counteracts voltage-dependent inactivation (Fig. 3A).
Figure 3.
Facilitation and inactivation of tail currents evoked by stimulus trains with 10 mM intracellular EGTA. (a–C) as in Figure 2, but with 10 mM intracellular EGTA (n = 6). In (A), Ca current data obtained with 0.5 mM EGTA from Figure 2 are included for comparison. Vertical scale bar in (B), 1 nA.
Indeed, in response to trains, the higher buffering conditions revealed a larger component of CDF than seen with low EGTA (Fig. 3B); for instance, the relative current carried by Ca exceeded that carried by Ba throughout the train for all frequencies, although it remained within 5% of the initial tail current at 50 and 100 Hz. At 200 Hz, the fifth step facilitated, reaching 106 ± 2% in Ca vs. 96 ± 1% with Ba (n = 6, p < 0.05 vs. Ba and vs. Ca with 1 mM EGTA, unpaired, Fig. 3C). At all three stimulus frequencies, the tail current at the end of the train was significantly larger in Ca than in Ba (p < 0.05). Together, these results suggest that CDI opposes CDF, at least in lower buffering conditions, preventing Ca currents evoked during trains from substantially exceeding the amplitude associated with a single action potential.
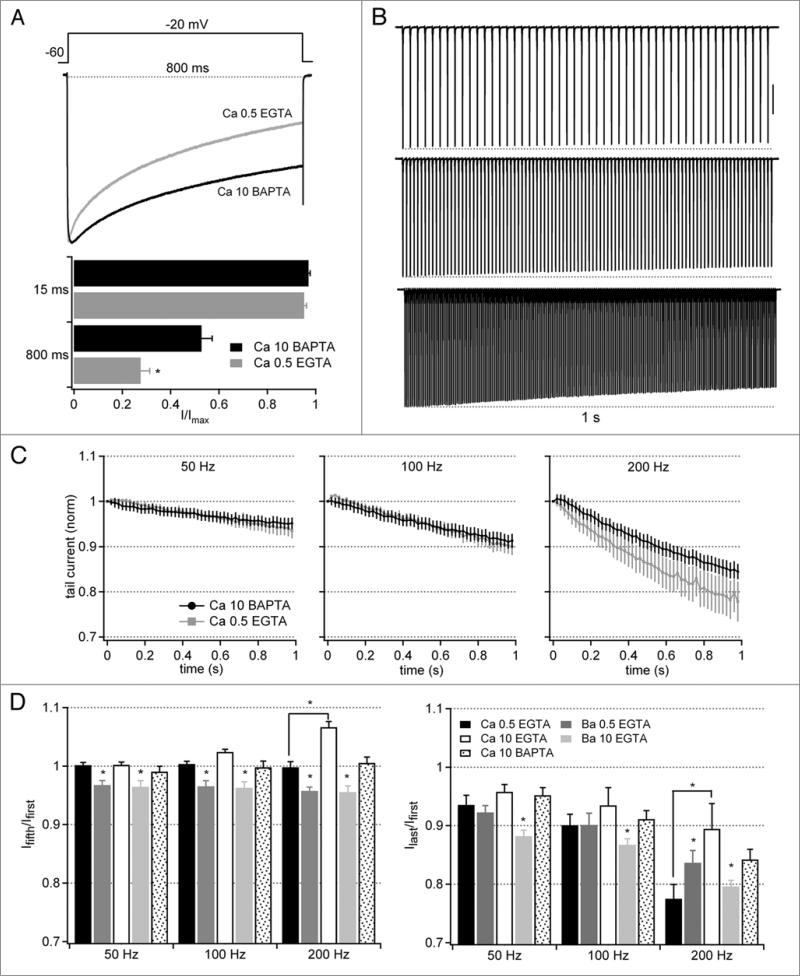
Ca currents recorded with modulation inhibited by 10 mM BAPTA
The relative stability of Ca currents in low buffering conditions, even with CDI and CDF occurring, raises the question of the precision with which these two processes balance each other. To address this issue, we minimized Ca-dependent modulation by using BAPTA (10 mM) as the buffer. In other preparations, BAPTA abolishes CDI and diminishes but does not always eliminate CDF.18,25,26 In our preparation, Ca current during the long depolarizing step decayed to a lesser extent with BAPTA (n = 6) than with 0.5 mM EGTA, consistent with a reduction of CDI (Fig. 4A). The profiles of Ca currents evoked by 50- and 100-Hz trains, however, nearly overlapped in 0.5 EGTA and 10 mM BAPTA, with only the loss of a tiny phase of facilitation early in the trains in BAPTA (Fig. 4B and C). Only after repeated stimuli at 200 Hz, did a substantial difference emerge, as currents with BAPTA remained larger than those with 0.5 EGTA. Thus, at stimulus rates that resemble firing rates in Purkinje neurons, CDI and CDF offset each other remarkably closely in the low buffering conditions.
Figure 4.
Facilitation and inactivation of tail currents evoked by stimulus trains with 10 mM intracellular BAPTA. (a–C) Ca currents as in Figure 2, but with 10 mM intracellular BAPTA (n = 6). In (A), Ca current data obtained with 0.5 mM EGTA from Figure 2 are included for comparison. Vertical scale bar in (B), 1 nA. (D) Summary plots of the different buffering conditions and charge carriers. Facilitation is expressed as the ratio of the fifth to the first tail current; inactivation is expressed as the ratio of the last to the first tail current. Asterisks over bars representing Ba currents indicate statistical differences between Ba and Ca with the same buffering condition (same cells), and over bars representing Ca currents indicate statistical differences between Ca with different buffering conditions (different cells).
The relative amplitudes of currents early in the train (at the fifth tail current) and late in the train (at the last tail current) are summarized in Figure 4D. Together, they illustrate that CDF consistently operates early in the train, occluding voltage-dependent inactivation, but generally keeping tail current amplitudes at or below the amplitude of the first current. Late in the train, CDI becomes evident, although inactivation by more than 20% occurs only with the highest frequency of stimulation.
Ca currents during transitions from basal to higher stimulation rates
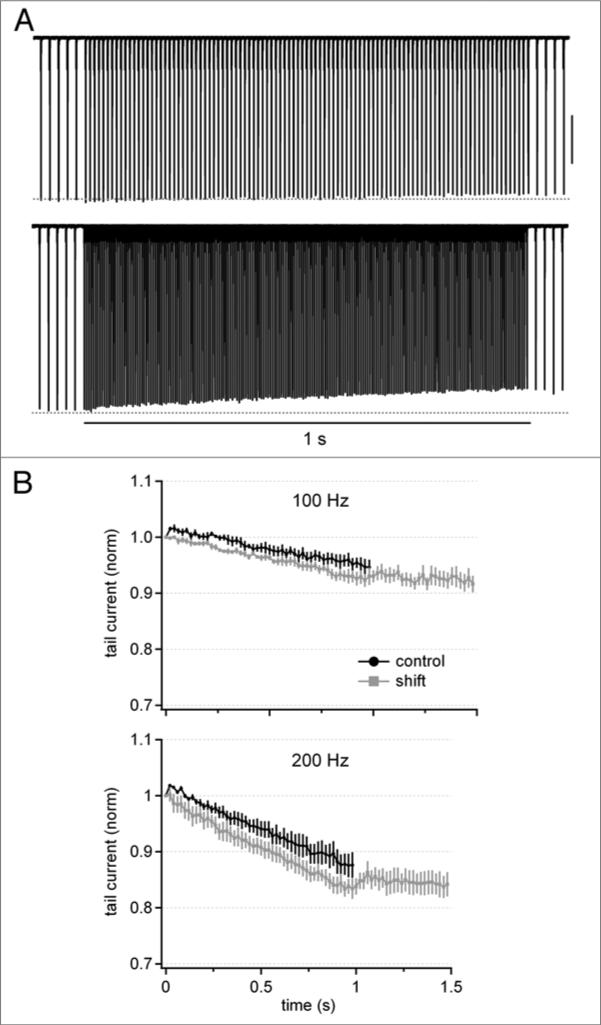
Because Purkinje cells fire spontaneously, the repeated depolarizations associated with each spike may present a tonic Ca load that affects the patterns of CDF and CDI, raising the question of the extent to which ongoing activity influences the profile of Ca currents evoked at high stimulation frequencies. To test how Ca currents are modulated during shifts from basal to elevated rates of stimulation, we evoked Ca currents (with buffering by 0.5 mM EGTA) with a 500-ms, 50-Hz train of depolarizing steps followed by a 1-sec train at 100 or 200 Hz, after which the stimulation was returned to 50 Hz for 500 ms. Consistent with the previous measurements, during the initial 50-Hz “conditioning” train, Ca currents remained within 2.5% of the first evoked current (n = 4). During the 100- or 200-Hz train, however, the small CDF in the control records was absent after conditioning (Fig. 5A and B). These data suggest that spontaneous firing elevates global intracellular Ca sufficiently to tip the balance away from CDF and toward CDI. Upon resumption of stimulation at 50 Hz, further inactivation did not occur. Ca currents did not, however, grow back to their original amplitude, suggesting that periods of reduced rates of stimulation are necessary to recover inactivated channels.
Figure 5.
Ca tail currents during transitions from basal to elevated rates of activation. (A) Cd-sensitive Ca currents (0.5 mM EGTA) elicited by a train of 1-ms pulses at 50 Hz for 500-ms, followed by a shift to 100 Hz (top) or 200 Hz (bottom) for 1 s, followed by a return to 50 Hz for 500 ms. Stimulation periods at 50 Hz are truncated for clarity.(B) Average tail current amplitudes normalized to the first tail current evoked by the initial pulse in the 100-hz or 200-hz train (n = 4), for cells with conditioning at 50 Hz (“shift”) and without conditioning at 50 Hz (“control”). shift and control data are from the same cells.
Discussion
These data demonstrate that, when tested at 30–33°C with near physiological concentrations of divalent cations, high-voltage-activated Ca currents in electrically mature Purkinje cells remain remarkably constant during patterns of activation that resemble high-frequency firing. This stability, however, does not result from an absence of modulation, or the gradual achievement of a steady-state level of channel availability. Instead, voltage-dependent inactivation, calcium-dependent facilitation, and calcium-dependent inactivation combine to yield a net negligible change in Ca current amplitude. These currents therefore appear well adapted for generating consistent Ca signals during spontaneous and driven high-frequency firing, and likely contribute to the maintenance of synaptic transmission.
Mechanisms of calcium-dependent facilitation and inactivation
Our results are consistent with previous work identifying the molecular mechanisms of calcium-dependent modulation of P-type (CaV2.1) Ca channels. These studies have demonstrated that facilitation of CaV2.1 is favored by increases in local Ca concentration, and therefore tends to be resistant to high intracellular EGTA, whereas inactivation requires an elevation of global Ca, and therefore is reduced by high EGTA.9,10,12,24 Facilitation and inactivation are mediated by interactions of calmodulin and other Ca binding proteins with intracellular domains of the CaV2.1 α subunit, and is also evident in neuronal preparations.13,18
In Purkinje neurons, although the dominant Ca current is P-type (Fig. 1),14,15 we find not only that the net modulation is small, but also that even the maximal modulation is modest; even with stimulation at 200 Hz, the largest facilitation was <8%. In contrast, superior cervical ganglion neurons expressing recombinant CaV2.1 show >20% facilitation, and even P10 Purkinje neurons can display up to 15% facilitation.13,18 Moreover, trains of action potential-like depolarizations applied to chromaffin cells (with high buffer) relieve a standing, G-protein dependent, voltage-dependent inactivation of P-type channels, yielding a net facilitation of about 15%.27 Inactivation in Purkinje cells reached a maximum of ~20%; this value is close to that seen in P-type channels in chromaffin cells (with low buffer).17
The comparatively low level of modulation that we observed in Purkinje cells may result in part from differences in recording conditions: To approximate physiological conditions, we recorded with 1 mM Ca, which reduces local Ca influx. In fact, in Purkinje cells in which currents failed to facilitate in 1 mM Ca, raising Ca to 5 or 10 mM revealed a facilitation of >10% (not shown). Other factors underlying the mild modulation of Purkinje P-type channels are likely to include subunit composition, splice variants, and/or additional protein members of the Ca channel complex. Previous work has demonstrated that CaV2.1 channels expressed with β2a subunits, but not β1b subunits, are highly sensitive to modulation by Ca.10 In situ hybridization studies of Purkinje neurons, however, indicate expression of Ca channel β2 and β4, with little β1,28,29 making it seem unlikely that the β1 subunit is responsible for the minor modulation in Purkinje cells. Nevertheless, subunits other than β1, or the presence (or absence) of regulatory proteins may contribute to the regulation of Ca currents in Purkinje cells.
In addition, both calcium- and voltage-dependent modulation are influenced by alternative splicing of CaV2.1 subunits.17,30,31 In fact, the increase in CDF in Purkinje cells over the second postnatal week is associated with a change in the expressed splice variant of CaV2.1.18 Our data suggest that further identification of the members of CaV2.1 channel complexes in Purkinje neurons from older animals will provide information about what subsets of subunits or splice variant combinations generate a minimal amount of modulation.
Ca buffering in Purkinje neurons
In intact neurons, the extent of Ca-dependent modulation will be set not only by the composition of the Ca channel complex but also by the endogenous Ca buffer. Although we have tried to bracket a range of Ca buffers by measuring currents with 0.5 EGTA, 10 EGTA and 10 mM BAPTA, it is likely that none of these precisely mimics the physiological situation. Purkinje cells express about 0.36 mM of a mobile endogenous buffer with an affinity of 370 nM, which is likely to be calbindin D28K, as well as the fixed buffer parvalbumin.32,33 Of the conditions we tested, therefore, experiments with 0.5 mM EGTA probably deviate the least from the physiological condition. The profile of Ca currents in 0.5 mM EGTA, however, closely resembles that in 10 mM BAPTA, despite the higher concentration and faster Ca binding rate of BAPTA. Therefore, if natural buffering in Purkinje cells is permissive for both inactivation and facilitation, the profile of Ca currents may be quite similar to a situation with minimal modulation. The kinetics and localization of calbindin and parvalbumin, however, differ from exogenous buffers, such that the inhibition of inactivation by endogenous buffers varies with the absolute amplitude of Ca current.34 It is therefore possible that a small facilitation emerges in nanodomains of the cell in which natural buffering permits local Ca accumulation while restricting more widespread Ca increases.
Ca currents during spontaneous and driven firing
In intact Purkinje neurons, the modest calcium-dependent modulation of Ca currents appears well suited for maintenance of firing, as well as for consistency of intracellular Ca transients per action potential. Somatic Ca currents in Purkinje cells couple strongly to KCa currents, such that the net effect of the total Ca influx is hyperpolarizing.4 KCa currents contribute to action potential repolarization via BK channels and setting of interspike intervals via SK channels; reducing P-type Ca influx decreases these currents and disrupts firing.4,35-37 Interestingly, CaV2.1 mutations underlying episodic ataxia type-2 irregularize Purkinje cell firing, and the cellular and behavioral symptoms can be counteracted by enhancers of SK channels.38 Thus, consistently large amplitude Ca currents not only contribute to regular firing but also appear necessary for normal motor coordination.
In this context, it is relevant that Ca currents in our experiments remained relatively constant in response to stimulus protocols that included transitions from silence to basal activation rates (roughly approximating synaptic inhibition followed by spontaneous firing) and from basal to elevated activation rates (roughly approximating spontaneous firing followed by synaptic excitation). If Ca currents instead facilitated or inactivated strongly upon changes activation rates, the resulting change in KCa currents recruited per action potential would modify firing patterns according to the dynamics of Ca channel modulation, adding an intrinsic component to the encoding of elevated excitation or inhibition. The lack of change reduces the intrinsic contribution to firing, and instead allows firing rates to represent the level of synaptic excitation directly.
Ca-dependent modulation and synaptic transmission
Recent studies support the idea that Ca-dependent modulation occurs at synaptic terminals and influences neurotransmitter release. In superior cervical ganglion cells made to express recombinant CaV2.1 channels, short-term facilitation and depression parallel the Ca-dependent modulation of the expressed subunits.13 Likewise, at the calyx of Held, Ca-dependent modulation regulates short-term synaptic plasticity.26,39 Moreover, recent work on CaV2.1 mutations associated with familial hemiplegic migraine and cortical spreading depression demonstrate that these disease states result from disruptions of synaptic transmission, which may originate in altered calcium-dependent modulation. Specifically, in R192Q CaV2.1 mice, P-type currents are enlarged, excitatory synaptic transmission is increased, and synaptic depression is intensified relative to wild-type.40,41 This mutant channel shows an increased Ca-dependent inactivation in heterologous expression systems,31 supporting the idea that Ca-dependent modulation affects synaptic transmission, and that disruption of this modulation correlates with pathophysiology.
Similarly, our measurements of somatic Ca-dependent modulation are consistent with the predicted behavior of channels at Purkinje cell synapses onto neurons of the cerebellar nuclei. These synapses have a number of specializations, including boutons with multiple release sites but no GABA transporters, which generate an efficient spillover-mediated transmission that maintains robust synaptic transmission even at high rates of activity.42,43 These specializations would be rendered ineffectual, however, if presynaptic Ca channels were subject to substantial Ca-dependent and/or voltage-dependent modulation: Facilitation would be expected to increase release probability but accelerate depletion, whereas inactivation would promote synaptic depression. Thus, it seems likely that the balanced facilitation and inactivation evident in Purkinje somata is reflected at the synapse, such that the Ca influx per action potential remains relatively constant, thereby contributing to the long-term efficacy of synaptic transmission.
Materials and Methods
Preparation of isolated Purkinje cells
All experimental procedures involving animals were carried out in accordance with institutional guidelines and were approved by the Northwestern University IACUC. Cerebellar Purkinje cells were acutely dissociated from P16-P21 C57BL6 mice (Charles River, Wilmington, MA) as in Raman and Bean.3 Mice were anesthetized with halothane and decapitated. The superficial layers of the cerebellum were removed and minced in an ice-cold dissociation solution containing (mM) 82 Na2SO4, 30 K2SO4, 5 MgCl2, 10 HEPES, 10 glucose and 0.001% phenol red (pH 7.4 with NaOH). The tissue was incubated in dissociation solution including 3 mg/mL protease XXIII (pH 7.4) for 7 min at 31°C with oxygen blowing over the surface of the fluid, then washed twice and microdissected in 1 mg/mL bovine serum albumin and trypsin inhibitor (pH 7.4) and finally transferred to Tyrode's solution, containing (mM) 150 NaCl, 4 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES and 10 glucose, pH 7.4. The tissue was then triturated with polished Pasteur pipettes. Cells were allowed to settle in the recording chamber, on a warming plate at 30–33°C, and recordings were made 1–6 hours after trituration. Purkinje somata were identified based on their size and morphology.
Electrophysiology
Borosilicate pipettes (2–4 MΩ, A-M Systems, Carlsborg, WA) were wrapped in parafilm to reduce capacitance and filled with an intracellular solution containing (mM): 120 TEA-CH3SO3, 10 NaCl, 2 MgCl2, 10 HEPES, 14 TrisCreatine PO4, 4 MgATP, 0.3 Tris-GTP, and 0.5 or 10 EGTA or 10 BAPTA, as noted, (pH to 7.4 with TEA-OH). Whole-cell voltage-clamp recordings were made with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA) and series resistance was compensated by more than 60%. Currents were evoked every 30 s, with the exception of current-voltage relationships, during which the inter-sweep interval was 5 s. Currents were low pass filtered at 5 kHz, digitized at 50 kHz, and recorded with a Digidata 1322A and pClamp 10. Recordings were made with cells positioned in front of an array of three gravity-driven flow pipes containing 150 mM TEA-Cl, 1 mM MgCl2, 10 mM HEPES, 300 nM TTX and 3 μM nimodipine (pH 7.4 with TEA-OH). The first pipe included 1 mM CaCl2, the second, 1 mM BaCl2, and the third, either 1 mM BaCl2 with 300 μM CdCl2, or 1 mM CaCl2 with 200 nM ω-agatoxin IVA. Recordings in Cd or ω-agatoxin were subtracted from records without blockers to isolate Ca channel currents. All comparisons of Ca and Ba currents are within-cell comparisons. All drugs were from Sigma-Aldrich, Inc., except TTX (Alomone Labs, Jerusalem) and ω-agatoxin IVA (Peptides International, Louisville, KY).
Analysis
Data were analyzed with IgorPro (Wavemetrics, Lake Oswego, OR) and are reported as mean ± SEM. Statistical significance was assessed with Student's two-tailed paired t-tests, except as noted. Significance was taken to be p < 0.05, and is indicated on figures with asterisks.
Acknowledgements
Supported by NIH grant RO1 NS39395 (IMR). Mark D. Benton was supported by T32 NS041234. We thank lab members Teresa Aman, Nan Zheng, Jason Bant and Abigail Person for discussions and comments on the data and manuscript.
Abbreviations
- CDF
calcium-dependent facilitation
- CDI
calcium-dependent inactivation
- EGTA
ethylene glycol tetraacetic acid
- BAPTA
1,2-bis(o-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid
References
- 1.Latham A, Paul DH. Spontaneous activity of cerebellar Purkinje cells and their responses to impulses in climbing fibres. J Physiol. 1971;213:135–56. doi: 10.1113/jphysiol.1971.sp009373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hausser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–78. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- 3.Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci. 1997;17:4517–26. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci. 1999;19:1663–74. doi: 10.1523/JNEUROSCI.19-05-01663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang H, DeMaria CD, Erickson MG, Mori MX, Alseikhan BA, Yue DT. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron. 2003;39:951–60. doi: 10.1016/s0896-6273(03)00560-9. [DOI] [PubMed] [Google Scholar]
- 6.Fox AP, Nowycky MC, Tsien RW. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987;394:149–72. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regan LJ. Voltage-dependent calcium currents in Purkinje cells from rat cerebellar vermis. J Neurosci. 1991;11:2259–69. doi: 10.1523/JNEUROSCI.11-07-02259.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imredy JP, Yue DT. Mechanism of Ca(2+)-sensitive inactivation of L-type Ca2+ channels. Neuron. 1994;12:1301–18. doi: 10.1016/0896-6273(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 9.Lee A, Wong ST, Gallagher D, Li B, Storm DR, Scheuer T, Catterall WA. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature. 1999;399:155–9. doi: 10.1038/20194. [DOI] [PubMed] [Google Scholar]
- 10.Lee A, Scheuer T, Catterall WA. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J Neurosci. 2000;20:6830–8. doi: 10.1523/JNEUROSCI.20-18-06830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee A, Westenbroek RE, Haeseleer F, Palczewski K, Scheuer T, Catterall WA. Differential modulation of CaV2.1 channels by calmodulin and Ca2+-binding protein 1. Nat Neurosci. 2002;5:210–7. doi: 10.1038/nn805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee A, Zhou H, Scheuer T, Catterall WA. Molecular determinants of Ca(2+)/calmodulin-dependent regulation of CaV2.1 channels. Proc Natl Acad Sci USA. 2003;100:16059–64. doi: 10.1073/pnas.2237000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mochida S, Few AP, Scheuer T, Catterall WA. Regulation of presynaptic CaV2.1 channels by Ca2+ sensor proteins mediates short-term synaptic plasticity. Neuron. 2008;57:210–6. doi: 10.1016/j.neuron.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 14.Mintz IM, Adams ME, Bean BP. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- 15.Erickson MA, Haburcak M, Smukler L, Dunlap K. Altered functional expression of Purkinje cell calcium channels precedes motor dysfunction in tottering mice. Neuroscience. 2007;150:547–55. doi: 10.1016/j.neuroscience.2007.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–6. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 17.Wykes RC, Bauer CS, Khan SU, Weiss JL, Seward EP. Differential regulation of endogenous N- and P/Q-type Ca2+ channel inactivation by Ca2+/calmodulin impacts on their ability to support exocytosis in chromaffin cells. J Neurosci. 2007;27:5236–48. doi: 10.1523/JNEUROSCI.3545-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaudhuri D, Alseikhan BA, Chang SY, Soong TW, Yue DT. Developmental activation of calmodulin-dependent facilitation of cerebellar P-type Ca2+ current. J Neurosci. 2005;25:8282–94. doi: 10.1523/JNEUROSCI.2253-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin SI, Khaliq ZM, Aman TK, Grieco TM, Kearney JA, Raman IM, Meisler MH. Impaired motor function in mice with cell-specific knockout of sodium channel Scn8a (NaV1.6) in cerebellar purkinje neurons and granule cells. J Neurophysiol. 2006;96:785–93. doi: 10.1152/jn.01193.2005. [DOI] [PubMed] [Google Scholar]
- 20.Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985;65:101–48. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- 21.McDonough SI, Bean BP. Mibefradil inhibition of T-type calcium channels in cerebellar purkinje neurons. Mol Pharmacol. 1998;54:1080–7. doi: 10.1124/mol.54.6.1080. [DOI] [PubMed] [Google Scholar]
- 22.Khaliq ZM, Raman IM. Axonal propagation of simple and complex spikes in cerebellar Purkinje neurons. J Neurosci. 2005;25:454–63. doi: 10.1523/JNEUROSCI.3045-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llinas R, Sugimori M, Simon SM. Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc Natl Acad Sci USA. 1982;79:2415–9. doi: 10.1073/pnas.79.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411:484–9. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- 25.Borst JG, Sakmann B. Facilitation of presynaptic calcium currents in the rat brainstem. J Physiol. 1998;513:149–55. doi: 10.1111/j.1469-7793.1998.149by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J, Wu LG. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron. 2005;46:633–45. doi: 10.1016/j.neuron.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 27.Currie KP, Fox AP. Differential facilitation of N- and P/Q-type calcium channels during trains of action potential-like waveforms. J Physiol. 2002;539:419–31. doi: 10.1113/jphysiol.2001.013206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka O, Sakagami H, Kondo H. Localization of mRNAs of voltage-dependent Ca2+-channels: four sub-types of alpha1- and beta-subunits in developing and mature rat brain. Brain Res Mol Brain Res. 1995;30:1–16. doi: 10.1016/0169-328x(94)00265-g. [DOI] [PubMed] [Google Scholar]
- 29.Ludwig A, Flockerzi V, Hofmann F. Regional expression and cellular localization of the alpha1 and beta subunit of high voltage-activated calcium channels in rat brain. J Neurosci. 1997;17:1339–49. doi: 10.1523/JNEUROSCI.17-04-01339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaudhuri D, Chang SY, DeMaria CD, Alvania RS, Soong TW, Yue DT. Alternative splicing as a molecular switch for Ca2+/calmodulin-dependent facilitation of P/Q-type Ca2+ channels. J Neurosci. 2004;24:6334–42. doi: 10.1523/JNEUROSCI.1712-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams PJ, Garcia E, David LS, Mulatz KJ, Spacey SD, Snutch TP. CaV2.1 P/Q-type calcium channel alternative splicing affects the functional impact of familial hemiplegic migraine mutations: implications for calcium channelopathies. Channels. 2009;3:110–21. doi: 10.4161/chan.3.2.7932. [DOI] [PubMed] [Google Scholar]
- 32.Fierro L, Llano I. High endogenous calcium buffering in Purkinje cells from rat cerebellar slices. J Physiol. 1996;496:617–25. doi: 10.1113/jphysiol.1996.sp021713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeda H, Ellis-Davies GC, Ito K, Miyashita Y, Kasai H. Supralinear Ca2+ signaling by cooperative and mobile Ca2+ buffering in Purkinje neurons. Neuron. 1999;24:989–1002. doi: 10.1016/s0896-6273(00)81045-4. [DOI] [PubMed] [Google Scholar]
- 34.Kreiner L, Lee A. Endogenous and exogenous Ca2+ buffers differentially modulate Ca2+-dependent inactivation of CaV2.1 Ca2+ channels. J Biol Chem. 2006;281:4691–8. doi: 10.1074/jbc.M511971200. [DOI] [PubMed] [Google Scholar]
- 35.Womack MD, Khodakhah K. Somatic and dendritic small-conductance calcium-activated potassium channels regulate the output of cerebellar purkinje neurons. J Neurosci. 2003;23:2600–7. doi: 10.1523/JNEUROSCI.23-07-02600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edgerton JR, Reinhart PH. Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function. J Physiol. 2003;548:53–69. doi: 10.1113/jphysiol.2002.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Womack MD, Chevez C, Khodakhah K. Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J Neurosci. 2004;24:8818–22. doi: 10.1523/JNEUROSCI.2915-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walter JT, Alvina K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci. 2006;9:389–97. doi: 10.1038/nn1648. [DOI] [PubMed] [Google Scholar]
- 39.Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron. 1998;20:797–807. doi: 10.1016/s0896-6273(00)81017-x. [DOI] [PubMed] [Google Scholar]
- 40.van den Maagdenberg AM, Pietrobon D, Pizzorusso T, Kaja S, Broos LA, Cesetti T, et al. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41:701–10. doi: 10.1016/s0896-6273(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 41.Tottene A, Pivotto F, Fellin T, Cesetti T, van den Maagdenberg AM, Pietrobon D. Specific kinetic alterations of human CaV2.1 calcium channels produced by mutation S218L causing familial hemiplegic migraine and delayed cerebral edema and coma after minor head trauma. J Biol Chem. 2005;280:17678–86. doi: 10.1074/jbc.M501110200. [DOI] [PubMed] [Google Scholar]
- 42.Telgkamp P, Padgett DE, Ledoux VA, Woolley CS, Raman IM. Maintenance of high-frequency transmission at purkinje to cerebellar nuclear synapses by spill-over from boutons with multiple release sites. Neuron. 2004;41:113–26. doi: 10.1016/s0896-6273(03)00802-x. [DOI] [PubMed] [Google Scholar]
- 43.Pugh JR, Raman IM. GABAA receptor kinetics in the cerebellar nuclei: evidence for detection of transmitter from distant release sites. Biophys J. 2005;88:1740–54. doi: 10.1529/biophysj.104.055814. [DOI] [PMC free article] [PubMed] [Google Scholar]