Abstract
Interleukin(IL)-1β is a proinflammatory cytokine implicated in several neurodegenerative disorders. Downstream actions of IL-1β include production of prostaglandin(PG)E2 by increasing expression of cyclooxygenase (COX) enzymes and prostaglandin E synthase (PGES) isoforms. We recently developed a transgenic mouse carrying a dormant human IL-1β eXcisional Activation Transgene (XAT) for conditional and chronic upregulation of IL-1β in selected brain regions. This model is characterized by regionally-specific glial activation, immune cell recruitment, and induction of cytokines and chemokines. Here, we aimed to elucidate the effects of long-term IL-1β expression on the PGE2 synthetic pathway and to determine the effects of prostaglandins on inflammation and memory in our model. As expected, PGE2 levels were significantly elevated after IL-1β upregulation. Quantitative real-time PCR analysis indicated significant induction of mRNAs for COX-1 and membranous PGES-1, but not COX-2 or other PGES isoforms. Immunohistochemistry revealed elevation of COX-1 but no change in COX-2 following sustained IL-1β production. Furthermore, pharmacological inhibition of COX-1 and use of COX-1 knockout mice abrogated IL-1β-mediated PGE2 increases. Although COX-1 deficient mice did not present a dramatically altered neuroinflammatory phenotype, they did exhibit improved contextual fear memory. This data suggests a unique role for COX-1 in mediating chronic neuroinflammatory effects through PGE2 production.
Keywords: prostaglandin E2, interleukin-1, cyclooxygenase-1, neuroinflammation, hippocampus
Introduction
Neuroinflammation is an innate response of glial cells, particularly microglia and astrocytes, to central nervous system (CNS) injury that protects and returns the environment to homeostasis. The proinflammatory cytokine interleukin(IL)-1β is a pivotal mediator of neuroinflammation and is rapidly induced in response to injury and disease (Basu et al. 2004, Lucas et al. 2006). Among its many actions within the CNS, IL-1β causes upregulation of prostaglandin(PG) E2, a key lipid mediator produced by the metabolism of arachidonic acid (AA) (O’Banion et al. 1996, Moore et al. 2004a). Two cyclooxygenase (COX) enzymes initiate the first step in the conversion of AA into PGE2 by catalyzing AA into the intermediate PGH2, which is subsequently converted into PGE2 by one of three PGE2 synthase enzymes: one cytosolic (cPGES), and two membranous (mPGES-1 and -2) isoforms (Choi et al. 2006, O’Banion 2009). COX-1 is considered to be responsible for homeostatic expression of PGs whereas COX-2, being rapidly induced in response to inflammatory stimuli, is thought to be responsible for inducible PG production (Kaufmann et al. 1997, O’Banion 1999).
Research suggests that an inappropriate or prolonged neuroinflammatory response can exacerbate injury or disease in the surrounding tissue (Lucas et al. 2006). COX-mediated PG production has been studied in many injury paradigms, and COX-2 has been implicated in promoting injury. For example, COX-2 inhibition can prevent neuronal death in ischemic brain injury, whereas overexpression of COX-2 leads to larger infarction volume following ischemia (Dore et al. 2003, Nakayama et al. 1998). Traumatic brain injury models show increased sparing of cortical tissue in COX-2 but not COX-1 KO mice (Kelso et al. 2009), and COX-2 inhibition may be beneficial in acute spinal cord injury (Resnick et al. 1998). Prolonged cyclooxygenase activity may also contribute to neurodegenerative disorders such as Alzheimer’s disease (AD) or amyotrophic lateral sclerosis (Kiaei et al. 2005, Hoozemans & O’Banion 2005), though clinical studies with nonsteroidal anti-inflammatory drugs (NSAIDs) have not shown efficacy in established disease (Aisen et al. 2003, Cudkowicz et al. 2006). Nevertheless, epidemiological studies suggest that long-term inhibition of the COX enzymes using NSAIDs may protect against AD (Vlad et al. 2008, McGeer & McGeer 2007). Better study of PG production in chronic neuroinflammatory settings is thus warranted to further elucidate the role PGs play in disease pathogenesis. To this end, we have utilized a novel mouse model capable of sustained IL-1β expression to examine downstream effects on the PG pathway.
The IL-1β XAT mouse employs eXcisional Activation Transgene (XAT) technology whereby a human IL-1β transgene can be activated with spatial and temporal control. Upon administration of a viral vector encoding Cre-recombinase, human IL-1β is produced by transduced astrocytes surrounding the site of injection. Human IL-1β binds to the murine IL-1R1 receptor and induces a chronic neuroinflammatory state observed for up to a year following injection (Shaftel et al. 2007a). At just two weeks following IL-1β upregulation, the inflammatory state includes glial activation, peripheral immune cell recruitment, and induction of cytokines and chemokines (Hein et al. 2010, Moore et al. 2009, Shaftel et al. 2007a, Shaftel et al. 2007b). Moreover, bilateral hippocampal activation of IL-1β expression leads to deficits in hippocampal-dependent memory (Hein et al. 2010, Moore et al. 2009). It is well known that IL-1β is capable of mediating behavioral impairments and research suggests that these are mediated by downstream PGs (Hein & O’Banion 2009). Thus, we hypothesized that PGs may be responsible for memory deficits in the IL-1β XAT mouse model.
To elucidate the effects of long-term hippocampal IL-1β expression on the PGE2 synthetic pathway we used the IL-1β XAT model and report that sustained overexpression of IL-1β in the hippocampus elevates PGE2 through a COX-1 dependent mechanism. Interestingly, experiments with COX-1 knockout (KO) mice harboring the IL-1β XAT transgene revealed that PGE2 is not necessary for most of the inflammatory phenotype; however COX-1 KO rescued IL-1-mediated deficits in a contextual fear conditioning task indicating a potential role for COX-1 in learning and memory.
Materials and methods
Animals
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee (University of Rochester Committee on Animal Resources) for compliance with federal regulations before the initiation of this study. Creation and genotyping of the IL-1β XAT mice on a C57BL/6 background have been described previously (Shaftel et al. 2007a). Briefly, the IL-1β XAT mice carry a transgenic construct containing a glial fibrillary acidic protein (GFAP) promoter, a loxP flanked transcriptional stop sequence, and downstream ssIL-1β transgene coding for the signal sequence from the human IL-1 receptor antagonist (75 bp) fused to the cDNA sequence of human mature IL-1β (464 bp). To achieve transcriptional activation of the transgene, a feline immunodeficiency virus encoding for Cre protein is directed to the area of interest for Cre-mediated excision of the transcriptional stop at the loxP elements.
COX-1 KO mice on a C57BL/6, 129P2 mixed background were purchased from Taconic Laboratories (Hudson, NY, USA) (model #002180). These mice were crossed to the IL-1β XAT mice (pure C57BL/6 background) and resulting COX-1 HET-IL-1β WT and COX-1 HET-IL-1β XAT progeny were used as breeders for COX-1 genetic studies. This cross allowed us to use littermates on the C57BL/6, 129P2 mixed background for all experimental groups, except for COX-1 WT-XAT mice, which this cross failed to produce. Consequently, for COX-1 genetic experiments, the COX-1 WT-XAT mice are on a C57BL/6 background and COX-1 KO-XAT mice are on a C57BL/6, 129P2 background. The similar inflammatory phenotypes seen in COX-1 WT-XAT mice and COX-1 KO-XAT mice and the intermediary PGE2 and behavioral response displayed by the COX-1 HET-XAT mice indicate that the strain difference did not significantly affect the main findings in this manuscript. Genotyping was performed on DNA isolated from tail snips using the Wizard SV Genomic kit (Promega, Madison, WI, USA) and screened by standard PCR using recommended primers (Taconic Laboratories).
Feline immunodeficiency Virus (FIV)
The construction and packaging of FIV-Cre has been described previously (Lai et al., 2006). Briefly, FIV-Cre encodes Cre recombinase protein (Cre) with a nuclear localization sequence and V5 epitope tag under the control of a cytomegalovirus promoter. FIV-Cre, and FIV-green fluorescent protein (GFP) (System Biosciences, Mountain View, CA, USA) were packaged to a final titer of ~1×107 infectious viral particles (IVP) per ml. In vivo stereotactic injections were performed from 8–16 weeks of age and used 1.5 μl of virus to deliver ~1.5×104 IVP either unilaterally or bilaterally to the dorsal hippocampus.
Stereotactic injections
Intrahippocampal injections were described previously (Shaftel et al. 2007b). Briefly, mice were anesthetized with 1.75% isoflurane in 30/70% oxygen/nitrogen gas and secured in a Kopf stereotax. A 0.5 mm burr hole was drilled in the skull at −1.8 mm caudal and 1.8 mm horizontal from bregma and a 33 GA needle pre-loaded with virus was lowered 1.8 mm from the brain surface over 2 minutes. A Micro-1 microsyringe pump controller (World Precision Instruments, Sarasota, FL, USA) was used to inject 1.5 μl of virus at a constant rate over 10 minutes. Following a 5 minute delay to allow viral diffusion, the needle was raised slowly over 2 minutes, the burr hole sealed with Ethicon bone wax and soft tissues sutured using 6-0 Dermalon suture (Ethicon, Somerville, NJ, USA).
Cyclooxygenase Inhibitor Study
Mice received stereotactic FIV-Cre injections unilaterally as described above. Eleven days after IL-1β transgene activation mice received 6 total i.p. injections of the COX-1 selective inhibitor, SC-560 (Cayman; 30 mg/kg), or drug control (40% DMSO in 0.1M PB) every 12 h over three days prior to sacrifice for two weeks total IL-1β upregulation. This dose of SC-560 has been shown to inhibit CNS PGE2 production following acute IL-1β injection, exposure to radiation, or central LPS injection (Moore et al. 2004a, Moore et al. 2005, Choi et al. 2008). Animals were sacrificed 4 hours after the final drug injection.
Quantitative real-time PCR
At several timepoints following FIV-Cre injections, mice were anesthetized with ketamine and xylazine (i.p., 60–90 and 4–8 mg/kg, respectively) and perfused with 0.15 M phosphate buffer (PB) containing 2 IU/ml heparin, 0.5% w/v sodium nitrite, and 1 μM indomethacin. Brains were removed and hippocampi microdissected, rapidly frozen in ice-cold isopentane, and stored at −80 °C. Detailed qRT-PCR procedures have been described previously (Shaftel et al. 2007a). Briefly, hippocampal RNA isolation was performed using the TRIzol (Invitrogen, Carlsbad, CA, USA) extraction protocol. RNA was quantified by UV spectrophotometric analysis at 260 nm, and cDNA generated using a Superscript III First Strand Synthesis kit (Invitrogen). Relative mRNA quantification was performed using the iCycler (Bio-Rad, Hercules, CA, USA) with custom designed primers and probes (Invitrogen). PCR reactions were performed in a final volume of 20 μl using iQ Supermix (Bio-Rad) and 5 nM FITC dye as follows: 95°C for 3 min, followed by 50 cycles of 95°C for 15 s, and 60°C for 1 min. The G3PDH housekeeping gene was used to normalize determinations of mRNA abundance. Sequences for all PCR reactions can be found in Table 1.
Table 1.
| Molecule | Upper Primer Sequence | Lower Primer Sequence | Probe Sequence |
|---|---|---|---|
| COX-1 | 5′ gtgccagaaccagggtgtct3′ | 5′ gtagcccgtgcgagtacaatc 3′ | 5′ cgctttggcctcgacaactaccagtg 3′ |
| COX-2 | 5′ tgacccccaaggctcaaata 3′ | 5′ cccaggtcctcgcttatgatc 3′ | 5′ ctttgcccagcacttcacccatcagtt 3′ |
| cPGES | 5′ cgcccacccgtttgtct 3′ | 5′ tctttactgtcttcaacacaaaattcaa 3′ | 5′ ccgttcaccatgcagcctgcttc 3′ |
| mPGES | 5′ gcccaccgcaacgacat 3′ | 5′ ggttgggtcccaggaatga 3′ | 5′ agacaatctatcctttcctcttcctcggcttc 3′ |
| GFAP | 5′ ctggaggtggagagggacaa 3′ | 5′ ggttggtttcatcttggagctt 3′ | 5′ tttgcacaggacctcggcaccc 3′ |
| MHC-II | 5′ agtcagtcgcagacggtgttt 3′ | 5′ gataagacagcttgtggaaggaatagt 3′ | 5′ tgagaccagcttcttcgtcaaccgtg 3′ |
| TNF-α | 5′ gacaaggctgccccgacta 3′ | 5′ tttctcctggtatgagatagcaaatc 3′ | 5′ ctcctcacccacaccgtcagcc 3′ |
| IL-1α | 5′ aaggagagccgggtgacagt 3′ | 5′ gaaactcagccgtctcttcttca 3′ | 5′ cagcaacgtcaagcaacgggaagattc 3′ |
| IL-1β | 5′ tcgctcagggtcacaagaaa 3′ | 5′ atcagaggcaaggaggaaacac 3′ | 5′ catggcacattctgttcaaagagagcctg 3′ |
| IL-6 | Applied Biosystems Kit | Applied Biosystems Kit | 5′ aatgagaaaagagttgtgcaatggc 3′ |
| CCR2 | 5′ agtaactgtgtgattgacaagcacttaga 3′ | 5′ caacaaaggcataaatgacaggat 3′ | 5′ acagagactcttggaatgacacactgctgc 3′ |
| CXCL1 | 5′ gctaaaaggtgtccccaagtaa 3′ | 5′ taggaccctcaaaagaaattgta 3′ | 5′ ctgctctgatggcaccgtctggt 3′ |
| CCL2 | 5′ ggctcagccagatgcagttaa 3′ | 5′ cctactcattgggatcatcttgct 3′ | 5′ ccccactcacctgctgctactcattca 3′ |
| CXCL2 | 5′ caagaacatccagagcttgagtgt 3′ | 5′ ttttgaccgcccttgagagt 3′ | 5′ cccactgcgcccagacagaagtcat 3′ |
| GAPDH | 5′ cccaatgtgtccgtcgtg 3′ | 5′ cctgcttcaccaccttcttg 3′ | 5′ tgtcatcatacttggcaggtttctccagg 3′ |
Immunoassay
Following two weeks of IL-1β upregulation, mice were sacrificed as described above. Frozen hippocampal samples were homogenized in tissue protein extraction reagent (Pierce, Rockford, IL, USA) with protease inhibitors (Sigma-Aldrich, St. Louis, MO, USA) at 1 mL per 50 mg tissue for a final dilution of 1:20. 700 μl methanol was added to 300 μl of homogenate in a 1.5 mL micro-centrifuge tube, vortexed briefly, then spun at 15,000 rpm for 20 minutes at room temperature. Supernatants were evaporated and reconstituted in 300 μl PGE2 EIA buffer. PGE2 concentrations were determined by ELISA according to the manufacturer’s suggested protocols (Cayman).
Immunohistochemistry
Two weeks following FIV-Cre injections, mice were anesthetized with ketamine and xylazine (i.p., 60–90 and 4–8 mg/kg, respectively) and intracardially flushed with a 0.15 M PB solution containing 0.5% w/v sodium nitrite, 2 IU/ml heparin, and 1 μM indomethacin followed immediately by an ice cold 4% paraformaldehyde solution. Brains were post-fixed for 3 h in 4% paraformaldehyde then immersed in 30% sucrose, snap frozen in isopentane and sectioned into 30 μm free floating slices. Antibody binding was visualized using either Elite avidin– biotin and 3,3-diaminobenzidine (Vector Laboratories, Burlingame, CA, USA) or secondary antibodies bound to Alexa 488 or 594 fluorophores (Invitrogen). Primary antibodies used were as follows: COX-1 Monoclonal FITC (Cayman; Catalog # 160111; 1:500), COX-2 (Cayman; Catalog # 160106; 1:1000), GFAP (Dako, Carpinteria, CA, USA; Catalog # Z0334; 1:5000), MHC-II (BD Pharmingen, San Jose, California, USA; Catalog # 556999; 1:6000), Neu-N (Chemicon, Temecula, CA, USA; Catalog # MAB377B; 1:2000), iba-1 (Wako Chemicals, Richmond, VA, USA; Catalog # 016-20001; 1:5000).
Fear Conditioning
For behavioral testing, mice received bilateral hippocampal injections of FIV-Cre. Two weeks later, mice underwent contextual and auditory fear conditioning to assess hippocampal-dependent and –independent memory processes. For 3 days before fear conditioning, mice were transported from the colony room to the testing room, handled for 2 min each, and returned to the colony room to acclimate to experimenter manipulation. On conditioning day, mice were allowed to explore the conditioning context, which consisted of a Plexiglas chamber and metal floor grid (model H10-11M; Coulbourn Instruments, Whitehall, PA, USA). After 3 min, 15 s of white noise (80 dB) was presented co-terminating with a 2 s, 0.75 mA foot shock. This noise-shock pairing was repeated twice for a total of 3 shocks with a 30 s interval between shocks. Twenty-four hours later, mice were re-exposed to the conditioning chamber for 5 min each to test long-term memory. Four hours later, mice were placed in a novel context consisting of a 15 cm open-topped plastic cylinder with bedding on the floor for 3 min followed by re-exposure to the white noise for 3 min, to test hippocampal-independent memory. All data were video recorded using FreezeFrame Video-Based Conditioned Fear System and analyzed by Actimetrics Software (Coulbourn Instruments). In pilot studies, both COX-1 WT and KO animals displayed similarly high levels of freezing. This evidence, combined with previous studies that found no effect of COX-1 inhibition in WT mice, led us to combine data from these two groups into one WT column for comparison to mice bearing the XAT transgene.
Data analysis and image capture
Confocal images were obtained using an Olympus FV1000 laser scanning confocal microscope in the Confocal and Conventional Microscopy Core of the URMC Core Facility Program. All images were acquired using sequential scanning and oversaturation was prevented by using the hi-lo feature of the FV1000 software. UPLAN objectives were used to acquire the images. Light microscopic images were acquired on an Axioplan IIi (Carl Zeiss, Oberkochen, Germany) microscope equipped with a Spot RT camera and software (version 4.5.9.8; Diagnostic Instruments, Burroughs, MI, USA). Fluorescence images for Figure 3 were captured with an AttoArc 2 mercury lamp (Zeiss), Sensicam QE camera (Cooke, Auburn Hills, MI, USA), and Slidebook software version 5.0.0.8 on Windows XP (Intelligent Imaging, Denver, CO, USA). For densitometric analysis of gliosis, light microscopy was used to capture 20x images from sections closest to the injection site followed by blinded analysis using ImageJ (http://rsb.info.nih.gov/ij/) to determine the area fraction of staining.
Figure 3. Effect of COX-1 inhibition or knockout on PGE2 levels in IL-1β XAT mice.
(a) PGE2 levels in IL-1β XAT mice treated for 3 days with saline control or SC-560 (COX-1 specific inhibitor; 30 mg/kg) following unilateral FIV-Cre injection. n=4–9 per group. (b) PGE2 levels in WT-XAT, COX-1 HET-XAT, and COX-1 KO-XAT mice after 2 weeks of unilateral IL-1β upregulation. Hippocampi for each drug group or genotype are shown separated into control (C) and injected (I) hemispheres. n=3–9 per group. (c) Representative photomicrographs of COX-1 staining (green) in WT, HET, and KO mice. Scale bars: 30 μm (c). Data are mean ± SEM. *p < 0.05, ***p < 0.001 versus control hemisphere.
Statistical analyses
Results are expressed as mean ± SEM. Analysis was performed with Prism (GraphPad Software, San Diego, CA, USA). Depending on the experiment, either paired t-test, 1-way or 2-way analyses of variance (ANOVAs) were carried out. Bonferroni post-hoc tests were used to further analyze significant findings in ANOVA analyses. Significance was established by p-values < 0.05.
Results
Sustained IL-1β expression in the mouse hippocampus leads to induction of PGE2
To determine whether the PG pathway is affected by IL-1β elevation in the XAT mouse model, we assessed PGE2 levels at two weeks following unilateral IL-1β induction in the hippocampus, caused by intrahippocampal injection of FIV-Cre. An identical group of animals was injected unilaterally with FIV-GFP to control for injection or viral-mediated immune activation. Repeated measures two-way ANOVA showed significant main effect of hemisphere (injected or non-injected) on PGE2 elevation (F(1,12)=13.16, p =0.0035). In animals with unilateral IL-1β transgene activation (FIV-Cre injected), PGE2 levels were elevated four-fold (p <0.01) over the non-injected hemisphere and more than two-fold (p <0.05) over the injected hemisphere of the control FIV-GFP group (Fig 1). These results indicate that IL-1β overexpression driven by FIV-Cre activation of the transgene causes significant induction of PGE2.
Figure 1. PGE2 levels following sustained IL-1β expression in the mouse hippocampus.
Levels of PGE2 in IL-1β XAT mice receiving control (FIV-GFP) or IL-1β-inducing (FIV-Cre) injections unilaterally. Hippocampi are further separated into control or injected hemispheres (C=Control, I=Injected). n = 4 per group. Data displayed as mean ± SEM. *p < 0.05 versus respective control hemisphere. NS = not significant.
COX-1 mRNA and cells expressing COX-1 protein are increased following IL-1β induction
To establish which PG biosynthetic pathway components were responsible for the observed rise in PGE2, we used quantitative real-time PCR to measure relative changes in copies of gene transcripts at various timepoints between WT and XAT animals injected with FIV-Cre (non-injected hemispheres not shown). Surprisingly, COX-2 mRNA was not elevated at any timepoint measured in either WT or XAT mice. Two-way ANOVA analysis indicated that there was an overall significant effect of genotype on COX-1 (F(1,21)=38.12, p <0.0001) and COX-1 mRNA transcripts were increased up to three-fold at one (p <0.05), four (p <0.05), and eight (p <0.01) weeks following IL-1β induction (Fig 2a and b). cPGES (Fig 2c) was only elevated significantly at the eight week timepoint (p <0.001) with an induction of less than two-fold while mPGES (Fig 2d) mRNA expression was significantly elevated at all timepoints measured including a six-fold induction at 8 weeks (p <0.001). No significant induction of mPGES-2 was observed (data not shown).
Figure 2. COX-1 and COX-2 levels following sustained IL-1 expression.
(a–d) qRT-PCR analysis comparing relative abundance of gene transcripts between IL-1β XAT and WT control hippocampi following unilateral FIV-Cre injection. Data represent gene transcript levels for COX-2 (a), COX-1 (b), mPGES (c), and cPGES (d) at three timepoints following FIV-Cre injection, n = 3–4 per group. (e) Representative photomicrographs of COX-2 protein (green) and the neuronal marker Neu-N (red) in CA3 of the hippocampus. (f) COX-1 protein (green) and the microglial marker iba-1 (red) shown in the dentate gyrus of the hippocampus. Scale bars: 20 μm (e) and (f). qRT-PCR data displayed as mean ± SEM. *p < 0.05, **p<0.01, ***p<0.001 versus respective WT injected hemisphere.
To further examine expression of the two COX isoforms, we carried out immunohistochemistry (IHC) in mice unilaterally injected with FIV-Cre. COX-2 IHC revealed neuronal staining in the hippocampus with no clear evidence of increased staining following IL-1β upregulation compared to the non-injected hemisphere (Fig 2e). In contrast, COX-1 stained cells were much more abundant on the inflamed side. To identify cells expressing COX-1, we carried out double immunofluorescence using the microglial marker iba-1. Many COX-1 cells co-localized with iba-1 stained microglia (Fig 2f). These results suggest a novel role for COX-1 in our chronic inflammatory paradigm.
IL-1β mediated PGE2 elevation is ameliorated by a COX-1 specific inhibitor and by COX-1 deletion
Due to the selective elevation of COX-1 mRNA and protein following chronic IL-1β stimulation, we aimed to assess whether COX-1 is responsible for the observed increases in PGE2. Following 11 days of unilateral IL-1β upregulation in the hippocampus, the COX-1 selective inhibitor SC-560 or a vehicle control was administered intraperitoneal twice daily for three days, and levels of PGE2 were measured in dissected hippocampal tissue. A repeated measures two-way ANOVA found a main effect of IL-1β upregulation (F(1,11)=14.49, p=0.0029) in the saline control group. As expected from our mRNA and IHC findings, post-hoc analyses showed significant reduction of PGE2 between IL-1β overexpressing hemispheres in saline control mice compared to COX-1 inhibited mice (p <0.01) (Fig 3a).
To further investigate the relationship between COX-1 and IL-1β mediated PGE2 increases, COX-1 KO-XAT, COX-1 HET-XAT, and WT-XAT mice were subject to 2 weeks of unilateral IL-1β overexpression and analyzed for PGE2 elevation. COX-1 staining was carried out in COX-1 WT, HET, and KO mice to confirm that the protein was deleted (Fig 3c). A repeated measures two-way ANOVA showed a significant main effect between IL-1β upregulated and control hemispheres (F(1,15)=59.45 p <0.0001); however, post-hoc analyses indicated that the injected hemisphere of COX-1 KO-XAT mice did not vary from the control non-injected hemisphere while it varied significantly from the injected hemisphere of WT-XAT mice (p <0.001). These results indicate that the absence of COX-1 is sufficient to completely abrogate IL-1 mediated PGE2 elevation (Fig 3b). In addition, the control hemispheres of the COX-1 KO-XAT mice showed baseline levels of PGE2 that were two-fold lower than both the HET-XAT and WT-XAT mice, suggesting a role for COX-1 in normal, endogenous production of PGE2.
The IL-1β XAT neuroinflammatory phenotype is not altered by COX-1 depletion
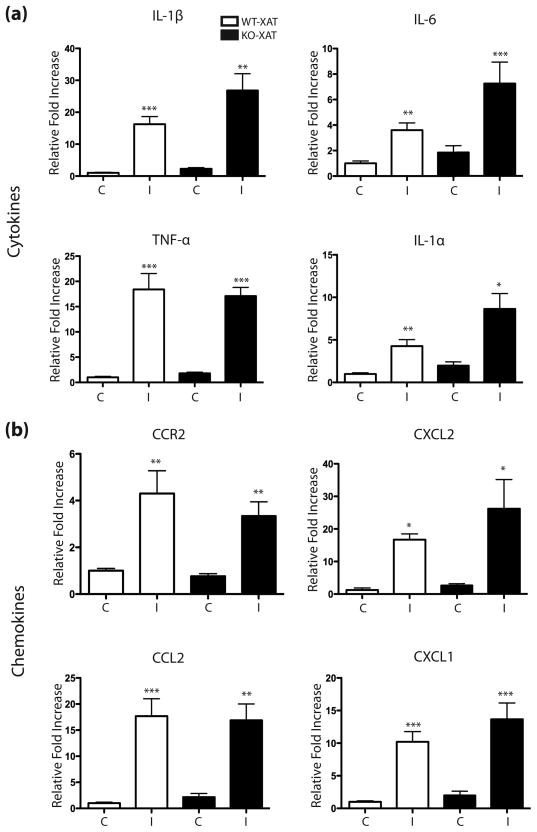
The IL-1β XAT model is characterized by regionally-specific glial activation, immune cell recruitment, and significant induction of a number of cytokines and chemokines (Hein et al. 2010, Shaftel et al. 2007a, Shaftel et al. 2007b). To determine whether COX-1 is necessary for the previously described neuroinflammatory phenotype following IL-1β upregulation IHC was carried out for GFAP and MHC-II to determine if glial reactivity was altered in COX-1 KO-XAT mice relative to WT-XAT controls. Densitometric analysis was performed using ImageJ to calculate the percent area covered by glial markers. One-way ANOVA indicated a significant effect of IL-1β upregulation for both GFAP (F(3,20)=35.21, p <0.0001) and MHC-II (F(3,16)=58.47, p <0.0001). Additional post-hoc analyses showed that GFAP elevation was diminished following IL-1β upregulation in the COX-1 KO-XAT mice compared to WT-XAT mice (p <0.001) while MHC-II was unchanged, indicating that COX-1 is involved in astrocyte reactivity in response to sustained IL-1β expression (Fig 4a–d). Using hippocampal lysates from unilaterally injected COX-1 KO-XAT and WT-XAT mice, we also examined several inflammatory mRNA transcripts including IL-1α, IL-1β, TNFα, IL-6, CCL2, CXCL2, CCR2, CXCL1, MHC-II, and GFAP. As previously reported, levels of all these inflammation-related mRNAs were elevated following IL-1β activation in XAT mice (Fig 4e,f & Fig 5). However, only GFAP showed an attenuated mRNA induction in the COX-1 KO-XAT mice compared to the COX-1 WT-XAT mice after unilateral IL-1β overexpression (Fig 4e).
Figure 4. Glial reactivity in WT and COX-1 KO animals following IL-1β overexpression.
(a–b) Representative light micrographs of control and IL-1β-induced hippocampi of COX-1 KO XAT and WT XAT mice following 2 weeks of unilateral IL-1β upregulation. GFAP was used to stain for astrocytes (a) and MHC-II was used to stain for activated microglia/monocytes (b). (c–d) Densitometric analysis of GFAP (c) and MHC-II (d) staining measured as percent area occupied by positively stained cells. (e–f) qRT-PCR analysis comparing relative abundance of GFAP (e) and MHC-II (f) between COX-1 KO XAT and WT XAT hippocampi normalized to G3PDH mRNA levels. Data are mean ± SEM (n=3–8). Scale bars: 50 μm (a) and (b). Statistically significant differences measured by two-way ANOVA between IL-1β and control hemispheres with *p <0.05, **p <0.01, ***p <0.001. Two-way ANOVA also measures †p<0.01 in COX-1 KO XAT IL-1β hemisphere versus WT XAT IL-1β hemisphere.
Figure 5. Cytokine and chemokine mRNA analysis in COX-1 KO and WT mice following IL-1β upregulation.
qRT-PCR analysis comparing relative mRNA abundance of selected transcripts to the housekeeping gene G3PDH in COX-1 KO-XAT and WT-XAT mice after 2 weeks of unilateral IL-1β induction. Cytokine (a) and chemokine (b) gene transcript levels were compared between genotypes and hemisphere (C=control, I=IL-1β) with data shown as mean ± SEM (n=3–8). Statistically significant difference measured by two-way ANOVA with *p <0.05, **p <0.01, ***p <0.001 versus WT-XAT control hemisphere.
COX-1 is involved in IL-1β mediated deficiency in contextual fear memory
PGE2 has been shown to play an important role in hindering learning and memory following acute IL-1β treatment (Hein & O’Banion 2009, Hein et al. 2007). To determine whether elevated PGE2 is associated with memory impairment in the IL-1β XAT mouse (Hein et al. 2010, Moore et al. 2009), we examined contextual fear memory 2 weeks following bilateral hippocampal induction of IL-1β in WT-XAT, COX-1 HET-XAT, and COX-1 KO-XAT mice. In our behavioral memory task, we found an overall significant one-way ANOVA (F(3,43)=6.535, p<0.001). As we have previously shown, WT-XAT animals with sustained hippocampal inflammation displayed significantly impaired contextual fear memory compared to WT controls (Hein et al. 2010). Similarly, post-hoc analyses reveal that COX-1 HET-XAT animals were also significantly impaired compared to WT controls (p<0.05) (Fig 6A). However, COX-1 KO-XAT animals had significantly better contextual fear memory compared to WT-XAT animals (p<0.05) (Fig 6A) indicating that COX-1 elimination is sufficient to rescue the IL-1β-mediated deficit. No differences between any groups were found in freezing to a novel context or an auditory stimulus, demonstrating the context and hippocampal specific nature of the memory impairment (Fig 6B). As expected, we observed elevated levels of hippocampal PGE2 in FIV-Cre injected WT-XAT mice which was absent in COX-1 KO-XAT mice from these studies (data not shown).
Figure 6. Contextual and auditory fear memory in COX-1 KO mice following IL-1β overexpression.
Freezing behavior to a conditioned context (a), novel context, and conditioned white noise (b) in WT, WT-XAT, COX-1 HET-XAT, and COX-1 KO-XAT mice 2 weeks following bilateral FIV-Cre injections in the hippocampus. Data shown as mean ± SEM (n=9–10). Significant differences measured by one-way ANOVA with *p <0.05, ***p <0.001.
Discussion
In this study, we confirmed our previous report that PGE2 levels are elevated following sustained IL-1β expression in the hippocampus (Hein et al. 2010). This was accompanied by a significant rise in COX-1 but not COX-2 mRNA and protein, suggesting a role for COX-1 in long-term PGE2 increases. Using COX-1 specific drug inhibition and KO mice we confirmed that COX-1 is responsible for the observed changes in PGE2. COX-1 deletion was also sufficient to rescue the IL-1β-mediated deficiency in contextual fear memory. The IL-1β XAT neuroinflammatory phenotype was largely unchanged in COX-1 KO-XAT mice; however GFAP mRNA and immunohistochemical staining were attenuated, indicating a possible role for COX-1 in mediating astrogliosis in this model.
In the CNS, COX-1 is expressed primarily in microglia (O’Banion 1999, Schwab et al. 2000, Yermakova et al. 1999) while COX-2 is principally localized to neurons, with some evidence for expression by other cell types including endothelial cells and astrocytes (Breder et al. 1995, Kaufmann et al. 1996, Takemiya et al. 2007, Yermakova & O’Banion 2001). There is much evidence to suggest COX-2 mediates acute inflammatory events in the CNS. Work in our laboratory has found that acute neuroinflammation following CNS radiation injury is propagated by COX-2 (Moore et al. 2005, Kyrkanides et al. 2002, Moore et al. 2004b), and that intraparenchymal injection of IL-1β causes an acute increase in PGE2 that is attenuated by a COX-2 selective inhibitor (Moore et al. 2004a). Other groups have demonstrated that COX-2 expression in the CNS is associated with excitotoxic injury from seizures and cerebral ischemia (Nakayama et al. 1998, Takemiya et al. 2003, Candelario-Jalil et al. 2006). However, in the current study, we found that COX-1 but not COX-2 mRNA and protein levels were elevated following sustained IL-1β upregulation. Furthermore, the fact that the COX-1 selective inhibitor SC-560 completely abrogated PGE2 elevation and COX-1 KO mice also showed no increase in PGE2 following IL-1β upregulation indicate that COX-1 is an important mediator of PG synthesis in long-term neuroinflammation.
Interestingly, a recent body of literature also describes COX-1 as a prominent player in CNS inflammation, particularly due to its expression pattern within inflammatory cells (Choi et al. 2009). Candelario-Jalil et al. provided the first experimental evidence that COX-1 is involved in secondary damage. This group used a model of cerebral ischemia to show that COX-1 inhibition rescued CA1 neurons after the insult (Candelario-Jalil et al. 2003). Subsequent studies by other groups found that COX-1 is involved in NMDA mediated PG production and excitotoxicity as well as lipopolysaccharide (LPS)-induced neuroinflammation and behavioral changes (Pepicelli et al. 2005, Choi et al. 2008, Teeling et al. 2009). COX-1 was also found to play a role in mediating PGE2 increases following brain irradiation suggesting that both cyclooxygenases are involved in post-radiation inflammatory processes (Moore et al. 2005).
Data from our laboratory and others suggest that COX-1 may also be involved in neural diseases or injury that are associated with long-term inflammatory features. A decade ago COX-1 was observed in plaque-associated microglia in human patients with AD and the density of these cells was increased in AD specific brain regions, suggesting that COX-1 may be involved in the neuroinflammatory reaction associated with AD pathology (Yermakova et al. 1999). Three years later Schwab and colleagues demonstrated that COX-1 expressing microglia accumulate in traumatic brain injury patients beginning just 6 hours after injury and remain for several months, leading to the speculation that COX-1 may play a role in secondary injury (Schwab et al. 2002). Our current findings lend further support to the idea that COX-1 is involved in chronic neuroinflammatory settings. We have shown that COX-1 but not COX-2 mRNA is elevated up to two months following IL-1β overexpression. These findings were confirmed by IHC, which showed elevated COX-1 in iba-1+ myeloid cells. As already mentioned, under normal conditions, COX-1 can be detected in parenchymal microglia (Yermakova et al. 1999). In the context of sustained IL-1β overexpression, the numbers of iba-1 positive myeloid cells increases dramatically (Shaftel et al. 2007a, Shaftel et al. 2007b). These cells are likely comprised of both endogenous microglia and recruited monocytes. Thus the observed elevation of COX-1 may be due to the larger number of myeloid cells rather than from increased expression within individual cells. We note that some cells staining for COX-1 did not appear to overlap with iba-1 staining. Whether these represent myeloid cells with low or undetectable iba-1 or cells of a different type is unknown. Since neutrophils are present in our IL-1β XAT model, we carried out double labeling for COX-1 and antibody 7/4. However, we did not detect overlap in antigen staining (data not shown). Although COX-1 expression has been reported in neurons and endothelial cells (Deininger et al. 2000, Yermakova et al. 1999), the morphology of COX-1 expressing cells in the current study is not consistent with these cell types. Regardless of the cell type(s) involved, overall expression of COX-1 is elevated in our model of chronic inflammation and COX-1 KO mice do not show elevated PGE2 levels after IL-1β upregulation. These findings indicate that the IL-1β XAT mouse is a good platform with which to test the effects of chronic COX-1 increases on brain pathology and injury.
IL-1β upregulation in the XAT model leads to induction of several cytokines and chemokines including murine IL-1β, IL-1α, TNF-α, IL-6, and CCL2, CXCL1, CXCL2, and CCR2. Gliosis is also a prominent feature including upregulation of MHC-II and iba-1 by microglia and GFAP by astrocytes (Moore et al. 2009, Shaftel et al. 2007b). We expected that COX-1 mediated induction of PGE2 might contribute to these neuroinflammatory features; however, we discovered that only GFAP levels were attenuated in the COX-1 KO XAT mice. Although many other inflammatory components are still elevated, our finding is supported by a report showing that COX-1 deletion attenuated astrocyte activation following LPS injection (Choi et al. 2008). Furthermore, others have shown that PGE2 production by activated microglia leads to astrocyte proliferation in vitro, which may explain why attenuating PGE2 in our model inhibits astrocyte activation (Zhang et al. 2009). While we did not assess time points earlier than 2 weeks, it is possible that COX-1 contributes to more acute phases of neuroinflammation in the XAT model. Furthermore, COX-1 and 2 contribute to the formation of other PGs, including PGD2, which plays a role in resolving neuroinflammation (Rajakariar et al. 2007). Additional studies will be required to ascertain whether such PGs are involved in this model of sustained neuroinflammation.
It is well known that IL-1β is capable of mediating memory deficits in animal models. Either peripheral administration or direct CNS injection or overexpression of IL-1β can impair spatial and contextual fear memory (Hein et al. 2007, Pugh et al. 1998, Oitzl et al. 1993, Moore et al. 2009, Hein et al. 2010). There is much evidence to suggest that IL-1β-mediated PG production may be involved in these impairments. Studies using acute LPS or IL-1β injections or models of traumatic brain injury to impair memory reveal that pharmacological inhibition of cyclooxygenases can improve outcomes (Shaw et al. 2005, Cernak et al. 2002, Hein et al. 2007). Furthermore, groups have shown beneficial effects of inhibition of both cyclooxygenases in models that mimic chronic neuroinflammatory states, including studies with long-term LPS infusion or models of AD (McKee et al. 2008, Hauss-Wegrzyniak et al. 1999, Jin et al. 2008, Cakala et al. 2007). In the current study, we have confirmed previous findings that chronic hippocampal IL-1β overexpression can mediate hippocampal memory deficits (Hein et al. 2010, Moore et al. 2009). Additionally, we show that COX-1 deletion is sufficient to significantly improve contextual fear learning, abrogating the effect of IL-1 overexpression. Therefore, elevated PGE2 in this model of chronic neuroinflammation likely contributes to the resulting contextual fear memory deficit. This finding is consistent with literature showing that PGE2 itself can impair memory and in fact may be a downstream mediator of IL-1-induced memory deficits (Hein et al. 2007, Matsumoto et al. 2004).
Overall, we have shown that COX-1 facilitates PGE2 upregulation following sustained IL-1β overexpression in the hippocampus and that COX-1 is involved in contextual fear memory deficits in these mice. Future experiments in additional disease models may help clarify the role of COX-1 in diseases such as Alzheimer’s that have a chronic neuroinflammatory component. COX-1 may represent a viable target to treat adverse events associated with sustained inflammation.
Acknowledgments
This work was supported by NIH RO1 NS33553, R21 NS048522 and RO1 AG030149 to MKO, and F31 AG031667 to SBM. The authors would like to thank Jen-nie Miller for FIV packaging, Jack Walter and Mallory Olschowka for animal colony maintenance and aid with experiments, Renee Johnson for ELISA expertise, and Linda Callahan for assistance with confocal microscopy.
Abbreviations Used
- AD
Alzheimer’s disease
- CNS
central nervous system
- COX
cyclooxygenase
- Cre
Cre recombinase protein
- FIV
feline immunodeficiency virus
- GFAP
glial fibrillary acidic protein
- GFP
green fluorescent protein
- IL-1α
interleukin-1α
- IL-1β
interleukin-1β
- LPS
lipopolysaccharide
- MHC-II
major histocompatibility complex II
- NSAIDs
non-steroidal anti-inflammatory drugs
- PG
prostaglandin
- PGES
prostaglandin E synthase
- TNF-α
tumor necrosis factor-α
- XAT
excisional activation transgene
References
- Aisen PS, Schafer KA, Grundman M, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78:151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- Breder CD, Dewitt D, Kraig RP. Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol. 1995;355:296–315. doi: 10.1002/cne.903550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakala M, Malik AR, Strosznajder JB. Inhibitor of cyclooxygenase-2 protects against amyloid beta peptide-evoked memory impairment in mice. Pharmacol Rep. 2007;59:164–172. [PubMed] [Google Scholar]
- Candelario-Jalil E, Akundi RS, Bhatia HS, Lieb K, Appel K, Munoz E, Hull M, Fiebich BL. Ascorbic acid enhances the inhibitory effect of aspirin on neuronal cyclooxygenase-2-mediated prostaglandin E2 production. J Neuroimmunol. 2006;174:39–51. doi: 10.1016/j.jneuroim.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Gonzalez-Falcon A, Garcia-Cabrera M, Alvarez D, Al-Dalain S, Martinez G, Leon OS, Springer JE. Assessment of the relative contribution of COX-1 and COX-2 isoforms to ischemia-induced oxidative damage and neurodegeneration following transient global cerebral ischemia. J Neurochem. 2003;86:545–555. doi: 10.1046/j.1471-4159.2003.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I, O’Connor C, Vink R. Inhibition of cyclooxygenase 2 by nimesulide improves cognitive outcome more than motor outcome following diffuse traumatic brain injury in rats. Exp Brain Res. 2002;147:193–199. doi: 10.1007/s00221-002-1245-z. [DOI] [PubMed] [Google Scholar]
- Choi SH, Aid S, Bosetti F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol Sci. 2009;30:174–181. doi: 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Langenbach R, Bosetti F. Cyclooxygenase-1 and -2 enzymes differentially regulate the brain upstream NF-kappa B pathway and downstream enzymes involved in prostaglandin biosynthesis. J Neurochem. 2006;98:801–811. doi: 10.1111/j.1471-4159.2006.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Langenbach R, Bosetti F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. Faseb J. 2008;22:1491–1501. doi: 10.1096/fj.07-9411com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz ME, Shefner JM, Schoenfeld DA, Zhang H, Andreasson KI, Rothstein JD, Drachman DB. Trial of celecoxib in amyotrophic lateral sclerosis. Ann Neurol. 2006;60:22–31. doi: 10.1002/ana.20903. [DOI] [PubMed] [Google Scholar]
- Deininger MH, Kremsner PG, Meyermann R, Schluesener HJ. Focal accumulation of cyclooxygenase-1 (COX-1) and COX-2 expressing cells in cerebral malaria. J Neuroimmunol. 2000;106:198–205. doi: 10.1016/s0165-5728(00)00187-9. [DOI] [PubMed] [Google Scholar]
- Dore S, Otsuka T, Mito T, Sugo N, Hand T, Wu L, Hurn PD, Traystman RJ, Andreasson K. Neuronal overexpression of cyclooxygenase-2 increases cerebral infarction. Ann Neurol. 2003;54:155–162. doi: 10.1002/ana.10612. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Vraniak P, Wenk GL. The effects of a novel NSAID on chronic neuroinflammation are age dependent. Neurobiol Aging. 1999;20:305–313. doi: 10.1016/s0197-4580(99)00028-7. [DOI] [PubMed] [Google Scholar]
- Hein AM, O’Banion MK. Neuroinflammation and memory: the role of prostaglandins. Mol Neurobiol. 2009;40:15–32. doi: 10.1007/s12035-009-8066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O’Banion MK. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 2010;24:243–253. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein AM, Stutzman DL, Bland ST, Barrientos RM, Watkins LR, Rudy JW, Maier SF. Prostaglandins are necessary and sufficient to induce contextual fear learning impairments after interleukin-1 beta injections into the dorsal hippocampus. Neuroscience. 2007;150:754–763. doi: 10.1016/j.neuroscience.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoozemans JJ, O’Banion MK. The role of COX-1 and COX-2 in Alzheimer’s disease pathology and the therapeutic potentials of non-steroidal anti-inflammatory drugs. Curr Drug Targets CNS Neurol Disord. 2005;4:307–315. doi: 10.2174/1568007054038201. [DOI] [PubMed] [Google Scholar]
- Jin DQ, Sung JY, Hwang YK, Kwon KJ, Han SH, Min SS, Han JS. Dexibuprofen (S(+)-isomer ibuprofen) reduces microglial activation and impairments of spatial working memory induced by chronic lipopolysaccharide infusion. Pharmacol Biochem Behav. 2008;89:404–411. doi: 10.1016/j.pbb.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Andreasson KI, Isakson PC, Worley PF. Cyclooxygenases and the central nervous system. Prostaglandins. 1997;54:601–624. doi: 10.1016/s0090-6980(97)00128-7. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso ML, Scheff SW, Pauly JR, Loftin CD. Effects of genetic deficiency of cyclooxygenase-1 or cyclooxygenase-2 on functional and histological outcomes following traumatic brain injury in mice. BMC Neurosci. 2009;10:108. doi: 10.1186/1471-2202-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiaei M, Kipiani K, Petri S, Choi DK, Chen J, Calingasan NY, Beal MF. Integrative role of cPLA with COX-2 and the effect of non-steriodal anti-inflammatory drugs in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurochem. 2005;93:403–411. doi: 10.1111/j.1471-4159.2005.03024.x. [DOI] [PubMed] [Google Scholar]
- Kyrkanides S, Moore AH, Olschowka JA, Daeschner JC, Williams JP, Hansen JT, Kerry O’Banion M. Cyclooxygenase-2 modulates brain inflammation-related gene expression in central nervous system radiation injury. Brain Res Mol Brain Res. 2002;104:159–169. doi: 10.1016/s0169-328x(02)00353-4. [DOI] [PubMed] [Google Scholar]
- Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147(Suppl 1):S232–240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Yamaguchi T, Watanabe S, Yamamoto T. Involvement of arachidonic acid cascade in working memory impairment induced by interleukin-1 beta. Neuropharmacology. 2004;46:1195–1200. doi: 10.1016/j.neuropharm.2004.02.012. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging. 2007;28:639–647. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- McKee AC, Carreras I, Hossain L, et al. Ibuprofen reduces Abeta, hyperphosphorylated tau and memory deficits in Alzheimer mice. Brain Res. 2008;1207:225–236. doi: 10.1016/j.brainres.2008.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AH, Olschowka JA, O’Banion MK. Intraparenchymal administration of interleukin-1beta induces cyclooxygenase-2-mediated expression of membrane- and cytosolic-associated prostaglandin E synthases in mouse brain. J Neuroimmunol. 2004a;148:32–40. doi: 10.1016/j.jneuroim.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Moore AH, Olschowka JA, Williams JP, Okunieff P, O’Banion MK. Regulation of prostaglandin E2 synthesis after brain irradiation. Int J Radiat Oncol Biol Phys. 2005;62:267–272. doi: 10.1016/j.ijrobp.2005.01.035. [DOI] [PubMed] [Google Scholar]
- Moore AH, Olschowka JA, Williams JP, Paige SL, O’Banion MK. Radiation-induced edema is dependent on cyclooxygenase-2 activity in mouse brain. Radiation Res. 2004b;161:153–159. doi: 10.1667/rr3116. [DOI] [PubMed] [Google Scholar]
- Moore AH, Wu M, Shaftel SS, Graham KA, O’Banion MK. Sustained expression of interleukin-1beta in mouse hippocampus impairs spatial memory. Neuroscience. 2009;164:1484–1495. doi: 10.1016/j.neuroscience.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Uchimura K, Zhu RL, Nagayama T, Rose ME, Stetler RA, Isakson PC, Chen J, Graham SH. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci U S A. 1998;95:10954–10959. doi: 10.1073/pnas.95.18.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Banion MK. Cyclooxygenase-2: molecular biology, pharmacology, and neurobiology. Crit Rev Neurobiol. 1999;13:45–82. doi: 10.1615/critrevneurobiol.v13.i1.30. [DOI] [PubMed] [Google Scholar]
- O’Banion MK. Prostaglandin E(2) synthases in neurologic homeostasis and disease. Prostaglandins Other Lipid Mediat. 2009 Apr 22; doi: 10.1016/j.prostaglandins.2009.04.008. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Banion MK, Dusel JC, Chang JW, Kaplan MD, Coleman PD. Interleukin-1β induces prostaglandin G/H synthase-2 (cyclooxygenase-2) in primary murine astrocyte cultures. J Neurochem. 1996;66:2532–2540. doi: 10.1046/j.1471-4159.1996.66062532.x. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, van Oers H, Schobitz B, de Kloet ER. Interleukin-1 beta, but not interleukin-6, impairs spatial navigation learning. Brain Res. 1993;613:160–163. doi: 10.1016/0006-8993(93)90468-3. [DOI] [PubMed] [Google Scholar]
- Pepicelli O, Fedele E, Berardi M, Raiteri M, Levi G, Greco A, Ajmone-Cat MA, Minghetti L. Cyclo-oxygenase-1 and -2 differently contribute to prostaglandin E2 synthesis and lipid peroxidation after in vivo activation of N-methyl-D-aspartate receptors in rat hippocampus. J Neurochem. 2005;93:1561–1567. doi: 10.1111/j.1471-4159.2005.03150.x. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Rajakariar R, Hilliard M, Lawrence T, Trivedi S, Colville-Nash P, Bellingan G, Fitzgerald D, Yaqoob MM, Gilroy DW. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proc Natl Acad Sci U S A. 2007;104:20979–20984. doi: 10.1073/pnas.0707394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick DK, Graham SH, Dixon CE, Marion DW. Role of cyclooxygenase 2 in acute spinal cord injury. J Neurotrauma. 1998;15:1005–1013. doi: 10.1089/neu.1998.15.1005. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Beschorner R, Meyermann R, Gozalan F, Schluesener HJ. Persistent accumulation of cyclooxygenase-1-expressing microglial cells and macrophages and transient upregulation by endothelium in human brain injury. J Neurosurg. 2002;96:892–899. doi: 10.3171/jns.2002.96.5.0892. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Nguyen TD, Postler E, Meyermann R, Schluesener HJ. Selective accumulation of cyclooxygenase-1-expressing microglial cells/macrophages in lesions of human focal cerebral ischemia. Acta Neuropathol (Berl) 2000;99:609–614. doi: 10.1007/s004010051170. [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O’Banion MK. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci. 2007a;27:9301–9309. doi: 10.1523/JNEUROSCI.1418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaftel SS, Kyrkanides S, Olschowka JA, Miller JH, Johnson RE, O’Banion MK. Sustained hippocampal Il-1β overexpression mediates chronic neuroinflammation and ameliorates Alzheimer’s plaque pathology. J Clin Invest. 2007b;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KN, Commins S, O’Mara SM. Cyclooxygenase inhibition attenuates endotoxin-induced spatial learning deficits, but not an endotoxin-induced blockade of long-term potentiation. Brain Res. 2005;1038:231–237. doi: 10.1016/j.brainres.2005.01.035. [DOI] [PubMed] [Google Scholar]
- Takemiya T, Matsumura K, Yamagata K. Roles of prostaglandin synthesis in excitotoxic brain diseases. Neurochem Internat. 2007;51:112–120. doi: 10.1016/j.neuint.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Takemiya T, Suzuki K, Sugiura H, Yasuda S, Yamagata K, Kawakami Y, Maru E. Inducible brain COX-2 facilitates the recurrence of hippocampal seizures in mouse rapid kindling. Prostaglandins Other Lipid Mediat. 2003;71:205–216. doi: 10.1016/s1098-8823(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Teeling JL, Cunningham C, Newman TA, Perry VH. The effect of non-steroidal anti-inflammatory agents on behavioural changes and cytokine production following systemic inflammation: implications for a role of COX-1. Brain Behav Immun. 2009 Dec 4; doi: 10.1016/j.bbi.2009.11.006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–1677. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yermakova AV, O’Banion MK. Downregulation of neuronal cyclooxygenase-2 expression in end stage Alzheimer’s disease. Neurobiol Aging. 2001;22:823–836. doi: 10.1016/s0197-4580(01)00303-7. [DOI] [PubMed] [Google Scholar]
- Yermakova AV, Rollins J, Callahan LM, Rogers J, O’Banion MK. Cyclooxygenase-1 in human Alzheimer’s and control brain: quantitative analysis of expression by microglia and CA3 hippocampal neurons. J Neuropathol Exp Neurol. 1999;58:1135–1146. doi: 10.1097/00005072-199911000-00003. [DOI] [PubMed] [Google Scholar]
- Zhang D, Hu X, Qian L, Wilson B, Lee C, Flood P, Langenbach R, Hong JS. Prostaglandin E2 released from activated microglia enhances astrocyte proliferation in vitro. Toxicol Appl Pharmacol. 2009;238:64–70. doi: 10.1016/j.taap.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]