Abstract
Somatosensory neurons detect environmental stimuli, converting external cues into neural activity that is relayed first to second-order neurons in the spinal cord. The detection of cold is proposed to be mediated by the ion channels TRPM8 and TRPA1. However, there is significant debate regarding the role of each channel in cold-evoked pain, complicating their potential as drug targets for conditions such as cold allodynia and hyperalgesia. To address this debate, we generated mice lacking functional copies of both channels and examined behaviors and neural activity in response to painful cold and noxious cooling compounds. Whereas normal mice display a robust preference for warmth over cold, both TRPM8-null (TRPM8−/−) and TRPM8/TRPA1 double knockout mice (DKO) display no preference until temperatures reach the extreme noxious range. Additionally, in contrast to wildtype mice that avoid touching cold surfaces, mice lacking TRPM8 channels display no such avoidance and explore noxious cold surfaces, even at 5°C. Furthermore, nocifensive behaviors to the cold mimetic icilin are absent in TRPM8−/− and DKO mice, but are retained in TRPA1-nulls (TRPA1−/−). Lastly, neural activity, measured by expression of the immediate early gene c-fos, evoked by hindpaw stimulation with noxious cold, menthol, or icilin is reduced in TRPM8−/− and DKO mice, but not in TRPA1−/− animals. Thus our results show that noxious cold signaling is exclusive to TRPM8, mediating neural and behavioral responses to cold and cold mimetics, and that TRPA1 is not required for acute cold pain in mammals.
Keywords: Cold, channel, somatosensory, nociception, temperature, spinal cord
1. Introduction
Changes in environmental temperature are detected by specialized somatosensory neurons whose somata reside in the dorsal root (DRG) or trigeminal (TG) ganglia and project afferent nerve fibers to peripheral tissues such as the skin [6]. When temperatures reach values that are considered noxious, 43°C for heat and 15°C for cold, nociceptors are activated and painful or aversive signals are transmitted to the central nervous system [6]. Cold-evoked pain is less robust than that produced by heat, but is a common complaint of patients suffering from complex regional pain syndrome (CRPS), diabetic neuropathy, peripheral or central nerve injury, and small fiber neuropathies [46; 11; 47; 49]. Whether the origin of such cold hypersensitivity is at the level of the peripheral nerve, or due to changes in higher processing of peripheral cold stimuli is not clear [49].
The ability of primary afferent neurons to respond to environmental temperatures is conferred by temperature-sensitive members of the transient receptor potential (TRP) family of ion channels. Two particular TRP channels, TRPM8 and TRPA1, have been linked to cold sensation [26]. TRPM8 was first identified through its sensitivity to cold and the cold-mimetics menthol and icilin in vitro [37; 42], and recent analyses of TRPM8-deficient (TRPM8−/−) mice suggest that it is the principle cold sensor in vivo [9; 15; 18]. While TRPM8 plays a significant role in injury-evoked hypersensitivity to cold following sciatic nerve injury or tissue inflammation [15], there is debate as to whether the channel mediates cold pain. Recombinant TRPA1 also responds to cold temperatures (<17°C) [48]. However, these findings have been difficult to reproduce in neurons, with the literature containing a relatively equal number of reports for or against TRPA1 conferring cold sensitivity in vitro [35; 12]. Furthermore, analyses of TRPA1-null (TRPA1−/−) mice has yielded conflicting data, with one group reporting no deficits in acute cold sensing, a second observing reduced cold sensitivity in female but not male mice, and a third showing cold deficits after prolonged exposure to cold [7; 30; 29]. Thus a vigorous debate remains on the roles of TRPM8 and TRPA1 in acute noxious cold sensing in vivo.
To firmly define the role of either channel in cold signaling, we have examined cellular, neural, and behavioral responses to noxious cold and pungent and irritating cooling compounds. In mice lacking both channels (DKO), we observed deficiencies in cold-evoked cell activity, preference behaviors and chemical nocifensive responses that were equivalent to TRPM8−/− mice. In addition, wildtype mice display robust noxious cold avoidance behaviors that are dependent on TRPM8 expression alone. Moreover, cold-evoked in vivo neural activity, measured by examining expression of the immediate-early gene c-fos (Fos) in spinal cord dorsal horn neurons [14], was dependent on TRPM8, but not TRPA1. These data show that TRPM8 is not solely a cool-sensor, but plays a key role in cold pain. Thus, future strategies to alleviate cold allodynia and hyperalgesia should target neurons and neural circuits that either express TRPM8 or are part of a larger TRPM8-mediated signaling pathway.
2. Methods
2.1. Animals
Adult (≥6 weeks old) wildtype, TRPM8−/− and TRPA1−/− mice on the C57/Bl6 background were used in all experiments [7; 9]. TRPM8−/−/TRPA1−/− double-knockout mice (DKO) were generated by crosses of TRPM8−/− and TRPA1−/− single-knockout mice. Breeding of the F2 generation produced mice normal in overall appearance and viability, and matings between heterozygous animals produced wild-type, heterozygous, and homozygous mutant male and female offspring with expected Mendelian ratios. Animals were genotyped by PCR screening using separate protocols for the TRPA1 and TRPM8 alleles. Tail DNA was amplified in a thermal cycler (Biorad), and amplified products were resolved by agarose gel electrophoresis.
TRPA1
Forward 5’-AGCTGCATGTGTGAATTAAATTCTTGACA-3’
Reverse 5’-ATCAACTACCAGTAAGTTCATGAGAACA-3’
Primers should yield a PCR product of 296 base pairs in wildtype animals (Supp. Fig. 1; lane 2), and a PCR product of 213 base pairs in TRPA1-deficient animals (Supp. Fig. 1, lane 3).
TRPM8
Forward 5’-CCTTGGCTGCTGGATTCACACAGC-3’
Reverse 5’CAGGCTGAGCGATGAAATGCTGATCTG-3’
Primers should yield a PCR product of 1000 basepairs in wildtype animals (Supp. Fig. 1; lane 5), and a PCR product of 500 basepairs in TRPM8-deficient animals (Supp. Fig. 1, lane 6).
The PCR products from a DKO mouse are displayed in Supplementary Figure 1 (lane 4 and 7).
All experiments were performed in accordance with the University of Southern California and the University of California, Berkeley Departments of Animal Resources and Institutional Animal Care and Use Committee guidelines and the ethical standards and guidelines of the International Association for the Study of Pain [54].
2.2. Peripheral stimulation
For cold stimulations, mice were deeply anesthetized with 50 mg/kg pentobarbital i.p. and one hindpaw dipped into an ice-water bath (~0°C) for 30 seconds of every two minutes, repeated 15 times over 30 minutes total. For all pharmacological experiments, mice were briefly anesthetized with isoflurane and given a 10µl intraplantar injection of one of the following solutions into one hindpaw using a 27 gauge needle: 2.4mg/ml icilin, 800µg/ml menthol, 500µg/ml capsaicin, 5% mustard oil, or vehicle (80% DMSO/20% PBS, pH 7.4).
2.3. Tissue preparation
Two hours after the initial stimulus, anesthetized mice were transcardially perfused with 30mL PBS (pH 7.4) followed by 30mL ice-cold 4% paraformaldehyde in PBS (PFA). Spinal cords were removed and L4–L6 tissue was post-fixed in 4% PFA for one hour at 4°C, cryoprotected in 30% sucrose in PBS overnight at 4°C, and then frozen in OCT medium (Sakura). Twenty micron thick sections were cut, mounted onto slides, and stored at −80°C.
2.4. Immunohistochemistry
Samples were thawed, washed with PBS, incubated in 1% H2O2 for 30 minutes, washed, and blocked in 10% normal goat serum (NGS) in 0.3% Triton X-100 in PBS (Tri-X) for one hour. Samples were then incubated with 1:1250 rabbit-anti-Fos antibody (K-25, Santa Cruz) in 1% NGS in Tri-X in a humidified chamber at room temperature overnight. Samples were processed with the Vector Labs Elite ABC kit (rabbit) according to the manufacturer’s instructions. Samples were incubated in 1:20 diaminobenzadine (DAB; Invitrogen) in PBS for 15 minutes, in 1:20 DAB in 0.003% H2O2 in PBS for five minutes, then in 1:20 DAB in 0.015% H2O2 in PBS for five minutes. Finally, slides were washed, cover-slipped, and analyzed.
Brightfield images were captured on an Olympus IX70 fluorescent microscope with Sutter Lambda LS light source, Roper CoolSnap ES camera, and the MetaImaging Software suite. Nuclei counts were obtained by using the ImageJ software (National Institutes of Health) with the Automatic Nuclei Counter plug-in (available at http://www.bioimage.ucsb.edu/downloads/automatic-nuclei-counter-plug-in-for-imagej), threshold values set between 7.0–10.0 and cell diameter of 7–9 pixels, counted by outlining the region of interest (dorsal horn) within the image. In addition, all computer generated date was verified manually on randomly picked samples. Differences between regions, stimuli, and genotypes were tested for significance using a two-sample independent t-test or one-way ANOVA followed by Tukey’s HSD post-hoc analysis, as appropriate. All data are listed means ± standard error of the mean (s.e.m.).
2.5. Behavioral analyses
Behaviors of wildtype, TRPM8−/−, TRPA1−/− and DKO mice after unilateral hind paw injections of 2.4mg/ml icilin (10 µl) were video-recorded over the first twenty minutes following injection. The number of paw licking, shaking, lifting, and other flinching behaviors were quantified from the videos, as was the cumulative time of the twenty minute period the mice spent attending to the injected paw. For the two-plate temperature choice test, wildtype, TRPM8−/− and DKO mice were placed in a chamber containing two identical, adjacent floor platforms with one set to 30°C and the other set to one of the following different temperatures: 30, 27, 25, 20, 15, 10 or 5°C. Mice were free to explore for five minutes and each test was videotaped for later analysis of the total time spent on each surface (surface preference) and the number of times an animal moved to an adjacent platform (surface avoidance) over the test period. Between trials, animals were rehabituated to the chamber with each surface set to 30°C, and the control surface was alternated between trials. All data sets were analyzed using two- or one-way ANOVA analysis followed by Tukey’s HSD post-hoc analysis.
2.6. Live-cell calcium imaging
Trigeminal ganglia were dissected from newborn mice of the various genotypes and dissociated with 0.25% collagenase P (Roche Applied Science, Indianapolis, IN) in a solution of 50% DMEM (Dulbecco’s Modification of Eagle’s Medium with 4.5g/L glucose, L-glutamine and sodium pyruvate, Mediatech, Inc., Manassas, VA), and 50% F-12 (HAM F-12 Nutrient Mixture with L-glutamine, Invitrogen Corporation, Carlsbad, CA) for 30 minutes. The ganglia were then pelleted and resuspended in 0.05% trypsin at 37°C for two minutes, and triturated gently with a fire-polished Pasteur pipette in culture medium (DMEM/F-12 with 10% FBS and penicillin-streptomycin). Cells were then resuspended in culture medium with 100ng/ml nerve growth factor 7S (Invitrogen Corporation, Carlsbad, CA) and plated onto coverslips coated with 20ul/ml Matrigel (BD Biosciences, Inc., San Jose, CA). Cultures were examined 16–20 hours after plating. Intracellular Ca2+ was determined with the cell-permeable form of Fura-2 (Invitrogen, Carlsbad, CA) as described [37] and pseudo-colored ratiometric images were captured on an Olympus IX70 fluorescent microscope with Sutter Lambda LS light source, Roper CoolSnap ES camera, and the MetaImaging Software suite. Data presented are means ± s.e.m.
3. Results
3.1. TRPM8−/− and TRPM8−/−/TRPA1−/− double knockout mice display similar deficits in cellular cold responses
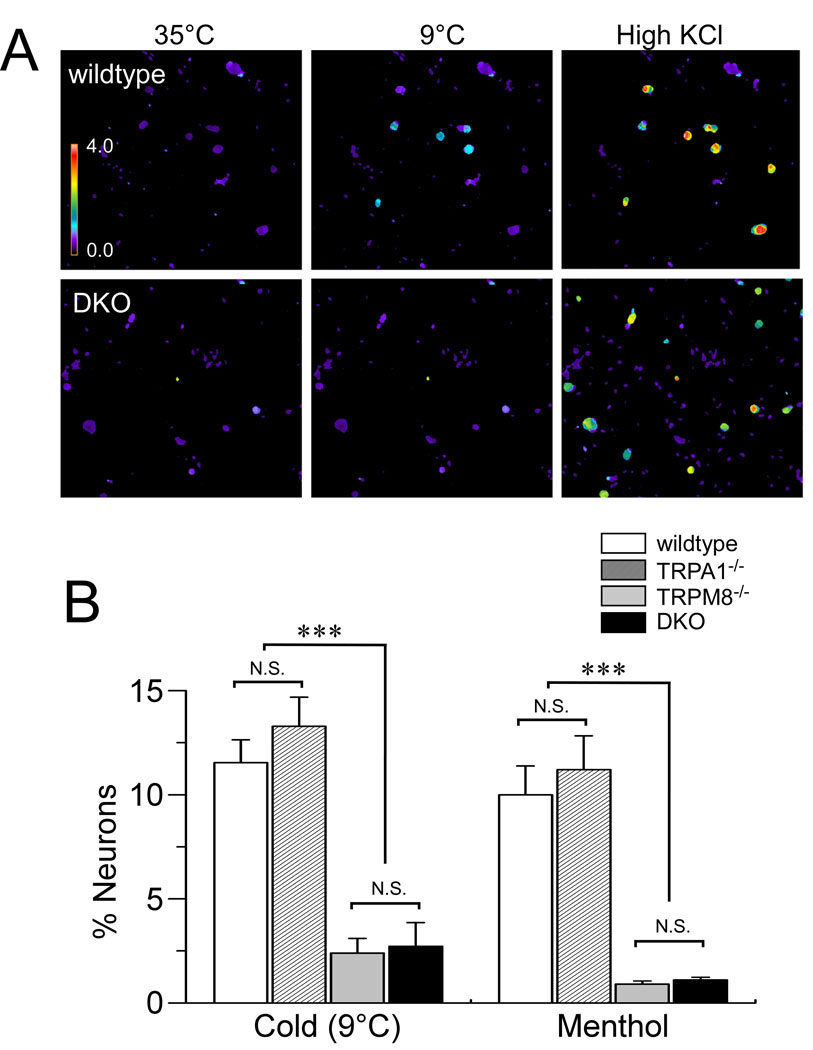
Both TRPM8 and TRPA1 have been linked to cold perception, yet the role of TRPA1 in cold sensing in vitro and in vivo remains controversial [16]. Some studies have shown deficits cellular cold responses in TRPA1-null mice [30; 29] whereas others have not [7; 31]. This may be due to presence of functional TRPM8 channels that compensate for the loss of TRPA1. In contrast, somatic neurons isolated from TRPM8-deficient mice display dramatic losses in cold- and menthol-sensitivity [7; 30; 34]. However, a small population of menthol-insensitive, cold sensitive neurons persist [7], and these responses have been attributed to TRPA1. To clearly establish a role for TRPA1 and TRPM8 in cellular cold sensitivity, we generated TRPM8−/−/TRPA1−/− DKO mice (Supplementary Figure 1) and examined the contribution of these channels to noxious cold signaling in vitro. When challenged with a cooling gradient from 35°C to 9°C, about 12% of trigeminal neurons from wildtype mice showed significant increases in intracellular calcium levels (Fig. 1). The majority of these cold-sensitive neurons were menthol-sensitive (86%). Neurons from TRPM8−/− and DKO mice showed a dramatic reduction in the total number of cold-sensitive neurons, with only ~3% of cells showing responses to cold (Fig. 1B) [9], and the residual cold-evoked responses in single and DKO animals were smaller in amplitude than those observed in wildtype neurons (not shown). Moreover, there were no differences in cellular cold sensitivity in neurons isolated from TRPA1−/− animals in comparison to wildtype mice, as has been shown previously (Fig. 1B) [7; 20; 34]. The reduction in the number and magnitude of cellular cold responses in TRPM8−/− animals supports a key role for TRPM8 in cold-evoked responses in vitro. Furthermore, as there were no additional deficits in cold responses in neurons obtained from DKO animals, these data suggest that TRPA1 does not mediate cold responses, even in the absence of TRPM8 expression in vitro.
Fig. 1.
Cellular cold sensitivity is similar in TRPM8−/−, TRPA1−/−, and DKO mice. (A) Trigeminal neurons from wildtype (top) and DKO (bottom) mice were challenged with cold (9°C), followed by high KCl (75 mM) to depolarize and excite all neurons. Responses were assessed by calcium imaging. The pseudocolor scale shows the F340/F380 Fura-2 ratio (from 0–4) which represents the relative calcium concentration in the cell. (B) Prevalence of neurons responding to cold or menthol (250µM) was determined for trigeminal cultures from wildtype (white), TRPA1−/− (dashed-lines), TRPM8−/− (gray) and DKO (black) mice. TRPM8−/− and DKO neurons showed a significant attenuation of cold- and menthol-evoked responses, whereas there were no differences (p>0.05) between responses in neurons from wildtype and TRPA1−/− mice (n≥400 neurons/genotype; ***p < 0.001.). Data are means ± s.e.m.
3.2. TRPM8−/− and DKO mice display similar deficits in the two-temperature preference assay
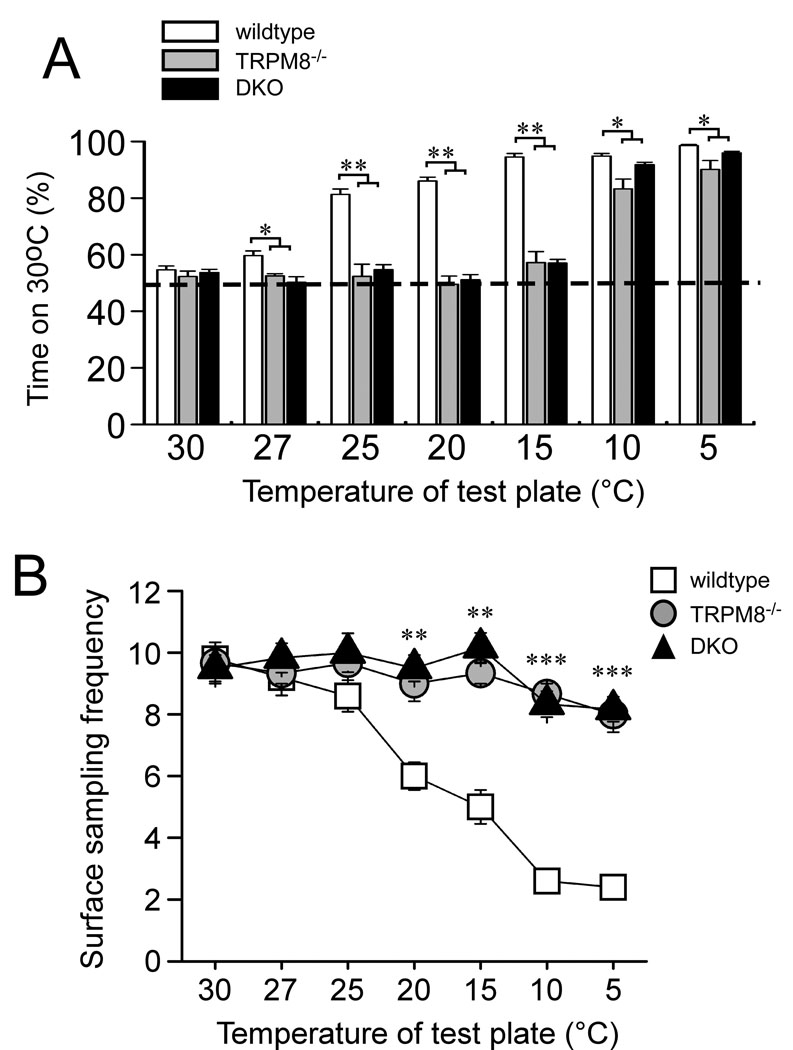
TRPM8−/− mice show a remarkable inability to discriminate between warm (30°C) and cold temperatures, down to 15°C, when assayed on the two-temperature preference test (Fig. 2A) [9]. However, when given the choice of 30°C versus more noxious cold temperatures, 10 or 5°C, TRPM8−/− mice do spend more time on the warmer side. Unlike TRPM8−/− mice, TRPA1−/− mice were previously shown to exhibit no significant differences in temperature preference at any temperature, as compared to wildtype mice [9]. It is possible that in TRPA1-deficient animals, TRPM8 expression may compensate for the lack of TRPA1, thus masking the normal contribution of the latter in cold sensing. In addition, TRPA1 has been proposed to mediate the preference for warmth over noxious cold temperatures observed in TRPM8-deficient mice. To address these questions, we tested the ability of DKO mice to detect cold using a temperature preference test.
Fig. 2.
Cold preference and avoidance behaviors are dependent on TRPM8 and not TRPA1. (A) Wildtype, TRPM8−/− and DKO mice were allowed to choose between adjacent surfaces adjusted to 30 °C versus a range of temperatures, as shown. The percentage of time spent on the 30°C plate over a 5 min period is shown and data are from 10 wildtype, 7 TRPM8−/−, and 6 DKO animals. (B) TRPM8−/− and DKO mice do not avoid contact with noxious cold temperatures. The number of times each animal made a full transition (crossing) between adjacent surfaces was counted over a 5 minute period. (n=5 for wildtype, 3 for TRPM8−/− and 7 for DKO). *p<0.05, **p<0.01, ***p<0.001. Data are means ± s.e.m.
Animals were allowed to freely explore adjacent surfaces, one held constantly at 30°C with the other variable surface set to temperatures ranging from 30 to 5°C, and the amount of time spent on the 30°C surface was measured over a five minute period (Fig. 2A). When placed on equivalent temperatures (30°C−30°C) wildtype and all mutant mice displayed no preference, spending statistically equal amounts of time on each side (percent time on the control 30°C plate 54.7±1.3% wildtype, n=10; 52.3±1.9% TRPM8−/−, n=7; 53.7±1.2% DKO, n=6; p>0.05). As the variable-temperature test plate was cooled, wildtype mice showed a clear preference for the 30°C side (98.6±0.4% by 5°C), as previously reported [9]. In contrast, DKO mice displayed similar behaviors to those observed in TRPM8-knockout mice, displaying no preference between 30°C and 15°C (57.1±1.3% time on 30°C; p>0.05). However, like TRPM8−/− mice, the DKO animals began to spend more time on the warm surface as temperatures approached 10°C. Interestingly, even though both TRPM8−/− and DKO mice do show a strong preference for the warm surface at 10 and 5°C, the time spent on the 30°C surface was slightly, but significantly, reduced compared to wildtype animals (Fig. 2A; p<0.05). For example, when preferences were examined at 10°C, wildtype mice spent 94.3±0.7% of the time on the 30°C plate while TRPM8−/− and DKO mice spent 83.3±3.5% and 91.8±0.8% of the time on the 30°C plate, respectively (p<0.05 compared to wildtype). These data suggest that while these mice do prefer warmer surfaces, this preference is still somewhat diminished compared to control mice, indicating that TRPM8 is involved in the detection of noxious cold (≤15°C) whereas TRPA1 appears to not be involved since the DKO mutants exhibit similar behavior. A lack of an additional deficit in the DKO mutants is consistent with previous analyses of TRPA1−/− mice in the two-temperature preference test in which no differences were observed between the mutants and wildtype animals [9].
3.3. TRPM8−/− mice are deficient in the detection of noxious cold
A caveat for the preference assay is that it does not distinguish between behaviors indicative of preference for warmth versus avoidance of cold. Thus, in order to directly measure cold avoidance, we also examined temperature sampling behaviors by quantifying the number of times animals crossed from one plate to another, finding that wildtype mice significantly reduced the number of crossings as temperature of the test plate decreased (Fig. 2B). When both plates were set to 30°C, wildtype mice had an average of 9.8±0.4 crossings during the five minute test period, whereas when the test plate was lowered to 5°C, this number was significantly reduced to 2.4±0.4 (p<0.001). Thus wildtype mice clearly avoid contact with noxious cold temperatures. However, even at an extreme cold temperature of 5°C, the average number of crossings made by TRPM8−/− and DKO mice were significantly different from wildtypes (8.0±0.6 for TRPM8−/− and 8.2±0.4 for DKO; p<0.01). Moreover, in both mutant genotypes, the number of crossings made when the variable plate was set to 5°C was not different from when it was at 30°C (p>0.05). These data clearly show that mice lacking TRPM8 are unable to perceive noxious cold temperatures as aversive. Furthermore, abolishing expression of both channels does not lead to additional deficiencies in cold-sensing beyond those observed in TRPM8-null animals.
3.4. Icilin-evoked nocifensive behaviors are lost in TRPM8−/− mice but retained in TRPA1−/− mice
Next we asked if chemical cooling agents can evoke a nocifensive behavior and if these were dependent on either TRPM8 or TRPA1. Intraperitoneal injection of the cold mimetic icilin induces robust shivering and shaking behaviors in rodents, referred to as “wet dog shakes” [52], and previous studies have shown that these behaviors are TRPM8-dependent [15; 18]. However, these responses relate to whole-animal effects reflective of changes in homeostatic thermal regulation likely related to controlling core body temperature [19], which cannot be classified as clearly nocifensive. Moreover, nocifensive-like behaviors to icilin have yet to be reported. Lastly, recombinant TRPA1 channels are also activated by icilin [48], in addition to a plethora of other reactive compounds [8; 33; 22; 32], but the dependence of icilin-evoked behaviors on TRPA1 has yet to be reported. Thus we used intraplantar injections of icilin to assess evoked nocifensive responses and their dependence on TRPM8 or TRPA1. Under this stimulation paradigm, whole animal behaviors were rarely observed and responses were largely limited to the injected hindpaw. Wildtype, TRPM8−/−, TRPA1−/−, and DKO mice were observed for twenty minutes following unilateral hindpaw injections of icilin. Wildtype (n=6) and TRPA1−/− (n=6) mice showed a high number of paw flinches following icilin injection (mean 40.8±8.1 and 40.8±5.8 behaviors, respectively), while TRPM8−/− (n=5) and DKO (n=8) mice exhibited a significantly reduced number of behaviors (5.6±1.9 and 9.6±1.9, respectively; p<0.01 compared to wildtype; Fig. 3A). Furthermore, the cumulative time over the twenty-minute observation period that the animals spent attending to their stimulated hindpaws followed the same pattern, with TRPM8−/− and DKO mice spending significantly less time on average (27.2±11.7 seconds and 38.0±9.5 seconds, respectively) than either wildtype (169.5±25.0 seconds) or TRPA1−/− mice (212.5±45.3 seconds; p<0.01; Fig. 3B). As in previous assays, we observed no additional deficits in DKO animals in comparison to TRPM8−/− mice (Fig. 3A & B; p>0.05). These data show that intraplantar injection of icilin can evoke a nocifensive response that resembles pain behaviors associated with noxious temperatures and that, consistent with icilin-evoked whole-animal behaviors [18], these reponses are dependent upon TRPM8 and not TRPA1.
Fig. 3.
Icilin-induced flinching behaviors are dependent on TRPM8 but not TRPA1. (A) Flinching behaviors recorded over a 20 min period after injection of icilin into the hindpaw were counted in wildtype (n=6), TRPM8−/− (n=5), TRPA1−/− (n=6), and DKO (n=8) mice. (B) The cumulative time animals spent attending to their stimulated paw was quantified. TRPM8−/− and DKO mice spent significantly less of the observation period than wildtype or TRPA1−/− mice. **p<0.01. Data are means ± s.e.m.
3.5. Peripheral stimulation with cold and cold-mimetics induces Fos expression in the spinal cord dorsal horn
Our cellular recordings and behavioral data clearly show that responses to cold and cold-mimetics such as icilin are dependent upon TRPM8. However, the complexity of animal behaviors combined with the indirectness of cellular recordings highlights the need for alternative assays that can measure changes in neural activity in vivo that result from peripheral stimulation. Therefore, to address the role of TRPM8 and TRPA1 in cold and cold-mimetic responses, we examined expression of the immediate early gene c-fos (Fos) in the mouse spinal cord dorsal horn following unilateral hindpaw stimulation [23]. Fos expression can be induced by a wide array of stimuli and is a reliable marker of elevated activity in spinal cord neurons following peripheral stimulation [14].
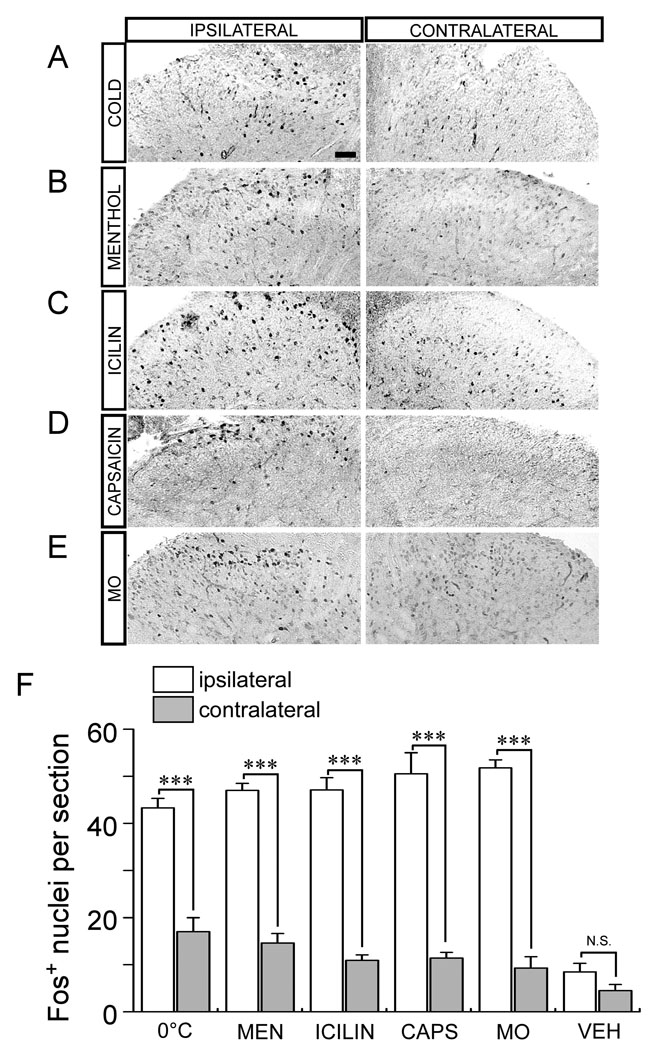
We first examined spinal Fos expression in anesthetized wildtype mice after noxious cold temperature stimulation of the hindpaw. Repeated unilateral exposure to 0°C over a 30 minute period induced robust Fos expression in the dorsal spinal cord ipsilateral to the site of stimulation (Fig. 4A & F), similar to the extreme-cold-induced expression previously observed in rats [1]. An average of 43.3±2.0 Fos-positive nuclei were observed per lumbar section on the ipsilateral side, while significantly less labeled nuclei (17.0±3.0 nuclei labeled per section) were found contralaterally (Fig. 4F; n=4; p<0.001). Fos-positive cells were localized primarily to lamina I and II of the dorsal horn (Supplementary Fig. 2), the central termination zone of thermosensitive and nociceptive sensory afferents [27]. Furthermore, the induction of Fos was exclusively neuronal as all positive nuclei co-labeled with immunoreactivity for the neuronal nuclear marker NeuN (data not shown). Thus peripheral cold stimulation at noxious, near-freezing temperatures robustly increases neuronal activity-related gene expression in second-order neurons within the cold neural circuitry of the spinal cord.
Fig. 4.
Cold, menthol and icilin induce Fos expression in the spinal cord dorsal horn. Unilateral stimulation of the mouse hindpaw with 0°C (A), intraplantar injection of menthol (B; MEN), icilin (C), capsaicin (D; CAPS) or mustard oil (E; MO) lead to increased levels of Fos protein in cell nuclei in the L4–L6 region of the spinal cord dorsal horn ipsilateral to the site of stimulation. Details of the specific stimulation conditions are listed in Methods. (F) All stimuli led to significantly more Fos+ nuclei in the ipsilateral dorsal horn compared to that contralateral. Vehicle injections induced a small but statistically insignificant amount of Fos in the same region compared to other regions. Scale bar = 50µm; ***p<0.001. Data are means ± s.e.m.
Next we compared the levels of cold-evoked Fos expression to those evoked by the cold-mimetics menthol and icilin. Intraplantar injection of menthol or icilin produced significant numbers of Fos-positive nuclei in the ipsilateral dorsal horn, an average of 47.0±1.5 ipsilateral cells and 14.6±2.0 contralateral cells (n=7; p<0.001) for menthol stimulation and 47.1±2.6 ipsilateral cells and 10.9±1.2 contralateral cells (n=12; p<0.001) per section for icilin (Fig. 4B, C & F). There were no significant differences in the laminar distribution of Fos-labeled nuclei across the different stimuli (Supplementary Fig. 2). Furthermore, the mean levels of Fos induced in the ipsilateral spinal cord by cold, menthol, or icilin were equivalent across each stimulus, suggesting that the extent of activation of these putative cold neural circuits was equal and saturated (Fig. 4F, p>0.05).
As control injections, we used the TRPV1 and TRPA1 agonists capsaicin and mustard oil, respectively, as well as vehicle alone, finding that the mean number of labeled nuclei was 50.5±4.5 ipsilateral and 11.4±1.2 contralateral for capsaicin (n=8), 51.8±1.7 ipsilateral and 9.3±2.4 contralateral for mustard oil (n=4), and 8.5±1.3 ipsilateral and 4.5±1.3 contralateral for vehicle (n=4) (Fig. 4D–F). Remarkably, a comparison of the levels of Fos induced by icilin, capsaicin, or mustard oil revealed no significant differences (p>0.05) between the groups (even in laminar distribution, Supplementary Fig. 2 & 3), indicating that each stimulus activated an equivalent number of neurons in the spinal cord (Fig. 4F). These data show that peripheral stimulation with agonists for TRPM8, TRPV1, or TRPA1 leads to robust and comparable Fos expression in the dorsal horn.
3.6. Cold- and cold-mimetic induced Fos expression is dependent on TRPM8
Next we set out to establish the genetic basis for the induction of Fos expression by cold and cold-mimetics by first repeating our cold stimulation paradigm in TRPM8−/− mice [9]. Unilateral cold stimulation of the hindpaw of TRPM8−/− mice resulted in an average of 18.3±0.3 cells ipsilateral and 8.0±1.0 cells contralateral to the stimulation site (n=3; p<0.001; Fig. 5A & D). Although the difference between ipsilateral and contralateral levels was significant, the levels of ipsilateral Fos were clearly reduced from wildtypes (p<0.001; Fig. 5D). That some cold-evoked neural activity is retained in TRPM8−/− mice is consistent with previous cellular studies and our current analyses (see Fig. 1) [9; 15; 18].
Fig. 5.
Cold- and cold-mimetic induced Fos expression is dependent on TRPM8. Stimulation with cold (A), or intraplantar injection of menthol (B) or icilin (C) failed to induce robust Fos expression only in mice lacking functional TRPM8 channels. (D) Fos levels ipsilateral to the stimulation site were significantly reduced in TRPM8−/− (n=3–5) and DKO (n=3–4) mice compared to TRPA1−/− (n=4–6) and wildtype (n=4–12) animals. Scale bar = 50µm; **p<0.01, N.S. = p>0.05. Data are means ± s.e.m.
We then tested menthol and icilin stimulation in TRPM8−/− mice, finding that menthol induced Fos expression in 14.3±1.2 ipsilateral nuclei on average (n=3, p<0.001 vs. wildtypes; Fig. 5B & D), while icilin injections induced expression in an average of 9.8±2.1 ipsilateral nuclei (n=5, p<0.001 vs. wildtypes; Fig. 5C & D). No significant differences were found between ipsilateral and contralateral levels in either the menthol or icilin stimulation conditions (p>0.05). Moreover, there were no statistical differences (p>0.05) in the levels of Fos expression in menthol- or icilin-injected TRPM8−/− mice compared to vehicle-injected controls (vehicle n=4; p>0.05; data not shown). We also examined Fos expression in mice heterozygous for TRPM8 (TRPM8+/−), finding that intraplantar injection of either menthol- or icilin-evoked Fos expression in the spinal cord dorsal horn at levels intermediate to both wildtype and TRPM8−/− mice (Supplementary Fig. 4). Lastly, to ensure that TRPM8−/− and TRPM8+/− mice are capable of mounting a wildtype-like Fos response upon peripheral stimulation, we performed intraplantar injections of capsaicin, finding no differences in Fos expression compared to wildtypes (p>0.05; Fig. 6). Taken together, these data indicate that activity-induced changes in gene expression in dorsal horn neurons resulting from peripheral cold and cold-mimetic stimuli are dependent upon TRPM8.
Fig. 6.
TRPM8-null mice are still capable of Fos-induction upon peripheral stimulation. TRPM8+/− (A) and TRPM8−/− (B) mice were given unilateral hindpaw injections of 10µl of 1.6mM capsaicin, showing robust Fos activation in the ipsilateral dorsal horn. (C) Both genotypes showed statistically more ipsilateral Fos-labeling than contralateral, identical to that found in capsaicin-injected wildtype mice (wildtype data taken from Fig. 4 and S2 for comparison purposes). Scale bar = 50µm; ***p<0.001. Data are means ± s.e.m.
3.7. TRPA1 is not involved in cold- or icilin-induced spinal Fos expression
In addition to robust activation of TRPM8 [37], cold, menthol, and icilin have been reported to also activate TRPA1 channels in heterologous expression systems under specific conditions [48; 28]. As TRPA1 is proposed to be involved in cold sensing in vivo [48; 30; 29], we asked if the small residual Fos expression we observed in cold-stimulated TRPM8−/− mice was due to activation of TRPA1, and if menthol or icilin-induced Fos expression is reduced in TRPA1-null mice [7]. We repeated our cold stimulus paradigm and the intraplantar menthol and icilin injections in TRPA1−/− mice, finding the average number of Fos-positive nuclei observed in animals stimulated with cold, menthol, or icilin was 43.3±1.3 ipsilateral and 15.5±3.5 contralateral (n=4), 40.3±1.0 ipsilateral and 19.0±4.1 contralateral (n=4), or 45.3±2.6 ipsilateral and 16.7±1.5 contralateral (n=3), respectively (all p<0.001 ipsi vs contra, Fig. 5A–D). The number of Fos-positive nuclei induced by cold, menthol, or icilin was indistinguishable from that observed in wildtype animals (p>0.05). In contrast, mustard oil-evoked Fos expression was significantly reduced in TRPA1−/− mice versus wildtype controls (average 16.0±0.6 ipsilateral nuclei, n=3, p<0.01; Supplementary Fig. 5).
The lack of deficits in cold-evoked Fos expression and cold behaviors observed in TRPA1−/− mice may result from compensation and activity of functional TRPM8 channels [7]. Thus we examined cold-evoked Fos expression in DKO mice. Stimulation with cold, menthol, or icilin resulted in mean levels of Fos-positive nuclei as follows: 21.5±1.0 ipsilateral nuclei and 13.0±2.1 contralateral nuclei for cold stimulation (n=4), 13.5±1.9 ipsilateral nuclei and 13.5±0.6 contralateral nuclei for menthol stimulation (n=4), and 15.2±1.4 ipsilateral nuclei and 12.3±1.8 contralateral nuclei for icilin stimulation (n=6) (Fig. 5A–D). In only the cold stimulation group did we find a significantly higher number of labeled nuclei (p<0.05; not shown) on the ipsilateral side compared to the contralateral side, as was observed before in cold-stimulated TRPM8−/− mice. Moreover, no additional deficits were observed in the DKO animals beyond those observed in TRPM8−/− mice. Thus these data show that, at the level of gene expression induced by neural activity, TRPM8 is required for acute sensing of cold and cold mimetics and further supports the findings that TRPA1 serves no role in acute cold detection.
4. Discussion
Despite extensive study there remains vigorous debate regarding the roles of TRPM8 and TRPA1 in cold sensing in vivo [12]. While it is clear that TRPM8 has a fundamental role in the detection of cold temperatures, a point of conjecture has been whether or not the channel is involved in the detection of innocuous cool, noxious cold, or both sensations. In vitro, cold-evoked TRPM8 currents occur at a threshold of ~26°C, with activity increasing in magnitude down to 8°C [37]. This high temperature activation threshold was thought to preclude the channel from involvement in cold nociception, despite the fact that channel activity covers both innocuous cool (~30−15°C) and noxious cold temperatures (<15°C). However, care must be taken when correlating psychophysical and behavioral responses to surface skin cooling to in vitro channel properties as there is little correlation between skin surface temperature and that at the subcutaneous location of peripheral nerve terminals where these channels reside. For example, Morin and Bushnell correlated surface temperatures to those recorded at a subcutaneous site 1 mm below the skin surface, near the epidermal-dermal boundary [38]. When a subfreezing temperature of −5°C was applied to the skin for 30 seconds, subcutaneous temperatures were reduced to approximately 17°C [38]. Thus when skin is exposed to temperatures well below the noxious threshold, subcutaneous temperatures are within the activation range of TRPM8, suggesting that the in vitro thermosensitivity range of the channel is more akin to a noxious cold sensor than one simply detecting innocuous cool.
Cellular assays reveal that approximately 10–20% of cultured sensory neurons respond with an increase in intracellular calcium when provoked by a cold stimulus [37; 50]. Of these, <20% are insensitive to menthol and appear to be present in TRPM8-null animals, suggesting a TRPM8-independent transduction mechanism [7; 9; 15; 18]. Our results show that these residual cellular responses are TRPA1-independent as there are no differences in neurons obtained from DKO mice compared to TRPM8−/− animals. The identity of the mechanism(s) responsible for this residual cold response in vitro is yet to be determined, but a few possibilities have been proposed [10]. For example, both potassium and sodium conductances have been shown to be involved in cold signaling [44; 50; 53; 2; 34; 40], although it is unlikely that these mediate cold detection and likely are essential for supporting and maintaining electrical impulses generated at cold temperatures.
Recent analyses of TRPM8-deficient rodents’ show that the channel mediates a variety of cold responses, including cold detection, injury evoked hypersensitivity to cold and, paradoxically, cooling-mediated analgesia [43; 9; 15; 16; 18]. However, these studies highlight the difficulty in measuring nocifensive responses to cold temperatures [3]. For example in two-temperature preference assays, TRPM8−/− mice prefer warmer temperatures to those ≤10°C [9; 18], again suggesting the existence of TRPM8-independent mechanisms at these temperatures [13]. Nonetheless, there are small but statistically significant differences in these behaviors when compared to wildtype mice, suggesting that TRPM8−/− mice are incapable of normal cold-evoked behaviors. What remains to be determined is what underlies the residual preference for warmth in TRPM8−/− mice at temperatures below 15°C. One hypothesis is that these preference behaviors result from a counter-balance of inputs from both cold and heat sensing afferent fibers. Here we present novel data in support of this hypothesis, clearly showing that when the assay is evaluated for cold avoidance instead of warm preference, TRPM8−/− mice are unable to appropriately detect temperatures down to 5°C. Indeed, there are no differences in the rate at which TRPM8−/− mice sample temperatures at 30 versus 5°C, suggesting these mice do not perceive these temperatures as noxious, and therefore still sample the cold surfaces without hesitation or aversion. However, in an extreme cold environment, a lack of input from heat sensors may promote innate warmth-seeking behaviors that drive the animals towards the warm surface and away from the cold. Indeed, such behaviors are commonly observed in invertebrates [36] and future analyses using operant behavioral paradigms may help tease apart cold-avoidant versus warm-seeking behaviors. Lastly, while it is not clear if these behaviors are indicative of aversion due to the sensation of cold pain or if they reflect an inability to avoid a non-preferred temperature, these results demonstrate the importance of TRPM8 in the proper detection of noxious cold temperatures.
TRPA1 was first reported to be activated by cold and icilin when expressed in heterologous systems [48]. However, several groups report no correlation between cold sensitivity and responsiveness to TRPA1 agonists in somatosensory neurons [4; 25; 39; 20], and several studies disagree as to whether recombinant TRPA1 channels are intrinsically cold sensitive [5; 39; 55; 29]. These conflicting data have muddled the role of TRPA1 in thermosensation and it was hoped that TRPA1-null mice would resolve these issues [35], but, as has often been the case for this enigmatic channel, the results were less than clear. Two independent knockout lines were characterized at the cellular, nerve fiber, and behavioral levels with only one found to have deficiencies in cold-evoked behaviors [7; 30]. Curiously, these deficiencies only reached statistical significance in female but not male mice [30], which is complicated by the fact that the estrus cycle, which can profoundly influence behaviors, was not reported [21; 41]. In contrast, a second study reported no deficits in TRPA1−/− mice in a variety of cold assays, including evaporative cooling, hindpaw lifts, shivering [7], and thermal preference [9], regardless of gender or estrus cycle stage. Similarly, recordings using the ex-vivo skin nerve preparation found no differences in cold sensitive fibers isolated from TRPA1−/− mice or wildtype littermates [31], whereas almost all cold-evoked responses were lost in TRPM8−/− mice using identical recordings [9]. Indeed, cold-evoked responses at all temperatures tested (30−0°C) were significantly reduced in TRPM8-nulls [9], further suggesting the channel mediates a broad range of cold responses [16].
The phenotype of TRPA1−/− mice was recently reassessed in a report showing deficiencies in escape behaviors in these animals when placed on a 0°C plate for a prolonged period of over two minutes [29]. Traditional measures of paw licking, guarding and whole-animal shivering were normal in TRPA1−/− mice and it is unclear whether a two minute exposure to 0°C induces tissue damage, as exposure times of animals to these extremes are typically limited to 5–60 seconds [53]. Since TRPA1 is a key mediator of inflammatory hypersensitivity, acting as a gate keeper to thermal and mechanical hypersensitivity, these altered escape behaviors could result from cold-evoked tissue damage, which was not measured. It would be interesting to probe escape behaviors of these mice exposed to other thermal and mechanical extremes to determine if this is a general phenomenon, or if it is specific to cold.
The confusing behavioral results from TRPA1−/− mice, along with the imprecision inherent in mouse behavioral testing, led us to conclude that an alternative method of testing in vivo cold sensation and response to cold mimetics was needed. Activation of the immediate-early gene c-fos upon peripheral stimulation was an appealing candidate as it is widely used as a measure of neural activity within the CNS [23; 14]. Surprisingly, neither menthol nor icilin has been applied to peripheral tissues such as the hindpaw to examine spinal Fos induction. When infused in the bladder or the nasal mucosa, menthol-evoked Fos expression has been reported [24; 51], yet only one study observed Fos in spinal neurons when the hindpaw was stimulated with extreme cold (<−10°C) for a period of three minutes [1]. We find that repeated and brief hindpaw exposures to 0°C can invoke robust Fos expression. It is likely that in the previous assay cold fibers adapted to this prolonged stimulus, thereby reducing overall neural activity [45; 17], whereas the current protocol allows continuous neuronal activity for 30 minutes. Remarkably, stimulation with both thermal and chemical stimuli led to equivalent numbers of Fos-labeled neurons in the spinal cord and at levels similar to that induced by the nociceptive agents’ capsaicin and mustard oil. These results are surprising given that capsaicin and mustard oil activate a larger percentage of afferent neurons and demonstrate that cold and cold-mimetics stimulation is sufficient to produce a vigorous Fos response.
With the establishment of a robust stimulation paradigm, we tested the genetic dependence of these responses in relation to TRPM8 and TRPA1 by examining the ability of cold, menthol, and icilin to induce spinal cord Fos in mice lacking either channel. TRPM8−/− mice are unable to mount a normal Fos response to these stimuli, although in the case of cold a small but statistically significant amount of Fos was found in the dorsal horn ipsilateral to the stimulation site. Interestingly, the residual Fos expression to cold seen in TRPM8−/− mice was preserved in DKO animals, thus excluding TRPA1 as a candidate for in vivo noxious cold signaling. However, our study clearly indicates that while TRPA1 may be a good clinical target for heat or mechanical-related symptoms, TRPM8 should be the primary target for cold pain management.
Supplementary Material
Acknowledgements
This work was supported by a National Institutes of Health Grant NS054069 (D.D.M.), a Burroughs Wellcome Fund Career Award in Biomedical Sciences and McKnight Endowment Fund Scholar Award (D.M.B) and a USC CBM NIH training grant GM067587-06 (W.M.K.). We thank David Julius and Sven-Eric Jordt for the TRPM8−/− and TRPA1−/− mice, Sarah Bottjer for advice on Fos labeling, Kavita Renduchintala for assistance with the preference assay, Farhan Baluch for assistance with computer-based nuclei counting, Takeshi Morita for assistance with calcium imaging, and the members of the McKemy and Bautista labs for advice and support during this work.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have declared that no conflicts of interest exist.
Summary
This study shows that TRPM8, but TRPA1, mediates behavioral and neuronal responses to noxious cold and pungent and irritating cooling compounds in vivo.
References
- 1.Abbadie C, Honore P, Besson JM. Intense cold noxious stimulation of the rat hindpaw induces c-fos expression in lumbar spinal cord neurons. Neuroscience. 1994;59(2):457–468. doi: 10.1016/0306-4522(94)90609-2. [DOI] [PubMed] [Google Scholar]
- 2.Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321(5889):702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- 3.Allchorne AJ, Broom DC, Woolf CJ. Detection of cold pain, cold allodynia and cold hyperalgesia in freely behaving rats. Mol Pain. 2005;1:36. doi: 10.1186/1744-8069-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci. 2004;20(9):2276–2282. doi: 10.1111/j.1460-9568.2004.03695.x. [DOI] [PubMed] [Google Scholar]
- 5.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41(6):849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 6.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124(6):1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102(34):12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448(7150):204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 10.Belmonte C, Brock JA, Viana F. Converting cold into pain. Exp Brain Res. 2009;196(1):13–30. doi: 10.1007/s00221-009-1797-2. [DOI] [PubMed] [Google Scholar]
- 11.Berglund B, Harju EL, Kosek E, Lindblom U. Quantitative and qualitative perceptual analysis of cold dysesthesia and hyperalgesia in fibromyalgia. Pain. 2002;96(1–2):177–187. doi: 10.1016/s0304-3959(01)00443-2. [DOI] [PubMed] [Google Scholar]
- 12.Caspani O, Heppenstall PA. TRPA1 and cold transduction: an unresolved issue? J Gen Physiol. 2009;133(3):245–249. doi: 10.1085/jgp.200810136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung MK, Caterina MJ. TRP channel knockout mice lose their cool. Neuron. 2007;54(3):345–347. doi: 10.1016/j.neuron.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Coggeshall RE. Fos, nociception and the dorsal horn. Progress in Neurobiology. 2005;77(5):299–352. doi: 10.1016/j.pneurobio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D'Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54(3):379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Daniels RL, McKemy DD. Mice left out in the cold: commentary on the phenotype of TRPM8-nulls. Mol Pain. 2007;3:23. doi: 10.1186/1744-8069-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniels RL, Takashima Y, McKemy DD. Activity of the Neuronal Cold Sensor TRPM8 Is Regulated by Phospholipase C via the Phospholipid Phosphoinositol 4,5-Bisphosphate. J Biol Chem. 2009;284(3):1570–1582. doi: 10.1074/jbc.M807270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54(3):371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Ding Z, Gomez T, Werkheiser JL, Cowan A, Rawls SM. Icilin induces a hyperthermia in rats that is dependent on nitric oxide production and NMDA receptor activation. Eur J Pharmacol. 2008;578(2–3):201–208. doi: 10.1016/j.ejphar.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Fajardo O, Meseguer V, Belmonte C, Viana F. TRPA1 channels mediate cold temperature sensing in mammalian vagal sensory neurons: pharmacological and genetic evidence. J Neurosci. 2008;28(31):7863–7875. doi: 10.1523/JNEUROSCI.1696-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev. 2000;24(4):485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- 22.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103(51):19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328(6131):632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 24.Jiang CH, Hermanson O. Cooling of the urinary bladder activates neurons in the dorsal horn of the spinal cord. Neuroreport. 2004;15(2):351–355. doi: 10.1097/00001756-200402090-00028. [DOI] [PubMed] [Google Scholar]
- 25.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427(6971):260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 26.Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol. 2003;13(4):487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 27.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 28.Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27(37):9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A. 2009;106(4):1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang D-S, Woolf CJ, Corey DP. TRPA1 Contributes to Cold, Mechanical, and Chemical Nociception but Is Not Essential for Hair-Cell Transduction. Neuron. 2006;50(2):277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 31.Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci. 2009;29(15):4808–4819. doi: 10.1523/JNEUROSCI.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445(7127):541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 33.Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15(10):929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 34.Madrid R, de la Pena E, Donovan-Rodriguez T, Belmonte C, Viana F. Variable threshold of trigeminal cold-thermosensitive neurons is determined by a balance between TRPM8 and Kv1 potassium channels. J Neurosci. 2009;29(10):3120–3131. doi: 10.1523/JNEUROSCI.4778-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKemy DD. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain. 2005;1(1):16. doi: 10.1186/1744-8069-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKemy DD. Temperature sensing across species. Pflugers Arch. 2007;454(5):777–791. doi: 10.1007/s00424-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 38.Morin C, Bushnell MC. Temporal and qualitative properties of cold pain and heat pain: a psychophysical study. Pain. 1998;74(1):67–73. doi: 10.1016/S0304-3959(97)00152-8. [DOI] [PubMed] [Google Scholar]
- 39.Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25(16):4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noel J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, Lazdunski M. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. Embo J. 2009;28(9):1308–1318. doi: 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paller CJ, Campbell CM, Edwards RR, Dobs AS. Sex-based differences in pain perception and treatment. Pain Med. 2009;10(2):289–299. doi: 10.1111/j.1526-4637.2008.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 43.Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, Fleetwood-Walker SM, Mitchell R. Analgesia Mediated by the TRPM8 Cold Receptor in Chronic Neuropathic Pain. Curr Biol. 2006;16(16):1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- 44.Reid G, Flonta M. Cold transduction by inhibition of a background potassium conductance in rat primary sensory neurones. Neurosci Lett. 2001;297(3):171–174. doi: 10.1016/s0304-3940(00)01694-3. [DOI] [PubMed] [Google Scholar]
- 45.Reid G, Flonta ML. Physiology. Cold current in thermoreceptive neurons. Nature. 2001;413(6855):480. doi: 10.1038/35097164. [DOI] [PubMed] [Google Scholar]
- 46.Rommel O, Malin JP, Zenz M, Janig W. Quantitative sensory testing, neurophysiological and psychological examination in patients with complex regional pain syndrome and hemisensory deficits. Pain. 2001;93(3):279–293. doi: 10.1016/S0304-3959(01)00332-3. [DOI] [PubMed] [Google Scholar]
- 47.Serra J, Sola R, Quiles C, Casanova-Molla J, Pascual V, Bostock H, Valls-Sole J. C-nociceptors sensitized to cold in a patient with small-fiber neuropathy and cold allodynia. Pain. 2009 doi: 10.1016/j.pain.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 48.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like Channel Expressed in Nociceptive Neurons, Is Activated by Cold Temperatures. Cell. 2003;112(6):819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 49.Viana F. Understanding the mechanisms of cold-evoked pain in humans. Pain. 2009;147(1):7–8. doi: 10.1016/j.pain.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Viana F, de la Pena E, Belmonte C. Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat Neurosci. 2002;5(3):254–260. doi: 10.1038/nn809. [DOI] [PubMed] [Google Scholar]
- 51.Wakai J, Yoshizaki K, Taniguchi K, Yamaguchi-Yamada M, Yamamoto Y. Expression of Fos protein in brainstem after application of l-menthol to the rat nasal mucosa. Neurosci Lett. 2008;435(3):246–250. doi: 10.1016/j.neulet.2008.02.043. [DOI] [PubMed] [Google Scholar]
- 52.Wei ET, Seid DA. AG-3–5: a chemical producing sensations of cold. J Pharm Pharmacol. 1983;35(2):110–112. doi: 10.1111/j.2042-7158.1983.tb04279.x. [DOI] [PubMed] [Google Scholar]
- 53.Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN, Reeh PW. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature. 2007;447(7146):855–858. doi: 10.1038/nature05880. [DOI] [PubMed] [Google Scholar]
- 54.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 55.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10(3):277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.