Abstract
Inflammation plays a central role in the manner that the nervous system responds to injury. These effects include vasodilatation, increased vascular permeability, plasma extravasation, cell migration, and pain. Extracellular signals associated with inflammation may also lead to increased levels of pro-nociceptive chemokines/receptors that directly contribute to persistent or chronic pain behavior. To date, research focused on improving the treatment of chronic pain has largely ignored the role of inflammation-associated transcription factors such as nuclear transcription factor in activated T cells (NFAT). Herein we discuss the idea that activation of this transcription factor may be responsible for the production of chemokines receptors in both neuronal and non-neuronal cells of the peripheral nervous system. Taken together, a better understanding of the transcription of these pro-nociceptive genes may lead to the development of novel analgesic targets.
Peripheral nerve injury triggers a wide variety of cellular changes in the neurons of the associated sensory ganglia and is frequently accompanied by nociceptive behavior such as tactile allodynia (pain in response to light touch/pressure) and hyperalgesia (an increased sensitivity to pain). It has been proposed that these ongoing symptoms are due to enhanced excitability or sensitization of primary afferents in the dorsal root ganglia (DRG) (Devor, 2009; Ma et al., 2003; Suter et al., 2009). In turn, signal amplification by hyperexcitable sensory neurons may trigger changes in the response pattern of spinal cord dorsal horn neurons, a mechanism also known as central sensitization (Woolf, 1983; Woolf and Thompson, 1991). Although it is clear that molecular and possibly anatomical changes in the spinal cord dorsal horn are responsible for some attributes of chronic pain, primary afferent neuron-associated discharge is critical to the development and maintenance of chronic inflammatory or neuropathic pain.
One mechanism in which somatosensory afferent neurons may be induced to exhibit enhanced excitability following nerve injury is through the action of proalgesic inflammatory mediators (Miller et al., 2009). These include pro-inflammatory cytokines such as tumor necrosis factor-α (TNFα) and interleukin-1β (IL-1β), growth factors such as nerve growth factor (NGF) and other mediators such as prostaglandins or adenosine triphosphate (ATP), all of which can induce peripheral sensitization. The release of these factors by cells such as leukocytes in damaged peripheral targets or glial cells in damaged nerves may directly excite sensory neurons or induce the production of further mediators of peripheral sensitization: the chemokines. As we shall discuss, numerous chemokines have been shown to produce excitatory effects on cultured (Oh et al., 2001) and previously injured adult DRG sensory neurons and may act as downstream mediators of increased neuronal excitability (Bhangoo et al., 2007a; Bhangoo et al., 2009; Jung et al., 2009; Sun et al., 2006; White et al., 2005).
Chemokines and Chemokine Receptors
Chemokines constitute a large family of relatively low molecular weight proteins classified by the presence of a cysteine motif in the N-terminal region of the protein. Initial characterization of chemokines divided the family into α- and β- chemokines. In α chemokines, one amino acid separates the first two cysteine residues (cysteine-X amino acid-cysteine or CXC), whereas in β-chemokines, the first two cysteine residues are adjacent to each other (cysteine-cysteine, or CC). Two additional classes were added for the chemokines, lymphotactin (single cysteine, XC) and fractalkine (first two cysteines are separated by three amino acids, CX3C). The chemokine nomenclature uses XC, CC, CXC and CX3C, indicating the class to which the chemokine belongs, followed by the letter “L” (for ligand) and then a number. The numbering system corresponds to that already in use to designate the genes encoding each chemokine. In addition all chemokines also have a “trivial” name that describes their possible function, localization or other property. So, for example, the chemokine stromal cell derived factor -1 (SDF1) is also CXCL12 and breast and kidney derived chemokine (BRAK) is also CXCL14. However, most current studies utilize the official numbering system.
All chemokines exert their biological effects through the activation of an extended family of seven transmembrane G-protein-coupled receptors (GPCRs). Nineteen chemokine receptors have been cloned including seven CXC receptors (CXCR1–7), 10 CC receptors (from CCR1–10) and two single receptors each for lymphotactin (XCR1) and fractalkine (CXC3CR1). Chemokines and their receptors are frequently promiscuous as single chemokines can often activate several different chemokine receptors. There are, however, instances when a chemokine receptor is uniquely activated by a single chemokine. For example, the CXCR4 receptor has only one known signaling ligand, the chemokine CXCL12/SDF1.
Chemokines and their receptors in the Immune System
Chemokine influences in the immune system can be largely separated into two functional roles; they act as chemoattractants during inflammation and as traffickers of hematopoietic stem cells during development and differentiation (Charo and Ransohoff, 2006; Lapidot et al., 2005). Chemokines associated with inflammation coordinate leukocyte migration following tissue injury and/or disease processes; actions typically affiliated with the innate immune cell response. For example, monocyte chemoattractant protein-1 (MCP1; also known as CCL2) acting via CCR2 predominantly attracts monocytes (Charo et al., 1994). Not surprisingly, MCP1 knockout mice exhibit severe defects in monocyte recruitment to sites of inflammation (Lu et al., 1998) and CCR2 knockout mice exhibit marked attenuation of monocyte recruitment in response to various inflammatory stimuli together with a reduction of inflammatory lesions (Boring et al., 1997; Kurihara et al., 1997). Alternatively some inflammatory chemokines also have broad target cell selectivity and act on cells of the innate and adaptive immune system (Forster et al., 2008). Chemokines that influence hematopoiesis, in contrast, enable immature myeloid cells and leukocytes to navigate through bone marrow and thymus (Lapidot et al., 2005). These hematopoietic chemokines also act during initiation of adaptive immune responses in the spleen and lymph nodes, and in immune surveillance of healthy peripheral tissues (Ma et al., 1998). Some chemokines also have recognized angiogenic properties associated with the neovascularization that occurs during cancer growth, trauma and disease (Ceradini and Gurtner, 2005; Kakinuma and Hwang, 2006). One clinically important viral disease that is served by the presence of the chemokine receptors on leukocytes is HIV-1. Co-expression of CXCR4 and CD4 on a cell allow T-tropic HIV isolates to fuse with and infect T cells. CCR5 (the chemokine receptor for Regulated upon Activation, Normal T-cell Expressed, and Secreted; RANTES; also known as CCL5) which is expressed on macrophages and on some populations of T cells, can also function in concert with CD4 to allow HIV-1 membrane fusion (Alkhatib, 2009). In addition to the direct effect of HIV-1 on cells of the immune system, the virus is also known to be neuroinvasive, neurotrophic, and neurovirulent with the most common CNS manifestation being HIV-1-associated neurocognitive disorders (Kraft-Terry et al., 2009). The most frequent neurological complication of HIV-1 in the peripheral nervous system is HIV-1 sensory neuropathy (Luciano et al., 2003). This peripheral neuropathy can be further divided into distal sensory polyneuropathy (DSP) and antiretroviral induced toxic neuropathy (ATN). Both forms involve sensory loss and neuropathic pain (Bouhassira et al., 1999; Hewitt et al., 1997).
Chemokine/Receptors and Neuropathic Pain
There is considerable data indicating that chemokines can act as pro-nociceptive mediators following tissue injury and disease in the nervous system. However, direct neuronal effects produced by chemokine receptor signaling remains an understudied field (Miller et al., 2009). From the point of view of the present discussion it should be noted that Oh and colleagues (Oh et al., 2001) first demonstrated that cultured neonatal rodent DRG neurons expressed numerous types of chemokine receptors. Addition of chemokines to these cultured neurons resulted in increased intracellular calcium ([Ca]i) signals and increased neuronal excitability. The authors therefore suggested that chemokine receptor expression by sensory neurons might act as a conduit allowing these inflammatory mediators to directly excite DRG neurons producing increased ectopic excitability and tactile allodynia. Also of importance, Oh and colleagues (Oh et al., 2001) observed that gp120, the viral coat protein of HIV-1, which interacts with either CCR5 or CXCR4 chemokine receptors (depending on the strain of the virus), could also produce DRG excitation. Considering that HIV-1 infection is associated with painful polyneuropathies (i.e. DSP and ATN) that affect up to 67% of infected patients, it is quite possible that direct interaction of HIV-1/gp120 with chemokine receptors expressed on sensory neurons may be central to the etiology of HIV-1 associated pain (Bhangoo et al., 2009; Oh et al., 2001; Wallace et al., 2007). Taken together, studies on HIV-1 associated painful neuropathies opened the door to the possibility that chemokines and their receptors represent a class of peptides that may directly or indirectly contribute to a number of acute and/or chronic pain conditions.
To better understand the degree to which chemokine/receptor signaling plays a functional role in acute and chronic nociceptive events requires a precise knowledge of the normal and injury-induced distribution of the peptides within the peripheral and central nervous system. Although there does appear to be active signaling by chemokine receptors in sensory neurons derived from neonatal animals (Oh et al., 2001), the naïve adult peripheral nervous system is largely devoid of functional neuronal chemokine receptor candidates. These include the chemokine receptors CCR2, CCR5, and CXCR4 (Bhangoo et al., 2007a; Bhangoo et al., 2007b; Sun et al., 2006; White et al., 2005). This observation is not surprising as there is relatively little evidence of pro-nociceptive chemokine receptor mRNA or protein in the naïve rodent PNS. One notable exception is the chemokine receptor, CXCR4. The distribution of CXCR4 mRNA and protein, however, is largely localized to non-myelinating satellite glial cells in the DRG under naïve and sham injury conditions (Bhangoo et al., 2007a; Bhangoo et al., 2007b; Bhangoo et al., 2009).
Following nerve or tissue injury and/or disease, functional chemokine receptor signaling in the adult peripheral nervous system appears to be upregulated [see review, (White et al., 2007)]. For example, chronic compression of the lumbar L4L5 DRG (CCD) produces increased MCP1/CCR2 dependent membrane depolarization in small, medium and large diameter neurons of intact DRGs derived from injured animals and action potential discharges in some large neurons (Sun et al., 2006; White et al., 2005); focal demyelination of the sciatic nerve upregulates CCR2, CCR5, CXCR3 and CXCR4 functional signaling in both nociceptive and non-nociceptive neurons (Bhangoo et al., 2007a; Jung et al., 2009); and both CCR2 and CXCR4 neuronal signaling is increased in nociceptive and non-nociceptive sensory neuron populations following a combination of gp120 to the sciatic nerve and systemic injection of the nucleoside reverse transcriptase inhibitor (NRTI), 2′,3′-dideoxycytidine two weeks later (Bhangoo et al., 2009).
Among the assayed chemokine receptors to date, CCR2 and CXCR4, appear to be well placed to play a major role in chronic pain behavior in rodents. Abbadie and colleagues (Abbadie et al., 2003) observed that while CCR2 knockout and wild-type mice exhibited equivalent responses to acute pain tests, CCR2 knockout mice exhibited a 70% reduction in phase 2 of an intraplantar formalin challenge and following partial nerve ligation these mice did not develop tactile allodynia, unlike their wildtype counterparts (Abbadie et al., 2003). Further demonstration of the importance of MCP1/CCR2 in pain behavior is observed in transgenic mice engineered to overexpress MCP1 using a glial fibillary acidic protein promoter. These transgenic mice, in contrast to wildtype controls, exhibited enhanced nociceptive behavior following acute pain challenges. Assayed behavioral changes included thermal (hot plate) and chemical (formalin test) stimulus modalities. However, whether this increased nociceptive behavior was due only to MCP1 acting through CCR2 or the increased levels of proinflammatory cytokines (IL-1β, IL-6, or TNFα) present in the skin, DRG or spinal cord is unclear (Menetski et al., 2007). Regardless, these data alone make a strong case for CCR2 regulation of elements of pain behavior in rodents.
The direct role of CXCR4 signaling on peripheral nervous injury and the resultant neuropathic pain behavior in rodent models is made more difficult to understand due to the lethality of CXCR4 or SDF1 gene deletion late in embryogenesis (Nagasawa et al., 1996; Zou et al., 1998). However, there is good evidence to suggest that NRTI injury-induced rodent pain behavior is due, in part, to increased SDF1/CXCR4 signaling. As noted previously, CXCR4 is restricted to satellite glial cells in the DRG. Following the administration of the NRTI, 2′-3′-dideoxycytidine, CXCR4 mRNA (Bhangoo et al., 2007) and protein is upregulated in mostly small TRPV1-immunopositive or IB4 populations of sensory neurons (Bhangoo et al., 2009). These neuronal changes in CXCR4 following NRTI administration was also accompanied by the presence of tactile allodynia. Subsequent systemic administration of the highly specific receptor antagonist for CXCR4, AMD3100, did not affect the pain behavior in vehicle-treated control animals, but convincingly reversed the bilateral tactile allodynia in animals previously exposed to NRTIs (Bhangoo et al., 2007b; Bhangoo et al., 2009).
The chemokine MCP1/CCL2
Not unlike the receptor, CCR2, an understanding of the functional role of its major agonist the chemokine MCP1 in different pain states is dependent on the tissue localization, before and after injury. Within the naïve DRG of rats and mice, MCP1 is largely absent (Bhangoo et al., 2007a; Bhangoo et al., 2009; Jeon et al., 2008; Jung et al., 2009; Tanaka et al., 2004; Thacker et al., 2009; White et al., 2005; Yang et al., 2007; Zhang and De Koninck, 2006). However, following the induction of different injury models, there is a robust and long lasting upregulation of MCP1 mRNA and protein by numerous small peptidergic and non-peptidergic subpopulations of sensory neurons in the DRG (Bhangoo et al., 2007a; Jeon et al., 2008; Tanaka et al., 2004; Thacker et al., 2009). Interestingly, following its injury induced de novo synthesis, neuronal MCP1 is packaged into large dense core vesicles that colocalize with the neuropeptide, calcitonin gene-related peptide (CGRP), and these peptides may function together in the transmission of pain (Jung et al., 2008). Moreover, when these MCP1 containing neurons were depolarized, the MCP1 could be released in a calcium dependent manner (Jung et al., 2008). The mere suggestion that vesicles containing both MCP1 and CGRP could be released following depolarization of the neurons indicates a potential neurotransmitter role for MCP1 expressed under these conditions (Jung et al., 2009; Jung et al., 2008). A working example of the role for MCP1 and CGRP is in the painful autoimmune disease, rheumatoid arthritis. Given the vesicular colocalization of neuropeptide and chemokine, perhaps it is not surprising that while CGRP knockout mice fail to demonstrate the development of tactile hyperalgesia following knee joint inflammation (murine model of arthritis), these same knockout mice continue to exhibit the expected decrease in paw withdrawal latency to thermal stimuli observed in CGRP wildtype mice (Zhang et al., 2001). The converse is difficult to prove as CCR2 and CCL2 knockout mice are protected from inflammatory diseases, including experimental autoimmune encephalomyelitis (EAE; a mouse model for multiple sclerosis), rheumatoid arthritis and neuropathic pain (Abbadie et al., 2003; Brodmerkel et al., 2005; Fife et al., 2000; Lu et al., 1998).
As activation of CCR2 receptors appears to be a critical event in the pathogenesis and maintenance of chronic pain, it is important to know the locus of MCP1/CCR2 signaling. Some evidence suggests that release of the MCP1 into the spinal cord dorsal horn via central projections of primary afferent neurons following nerve injury is an important element of mechanical allodynia (Dansereau et al., 2008; Thacker et al., 2009; Zhang and De Koninck, 2006). However, in order for activity dependent release of MCP1 to facilitate either second order neuron excitability or microglial activation, one must assume that CCR2 necessarily needs to be present in the CNS parenchyma. There are several publications that support CCR2 dependent microglial activation in rodents (Abbadie et al., 2003; Dansereau et al., 2008; Menetski et al., 2007; Thacker et al., 2009), or CCR2 positive neurons in the dorsal horn (Gao et al., 2009). However, a recent publication using bitransgenic reporter mice in which the chemokine receptor CCR2 and its ligand MCP1 were labeled by the fluorescent proteins enhanced green fluorescent protein and monomeric red fluorescent protein-1 failed to yield evidence of CCR2 positive cells in the spinal cord (neuronal or glial) following peripheral nerve injury (see Fig. 1), acute inflammation or EAE (see Fig. 2) (Jung et al., 2009). This experimental outcome was also supported by a previous publication by Mahad and colleagues (Mahad et al., 2006) which demonstrated that as CCR2-positive leukocytes crossed the blood-brain barrier in response to MCP1, these cells downregulated CCR2 receptor expression. Perhaps more importantly, the rapid antinociceptive effects of peripherally administered receptor antagonists for CCR2 (CCR2 RA) that have been observed under these circumstances strongly suggest that CCR2 RA may ameliorate tactile allodynia by inhibiting CCR2 receptor activation in the periphery (see Fig. 3) (Bhangoo et al., 2007a; Bhangoo et al., 2009; Jung et al., 2009). Taken together, CCL2/CCR2 signaling and/or CXCL12/CXCR4 signaling by sensory neurons may be critical events in the pathogenesis and maintenance of neuropathic pain states in rodents and one might hypothesize, in humans as well.
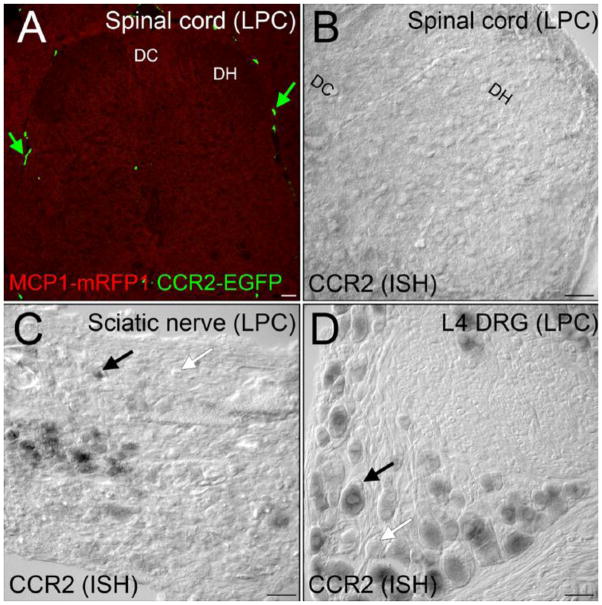
Figure 1.
After demyelination injury, CCR2 signaling was not activated in the spinal cord. A, MCP1::MCP1–mRFP1; CCR2::CCR2–EGFP mice were subjected to lysophosphatidylcholine (LPC)-induced focal demyelination of the sciatic nerve. There was no expression of MCP1 or CCR2 in the associated lumbar spinal cord level at detectable levels. Leukocytes outside the spinal cord were clearly visible (green arrow). B, CCR2 expression was also examined at the mRNA level by in situ hybridization. The spinal cord does not contain significant CCR2 expressing cellular components, whereas many cells in the sciatic nerve (C) express and DRG (D) express CCR2 receptors in the LPC group. DH, dorsal horn; DC, dorsal column. Scale bars 60μm. Courtesy of the Society for Neuroscience.
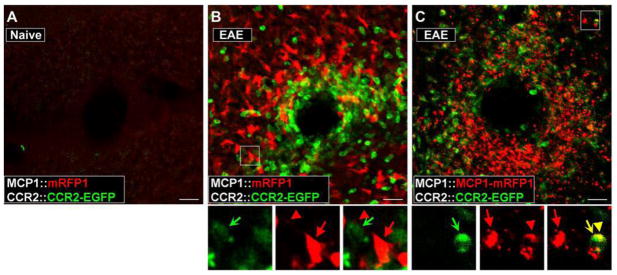
Figure 2.
Activation of CCR2 receptors in peripheral leukocytes during the pathogenesis of EAE can be visualized in the BAC transgenic mice. EAE was induced in MCP1:mRFP1; CCR2::CCR2–EGFP mice (B) and MCP1::MCP1-mRFP1; CCR2::CCR2-EGFP mice (C). The pictures were taken from the white matter from coronal cerebellar sections. Unlike control conditions (A), there was a marked infiltration of leukocytes (B, C; CCR2-EGFP+) into the parenchyma of the cerebellum (examined on postoperative day 14). Magnified views of the boxed areas are shown below B and C. Although there were close interactions between MCP1 (red arrow, cell body; red arrowhead, cellular process)- and CCR2-expressing cells (green concave arrow) (B, C), onlyMCP1–mRFP1(C), but not mRFP1 alone (B), was transferred to CCR2–EGFP-expressing leukocytes as indicated from the appearance of yellow internal vesicles (yellow arrow and arrowhead). Scale bars, 20μm. Courtesy of the Society for Neuroscience.
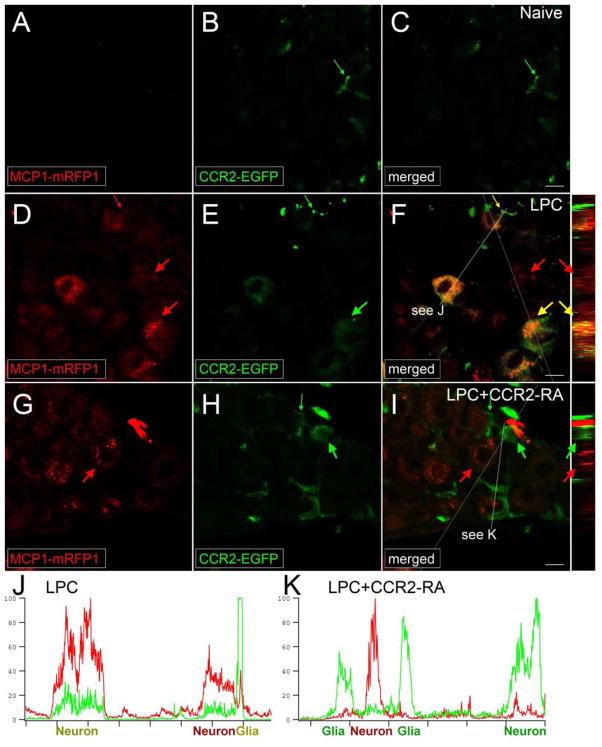
Figure 3.
CCR2 signaling is activated in the DRG. MCP1::MCP1–mRFP1; CCR2::CCR2–EGFP mice were subjected to lysophosphatidylcholine (LPC)-induced demyelination of the sciatic nerve. A–F, In the DRG ipsilateral to the injury, expression of both MCP1 and CCR2 increased (D–F), whereas there was little expression of MCP1 or CCR2 under naive conditions (A–C). MCP1-mRFP1 mainly localized to neurons (large red arrow) and, to some extent, to satellite glia (small red arrow; D). CCR2–EGFP localized to neurons (large green arrow) and satellite glia (small green arrow; E). Most CCR2–EGFP-expressing neurons and satellite glia also contained MCP1-mRFP1 (yellow arrow; F). Systemic injection of a receptor antagonist for CCR2 (CCR2-RA) eliminated MCP1-mRFP1 in satellite glia (G–I; small green arrow). Also, after CCR2-RA treatment, MCP1-mRFP1- and CCR2-EGFP-expressing cells existed as separate populations (G–I; large green and red arrows). Cross-sectional images across the white lines are shown right to F and I. J, K, Intensities of mRFP1 and EGFP in shorter white lines in F and I are expressed in arbitrary units to compare relative signal intensities among different cells. J, In the LPC group, most neurons which express CCR2–EGFP also contain MCP1 mRFP1. Also, the CCR2–EGFP signal in neurons is relatively weaker than the signal in satellite glia (J). K, In the LPC plus CCR2–RA group, however, most CCR2–EGFP-expressing cells do not contain significant amount of MCP1 mRFP1 signal. In addition, the CCR2–EGFP signal in neurons is now as strong as the signal in satellite glia (K). Scale bars, 15μm. Courtesy of the Society for Neuroscience.
Transcription factors in chemokine receptor mediated pain behavior in rodents
Given the above discussion, it is clear that chemokines and their respective receptors may be attractive therapeutic targets for modulating both inflammatory responses to injury and disease processes in all areas of medicine including chronic pain syndromes. Understanding the underlying mechanism(s) leading to the upregulated expression of both chemokines and their receptors in the peripheral nervous system, and the identification of the signaling cascades responsible, may provide new strategies for effectively diminishing or eliminating chronic pain syndromes.
The regulation of CCR2 receptor expression serves as a good example, particularly considering the evidence discussed above that CCR2 signaling is frequently involved in chronic pain behavior in rodents. Recently published data have demonstrated that the upregulated expression of CCR2 in DRG neurons is mediated by the Ca dependent transcription factor nuclear factor of activated T cells (NFAT) (Jung and Miller, 2008). Both the Ca dependent phosphatase calcineurin and its target NFAT were detected in these neurons and calcineurin was shown to be activated by Ca influx. Furthermore, the observed up modulation of CCR2 expression was inhibited by drugs such as FK506 and cyclosporine which inhibit NFAT signaling. Indeed, we have observed that administration of FK506 inhibits the development of neuropathic pain in some models (Jung et al, unpublished observations). These data indicate that levels of chemokine receptor expression, including CCR2 receptors, may respond to the overall electrical activity of DRG neurons. Increased action potential generation would result in increased neuronal Ca levels leading to increased NFAT activity and upregulated expression of CCR2. It is possible that some upstream cytokine such as TNFα or IL-1β is generated by events that initiate the signaling that leads to chronic pain. These agents can directly excite sensory neurons (Inoue et al., 1999; Jin and Gereau, 2006; Miller et al., 2009; Obreja et al., 2002; Opree and Kress, 2000) leading to increased Ca influx and also the upregulation of genes such as those for CCR2. The action of CCL2 on upregulated CCR2 is also excitatory and would lead to further increases and maintenance of excitation.
CONCLUSIONS
The above discussion indicates that upregulation of chemokine signaling in the DRG and peripheral nerve may be one common pathway through which many agents can produce chronic pain behaviors. The general model we suggest is that different kinds of stimuli such as those resulting from tissue damage, administration of NRTIs or other means would lead to the synthesis of “upstream” cytokines. The action of these molecules on DRG neurons may lead to direct depolarization, Ca influx and/or other signaling molecules and the activation of transcriptional pathways that upregulate chemokine and chemokine receptor expression by DRG neurons. The activation of these receptors following the local release of chemokines will then produce enhanced neuronal excitation (White et al., 2009). Indeed current studies with CCR2 receptor antagonists appear to support this hypothesis. Thus targeting chemokine signaling may result in novel approaches to numerous types of previously intractable chronic pain syndromes.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant NS049136 and DA026040 (to F.A.W.); and National Institutes of Health Grants NS043095, DA013141, and MH040165 (to R.J.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Fletcher A. White, Email: fawhite@iupui.edu.
Richard J. Miller, Email: r-miller10@northwestern.edu.
References
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhatib G. The biology of CCR5 and CXCR4. Curr Opin HIV AIDS. 2009;4:96–103. doi: 10.1097/COH.0b013e328324bbec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhangoo S, Ren D, Miller RJ, Henry KJ, Lineswala J, Hamdouchi C, Li B, Monahan PE, Chan DM, Ripsch MS, White FA. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: a mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain. 2007a;3:38. doi: 10.1186/1744-8069-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhangoo SK, Ren D, Miller RJ, Chan DM, Ripsch MS, Weiss C, McGinnis C, White FA. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav Immun. 2007b;21:581–591. doi: 10.1016/j.bbi.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhangoo SK, Ripsch MS, Buchanan DJ, Miller RJ, White FA. Increased chemokine signaling in a model of HIV1-associated peripheral neuropathy. Mol Pain. 2009;5:48. doi: 10.1186/1744-8069-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF. Impaired Monocyte Migration and Reduced Type 1 (Th1) Cytokine Responses in C-C Chemokine Receptor 2 Knockout Mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhassira D, Attal N, Willer JC, Brasseur L. Painful and painless peripheral sensory neuropathies due to HIV infection: a comparison using quantitative sensory evaluation. Pain. 1999;80:265–272. doi: 10.1016/s0304-3959(98)00227-9. [DOI] [PubMed] [Google Scholar]
- Brodmerkel CM, Huber R, Covington M, Diamond S, Hall L, Collins R, Leffet L, Gallagher K, Feldman P, Collier P, Stow M, Gu X, Baribaud F, Shin N, Thomas B, Burn T, Hollis G, Yeleswaram S, Solomon K, Friedman S, Wang A, Xue CB, Newton RC, Scherle P, Vaddi K. Discovery and Pharmacological Characterization of a Novel Rodent-Active CCR2 Antagonist, INCB3344. J Immunol. 2005;175:5370–5378. doi: 10.4049/jimmunol.175.8.5370. [DOI] [PubMed] [Google Scholar]
- Ceradini DJ, Gurtner GC. Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc Med. 2005;15:57–63. doi: 10.1016/j.tcm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci U S A. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Dansereau MA, Gosselin RD, Pohl M, Pommier B, Mechighel P, Mauborgne A, Rostene W, Kitabgi P, Beaudet N, Sarret P, Melik-Parsadaniantz S. Spinal Ccl2 Pronociceptive Action Is No Longer Effective in Ccr2 Receptor Antagonist-Treated Rats. J Neurochem. 2008 Jul;106(2):757–69. doi: 10.1111/j.1471-4159.2008.05429.x. Epub 2008 Apr 17. [DOI] [PubMed] [Google Scholar]
- Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res. 2009 doi: 10.1007/s00221-009-1724-6. [DOI] [PubMed] [Google Scholar]
- Fife B, Huffnagle G, Kuziel W, Karpus WJ. CC Chemokine Receptor 2 Is Critical for Induction of Experimental Autoimmune. J Exp Med. 2000;192:899–906. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt DJ, McDonald M, Portenoy RK, Rosenfeld B, Passik S, Breitbart W. Pain syndromes and etiologies in ambulatory AIDS patients. Pain. 1997;70:117–123. doi: 10.1016/s0304-3959(96)03281-2. [DOI] [PubMed] [Google Scholar]
- Inoue A, Ikoma K, Morioka N, Kumagai K, Hashimoto T, Hide I, Nakata Y. Interleukin-1beta induces substance P release from primary afferent neurons through the cyclooxygenase-2 system. J Neurochem. 1999;73:2206–2213. [PubMed] [Google Scholar]
- Jeon SM, Lee KM, Park ES, Jeon YH, Cho HJ. Monocyte chemoattractant protein-1 immunoreactivity in sensory ganglia and hindpaw after adjuvant injection. Neuroreport. 2008;19:183–186. doi: 10.1097/WNR.0b013e3282f3c781. [DOI] [PubMed] [Google Scholar]
- Jin X, Gereau RWt. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci. 2006;26:246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Bhangoo S, Banisadr G, Freitag C, Ren D, White FA, Miller RJ. Visualization of chemokine receptor activation in transgenic mice reveals peripheral activation of CCR2 receptors in states of neuropathic pain. J Neurosci. 2009;29:8051–8062. doi: 10.1523/JNEUROSCI.0485-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Miller RJ. Activation of the nuclear factor of activated T-cells (NFAT) mediates upregulation of CCR2 chemokine receptors in dorsal root ganglion (DRG) neurons: A possible mechanism for activity-dependent transcription in DRG neurons in association with neuropathic pain. Mol Cell Neurosci. 2008;37:170–177. doi: 10.1016/j.mcn.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma T, Hwang ST. Chemokines, chemokine receptors, and cancer metastasis. J Leukoc Biol. 2006;79:639–651. doi: 10.1189/jlb.1105633. [DOI] [PubMed] [Google Scholar]
- Kraft-Terry SD, Buch SJ, Fox HS, Gendelman HE. A coat of many colors: neuroimmune crosstalk in human immunodeficiency virus infection. Neuron. 2009;64:133–145. doi: 10.1016/j.neuron.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot T, Dar A, Kollet O. How do stem cells find their way home? 10.1182/blood-2005-04-1417. Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano CA, Pardo CA, McArthur JC. Recent developments in the HIV neuropathies. Curr Opin Neurol. 2003;16:403–409. doi: 10.1097/01.wco.0000073943.19076.98. [DOI] [PubMed] [Google Scholar]
- Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar Electrophysiological Changes in Axotomized and Neighboring Intact Dorsal Root Ganglion Neurons. J Neurophysiol. 2003;89:1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad D, Callahan MK, Williams KA, Ubogu EE, Kivisakk P, Tucky B, Kidd G, Kingsbury GA, Chang A, Fox RJ, Mack M, Sniderman MB, Ravid R, Staugaitis SM, Stins MF, Ransohoff RM. Modulating CCR2 and CCL2 at the blood-brain barrier: relevance for multiple sclerosis pathogenesis. Brain. 2006;129:212–223. doi: 10.1093/brain/awh655. [DOI] [PubMed] [Google Scholar]
- Menetski J, Mistry S, Lu M, Mudgett JS, Ransohoff RM, Demartino JA, Macintyre DE, Abbadie C. Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience. 2007;149:706–714. doi: 10.1016/j.neuroscience.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009:417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Obreja O, Rathee PK, Lips KS, Distler C, Kress M. IL-1 beta potentiates heat-activated currents in rat sensory neurons: involvement of IL-1RI, tyrosine kinase, and protein kinase C. Faseb J. 2002;16:1497–1503. doi: 10.1096/fj.02-0101com. [DOI] [PubMed] [Google Scholar]
- Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and Glycoprotein120 Produce Pain Hypersensitivity by Directly Exciting Primary Nociceptive Neurons. J Neurosci. 2001;21:5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opree A, Kress M. Involvement of the Proinflammatory Cytokines Tumor Necrosis Factor-alpha, IL-1beta, and IL-6 But Not IL-8 in the Development of Heat Hyperalgesia: Effects on Heat-Evoked Calcitonin Gene-Related Peptide Release from Rat Skin. J Neurosci. 2000;20:6289–6293. doi: 10.1523/JNEUROSCI.20-16-06289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JH, Yang B, Donnelly DF, Ma C, LaMotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol. 2006;96:2189–2199. doi: 10.1152/jn.00222.2006. [DOI] [PubMed] [Google Scholar]
- Suter MR, Berta T, Gao YJ, Decosterd I, Ji RR. Large A-fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. Mol Pain. 2009;5:53. doi: 10.1186/1744-8069-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Minami M, Nakagawa T, Satoh M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neurosci Res. 2004;48:463–469. doi: 10.1016/j.neures.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain. 2009;13:263–272. doi: 10.1016/j.ejpain.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Wallace VC, Blackbeard J, Pheby T, Segerdahl AR, Davies M, Hasnie F, Hall S, McMahon SB, Rice AS. Pharmacological, behavioural and mechanistic analysis of HIV-1 gp120 induced painful neuropathy. Pain. 2007;133:47–63. doi: 10.1016/j.pain.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Feldman P, Miller RJ. Chemokine signaling and the management of neuropathic pain. Mol Interv. 2009;9:188–195. doi: 10.1124/mi.9.4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:20151–20158. doi: 10.1073/pnas.0709250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, Lamotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A. 2005;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Yang HY, Mitchell K, Keller JM, Iadarola MJ. Peripheral inflammation increases Scya2 expression in sensory ganglia and cytokine and endothelial related gene expression in inflamed tissue. J Neurochem. 2007;103:1628–1643. doi: 10.1111/j.1471-4159.2007.04874.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772–783. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hoff AO, Wimalawansa SJ, Cote GJ, Gagel RF, Westlund KN. Arthritic calcitonin/alpha calcitonin gene-related peptide knockout mice have reduced nociceptive hypersensitivity. Pain. 2001;89:265–273. doi: 10.1016/s0304-3959(00)00378-x. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.