Abstract
Some Lyme disease patients report debilitating chronic symptoms of pain, fatigue, and cognitive deficits despite recommended courses of antibiotic treatment. The mechanisms responsible for these symptoms, collectively referred to as post-Lyme disease syndrome (PLS) or chronic Lyme disease, remain unclear. We investigated the presence of immune system abnormalities in PLS by assessing the levels of antibodies to neural proteins in patients and controls. Serum samples from PLS patients, post-Lyme disease healthy individuals, patients with systemic lupus erythematosus, and normal healthy individuals were analyzed for anti-neural antibodies by immunoblotting and immunohistochemistry. Anti-neural antibody reactivity was found to be significantly higher in the PLS group than in the post-Lyme healthy (p<0.01) and normal healthy (p<0.01) groups. The observed heightened antibody reactivity in PLS patients could not be attributed solely to the presence of cross-reactive anti-borrelia antibodies, as the borrelial seronegative patients also exhibited elevated anti-neural antibody levels. Immunohistochemical analysis of PLS serum antibody activity demonstrated binding to cells in the central and peripheral nervous systems. The results provide evidence for the existence of a differential immune system response in PLS, offering new clues about the etiopathogenesis of the disease that may prove useful in devising more effective treatment strategies.
Keywords: post-Lyme disease syndrome, chronic Lyme disease, Borrelia burgdorferi, immune dysregulation, antibody
1. Introduction
Lyme disease is a multisystem infection, caused by bacteria of the Borrelia burgdorferi species complex and transmitted by Ixodes ticks (Stanek and Strle, 2003). It is the most commonly reported tick-borne disease in the northern hemisphere, widespread in Europe and endemic in more than 15 states in the United States (Stanek and Strle, 2008; Steere, 2001). The initial skin rash (erythema migrans) may be followed by complications affecting joints, heart, and the nervous system (Stanek and Strle, 2003; Wormser et al., 2006). The neurologic complications involve both the central and peripheral nervous systems. These include lymphocytic meningitis, encephalitis, cranial neuropathy, radiculopathy, and alterations of mental status, all of which usually respond well to antibiotic treatment (Halperin, 2008). However, some patients with Lyme disease continue to have persistent complaints despite treatment and in the absence of objective evidence of infection, as determined by currently available methods (Feder et al., 2007; Marques, 2008). The symptoms in these patients are generally accepted to include mild to severe musculokeletal pain, fatigue, and/or difficulties with concentration and memory (Feder et al., 2007; Marques, 2008). The condition, variably referred to as chronic Lyme disease, post-treatment Lyme disease syndrome (PTLDS), and post-Lyme disease syndrome (PLDS or PLS), is associated with considerable impairment in the health-related quality of life in some patients (Klempner et al., 2001).
Considering the lack of evidence for the presence of live spirochetes in PLS patients who have received recommended antibiotics, persistent infection is currently not thought to account for the symptoms of PLS by most investigators (Baker, 2008; Feder et al., 2007). However, despite several years of debate and a number of treatment clinical trials (Fallon et al., 2008; Klempner et al., 2001; Krupp et al., 2003), few clues to the causes of the symptoms have emerged. Lack of any biomarkers to aid in the diagnosis and follow up has also compounded the problem of understanding the disease. Mechanisms other than active infection, including the possibility of involvement of adaptive or innate immune system abnormalities, have been suggested, but experimental evidence has been scarce (Marques, 2008; Sigal, 1997). The aim of this study was to characterize the level and specificity of antibody reactivity to neural antigens in PLS patients. Here, we show evidence of heightened anti-neural antibody levels in PLS, indicating the presence of objective immunologic abnormalities in affected patients that may be relevant to the pathogenic mechanism of the disease.
2. Methods
2.1. Subjects
Serum samples from 83 individuals with a history of Lyme borreliosis and persistent symptoms, recruited as part of a previous clinical trial (Klempner et al., 2001), were used in this study (37 female, 46 male; mean age 55.6 ± 12.0 y (SD); mean elapsed time since the original diagnosis of Lyme disease 5.0 ± 2.9 y (SD)). Selection of these specific specimens from the original cohort was based on limiting the elapsed time between diagnosis of acute Lyme disease and serum specimen collection to between 1 and 12 years. Patients had at least one of the following: a history of erythema migrans (EM) skin lesion, early neurologic or cardiac symptoms attributed to Lyme disease, radiculoneuropathy, or Lyme arthritis. Documentation by a physician of previous treatment of acute Lyme disease with a recommended antibiotic regimen was also required. Patients had one or more of the following symptoms at the time of enrollment: widespread musculoskeletal pain, cognitive impairment, radicular pain, paresthesias, or dysesthesias. Fatigue often accompanied one or more of these symptoms. The chronic symptoms had to have begun within 6 months after the infection with B. burgdorferi. Control subjects included 27 individuals who had been treated for early localized or disseminated Lyme disease associated with single (n=18) or multiple (n=9) EM, but had no post-Lyme symptoms after at least 2 years of follow-up (12 female, 15 male; mean age 54.4 ± 14.7 y (SD); mean elapsed time since the original diagnosis of Lyme disease 5.4 ± 3.8 y (SD)). The diagnosis of acute Lyme disease in control subjects was confirmed by recovery of B. burgdorferi in cultures of skin and/or blood sample. The elapsed time between diagnosis of acute Lyme disease and serum specimen collection was limited to between 1 and 12 years for post-Lyme healthy subjects. In addition to the above, serum samples from 15 patients with systemic lupus erythematosus (SLE) and 20 healthy individuals were analyzed in the study. All SLE patients met four or more of the American College of Rheumatology classification criteria for diagnosis (Tan et al., 1982). Serum specimens were stored at −80 °C prior to use. This study was approved by the Institutional Review Board of the Weill Medical College of Cornell University.
2.2. Total IgG
Total IgG concentration of serum specimens was measured with an ELISA kit (ICL), according to the manufacturer’s instructions.
2.3. Anti-borrelia antibodies
IgG anti-borrelia antibody levels were determined by ELISA. 96-well polystyrene plates (BD Biosciences) were incubated overnight with 0.5 µg/well of B. burgdorferi B31 antigen (Meridian) in 0.1 M carbonate buffer (pH 9.6). Blocking of wells was done with 1% BSA in phosphate-buffered saline containing 0.05% Tween-20 (PBST) for 1.5 h. Incubation with diluted serum samples (50 µL/well at 1:800 in blocking buffer) was done for 1 h. Each plate contained one negative and two positive controls. Incubation with HRP-conjugated sheep anti-human IgG (Amersham) secondary antibody was for 1 h. Incubation with developing solution, comprising 27 mM citric acid, 50 mM Na2HPO4, 5.5 mM o-phenylenediamine, and 0.01% H2O2 (pH 5), was for 20 min. Absorbance was measured at 450 nm and corrected for non-specific binding by subtraction of the mean absorbance of corresponding wells not coated with the borrelia antigen. Absorbance values were normalized based on the mean for the positive controls on each plate. Cutoff for positivity was assigned as two standard deviations above the mean for the healthy control group results.
2.4. Anti-neural antibodies
2.4.1. Immunoblotting
Antibodies to brain proteins were detected by immunoblotting for all specimens as follows. Mouse brain was utilized in order to avoid artifactual bands that result from the binding of secondary anti-human antibodies to endogenous immunoglobulins when using the sensitive chemiluminescence method of detection. Mouse tissue was specifically chosen among non-primate sources due to the high level of known homology and orthology between human and mouse proteomes (Southan, 2004), a strategy that has been used in other studies as well (Maruyama et al., 2004; Shoenfeld et al., 2003; Tin et al., 2005). Mouse (C57BL/6J strain) brain lysate was prepared as previously described (Alaedini et al., 2007). SDS-PAGE (4–15% pre-cast 2D-prep gel from Bio-Rad) was carried out on 400 µg protein aliquots of lysate at 200 V in Tris-glycine-SDS buffer for 35 min, followed by transfer to nitrocellulose membrane at 33 V in Tris-glycine buffer containing 20% methanol for 16 h. Each gel contained the Precision Plus molecular weight marker mix (Bio-Rad) in one lane. The membrane was incubated in blocking buffer, containing 5% milk and 0.5% BSA in Tris-buffered saline containing 0.05% Tween-20 (TBST) for 2 h. Incubation with patient serum (1:2000 in dilution buffer containing 10% blocking buffer and 10% fetal bovine serum in TBST) was carried out for 1 h in a Mini-PROTEAN II Multiscreen apparatus (Bio-Rad). A positive control sample was included on every membrane. HRP-conjugated sheep anti-human IgG (Amersham) was used as the secondary antibody. Detection of bound antibodies was by the ECL system (Millipore) and BioMax MR film (Kodak) after 10s exposure. Each membrane was treated with stripping buffer (Pierce) at 58 °C for 30 min, and reblotted with HRP-conjugated rabbit anti-β tubulin antibody (Novus). Detection of bound antibodies was as before. Conversion of immunoblots to line graph, density analysis, and subtraction of background were performed by the Unscan-It program (Silk Scientific). Measurement of total antibody reactivity towards neural proteins in each sample was done by calculating the sum of gray-level intensities for all software-assigned and background-subtracted reactive bands. Total gray-level intensity for each specimen was corrected for 1) inconsistencies within each membrane (e.g., for variation in sample loading and efficiency of protein transfer) according to the gray-level intensity of the tubulin band for each lane, and 2) inconsistencies in experimental conditions between membranes (e.g., for variation in sample loading, efficiency of protein transfer, and autoradiography exposure time) according to the total gray-level intensity for the positive control on each membrane.
2.4.2. Immunohistochemistry
Immunohistochemical analysis was similar to previously described procedure (Alaedini et al., 2008). Formaldehyde-fixed and paraffin-embedded sections of human cerebral cortex and dorsal root ganglia (DRG), obtained at post mortem, were cut (10 µm thickness) and placed on slides. Sections were deparaffinized and rehydrated by sequential incubation in xylene, ethanol (100%, 90%, 80%, and 70%), and PBS. Antigen retrieval was done by incubation in 0.05% citraconic anhydride buffer (pH 6.0) for 20 min at 95 °C. Endogenous peroxidase was quenched with 1% H2O2. Tissue sections were blocked for 30 min with 15% sheep serum (Sigma-Aldrich) in PBS. Sections were then incubated for 1.5 h with 1:100 dilutions of representative serum specimens from each group in duplicate. HRP-conjugated goat anti-human IgG was used as secondary antibody. Tissues were washed and colorimetric detection was carried out using the metal-enhanced DAB (3,3’-diaminobenzidine) system (Pierce).
2.5. Cross-reactivity of anti-borrelia antibodies towards neural antigens
2.5.1. Affiinity-purification of antibodies
Anti-borrelia antibodies were obtained from the pooled serum IgG fraction of rabbits immunized with B. burgdorferi B31 strain (Virostat). The antibodies were affinity purified with a column coupled with B. burgdorferi proteins as follows. The affinity column was prepared using the AminoLink activated agarose gel bead support (Pierce). After packing the column with slurry, it was equilibrated with PBS, followed by the addition of 2 mL of a 0.9 mg/mL solution of desalted proteins from a B. burgdorferi B31 lysate (Meridian) and 200 µL of 1 M NaCNBH3 in 10 mM NaOH. The coupling reaction was allowed to continue while gently rotating the column (6 h, room temperature). Remaining reactive sites were blocked by incubation with 1 M Tris (pH 7.4). Affinity purification was initiated by the introduction of antibody solution into the column and continuous flow for 1 h. The column was washed and bound antibodies were eluted with 100 mM glycine buffer (pH 3.0). The eluted antibody fraction was neutralized with 1 M Tris (pH 7.5) and concentrated by centrifugal filtration.
2.5.2. Binding of anti-borrelia antibodies to neural antigens
The interaction of the anti-borrelia antibodies with neural proteins were characterized by Western blotting and immunohistochemistry. One- and two-dimensional electrophoresis was carried out on 40–80 µg aliquots of mouse brain lysate protein. The two-dimensional electrophoresis was based on previously described procedure (O'Farrell, 1975) in which isoelectric focusing was carried out in glass tube using pH 3.5–10 ampholines (GE Healthcare) and SDS slab gel electrophoresis was done for 4 h at 15 mA/gel. The proteins were transferred to nitrocellulose membrane. The membrane was blocked as before and then incubated with the prepared affinity-purified anti-borrelia antibody or with non-immunized rabbit serum IgG (Sigma-Aldrich) (0.5 µg/mL) for 1 h. The HRP-conjugated secondary antibody used was anti-rabbit IgG (Amersham). Detection of bound antibodies was as before. Immunohistochemical analysis was as was described above for human samples, but instead using the affinity-purified anti-borrelia antibodies or rabbit IgG at 0.01 mg/mL as primary antibody, and HRP-conjugated donkey anti-rabbit IgG (Amersham) as secondary antibody.
2.6. Data analysis
Group differences were analyzed by two-tailed Welch’s t-test (continuous data with unequal variances), and Chi-square test or Fisher’s exact test (nominal data). Calculated gray-level intensity data were normalized by square root transformation prior to statistical analysis. Adjustment for covariate effect was carried out by analysis of covariance (ANCOVA), using the general linear model. Differences with p<0.05 were considered to be significant.
3. Results
3.1. Total IgG
The mean total IgG concentration (± standard error of mean) for the PLS group (14.1 ± 0.35 mg/mL) was not significantly different from that of the post-Lyme healthy (13.0 ± 0.50 mg/mL), SLE (14.7 ± 0.67 mg/mL), and normal healthy (12.8 ± 0.68 mg/mL) groups.
3.2. Anti-borrelia antibodies
Serum samples from 54 of 83 PLS and 14 of 27 post-Lyme healthy individuals were found to be positive for IgG anti-borrelia antibodies by ELISA. None of the SLE and healthy control samples were positive for anti-borrelia antibodies.
3.3. Anti-neural antibodies
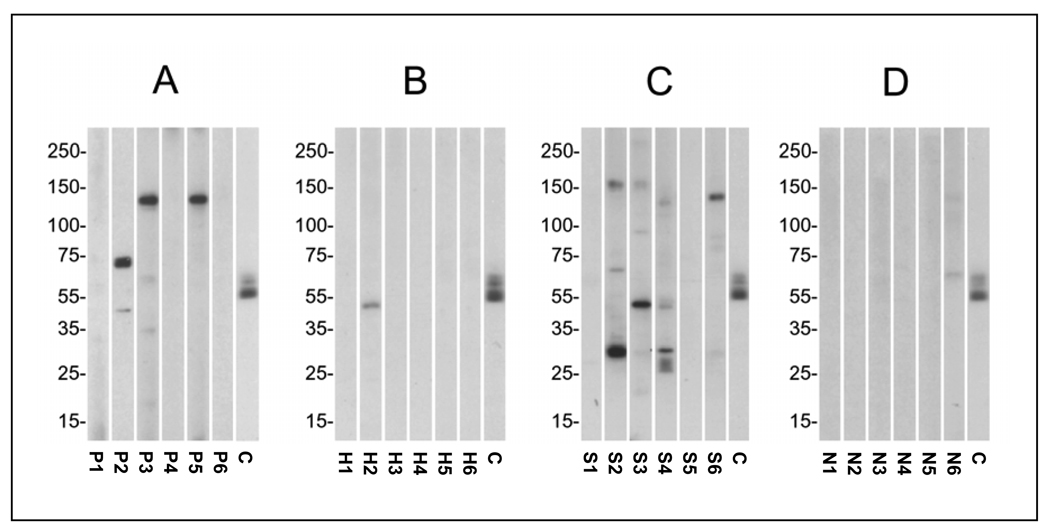
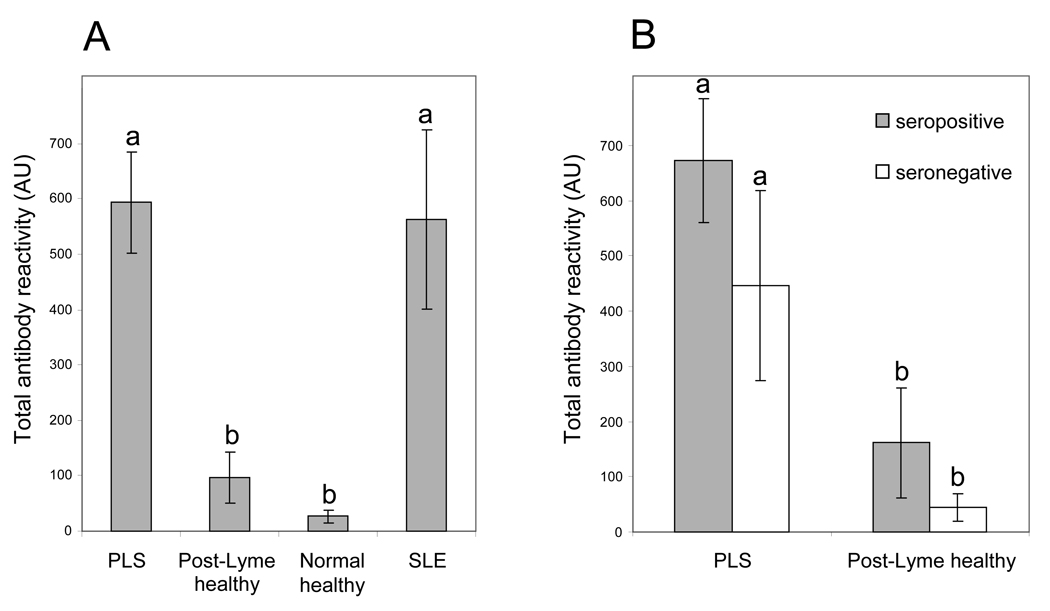
At the dilution and exposure used in this study, anti-neural antibody reactivity (as represented by the presence of one or more reactive protein bands on Western blots) was seen in serum specimens from 41 of 83 (49.4%) PLS patients, 5 of 27 (18.5%) post-Lyme healthy individuals, 11 of 15 (73.3%) patients with SLE, and 3 of 20 (15.0%) normal healthy subjects. A significantly higher number of PLS patients exhibited anti-neural antibody reactivity to one or more protein bands than post-Lyme healthy (p<0.01) and normal healthy (p <0.01) individuals. The anti-neural antibody reactivities in PLS and SLE patients were directed at multiple protein bands (Fig. 1). The mean number of reactive protein bands per specimen (± standard error of mean) for the PLS group (1.2 ± 0.16) was similar to that for the SLE group (1.6 ± 0.34), but significantly higher than the post-Lyme healthy (0.22 ± 0.10) (p<0.005) and normal healthy (0.10 ± 0.10) (p<0.005) groups. The differences in antibody reactivity were even more significant when taking into account both the number and intensity of bands (total antibody reactivity), measured as described in the methods section. The total antibody reactivity was significantly higher in the PLS group in comparison to the post-Lyme healthy (p<0.001) and normal healthy (p<0.001) groups (Fig. 2A). The difference between PLS and post-Lyme healthy groups remained significant, even after adjusting for differences in age, gender, and elapsed time since exposure to pathogen (p<0.001). The differences in the frequency and level of total antibody reactivity between PLS and SLE groups did not reach statistical significance.
Figure 1.
Pattern of antibody reactivity in the serum of representative PLS patients and control subjects towards electrophoresis-separated and transferred brain proteins. A) PLS patients P1-P6; B) post-Lyme healthy individuals H1-H6 (H1-H4 had presented with single EM, while H5 and H6 had presented with multiple EM); C) systemic lupus erythematosus patients S1-S6; D) normal healthy individuals N1-N6. Lane C in each panel is the positive control. Molecular weight markers are indicated to the left of each panel (kDa).
Figure 2.
Mean total anti-brain antibody reactivity in patient and control groups. A) Comparison between PLS patients, post-Lyme healthy subjects, normal healthy subjects without serologic evidence of prior Lyme disease, and patients with systemic lupus erythematosus. Reactivity was significantly higher in the PLS group than in post-Lyme healthy (p<0.001) and normal healthy (p<0.001) groups. B) Comparison between seropositive and seronegative patients in PLS and post-Lyme healthy groups. PLS seropositive and seronegative subgroups had significantly higher anti-brain antibody reactivity than their counterparts in the post-Lyme healthy group (p<0.005). The difference between PLS seropositive and seronegative patients did not reach statistical significance. Error bars represent the standard error of the mean. Groups indicated by different superscripts are significantly different from one another.
When considering only the borrelial seropositive subjects in the study, total anti-neural antibody reactivity was significantly higher in the PLS group than the post-Lyme healthy group (p<0.005) (Fig. 2B). Similarly, total anti-neural antibody reactivity was higher in the PLS seronegative group than the post-Lyme healthy seronegative group (p<0.005) (Fig. 2B). On the other hand, the difference in the anti-neural antibody reactivity between borrelial seropositive and seronegative patients in either the PLS group or the post-Lyme healthy group did not reach the level of significance (Fig. 2B).
One of 9 post-Lyme healthy subjects with multiple EM was positive for anti-neural antibodies (11.1%), a rate that was even lower (though not statistically significant) than that for those with single EM (4 of 18; 22.2%), indicating a lack of correlation between dissemination of B. burgdorferi infection and anti-neural antibodies in the post-Lyme healthy group.
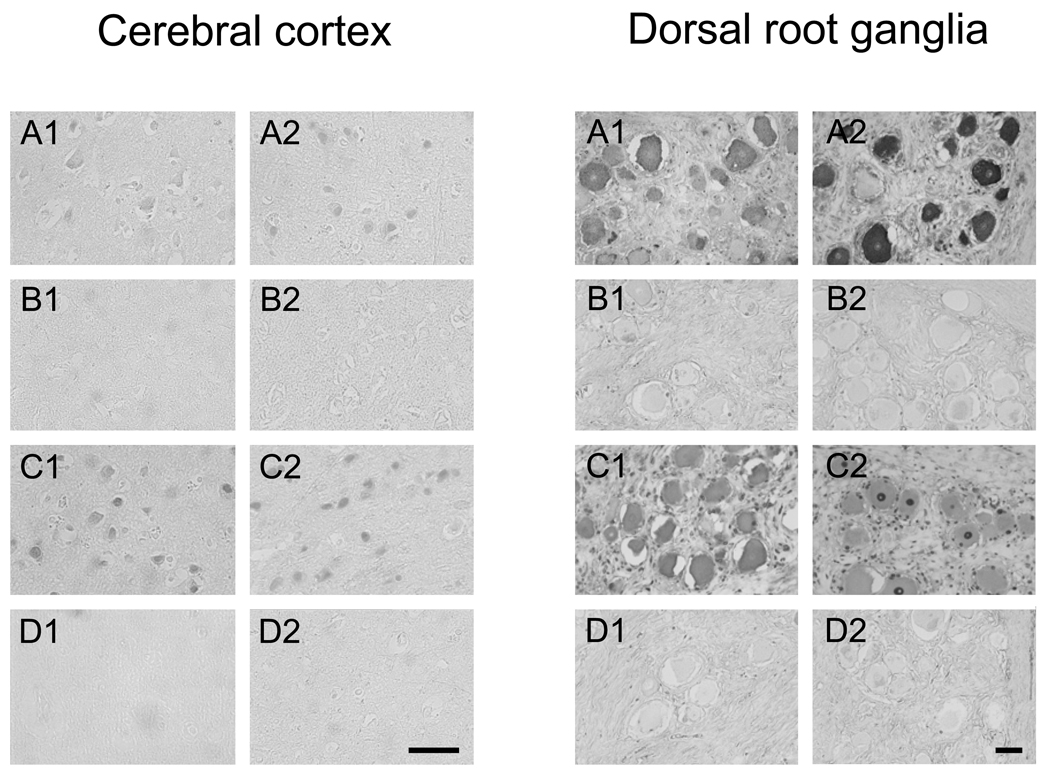
In order to ascertain the presence of antibodies against human central and peripheral nervous system tissue and assess target cell specificity, reactivity of serum antibodies from representative patients in each group was also analyzed by immunohistochemistry. Serum antibodies from PLS patients found to be positive for anti-neural antibody reactivity by immunoblotting (both borrelial seropositive and seronegative specimens) stained cortical pyramidal neurons, as well as neurons of the DRG (Fig. 3). Antibody binding to some glial cells of the brain and DRG was also observed. Patterns of staining varied for different PLS patients, with preferential binding to cell membrane seen in some cases. Serum specimens from borrelial seropositive and seronegative post-Lyme healthy individuals with anti-neural antibody reactivity showed faint or no binding of antibodies to neural tissues. Serum antibodies from control SLE patients with anti-neural antibody reactivity bound strongly to neurons and glial cells in the cerebral cortex and the DRG, with preferential staining of the nuclei in some cases. Sera from normal healthy subjects, however, did not stain tissues specifically.
Figure 3.
Immunohistochemical analysis of serum antibody reactivity towards cells in the brain cerebral cortex (left panel) and DRG (right panel). A) Staining of sections with serum from borrelial seropositive (A1) and borrelial seronegative (A2) patients with anti-neural antibody reactivity (as determined by immunoblotting) showed specific binding to neurons of the cerebral cortex and the DRG. B) Staining of sections with serum from borrelial seropositive (B1) and borrelial seronegative (B2) post-Lyme healthy individuals with anti-neural antibody reactivity showed faint or no specific binding of antibodies to cerebral cortex and DRG tissues. C) Serum antibodies from two representative SLE patients (C1 and C2) with anti-neural antibody reactivity bound strongly to neurons and glial cells in the cerebral cortex and the DRG. D) Sera from two normal healthy subjects (D1 and D2) did not stain tissues specifically. Bars = 50 µm.
3.4. Cross-reactivity of anti-borrelia antibodies toward neural proteins
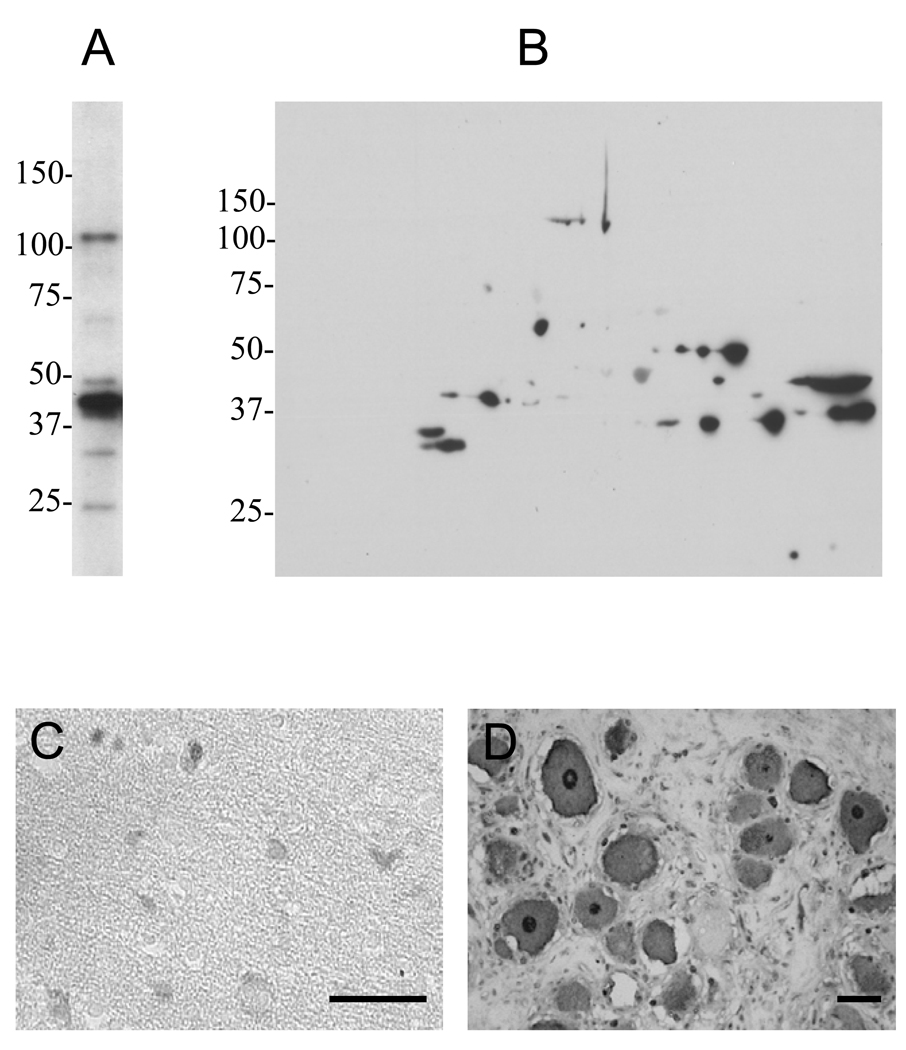
In order to assess the extent of cross-reactivity of the anti-borrelia antibodies towards brain proteins using our system of anti-neural antibody detection, we examined the binding of affinity-purified anti-borrelia antibodies to brain proteins by one- and two-dimensional immunoblotting. The purified antibodies bound to approximately 20 different protein bands (Fig. 4A), demonstrating the potential for substantial cross-reactivity of the anti-borrelia antibody response towards neural proteins. The cross-reactivity was confirmed by immunohistochemical analysis, which showed anti-borrelia antibody binding to neurons and glial cells of the cerebral cortex and the DRG (Fig. 4B).
Figure 4.
Cross-reactivity of the anti-borrelia immune response in immunized rabbits towards neural proteins. A, B) One- and two-dimensional immunoblots of mouse brain lysate with rabbit affinity-purified anti-borrelia antibody indicated cross-reactivity towards several neural proteins. Numbers to the left of each panel indicate molecular weight markers (kDa). C, D) Immunohistochemical analysis of the interaction of affinity-purified anti-borrelia antibodies with human cerebral cortex (C) and DRG (D) showed binding to neurons and glial cells. Bars = 50 µm.
Discussion
Much of the controversy surrounding PLS arises from the lack of sufficient knowledge about the etiology and pathology of the disease. This is compounded by the fact that there are few or no objective methods available for diagnosis and follow-up of affected individuals. In light of the results from the aforementioned clinical trials of antibiotic treatment and the lack of convincing evidence for active infection in PLS, other hypotheses, including a role for involvement of the immune system, have been suggested (Bolz and Weis, 2004; Marques, 2008). If present, immune abnormalities—possibly triggered by the original infection—may offer clues about the disease (Jarefors et al., 2007; Segal and Logigian, 2005). Considering the neurologic and psychiatric nature of post-Lyme symptoms, we sought to assess the presence of nervous system-specific antibodies in patients and control subjects. Approximately half of the examined PLS patients had heightened levels of antibodies to neural proteins, compared with 18.5% of post-Lyme healthy subjects and 15% of normal healthy controls. In fact, the heightened antibody response level in PLS was statistically similar to that in SLE, a multisystem autoimmune disease. Immunohistochemical analysis with representative PLS patient sera demonstrated binding of the antibodies to pyramidal neurons in the cerebral cortex and neurons of the DRG, highlighting their relevance in the context of central and peripheral nervous system disease.
It is important to note that our method of analysis only detected antibodies against prominently expressed proteins. Elevated antibodies to minor proteins or non-protein antigens might also exist in some cases that were reported to be negative. Therefore, examination of antibody binding to antigens in specific regions of the nervous system might reveal reactivity in more individuals. In addition, although this work focused on antibodies against neural proteins, antibodies to specific antigens in other tissues (e.g. muscle, thyroid, etc.) may also be found in some patients and could be relevant to PLS. At the same time, the absence of anti-neural antibodies in many patients might provide evidence for the heterogeneous nature of the population under study.
We can make some conjectures about the possible reasons for the observed increased antibody reactivity to self antigens in PLS. First, our experiments with affinity-purified antibodies generated in rabbits against B. burgdorferi antigens clearly show that anti-borrelia antibodies can cross-react with several neural proteins. A number of earlier studies have also demonstrated the potential for cross-reactivity of the anti-borrelia immune response towards neural antigens (Alaedini and Latov, 2005; Dai et al., 1993; Garcia-Monco et al., 1995; Maier et al., 2000; Sigal and Tatum, 1988). A portion of the detected anti-neural antibody reactivity in PLS patients is, therefore, likely to be the result of such cross-reactivity. However, the observed anti-neural antibody reactivity cannot be attributed solely to positive anti-borrelia serology, as increased anti-neural antibody reactivity was also seen in the borrelial seronegative PLS group. Second, considering the non-specific pattern of immunologic reactivity, the presence of these antibodies might signify an activated immunologic response to neural injury caused by the original borrelial infection or another disease. Tissue injury can, in fact, result in the release of autoantigens and lead to an increase in post-translational modification of proteins and production of novel self-epitopes that elicit a strong immune response (Doyle and Mamula, 2005). Third, borrelial infection has been shown to be a potent polyclonal B cell activator, capable of inducing the non-specific proliferation and differentiation of antibody-secreting cells (Ma and Weis, 1993; Yang et al., 1992). The ability of borrelia to act as a B cell activator is likely to be enhanced the longer the infection is left untreated (Soulas et al., 2005). Therefore, the observed non-specific increase in autoreactive antibodies in PLS may be due to the mitogenic effect of the borrelial antigens, including OspA and OspB, and point to a possible association between post-Lyme disease symptoms and the duration of the course of active infection prior to treatment. Finally, immune abnormalities stemming from genetic predisposition might also play a significant role in the form of B cell and effector cell dysregulation that leads to elevated levels of released autoantibodies (Hostmann et al., 2008).
At this point, it is difficult to know what role, if any, the anti-neural antibodies might play in the pathogenesis of PLS. Several immune-mediated diseases of the nervous system, including multiple sclerosis, paraneoplastic nervous system disorders, autoimmune neuropathies, myasthenia gravis, and stiff-person syndrome, are associated with elevated levels of antibodies to neural antigens. A disease-causing role for such antibodies has been demonstrated in some of these disorders (Dalakas, 2008). In general, antibodies might have a pathogenic effect in the body through direct binding to a molecule and interference with its function, by activation of complement and initiation of an inflammatory response, or by inducing tissue injury through binding to Fc receptors on macrophages, neutrophils, and NK cells (Diamond et al., 2009). Considering the non-specific antibody response seen in the examined PLS cohort, however, a direct pathogenic role for the antibodies is doubtful. Nevertheless, even without a direct role, antibodies have the potential to be involved in disease mechanism through the activation of toll-like receptor pathways and secretion of various inflammatory molecules, which can affect the function of other cells responsible for neuropsychiatric defects (Crow, 2007; Halperin, 2008; Nawa and Takei, 2006).
The aim of this study was to begin a process of examining potential immune abnormalities in PLS that would be relevant to the reported neurologic and cognitive symptoms of affected patients. Results of the antibody analysis demonstrate the presence of a heightened, but apparently non-specific, production of antibodies to neural antigens in PLS. We speculate that these antibodies may either 1) be indicative of past injury to the nervous system during the active phase of the Lyme disease infection, resulting in the immune system being exposed to and activated by novel self antigens, or 2) point to the enhanced B cell mitogenic effect of the borrelia pathogen in cases of delayed treatment and prolonged infection in genetically predisposed individuals. As such, this study points to the presence of a differential immune response in PLS in comparison to healthy individuals. Obviously, these findings are preliminary and must be extended in future studies using a larger number of subjects and additional cohorts, including healthy individuals with past Lyme arthritis and neurologic Lyme, as well as patients with similar complaints and no history of Lyme disease. At this juncture, it is logical to assume that further study of immune system response in PLS is likely to yield more clues about the etiopathogenesis of the disease and provide insights that may pave the way for developing safe and effective treatments.
Acknowledgment
This work was supported by the National Institutes of Health (NIH) [grant number AI071180-02 to A. Alaedini] and involved the use of specimens derived from an NIH-supported repository [contract number N01-AI-65308]. We are indebted to Dr. Phillip J. Baker at the National Institutes of Health for his invaluable support and guidance. We thank the New York Brain Bank for the human neural tissues [5 P50 AG08702-15 and NS 16367-24] and the HSS Core Center for Musculoskeletal Repair and Regeneration for access to microscopy facilities [NIH AR 46121]. We would also like to thank Ms. Diane Holmgren, Ms. Donna McKenna, and Ms. Susan Bittker for their assistance with specimen collection and organization. We are grateful to all of the research participants involved in this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
All authors declare that there are no conflicts of interest.
References
- Alaedini A, Latov N. Antibodies against OspA epitopes of Borrelia burgdorferi cross-react with neural tissue. J Neuroimmunol. 2005;159:192–195. doi: 10.1016/j.jneuroim.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Alaedini A, Okamoto H, Briani C, et al. Immune cross-reactivity in celiac disease: anti-gliadin antibodies bind to neuronal synapsin I. J Immunol. 2007;178:6590–6595. doi: 10.4049/jimmunol.178.10.6590. [DOI] [PubMed] [Google Scholar]
- Alaedini A, Xiang Z, Kim H, Sung YJ, Latov N. Up-regulation of apoptosis and regeneration genes in the dorsal root ganglia during cisplatin treatment. Exp Neurol. 2008;210:368–374. doi: 10.1016/j.expneurol.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Baker PJ. Perspectives on "chronic Lyme disease". Am J Med. 2008;121:562–564. doi: 10.1016/j.amjmed.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Bolz DD, Weis JJ. Molecular mimicry to Borrelia burgdorferi: pathway to autoimmunity? Autoimmunity. 2004;37:387–392. doi: 10.1080/08916930410001713098. [DOI] [PubMed] [Google Scholar]
- Crow MK. Type I interferon in systemic lupus erythematosus. In: Pitha PM, editor. CTMI. Vol. 316. Berlin Heidelberg: Springer; 2007. pp. 359–386. [DOI] [PubMed] [Google Scholar]
- Dai Z, Lackland H, Stein S, et al. Molecular mimicry in Lyme disease: monoclonal antibody H9724 to B. burgdorferi flagellin specifically detects chaperonin-HSP60. Biochim Biophys Acta. 1993;1181:97–100. doi: 10.1016/0925-4439(93)90096-j. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. B cells as therapeutic targets in autoimmune neurological disorders. Nat Clin Pract Neurol. 2008;4:557–567. doi: 10.1038/ncpneuro0901. [DOI] [PubMed] [Google Scholar]
- Diamond B, Huerta PT, Mina-Osorio P, Kowal C, Volpe BT. Losing your nerves? Maybe it's the antibodies. Nat. Rev. Immunol. 2009;9:449–456. doi: 10.1038/nri2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle HA, Mamula MJ. Posttranslational modifications of self-antigens. Ann N Y Acad Sci. 2005;1050:1–9. doi: 10.1196/annals.1313.001. [DOI] [PubMed] [Google Scholar]
- Fallon BA, Keilp JG, Corbera KM, et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology. 2008;70:992–1003. doi: 10.1212/01.WNL.0000284604.61160.2d. [DOI] [PubMed] [Google Scholar]
- Feder HM, Jr, Johnson BJ, O'Connell S, et al. A critical appraisal of "chronic Lyme disease". N Engl J Med. 2007;357:1422–1430. doi: 10.1056/NEJMra072023. [DOI] [PubMed] [Google Scholar]
- Garcia-Monco JC, Seidman RJ, Benach JL. Experimental immunization with Borrelia burgdorferi induces development of antibodies to gangliosides. Infect Immun. 1995;63:4130–4137. doi: 10.1128/iai.63.10.4130-4137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JJ. Nervous system Lyme disease. Infect Dis Clin North Am. 2008;22:261–274. doi: 10.1016/j.idc.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Hostmann A, Jacobi AM, Mei H, Hiepe F, Dorner T. Peripheral B cell abnormalities and disease activity in systemic lupus erythematosus. Lupus. 2008;17:1064–1069. doi: 10.1177/0961203308095138. [DOI] [PubMed] [Google Scholar]
- Jarefors S, Janefjord CK, Forsberg P, Jenmalm MC, Ekerfelt C. Decreased up-regulation of the interleukin-12Rbeta2-chain and interferon-gamma secretion and increased number of forkhead box P3-expressing cells in patients with a history of chronic Lyme borreliosis compared with asymptomatic Borrelia-exposed individuals. Clin Exp Immunol. 2007;147:18–27. doi: 10.1111/j.1365-2249.2006.03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempner MS, Hu LT, Evans J, et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. 2001;345:85–92. doi: 10.1056/NEJM200107123450202. [DOI] [PubMed] [Google Scholar]
- Krupp LB, Hyman LG, Grimson R, et al. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology. 2003;60:1923–1930. doi: 10.1212/01.wnl.0000071227.23769.9e. [DOI] [PubMed] [Google Scholar]
- Ma Y, Weis JJ. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect Immun. 1993;61:3843–3853. doi: 10.1128/iai.61.9.3843-3853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier B, Molinger M, Cope AP, et al. Multiple cross-reactive self-ligands for Borrelia burgdorferi-specific HLA-DR4-restricted T cells. Eur J Immunol. 2000;30:448–457. doi: 10.1002/1521-4141(200002)30:2<448::AID-IMMU448>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Marques A. Chronic Lyme disease: a review. Infect Dis Clin North Am. 2008;22:341–360. doi: 10.1016/j.idc.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Saito I, Hayashi Y, et al. Molecular analysis of the human autoantibody response to alpha-fodrin in Sjogren's syndrome reveals novel apoptosis-induced specificity. Am J Pathol. 2004;165:53–61. doi: 10.1016/s0002-9440(10)63274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawa H, Takei N. Recent progress in animal modeling of immune inflammatory processes in schizophrenia: implication of specific cytokines. Neurosci Res. 2006;56:2–13. doi: 10.1016/j.neures.2006.06.002. [DOI] [PubMed] [Google Scholar]
- O'Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Segal BM, Logigian EL. Sublime diagnosis of Lyme neuroborreliosis. Neurology. 2005;65:351–352. doi: 10.1212/01.wnl.0000174140.95685.11. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y, Nahum A, Korczyn AD, et al. Neuronal-binding antibodies from patients with antiphospholipid syndrome induce cognitive deficits following intrathecal passive transfer. Lupus. 2003;12:436–442. doi: 10.1191/0961203303lu409oa. [DOI] [PubMed] [Google Scholar]
- Sigal LH. Lyme disease: a review of aspects of its immunology and immunopathogenesis. Annu Rev Immunol. 1997;15:63–92. doi: 10.1146/annurev.immunol.15.1.63. [DOI] [PubMed] [Google Scholar]
- Sigal LH, Tatum AH. Lyme disease patients' serum contains IgM antibodies to Borrelia burgdorferi that cross-react with neuronal antigens. Neurology. 1988;38:1439–1442. doi: 10.1212/wnl.38.9.1439. [DOI] [PubMed] [Google Scholar]
- Soulas P, Woods A, Jaulhac B, et al. Autoantigen, innate immunity, and T cells cooperate to break B cell tolerance during bacterial infection. J Clin Invest. 2005;115:2257–2267. doi: 10.1172/JCI24646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C. Has the yo-yo stopped? An assessment of human protein-coding gene number. Proteomics. 2004;4:1712–1726. doi: 10.1002/pmic.200300700. [DOI] [PubMed] [Google Scholar]
- Stanek G, Strle F. Lyme borreliosis. Lancet. 2003;362:1639–1647. doi: 10.1016/S0140-6736(03)14798-8. [DOI] [PubMed] [Google Scholar]
- Stanek G, Strle F. Lyme disease: European perspective. Infect Dis Clin North Am. 2008;22:327–339. doi: 10.1016/j.idc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Steere AC. Lyme disease. N Engl J Med. 2001;345:115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Tin SK, Xu Q, Thumboo J, Lee LY, Tse C, Fong KY. Novel brain reactive autoantibodies: prevalence in systemic lupus erythematosus and association with psychoses and seizures. J Neuroimmunol. 2005;169:153–160. doi: 10.1016/j.jneuroim.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- Yang L, Ma Y, Schoenfeld R, et al. Evidence for B-lymphocyte mitogen activity in Borrelia burgdorferi-infected mice. Infect Immun. 1992;60:3033–3041. doi: 10.1128/iai.60.8.3033-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]