Abstract
Glioblastoma is the most common and lethal type of primary brain tumor. Despite recent therapeutic advances in other cancers, the treatment of glioblastomas remains ineffective and essentially palliative. The treatment failure is a result of a number of causes, but we and others have demonstrated that a highly tumorigenic subpopulation of cancer cells called glioblastoma stem cells (GSCs) display relative resistance to radiation and chemotherapy. GSCs also contribute to tumor growth through the stimulation of angiogenesis, which has been shown to be a useful therapeutic target in the treatment of recurrent or progressive malignant gliomas. Cancer stem cells also have been hypothesized as a contributor to systemic metastases. While glioblastomas rarely metastasize beyond the central nervous system, glioblastomas invade into brain structures to prevent surgical cure and GSCs have an extremely invasive phenotype. Collectively, these studies and others suggest that GSCs may be important therapeutic targets not only to achieve cure but even reduce tumor relapse and improve overall survival. Many recent studies suggest that GSCs share core regulatory pathways with normal embryonic and somatic stem cells, but display important distinctions that provide clues into useful treatment targets. The cancer stem cell hypothesis may also modify our approaches in tumor imaging and biomarker development, but clinical validation waits. In this review, we summarize the current understanding of GSC biology with a focus on potential anti-GSC therapies.
Keywords: Cancer stem cells; Glioblastoma; Molecular targeting; Tumor angiogenesis, Therapeutic resistance; Hypoxia response
1. Introduction
Glioblastomas (GBMs, World Health Organization grade IV gliomas) are the most common type of primary brain tumors in adults. GBMs are among the most lethal and least successfully treated solid tumors [1]. Median survival of GBM patients treated with aggressive multimodal therapy, including maximal surgical resection, combined radiation and chemotherapy, and adjuvant chemotherapy is only 12 - 15 months [2]. Compared to the advances in the treatment of other types of tumors, the poor prognosis for GBM patients has improved minimally over decades, underscoring the challenges and difficulties in effectively detecting and treating these fatal cancers. Metastatic spread is responsible for deaths in many cancer patients, but GBMs rarely metastasize out of the central nervous system. In contrast, GBMs are highly infiltrative into the brain and spinal cord preventing surgical cure even with heroic resections. Invading tumor cells appear to be particularly resistant to cytotoxic therapy and are often protected by an intact neurovascular unit [1]. Additionally the majority of patients suffer primary treatment failure within 2-3 cm of the original resection cavity suggesting that therapeutic resistance is a common feature of the entire tumor. Collectively, these difficulties have propelled the refinement of therapy to achieve maximal efficacy with minimized toxicities. Improved intra-operative imaging and advanced surgical techniques have increased the ability to remove tumors safely as extent of surgical resection may be associated with increased patient survival [3]. After resection, patients undergo focused radiation at the primary tumor site instead of whole brain radiotherapy. Novel radiation technologies, such as intensity-modulated radiation therapy (IMRT) and proton therapy, permit delivery of higher radiation doses to tumor-bearing brain while relatively sparing normal brain. While chemotherapy has been used for decades in neuro-oncology, the oral methylator temozolomide (TMZ) has shown benefit when used concurrently with radiation and then as adjuvant chemotherapy such that it is now standard practice [2]. Many molecularly targeted therapies have been investigated in trials of malignant gliomas (glioblastomas and anaplastic gliomas) but to date only bevacizumab (Avastin) has been approved by the FDA, unfortunately lacking a definitive impact on survival [4-6]. Immunotherapies and toxin-ligand conjugates have shown promise in early trials but so far lack definitive efficacy in larger (phase III) trials. Transforming glioblastoma into a treatable entity will require new paradigms in cancer biology and the mechanisms underlying the GBM invasion, resistance and recurrence.
Most solid tumors consist of heterogeneous cancer cells, as well as recruited vasculature, inflammatory cells and stromal elements [7]. As the name indicates, glioblastoma multiforme displays striking intratumoral heterogeneity not only morphologically but also in differentiation status. Tumor heterogeneity may be derived from both genetic and non-genetic/epigenetic causes. Growing evidence from hematopoietic malignancies and solid tumors (including breast, brain, head and neck and colorectal cancers) has supported the hypothesis that a subpopulation of cancer cells in each malignancy has greater potential of tumor initiation and repopulation [8-19]. The nomenclature of these cells has been highly controversial due to the lack of definitive criteria (it is worth noting that we lack absolute definitions for somatic stem cells as well). The term tumor-initiating cell is commonly used but the evidence that these are the cells at tumor initiation is unclear. Tumor propagating cells has been proposed [20] as the defining assays involve tumor propagation into secondary hosts. Cancer stem cell or stem cell-like cancer cell terminology is imperfect due to distinct differences from stem cells and frequent confusion over a stem cell cell-of-origin but does capture the shared characteristics with normal stem cells especially somatic stem cells, including the capacities for self-renewal, differentiation, and maintained proliferation. Based on these concerns, we employ the term cancer stem cell with a firm recognition of the challenges of the name.
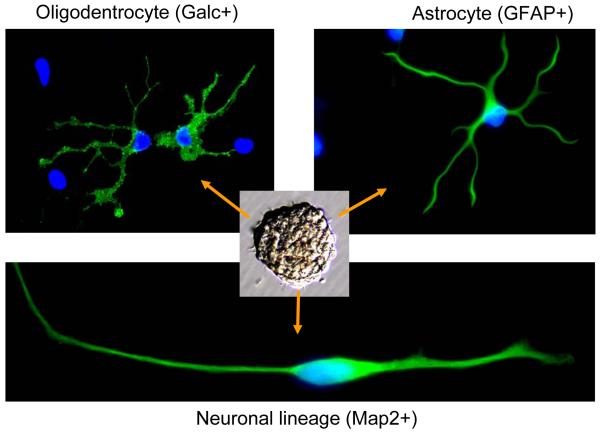
Glioma stem cells (GSCs) have been described by several groups [10, 11, 17, 18]. These cells are functionally defined with self-renewal measured by serial neurosphere assay and tumor propagation by in vivo intracranial limiting dilution assays. Although cancer stem cells need not recapitulate normal differentiation cascades and commonly display aberrant differentiation signatures with multiple lineage markers, GSCs have been shown to differentiate into astrocytes, oligodendrocytes and neurons [10, 11, 17, 18, 21] (Fig. 1). Studies from a number of groups including ours have demonstrated that GSCs display much greater tumorigenic potential than matched non-stem tumor cells when xenotransplanted into brains of immunocompromised rodents [10, 11, 17, 18, 21]. Although the origin of GSCs is not defined, GSCs share developmental programs with normal neural stem cells (NSCs) that endow these cells with key traits in carcinogenesis. Finally, we posit that the definition of a cancer stem cell will likely encompass a broader spectrum of behaviors in parallel to those of somatic stem cells, including the ability to modulate immune responses, interact and support the vasculature, and disperse into new locations.
Fig. 1.
Glioma stem cells display multiple-lineage differentiation potential. Immunofluorescent staining demonstrated that glioblastoma stem cells derived from a tumor specimen (T3359) formed neurospheres and differentiated into cells expressing markers for astrocytes (GFAP+), oligodentrocytes (Galc+) and neuron (Map2+) lineages upon induction of differentiation.
GSCs have been implicated in several malignant behaviors. We found that GSCs express elevated levels of vascular endothelial growth factor (VEGF) to promote tumor angiogenesis [22]. This finding has been confirmed by others with an additional determination that stromal-derived factor-1 (SDF-1, CXCL12) is another pro-angiogenic ligand expressed by GSCs [23, 24]. This is of particular significance as bevacizumab (Avastin), a humanized neutralizing anti-VEGF antibody, has demonstrated activity against GBMs and was recently approved by the United States Food and Drug Administration for the treatment of recurrent or progressive GBMs [4-6, 25]. We also demonstrated that GSCs are relatively resistant to radiation due to preferential activation of the DNA damage checkpoint and lesion repair [21], while other groups have described relative resistance of GSCs to chemotherapies [26-28]. These studies may explain in part how even patients with a promising radiographic response universally suffer recurrence and/or progression of their cancers. Therefore, direct targeting of GSCs may improve the efficacy of conventional cytotoxic therapies as well as anti-angiogenic therapies. In this review, we summarize the roles of GSCs in the malignant behavior of these cancers with attention to signaling pathways involved in GSC maintenance and growth, and discuss molecular strategies for development of novel therapeutics targeting GSCs.
2. Targeting vascular niche of glioma stem cells
Florid angiogenesis is one of hallmarks of malignant gliomas [1]. Tumor growth is limited by constraints of the supportive vasculature to feed the tumor and remove waste products. Initial tumor growth occurs through vessel cooption but eventually neoangiogenesis is required although the vessels formed are abnormal and often inefficient. The degree of vascularization is significantly correlated with the glioma malignancy, tumor aggressiveness, and clinical prognosis [29]. Based on this background, a number of laboratories have aggressively investigated the relationship between the tumor vasculature and GSCs. Characterizing in vitro and in vivo growth of GSCs in relation to matched non-stem glioma cells, we determined that GSCs formed tumors with greater vascularity than the non-stem tumor cells [22]. The mechanism of this hypervascularity appeared in part due to a secreted factor from GSCs induced microvascular endothelial cell migration and vessel formation. We examined the conditioned media for expression of a series of angiogenesis regulators and noted that markedly higher levels of VEGF were the most consistent findings. Targeting VEGF effects through bevacizumab specifically blocked GSC pro-angiogenic effects both in vitro and in vivo. Using the C6 cell line, Folkins and co-workers showed that GSCs promote both tumor angiogenesis and vasculogenesis via VEGF and SDF-1 [24]. To understand the upstream mechanisms that drive VEGF expression in GSCs, we interrogated the role of hypoxia and the hypoxia inducible factors (HIFs). As expected, hypoxia treatment induced VEGF expression in both GSCs and non-stem glioma cells but the levels were consistently higher in GSCs [22, 30]. Interestingly, HIF-1α and HIF-2α specifically controlled VEGF expression in GSCs in a non-redundant manner. Hypoxia can also expand the GSC fraction and regulate stem cell marker expression [31-33]. Therefore, hypoxia may function in tumor progression and therapeutic resistance through its promotion of a cancer stem cell phenotype.
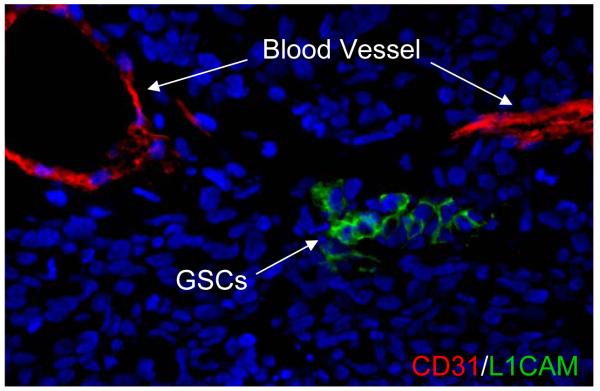
The relationship between GSCs and the vasculature is complex and bi-directional. Normal neural stem cells (NSCs) reside in perivascular locations that provide essential pro-survival and maintenance cues [34-36]. In a seminal study, Gilbertson and co-workers demonstrated that brain tumor cells that express stem cell markers reside in a perivascular niche [23]. They further showed that endothelial cells increase brain tumor stem cell survival and targeting the tumor vasculature with bevacizumab reduces the number of cancer stem cells in treated tumors. They also found that co-transplantation of GSCs with endothelial cells accelerates tumor initiation and progression [23]. Another group showed that a metronomic chemotherapy regimen decreased the growth of sphere forming C6 brain tumor cells [37]. We have observed that glioma cells bearing cancer stem cell markers, CD133, HIF2α or L1CAM (CD171), are localized near blood vessels [21, 22, 30] (Fig. 2). Thus, the symbiotic relationship between GSCs and vasculatures may explain the promising efficacy of anti-angiogenesis therapy with bevacizumab [4-6, 25] or cediranib (AZD2171, a VEGFR inhibitor) for GBM patients in the clinical trials [38]. Thus, anti-angiogenic therapy may function as an anti-GSCs therapy. A further nuance has come from early studies that suggest that glioblastoma cells can form parts of the tumor vasculature [39]. It is likely that anti-angiogenic drugs might not only inhibit tumor vascularization to suppress GBM growth, but also directly disrupt the niches for the maintenance of GSCs, therefore weakening the “tumor roots”.
Fig. 2.
Glioma stem cells are localized in vascular niches. The frozen section of GBM tumor was immunostained with anti-L1CAM antibody for glioblastoma stem cells (shown in green) and anti-CD31 antibody for endothelial cells (shown in red), and counterstained with DAPI for DNA (blue). L1CAM-positive cells (green) are located near the blood vessels (CD31+, red).
3. Targeting therapeutic resistance of glioma stem cells
Glioblastoma remains to be one of the most fatal cancers despite optimal therapies. These diffusely infiltrative tumors are highly resistant to conventional radiotherapy and chemotherapy, and often recur in a local fashion despite maximal surgical resection [1]. Studies from our group and others have demonstrated that GSCs promote the therapeutic resistance and likely are responsible for the relapse of GBM [21, 27, 28]. The presence of a subpopulation of cancer cells within GBM possibly responsible for generating the entire mass of cancer cells has important implications for the understanding of the efficacy of current therapies and the resistance issue. It should be noted that the hierarchical relationship between the stem-like population and bulk tumor remains controversial [40], but it is possible that cancer stem cells may contribute to tumor repopulation after therapy. Radiation is the most effective non-surgical therapy for GBMs but is palliative suggesting that tumors contain resistant populations. Indeed, we found that GSCs are more resistant to radiation than the matched non-stem glioma cells [21]. In response to radiation-induced DNA damage, GSCs preferentially activate several critical checkpoint proteins (ATM, Rad17, Chk2 and Chk1). As a result of the preferential DNA damage checkpoint activation, GSCs are more efficient in repairing the damaged DNA and more rapidly recover from the DNA damage than the matched non-stem tumor cells. Thus, GSCs are more resistant to radiation-induced apoptosis than the non-stem tumor cells in vitro and in vivo. Further, a low molecular weight inhibitor (Debromohymenialdisine, DBH) of Chk2 and Chk1 checkpoint kinases abolished the radioresistance of GSCs, suggesting that targeting the DNA damage checkpoint response may sensitize GSCs to radiotherapy and thus overcome the radioresistance of GBM during treatment. Although inhibitors of checkpoint activation may be used as radiosensitizers of GSCs, consideration of the effects of the therapy on normal stem cells must also be considered, as inhibition of checkpoint activation in normal cells may lead to oncogenesis. Recently, we also showed that inhibition of the Notch pathway by the γ-secretase inhibitor or Notch shRNA renders GSCs more sensitive to radiation [41], suggesting that Notch pathway may serve as another potential therapeutic target for reducing GBM radioresistance. Additional recent studies suggest that targeting SirT1 expression or HSP90 activity can also attenuate GSC radioresistance [42, 43]. It is likely that multiple mechanisms regulate cancer stem cell radioresistance, perhaps with intertumoral variation. In breast cancer stem cells, decreased radiosensitivity may be due to lower levels reactive oxygen species (ROS) [44] or activity of Wnt/β-catenin signaling [45]. To date these mechanisms have not been described in GSCs, but they give hope that several druggable targets may be targeted in GSCs to overcome radioresistance.
Besides radiotherapy, the current standard of care for newly diagnosed GBM includes adjuvant chemotherapy with temozolomide (TMZ), an oral methylating chemotherapeutic agent [2]. TMZ induces DNA alterations at several locations but achieves significant cytotoxic effect by methylating the O6 position of guanine in DNA. This DNA adduct can be removed by the repair enzyme O6-methylguanine-DNA-methyltransferase (MGMT) that is expressed in graded levels in GBM. MGMT expression is likely regulated at several levels but recent attention has focused on its promoter methylation that has been linked to reduced MGMT levels and greater sensitivity to TMZ treatment [46]. The addition of TMZ to GBM therapy has been potentially most effective by a radiosensitization effect [2], but TMZ is commonly used as adjuvant therapy as well. Although therapy with TMZ may slow GBM tumor growth and increases the proportion of patients surviving for two years, long-term survivors are still rare due to drug resistance and GBM recurrence. Invariable tumor recurrence after TMZ therapy indicates the presence of TMZ-resistant cancer cells in GBM. Recently, it was shown that GSCs also contribute to the chemoresistance to TMZ [26-28]. In a genetically engineered glioma mouse model, TMZ treatment increased the side population (SP), a potential measure of cancer stem cells [47] and TMZ does not inhibit GSC self-renewal [48], although it has been shown that TMZ can eliminate MGMT-negative GSCs [49]. Several other potential mechanisms of GSC drug resistance have also been reported. Increased expression of drug transporters that pump out chemotherapeutic agents may be one of critical mechanisms, including the ABC (ATP-binding cassette) drug transporters. Some studies suggest that ABC expression may enrich for cancer stem cells [16]. A side population (SP) of cancer cells isolated from tumors may represent a class of cancer stem cells with high drug efflux capacity and thus show inherently high resistance to chemotherapeutic agents [50]. SP cells express high levels of the ABC drug transporters such as ABCG2 and ABCA3 in GBM cell lines [50], suggesting that targeting these drug transporters may reduce drug resistance of GSCs. In addition, several studies have shown that the poly(ADP-ribose) polymerase-1 (PARP-1) inhibitors such as CEP-8983 and E7016 can increase sensitivity of chemoresistant GBM tumor cells to TMZ and radiation [51-53]. PARP-1 plays a critical role in DNA repair, particularly in the base excision repair of DNA strand breaks caused by ionizing radiation or DNA lesions induced by methylating agents such as TMZ [54-57]. In vivo studies have demonstrated PARP-1 inhibitors enhance the anti-tumor effects of radiation or chemotherapy and sensitize glioma cells to TMZ treatment [23, 53]. As GSCs display preferential DNA repair capacity, it is worth of testing whether PARP-1 inhibitors can be used to overcome the radio- or chemo-resistance of GSCs.
4. Targeting glioma stem cells through specific cell surface molecules
Cell surface molecules differentially expressed in GSCs and functionally associated with the maintenance of GSC may be ideal targets. Several molecules, including CD133 [17, 18], CD15 [58-60], L1CAM [61], A2B5 [62, 63], have been identified on cell surface of brain tumor stem cells. Although CD133 (prominin-1) has been widely used as a marker for enrichment of GSC population from GBM tumors or xenografts, many normal cells express CD133 potentially limiting its utility as a target and the reliability of CD133 to discriminate GSCs is not absolute [64]. CD15 (stage-specific embryonic antigen-1, SSEA-1; Lewis X antigen) originally identified as a marker of mouse embryonic stem cells [65, 66] has recently been used as an alternative marker to enrich GSCs from some GBM tumors (and medulloblastoma stem cells as well) in which CD133 marker is not a informative maker for GSC population [58-60]. But whether CD15 can be used as a target for GSCs is not clear because CD15 is a carbohydrate antigen rather than a distinct protein target, expressed in the normal brain including normal neural and progenitor cells [67], and the function of CD15 in normal stem cells and cancer stem cells remains poorly understood. Other surface markers such as A2B5 have been used for the enrichment of GSC population [62, 63], but whether these surface markers can be used for targeting GSCs need further investigations.
In the search for specific functional surface targets for GSCs, we have identified L1CAM as a cell surface molecule that is differentially expressed in GSCs and plays critical roles in the maintenance, survival and functions of GSCs [61]. L1CAM was originally identified as a neural cell adhesion molecule in the nervous system and plays important roles in the development of nervous system [68]. This glycoprotein contains a cytoplasmic tail, a transmembrane domain and an extracellular domain that can interact with another L1CAM molecule through homophilic interaction, or binds to EGFR and FGFR, α5β1 and αvβ3 integrins, Neuropilin-1, and a number of extracellular matrix proteins through heterophilic interaction (reviewed in [69]). L1CAM mediated intra- and inter-cellular signaling plays important roles in regulating cell adhesion, migration, survival, growth and cancer cell invasion. We have found that L1CAM is highly expressed in GSCs relative to non-stem GBM tumor cells and normal neural progenitor cells [61]. Knockdown of L1CAM using specific shRNA specifically disrupts neurosphere formation and growth of GSCs in vitro. Targeting L1CAM in GSCs remarkably suppressed the tumor growth and increased the survival of mice bearing intracranial GBM xenografts [61]. We have determined that the molecular mechanism by which L1CAM promotes GSC maintenance and tumor growth is through up-regulation of Olig2 to suppress expression of p21WAF1/CIP1. Our data indicate that L1CAM as a functional surface molecule may represent a novel target for development of anti-GSC specific therapeutics.
5. Targeting glioma stem cells through blocking specific signaling pathways
Although the therapeutic targeting of cancer stem cells has generated excitement [70], the understanding on the molecular signaling of cancer stem cells is in early development. While cancer stem cells share some properties with normal somatic stem or progenitor cells, they are distinct from the normal stem cells at genetic and molecular signaling levels. The identification of specific signaling pathways involved in the maintenance and functions of GSCs may be useful to develop novel strategies to improve GBM treatment. A number of singling pathways associated with cancer stem cell maintenance have been reported [70]. Here we discuss a few of the critical signaling transduction pathways mediated from external signals to nucleus in GSCs.
5.1. RTK-Akt signaling
Receptor Tyrosine Kinases (RTKs) transduce signaling of multiple oncogenic growth factors, including the epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) that are used in culturing GSCs [71]. Among these RTK pathways, the EGFR-mediated growth signaling through phosphoinositide 3-kinase (PI3K)/Akt is one of the most important and best characterized pathways in gliomas. Malignant gliomas, particularly GBMs, frequently display EGFR amplification and/or expression of the constitutively active variant EGFRvIII that leads to increased EGFR-Akt signaling in cancer cells [72, 73]. Overexpression of EGFRvIII in genetically engineered models induces glioma-like tumors [74, 75]. It is not then surprising that EGFR activity is required for maintenance of GSCs as EGFR kinase inhibitors attenuates in vitro GSC proliferation and neurosphere formation [76, 77]. The intracellular pathways that are activated in turn upon EGFR activation are numerous but prominently the PI3K-Akt axis has been strongly linked to glioma stem cell biology [78]. Our group recently demonstrated that GSCs are more dependent on Akt signaling than non-stem cancer cells [79]. Inhibition of Akt with the pharmacologic inhibitors (SH-6 or LY294002) disrupts GSC neurosphere formation, induces apoptosis, reduces migration and invasion in vitro, and significantly delays intracranial tumor formation of GSCs. These results have been validated by other groups, including in a genetically engineered mouse model [47, 80]. Although targeting EGFR-PI3K-Akt signaling pathway may have specific effects on GSC population to reduce tumorigenic potential, the results to date in clinical trials of EGFR inhibitors have been disappointing suggesting that alone this is an insufficient therapeutic paradigm and prompting greater focus on PI3K inhibitors.
5.2. Notch signaling
Notch proteins including four members (Notch 1-4) are transmembrane receptors that mediate short-range cellular communication through interaction with ligands (Jagged-1, -2, and Delta-like-1, -3, and -4). The Notch signaling pathway is essential for the maintenance and fate determination in somatic stem and progenitor cells by promoting self-renewal and repressing differentiation. The critical roles of Notch signaling in regulating self-renewal and determining cell fate have been well established in neural stem cells (reviewed in [81]). The activation of Notch requires sequential proteolytic cleavages by the γ-secretase complex to release its intracellular domain from membrane to nucleus. The nuclear translocation of the cleaved Notch leads to Notch-dependent transcription. Notch signaling promotes the proliferation of normal neural stem cells and is required for the maintenance of neural progenitors both in vitro and in vivo [82]. Aberrant Notch signaling has been found in several types of tumors including gliomas [83, 84]. The role of Notch signaling in brain tumor stem cells was initially identified in medulloblastomas. Inhibition of Notch signaling by a γ-secretase inhibitor (GSI-18) induces differentiation and apoptosis of CD133+ stem-like cells derived from medulloblastoma and impairs the tumorigenic potential of these cells [85]. Recently, the function of Notch signaling has been linked to GSCs, as blockade of Notch signaling in GSCs attenuates the formation of neurosphere-like colonies [86]. In addition, Notch overexpression in a K-ras-induced glioblastoma mouse model increased expression of NSC marker Nestin and induced glioma formation in the NSC-rich subependymal zone [87]. As mentioned above, Notch signaling has been linked to radioresistance of GSCs by our group [88], suggesting that inhibition of Notch signaling may not only disrupt the maintenance of GSCs but also reduce the radioresistance of GSCs. Other regulators of Notch signaling, including Delta/Notch-like epidermal growth factor-related receptor (DNER) and the Notch ligand Delta-like 4 (DLL4), can also regulate glioblastoma growth [89, 90]. Further, other signaling pathways – inhibitor of differentiation 4 (ID4) and CXCR4 – functionally interact with Notch signaling in brain tumors as well [91, 92]. Anti-DLL4 therapies have demonstrated anti-cancer stem cell activity [93] but concern has been raised as chronic DLL4 targeting can induce neoplasia as well [94]. Although blocking Notch signaling may be a good strategy targeting GSCs, γ-secretase inhibitors are still in early clinical development for brain cancers.
5.3. Bone morphogenetic proteins (BMPs)/transforming growth factor-β (TGF-β)
The BMPs and TGFβ superfamily regulates a large number of cellular processes during development and injury responses. The BMPs instruct cell fate during neural development. Based on this background, the Vescovi group performed a seminal study that demonstrated the ability of BMP ligands to activate their canonical receptors on GSCs to induce differentiation and inhibit tumor growth [95]. This study demonstrated that direct implantation of BMP-bearing beads into glioblastomas slowed tumor growth laying the foundation for a potential therapy. The role of BMPs in GSCs became more nuanced after the Fine group showed that some GSCs epigenetically regulate BMP receptors to shift towards a fetal phenotype to escape the pro-differentiation effects of BMPs [96]. In contradistinction, TGF-β serves as a largely oncogenic stimulus in glioblastoma growth through induction of angiogenesis, immune evasion, and invasion [97]. Recent studies have added a new dimension in TGF-β oncogenesis as autocrine and paracrine loops function to maintain GSCs through induction of leukemia inhibitory factor (LIF) [98] and the SOX family members [99]. TGF-β inhibitors have already entered into clinical trial and BMPs are being considered.
5.4. Hedgehog-Gli signaling
The Sonic Hedgehog-Gli signaling is one of the key regulatory pathway during embryogenesis and is critical for the maintenance of several types of adult stem cells, including neural stem cells [100]. The binding of Hedgehog ligands to the PTCH receptor activates Gli signal transducers that then translocate into the nucleus to activate or repress transcription of downstream genes. Aberrant Hedgehog signaling has been associated with medulloblastomas, the common childhood tumors [100, 101]. Hedgehog signaling is mediated not only through specific signaling molecules but also in association with a physical organelle, primary cilia, that function in medulloblastoma development [102]. Active Hedgehog-Gli signaling is also found in gliomas [103], in fact the key intracellular mediator Gli was originally discovered in gliomas [104]. Gli activity correlates with tumor grade in a genetically engineered mouse model [105]. Several groups have studied the role of Hedgehog-Gli signaling in GSCs and found that this signaling pathway regulates self-renewal and tumorigenic potential of GSCs [48, 106-108]. Treatment of GSCs with the hedgehog inhibitor cyclopamine or Gli RNA interference suppresses self-renewal and proliferation while increases apoptotic cell death. Importantly, inhibition of Hedgehog-Gli signaling enhances the efficacy of TMZ to inhibit GSC proliferation and induce cell death [109]. In vivo studies showed that the inhibition of the Hedgehog signaling pathway blocks GSC tumor growth, and the viable neoplastic cells after the cyclopamine treatment failed to propagate tumors in vivo [109]. Furthermore, cyclopamine treatment has been shown to improve the effect of radiation on GSC cell survival. Collectively, these studies indicated that Hedgehog-Gli signaling pathway is critical for GSC maintenance and targeting this pathway with pharmacologic inhibitors may suppress GSC growth and improve the efficacy of conventional therapies against malignant gliomas. Although the side effect of these inhibitors on normal stem cells needs to be carefully evaluated, recent clinical studies with the Hedgehog inhibitor GDC-0449 have shown promising responses with acceptable toxicity [110, 111].
5.5. Wnt-β-catenin signaling
The canonical Wnt cascade is one of critical regulators in embryonic stem cells and adult stem cells. Wnt-β-catenin signaling has clearly defined roles in both normal stem cells and cancer stem cells (reviewed in [112]). In brain, the Wnt signaling pathway regulates brain development as well as proliferation and self-renewal of NSCs or neural progenitor cells in the fetal ventricular zone, the postnatal subventricular zone and hippocampus [113-117] and alterations have been linked to medulloblastoma [118, 119]. Wnt signaling is activated predominantly in medulloblastoma of the classic subtype [120]. Recent studies indicate that Wnt-β-catenin signaling may contribute to radioresistance in cancer stem cells [45]. Whether Wnt-β-catenin signaling is associated with GSC maintenance and radioresistance requires further investigation, but it is possible that Wnt blockade can effectively target GSCs.
5.6. STAT3 signaling
The signal transducer and activator of transcription 3 (STAT3) is a critical transcriptional regulator involved in a wide range of cellular activities in the immune response, central nervous system development, stem cell maintenance and tumorigenesis. The link between the activation of STAT3 and glioblastoma biology has become increasingly evident (reviewed in [121]). Abnormal STAT3 activation has been detected in many types of cancers including solid tumors and hematopoietic malignancies. The canonical oncogenic function of STAT3 depends on its phosphorylation on Tyr705 that can be attributed to aberrant activity of various upstream kinases. STAT3 in conjunction with C/EBP-beta correlates with mesenchymal transformation of glioblastomas and inversely related to patient outcome [122]. Based on this background, several groups have interrogated STAT3 in GSCs. Genetic knockdown of STAT3 or inhibition of STAT3 with specific inhibitors disrupts proliferation and maintenance of GSCs [123, 124]. Moreover, the phosphorylated active form of STAT3 on tyrosine-705 and serine-727 is present in GSC population and the active form of STAT3 decreases to undetectable level after differentiation induction of GSCs [124]. Several pathways upstream of STAT3 are active in GSCs – interleukin-6 (IL6), erythropoietin, and Notch – and targeting these pathways inhibits STAT3 activation and GSC growth and self renewal [85, 123, 125]. STAT3 also contributes to the immune regulation by GSCs [126]. Since STAT3 is involved in many cellular activities in a wide range of cancer types, STAT3 inhibitors are undergoing clinical development. However, as STAT3 is also important for the maintenance of normal stem cells and required for critical immune responses and other normal cellular activities, targeting STAT3 may display significant side effects and is unlikely to be specific for GSCs.
5.7. GSK3-β signaling
GSK3-β is one of isoforms of glycogen synthase kinase 3 (GSK3) and involved in different signaling pathways regulating cell cycle, proliferation, differentiation and apoptosis. GSK3-β has been implicated in the regulation of neural stem cell differentiation and proliferation [127-130]. Recently, it has been shown that GSK3-β is involved in maintaining GSCs, as reduced GSK3-β activity either by shRNA or the specific inhibitor SB216763 or lithium chloride (LiCl) induces GSC differentiation and reduces expression of stem cell marker Sox2 and Myc [131]. In addition, down regulation of the stem cell factor Bmi1 (a member of polycomb group of proteins) reduces GSK3-β expression in GSCs [131]. Since GSK3-β signaling is very complex, the role of GSK3-β in GSCs needs further investigation, but the number of specific GSK3-β inhibitors available provides an additional therapeutic strategy for treating malignant gliomas.
6. Targeting hypoxic responses of glioma stem cells
Hypoxic conditions are commonly present in solid tumors including malignant gliomas. Hypoxia was thought to have a negative impact for tumor growth, but hypoxia actually promotes tumor angiogenesis, cancer dispersal and therapeutic resistance such as radioresistance in GBM [132]. Furthermore, recent work from our group and others has suggested that hypoxic niches play critical roles in the maintenance of cancer stem cells in tumor tissue [30, 31, 133, 134]. Similarly, hypoxic niches are also involved in the maintenance of normal stem cells. For example, hematopoietic stem cells are maintained in hypoxic niches in bone marrow [135]. Hypoxia also prevents the differentiation of neural stem cells and promotes the self-renewal of embryonic stem (ES) cells [136-139]. Restricted oxygen concentrations also enhance the production of induced pluripotent stem cells (iPSC) [140]. GBMs frequently display areas of necrosis – necrosis serves as a grading criterium for GBM – that occur in avascular and low oxygen regions. While brain tumor stem cells have been linked to a perivascular niche, we have found an additional enrichment of GSCs around necrotic regions [30]. Other groups have found that restricted oxygen promotes a GSC phenotype [32, 33]. We also found that restricted oxygen conditions increase expression of GSC markers and indicator of self-renewal and tumor growth, suggesting that the GSC state may be plastic and that microenvironmental conditions can promote the acquisition of a stem cell-like state [31, 141]. These studies suggest that disrupting the microenvironment of GSCs, like the hypoxic niches, may provide a new approach targeting GSCs in malignant gliomas.
In response to hypoxia, cells undergo significant transcription modification that leads to alterations of cellular function. The cellular responses to hypoxia are mainly mediated through the hypoxia inducible factors (HIFs). We recently demonstrated that hypoxia responses in GSCs differ from non-stem cancer cells. Hypoxia differentially induces HIF2α in GSCs, while HIF1α is induced in both GSCs and non-stem GBM tumor cells by hypoxia [30]. HIF2α was essential only in GSCs and was not expressed by normal neural progenitors, suggesting that HIF2α may represent a specific target for GSCs or other cancer stem cells. Under hypoxic conditions, GSCs display a specific gene expression profile relative to non-stem cancer cells. In addition to the increased VEGF expression, GSCs specifically up-regulates HIF2α and several HIF2α transcriptional targets such as Oct4, Glut1 and Serpin B9 under hypoxia [30]. GSCs display high levels of HIF2α under oxygen concentration as high as 5% that is within the physiologic range of oxygen in the brain and most tumor tissues [30], whereas HIF1α is induced in both GSCs and the non-stem tumor cells only at severely hypoxic conditions (<1% oxygen). Functional studies through RNA interference demonstrated that both HIF1α and HIF2α are required for GSC growth in vitro and GSC tumor formation in vivo, but only HIF1α is required for non-stem GBM tumor cell growth, suggesting that GSCs use both HIF1α and HIF2α for the hypoxia response and may be able to survive better under stress conditions. Other groups have found that HIF1α functions in the hypoxia driven expansion of GSCs [33]. Moreover, the silico analysis of HIFs expression in the REMBRANDT National Cancer Institute patient database showed that HIF2α but not HIF1α levels informed negative survival of patients. In addition, overexpression of HIF2α promotes cancer stem state in GBM [31]. These data indicate that HIF2α is a potential target specific for GSCs since HIF2α is not expressed in normal neural progenitors. However, the role HIF2α in other normal stem cells needs to be elucidated, in order to understand whether targeting HIF2α have negative impact on other normal stem cells.
7. Targeting glioma stem cell maintenance through specific transcription factors
Since GSCs share some critical characteristics with normal neural stem cells and embryonic stem cells, some important stem cell transcription factors (SCTFs) are also involved in the maintenance and functions of GSCs. These SCTFs such as Sox2, Oct4, Nanog, c-Myc, Olig2 and Bmi1 are critical for the self-renewal, proliferation, survival, and maintenance of multi-lineage differentiation potential of GSCs.
Sox2, Oct4 and Nanog are core components in maintaining embryonic stem cells and somatic stem cells [142-145]. They are also critical factors for cell reprogram and generation of inducible pluripotent stem cells (iPS) [146, 147]. These SCTFs are highly expressed in GSCs and may be important for GSC maintenance [21, 99]. Although targeting these SCTFs induces differentiation of GSCs, they present challenging targets for GSCs because these SCTFs are also highly expressed in normal somatic stem cells and are critical for maintaining normal stem cells, such as neural stem cells and hematopoietic stem cells.
c-Myc is a well known oncoprotein that has been extensively studied for its crucial role in the proliferation of both normal stem cells and cancer cells. c-Myc may be a critical link to study the relationship between “stemness” and tumorigenicity. c-Myc expression levels correlate with tumor grade in gliomas [140]. Recently, our group and others demonstrated that c-Myc expression is elevated in GSCs and it is required for maintaining GSCs in vitro and their tumorigenic potential in vivo [148]. Conditional overexpression of c-Myc in mouse astroglia leads to brain tumors resembling human malignant gliomas [149]. In addition, c-Myc prevents cell differentiation and promotes self-renewal of tumor cells derived from the pten/p53 double null mouse model [150]. These studies support an important role of c-Myc in GSC maintenance. However, the widespread effects of c-Myc in normal physiology must be considered. Although c-Myc plays crucial roles in tumorigenesis and tumor progression, it is unlikely a specific target.
Olig2 is a unique transcription factor that is almost exclusively expressed in the stem cells or progenitors in the CNS (central nervous system). Olig2 is expressed in neural progenitors fated to give rise to oligodendrocytes and subtypes of neurons [151]. Several studies have revealed that Olig2 is widely expressed in astrocytomas and is required for tumor initiation and growth [152, 153], suggesting a link between Olig2 expression and GSCs. Indeed, Olig2 differential expression was found in GSC populations isolated from most cases of GBM tumor specimens obtained in our group, suggesting that Olig2 is a common marker for GSCs. This transcription factor is required for maintaining the multi-lineage differentiation potential of neural progenitors and GSCs. Olig2 mediates GSC proliferation in part through suppression of p21WAF1/CIP1, a key cell cycle regulator [61]. Since Olig2 expression is limited in GSCs and CNS stem cells/progenitors, Olig2 could be a potential target for GSCs even it is not an ideal target.
REST (repressor element 1-silencing transcription factor) or NRSF (neuron-restricted silencing factor) is a master neuronal repressor that plays a critical role in maintaining neural stem cells by suppressing neuronal differentiation [154]. The zinc-finger domain of REST recognizes a conserved RE-1 element (21-23 base pairs) within regulatory regions of target genes to repress the transcription of the differentiation-associated genes. REST promotes oncogenesis in medulloblastomas and neuroblastomas that usually arise from neural progenitors [155, 156]. This transcription repressor is also highly expressed in human glioblastomas and neuroblastomas [155]. REST is targeted for proteasomal degradation by the ubiquitin E3 ligase SCFβ-TRCP to promote neural differentiation [157]. We have observed that REST is differentially expressed in GSCs isolated from some cases of GBM tumor samples (unpublished data), suggesting that targeting REST may induce GSC differentiation.
Bmi1 is one of Polycomb group genes that normally function as epigenetic silencers. Bmi1 is a positive regulator of neural stem cells and has been implicated in stem cell fate determination in several tissues [158]. Bmi1 is required for the malignant transformation of both neural stem cells and differentiated astrocytes [159]. Transformed Bmi1 wild-type neural stem cells give rise to high grade gliomas in vivo, but Bmi1-deficient neural stem cells only initiate less malignant type of gliomas with fewer cells expressing stem cell markers. Bmi1 is frequently overexpressed in several types of cancer including gliomas. Interestingly, Bmi1 is also highly expressed in GSCs and required for GSC self-renewal [160]. Similar finding for an essential role of Bmi1 in maintaining cancer stem cells has been shown in hepatocellular carcinomas [161].
8. Targeting glioma stem cells through induction of differentiation
One of important properties that GSCs share with normal stem cells is their multi-lineage differentiation potential, although differentiation potential is not one of essential characteristics to define a cancer stem cell. GSCs isolated from primary GBM tumors or xenografts have the capacity to differentiate into cells with the morphologies and marker profiles of astrocytes, oligodendrocytes and neurons (ref [21] and Fig. 1). These differentiated cells lose long-term repopulation potential in vitro and fail to propagate tumors in vivo, suggesting that inducing GSC differentiation may be a practical strategy to deplete the GSC population. Several signaling pathways involved in differentiation induction of stem cells have been identified. As noted above, the BMPs inhibit GSC proliferation and deplete GSC population by inducing the differentiation of GSCs into astroglial and neuron-like cells [95]. Targeting GSCs with BMP4 in vivo significantly inhibits GBM tumor growth and reduces tumor invasion [95]. Recent study by Lee et al has confirmed that BMPs promote glial differentiation of GSCs [96], but they also found that GSCs derived from one GBM sample showed enhanced cell proliferation rather than differentiation in response to BMP treatment. This is because GSCs from this sample lost BMPR1B expression due to epigenetic silencing by an EZH2-dependent mechanism [96]. Restoration of BMPR1B expression rescued the BMP4-induced differentiation in these GSCs. These studies suggested that individual epigenetic characteristics may determine GSC response to the differentiation-inducing agents, and BMPs in combination with epigenetic modulators may be critical to induce GSC differentiation.
In addition, there are other factors that have been implicated to promote differentiation of GSCs. Recent study showed that inactivation of PTEN (a well-known tumor suppressor) promotes undifferentiated state of glioma stem cells [162], suggesting that PTEN may promote GSC differentiation. PTEN is a phosphatase with dual-specificity for both protein and lipid. PTEN deletion or functional loss has been linked to initiation and/or progression of malignant gliomas. PTEN inactivation leads to increased expression of Myc that is critical for maintaining GSC proliferation and self-renewal, suggesting promoting PTEN function may suppress the “stemness” of GSCs. In another study, Sox11 was shown to promote GSC differentiation [163]. Overexpression of Sox11 inhibits tumorigenic potential of GSCs by promoting neuronal differentiation. Furthermore, inactivated Sox11 expression was detected in GSCs derived from some cases of GBM when a gene expression profile was analyzed between tumorigenic and non-tumorigenic clones of glioma cells. Although these factors have been shown to promote GSC differentiation, whether these factors can be used for targeting GSC in clinical applications needs further studies.
9. Regulation of glioma stem cells by micro RNAs (miRNA)
The roles of miRNAs in regulating embryonic stem cells, somatic stem cells or cancer stem cells have received much attention in recent years. miRNAs are a group of small non-coding RNAs that potently silence gene expression through post-transcriptional modification on target mRNAs. Since a single miRNA may regulate several or many distinct mRNAs, miRNAs are powerful regulators of gene expression. miRNAs are emerging as crucial regulators of cellular proliferation and differentiation. They can function as either oncogenes or tumor suppressors in various tissues or tumors. miRNA has been shown to be critical in the regulation of glioma cell functions. For example, miRNA-21 is overexpressed in GBM tumors and blocking its function induces apoptotic cell death [164]. The roles of miRNA in GSCs have been demonstrated in two recent reports [165, 166]. Levels of miR-124, miR-137 and miR-451 are significantly reduced in malignant gliomas (both grade III and grade IV) relative to normal brain and in GSCs relative to non-stem tumor cells. Overexpression of these miRNAs in GSCs inhibits proliferation and induces differentiation of GSCs, suggesting that these miRNAs have an important role in maintaining GSCs. Furthermore, external expression of miR-451 disrupts neurosphere formation and suppresses tumor growth of GSCs, indicating a tumor suppressor role of miR-451 in gliomas. These studies suggest that some critical miRNAs can be potentially used as therapeutic agents for targeting GSCs. However, how we deliver these miRNAs into GSCs or non-stem tumor cells and how we make these miRNAs to be stable targeting agents may face a great challenge in the future.
10. Conclusions
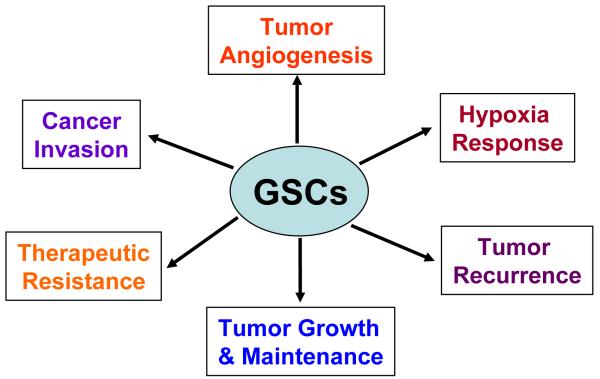
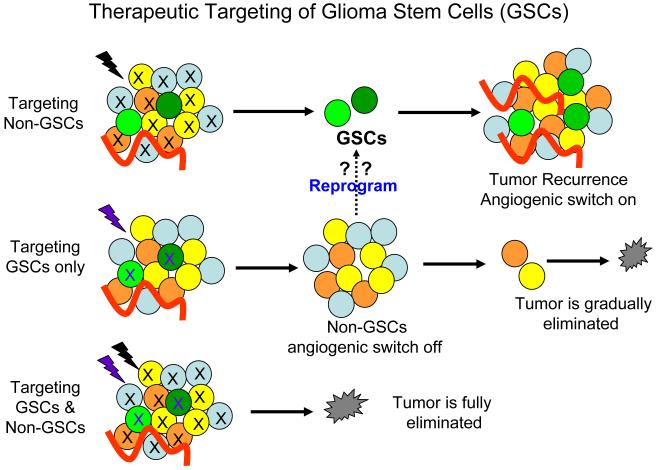
The identification of cancer stem cells and their roles in GBM progression and therapeutic resistance has altered our understanding of glioma tumor biology and causing a reevaluation of current therapies for malignant gliomas. Cancer cure requires elimination of all tumor cells, even small fractions like GSCs. Novel therapies directed against GSCs may help improve the currently dreadful record of clinical activity with conventional therapy. Although controversy still exists as to the methods for isolating and characterizing GSCs and defining the role of non-stem cancer population, GSCs represent a subpopulation of cancer cells with extraordinary capacities to promote tumor repopulation, angiogenesis, invasion and therapeutic resistance (summarized in Fig. 3), making them a critical cell population that should be targeted for anti-glioma therapies. A recent report showed that a lentival vector-mediated suicide gene therapy can eliminate invasive GSCs in xenograft models [167]. Since non-stem tumor cells (non-GSCs) may be able to reprogram into GSCs under certain conditions [31, 141], we believe that eliminating both GSC and non-GSC cancer cell populations is essential to achieve therapeutic success (Fig. 4). Recent research advances in this exciting area have allowed us to gain remarkable insights into the signaling pathways that are differentially present or regulated in GSCs or non-stem cancer cells. As GSCs share critical signaling pathways with normal neural stem/progenitor cells but also significantly distinct from normal stem cells in many aspects, identification of the unique signaling pathways or molecular regulators that differentially control the phenotypes and tumorigenic potential of GSCs might offer new avenues for developing novel therapeutics against GSCs to significantly improve GBM treatment. We have discussed a number of signaling pathways or molecular targets that are potentially useful for the future development of anti-GSC therapeutics, but most of them are still far away from the clinical application except the anti-vascular niche treatment that has shown promising results in clinical trials leading to preliminary FDA approval for bevacizumab for the treatment of recurrent or progressive GBM. Some molecular regulators such as L1CAM and HIF2α that are preferentially expressed in GSCs are likely to be specific targets for GSCs, as targeting other critical pathways such as Notch, Hedgehog-gli, Wnt or STAT3 signaling pathways that are shared by normal stem cells may display significant side effect on normal cells. Additional research is certainly needed to confirm the clinical relevance of these laboratory findings and better apply these concepts to clinical practice. For example, biomarker development and the application of personalized medical therapy may be accelerated with analysis of tumor heterogeneity. However, great challenges lay ahead as GSC populations themselves are heterogeneous [168] and the GSCs may evolve over time within a patient. As the genetics of glioblastomas are becoming increasingly defined with clear subgroups of tumors evolving, our understanding of GSC diversity will certainly become more nuanced. GSCs are also a product of their environment and almost certainly not only interact with vascular niche but also with non-stem tumor cells, stromal elements and immune cells. The emerging concepts and roles of cancer stem cells are still rapidly evolving. The road forward will likely be bumpy but these paradigms are exciting as they may bring new opportunities to a group of patients sadly lacking in effective treatment options. Since the origin of cancer stem cells in GBM from different patient may vary and they may display different genetic changes in complex tumor tissues, future treatment for GBM may rely on a unique combination of several targeted therapies based on the cellular, genetic and molecular information of the tumor in the individual patient.
Fig. 3.
A summary of roles of glioma stem cells (GSCs) in glioblastoma tumor progression and therapeutic resistance.
Fig. 4.
Therapeutic targeting of glioma stem cells (GSCs) and non-stem cancer cells (Non-GSCs). Targeting both populations of glioblastoma cancer cells is important to eliminate the tumor, since non-stem cancer cells may be able to reprogram into GSCs under certain conditions.
Acknowledgements
This work was supported by the Cleveland Clinic Foundation and an NIH grant NS070315 to S.B., and the National Brain Tumor Society, Goldhirsh Foundation, and NIH grants NS047409, NS054276, CA112958, and CA116659 to J.R. Many investigators have contributed to the advances in the related fields described in this review. We apologize for not including all references in this review due to space limit.
References
- [1].Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- [2].Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- [3].McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65:463–9. doi: 10.1227/01.NEU.0000349763.42238.E9. discussion 9-70. [DOI] [PubMed] [Google Scholar]
- [4].Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- [5].Vredenburgh JJ, Desjardins A, Herndon Ii JE, Dowell JM, Reardon DA, Quinn JA, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–9. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- [6].Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–9. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- [7].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [8].Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- [10].Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- [11].Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–83. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- [13].O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- [14].Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- [16].Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–9. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- [18].Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- [19].Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- [21].Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- [22].Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–8. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- [23].Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A Perivascular Niche for Brain Tumor Stem Cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- [24].Folkins C, Shaked Y, Man S, Tang T, Lee CR, Zhu Z, et al. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res. 2009;69:7243–51. doi: 10.1158/0008-5472.CAN-09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Miletic H, Niclou SP, Johansson M, Bjerkvig R. Anti-VEGF therapies for malignant glioma: treatment effects and escape mechanisms. Expert Opin Ther Targets. 2009;13:455–68. doi: 10.1517/14728220902806444. [DOI] [PubMed] [Google Scholar]
- [26].Chekenya M, Krakstad C, Svendsen A, Netland IA, Staalesen V, Tysnes BB, et al. The progenitor cell marker NG2/MPG promotes chemoresistance by activation of integrin-dependent PI3K/Akt signaling. Oncogene. 2008;27:5182–94. doi: 10.1038/onc.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Johannessen TC, Wang J, Skaftnesmo KO, Sakariassen PO, Enger PO, Petersen K, et al. Highly infiltrative brain tumours show reduced chemosensitivity associated with a stem cell-like phenotype. Neuropathol Appl Neurobiol. 2009;35:380–93. doi: 10.1111/j.1365-2990.2008.01008.x. [DOI] [PubMed] [Google Scholar]
- [28].Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5 doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Plate KH, Risau W. Angiogenesis in malignant gliomas. Glia. 1995;15:339–47. doi: 10.1002/glia.440150313. [DOI] [PubMed] [Google Scholar]
- [30].Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, et al. Hypoxia-Inducible Factors Regulate Tumorigenic Capacity of Glioma Stem Cells. Cancer Cell. 2009;15:501–13. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274–84. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McCord AM, Jamal M, Shankavaram UT, Lang FF, Camphausen K, Tofilon PJ. Physiologic oxygen concentration enhances the stem-like properties of CD133+ human glioblastoma cells in vitro. Mol Cancer Res. 2009;7:489–97. doi: 10.1158/1541-7786.MCR-08-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene. 2009;28:3949–59. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- [34].Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–78. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–4. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- [38].Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shaifer CA, Huang J, Lin PC. Glioblastoma cells incorporate into tumor vasculature and contribute to vascular radioresistance. Int J Cancer. doi: 10.1002/ijc.25249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Park SY, Gonen M, Kim HJ, Michor F, Polyak K. Cellular and genetic diversity in the progression of in situ human breast carcinomas to an invasive phenotype. J Clin Invest. 120:636–44. doi: 10.1172/JCI40724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang J, Wakeman TP, Lathia JD, Hjelmeland AB, Wang XF, White RR, et al. Notch promotes radioresistance of glioma stem cells. Stem Cells. 28:17–28. doi: 10.1002/stem.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chang CJ, Hsu CC, Yung MC, Chen KY, Tzao C, Wu WF, et al. Enhanced radiosensitivity and radiation-induced apoptosis in glioma CD133-positive cells by knockdown of SirT1 expression. Biochem Biophys Res Commun. 2009;380:236–42. doi: 10.1016/j.bbrc.2009.01.040. [DOI] [PubMed] [Google Scholar]
- [43].Sauvageot CM, Weatherbee JL, Kesari S, Winters SE, Barnes J, Dellagatta J, et al. Efficacy of the HSP90 inhibitor 17-AAG in human glioma cell lines and tumorigenic glioma stem cells. Neuro Oncol. 2009;11:109–21. doi: 10.1215/15228517-2008-060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–3. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci U S A. 2007;104:618–23. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hegi ME, Diserens AC, Gorlia T, Hamou MF, De Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- [47].Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, et al. PTEN/PI3K/Akt Pathway Regulates the Side Population Phenotype and ABCG2 Activity in Glioma Tumor Stem-like Cells. Cell Stem Cell. 2009;4:226–35. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 Signaling Regulates Human Glioma Growth, Cancer Stem Cell Self-Renewal, and Tumorigenicity. Curr Biol. 2007;17:165–72. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Beier D, Rohrl S, Pillai DR, Schwarz S, Kunz-Schughart LA, Leukel P, et al. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer Res. 2008;68:5706–15. doi: 10.1158/0008-5472.CAN-07-6878. [DOI] [PubMed] [Google Scholar]
- [50].Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101:14228–33. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dungey FA, Caldecott KW, Chalmers AJ. Enhanced radiosensitization of human glioma cells by combining inhibition of poly(ADP-ribose) polymerase with inhibition of heat shock protein 90. Mol Cancer Ther. 2009;8:2243–54. doi: 10.1158/1535-7163.MCT-09-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Miknyoczki S, Chang H, Grobelny J, Pritchard S, Worrell C, McGann N, et al. The selective poly(ADP-ribose) polymerase-1(2) inhibitor, CEP-8983, increases the sensitivity of chemoresistant tumor cells to temozolomide and irinotecan but does not potentiate myelotoxicity. Mol Cancer Ther. 2007;6:2290–302. doi: 10.1158/1535-7163.MCT-07-0062. [DOI] [PubMed] [Google Scholar]
- [53].Russo AL, Kwon HC, Burgan WE, Carter D, Beam K, Weizheng X, et al. In vitro and in vivo radiosensitization of glioblastoma cells by the poly (ADP-ribose) polymerase inhibitor E7016. Clin Cancer Res. 2009;15:607–12. doi: 10.1158/1078-0432.CCR-08-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Beneke S, Diefenbach J, Burkle A. Poly(ADP-ribosyl)ation inhibitors: promising drug candidates for a wide variety of pathophysiologic conditions. Int J Cancer. 2004;111:813–8. doi: 10.1002/ijc.20342. [DOI] [PubMed] [Google Scholar]
- [55].Delaney CA, Wang LZ, Kyle S, White AW, Calvert AH, Curtin NJ, et al. Potentiation of temozolomide and topotecan growth inhibition and cytotoxicity by novel poly(adenosine diphosphoribose) polymerase inhibitors in a panel of human tumor cell lines. Clin Cancer Res. 2000;6:2860–7. [PubMed] [Google Scholar]
- [56].Masutani M, Nozaki T, Nakamoto K, Nakagama H, Suzuki H, Kusuoka O, et al. The response of Parp knockout mice against DNA damaging agents. Mutat Res. 2000;462:159–66. doi: 10.1016/s1383-5742(00)00033-8. [DOI] [PubMed] [Google Scholar]
- [57].Southan GJ, Szabo C. Poly(ADP-ribose) polymerase inhibitors. Curr Med Chem. 2003;10:321–40. doi: 10.2174/0929867033368376. [DOI] [PubMed] [Google Scholar]
- [58].Read TA, Fogarty MP, Markant SL, McLendon RE, Wei Z, Ellison DW, et al. Identification of CD15 as a marker for tumor-propagating cells in a mouse model of medulloblastoma. Cancer Cell. 2009;15:135–47. doi: 10.1016/j.ccr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Son MJ, Woolard K, Nam DH, Lee J, Fine HA. SSEA-1 Is an Enrichment Marker for Tumor-Initiating Cells in Human Glioblastoma. Cell Stem Cell. 2009;4:440–52. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ward RJ, Lee L, Graham K, Satkunendran T, Yoshikawa K, Ling E, et al. Multipotent CD15+ cancer stem cells in patched-1-deficient mouse medulloblastoma. Cancer Res. 2009;69:4682–90. doi: 10.1158/0008-5472.CAN-09-0342. [DOI] [PubMed] [Google Scholar]
- [61].Bao S, Wu Q, Li Z, Sathornsumetee S, Wang H, McLendon RE, et al. Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res. 2008;68:6043–8. doi: 10.1158/0008-5472.CAN-08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ogden AT, Waziri AE, Lochhead RA, Fusco D, Lopez K, Ellis JA, et al. Identification of A2B5+CD133− tumor-initiating cells in adult human gliomas. Neurosurgery. 2008;62:505–14. doi: 10.1227/01.neu.0000316019.28421.95. [DOI] [PubMed] [Google Scholar]
- [63].Tchoghandjian A, Baeza N, Colin C, Cayre M, Metellus P, Beclin C, et al. A2B5 Cells from Human Glioblastoma have Cancer Stem Cell Properties. Brain Pathol. 2009 doi: 10.1111/j.1750-3639.2009.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, et al. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–5. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- [65].Solter D, Knowles BB. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1) Proc Natl Acad Sci U S A. 1978;75:5565–9. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Damjanov I, Fox N, Knowles BB, Solter D, Lange PH, Fraley EE. Immunohistochemical localization of murine stage-specific embryonic antigens in human testicular germ cell tumors. Am J Pathol. 1982;108:225–30. [PMC free article] [PubMed] [Google Scholar]
- [67].Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–75. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- [68].Salton SR, Richter-Landsberg C, Greene LA, Shelanski ML. Nerve growth factor-inducible large external (NILE) glycoprotein: studies of a central and peripheral neuronal marker. J Neurosci. 1983;3:441–54. doi: 10.1523/JNEUROSCI.03-03-00441.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Schmid RS, Maness PF. L1 and NCAM adhesion molecules as signaling coreceptors in neuronal migration and process outgrowth. Curr Opin Neurobiol. 2008;18:245–50. doi: 10.1016/j.conb.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8:806–23. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
- [71].Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- [72].Choe G, Horvath S, Cloughesy TF, Crosby K, Seligson D, Palotie A, et al. Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–6. [PubMed] [Google Scholar]
- [73].Moscatello DK, Holgado-Madruga M, Emlet DR, Montgomery RB, Wong AJ. Constitutive activation of phosphatidylinositol 3-kinase by a naturally occurring mutant epidermal growth factor receptor. J Biol Chem. 1998;273:200–6. doi: 10.1074/jbc.273.1.200. [DOI] [PubMed] [Google Scholar]
- [74].Ding H, Shannon P, Lau N, Wu X, Roncari L, Baldwin RL, et al. Oligodendrogliomas result from the expression of an activated mutant epidermal growth factor receptor in a RAS transgenic mouse astrocytoma model. Cancer Res. 2003;63:1106–13. [PubMed] [Google Scholar]
- [75].Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12:3675–85. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Griffero F, Daga A, Marubbi D, Capra MC, Melotti A, Pattarozzi A, et al. Different response of human glioma tumor-initiating cells to epidermal growth factor receptor kinase inhibitors. J Biol Chem. 2009;284:7138–48. doi: 10.1074/jbc.M807111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Soeda A, Inagaki A, Oka N, Ikegame Y, Aoki H, Yoshimura S, et al. Epidermal growth factor plays a crucial role in mitogenic regulation of human brain tumor stem cells. J Biol Chem. 2008;283:10958–66. doi: 10.1074/jbc.M704205200. [DOI] [PubMed] [Google Scholar]
- [78].Dreesen O, Brivanlou AH. Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev. 2007;3:7–17. doi: 10.1007/s12015-007-0004-8. [DOI] [PubMed] [Google Scholar]
- [79].Eyler CE, Foo WC, LaFiura KM, McLendon RE, Hjelmeland AB, Rich JN. Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells. 2008;26:3027–36. doi: 10.1634/stemcells.2007-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gallia GL, Tyler BM, Hann CL, Siu IM, Giranda VL, Vescovi AL, et al. Inhibition of Akt inhibits growth of glioblastoma and glioblastoma stem-like cells. Mol Cancer Ther. 2009;8:386–93. doi: 10.1158/1535-7163.MCT-08-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lathia JD, Mattson MP, Cheng A. Notch: from neural development to neurological disorders. J Neurochem. 2008;107:1471–81. doi: 10.1111/j.1471-4159.2008.05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–5. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- [83].Kanamori M, Kawaguchi T, Nigro JM, Feuerstein BG, Berger MS, Miele L, et al. Contribution of Notch signaling activation to human glioblastoma multiforme. J Neurosurg. 2007;106:417–27. doi: 10.3171/jns.2007.106.3.417. [DOI] [PubMed] [Google Scholar]
- [84].Purow BW, Haque RM, Noel MW, Su Q, Burdick MJ, Lee J, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353–63. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- [85].Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445–52. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- [86].Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 28:5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Shih AH, Holland EC. Notch signaling enhances nestin expression in gliomas. Neoplasia. 2006;8:1072–82. doi: 10.1593/neo.06526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wang J, Wakeman TP, Lathia JD, Hjelmeland AB, Wang XF, White RR, et al. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2009;28:17–28. doi: 10.1002/stem.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Li JL, Sainson RC, Shi W, Leek R, Harrington LS, Preusser M, et al. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 2007;67:11244–53. doi: 10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- [90].Sun P, Xia S, Lal B, Eberhart CG, Quinones-Hinojosa A, Maciaczyk J, et al. DNER, an epigenetically modulated gene, regulates glioblastoma-derived neurosphere cell differentiation and tumor propagation. Stem Cells. 2009;27:1473–86. doi: 10.1002/stem.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Jeon HM, Jin X, Lee JS, Oh SY, Sohn YW, Park HJ, et al. Inhibitor of differentiation 4 drives brain tumor-initiating cell genesis through cyclin E and notch signaling. Genes Dev. 2008;22:2028–33. doi: 10.1101/gad.1668708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Williams CK, Segarra M, Sierra Mde L, Sainson RC, Tosato G, Harris AL. Regulation of CXCR4 by the Notch ligand delta-like 4 in endothelial cells. Cancer Res. 2008;68:1889–95. doi: 10.1158/0008-5472.CAN-07-2181. [DOI] [PubMed] [Google Scholar]
- [93].Hoey T, Yen WC, Axelrod F, Basi J, Donigian L, Dylla S, et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5:168–77. doi: 10.1016/j.stem.2009.05.019. [DOI] [PubMed] [Google Scholar]
- [94].Yan M, Callahan CA, Beyer JC, Allamneni KP, Zhang G, Ridgway JB, et al. Chronic DLL4 blockade induces vascular neoplasms. Nature. 463:E6–7. doi: 10.1038/nature08751. [DOI] [PubMed] [Google Scholar]
- [95].Piccirillo SGM, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–5. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- [96].Lee J, Son MJ, Woolard K, Donin NM, Li A, Cheng CH, et al. Epigenetic-Mediated Dysfunction of the Bone Morphogenetic Protein Pathway Inhibits Differentiation of Glioblastoma-Initiating Cells. Cancer Cell. 2008;13:69–80. doi: 10.1016/j.ccr.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wick W, Naumann U, Weller M. Transforming growth factor-beta: a molecular target for the future therapy of glioblastoma. Curr Pharm Des. 2006;12:341–9. doi: 10.2174/138161206775201901. [DOI] [PubMed] [Google Scholar]
- [98].Penuelas S, Anido J, Prieto-Sanchez RM, Folch G, Barba I, Cuartas I, et al. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009;15:315–27. doi: 10.1016/j.ccr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- [99].Ikushima H, Todo T, Ino Y, Takahashi M, Miyazawa K, Miyazono K. Autocrine TGF-beta signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell. 2009;5:504–14. doi: 10.1016/j.stem.2009.08.018. [DOI] [PubMed] [Google Scholar]
- [100].Ruiz i, Altaba A, Mas C, Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 2007;17:438–47. doi: 10.1016/j.tcb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Vorechovsky I, Tingby O, Hartman M, Stromberg B, Nister M, Collins VP, et al. Somatic mutations in the human homologue of Drosophila patched in primitive neuroectodermal tumours. Oncogene. 1997;15:361–6. doi: 10.1038/sj.onc.1201340. [DOI] [PubMed] [Google Scholar]
- [102].Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med. 2009;15:1062–5. doi: 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Shahi MH, Lorente A, Castresana JS. Hedgehog signalling in medulloblastoma, glioblastoma and neuroblastoma. Oncol Rep. 2008;19:681–8. [PubMed] [Google Scholar]
- [104].Kinzler KW, Bigner SH, Bigner DD, Trent JM, Law ML, O’Brien SJ, et al. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987;236:70–3. doi: 10.1126/science.3563490. [DOI] [PubMed] [Google Scholar]
- [105].Becher OJ, Hambardzumyan D, Fomchenko EI, Momota H, Mainwaring L, Bleau AM, et al. Gli activity correlates with tumor grade in platelet-derived growth factor-induced gliomas. Cancer Res. 2008;68:2241–9. doi: 10.1158/0008-5472.CAN-07-6350. [DOI] [PubMed] [Google Scholar]
- [106].Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–33. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Ehtesham M, Sarangi A, Valadez JG, Chanthaphaychith S, Becher MW, Abel TW, et al. Ligand-dependent activation of the hedgehog pathway in glioma progenitor cells. Oncogene. 2007;26:5752–61. doi: 10.1038/sj.onc.1210359. [DOI] [PubMed] [Google Scholar]
- [108].Xu Q, Yuan X, Liu G, Black KL, Yu JS. Hedgehog signaling regulates brain tumor-initiating cell proliferation and portends shorter survival for patients with PTEN-coexpressing glioblastomas. Stem Cells. 2008;26:3018–26. doi: 10.1634/stemcells.2008-0459. [DOI] [PubMed] [Google Scholar]
- [109].Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–72. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–8. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–72. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- [112].Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–41. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–36. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- [114].Kalani MY, Cheshier SH, Cord BJ, Bababeygy SR, Vogel H, Weissman IL, et al. Wnt-mediated self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci U S A. 2008;105:16970–5. doi: 10.1073/pnas.0808616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–5. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- [116].McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–85. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- [117].Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990;346:847–50. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- [118].Koch A, Waha A, Tonn JC, Sorensen N, Berthold F, Wolter M, et al. Somatic mutations of WNT/wingless signaling pathway components in primitive neuroectodermal tumors. Int J Cancer. 2001;93:445–9. doi: 10.1002/ijc.1342. [DOI] [PubMed] [Google Scholar]
- [119].Yokota N, Nishizawa S, Ohta S, Date H, Sugimura H, Namba H, et al. Role of Wnt pathway in medulloblastoma oncogenesis. Int J Cancer. 2002;101:198–201. doi: 10.1002/ijc.10559. [DOI] [PubMed] [Google Scholar]
- [120].Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24:1924–31. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- [121].de la Iglesia N, Puram SV, Bonni A. STAT3 regulation of glioblastoma pathogenesis. Curr Mol Med. 2009;9:580–90. doi: 10.2174/156652409788488739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 463:318–25. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Cao Y, Lathia JD, Eyler CE, Wu Q, Li Z, Wang H, et al. Erythropoietin Receptor Signaling through STAT3 Is Required for Glioma Stem Cell Maintenance. Genes & Cancer. 2010;1:50–61. doi: 10.1177/1947601909356352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Sherry MM, Reeves A, Wu JK, Cochran BH. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells. 2009;27:2383–92. doi: 10.1002/stem.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Wang H, Lathia JD, Wu Q, Wang J, Li Z, Heddleston JM, et al. Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells. 2009;27:2393–404. doi: 10.1002/stem.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, et al. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther. 9:67–78. doi: 10.1158/1535-7163.MCT-09-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Jiang H, Guo W, Liang X, Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell. 2005;120:123–35. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- [128].Kim WY, Wang X, Wu Y, Doble BW, Patel S, Woodgett JR, et al. GSK-3 is a master regulator of neural progenitor homeostasis. Nat Neurosci. 2009;12:1390–7. doi: 10.1038/nn.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–31. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Maurer MH, Brömme JO, Feldmann RE, Jr, Järve A, Sabouri F, Bürgers HF, et al. Glycogen synthase kinase 3β (GSK3β) regulates differentiation and proliferation in neural stem cells from the rat subventricular zone. J Proteome Res. 2007;6:1198–208. doi: 10.1021/pr0605825. [DOI] [PubMed] [Google Scholar]
- [131].Korur S, Huber RM, Sivasankaran B, Petrich M, Morin P, Jr., Hemmings BA, et al. GSK3beta regulates differentiation and growth arrest in glioblastoma. PLoS ONE. 2009;4:e7443. doi: 10.1371/journal.pone.0007443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Jensen RL. Brain tumor hypoxia: tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a therapeutic target. J Neurooncol. 2009;92:317–35. doi: 10.1007/s11060-009-9827-2. [DOI] [PubMed] [Google Scholar]
- [133].Pietras A, Gisselsson D, Ora I, Noguera R, Beckman S, Navarro S, et al. High levels of HIF-2alpha highlight an immature neural crest-like neuroblastoma cell cohort located in a perivascular niche. J Pathol. 2008;214:482–8. doi: 10.1002/path.2304. [DOI] [PubMed] [Google Scholar]
- [134].Pietras A, Hansford LM, Johnsson AS, Bridges E, Sjolund J, Gisselsson D, et al. HIF-2alpha maintains an undifferentiated state in neural crest-like human neuroblastoma tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106:16805–10. doi: 10.1073/pnas.0904606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104:5431–6. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102:4783–8. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20:7370–6. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Santilli G, Lamorte G, Carlessi L, Ferrari D, Rota Nodari L, Binda E, et al. Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PLoS ONE. 5:e8575. doi: 10.1371/journal.pone.0008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–83. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–41. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- [141].Wang J, Sakariassen PO, Tsinkalovsky O, Immervoll H, Boe SO, Svendsen A, et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122:761–8. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- [142].Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–40. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- [143].Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124–8. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- [144].Fong H, Hohenstein KA, Donovan PJ. Regulation of self-renewal and pluripotency by Sox2 in human embryonic stem cells. Stem Cells. 2008;26:1931–8. doi: 10.1634/stemcells.2007-1002. [DOI] [PubMed] [Google Scholar]
- [145].Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–65. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- [146].Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- [147].Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- [148].Wang J, Wang H, Li Z, Wu Q, Lathia JD, McLendon RE, et al. c-Myc is required for maintenance of glioma cancer stem cells. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Jensen NA, Pedersen KM, Lihme F, Rask L, Nielsen JV, Rasmussen TE, et al. Astroglial c-Myc overexpression predisposes mice to primary malignant gliomas. J Biol Chem. 2003;278:8300–8. doi: 10.1074/jbc.M211195200. [DOI] [PubMed] [Google Scholar]
- [150].Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, et al. Pten and p53 converge on c-Myc to control differentiation, self-renewal, and transformation of normal and neoplastic stem cells in glioblastoma. Cold Spring Harb Symp Quant Biol. 2008;73:427–37. doi: 10.1101/sqb.2008.73.047. [DOI] [PubMed] [Google Scholar]
- [151].Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- [152].Ligon KL, Alberta JA, Kho AT, Weiss J, Kwaan MR, Nutt CL, et al. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol. 2004;63:499–509. doi: 10.1093/jnen/63.5.499. [DOI] [PubMed] [Google Scholar]
- [153].Ligon KL, Huillard E, Mehta S, Kesari S, Liu H, Alberta JA, et al. Olig2-Regulated Lineage-Restricted Pathway Controls Replication Competence in Neural Stem Cells and Malignant Glioma. Neuron. 2007;53:503–17. doi: 10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–57. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- [155].Blom T, Tynninen O, Puputti M, Halonen M, Paetau A, Haapasalo H, et al. Molecular genetic analysis of the REST/NRSF gene in nervous system tumors. Acta Neuropathol (Berl) 2006;112:483–90. doi: 10.1007/s00401-006-0102-8. [DOI] [PubMed] [Google Scholar]
- [156].Lietz M, Cicchetti F, Thiel G. Inverse expression pattern of REST and synapsin I in human neuroblastoma cells. Biol Chem. 1998;379:1301–4. [PubMed] [Google Scholar]
- [157].Zhang P, Lathia JD, Flavahan WA, Rich JN, Mattson MP. Squelching glioblastoma stem cells by targeting REST for proteasomal degradation. Trends Neurosci. 2009;32:559–65. doi: 10.1016/j.tins.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–7. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Bruggeman SWM, Hulsman D, Tanger E, Buckle T, Blom M, Zevenhoven J, et al. Bmi1 Controls Tumor Development in an Ink4a/Arf-Independent Manner in a Mouse Model for Glioma. Cancer Cell. 2007;12:328–41. doi: 10.1016/j.ccr.2007.08.032. [DOI] [PubMed] [Google Scholar]
- [160].Abdouh M, Facchino S, Chatoo W, Balasingam V, Ferreira J, Bernier G. BMI1 sustains human glioblastoma multiforme stem cell renewal. J Neurosci. 2009;29:8884–96. doi: 10.1523/JNEUROSCI.0968-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Chiba T, Miyagi S, Saraya A, Aoki R, Seki A, Morita Y, et al. The polycomb gene product BMI1 contributes to the maintenance of tumor-initiating side population cells in hepatocellular carcinoma. Cancer Res. 2008;68:7742–9. doi: 10.1158/0008-5472.CAN-07-5882. [DOI] [PubMed] [Google Scholar]
- [162].Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455:1129–33. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Hide T, Takezaki T, Nakatani Y, Nakamura H, Kuratsu JI, Kondo T. Sox11 prevents tumorigenesis of glioma-initiating cells by inducing neuronal differentiation. Cancer Res. 2009;69:7953–9. doi: 10.1158/0008-5472.CAN-09-2006. [DOI] [PubMed] [Google Scholar]
- [164].Conti A, Aguennouz M, La Torre D, Tomasello C, Cardali S, Angileri FF, et al. miR-21 and 221 upregulation and miR-181b downregulation in human grade II-IV astrocytic tumors. J Neurooncol. 2009;93:325–32. doi: 10.1007/s11060-009-9797-4. [DOI] [PubMed] [Google Scholar]
- [165].Gal H, Pandi G, Kanner AA, Ram Z, Lithwick-Yanai G, Amariglio N, et al. MIR-451 and Imatinib mesylate inhibit tumor growth of Glioblastoma stem cells. Biochem Biophys Res Commun. 2008;376:86–90. doi: 10.1016/j.bbrc.2008.08.107. [DOI] [PubMed] [Google Scholar]
- [166].Silber J, Lim DA, Petritsch C, Persson AI, Maunakea AK, Yu M, et al. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6 doi: 10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Huszthy PC, Giroglou T, Tsinkalovsky O, Euskirchen P, Skaftnesmo KO, Bjerkvig R, et al. Remission of invasive, cancer stem-like glioblastoma xenografts using lentiviral vector-mediated suicide gene therapy. PLoS ONE. 2009;4:e6314. doi: 10.1371/journal.pone.0006314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Piccirillo SG, Combi R, Cajola L, Patrizi A, Redaelli S, Bentivegna A, et al. Distinct pools of cancer stem-like cells coexist within human glioblastomas and display different tumorigenicity and independent genomic evolution. Oncogene. 2009;28:1807–11. doi: 10.1038/onc.2009.27. [DOI] [PubMed] [Google Scholar]