Abstract
The observation that maternal infection increases the risk for schizophrenia in the offspring suggests that the maternal immune system plays a key role in the etiology of schizophrenia. In a mouse model, maternal immune activation (MIA) by injection of poly(I:C) yields adult offspring that display abnormalities in a variety of behaviors relevant to schizophrenia. As abnormalities in the hippocampus are a consistent observation in schizophrenia patients, we examined synaptic properties in hippocampal slices prepared from the offspring of poly(I:C)- and saline-treated mothers. Compared to controls, CA1 pyramidal neurons from adult offspring of MIA mothers display reduced frequency and increased amplitude of miniature excitatory postsynaptic currents. In addition, the specific component of the temporoammonic pathway that mediates object-related information displays increased sensitivity to dopamine. To assess hippocampal network function in vivo, we used expression of the immediate early gene, c-Fos, as a surrogate measure of neuronal activity. Compared to controls, the offspring of poly(I:C)-treated mothers display a distinct c-Fos expression pattern in area CA1 following novel object, but not novel location, exposure. Thus, the offspring of MIA mothers may have an abnormality in modality-specific information processing. Indeed, the MIA offspring display enhanced discrimination in a novel object recognition, but not in an object location, task. Thus, analysis of object and spatial information processing at both synaptic and behavioral levels reveals a largely selective abnormality in object information processing in this mouse model. Our results suggest that altered processing of object-related information may be part of the pathogenesis of schizophrenia-like cognitive behaviors.
Keywords: dopamine, hippocampus, schizophrenia, poly(I:C), temporoammonic pathway
INTRODUCTION
Schizophrenia is a major psychiatric disorder with a strong genetic contribution (Burmeister et al., 2003; Bertolino and Blasi, 2009). Nonetheless, epidemiologic evidence indicates that genetic factors alone cannot explain the pathogenesis. For example, the concordance for schizophrenia in monozygotic twins is approximately 48% (Gottesman, 1991). Furthermore, while the concordance between monozygotic twins who share a placenta is 60%, the concordance rate between such twins who do not share a placenta is only 10% (Davis et al., 1995; Patterson, 2007). These studies suggest the importance of the fetal environment. Supporting this idea, Mednick et al. (1988) reported that fetuses gestating during a viral epidemic are at elevated risk for developing schizophrenia. Subsequent prospective studies have shown that maternal infections of various types increase the risk for schizophrenia in the offspring 3–7 fold (reviewed by Patterson, 2009; Brown and Derkits, 2010).
Based on this evidence, several animal models of MIA have been established (reviewed by Meyer et al., 2009; Patterson, 2009). Among these, administration of the synthetic dsRNA, poly(I:C) can effectively induce MIA, resulting in offspring that display a variety of behaviors and neuropathologies that are consistent with those seen in schizophrenia patients (Meyer and Feldon, 2009; Patterson, 2009). Thus, this animal model is useful for investigating the pathophysiology of schizophrenia.
Clinical studies reveal an important role for dopamine (DA)-mediated signaling in the pathophysiology of schizophrenia. For example, drugs that increase DA release induce several aspects of schizophrenic psychosis in normal adults, and exacerbate psychotic symptoms in patients with schizophrenia (Angrist and Vankammen, 1984; Lieberman et al., 1987). Moreover, all drugs currently in widespread use for the treatment of schizophrenia block DA D2 receptors (Creese et al., 1976). Other studies suggest that there is a deficit in DA D1 receptor-mediated transmission in prefrontal areas of schizophrenic patients (Davis et al, 1991; Toda and Abi-Dargham, 2007). Indeed, imaging studies of patients reveal an increased D2 receptor density in the striatum (Weinberger and Laruelle, 2001) and a decreased D1 receptor density in the prefrontal cortex (Okubo et al., 1997).
Deficits in other cortical regions may also play a key role in the pathophysiology of schizophrenia. Among them, hippocampal abnormalities are commonly found (Heckers and Konradi, 2002). Lipska et al. (1993) suggested that the important variable is the developmental period during which the hippocampal damage takes place, because lesion of the adult rat hippocampus fails to produce schizophrenia-like behaviors, while hippocampal disruption in neonatal stages causes these behavioral alterations to emerge in adulthood. Most importantly in the present context are recent studies showing a reciprocal functional interaction between the DA system and the hippocampus (Lisman and Grace, 2005), and DA D1- and D2-like receptors are highly expressed in the hippocampus (Wamsley et al. 1989; Meador-Woodruff et al., 1994; Khan et al., 1997). Together, these studies indicate that hippocampal dysfunction participates in the pathogenesis of schizophrenia.
Considering these findings, we used the poly(I:C) MIA mouse model to investigate the pathogenesis of schizophrenia-like behaviors. We focus on the hippocampal network and the influence of DA, conducting experiments at the synaptic as well as the behavioral level. The data suggest a link between synaptic dysfunction, DA and altered behavior.
MATERIALS AND METHODS
Animals
Pregnant C57BL/6J mice were injected either i.v. with 5 mg/kg or i.p. with 20 mg/kg poly(I:C) potassium salt freshly dissolved in 0.9% sterile saline on E12.5. Both doses and routes of administration have been used to induce MIA and behaviors relevant to schizophrenia in adult offspring (Meyer et al., 2006; Smith et al., 2007). Control females were injected with the same volume of saline. The offspring were undisturbed until weaning on P21. Offspring were behaviorally tested from 6 to 11 weeks for pre-pulse inhibition (PPI), latent inhibition and open field exploration, to confirm behavioral deficits observed in previous studies (Shi et al., 2003; Smith et al., 2007; data not shown), and for Morris water maze, object location and novel object recognition. Five litters each from saline- and poly(I:C)-treated mothers were generated (3 by i.p. injection and 2 by i.v. injection), and four litters each were used for analysis in the present study. One pair of litters generated by i.p injection was not included because control and MIA offspring did not show significant differences in PPI and latent inhibition. The other excluded pair was used for other experiments. The litters generated by i.p. injection (24 offspring pairs in total) were used for the analysis in Figs. 1A, 1B, 2D, 2E and for all of the behavioral analysis in Fig. 5. The rest of experiments, including those in supplemental figures, were conducted using the litters generated by i.v. injection (28 offspring pairs in total). No striking differences in PPI, latent inhibition or open field behavior was observed between the offspring of i.p. and i.v. injected mothers. To avoid influences of prior behavioral testing, animals used for electrophysiology, immunohistochemisty and c-Fos expression analysis were sacrificed at least 3 days after the last behavioral test.
Figure 1. CA1 pyramidal neurons in MIA offspring display a reduced frequency and an increased amplitude of mEPSCs, but no significant difference in mIPSCs.
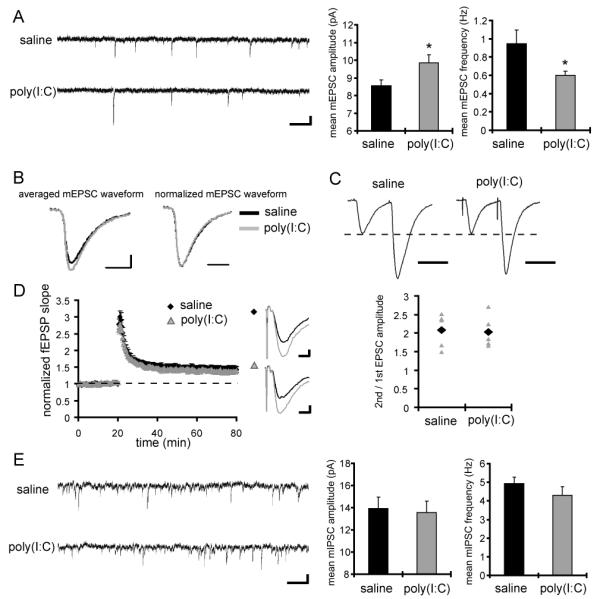
(A) Typical recordings and quantification of mEPSCs from CA1 pyramidal neurons are shown (saline: n = 11 neurons, poly(I:C): n = 12 neurons, 4 pairs of animals) (scale bar = 500 ms, 10 pA) (*p < 0.05 relative to control). (B) Averaged mEPSC waveforms from animals used for the analysis in A are shown on the left. The mEPSC waveform in MIA offspring shows larger amplitude compared to that in control animals. To examine the kinetics of the waveforms, the amplitudes of mEPSC waveforms are normalized on the right. There is no significant difference in kinetics of mEPSCs between the groups. (C) Whole-cell voltage-clamp recordings were performed in CA1 pyramidal neurons under inhibitory blockade with the GABA receptor antagonists bicuculline (10 μM) and CGP55845 (1 μM). Membrane potential was clamped at −60 mV. Paired-pulse facilitation (PPF) was analyzed at Schaffer-collateral-CA1 synapses. The left panel shows representative waveforms (interstimulus interval is 50 ms; scale bar = 50 ms), and the right panel shows the PPF ratio (2nd/1st EPSC amplitude) of individual recordings (gray triangles), and the mean values (black diamonds) (n = 5 neurons for each group, 3 pairs of animals). (D) Extracellular field recordings were performed at Schaffer-collateral-CA1 synapses under fast inhibitory transmission block with the GABAA receptor antagonist bicuculline. The left panel shows the normalized fEPSP slope from control animals (black diamonds) and MIA offspring (gray triangles) and LTP was induced at 20 min by a single train of 100 stimuli at 100 Hz. The right panel shows representative waveforms before (black) and after (gray) LTP induction (scale bar = 0.2 mV, 5 ms). No significant difference is observed in LTP magnitude between animal groups (n = 8 slices for each group, 3 pairs of animals). (E) Typical recordings and quantification of mIPSCs from CA1 pyramidal neurons are shown (n = 12 neurons for each group, 3 pairs of animals). A cesium chloride-based solution was used as the internal solution of patch pipettes and recordings were made at 28 °C in the presence of TTX (1 μM), NBQX (20 μM) and APV (50 μM) to block excitatory synaptic transmission. The extracellular potassium concentration was increased from 2.5 mM to 5 mM to enhance the frequency of miniature synaptic events. No significant difference is observed in mIPSC amplitude or frequency between the groups.
Figure 2. The offspring of poly(I:C)-treated mothers display increased DA-induced depression at distal temporoammonic-CA1 synapses.
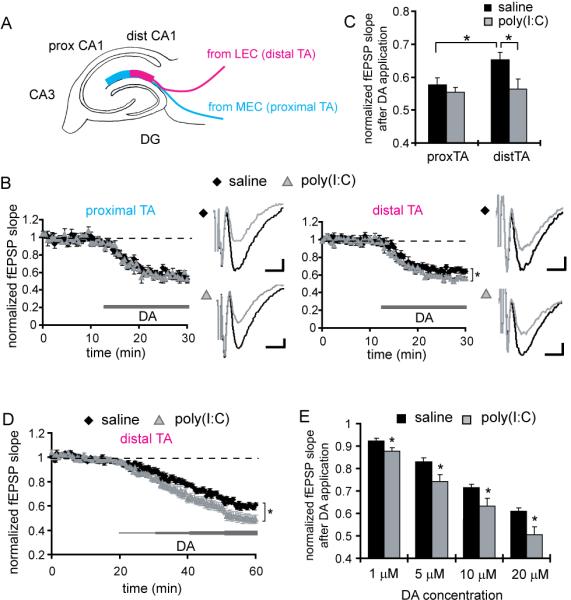
(A) A schematic diagram is shown depicting the two distinct axonal projections in the TA pathway from MEC and LEC. (B) Extracellular field recordings are obtained simultaneously from proximal and distal TA-CA1 synapses. For each recording from proximal or distal TA-CA1 synapses, the left panels show the normalized fEPSP slopes from control (black diamonds) and MIA offspring (gray triangles), and the right panels show representative traces of fEPSP waveforms before (black) and after (gray) DA application (scale bar = 0.05 mV, 5 ms). The test pulse was applied every 30 sec and DA (20 μM) was bath applied during the time indicated by the thick line (*p < 0.05 relative to control) (saline: n = 7 slices, poly(I:C): n = 6 slices, 4 pairs of animals). (C) In hippocampal slices prepared from control animals, DA induces a significantly larger depression at proximal compared to distal TA-CA1 synapses. While the slices prepared from MIA offspring show a normal DA-induced depression at proximal synapses, they exhibit a significantly larger depression at distal TA-CA1 synapses, compared to controls (*p < 0.05). (D) Hippocampal slices prepared from MIA offspring show increased DA-induced depression at distal TA-CA1 synapses. The DA concentration was increased every 10 min, from 1 to 20 μM (*p < 0.05 relative to control). (E) Analysis of the data in D is shown. The mean fEPSP slopes at 5–10 min after the application of each designated concentration of DA were analyzed. Slices prepared from MIA offspring show a significantly larger depression at each DA concentration examined (n = 12 slices for each group, 8 pairs of animals) (*p < 0.05 relative to control).
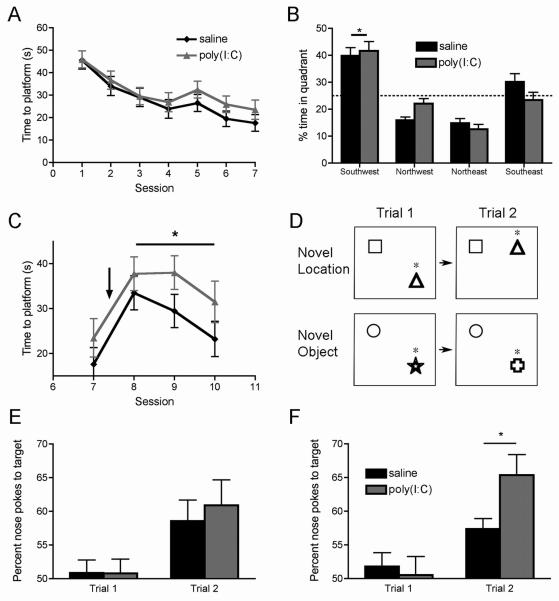
Figure 5. The offspring of poly(I:C)-treated mothers display behavioral inflexibility in platform relocation in the Morris water maze task, and abnormal preference in the novel object recognition task.
(A) In the Morris water maze, the latency to find the platform is similar in the MIA offspring compared to controls (saline: n = 16, poly(I:C): n= 17). (B) Both control and MIA offspring show a significant learned preference for the target quadrant in the session 7 probe trial, which was not present before training (*p < 0.05 vs. all other quadrants). (C) When the location of the platform was moved after training (indicated by arrow), the MIA offspring do not learn the new location as quickly as controls (*p < 0.05). The experimental groups diverge significantly after the platform is moved, at the point indicated by the arrow. (D) A graphical representation of the object location and novel object recognition tests illustrates how the location of the target object, or the type of target object itself, is changed in the 5 min interval between trial 1 and 2. Asterisks indicate the target objects. (E) In the novel location test, both control and MIA offspring show a significant preference for the target object in trial 2, compared to trial 1 (p < 0.05), but there is no difference between control and MIA offspring. (F) In the novel object recognition test, both groups also display a significant preference for the target object in trial 2 compared to trial 1 (p < 0.05). Moreover, compared to control animals, the MIA offspring show a significantly greater preference for the target object in trial 2 (n = 16 animals per group) (*p < 0.05).
Hippocampal slice preparation
For each experiment, hippocampal slices were made from paired adult offspring (7–15 week old, a pair of the same age and sex for each set of experiments were randomly selected from each litter without regard to the results of behavioral tests) from saline- and poly(I:C)-treated mothers. In brief, a vibrating microtome (Leica VT1000S) was used to cut transverse hippocampal sections from the intermediate part of a dorsoventral axis of the hippocampus (400 μm thickness) in ice-cold, oxygenated artificial cerebrospinal fluid (ACSF) containing (in mM) 119 NaCl, 2.5 KCl, 1.3 MgSO4, 2.5 CaCl, 1.0 NaH2PO4, 26.2 NaHCO3, 11.0 glucose. Slices were recovered at room temperature for at least 2 hours in an interface chamber, and then transferred to a submerged recording chamber perfused with ACSF. Concentric bipolar tungsten electrodes (FHC) and stimulus isolators (Axon Instruments) were used for the stimulation.
Electrophysiology
Extracellular field potential recordings were made with 1–3 MΩ resistance microelectrodes filled with 3 M NaCl using a bridge amplifier (Axoclamp 2B, Molecular Devices). The recordings were made at 25°C. Whole-cell voltage-clamp recordings from CA1 pyramidal neurons were obtained without visualization with an Axopatch 200B (Molecular Devices). The internal solution of whole-cell patch pipettes contained (in mM) 115 cesium gluconate, 20 cesium chloride, 10 sodium phosphocreatine, 10 HEPES, 0.2 EGTA, 2 MgATP, 0.3 NaGTP (pH 7.3). The membrane capacitance was cancelled and series resistance was compensated (60–70%) for paired-pulse facilitation experiments, but uncompensated for miniature recordings. Recordings were discarded when the series resistance was over 20 MΩ or either series or membrane resistance changed more than 20% (30% for mIPSC recordings) during data acquisition. The amplitude of test pulses was 20–40 A for recordings from SC-CA1 synapses and 50–150 A for recordings from TA-CA1 synapses, and the duration of pulse was 100 μs. The test pulses were applied once every 30 s. Dopamine was obtained from Sigma. All other drugs were obtained from Tocris. For mEPSC recordings, whole-cell patch clamp recordings were obtained from CA1 pyramidal neurons in extracellular solution containing TTX (1 μM) and bicuculline (10 μM) at 25 °C. For mIPSC recordings, the internal solution of patch pipettes was a cesium chloride-based solution (in mM): 115 cesium chloride, 20 KCl, 10 sodium phosphocreatine, 10 HEPES, 0.2 EGTA, 2 MgATP, 0.3 NaGTP (pH 7.3). The recordings were made at 28 °C, using TTX (1 μM), NBQX (20 μM) and APV (50 μM) to block excitatory synaptic transmission, and the extracellular potassium concentration was increased from 2.5 mM to 5 mM to enhance the frequency of miniature synaptic events. Membrane voltage was clamped at −70 mV. For the analysis of paired-pulse facilitation, whole-cell patch clamp recordings were obtained from CA1 pyramidal neurons. The membrane potential was clamped at −60 mV, and recordings were made at 25 °C under bicuculline (10 μM) and CGP55845 (1 μM) to block inhibitory synaptic transmission. The interstimulus interval was 50 msec. The LTP induction protocol was a single train of 100 pulse stimuli at 100 Hz. All stimulus pulses were of the same length and amplitude as test pulses. The baseline fEPSP slope prior to LTP induction was adjusted within the range of 0.2–0.3 mV/ms.
Behavioral manipulations for c-Fos expression analysis
All the behavioral manipulations were carried out at night (12–4 am) to maximize active exploration of the environment. The objects used for novel object exposure were two small children’s toys, made of either plastic or wood. The new cage for novel place exposure was in the same color and dimensions as the original cage, but had new woodchip bedding of a different scent and texture than the prior bedding, and the new cage did not have a food box on the ceiling. Such sensory cue changes in the environmental context, without geometric changes in the cage configuration, are sufficient for remapping of hippocampal place cell activities (Anderson and Jeffery, 2003), implying that animals should recognize the new cage as a different context. More radical changes in the new cage configuration were not made in an effort not to introduce fear or anxiety in the mice.
Behavioral tests
Water maze task
Water maze testing followed previously published procedures (Zuckerman and Weiner 2005). Briefly, mice were introduced to the maze (water made opaque with non-toxic latex paint) in a random spot and allowed 60 s to find a platform submerged 1 cm under the water. The walls of the room contained visual cues (four 0.5 m diameter pieces of different color paper cut into different shapes). Mice were given four trials per session, two sessions per day separated by approx 4 hours. In between trials mice were allowed to recover for approx 5 min in a cage lined with paper towels and warmed with an electric heating pad. If the mice found the platform within 60 s, the time to find the platform was recorded and the mouse was removed after 10 s on the platform; if it did not find the platform it was placed on the platform for 10 s and the time was recoded as 60 s. On the afternoon of the 4th day, the platform was removed and swim pattern was recorded for 60 s (probe trial). On the day following the probe trial, the platform was placed in the opposite quadrant of the pool, and three more sessions were conducted with the platform in the novel location. Finally, the water level was dropped and four visible platform trials were conducted.
Novel object recognition and object location recognition tasks
Mice were first allowed to explore the environment (a 50 cm-square white plastic box) for 10 min. They were then removed for 5 minutes, during which time two different objects were placed in opposite corners of the box, and the mice were allowed to explore the objects for 5 min. The mice were again removed from the box for five minutes, and for the novel object recognition test, one of the objects was replaced with a novel object (the target object), and the mice were allowed to explore for another 5 min. On the following day, the object location test was administered. The procedure was identical to the previous one except that new objects were used, and rather than replacing the target object during the second phase of the test, it was moved 90 degrees to a different corner of the box (see fig. 7D). To eliminate experimental bias due to innate preference for an object, the five objects used (a plastic pickle, a “mini-koosh” ball, a plastic top, a small metal knife, and a small candle) were pseudo-randomized such that different mice saw different combinations of objects at each stage of the test; however no innate preferences for an object were observed.
Data analysis
Electrophysiology
Data were collected using a custom-written program (LabView data acquisition system; National Instruments) for extracellular recordings, or DigiData 1200 and pClamp 9 (Molecular Devices) for whole-cell recordings. After recordings were stabilized, current traces were acquired for 3 min. The mEPSCs were detected using a template-matching algorithm of Clampfit 9 (Molecular Devices). All numerical values listed represent mean ± s.e.m. Student’s t-test was performed to analyze the signficance of the data.
Immunohistochemistry
Slices (400 μm thickness) were prepared from randomly selected pairs of animals using the same procedures as for electrophysiology recordings. After cutting, slices were quickly fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for at least 2 days. Thin (50 μm) sections were cut with a vibrating microtome (Leica VT1000S).
The sections were incubated overnight with either of 1:250 concentration of anti-c-Fos (sc-52) (Santa Cruz), 1:1000 of anti-synaptophysin (Millipore), 1:1000 of anti-synapsin I (Millipore), 1:1000 of anti-Bassoon (Stressgen), 1:1000 of NeuN (Millipore), 1:1000 of GluR1 (Millipore), or 1:1000 of anti-MAP2 (Sigma) antibodies. The incubation was carried out at room temperature in Tris-buffered saline containing 0.2% Triton X-100, BSA 2%, NGS 4%, followed by 4 hrs of secondary-antibody incubation with 1:1000 of Alexa 488-conjugated anti-rabbit and 1:1000 of Alexa 543-conjugated anti-mouse antibodies (Invitrogen).
For the analysis of immunohistochemistry experiments, images were obtained with Zeiss LSM 510 laser scanning confocal microscopes using a Plan-Neofluor 10×/0.3 air objective. Alexa 488 and 546 were visualized by excitation with the 488 line of an argon ion laser and the 543 nm line of a HeNe laser, respectively. The optical section was 20 μm and fluorescent signals were acquired throughout the section thickness. Each 50 μm section was obtained from a different 400 μm slice and two sections were analyzed from each animal. Slices were obtained from the same septo-temporal position in all experiments. To count the number of c-Fos positive neurons, fluorescent signals of less than the mean + 2 SD were excluded. Then automated particle analysis was carried out using ImageJ (NIH) based on the following criteria: the particle size was larger than 39 μm2 and the circularity was larger than 0.5. Statistical differences between animals groups were assessed by ANOVA.
Behavioral analysis
Water maze task
All trials were recorded by a camera suspended over the pool, and data was analyzed with Ethovision (Noldus). Swim speed, mean distance swam and performance in the visible platform task was not significantly different between groups (data not shown).
Novel object recognition and object location tasks
The trials were recorded by an overhead camera, and object investigation was measured as the number of times a mouse brought its nose within 2 cm of the object, by an observer blind to the identification of each mouse. Data was expressed as percent nose pokes to the target object (nose-pokes to target / [nose-pokes to target + nose-pokes to non-target object] × 100), where the target object is represented by the novel object during the novel object recognition task and by the re-located object during the object location task.
RESULTS
CA1 pyramidal neurons in the offspring of poly(I:C)-treated mothers display a reduced frequency and increased amplitude of mEPSCs
A number of studies indicate that the brains of schizophrenia patients exhibit a reduction in the volume of the hippocampus (Nelson et al., 1998; Heckers and Konradi, 2002). Although most studies on schizophrenia patients report no significant change in neuronal density in the hippocampus (Dwork, 1997; Harrison, 1999), many post-mortem studies have reported an abnormal expression of synaptic proteins, including synaptophysin (Eastwood and Harrison, 1995; Davidsson et al., 1999), synapsin I (Browning et al., 1993), SNAP-25 (Young et al., 1998) and spinophilin (Law et al., 2004).
Therefore, we asked whether the MIA offspring display abnormalities in synaptic number or efficacy in CA1 pyramidal neurons. We observe an increased amplitude, but decreased frequency in miniature excitatory postsynaptic currents (mEPSCs) (amplitude: saline 8.5 ± 0.3 pA, poly(I:C) 9.9 ± 0.5 pA; frequency: saline 0.95 ± 0.15 Hz, poly(I:C) 0.60 ± 0.05 Hz) (Fig. 1A). There is no significant difference in the kinetics of mEPSC waveforms (Fig. 1B) or membrane properties (membrane capacitance: saline 220 ± 36 pF, poly(I:C) 210 ± 33 pF, membrane resistance: saline 194 ± 62 MΩ, poly(I:C) 149 ± 55 MΩ, in mean ± SD), suggesting that the observed differences in mEPSC amplitude and frequency are primarily due to altered synaptic properties. The decrease in mEPSC frequency suggests either presynaptic dysfunction or a reduction in excitatory synapse number per neuron. To assess presynaptic function, we analyzed paired-pulse facilitation but do not find a significant difference between the groups (saline: 2.1 ± 0.2, poly(I:C): 2.0 ± 0.2; ratio of 2nd to 1st EPSC amplitude) (Fig. 1C), suggesting that presynaptic function in the experimental group is normal and that the reduced mEPSC frequency is likely due to a reduction in excitatory synapse number. The increase in mEPSC amplitude in the offspring of poly(I:C)-treated mothers may be a compensatory response for the reduction of mEPSC frequency (Turrigiano et al., 1998; Sutton et al., 2006). We also tested synaptic plasticity at Schaffer-collateral-CA1 synapses. When LTP was induced by a single train of 100 stimuli at 100 Hz, the magnitude of LTP in slices prepared from the offspring of poly(I:C)-treated mothers is similar to controls (saline: 1.45 ± 0.05, poly(I:C): 1.36 ± 0.07; mean fEPSP slope at 55 – 60 min after LTP induction relative to the baseline) (Fig. 1D). Taken together, these results indicate that the adult offspring of poly(I:C)-treated mothers display a reduced number of normally functioning excitatory synapses on CA1 pyramidal neurons.
We next performed immunohistochemistry of the synaptic proteins synaptophysin and GluR1 in area CA1. Dendritic distribution of these proteins is similar between control and MIA offspring. In contrast, we found that a cytoskeletal protein, MAP2, shows a distinct distribution along the dendritic axis of CA1 pyramidal neurons in MIA offspring (Supplemental Fig. 1), which is consistent with a study on schizophrenia patients showing an elevated expression of MAP2 in the hippocampus (Cotter et al., 2000).
To examine inhibitory synaptic transmission, we recorded mIPSCs from CA1 pyramidal neurons. We do not find a significant difference in either amplitude or frequency of mIPSCs between groups (amplitude: saline 13.9 ± 1.1 pA, poly(I:C) 13.6 ± 1.0 pA, frequency: saline 4.9 ± 0.3 Hz, poly(I:C) 4.3 ± 0.5 Hz) (Fig. 1E), suggesting that the function of inhibitory synapses is normal in area CA1.
Temporoammonic-CA1 synapses in MIA offspring display increased sensitivity to dopamine
Many lines of evidence indicate that DA signaling plays an important role in hippocampal function, as well as in the pathophysiology of schizophrenia. For example, DA neurons in the substantia nigra compacta and ventral tegmental area innervate the hippocampus (Swanson, 1987; Gasbarri et al., 1997) and release DA when animals are exposed to novel environments (Ihalainen et al., 1999). This neurotransmitter influences hippocampal synaptic plasticity (Huang and Kandel, 1995; Otmakhova and Lisman, 1996; Li et al., 2003) and frequency-dependent synaptic transmission (Ito and Schuman, 2007). Furthermore, disruption of DA-mediated signaling impairs hippocampal-dependent learning (Gasbarri et al. 1996; El-Ghundi et al., 1999; Rossato et al., 2009). Therefore, we investigated DA-mediated synaptic control in hippocampal area CA1. As previous studies showed a selective influence of DA on the temporoammonic (TA)-CA1 synapses compared to Schaffer-collateral-CA1 synapses (Otmakhova and Lisman, 1999; Ito and Schuman, 2007; Supplemental Fig. 2), we examined DA modulation of TA-CA1 synapses. The TA pathway includes axonal populations from both the medial (MEC) and lateral (LEC) entorhinal cortexes (Steward, 1976; Witter and Amaral, 2004). These projections are topographically organized along the transverse axis of area CA1, such that the projections from MEC make synapses at proximal CA1 (close to CA3), while those from the LEC project to distal CA1 (close to subiculum) (Fig. 2A; Steward, 1976; Witter and Amaral, 2004). Previous anatomical studies in the mouse brain showed a topographic projection of the TA pathway similar to that observed in the rat brain (van Groen et al., 2003). Our immunohistochemical analysis of the presynaptic proteins synapsin I and bassoon in the stratum lacunosum-moleculare indeed indicates expression differences between distal and proximal regions of area CA1 (Supplemental Fig. 3). Thus, the presynaptic properties of the proximal and distal TA-CA1 synapses in the topographic projection of entorhinal-cortical fibers may be distinct. This organization of presynaptic proteins is observed in both MIA and control offspring (Supplemental Fig. 3).
We recorded fEPSPs simultaneously from proximal and distal TA-CA1 synapses. The application of DA (20 μM; Otmakhova and Lisman 1999) to slices prepared from control mice induces a significantly larger depression at proximal TA-CA1 synapses compared to distal TA-CA1 synapses (proximal TA: 57.6 ± 2.2%; distal TA: 65.3 ± 2.3%, relative to baseline; Fig. 2B and 4C). Furthermore, we found that another neuromodulator, norepinephrine (NE), also differentially controls TA synapses made by MEC and LEC inputs (Supplemental Fig. 4). These data indicate that the neuromodulators, DA and NE, can differentially control MEC and LEC inputs to area CA1 of the mouse hippocampus.
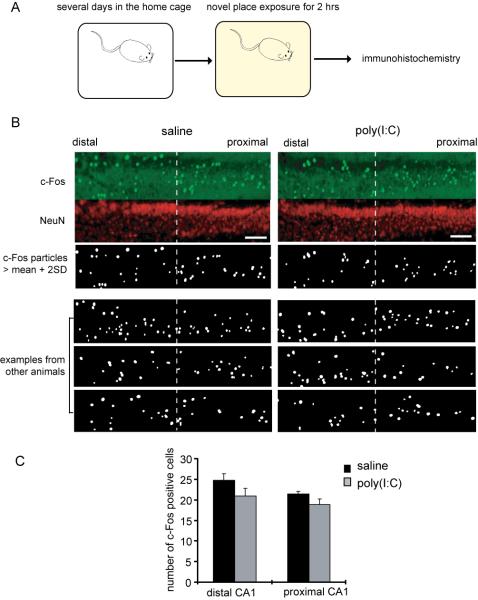
Figure 4. The offspring of poly(I:C)-treated mothers display comparable c-Fos expression in area CA1 pyramidal neurons following novel place exposure.
(A) A schematic diagram of the behavioral procedure is shown. After mice were accommodated to the new home cage for a few days, they were moved to a novel cage. After a 2 hr exposure, immunohistochemistry on the hippocampus was carried out. (B) Examples of c-Fos expression in the pyramidal layer of area CA1 are shown. The pyramidal layer was divided into equal proximal and distal regions. The c-Fos signals that are larger than the mean + two times the standard deviation, were quantified using ImageJ (see Methods for details) (scale bar = 100 μm). (C) The number of c-Fos signals was quantified in proximal and distal regions of CA1 pyramidal layer (n = 5 pairs of animals, 2 sections analyzed from each).
In slices prepared from MIA offspring, DA induces a depression comparable to that observed in control slices at proximal TA-CA1 synapses (proximal TA: 55.4 ± 3.1%, relative to baseline; Fig. 2C). At distal TA-CA1 synapses, however, the slices prepared from MIA offspring show a significantly larger depression compared to controls (distal TA: 56.3 ± 1.4%, relative to baseline; Fig. 2C). To further examine DA sensitivity at distal TA-CA1 synapses, the neurotransmitter was applied sequentially from low to high concentration to acute slices (Fig. 2D) and DA-mediated depression quantified. We find that, compared to controls, the amount of depression is significantly larger at each DA concentration in the slices prepared from the MIA group (Fig. 2E). These results indicate that the adult offspring of poly(I:C)-treated mothers display an enhanced sensitivity to DA selectively at LEC inputs.
The offspring of poly (I:C)-treated mothers display a distinct c-Fos expression pattern in hippocampal area CA1 following novel object exposure
While the major afferent inputs to the hippocampus are provided by the entorhinal cortex (Cajal, 1911), recent studies demonstrated that two subdivisions of the entorhinal cortex, MEC and LEC, provide distinct information modalities to the hippocampus. Spatial information is carried by axons from the MEC, whereas nonspatial, or object information is carried by axons from the LEC (Haagreaves et al., 2005; Knierim et al., 2006; Manns and Eichenbaum, 2006). Because MIA offspring display higher sensitivity to DA in the LEC projection to area CA1, these animals may exhibit abnormal object information processing.
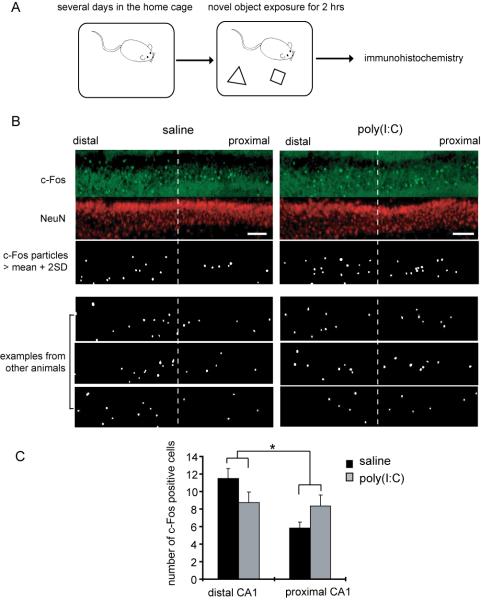
One of the major features shared by hippocampal and DA-releasing neurons in vivo is the modulation of neuronal activity by stimulus novelty (Knight, 1996; Schultz, 1998; Horvitz, 2000; Rutishauser et al., 2006). Therefore, we examined how hippocampal neurons are activated in vivo during novel object exposure using immunostaining for an immediate-early gene product, c-Fos (Morgan and Curran, 1991). Immediate early gene expression in resting animals is very low (e.g. Supplemental Fig. 5C), but rapidly increases following patterned neuronal activity that induces synaptic plasticity (Cole et al., 1989), suggesting that c-Fos expression can be used as a surrogate marker for synaptic modification (Guzowski et al., 2005). Following accomodation to the home cage for several days, control and experimental mice were exposed to novel objects in the home cage (Fig. 3A). After 2 hrs of exposure, animals were sacrificed and immunohistochemistry performed. Control mice show differential c-Fos expression between proximal and distal CA1 pyramidal neurons (Figs. 3B, C and Supplemental Fig. 5A). In contrast, MIA offspring do not show clear differential c-Fos activation between proximal and distal CA1 pyramidal neurons (Figs. 3B, C and Supplemental Fig. 5A). These results suggest that MIA offspring display abnormal object information processing in the hippocampus.
Figure 3. The offspring of poly(I:C)-treated mothers display abnormal c-Fos expression in area CA1 pyramidal neurons following novel object exposure.
(A) A schematic diagram of the behavioral procedure is shown. After mice were accommodated to their new home cages for a few days, two novel objects were placed in the cage. After a 2 hr exposure to allow c-Fos gene activation to be expressed as protein, the hippocampus was processed for immunohistochemistry. (B) Examples of c-Fos expression in the pyramidal layer of area CA1 are shown. The pyramidal layer was divided into equal proximal and distal regions. The c-Fos signals that are larger than the mean + two times the standard deviation, were quantified using ImageJ (see Methods for details) (scale bar = 100 μm). (C) The number of c-Fos signals was quantified in proximal and distal regions of the CA1 pyramidal layer. A two-way ANOVA was performed with 2 variables: animal group (saline vs. poly(I:C)) and CA1 subregion (distal vs. proximal), and revealed a significant interaction (*p < 0.05) (n = 6 pairs of animals, 2 sections analyzed and averaged from each).
We also examined c-Fos expression after animals were exposed to a novel cage environment. Following accommodation to the home cage for several days, animals were placed in a new cage, which lacked a food box and contained new bedding with a different texture and scent than the prior bedding. After 2 hrs of such novel location exposure, animals were sacrificed and immunohistochemistry performed (Fig. 4A). In contrast to the results after novel object exposure (Fig. 3), we observe a similar c-Fos expression pattern in the transverse-axis of area CA1 between the offspring of saline- and poly(I:C)-treated mothers (Figs. 4B, C and Supplemental Fig. 5B). Thus, MIA offspring appear to have a selective abnormality in object, but not spatial, information processing. This could be due to hyper-DA sensitivity in LEC inputs at TA-CA1 synapses because our previous studies indicate that neuromodulators play a key role in novel object-driven differential c-Fos expression between proximal and distal CA1 (Ito and Schuman, submitted).
The offspring of poly(I:C)-treated mothers display behavioral inflexibility and abnormal novel object recognition
Our slice recording and c-Fos expression analyses indicate that MIA offspring have a selective abnormality in nonspatial information processing in the hippocampus. To examine if these animals display a corresponding behavioral abnormality, we tested the performance of hippocampus-dependent behavior using the Morris water maze task (Morris, 1984).
We do not find a significant difference in the learning of the initial platform location between experimental and control groups (Fig. 5A, B), suggesting that the MIA offspring have normal ability to acquire spatial navigation memory. After animals learned the initial platform location, we moved the platform to a different location. Two-way ANOVA with session number and prenatal treatment as variables reveals a significant effect of prenatal treatment (T1,222=5.693, p < 0.05), as well as a significant effect of session number (T3,222=5.875, p < 0.01) with no interaction between the two variables, indicating that while both groups improve their performance over time, the MIA offspring display a significantly slower learning of the new platform location (Fig. 5C). Thus, although the MIA offspring have normal ability to learn a spatial context per se, they have difficulty in adapting to a change introduced in a previously-learned context.
To further test this idea, we examined how these animals perform in novel object recognition and object location tasks. We placed two different objects in a test cage and allowed animals become familiar with them. One object was subsequently moved to a new location (object location test) (Fig. 5D). Both groups of animals display a higher preference for the moved object, and we do not find a significant difference in the magnitude of preference between groups (Fig. 5E). In another set of experiments, after familiarization with the two objects, a novel object replaced one of the familiar ones at the same location (novel object recognition test) (Fig. 5D). Although both groups of animals display preference for the new object, the MIA offspring display a significantly stronger preference than control animals (Fig. 5F). Thus, the MIA offspring display abnormally high sensitivity to a novel object, but not to a novel location, which may be due to altered processing of nonspatial information in the hippocampus of these animals. This is consistent with the slice recording and c-Fos findings.
DISCUSSION
Reduced excitatory input on CA1 pyramidal neurons
Our electrophysiological studies demonstrate that the offspring of poly(I:C)-treated mothers display a decreased number, but enhanced efficacy, of excitatory synapses on CA1 pyramidal neurons. Synaptic abnormalities have also been observed in area CA3 in schizophrenia patients, including abnormal mRNA expression of presynaptic proteins (Harrison and Eastwood, 2001).
We do not observe a significant abnormality in the mIPSC amplitude or frequency in the offspring of poly(I:C)-treated animals. Although several studies have reported a decreased number of GABAergic neurons in the hippocampus, most of these differences were observed in area CA2/3, not in area CA1 (Benes et al., 1996; Benes et al., 1997; Heckers and Konradi, 2002). Thus, GABAergic input to area CA1 pyramidal neurons appears normal in both our model and in patients.
Increased dopamine sensitivity in the temporoammonic pathway
Compared to the Schaffer-collateral pathway in area CA1, the TA pathway has been a relatively unexplored circuit in the hippocampus. Many recent findings highlight its unique role in hippocampal function, however (Brun et al., 2002; Remondes and Schuman, 2004; Nakashiba et al., 2008). Some interesting features of this pathway include its topographic projection pattern and its sensitivity to neuromodulators.
A topographic projection in which LEC- or MEC-derived axons terminate in different regions allows the EC to send nonspatial and spatial information to distinct neuronal populations in area CA1 (Steward, 1976; Witter and Amaral, 2004). The efferents from area CA1 are also topographically organized such that neurons in proximal CA1 send projections back to the MEC, while neurons in distal CA1 project back to the LEC (Tamamaki and Nojyo, 1995). Thus, two independent circuit loops for nonspatial and spatial information exist between the EC and CA1. This architecture, based on the TA pathway, may allow the hippocampus to independently process nonspatial and spatial information, providing a unique role for the TA pathway in the hippocampus.
Sensitivity to neuromodulators is another feature of the TA pathway. In recordings from control mouse hippocampal slices, DA induces a larger depression at proximal compared to distal TA-CA1 synapses. However, hippocampal slices prepared from offspring of poly(I:C)-treated mothers exhibit a significantly larger DA-induced depression, selectively at distal TA-CA1 synapses compared to controls, which may abolish the differential control of LEC and MEC inputs by DA. Our present observations, based on both electrophysiology and c-Fos expression, suggest that the offspring of poly(I:C)-treated mothers have altered DA-mediated control of the TA pathway and may have an abnormality in object information processing. This altered DA signaling may be due to the abnormal development of the DA system recently observed in MIA offspring (Vuillermot, 2010), although the hippocampus and EC in this animal model have not yet been examined for expression of DA receptor or DA transporter expression with regard to the TA pathway. It is worth noting that the antipsychotic drug, clozapine, effectively blocks DA-induced depression at TA-CA1 synapses (Otmakhova and Lisman, 1999), indicating that the TA pathway may be a locus for clozapine action in schizophrenia.
The proper integration between Schaffer-collateral- and TA-CA1 synapses is critical for controlling spike initiation and synaptic plasticity in CA1 pyramidal neurons (Dvorak-Carbone and Schuman, 1999; Remondes and Schuman, 2002; Jarsky et al., 2005; Ang et al., 2005; Dudman et al., 2007). Thus, abnormal DA-mediated control of TA-CA1 synapses, together with reduced number of excitatory inputs on CA1 pyramidal neurons, will likely lead to altered synaptic plasticity or information integration in CA1 pyramidal neurons in the MIA offspring.
Perseveration behavior and hypersensitivity to a novel object
Our behavioral analyses indicate that the offspring of poly(I:C)-treated mothers show behavioral inflexibility in the Morris water maze task and increased sensitivity in the novel object recognition task. The normal acquisition of an initial platform location in the water maze task indicates that MIA offspring have normal spatial navigation, such as in self-localization, route learning and motor function for swimming (Redish and Touretzky, 1998). A selective deficit in learning a moved platform location suggests that these animals have difficulty in adapting to a modification introduced in previously acquired information, which may correspond to perseveration behavior. Such behavior is defined as contextually inappropriate and unintentional repetition, and is often observed in schizophrenia patients (Crider, 1997). These patients have difficulty in switching behavioral-strategy, or reversal learning, and tend to repeat the same response or strategy (Fey, 1951; Nolan, 1974; Floresco et al., 2008). Interestingly, a recent human study indicates that maternal infection is correlated in the offspring with impaired performance in the Wisconsin card sorting test, which provides a good measure of perseveration behavior (Brown et al., 2009). That is, the subset of schizophrenia subjects born to infected mothers display a more severe behavioral deficit in this test than schizophrenia subjects born to non-infected mothers. In experimental animals, the offspring of poly(I:C)-treated mothers display deficits in reversal learning in a left-right discrimination task (Meyer et al., 2006). Perseveration behavior is also observed in hippocampus-lesioned animals in the Morris water maze task (Whishaw and Tomie, 1997). Both hippocampus-lesioned animals (Kim and Frank, 2009) and rodents injected with a DA receptor agonist (Boulougouris et al., 2009) exhibit impaired reversal learning. Thus, abnormalities in the hippocampus and the DA system may play a key role in perseveration behavior.
In light of perseveration behavior in the water maze, the increased sensitivity to a novel object that we observe in the MIA offspring may be due to an enhanced expectation for the original object, which will increase novelty for a switched object. Importantly, the abnormal preference in the MIA offspring was observed selectively in the novel object recognition (nonspatial information), but not in the object location (spatial information), task. These altered behaviors in the MIA offspring may be due to abnormal processing of nonspatial information in the hippocampus, as observed in our slice physiology and c-Fos expression analyses. Contrary to our results, Ozawa et al. (2006) and Ibi et al. (2009) reported a deficit in a novel object recognition test. However, they used a more severe regimen of poly(I:C) treatment for 5-6 consecutive days at a different time during development, and they employed a longer time away from the object, making the test more of a memory test than a novelty preference test. Moreover, Golan et al. (2005) obtained a result similar to ours in a novel object recognition test using maternal injection of lipopolysaccharide to mimic maternal bacterial infection. It should also be noted that, in the water maze test, Zuckerman and Weiner (2005) reported some similarities and some differences in results compared to ours. However, they used rats and administered poly(I:C) at a much later time in gestation.
Integration of information processed in parallel
A major feature of brain function is parallel information processing. For example, visual information is processed in two distinct information streams: a ventral stream that subserves object recognition, or “what” perception, and a dorsal stream that primarily subserves spatial information, or “where” perception (Ungerleider and Haxby, 1994). The distributed information, processed in different brain areas, must be integrated for coherent perception.
Recent imaging and physiology studies report abnormal visual object recognition in schizophrenia patients (Doniger et al., 2002; Wynn et al., 2008). Wynn et al. (2008) measured activity in early retinotopically organized areas (V1–V4), motion-sensitive areas (human area MT) and object-recognition areas (lateral occipital complex), and found that schizophrenia patients display more widely-distributed activation in areas involved in object-recognition than controls. Thus, the abnormal distribution of activity in object-selective cortex in schizophrenia patients may indicate a problem in the integration of spatial and nonspatial information.
In the hippocampus, the integration of spatial and nonspatial information is critical for constructing a neural representation of environmental context. As such, the hippocampus plays an essential role in contextual memory formation. Interestingly, several studies indicate that schizophrenia patients have a severe problem in contextual memory formation (Boyer et al., 2007; Rizzo et al., 1996; Danion et al., 1999), although other types of memory, which do not require contextual information, are relatively intact. Our findings raise the question of whether this memory deficit could be due to abnormal DA-mediated control of the TA pathway in the hippocampus. Interestingly, a recent study using model simulation predicts a dominance of object over spatial information processing in the medial temporal lobe of schizophrenia patients (Talamini and Meeter, 2009). Thus, the altered hippocampal information processing we observe in MIA offspring may underlie some schizophrenia-like behaviors of these animals, such as in prepulse inhibition, latent inhibition and perseverative behavior. Further investigation based on this perspective may shed new light on the pathophysiology of schizophrenia.
Supplementary Material
Acknowledgements
We thank Prof. Erin M. Schuman (Caltech/Howard Hughes Medical Institute) and the HHMI for support in the electrophysiology and c-Fos experiments. The other experiments were supported by grants to P.H.P. from the National Institute of Mental Health and the McKnight Foundation. H.T.I. was supported by the Nakajima Foundation. S.E.P.S. was supported by the Autism Speaks Foundation. E.H. was supported by an NIH training grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson MI, Jeffery KJ. Heterogeneous modulation of place cell firing by changes in context. J Neurosci. 2003;23:8827–8835. doi: 10.1523/JNEUROSCI.23-26-08827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang CW, Carlson GC, Coulter DA. Hippocampal CA1 circuitry dynamically gates direct cortical inputs preferentially at theta frequencies. J Neurosci. 2005;25:9567–9580. doi: 10.1523/JNEUROSCI.2992-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrist B, Vankammen DP. CNS stimulants as tools in the study of schizophrenia. Trends Neurosci. 1984;7:388–390. [Google Scholar]
- Benes FM, Khan Y, Vincent SL, Wickramasinghe R. Differences in the subregional and cellular distribution of GABAA receptor binding in the hippocampal formation of schizophrenic brain. Synapse. 1996;22:338–349. doi: 10.1002/(SICI)1098-2396(199604)22:4<338::AID-SYN5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Benes FM, Wickramasinghe R, Vincent SL, Khan Y, Todtenkopf M. Uncoupling of GABA(A) and benzodiazepine receptor binding activity in the hippocampal formation of schizophrenic brain. Brain Res. 1997;755:121–129. doi: 10.1016/s0006-8993(97)00113-3. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Blasi G. The genetics of schizophrenia. Neurosci. 2009 doi: 10.1016/j.neuroscience.2009.04.038. in press. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Castane A, Robbins TW. Dopamine D2/D3 receptor agonist quinpirole impairs spatial reversal learning in rats: investigation of D3 receptor involvement in persistent behavior. Psychopharmacol (Berl.) 2009;202:611–620. doi: 10.1007/s00213-008-1341-2. [DOI] [PubMed] [Google Scholar]
- Boyer P, Phillips JL, Rousseau FL, Ilivitsky S. Hippocampal abnormalities and memory deficits: new evidence of a strong pathophysiological link in schizophrenia. Brain Res Rev. 2007;54:92–112. doi: 10.1016/j.brainresrev.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Brown AS, Vinogradov S, Kremen WS, Poole JH, Deicken RF, Penner JD, McKeague IW, Kochetkova A, Kern D, Schaefer CA. Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. Am J Psychiat. 2009;166:683–690. doi: 10.1176/appi.ajp.2008.08010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: A review of epidemiologic and translational studies. Amer J Psychiat. 2010 doi: 10.1176/appi.ajp.2009.09030361. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning MD, Dudek EM, Rapier JL, Leonard S, Freedman R. Significant reductions in synapsin but not synaptophysin specific activity in the brains of some schizophrenics. Biol Psychiat. 1993;34:529–535. doi: 10.1016/0006-3223(93)90195-j. [DOI] [PubMed] [Google Scholar]
- Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- Burmeister M, McInnis MG, Zollner S. Psychiatric genetics: progress amid controversy. Nat Rev Genet. 2008;9:527–540. doi: 10.1038/nrg2381. [DOI] [PubMed] [Google Scholar]
- Cajal SRY. Histologie du Systeme nerveux de l’hommes et des vertebres. Maloine; Paris: 1911. [Google Scholar]
- Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340:474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- Cotter D, Wilson S, Roberts E, Kerwin R, Everall IP. Increased dendritic MAP2 expression in the hippocampus in schizophrenia. Schizophr Res. 2000;41:313–323. doi: 10.1016/s0920-9964(99)00068-7. [DOI] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- Crider A. Perseveration in schizophrenia. Schizophr Bull. 1997;23:63–74. doi: 10.1093/schbul/23.1.63. [DOI] [PubMed] [Google Scholar]
- Danion JM, Rizzo L, Bruant A. Functional mechanisms underlying impaired recognition memory and conscious awareness in patients with schizophrenia. Arch Gen Psychiat. 1999;56:639–644. doi: 10.1001/archpsyc.56.7.639. [DOI] [PubMed] [Google Scholar]
- Davidsson P, Gottfries J, Bogdanovic N, Ekman R, Karlsson I, Gottfries CG, Blennow K. The synaptic-vesicle-specific proteins rab3a and synaptophysin are reduced in thalamus and related cortical brain regions in schizophrenic brains. Schizophr Res. 1999;40:23–29. doi: 10.1016/s0920-9964(99)00037-7. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiat. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Davis JO, Phelps JA, Bracha HS. Prenatal development of monozygotic twins and concordance for schizophrenia. Schizophr Bull. 1995;21:357–366. doi: 10.1093/schbul/21.3.357. [DOI] [PubMed] [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiat. 2002;59:1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- Dudman JT, Tsay D, Siegelbaum SA. A role for synaptic inputs at distal dendrites: instructive signals for hippocampal long-term plasticity. Neuron. 2007;56:866–879. doi: 10.1016/j.neuron.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak-Carbone H, Schuman EM. Patterned activity in stratum lacunosum moleculare inhibits CA1 pyramidal neuron firing. J Neurophysiol. 1999;82:3213–3222. doi: 10.1152/jn.1999.82.6.3213. [DOI] [PubMed] [Google Scholar]
- Dwork AJ. Postmortem studies of the hippocampal formation in schizophrenia. Schizophr Bull. 1997;23:385–402. doi: 10.1093/schbul/23.3.385. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Decreased synaptophysin in the medial temporal lobe in schizophrenia demonstrated using immunoautoradiography. Neurosci. 1995;69:339–343. doi: 10.1016/0306-4522(95)00324-c. [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, Fletcher PJ, Drago J, Sibley DR, O’Dowd BF, George SR. Spatial learning deficit in dopamine D(1) receptor knockout mice. Eur J Pharmacol. 1999;383:95–106. doi: 10.1016/s0014-2999(99)00573-7. [DOI] [PubMed] [Google Scholar]
- Fey ET. The performance of young schizophrenics and young normals on the Wisconsin Card Sorting Test. J Consult Psychol. 1951;15:311–319. doi: 10.1037/h0061659. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Innocenzi R, Pacitti C, Brioni JD. Spatial memory impairment induced by lesion of the mesohippocampal dopaminergic system in the rat. Neuroscience. 1996;74:1037–1044. doi: 10.1016/0306-4522(96)00202-3. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Sulli A, Packard MG. The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1–22. doi: 10.1016/s0278-5846(96)00157-1. [DOI] [PubMed] [Google Scholar]
- Golan HM, Lev V, Hallak M, Sorokin Y, Huleihel M. Specific neurodevelopmental damage in mice offspring following maternal inflammation during pregnancy. Neuropharmacology. 2005;48:903–917. doi: 10.1016/j.neuropharm.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Gottesman II. Schizophrenia Genesis: The Origins of Madness. W.H. Freeman & Company; New York, NY: 1991. [Google Scholar]
- Guzowski JF, Timlin JA, Roysam B, McNaughton BL, Worley PF, Barnes CA. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol. 2005;15:599–606. doi: 10.1016/j.conb.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308:1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Eastwood SL. Neuropathological studies of synaptic connectivity in the hippocampal formation in schizophrenia. Hippocampus. 2001;11:508–519. doi: 10.1002/hipo.1067. [DOI] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal neurons in schizophrenia. J Neural Transm. 2002;109:891–905. doi: 10.1007/s007020200073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci U S A. 1995;92:2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi D, Nagai T, Kitahara Y, Mizoguchi H, Koike H, Shiraki A, Takuma K, Kamei H, Noda Y, Nitta A, Nabeshima T, Yoneda Y, Yamada K. Neonatal polyI:C treatment in mice results in schizophrenia-like behavioral and neurochemical abnormalities in adulthood. Neurosci Res. 2009;64:297–305. doi: 10.1016/j.neures.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Ihalainen JA, Riekkinen P, Jr., Feenstra MG. Comparison of dopamine and noradrenaline release in mouse prefrontal cortex, striatum and hippocampus using microdialysis. Neurosci Lett. 1999;277:71–74. doi: 10.1016/s0304-3940(99)00840-x. [DOI] [PubMed] [Google Scholar]
- Ito HT, Schuman EM. Frequency-dependent gating of synaptic transmission and plasticity by dopamine. Front Neural Circuits. 2007;1:1. doi: 10.3389/neuro.04.001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Roxin A, Kath WL, Spruston N. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat Neurosci. 2005;8:1667–1676. doi: 10.1038/nn1599. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Gutierrez A, Martin R, Penafiel A, Rivera A, De La Calle A. Differential regional and cellular distribution of dopamine D2-like receptors: an immunocytochemical study of subtype-specific antibodies in rat and human brain. J Comp Neurol. 1998;402:353–371. doi: 10.1002/(sici)1096-9861(19981221)402:3<353::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Kim SM, Frank LM. Hippocampal lesions impair rapid learning of a continuous spatial alternation task. PLoS One. 2009;4:e5494. doi: 10.1371/journal.pone.0005494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Lee I, Hargreaves EL. Hippocampal place cells: parallel input streams, subregional processing, and implications for episodic memory. Hippocampus. 2006;16:755–764. doi: 10.1002/hipo.20203. [DOI] [PubMed] [Google Scholar]
- Knight R. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Law AJ, Weickert CS, Hyde TM, Kleinman JE, Harrison PJ. Reduced spinophilin but not microtubule-associated protein 2 expression in the hippocampal formation in schizophrenia and mood disorders: molecular evidence for a pathology of dendritic spines. Am J Psychiat. 2004;161:1848–1855. doi: 10.1176/ajp.161.10.1848. [DOI] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Li XG, Somogyi P, Ylinen A, Buzsaki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Kane JM, Alvir J. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacol (Berl.) 1987;91:415–433. doi: 10.1007/BF00216006. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacol. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16:795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Grandy DK, Van Tol HH, Damask SP, Little KY, Civelli O, Watson SJ., Jr. Dopamine receptor gene expression in the human medial temporal lobe. Neuropsychopharmacology. 1994;10:239–248. doi: 10.1038/npp.1994.27. [DOI] [PubMed] [Google Scholar]
- Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiat. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- Megias M, Emri Z, Freund TF, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neurosci. 2001;102:527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Prenatal exposure to infection: a primary mechanism for abnormal dopaminergic development in schizophrenia. Psychopharmacol (Berl) 2009 doi: 10.1007/s00213-009-1504-9. in press. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Fatemi SH. In vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Behav Rev. 2009 doi: 10.1016/j.neubiorev.2009.05.001. in press. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319:1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiat. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Nolan JD. A within-subjects analysis of discrimination shift behavior in schizophrenics. J Abnorm Psychol. 1974;83:497–511. doi: 10.1037/h0037068. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. D1/D5 dopamine receptor activation increases the magnitude of early long-term potentiation at CA1 hippocampal synapses. J Neurosci. 1996;16:7478–7486. doi: 10.1523/JNEUROSCI.16-23-07478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. Dopamine selectively inhibits the direct cortical pathway to the CA1 hippocampal region. J Neurosci. 1999;19:1437–1445. doi: 10.1523/JNEUROSCI.19-04-01437.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiat. 2006;59:546–54. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Maternal effects on schizophrenia risk. Science. 2007;318:576–577. doi: 10.1126/science.1150196. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav Brain Res. 2009;204:313–324. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Redish AD, Touretzky DS. The role of the hippocampus in solving the Morris water maze. Neural Comput. 1998;10:73–111. doi: 10.1162/089976698300017908. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Direct cortical input modulates plasticity and spiking in CA1 pyramidal neurons. Nature. 2002;416:736–740. doi: 10.1038/416736a. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431:699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- Rizzo L, Danion JM, Van Der Linden M, Grangé D, Rohmer JG. Impairment of memory for spatial context in schizophrenia. Neuropsychol. 1996;10:376–384. [Google Scholar]
- Rossato JI, Bevilaqua LR, Izquierdo I, Medina JH, Cammarota M. Dopamine controls persistence of long-term memory storage. Science. 2009;325:1017–1020. doi: 10.1126/science.1172545. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Mamelak AN, Schuman EM. Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex. Neuron. 2006;49:805–813. doi: 10.1016/j.neuron.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Sorra KE, Harris KM. Occurrence and three-dimensional structure of multiple synapses between individual radiatum axons and their target pyramidal cells in hippocampal area CA1. J Neurosci. 1993;13:3736–3748. doi: 10.1523/JNEUROSCI.13-09-03736.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol. 1976;167:285–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Swanson L. Handbook of Chemical neuroanatomy. Elsevier; Amsterdam: 1987. The limbic region I. The septohippocampal system; pp. 125–227. [Google Scholar]
- Talamini LM, Meeter M. Dominance of objects over context in a mediotemporal lobe model of schizophrenia. PLoS One. 2009;4:e6505. doi: 10.1371/journal.pone.0006505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Nojyo Y. Preservation of topography in the connections between the subiculum, field CA1, and the entorhinal cortex in rats. J Comp Neurol. 1995;353:379–390. doi: 10.1002/cne.903530306. [DOI] [PubMed] [Google Scholar]
- Toda M, Abi-Dargham A. Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiat Rep. 2007;9:329–336. doi: 10.1007/s11920-007-0041-7. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- van Groen T, Miettinen P, Kadish I. The entorhinal cortex of the mouse: organization of the projection to the hippocampal formation. Hippocampus. 2003;13:133–149. doi: 10.1002/hipo.10037. [DOI] [PubMed] [Google Scholar]
- Vuillermot S, Weber L, Feldon J, Meyer U. A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. J Neurosci. 2010;30:1270–1287. doi: 10.1523/JNEUROSCI.5408-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley JK, Gehlert DR, Filloux FM, Dawson TM. Comparison of the distribution of D-1 and D-2 dopamine receptors in the rat brain. J Chem Neuroanat. 1989;2:119–137. [PubMed] [Google Scholar]
- Weinberger D, Laruelle M. Neuropsychopharmacology. Lippincott Williams & Wilkins; Philadelphia: 2001. Neurochemical and neuropharmacological imaging in schizophrenia. [Google Scholar]
- Whishaw IQ, Tomie JA. Perseveration on place reversals in spatial swimming pool tasks: further evidence for place learning in hippocampal rats. Hippocampus. 1997;7:361–370. doi: 10.1002/(SICI)1098-1063(1997)7:4<361::AID-HIPO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Hippocampal Formation. In: Paxinos G, editor. The Rat Nervous System. Elsevier academic press; Amsterdam: 2004. [Google Scholar]
- Wynn JK, Green MF, Engel S, Korb A, Lee J, Glahn D, Nuechterlein KH, Cohen MS. Increased extent of object-selective cortex in schizophrenia. Psychiat Res. 2008;164:97–105. doi: 10.1016/j.pscychresns.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CE, Arima K, Xie J, Hu L, Beach TG, Falkai P, Honer WG. SNAP-25 deficit and hippocampal connectivity in schizophrenia. Cereb Cortex. 1998;8:261–268. doi: 10.1093/cercor/8.3.261. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.