Abstract
Connective tissue growth factor (CTGF/CCN2) is induced by transforming growth factor beta 1(TGF-β1) where it acts as a downstream mediator of TGF-β1 induced matrix production in osteoblasts. We have shown the requirement of Src, Erk and Smad signaling for CTGF induction by TGF-β1 in osteoblasts, however the potential interaction among these signaling pathways remains undetermined. In this study we demonstrate that TGF-β1 activates Src kinase in ROS17/2.8 cells and that treatment with the Src family kinase inhibitor PP2 prevents Src activation and CTGF induction by TGF-β1. Additionally, inhibiting Src activation prevented Erk activation, Smad 2 & 3 activation and nuclear translocation by TGF-β1, demonstrating that Src is an essential upstream signaling partner of both Erk and Smads in osteoblasts. MAPKs such as Erk can modulate the Smad pathway through directly mediating the phosphorylation of Smads or indirectly through activation/inactivation of required nuclear co-activators that mediate Smad DNA binding. When we treated cells with the Erk inhibitor, PD98059 it inhibited TGF-β1-induced CTGF protein expression but had no effect on Src activation, Smad activation or Smad nuclear translocation. However PD98059 impaired transcriptional complex formation on the Smad binding element (SBE) on the CTGF promoter, demonstrating that Erk activation was required for SBE transactivation. This data demonstrates that Src is an essential upstream signaling transducer of Erk and Smad signaling with respect to TGF-β1 in osteoblasts and that Smads and Erk function independently but are both essential for forming a transcriptionally active complex on the CTGF promoter in osteoblasts.
Keywords: SRC, Osteoblasts, CTGF, TGF-β1, MAPK, Smads
Introduction
Osteoblasts are highly differentiated, biosynthetic cells that form bone through production and secretion of extracellular matrix (ECM) that becomes mineralized to form mature bone tissue (Sims, 2000). Osteoblast growth, differentiation and biosynthetic activity are initiated and tightly regulated by systemic and locally-produced growth factors. Recently connective tissue growth factor (CTGF/CCN2) has emerged as an important growth factor in osteogenesis. CTGF is produced and secreted by osteoblasts where it acts in an autocrine fashion as an anabolic growth factor to regulate osteoblast differentiation and function (Safadi et al., 2003; Xu et al., 2000). In cultured osteoblasts, CTGF induces pro-osteogenic cellular activity including osteoblast proliferation, matrix production and terminal differentiation (mineralization) (Arnott et al., 2007; Nishida et al., 2000; Safadi et al., 2003; Takigawa et al., 2003; Xu et al., 2000). Transforming growth factor-β1 (TGF-β1) is a potent, multifunctional, osteogenic growth factor that also regulates osteoblast differentiation and function (Bonewald, 2002). One of TGF-β1 major effects on osteoblasts is its ability to stimulate the production and secretion of ECM (Bonewald and Dallas, 1994; Centrella and Canalis, 1987; Hock et al., 1990; Wrana et al., 1988) however the mechanisms or downstream effector genes that facilitate this response are not understood. We recently demonstrated that in osteoblasts CTGF is stimulated by TGF-β1 and that CTGF is a downstream effector for TGF-β1 induced ECM synthesis (Arnott et al., 2007; Nakanishi et al., 1997; Parisi et al., 2005). The signaling pathways that mediate TGF-β1 induction of CTGF vary depending on the cell type being examined (Blom et al., 2002), and we recently demonstrated that in osteoblasts CTGF up-regulation by TGF-β1 required Smads, the mitogen-activated protein kinase (MAPK) Erk and Src signaling (Arnott et al., 2008).
In general, TGF-β1 signals through a generic Smad mediated pathway involving Smads 2, 3 and 4 (Shi and Massague, 2003). Smads 2 and 3 are phosphorylated by active transmembrane serine/threonine TGF-β1 receptors (Massague and Chen, 2000). Following activation, Smad 2 and 3 form a trimeric complex with Smad 4, and this complex subsequently translocates to the nucleus, where it binds to Smad binding elements (SBE) in promoters of TGF-β1-responsive genes (Derynck and Zhang, 2003; Shi and Massague, 2003). Transcriptional activation by Smads is not limited to the Smad-SBE interaction alone but requires additional association of Smads with other transcription factors and co-factors that together bind the SBE and adjacent cis-regulatory binding elements (DNA motifs) (Feng and Derynck, 2005). Thus, Smad signaling is required, but in most cases it is not sufficient by itself to achieve target gene activation. The requisite additional transcription factors, co-factors and DNA motifs required for Smad transcriptional activation of the CTGF promoter are cell type dependent and have not been elucidated in osteoblasts. There are a number of studies to date that have shown there a considerable amount of crosstalk between Smads and other signaling pathways. TGF-β receptors activate Smad-independent signaling pathways that can regulate Smad activation and function (Derynck and Zhang, 2003). MAPKs (Erk1/2, p38 and Jnk) represent one group of downstream signaling transducers of TGF-β1 that can regulate Smad activation and function (Derynck and Zhang, 2003). MAPKs have been shown to directly regulate TGF-β1 induction of CTGF expression in some cell types (Abdollah et al., 1997; Chen et al., 2002; Leask and Abraham, 2003; Leask et al., 2003; Leivonen et al., 2005; Utsugi et al., 2003; Xie et al., 2005). We have recently demonstrated that Erk and not p38 or Jnk, are required for CTGF induction by TGF-β1 in osteoblasts (Arnott et al., 2008). Erk can potentiate the TGF-β1/Smad pathway via direct phosphorylation of Smads, or indirectly through activation/inactivation of co-activators/co-repressors that mediate Smad DNA binding (Kretzschmar et al., 1999; Mori et al., 2004). However, the potential interaction between Erk and Smads for CTGF induction in osteoblast remains unexplored and is the focus of this study.
We have also previously demonstrated that Src signaling is required for CTGF induction by TGF-β1 in osteoblasts, and that Src is activated upon TGF-β1 treatment (Arnott et al., 2008). Src activation following TGF-β1 treatment can occur as a direct result of TGF-β receptor activation (Sato et al., 2005; Tanaka et al., 2004). Studies have shown that Src can act as a downstream signaling effector for TGF-β1 and can function upstream of Erk in some cell types (Galliher and Schiemann, 2006; Kim et al., 2004; Kim and Joo, 2002; Sato et al., 2005; Tanaka et al., 2004; Varon et al., 2006). Although our previous studies have demonstrated that Smad, Erk and Src play important roles in TGF-β1 induction of CTGF expression in osteoblasts, their potential interactions with one another have not been investigated. It is not known whether Erk and Src family kinases cooperate with one another, function independent of each other, or how they regulate Smad signaling. Therefore, the focus of this study is to investigate these potential interactions between Src family kinases, Erk and Smad signaling in osteoblast.
MATERIALS AND METHODS
Source of Animals
Rat primary osteoblasts were isolated from calvaria of 3~5 day-old Sprague-Dawley (SD) rats. All animals were managed and handled following the principles in the NIH Guide for the Care and Use of Laboratory Animals (U.S. Department of Health and Human Services, Publ. No. 86-23, 1985) and guidelines established by the IACUC of Temple University and The Commonwealth Medical College.
Reagents
Transforming Growth Factor-β1 (rhTGF-β1) was purchased from Calbiochem (Gibbstown, NJ) and reconstituted as 2μg/ml in 4mM HCl with 0.1% bovine serum albumin. The Src family kinase inhibitor (PP2), an inactive analog of this inhibitor (PP3), the MEK1/2 inhibitor (U0126), and the anti-DAPI antibody were purchased from Calbiochem. The MEK1/2 inhibitor (PD98059) and the anti-b-actin antibody were purchased from Sigma (St. Louis, MO). The anti-phospho-Tyrosine 416 Src family, anti-phospho-Smad2 (Ser465/467), anti-total Smad2/3, anti-phospho-Erk1/2 (Thr202/Tyr204), and anti-total Erk1/2 antibodies were purchased from Cell Signaling (Danvers, MA). The anti-phospho-Smad3 (Ser423/425) and anti-phospho-Serine 213 Smad3 antibodies were purchased from Abcam (Cambridge, MA). The anti-total Src antibody, avian Src antibody, Hck antibody and LaminA/C antibody were purchased from Millipore (Billerica, MA). The anti-CTGF antibody, Fyn antibody, and Yes antibody were from Santa Cruz (Santa Cruz, CA). The horseradish peroxidase conjugated anti-rabbit and anti-mouse IgG antibodies were purchased from Pierce (Rockford, IL). The fluorescein conjugated anti-rabbit IgG antibody was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Infrared-conjuated secondary antibodies were from LI-COR Biosciences (Lincoln, NE).
Cell Culture
Rat osteosarcoma osteoblast cells (ROS 17/2.8) were provided by Dr. Archana Sanjay (Temple University, PA). Cells were cultured in α-MEM (Mediatech, Manassas, VA) supplemented with 10% heat-inactivated fetal bovine serum (Biowest, France), 100 IU/ml penicillin, and 100μg/ml streptomycin (Gibco, Carlsbad, CA) and maintained in 37°C with 5% CO2 incubator. When they are ~80% confluent, cells were trypsinized and re-plated at 1:10 ratio.
TGF-β1 Treatment
Cells were cultured in 10% serum-supplemented media until they were 80% confluent and serum starved for an additional 24 hours. TGF-β1 at the final concentration of 5ng/ml was applied to serum-free cells and incubated for indicated time period.
Pharmacological Inhibitor Treatment
Cells were cultured in 10% serum-supplemented media until they were 80% confluent and serum starved for an additional 24 hours. Pharmacological inhibitor of Src (PP2) or Erk (PD98059 or U0126) or inactive analog of PP2 (PP3) or equal volume of diluent (DMSO) was added to cells at indicated dose for 30 minutes followed by 5ng/ml TGF-β1 treatment for indicated time period.
Dominant-negative Src Constructs Transfection
Primary osteoblasts were cultured in 6-well plate with EMEM supplemented with 10% fetal bovine serum but without antibiotics one day before transfection. Next day the cells were 90%~95% confluent and serum supplemented media was replaced by OPT-MEM without serum before transfection. The transfection was carried out using kinase-dead dominant-negative Src constructs (KD or KDYF) or empty vector (PKB), which are provided by Dr. Archana Sanjay (Temple University, PA). Briefly, 4μg plasmid DNA was dissolved in 250μl OPT-MEM (Invitrogen, Carlsbad, CA) and incubated at room temperature for 5 minutes. 10μl Lipofectamine 2000 (Invitrogen, Carlsbad, CA) was diluted in 250μl OPT-MEM. The dissolved DNA and the diluted Lipofectamine 2000 were mixed together and incubated at room temperature for 20 minutes. The mixture of DNA and Lipofectamine was added to cell culture drop by drop. Twenty-four hours after transfection, cells were treated with 5ng/ml TGF-β1 for 8 hours and collected in RIPA buffer.
Src siRNA Transfection
ROS17/2.8 cells were cultured in 6-well plate with α-MEM supplemented with 10% fetal bovine serum but without antibiotics one day before transfection. Next day cells were transfected with Src siRNA or non-targeting scrambled siRNA control (Dharmacon, Lafayette, CO) by Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA) following the company's protocol. A Src specific siRNA SMARTpool consisting of four target-specific 19-nucleotide siRNA duplexes was manufactured from the open reading frame of rat Src (accession number in GenBank: NM_031977) by Dharmacon Research (Lafayette, CO). siGENOME non-targeting siRNA pool from the Dharmacon research was used as a negative control. Briefly, final concentration of 60nM siRNA was dissolved in 250μl OPT-MEM (Invitrogen, Carlsbad, CA) for 5 minutes at room temperature. 5μl Lipofectamine RNAiMAX was diluted in 250μl OPT-MEM. The dissolved siRNA and diluted Lipofectamine RNAiMAX were mixed and incubated at room temperature for 20 minutes. Then the mixture was added to cell culture drop by drop. After transfected for indicated time period, cells were serum starved for additional 24 hours followed by indicated treatment.
Protein Isolation and Western Blotting
2 × 106 cells/100mm culture dish were washed twice in PBS and harvested from culture dishes in protein extraction buffer (RIPA buffer) consisting of 50mM Tris-HCl (pH 7.5), 135 mM NaCl, 1% Triton X-100, 0.1% sodium deoxycholate, 2mM EDTA, 50mM NaF, 2mM sodium orthovanadate, 10μg/ml aprotinin, 10μg/ml leupeptin and 1mM PMSF. Cell lysates were agitated for 24 hours in 4°C and centrifuged at 14,000×g for 10min at 4°C. The supernatant was stored in -80°C for later Western blot studies. The total protein concentration was measured using the BCA Protein Assay Reagent Kit (Pierce, Rockford, IL) according to the manufacturer's instructions. Twenty or fifty μg proteins from each sample were mixed with equal volume of 2×Laemmli loading buffer and boiled at 100°C for 3 minutes. Samples were subjected to electrophoresis on 10% Tris-Glycine ready gels (Bio-Rad, Hercules, CA) and transferred to PVDF filters or nitrocellular members (Bio-Rad, Hercules, CA) by electroblotting. After 1 hour blocking in 5% BSA or 3% dry milk/0.5% BSA (per antibody instructions) at room temperature, blots were incubated with one of the following primary antibodies: p-Erk (1:1000), p-Smad2 (Serine 465/467) (1:1000), p-Smad3 (Serine 423/425)(1:1000), p-Smad3 (Serine 213) (1:1000), p-Src (Tyr 416) (1:1000), total Erk (1:1000), total Smad2/3 (1:1000), total Src (1:1000), Avian Src (1:1000), Lamin A/C (1:500), b-actin (1:5000), or CTGF (1:200), and then with the corresponding HRP-conjugated secondary antibody (1:10,000) or Infrared-conjuated secondary antibody (1:10,000). Antigens were detected using the Pierce supersignal west pico chemiluminescent substrate system for HRP conjugated secondary antibodies or Li-Cor Odessey system for infrared-conjugated secondary antibodies.
Nuclear Protein Separation
The nuclear protein separation was carried out using the protocol described by Dignam et al. (Dignam et al., 1983). Cells (3 × 106) were harvested after treatment and washed in PBS. After centrifugation, the cell pellet was resuspended in 50μl sucrose buffer containing 0.32 M Sucrose, 10mM Tris HCl (pH 8.0), 3mM CaCl2, 2mM MgOAc, 0.1mM EDTA, 0.5% NP-40, 1mM DTT and 0.5mM PMSF. The lysates were centrifuged at 500×g for 5 minutes at 4°C. The nuclear pellet was washed in sucrose buffer without NP-40. After centrifugation, the nuclear pellets were resuspended in 15μl low salt buffer containing 20mM HEPES (pH 7.9), 1.5mM MgCl2, 20mM KCl, 0.2mM EDTA, 25% glycerol, 1% NP-40, 0.5mM DTT, 0.5mM PMSF and in an equal volume (15μl) of high salt buffer containing 20mM HEPES (pH 7.9), 1.5mM MgCl2, 800mM KCl, 0.2mM EDTA, 25% glycerol, 1% NP-40, 0.5mM DTT, 0.5mM PMSF, and 4μg/ml aprotinin. Lysates were incubated at 4°C for 30min with agitation followed by centrifuge at 14,000×g for 10min at 4°C. The supernatants containing the nuclear protein lysate were then used for Western blot analysis and protein concentrations were determined using the Bradford protein assay.
Immunofluorescence Staining
Cells were plated at 5000/chamber in chamber slides (Nunc, Rochester, NY) in serum supplemented medium for 24 hours. Cells were then serum deprived for 24 hours prior to treatment. Some chambers were pretreated with 20μM PP2 (Src kinase inhibitor), 20μM PD98059 (Erk inhibitor), or equal volume of DMSO (diluent control) for 30 minutes prior to TGF-β1 treatment (5ng/ml) for an additional 30 minutes. Following treatment, cells were washed twice in PBS and fixed in 4% paraformaldehyde for 15 minutes at room temperature. The cells were washed again in PBS and permeabilized with 0.1% Triton X-100 in PBS for 5 min at room temperature. After three washes in PBS, slides were blocked using 1% BSA in PBS for 1 hour at room temperature. Cells were incubated with either anti-phospho Smad2 (1:100) and anti-phospho Smad3 (1:250) overnight at 4°C. After three washes in PBS-0.1% Tween 20, cells were incubated with anti-DAPI antibody (1:1000; Calbiochem, Gibbstown, NJ) and fluorescein-conjugated secondary antibody (1:1000; West Grove, PA) for 1 hour at room temperature. The cells were washed again with PBS-T for three times, mounted using the mounting fluid (Light Diagnostics, Murray, Utah), and examined with a Nikon Eclipse E800 epifluorescent microscope. All images were captured using a Retiga EXi digital camera.
Electro-Mobility Shift Assay
Nuclear extracts from TGF-β1 treated cells with or without PD98059 pretreatment were prepared following the nuclear protein separation protocol described above. The electro-mobility shift assays and oligonucletide probes used in this study were prepared as previously described (Arnott et al., 2008). Briefly, probes were synthesized that were homologous to the native sequence found in the CTGF promoter and labeled with [γ-32P] ATP (Amersham, Louisville, CO) and T4 polynucleotide kinase (NEB, Ipswich, MA). The binding reaction is composed of 5 μg of nuclear extract, 1× binding buffer [5× binding buffer: 50 mM Tris–HCL (pH8.0), 750 mM KCL, 2.5 mM EDTA, 0.5% Triton X-100, 62.5% glycerol and 1 mM DTT], poly-dldc (1 μg/ml), and 10,000 cpm of labeled probe. After incubating at room temperature for 30 minutes the entire sample was loaded on a 4% acrylamide, 60:1 acrylamide:bisacrylamide gel using 0.5× TBE.
Results
Src is required for CTGF Induction by TGF-β1 in Osteoblasts
Recently, we found that in primary osteoblasts CTGF could be induced by TGF-β1 in a time- and dose-dependent fashion (Arnott et al., 2007). In this study we first examined whether CTGF could be induced by TGF-β1 in rat osteosarcoma osteoblast-like cells (ROS17/2.8). ROS osteoblast-like cells are a transformed osteoblast cell line that are widely used for studies of osteoblast differentiation and function and are considered as more differentiated osteoblasts relative to primary osteoblasts (Granet et al., 2002; Kartsogiannis and Ng, 2004; Majeska and Rodan, 1982; Noda, 1989; Rodan and Majeska, 1982; Takai et al., 2008; Zirngibl et al., 2008). The TGF-β1 dose of 5ng/ml was used for this and all subsequent experiments since we had previously demonstrated that this was the minimal dose required for maximal induction of CTGF (Arnott et al., 2007). Time course analysis of CTGF expression following TGF-β1 stimulation in ROS osteoblast-like cells, demonstrated that CTGF expression was evident as early as 2 hours and reached maximal levels after 4 to 8 hours stimulation with TGF-b1 (Figure 1A). After 24 hours treatment, CTGF up-regulation abated (Figure 1A). Therefore, we chose the 4 hour time point for all subsequent studies involving induction of CTGF expression by TGF-b1 using ROS osteoblast-like cells.
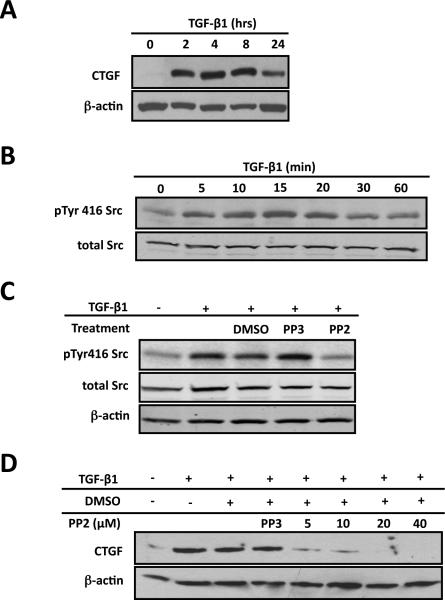
Figure 1. Src signaling is required for CTGF induction in ROS cells.
ROS osteoblast-like cells were cultured until they were 80% confluent and serum deprived for 24 hours before any treatment. After all treatments, whole-cell protein lysates were used for Western blot analysis. Each experiment was repeated a minimum of three times with similar results. (A) To determine if TGF-β1 induces CTGF expression cells were treated with TGF-β1 (5ng/ml) for indicated times. Whole cells lysates were extracted and used for Western blot. (B) To determine the time course for Src family kinase activation, serum-deprived cells were treated with TGF-β1 (5ng/ml) for 0, 5, 10, 15, 20, 30 or 60 minutes. Src kinase activation reaches maximal level 20 minutes following TGF-β1 treatment. (C) To determine if PP2 blocks Src activation in ROS cells, cells were serum-starved cells and pre-treated with 20mM PP2 (Src kinase inhibitor) or PP3 (inactive analog, negative control), or with DMSO (the diluent for PP2 and PP3) for 30 minutes and then treated with TGF-b1 (5ng/ml) for 20 minutes. Western blot analysis demonstrated that PP2 prevents TGF-β1 induced Src activation compared to controls. (D) To determine if Src signaling is required for CTGF induction cells were pre-treated with PP2 (at indicated concentrations), DMSO (the diluent for PP2) or PP3 (20mM; the inactive analog of PP2) for 30 minutes followed by TGF-β1 (5ng/ml) 4 hours. PP2 blocks the induction of CTGF expression by TGF-β1 (maximal effect at a dose of 20 μM) in primary and ROS osteoblasts.
Studies have shown that Src family kinase can act as a downstream signaling effector for TGF-β1 in different cells types (Kutz et al., 2006; Samarakoon et al., 2009; Samarakoon and Higgins, 2008) (Wang et al., 2009b) (Galliher and Schiemann, 2006; Galliher and Schiemann, 2007; Galliher-Beckley and Schiemann, 2008) (Mishra et al., 2007) (Murillo et al., 2005). To determine whether Src kinase could be activated by TGF-β1 in ROS osteoblast-like cells, we performed Western blot analysis using the phosphorylated tyrosine 416 antibody. The phosphorylated tyrosine 416 antibody can potentially detect all activated Src family members if they are phosphorylated at equivalent sites, so this antibody is not specific for Src activation alone. Western blot analysis demonstrated a time-dependent activation of Src family kinase following TGF-β1 stimulation with maximal activation occurring at 20 minutes post-treatment (Figure 1B). After 60 minutes the phosphorylation level was comparable to the basal pre-treatment level (Figure 1B). Total Src levels remained constant at all time points. PP2, a Src family kinase inhibitor, has been widely used to evaluate the physiological and pathological roles of Src family kinases. PP3 is the inactive analog of PP2 and is used as a negative control. When we pre-treated cells by PP2, PP3 or DMSO (the diluent for PP2 and PP3), we found that PP2 could effectively block TGF-β1 induced Src kinase activation, while PP3 and DMSO did not have an inhibitory effect on Src activation (Figure 1C). Based on these results, PP2 and PP3 were used in subsequent studies. In the previous experiments we showed that CTGF was up-regulated and that Src kinase was activated by TGF-β1. Next we determined whether TGF-β1 induced activation of Src kinase activation was important for TGF-β1 induced CTGF expression. ROS osteoblast-like cells were pre-treated with the indicated doses of PP2 for 30 minutes, followed by 8 hours treatment with TGF-β1. Western blot analysis demonstrated a dose-dependent inhibition of TGF-β1 stimulated CTGF expression by PP2 with maximal effect at the 20μM dose, while PP3 and DMSO had no inhibitory effect on CTGF up-regulation (Figure 1D). Since 20μM PP2 was the optimal dose for inhibiting CTGF expression, this dose was used in all subsequent experiments.
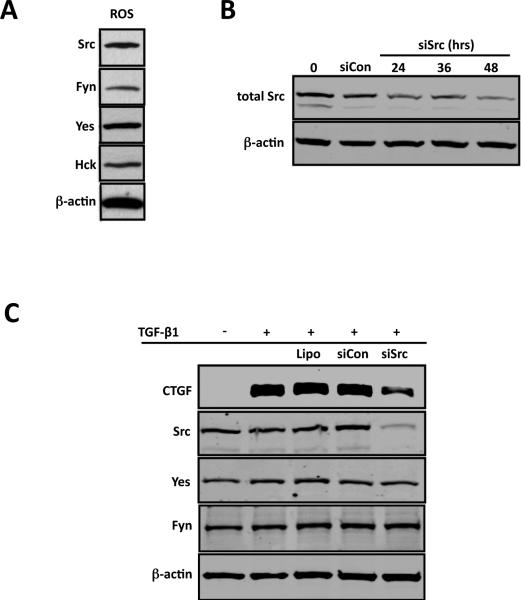
Src is the prototype of the Src family kinases and Src has been shown to play an important role in regulating osteoblast function (Marzia et al., 2000). Since PP2 is a broad-spectrum inhibitor for all Src family members, we wanted to confirm that the effect of PP2 on CTGF up-regulation was specific to Src. We first assessed the endogenous expression of the four major members of the Src kinase family Fyn, Yes, Hck and Src, and found that all four family members are expressed in ROS osteoblast-like cells (Figure 2A). To determine whether Src specifically is required for TGF-β1 induced CTGF expression, we utilized an siRNA technique to knockdown Src. First we initially conducted a time course study to determine the conditions for maximal knockdown of Src. As early as 24 hours after transfection, total Src levels decreased approximately 50% compared to the scrambled non-targeted control siRNA transfection (Figure 2B). At 48 hours post-transfection, total Src levels were maximally decreased up to 90% compared to the scrambled non-targeting siRNA control (Figure 2B). Therefore, in subsequent experiments, a 48-hour-transfection was used. To determine if Src was the major Src Family kinase involved in CTGF induction by TGF-β1 in ROS osteoblast-like cells, we transfected cells with Src siRNA for 48 hours followed by 4 hours TGF-β1 treatment. Knockdown of Src (>90%) prevented CTGF induction (~70%) after TGF-β1 stimulation (Figure 2C). To confirm that the Src siRNA we used was specific to Src, we also assessed the level of Fyn and Yes after Src siRNA transfection. There was no change in Fyn and Yes levels after Src siRNA transfection, confirming that Src siRNA we used is specific to Src (Figure 2C).
Figure 2. Src expression is required for CTGF induction by TGF-β1 in ROS cells.
ROS osteoblast-like cells were cultured until 80% confluent. Following treatment whole-cell lysates were analyzed for Src expression using Western blot. (A) Src, Fyn, Yes and Hck. Src, Fyn, Yes and Hck, are expressed in ROS osteoblasts. (B) ROS osteoblast-like cells were cultured until 50~60% confluence and transfected with Src-specific siRNA (siSrc) (60nM) for 0, 24, 36 or 48 hours using the Lipofectamine RNAiMax kit. A non-targeting scrambled siRNA (siCon) was used as a negative control. Following transfection for the indicated times, whole-cell lysates were analyzed for Src expression using Western blot. The time course demonstrates maximal inhibition of Src expression at 48 hours post-transfection. (C) ROS osteoblast-like cells were transfected with Src-specific siRNA (siSrc) (60nM) or non-targeting scrambled siRNA (siCon) for 48 hours as described above, serum starved for an additional 24 hours and treated with TGF-b1 (5ng/ml) for 4 hours. Whole-Cell lysates were analyzed for Src, Yes and Fyn expression by Western blot. Note that the knockdown of Src by the Src specific siRNA blocks CTGF induction by TGF-β1 in ROS osteoblast-like cells but doesn't affect the protein expression of Fyn and Yes. Experiments were repeated 4 times with similar results.
Erk is Required for CTGF Induction by TGF-β1
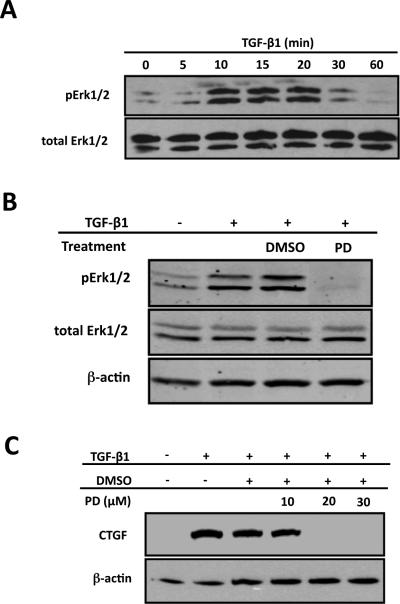
Studies have shown that MAPKs can be activated by TGF- β1 in numerous cell types (Blanchette et al., 2001; Hayashida et al., 2003; Hayashida et al., 1999; Hayashida et al., 2007; Liu et al., 2008; Massague, 2003; Watanabe et al., 2001; Yang et al., 2007). The identity of the specific MAPK(s) that are activated, including Erk1/2, p38 and JNK1/2/3, is cell-type dependent. We previously showed in primary osteoblasts, using a luciferase reporter analyses, that both the MEK inhibitor PD98059 and dominant negative Erk construct blocked TGF-β1 induced CTGF promoter activity (Arnott et al., 2008). In this series of experiments, we wanted to examine if Erk signaling plays a role for CTGF induction by TGF-β1 in ROS cells. We conducted an initial time course study of Erk activation after TGF-β1 treatment in ROS osteoblast-like cells. Western blot analysis revealed time-dependent activation of Erk with maximal activation occurring at 20 minutes post-treatment (Figure 3A). At 60 minutes post-treatment, Erk phosphorylation was comparable to basal pre-treatment levels (Figure 3A). The total Erk levels remained constant at all time points (Figure 3A). Therefore, the 20 minutes post-treatment time point was selected for subsequent analysis of Erk activation. Next we tested various doses of the MEK1/2 inhibitor, PD98059, to determine the optimal dose of each that could block Erk activation. PD98059 is one of the first discovered pharmacological inhibitors targeting Erk. They exert their effects by preventing the phosphorylation of MEK1/2 and/or conformation transition that generate active enzymes (Favata et al., 1998). In ROS osteoblast-like cells, 20μM of PD98059 could effectively prevent TGF-β1 induced Erk activation (Figure 3B). In order to determine whether Erk plays a role in TGF-β1 induced CTGF induction, ROS osteoblast-like cells were pre-treated with PD98059 at the indicated doses followed by TGF-β1 treatment for 4 hours. PD98059 dose-dependently blocked TGF-β1 induced CTGF (Figure 3C) demonstrating that Erk signaling is essential for CTGF induction in ROS cells. The dose of 20mM for PD98059 was used for subsequent studies.
Figure 3. Erk is essential for CTGF induction by TGF-β1 in ROS cells.
ROS osteoblast-like cells were cultured until 80% confluent and serum starved for an additional 24 hours prior to treatment. After treatment, whole-cell protein extracts were analyzed by Western blot analysis. Experiments were repeated a minimum of 3 times with similar results. (A) To determine the time course for Erk activation by TGF-β1, cells were treated with TGF-β1 (5ng/ml) for 0, 5, 10, 15, 20, 30 or 60 minutes. Erk activation reaches maximal levels after 20 minutes TGF-β1 treatment. (B) Serum-starved cells were pre-treated with PD98059 (PD; 20mM; a MEK1/2 inhibitor) or DMSO (the diluent for PD98059) for 30 minutes followed by TGF-β1 (5ng/ml) treatment for another 20 minutes. The MEK1/2 inhibitor, PD98059, blocks TGF-β1 induced Erk activation. (C) Cells were pre-treated with the MEK1/2 inhibitors, PD98059 at indicated concentrations or DMSO (the diluent for PD98059 or U0126) prior to TGF-b1 (5ng/ml) treatment for 8 hours. The inhibition of Erk in ROS osteoblasts prevents TGF-β1 induction of CTGF expression.
Cross-talk between Src, Erk and Smads in ROS Osteoblast-like Cells
In general, TGF-β1 exerts its functions through Smad signaling. In addition to the canonical Smad signaling pathway, recent studies have demonstrated that other signaling effectors, including Src family kinases and MAPKs, can be activated by TGF-β1 in some cells and that these non-canonical signaling molecules may function as important regulators of Smad signaling (Joo et al., 2008; Samarakoon et al., 2009; Samarakoon et al., 2008; Sato et al., 2005) (Hayashida et al., 2003; Imamichi et al., 2005; Kretzschmar et al., 1999; Matsuura et al., 2005; Wang et al., 2009a; Watanabe et al., 2001; Yang et al., 2007). In our preceding studies we demonstrated that Src and Erk are required for CTGF induction by TGF-β1 in ROS osteoblast-like cells, so the next approach was aimed at delineating any potential cross-talk between Src, Erk and Smad signaling in osteoblasts. In some signaling paradigms, Src has been shown to be upstream of Erk (Mishra et al., 2007) (Tanaka et al., 2004). First we wanted to determine whether Src could regulate the activation of Erk. In these experiments, we pre-treated ROS osteoblast-like cells with the Src inhibitor, PP2, or the Erk inhibitor, PD98059 (PD), for 30 minutes and then stimulated them with TGF-β1 for an additional 20 minutes. Previous studies revealed that 20 minutes post-TGF-β1 treatment caused maximal Erk activation (Figure 3A). Western blot analysis of cell lysates demonstrated that the activation of Erk was completely blocked following Src kinase inhibition (Figure 4). The Erk inhibitor, PD98059, was used as a control, and effectively blocked Erk activation subsequent to TGF-β1 treatment (Figure 4). More importantly, the Erk inhibitor had no effect on Src activation induced by TGF-β1, demonstrating that Erk was not upstream of Src. Total Src and total Erk levels were not affected by any of these treatments (Figure 4). These results demonstrate that Src functions upstream of Erk in osteoblasts.
Figure 4. Src is an upstream signaling partner of Erk.
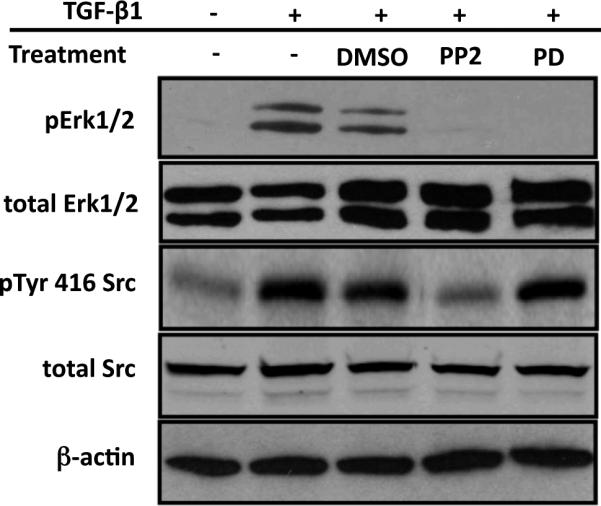
ROS osteoblast-like cells were cultured until they were 80% confluent and serum starved for 24 hours. Cells were pre-treated with PP2 (20mM), PD98059 (PD; 20mM) or DMSO (the diluent for PP2 or PD98059) for 30 minutes, and then treated with TGF-b1 (5ng/ml) for 20 minutes. After treatment, whole-cell protein extracts were analyzed by Western blot analysis. The inhibition of Src activation by PP2 also blocks the activation of Erk, whereas inhibition of Erk has no effect on Src activation by TGF-β1. Experiment was repeated 4 times with similar results.
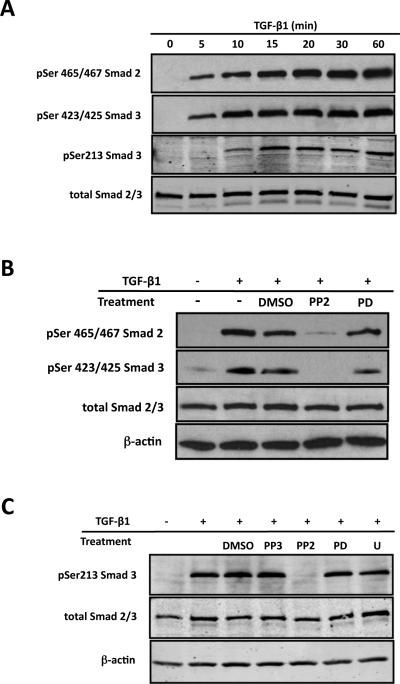
Since Smad signaling is essential for TGF-β1 induction of CTGF in osteoblasts (Arnott et al., 2008), we were interested in examining whether Src or Erk could affect Smad activation and/or nuclear translocation subsequent to TGF-β1 treatment in ROS osteoblast-like cells. We conducted an initial time course study to assess the activation of Smad 2 and Smad 3 by TGF-β1. In the canonical TGF-β1 signaling pathway, the SSXS motif at the C-terminus of Smad 2/3 is phosphorylated by the type I receptor (Chacko et al., 2004). The antibodies against phosphorylated serine 465/467 of Smad 2 or serine 423/425 of Smad 3 at the C-terminus can only recognize the receptor phosphorylated Smad 2 and Smad 3, respectively. Western blot analysis of cell lysates using these antibodies revealed a time-dependent activation of Smad 2 and Smad 3 (Figure 5A), while there was no change in total Smad 2/3 level in ROS osteoblast-like cells. Recent studies have shown that Erk could modify the linker region at Seine 213 between the MH1 and MH2 domain of R-Smads (Furukawa et al., 2003; Kamaraju and Roberts, 2005; Kretzschmar et al., 1999; Matsuura et al., 2005; Wang et al., 2009a). Therefore, we determined the phosphorylation status of linker region of Smad 3 under different conditions. Western blot analysis of whole cell lysates demonstrated a time-dependent phosphorylation of serine 213 at the linker region of Smad 3 (Figure 5A). Phosphorylation of serine 213 could be detected as early as 15 minutes post-treatment and it increased with time (Figure 5A). Interestingly, the phosphorylation of the linker region occurred later than the phosphorylation of the conserved SSXS motif.
Figure 5. Src regulates Smad activation in an Erk independent fashion.
ROS osteoblast-like cells were cultured until they were 80% confluent and serum starved for 24 hours prior to any treatment. After treatment, whole-cell protein extracts were analyzed by Western blot analysis. Experiment were repeated 4 times with similar results. (A) To determine the time course for TGF-β1 induced Smad2 and Smad3 activation, cells were treated with TGF-β1 (5ng/ml) for 0, 5, 10, 15, 30 or 60 minutes. Both Smad2 and Smad3 demonstrate the robust activation following TGF-β1 treatment with maximal activation occurring 20 minutes post-treatment (B) Serum-starved cells were pre-treated with the Src kinase inhibitor, PP2 (20mM), the Erk inhibitor, PD98059 (PD; 20mM), or diluent (DMSO) alone for 30 minutes followed by treatment with TGF-b1 (5ng/ml) for 20 minutes. While Src inhibition blocks activation of Smad2 and Smad3, Erk inhibition has no effect on Smad activation. (C) Serum-deprived cells were pre-treated with PP2 (20mM; Src kinase inhibitor), PD98059 (PD; 20mM; Erk inhibitor), U0126 (U; 30mM; Erk inhibitor), PP3 (an inactive analog of PP2), or DMSO (diluent only) for 30 minutes followed by TGF-b1 stimulation (5ng/ml) for 20 minutes. The results demonstrated that inhibition of Src, but not Erk, blocks linker region phosphorylation of Smad3.
Next we investigated how Src and Erk affect Smad 2 and Smad 3 activation after TGF-β1 stimulation. ROS osteoblast-like cells were pre-treated with 20μM of PP2 or PD98059 for 30 minutes followed by 5ng/ml TGF-β1 for 20 minutes. Western blot analysis using the phosphorylated serine465/467 Smad 2 or serine 423/425 Smad 3 antibodies revealed that pre-treatment of PP2 blocked the phosphorylation of Smad 2 and Smad 3 induced by TGF-β1 (Figure 5B). DMSO, the diluent for PP2, had no inhibitory effect on Smad 2 or Smad 3 phosphorylation (Figure 5B). On the contrary, the Erk inhibitor, PD98059, did not prevent Smad 2 or Smad 3 phosphorylation after TGF-β1 stimulation (Figure 5B). Our previous results demonstrated that Erk couldn't affect the phosphorylation of Smad 2 or Smad 3 at the conserved C-terminal SSXS motif, so next we wanted to determine whether Src inhibitor, PP2, or the Erk inhibitors, PD98059 could affect the phosphorylation of linker region. We used ROS osteoblast-like cells treated with 5ng/ml TGF-β1 for 20 minutes with or without pre-treatment with each of the pharmacological inhibitors. Once again, only the Src inhibitor blocked TGF-β1 induced serine 213 phosphorylation, while the Erk inhibitors did not inhibit TGF-β1 induced serine 213 phosphorylation (Figure 5C). Controls included PP3, the inactive analog of PP2, or DMSO, the diluent for the pharmacological inhibitors, and they had no inhibitory effect on TGF-β1 induced serine 213 phosphorylation (Figure 5C). This experiment demonstrated that Src positively regulates Smad phosphorylation, while Erk does not regulate Smad phosphorylation.
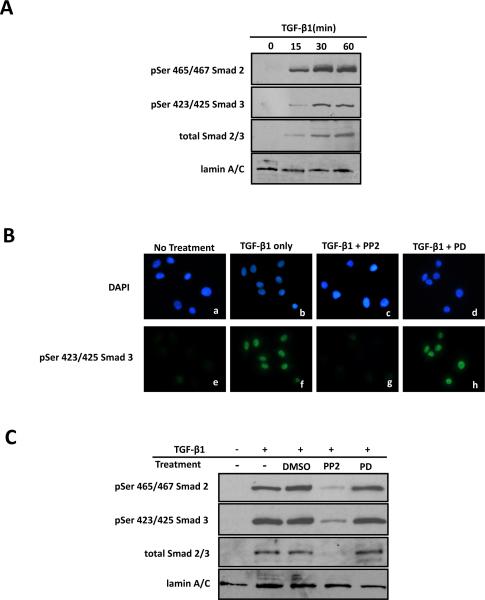
Although the inhibition of Erk did not affect TGF-β1 induced Smad phosphorylation, it is possible that Erk inhibition could affect the nuclear translocation of phosphorylated Smads, the immediate downstream event after phosphorylation. In order to examine the effects of Erk inhibition on nuclear translocation, we first established the time course for the nuclear translocation of Smad complex. ROS osteoblast-like cells were treated with 5ng/ml TGF-β1 for various times after which nuclear proteins were isolated and used for Western blot analysis. In the nuclear protein extract, phosphorylated Smad 2 and Smad 3 were detected as early as 15 minutes post-treatment and accumulated with time (Figure 6A). LaminA/C, a nuclear marker, was used as a control for sample loading and transfer (Figure 6A).
Figure 6. Src regulates Smad nuclear translocation.
ROS osteoblast-like cells were plated and serum starved for 24 hours prior to any treatment. (A) To determine the time course for nuclear translocation of activated Smad2 and Smad3, cells were treated with TGF-b1 (5ng/ml) for 0, 15, 30 or 60 minutes. Nuclear protein was separated from cytoplasmic protein as described in Material and Methods and nuclear lysate (20mg) was used for Western blot analysis. Blots were probed with antibodies for phosphor-Ser465/467 Smad2 or phosphor-Ser423/425 Smad3, and then reprobed for total Smad2/3. Lamin A/C was used as a control for nuclear proteins. Experiment was repeated 4 times with similar results. Nuclear translocation of activated Smad2 and Smad3 reaches maximal levels 30 post-treatment. (B) To determine if Src regulates Smad nuclear translocation all cells except for the negative controls (a, e) were treated with 5ng/ml TGF-b1 for 30 minutes; some were pre-treated for 30 minutes with 20mM PP2 (c, g), a Src kinase inhibitor, or with 20mM PD98059 (PD; d, h), an Erk inhibitor. Following treatment, all cells were fixed, permeabilized and incubated with anti-phospho Ser 423/425 Smad3 primary antibody followed by the appropriate fluorescein-conjugated secondary antibody and DAPI staining. Fields of cells with DAPI-stained nuclei (blue) are shown in a-d, while the corresponding fluorescein-stained (p-Smad2 or p-Smad3, green) cells are shown in e-h. Experiment was repeated 3 times with similar results. Note that nuclear accumulation of activated Smad2 (A panel) or Smad 3 (B panel) following TGF-β1 treatment (compare f with e) is blocked by PP2 (g) but not by PD98059 (h). (C) Additionally nuclear translocation was examined using Western blot analysis. Serum-starved cells were pre-treated with PP2 (20mM), PD98059 (PD; 20mM) or DMSO (diluent for PP2 and PD98059) for 30 minutes followed by TGF-b1 (5ng/ml) stimulation for another 30 minutes. Nuclear protein was separated from cytoplasmic protein and total nuclear protein (20mg) lysate was used for Western blot analysis. The inhibition of Src, but not Erk, blocks the nuclear translocation of activated Smads. Experiment was repeated 3 times with similar results.
Subsequent immunofluorescent experiments were conducted to visualize the localization of activated Smad 3 following TGF-β1 treatment and to examine the effects of Src or Erk inhibition on phosphorylated Smad 3 localization. Cells treated with TGF-β1 for 30 minutes (positive control) showed an intense fluorescent signal for activated Smad 3 in their nuclei compared to negative controls in which the fluorescent signal for activated Smad 3 was undetectable or very weak (Figure 6B). Nuclei were also stained with DAPI (blue) to correlate with the nuclear localization of activated Smad 3 (green) (Figure 6B). Similar result were found using Smad 2 (data not shown). Cells pre-treated with the Src inhibitor, PP2, did not exhibit a detectable fluorescent signal for activated Smad 3 (similar to negative control), while the Erk inhibitor had no effect on the nuclear localization of activated Smad 3 (similar to positive control) (Figure 6B). These results demonstrate that Smad activation and nuclear translocation is directly affected by the inhibition of Src but not Erk.
In addition to the immunofluorescence studies we also assessed nuclear translocation using Western blot. Based on this time course experiment, ROS osteoblast-like cells were treated with TGF-β1 for 30 minutes with or without pre-treatment with the Src or Erk inhibitors. Blocking Src activation with PP2 prevented the nuclear accumulation of phosphorylated Smad 2 and Smad 3, confirming the inhibitory effects of PP2 on the phosphorylation of Smads (Figure 6C). However, blocking Erk activation with PD98059 did not prevent the nuclear accumulation of phosphorylated Smads, which was similar to TGF-β1 only treatment (Figure 6C). Cells pre-treated with the diluent, DMSO, served as a negative control and showed the same response as cells treated with TGF-β1, and none of the experimental conditions had any effect on total Smad 2/3 levels (Figure 6C).
Erk is Required for Transcriptional Complex Formation on the CTGF Promoter
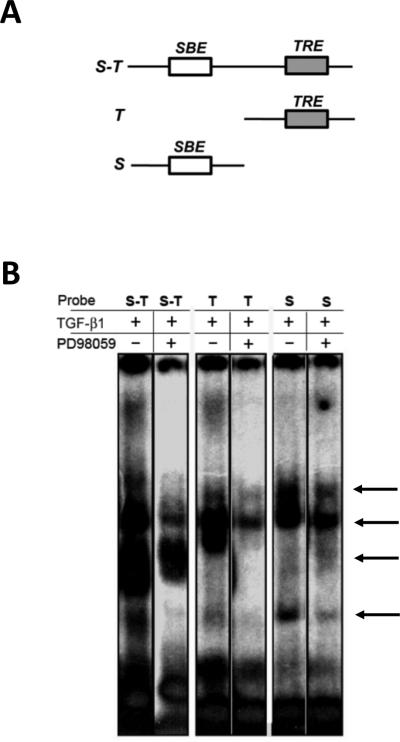
Thus far we demonstrated that Erk is required for TGF-β1 induced CTGF expression, but that Erk inhibition does not regulate Smad phosphorylation or nuclear translocation. Therefore, we conducted additional studies to investigate how Erk can affect CTGF expression. Studies in other cell types have shown that MAPKs, such as Erk, can modulate the TGF-β1/Smad signaling pathway through activation/inactivation of required nuclear co-activators/co-repressors that mediate Smad DNA binding (Kretzschmar et al., 1999). To determine how Erk signaling functions to potentiate CTGF induction by TGF-β1, we examined if Erk was required for transcriptional complex formation on the CTGF promoter in osteoblasts after TGF-β1 treatment. Previous studies have identified functional regulatory motifs in the CTGF proximal promoter that are required to confer TGF-β1 responsiveness (Grotendorst et al., 1996; Holmes et al., 2001). We previously demonstrated that CTGF induction by TGF-β1 in osteoblasts is dependent on two proximal promoter elements, the TGF-β1 response element (TRE) and the Smad binding element (SBE) (Arnott et al., 2008). For these studies, probes were generated that contained both the SBE and TRE (S-T), the TRE alone (T) or the SBE alone (S) (Figure 7A). To determine if Erk signaling was required to facilitate TGF-β1 induced complex formation/binding to the CTGF promoter, we used the Erk inhibitor, PD98059, to block the Erk signaling pathway. Nuclear lysates were prepared from osteoblasts treated with TGF-β1 that were either pretreated with the inhibitor (PD98059) or mock-treated (DMSO). We assessed the ability of Erk inhibitor/TGF-β1 treated nuclear lysates versus TGF-β1 treated alone nuclear lysates to bind to each of the probes (Figure 7B). We found that blocking Erk activation impaired the ability of nuclear protein complexes to bind to the S-T, T and S probes (Figure 7B). In addition, blocking Erk completely abolished the binding of specific complexes on all three probes (Figure 7B), suggesting that Erk signaling is required to facilitate proper CTGF promoter complex formation/binding resulting from TGF-β1 induction in osteoblasts. Additional studies are required to determine the identity of the nuclear proteins that form a complex with activated Smads on these regulatory elements of the CTGF promoter, and to determine which of these nuclear proteins are regulated by Erk signaling
Figure 7. Inhibition of Erk alters the binding of nuclear proteins to the CTGF promoter.
(A) Probes were created from the CTGF promoter that contained both the Smad binding element (SBE) and the TGF-b responsive element (TRE) (S-T probe), the TRE alone (T probe) or the SBE alone (S probe). These probes were subsequently utilized to determine the ability of TGF-b1 induced nuclear protein to bind to the promoter element. (B) Electro-mobility shift assays were performed using nuclear lysates generated from osteoblasts pre-treated for 30 minutes with the Erk inhibitor PD98059 (+) or diluent (DMSO, -), then treated with TGF-b1 (5ng/ml) for 24 hours. The binding of nuclear proteins to the SBE and/or the TRE in the CTGF promoter was assessed using 5mg of indicated nuclear lysates. In the presence of the Erk inhibitor the proteins that bind to each of the probes are significantly reduced in quantity or eliminated (see arrows for examples). Experiment was repeated 3 times with similar results.
Discussion
Connective tissue growth factor (CTGF/CCN2) is a cysteine rich, extracellular matrix protein that acts as an anabolic growth factor to regulate osteoblast differentiation and function (Safadi et al., 2003; Xu et al., 2000). We have previously shown that in primary osteoblasts, CTGF was induced by TGF-β1 where it acted as a downstream mediator of TGF-β1 induced matrix production (Arnott, Nuglozeh et al. 2007). We recently tested the requirements of Smads and MAPKs signaling for TGF-β1 induced CTGF promoter activity in osteoblasts, and demonstrated that Smads (3 and 4) and Erk (not p38 or Jnk) were required for CTGF induction by TGF-β1 (Arnott et al., 2008). Interestingly in the same study, we also demonstrated that the non-receptor tyrosine kinase, Src, was also an essential signal molecule for TGF-β1 induced CTGF induction. Although these findings suggest that Smads, Erk and Src play an important role in TGF-β1 induced CTGF promoter activity and expression in osteoblasts, the detailed molecular mechanisms required for CTGF induction by TGF-β1 remain undetermined. In this study, we examined the potential interaction between Erk and Src for CTGF induction by TGF-β1 in ROS osteoblast-like cells and whether they function to modulate Smad signaling.
In this study we demonstrated a time dependent activation of Src following TGF-β1 treatment in rat osteosarcoma osteoblast-like cells (ROS 17/2.8) (Figure 1B). When osteoblasts were pre-treated with the Src family kinase inhibitor, PP2, Src activation induced by TGF-β1 was inhibited. Importantly, TGF-β1 induced CTGF expression is impaired by PP2 in a dose-dependent manner in ROS cells (Figure 1D), demonstrating that Src family kinases are an essential signaling component of TGF-β1 induced CTGF expression in osteoblasts. We found that in addition to Src, Ros cells also express other Src family members, including Fyn, Yes or Hck (Figure 2A). In order to confirm the role of Src for CTGF expression, we used Src siRNA to knockdown Src expression in ROS osteoblast-like cells (Figure 2B). This approach specifically blocks Src expression by ~90% without affecting the expression of other Src family kinase members, such as Fyn and Yes (Figure 2C). Interestingly, TGF-β1 induction of CTGF expression is abolished by >70% with Src siRNA transfection, demonstrating that Src appears to be the major Src Family kinase involved in CTGF induction be TGF-β1 in osteoblasts.
The critical role that Src plays in bone was first demonstrated through the generation of the Src knockout mouse model that exhibited osteopetrosis due to impared osteoclast function (Soriano et al., 1991) (Boyce et al., 1992) (Boyce et al., 1993). A role for Src in osteoblast regulation was first seen when osteoblasts from Src knockout mice had enhanced osteoblast differentiation and bone formation with an up-regulation of bone matrix related proteins, osteocalcin and pro-alpha 2(I) collagen (Marzia et al., 2000). On the contrary, other in-vitro studies demonstrated that Src was activated in osteoblasts by fibroblast growth factor and that blocking Src expression prevented expression of the extracellular matrix protein, fibronectin (Tang et al., 2007), demonstrating that Src is a positive regulator of extracellular matrix production in osteoblasts. While the discrepancy among some of these findings regarding the role of Src is not understood, the role for Src as a regulator of matrix production is consistent with its role as a regulator of CTGF as we have previously demonstrated that CTGF is a downstream mediator of TGF-β1 induced matrix production in osteoblasts (Arnott, Nuglozeh et al. 2007). One possibility for these discrepant findings is compensatory effects of other Src family members. For example, Hck was up-regulated in Src-/- osteoclasts and Src and Hck double knockout mice showed a much more severe osteopetrosis than the Src knockout mice alone (Lowell et al., 1996). The role of other Src family members in osteoblast regulation is not well understood. One report has demonstrated that the up-regulation of alkaline phosphatase expression by fibroblast growth factor receptor activation was mediated by proteasome degradation of Fyn in human calvarial osteoblasts (Kaabeche et al., 2004), however there is no report of any bone phenotype in knockout models of Fyn, Yes and Hck. In this study, we did demonstrate that Fyn, Yes and Hck are expressed in osteoblasts, however in these cells Src accounts for >70-% of CTGF induction by TGF-β1 and appears to be the major downstream signal effecter for CTGF induction. Additional studies are warranted to examine the effects of Fyn, Yes, or Hck in osteoblasts.
The role for Src as a signal transducer of TGF-β1 in osteoblasts is consistent with other published reports that have also implicated Src as a downstream signaling effector of TGF-β1 in certain cell types (Galliher and Schiemann, 2006; Kim et al., 2005; Mishra et al., 2007; Tanaka et al., 2004; Varon et al., 2006), although there is no published report on TGF-β1 induced Src activation in osteoblasts. We used an approach to inhibit Src using the Src family kinase inhibitor, PP2, which blocks Src activation and CTGF induction by TGF-β1 (Zhu et al., 1999). This finding is consistent with studies in fibroblasts where PP2 blocked CTGF induction, demonstrating that Src activity is necessary for CTGF expression (Graness et al., 2006). Src activation following TGF-β1 treatment can occur as a direct result of TGF-β receptor activation (Sato et al., 2005; Tanaka et al., 2004) or indirectly as a result of enhanced integrin-mediated cell attachment induced by TGF-β1 (Galliher and Schiemann, 2006; Kim et al., 2004; Kim and Joo, 2002; Varon et al., 2006). In a study using mammary epithelial cells it was shown that PP1, and to a lesser extent, PP2, significantly inhibited TGF receptor kinase activity and blocked subsequent downstream signal transduction (Maeda et al., 2006) however in our cells PP2 did not inhibit TGF receptor kinase activity (data not shown). Further, our results demonstrate a time dependent activation of Src that is consistent with direct activation by the TGF-β1 receptor, however these results do not rule out the possibility that other proteins may be involved. Future studies will address the interaction between Src and the TGF-β1 receptor, and the potential requirement of other proteins in this process
Src can function as an upstream signaling partner of Erk (Katz et al., 2006; Tsuruda et al., 2004) and more recently Src was found as an upstream signaling partner of Erk in osteoblastic cell lines (Katz et al., 2006). In this study, we demonstrated that activation of Src is required for TGF-β1 induced Erk activation, indicating that Src functions upstream of Erk with regard to TGF-β1 in Ros cells. It is also important to note that inhibition of Erk activation using the MEK inhibitor, PD98059, had no effect on Src activation following TGF-β1 treatment. However, the most interesting observation was the novel requirement of Src for Smad activation and nuclear translocation. Our results demonstrated that inhibition of Src activation completely blocked TGF-β1 induced activation of Smads 2 and 3 as well as their nuclear translocation. Such a role for Src in regulating Smad activation downstream of TGF-β1 has not been identified previously in any cell type. To date there has been one report demonstrating a role for Src mediated Smad 1/5 signaling downstream of BMP where Src complexed with Smad 1/5 to facilitate Smad nuclear translocation (Gautschi et al., 2008). A more detailed investigation of the Smad-Src interaction in osteoblasts is warranted.
Recent studies examining the interactions between MAPKs and Smad signaling demonstrated that TGF-β1 induced Smad signaling is regulated by MAPKs in osteoblasts and the cellular responses induced by TGF-β1 are determined by this interaction (Sowa et al., 2002a; Sowa et al., 2002b). MAPKs, such as Erk, can modulate the TGF-β1/Smad pathway through direct effects on the phosphorylation of Smads or indirectly through activation/inactivation of required nuclear co-activators/co-repressors that mediate Smad DNA binding (Kretzschmar et al., 1999). Using the ROS osteoblast-like cells, we demonstrated that TGF-β1 activates Smad2 and Smad3 at the conserved TGF-β receptor phosphorylation motif (SSXS) and the nuclear accumulation of activated Smad2 and Smad3 occurs in a time-dependent fashion. In addition to the conserved SSXS motif, the non-conserved linker region is emerging as an important site for Smad activation. In the linker region of Smad 3, four phosphorylation sites, Threonine 179, Serine 204, Serine 208, and Serine 213 have been identified which can be phosphorylated by MAPKs, although the utility of these sites for Smad activation is cell type dependent (Kretzschmar et al., 1999) (Matsuura et al., 2005). For example, in human HaCaT keratinocytes, Erk phosphorylates Serine 204 and Serine 208 while in mink lung epithelial cells Serine 208 was the preferred phosphorylation site (Matsuura et al., 2004; Matsuura et al., 2005). In our study, we found that the Serine 213 residue at the linker region of Smad3 is phosphorylated by TGF-β1 in a time-dependent manner in addition to the SSXS motif. This is the first report of TGF-β1 activation of Smads at this site. While this study did not assess activation of other residues in the linker region, the emerging importance of this region for Smad activation warrants future studies in osteoblasts.
In contrast to Src family kinases inhibition of Smad activation, this study demonstrated that the inhibition of Erk using the MEK inhibitor, PD98059, did not prevent activation (phosphorylation) of Smads 2 and 3 or the translocation of the activated Smad2/3/4 complex into the nucleus. To assess if Erk regulates Smad signaling indirectly through activation/inactivation of required nuclear co-activators/co-repressors to mediate Smad DNA binding, we employed electro-mobility shift assays. In these assays we demonstrated that inhibition of Erk activation causes a significant reduction in the binding of trans-acting protein complexes to the TRE and/or SBE in the CTGF promoter. Previous studies have shown that activated Erk can translocate to the nucleus where it activates (phosphorylates) downstream transcription factors (Chang et al., 2003) that can form a transcriptionally active complex with Smad transcriptional co- activators such as p300 and CBP (Foulds et al., 2004; Pei et al., 2003). Based on our results, we hypothesize that Erk mediates Smad signaling through activation of nuclear transcription factors that enhance Smad DNA binding and are necessary for transcriptional activation of the CTGF promoter. However, the identity of these nuclear transcription factors (proteins) through which Erk functions to regulate Smad binding remain unknown and are the focus of current investigation.
In this study, we examined the interaction between Src and Erk for CTGF induction by TGF-β1 in osteoblasts and whether they regulate Smad signaling. Our results demonstrate a new paradigm where Src family kinases play a role as a major downstream signal transduction conduit for TGF-β1 signaling in osteoblasts in the context of CTGF regulation. Upon TGF-β1 stimulation, Src functions to distinctly regulate both Erk and Smad pathways. It appears from our results that these two pathways synergize at the promoter level and both pathways stimulate transcription factor binding for combinatorial CTGF promoter transactivation. Future studies will focus on the interaction between Src and the TGF-β1 receptor and identification of the transcription factors that function downstream of Erk and Smads that are required to achieve CTGF induction.
Footnotes
Contract grant sponsor: NIH; Contract grant number: 2R01AR047432-06.
Contract grant sponsor: NIH; Contract grant number: 1R03AR057193-01.
Contract grant sponsor: The State of Pennsylvania Department of Health
Contract grant sponsor: The Commonwealth Medical College
References
- Abdollah S, Macias-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana JL. TbetaRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2-Smad4 complex formation and signaling. The Journal of biological chemistry. 1997;272(44):27678–27685. doi: 10.1074/jbc.272.44.27678. [DOI] [PubMed] [Google Scholar]
- Arnott JA, Nuglozeh E, Rico MC, Arango-Hisijara I, Odgren PR, Safadi FF, Popoff SN. Connective tissue growth factor (CTGF/CCN2) is a downstream mediator for TGF-beta1-induced extracellular matrix production in osteoblasts. J Cell Physiol. 2007;210(3):843–852. doi: 10.1002/jcp.20917. [DOI] [PubMed] [Google Scholar]
- Arnott JA, Zhang X, Sanjay A, Owen TA, Smock SL, Rehman S, DeLong WG, Safadi FF, Popoff SN. Molecular requirements for induction of CTGF expression by TGF-beta1 in primary osteoblasts. Bone. 2008;42(5):871–885. doi: 10.1016/j.bone.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atfi A, Drobetsky E, Boissonneault M, Chapdelaine A, Chevalier S. Transforming growth factor beta down-regulates Src family protein tyrosine kinase signaling pathways. J Biol Chem. 1994;269(48):30688–30693. [PubMed] [Google Scholar]
- Blanchette F, Rivard N, Rudd P, Grondin F, Attisano L, Dubois CM. Cross-talk between the p42/p44 MAP kinase and Smad pathways in transforming growth factor beta 1-induced furin gene transactivation. J Biol Chem. 2001;276(36):33986–33994. doi: 10.1074/jbc.M100093200. [DOI] [PubMed] [Google Scholar]
- Blom IE, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy? Matrix Biol. 2002;21(6):473–482. doi: 10.1016/s0945-053x(02)00055-0. [DOI] [PubMed] [Google Scholar]
- Bonewald LF. Transforming Growth Factor-B. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. Academic Press; New York: 2002. pp. 903–918. [Google Scholar]
- Bonewald LF, Dallas SL. Role of active and latent transforming growth factor beta in bone formation. J Cell Biochem. 1994;55(3):350–357. doi: 10.1002/jcb.240550312. [DOI] [PubMed] [Google Scholar]
- Boyce BF, Chen H, Soriano P, Mundy GR. Histomorphometric and immunocytochemical studies of src-related osteopetrosis. Bone. 1993;14(3):335–340. doi: 10.1016/8756-3282(93)90161-3. [DOI] [PubMed] [Google Scholar]
- Boyce BF, Yoneda T, Lowe C, Soriano P, Mundy GR. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J Clin Invest. 1992;90(4):1622–1627. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centrella M, Canalis E. Isolation of EGF-dependent transforming growth factor (TGF beta-like) activity from culture medium conditioned by fetal rat calvariae. J Bone Miner Res. 1987;2(1):29–36. doi: 10.1002/jbmr.5650020106. [DOI] [PubMed] [Google Scholar]
- Chacko BM, Qin BY, Tiwari A, Shi G, Lam S, Hayward LJ, De Caestecker M, Lin K. Structural basis of heteromeric smad protein assembly in TGF-beta signaling. Molecular cell. 2004;15(5):813–823. doi: 10.1016/j.molcel.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17(7):1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- Chen Y, Blom IE, Sa S, Goldschmeding R, Abraham DJ, Leask A. CTGF expression in mesangial cells: involvement of SMADs, MAP kinase, and PKC. Kidney Int. 2002;62(4):1149–1159. doi: 10.1111/j.1523-1755.2002.kid567.x. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Dignam JD, Martin PL, Shastry BS, Roeder RG. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273(29):18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annual review of cell and developmental biology. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Foulds CE, Nelson ML, Blaszczak AG, Graves BJ. Ras/mitogen-activated protein kinase signaling activates Ets-1 and Ets-2 by CBP/p300 recruitment. Molecular and cellular biology. 2004;24(24):10954–10964. doi: 10.1128/MCB.24.24.10954-10964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa F, Matsuzaki K, Mori S, Tahashi Y, Yoshida K, Sugano Y, Yamagata H, Matsushita M, Seki T, Inagaki Y, Nishizawa M, Fujisawa J, Inoue K. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology. 2003;38(4):879–889. doi: 10.1053/jhep.2003.50384. [DOI] [PubMed] [Google Scholar]
- Galliher AJ, Schiemann WP. Beta3 integrin and Src facilitate transforming growth factor-beta mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2006;8(4):R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliher AJ, Schiemann WP. Src phosphorylates Tyr284 in TGF-beta type II receptor and regulates TGF-beta stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res. 2007;67(8):3752–3758. doi: 10.1158/0008-5472.CAN-06-3851. [DOI] [PubMed] [Google Scholar]
- Galliher-Beckley AJ, Schiemann WP. Grb2 binding to Tyr284 in TbetaR-II is essential for mammary tumor growth and metastasis stimulated by TGF-beta. Carcinogenesis. 2008;29(2):244–251. doi: 10.1093/carcin/bgm245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautschi O, Tepper CG, Purnell PR, Izumiya Y, Evans CP, Green TP, Desprez PY, Lara PN, Gandara DR, Mack PC, Kung HJ. Regulation of Id1 expression by SRC: implications for targeting of the bone morphogenetic protein pathway in cancer. Cancer Res. 2008;68(7):2250–2258. doi: 10.1158/0008-5472.CAN-07-6403. [DOI] [PubMed] [Google Scholar]
- Graness A, Cicha I, Goppelt-Struebe M. Contribution of Src-FAK signaling to the induction of connective tissue growth factor in renal fibroblasts. Kidney Int. 2006;69(8):1341–1349. doi: 10.1038/sj.ki.5000296. [DOI] [PubMed] [Google Scholar]
- Granet C, Vico AG, Alexandre C, Lafage-Proust MH. MAP and src kinases control the induction of AP-1 members in response to changes in mechanical environment in osteoblastic cells. Cell Signal. 2002;14(8):679–688. doi: 10.1016/s0898-6568(02)00008-6. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996;7(4):469–480. [PubMed] [Google Scholar]
- Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. Faseb J. 2003;17(11):1576–1578. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- Hayashida T, Poncelet AC, Hubchak SC, Schnaper HW. TGF-beta1 activates MAP kinase in human mesangial cells: a possible role in collagen expression. Kidney Int. 1999;56(5):1710–1720. doi: 10.1046/j.1523-1755.1999.00733.x. [DOI] [PubMed] [Google Scholar]
- Hayashida T, Wu MH, Pierce A, Poncelet AC, Varga J, Schnaper HW. MAP-kinase activity necessary for TGFbeta1-stimulated mesangial cell type I collagen expression requires adhesion-dependent phosphorylation of FAK tyrosine 397. J Cell Sci. 2007;120(Pt 23):4230–4240. doi: 10.1242/jcs.03492. [DOI] [PubMed] [Google Scholar]
- Hock JM, Canalis E, Centrella M. Transforming growth factor-beta stimulates bone matrix apposition and bone cell replication in cultured fetal rat calvariae. Endocrinology. 1990;126(1):421–426. doi: 10.1210/endo-126-1-421. [DOI] [PubMed] [Google Scholar]
- Holmes A, Abraham DJ, Sa S, Shiwen X, Black CM, Leask A. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem. 2001;276(14):10594–10601. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]
- Imamichi Y, Waidmann O, Hein R, Eleftheriou P, Giehl K, Menke A. TGF beta-induced focal complex formation in epithelial cells is mediated by activated ERK and JNK MAP kinases and is independent of Smad4. Biol Chem. 2005;386(3):225–236. doi: 10.1515/BC.2005.028. [DOI] [PubMed] [Google Scholar]
- Joo CK, Kim HS, Park JY, Seomun Y, Son MJ, Kim JT. Ligand release-independent transactivation of epidermal growth factor receptor by transforming growth factor-beta involves multiple signaling pathways. Oncogene. 2008;27(5):614–628. doi: 10.1038/sj.onc.1210649. [DOI] [PubMed] [Google Scholar]
- Kaabeche K, Lemonnier J, Le Mee S, Caverzasio J, Marie PJ. Cbl-mediated degradation of Lyn and Fyn induced by constitutive fibroblast growth factor receptor-2 activation supports osteoblast differentiation. J Biol Chem. 2004;279(35):36259–36267. doi: 10.1074/jbc.M402469200. [DOI] [PubMed] [Google Scholar]
- Kamaraju AK, Roberts AB. Role of Rho/ROCK and p38 MAP kinase pathways in transforming growth factor-beta-mediated Smad-dependent growth inhibition of human breast carcinoma cells in vivo. J Biol Chem. 2005;280(2):1024–1036. doi: 10.1074/jbc.M403960200. [DOI] [PubMed] [Google Scholar]
- Kartsogiannis V, Ng KW. Cell lines and primary cell cultures in the study of bone cell biology. Molecular and cellular endocrinology. 2004;228(1-2):79–102. doi: 10.1016/j.mce.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Katz S, Boland R, Santillan G. Modulation of ERK 1/2 and p38 MAPK signaling pathways by ATP in osteoblasts: involvement of mechanical stress-activated calcium influx, PKC and Src activation. Int J Biochem Cell Biol. 2006;38(12):2082–2091. doi: 10.1016/j.biocel.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Kim HP, Kim TY, Lee MS, Jong HS, Lee JW, Bang YJ. TGF-beta1-mediated activations of c-Src and Rac1 modulate levels of cyclins and p27(Kip1) CDK inhibitor in hepatoma cells replated on fibronectin. Biochim Biophys Acta. 2005;1743(1-2):151–161. doi: 10.1016/j.bbamcr.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Kim HP, Lee MS, Yu J, Park JA, Jong HS, Kim TY, Lee JW, Bang YJ. TGF-beta1 (transforming growth factor-beta1)-mediated adhesion of gastric carcinoma cells involves a decrease in Ras/ERKs (extracellular-signal-regulated kinases) cascade activity dependent on c-Src activity. Biochem J. 2004;379(Pt 1):141–150. doi: 10.1042/BJ20031408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JT, Joo CK. Involvement of cell-cell interactions in the rapid stimulation of Cas tyrosine phosphorylation and Src kinase activity by transforming growth factor-beta 1. J Biol Chem. 2002;277(35):31938–31948. doi: 10.1074/jbc.M201178200. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Timokhina I, Massague J. A mechanism of repression of TGFbeta/ Smad signaling by oncogenic Ras. Genes Dev. 1999;13(7):804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutz SM, Higgins CE, Samarakoon R, Higgins SP, Allen RR, Qi L, Higgins PJ. TGF-beta 1-induced PAI-1 expression is E box/USF-dependent and requires EGFR signaling. Experimental cell research. 2006;312(7):1093–1105. doi: 10.1016/j.yexcr.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2003;81(6):355–363. doi: 10.1139/o03-069. [DOI] [PubMed] [Google Scholar]
- Leask A, Holmes A, Black CM, Abraham DJ. Connective tissue growth factor gene regulation. Requirements for its induction by transforming growth factor-beta 2 in fibroblasts. The Journal of biological chemistry. 2003;278(15):13008–13015. doi: 10.1074/jbc.M210366200. [DOI] [PubMed] [Google Scholar]
- Leivonen SK, Hakkinen L, Liu D, Kahari VM. Smad3 and extracellular signal-regulated kinase 1/2 coordinately mediate transforming growth factor-beta-induced expression of connective tissue growth factor in human fibroblasts. J Invest Dermatol. 2005;124(6):1162–1169. doi: 10.1111/j.0022-202X.2005.23750.x. [DOI] [PubMed] [Google Scholar]
- Liu P, Zhang C, Feng JB, Zhao YX, Wang XP, Yang JM, Zhang MX, Wang XL, Zhang Y. Cross talk among Smad, MAPK, and integrin signaling pathways enhances adventitial fibroblast functions activated by transforming growth factor-beta1 and inhibited by Gax. Arterioscler Thromb Vasc Biol. 2008;28(4):725–731. doi: 10.1161/ATVBAHA.107.159889. [DOI] [PubMed] [Google Scholar]
- Lowell CA, Niwa M, Soriano P, Varmus HE. Deficiency of the Hck and Src tyrosine kinases results in extreme levels of extramedullary hematopoiesis. Blood. 1996;87(5):1780–1792. [PubMed] [Google Scholar]
- Maeda M, Shintani Y, Wheelock MJ, Johnson KR. Src activation is not necessary for transforming growth factor (TGF)-beta-mediated epithelial to mesenchymal transitions (EMT) in mammary epithelial cells. PP1 directly inhibits TGF-beta receptors I and II. J Biol Chem. 2006;281(1):59–68. doi: 10.1074/jbc.M503304200. [DOI] [PubMed] [Google Scholar]
- Majeska RJ, Rodan GA. The effect of 1,25(OH)2D3 on alkaline phosphatase in osteoblastic osteosarcoma cells. J Biol Chem. 1982;257(7):3362–3365. [PubMed] [Google Scholar]
- Marzia M, Sims NA, Voit S, Migliaccio S, Taranta A, Bernardini S, Faraggiana T, Yoneda T, Mundy GR, Boyce BF, Baron R, Teti A. Decreased c-Src expression enhances osteoblast differentiation and bone formation. The Journal of cell biology. 2000;151(2):311–320. doi: 10.1083/jcb.151.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. Integration of Smad and MAPK pathways: a link and a linker revisited. Genes Dev. 2003;17(24):2993–2997. doi: 10.1101/gad.1167003. [DOI] [PubMed] [Google Scholar]
- Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14(6):627–644. [PubMed] [Google Scholar]
- Matsuura I, Denissova NG, Wang G, He D, Long J, Liu F. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature. 2004;430(6996):226–231. doi: 10.1038/nature02650. [DOI] [PubMed] [Google Scholar]
- Matsuura I, Wang G, He D, Liu F. Identification and characterization of ERK MAP kinase phosphorylation sites in Smad3. Biochemistry. 2005;44(37):12546–12553. doi: 10.1021/bi050560g. [DOI] [PubMed] [Google Scholar]
- Mishra R, Zhu L, Eckert RL, Simonson MS. TGF-beta-regulated collagen type I accumulation: role of Src-based signals. Am J Physiol Cell Physiol. 2007;292(4):C1361–1369. doi: 10.1152/ajpcell.00370.2006. [DOI] [PubMed] [Google Scholar]
- Mori Y, Ishida W, Bhattacharyya S, Li Y, Platanias LC, Varga J. Selective inhibition of activin receptor-like kinase 5 signaling blocks profibrotic transforming growth factor beta responses in skin fibroblasts. Arthritis and rheumatism. 2004;50(12):4008–4021. doi: 10.1002/art.20658. [DOI] [PubMed] [Google Scholar]
- Murillo MM, del Castillo G, Sanchez A, Fernandez M, Fabregat I. Involvement of EGF receptor and c-Src in the survival signals induced by TGF-beta1 in hepatocytes. Oncogene. 2005;24(28):4580–4587. doi: 10.1038/sj.onc.1208664. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Kimura Y, Tamura T, Ichikawa H, Yamaai Y, Sugimoto T, Takigawa M. Cloning of a mRNA preferentially expressed in chondrocytes by differential display-PCR from a human chondrocytic cell line that is identical with connective tissue growth factor (CTGF) mRNA. Biochem Biophys Res Commun. 1997;234(1):206–210. doi: 10.1006/bbrc.1997.6528. [DOI] [PubMed] [Google Scholar]
- Nishida T, Nakanishi T, Asano M, Shimo T, Takigawa M. Effects of CTGF/Hcs24, a hypertrophic chondrocyte-specific gene product, on the proliferation and differentiation of osteoblastic cells in vitro. Journal of cellular physiology. 2000;184(2):197–206. doi: 10.1002/1097-4652(200008)184:2<197::AID-JCP7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Noda M. Transcriptional regulation of osteocalcin production by transforming growth factor-beta in rat osteoblast-like cells. Endocrinology. 1989;124(2):612–617. doi: 10.1210/endo-124-2-612. [DOI] [PubMed] [Google Scholar]
- Parisi MS, Gazzerro E, Rydziel S, Canalis E. Expression and regulation of CCN genes in murine osteoblasts. Bone. 2005 doi: 10.1016/j.bone.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Pei H, Yordy JS, Leng Q, Zhao Q, Watson DK, Li R. EAPII interacts with ETS1 and modulates its transcriptional function. Oncogene. 2003;22(18):2699–2709. doi: 10.1038/sj.onc.1206374. [DOI] [PubMed] [Google Scholar]
- Rodan GA, Majeska RJ. Phenotypic maturation of osteoblastic osteosarcoma cells in culture. Prog Clin Biol Res. 1982;110(Pt B):249–259. [PubMed] [Google Scholar]
- Safadi FF, Xu J, Smock SL, Kanaan RA, Selim AH, Odgren PR, Marks SC, Jr., Owen TA, Popoff SN. Expression of connective tissue growth factor in bone: its role in osteoblast proliferation and differentiation in vitro and bone formation in vivo. J Cell Physiol. 2003;196(1):51–62. doi: 10.1002/jcp.10319. [DOI] [PubMed] [Google Scholar]
- Samarakoon R, Higgins CE, Higgins SP, Higgins PJ. Differential requirement for MEK/ERK and SMAD signaling in PAI-1 and CTGF expression in response to microtubule disruption. Cell Signal. 2009;21(6):986–995. doi: 10.1016/j.cellsig.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarakoon R, Higgins PJ. Integration of non-SMAD and SMAD signaling in TGF-beta1-induced plasminogen activator inhibitor type-1 gene expression in vascular smooth muscle cells. Thrombosis and haemostasis. 2008;100(6):976–983. [PMC free article] [PubMed] [Google Scholar]
- Samarakoon R, Higgins SP, Higgins CE, Higgins PJ. TGF-beta1-induced plasminogen activator inhibitor-1 expression in vascular smooth muscle cells requires pp60(c-src)/EGFR(Y845) and Rho/ROCK signaling. Journal of molecular and cellular cardiology. 2008;44(3):527–538. doi: 10.1016/j.yjmcc.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Kawai-Kowase K, Sato H, Oyama Y, Kanai H, Ohyama Y, Suga T, Maeno T, Aoki Y, Tamura J, Sakamoto H, Nagai R, Kurabayashi M. c-Src and hydrogen peroxide mediate transforming growth factor-beta1-induced smooth muscle cell-gene expression in 10T1/2 cells. Arterioscler Thromb Vasc Biol. 2005;25(2):341–347. doi: 10.1161/01.ATV.0000152608.29351.8f. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Sims N, Baron R. Bone Cells and Their Function. In: Canalis E, editor. Skeletal Growth Factors. Lippincott Williams and Wilkins; Philadelphia, PA: 2000. pp. 1–17. [Google Scholar]
- Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Sowa H, Kaji H, Yamaguchi T, Sugimoto T, Chihara K. Activations of ERK1/2 and JNK by transforming growth factor beta negatively regulate Smad3-induced alkaline phosphatase activity and mineralization in mouse osteoblastic cells. The Journal of biological chemistry. 2002a;277(39):36024–36031. doi: 10.1074/jbc.M206030200. [DOI] [PubMed] [Google Scholar]
- Sowa H, Kaji H, Yamaguchi T, Sugimoto T, Chihara K. Smad3 promotes alkaline phosphatase activity and mineralization of osteoblastic MC3T3-E1 cells. J Bone Miner Res. 2002b;17(7):1190–1199. doi: 10.1359/jbmr.2002.17.7.1190. [DOI] [PubMed] [Google Scholar]
- Takai H, Araki S, Mezawa M, Kim DS, Li X, Yang L, Li Z, Wang Z, Nakayama Y, Ogata Y. AP1 binding site is another target of FGF2 regulation of bone sialoprotein gene transcription. Gene. 2008;410(1):97–104. doi: 10.1016/j.gene.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Takigawa M, Nakanishi T, Kubota S, Nishida T. Role of CTGF/HCS24/ecogenin in skeletal growth control. Journal of cellular physiology. 2003;194(3):256–266. doi: 10.1002/jcp.10206. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kobayashi H, Suzuki M, Kanayama N, Terao T. Transforming growth factor-beta1-dependent urokinase up-regulation and promotion of invasion are involved in Src-MAPK-dependent signaling in human ovarian cancer cells. J Biol Chem. 2004;279(10):8567–8576. doi: 10.1074/jbc.M309131200. [DOI] [PubMed] [Google Scholar]
- Tang CH, Yang RS, Chen YF, Fu WM. Basic fibroblast growth factor stimulates fibronectin expression through phospholipase C gamma, protein kinase C alpha, c-Src, NF-kappaB, and p300 pathway in osteoblasts. J Cell Physiol. 2007;211(1):45–55. doi: 10.1002/jcp.20896. [DOI] [PubMed] [Google Scholar]
- Tsuruda A, Suzuki S, Maekawa T, Oka S. Constitutively active Src facilitates NGF-induced phosphorylation of TrkA and causes enhancement of the MAPK signaling in SK-N-MC cells. FEBS Lett. 2004;560(1-3):215–220. doi: 10.1016/S0014-5793(04)00115-2. [DOI] [PubMed] [Google Scholar]
- Utsugi M, Dobashi K, Ishizuka T, Masubuchi K, Shimizu Y, Nakazawa T, Mori M. C-Jun-NH2-terminal kinase mediates expression of connective tissue growth factor induced by transforming growth factor-beta1 in human lung fibroblasts. Am J Respir Cell Mol Biol. 2003;28(6):754–761. doi: 10.1165/rcmb.4892. [DOI] [PubMed] [Google Scholar]
- Varon C, Tatin F, Moreau V, Van Obberghen-Schilling E, Fernandez-Sauze S, Reuzeau E, Kramer I, Genot E. Transforming growth factor beta induces rosettes of podosomes in primary aortic endothelial cells. Mol Cell Biol. 2006;26(9):3582–3594. doi: 10.1128/MCB.26.9.3582-3594.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Matsuura I, He D, Liu F. TGF-beta -inducible phosphorylation of Smad3. J Biol Chem. 2009a doi: 10.1074/jbc.M809281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SE, Xiang B, Zent R, Quaranta V, Pozzi A, Arteaga CL. Transforming growth factor beta induces clustering of HER2 and integrins by activating Src-focal adhesion kinase and receptor association to the cytoskeleton. Cancer Res. 2009b;69(2):475–482. doi: 10.1158/0008-5472.CAN-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, de Caestecker MP, Yamada Y. Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-beta-induced aggrecan gene expression in chondrogenic ATDC5 cells. J Biol Chem. 2001;276(17):14466–14473. doi: 10.1074/jbc.M005724200. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Maeno M, Hawrylyshyn B, Yao KL, Domenicucci C, Sodek J. Differential effects of transforming growth factor-beta on the synthesis of extracellular matrix proteins by normal fetal rat calvarial bone cell populations. J Cell Biol. 1988;106(3):915–924. doi: 10.1083/jcb.106.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Sukkar MB, Issa R, Oltmanns U, Nicholson AG, Chung KF. Regulation of TGF-beta 1-induced connective tissue growth factor expression in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2005;288(1):L68–76. doi: 10.1152/ajplung.00156.2004. [DOI] [PubMed] [Google Scholar]
- Xu J, Smock SL, Safadi FF, Rosenzweig AB, Odgren PR, Marks SC, Jr., Owen TA, Popoff SN. Cloning the full-length cDNA for rat connective tissue growth factor: implications for skeletal development. Journal of cellular biochemistry. 2000;77(1):103–115. doi: 10.1002/(sici)1097-4644(20000401)77:1<103::aid-jcb11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Yang C, Patel K, Harding P, Sorokin A, Glass WF., 2nd Regulation of TGF-beta1/MAPK-mediated PAI-1 gene expression by the actin cytoskeleton in human mesangial cells. Exp Cell Res. 2007;313(6):1240–1250. doi: 10.1016/j.yexcr.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Kim JL, Newcomb JR, Rose PE, Stover DR, Toledo LM, Zhao H, Morgenstern KA. Structural analysis of the lymphocyte-specific kinase Lck in complex with non-selective and Src family selective kinase inhibitors. Structure. 1999;7(6):651–661. doi: 10.1016/s0969-2126(99)80086-0. [DOI] [PubMed] [Google Scholar]
- Zirngibl RA, Chan JS, Aubin JE. Estrogen receptor-related receptor alpha (ERRalpha) regulates osteopontin expression through a non-canonical ERRalpha response element in a cell context-dependent manner. J Mol Endocrinol. 2008;40(2):61–73. doi: 10.1677/JME-07-0114. [DOI] [PubMed] [Google Scholar]