Abstract
Loss of functional β-cells is a primary mechanism of type 2 diabetes creating an acute need for understanding how β-cell number and function are regulated in adults under normal physiological conditions. Recent studies suggest a role for TGFβ family ligands in regulating β-cell function and glucose homeostasis. These ligands might influence β-cell proliferation and/or incorporation of new β-cells from progenitors in adults. Soluble antagonists of these ligands also appear to have important roles in regulating ligand activity to maintain homeostasis. These studies suggest that the coordinated activity of several TGFβ ligands might have important regulatory actions in adult β-cells and raise the possibility of developing new therapies for diabetes based on using agonists or antagonists of these ligands.
TGFβ family, β-cells and diabetes
The recent obesity epidemic in the US and other developed countries has contributed to an increased incidence of insulin resistance and diabetes, resulting in a more urgent search for novel therapeutic approaches. Loss of β-cell number and/or function results in reduced ability to control blood glucose concentrations, hyperglycemia, and diabetes – a process often exacerbated by peripheral insulin resistance that requires ever increasing insulin output from remaining β-cells. Indeed, recent GWAS studies have identified a number of genetic polymorphisms associated with increased diabetes risk, the majority of which implicate genes important for proper β-cell function. [1-3]. The critical nature of functional β-cells to glucose control has focused research on finding new sources for β-cells for transplantation or on factors that can lead to enhanced β-cell function and/or mass. Recent studies suggest that several members of the TGFβ family of growth factors might fill this role under normal physiological conditions and thus, might also be exploited to develop new therapies for diabetes. Although these growth factors are most widely known for their critical roles in development and tissue specification [4], more recent evidence suggests they also mediate numerous actions in adults involving homeostatic control of normal physiological processes, as well as roles in pathology, such as cancer [5]. Although potential actions of some TGFβ family members in glucose regulation were suggested more than 20 years ago, a specific role for a subset of these ligands has been only recently described, including regulation of glucose homeostasis in peripheral tissues and modulation of β-cell function (Table 1).
Table 1.
Summary of genetic mouse models used to identify in vivo roles of TGFβ signaling in glucose homeostasis.
| MOUSE MODEL | PHENOTYPE | REFERENCE |
|---|---|---|
| ACTIVIN: | ||
| ActRIIA and IIB knock out | Hypoplastic islets, impaired glucose tolerance |
[13] |
| ActRIIB knock out | Hypoplastic islets | [22] |
| Double ActRIIB/Smad 2 heterozygotes | Hypoplastic islets, decreased islet area, decreased insulin content, impaired glucose tolerance |
[22] |
| Dominant negative Activin Type IIR | β-cell hypoplasia, impaired glucose tolerance |
[12,23] |
| Activin B knock out | Hyperinsulinemia | [17] |
| ALK7 knock out | Hyperinsulinemia, decreased insulin sensitivity, impaired glucose tolerance, enlarged islets |
[17] |
| TGF-β: | ||
| Transgenic overexpression of TGF-β through insulin promoter |
Decreased development of exocrine pancreas and islets, maintenance of glucose control |
[41,42] |
| Transgenic overexpresssion of TGF-β through glucagon promoter |
B-cell hypoplasia, decreased insulin secretion, impaired glucose tolerance |
[43] |
| MYOSTATIN: | ||
| Myostatin knock out | Increase in skeletal muscle mass, decrease in fat mass, increased glucose utilization, increased insulin sensitivity |
[46-48] |
| GDF11: | ||
| GDF11 knock out | Increase in NGN+ islet precursor cells that did not develop into mature β-cells |
[45] |
| BMP: | ||
| Transgenic overexpression of BMP4 | Increased glucose tolerance, increased insulin secretion |
[49] |
| ALK3 knock out | Impaired glucose tolerance, diabetes | [49] |
| FSTL3: | ||
| FSTL3 knock out | Enlarged islets, β-cell hyperplasia, increased glucose tolerance, slight hyperinsulinemia |
[53] |
| SMAD 2: | ||
| Smad 2 heterozygote | Hypoplastic islets | [22] |
| Smad 2 conditional mice | Increase in NGN+ cells and decreased β-cell mass |
[45] |
| SMAD 3: | ||
| Smad 3 knock out | Increased insulin production and release, enhanced glucose tolerance, no change in β-cell mass |
[40] |
| SMAD 6: | ||
| Transgenic overexpression of noggin and Smad 6 |
Impaired glucose tolerance, diabetes | [49] |
| SMAD 7: | ||
| Conditional transgenic overexpression of Smad 7 |
Hypoplastic islets, decreased insulin content, impaired glucose tolerance, increased serum glucose (reversible condition by removal of the Smad7 overexpression) |
[44] |
In this review, we examine data from both in vitro and in vivo approaches supporting a role for TGFβ family ligands in glucose homeostasis in adults. These actions of TGFβ family members might thus be part of an as yet incompletely described system responsible for influencing β-cell mass and/or function in response to changing insulin demand. If so, this system would be of importance in understanding pathogenesis of type 2 diabetes, and might also provide new directions for developing therapies for β-cell dysfunction.
TGFβ family signaling
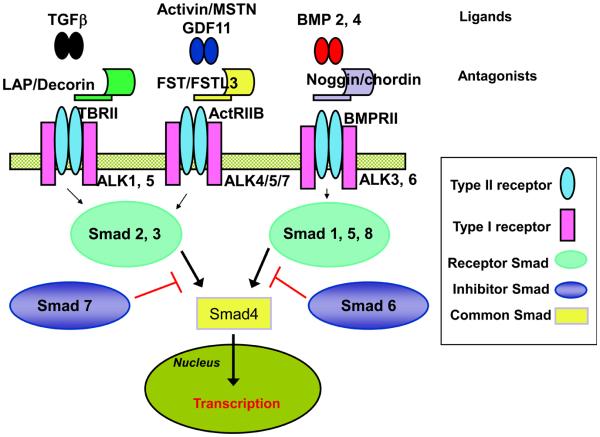
The TGFβ family of growth and differentiation factors contains more than 40 structurally related members that share biochemical and functional features including cleavage from a larger precursor, dimeric composition, heterotetrameric receptor complexes, and a canonical second messenger pathway (see [6,7] for review). All members act by binding a complex of cell surface receptors comprised of two Type I and two Type II receptors, with the Type I receptor transducing this signal to the intracellular environment after being phosphorylated and activated by the Type II receptor (Figure 1). Signal transduction is relayed through the serine-threonine kinase actions of the activated Type I receptor via a family of Smad second messenger proteins. Activated receptor Smads (Smads 1, 2, 3, 5, and 8) then complex with a common Smad (Smad 4) and translocate to the nucleus to alter gene transcription, often in association with other transcriptional modifiers. Smads 6 and 7, known as inhibitory Smads, suppress phosphorylation of the signaling Smads, thereby blocking signal transduction through the Smad 2/3 or Smad 1/5/8 pathways, respectively.
Figure 1.
Schematic of TGFβ family signaling. (a) TGFβ and Activin form one subfamily that have separate receptors and antagonists, but common 2nd messenger Smads. (b) BMPs form a second subfamily with their own receptors and antagonists, as well as a different set of 2nd messengers. All receptor Smads dimerize with the common Smad (Smad4) and the complex translocates to the nucleus to alter gene transcription. MSTN, myostatin; LAP, latency protein; FST, follistatin; FSTL3, follistatin like-3; ALK, activin like kinase.
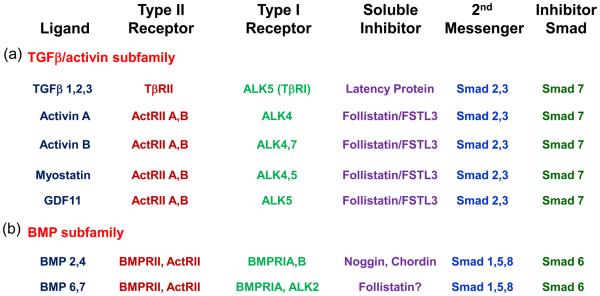
The TGFβ family can be broadly divided into two subfamilies, the TGFβ-activin subfamily and the BMP subfamily, each of which shares a subset of receptors and Smad second messengers (Figure 2). However, given that there are more than 40 ligands in this family and only 5 Type II and 7 Type I receptors [7], there is substantial promiscuity in receptor usage among the subfamilies [8], raising as yet unanswered questions of how specificity in biological response is maintained among the different ligands.
Figure 2.
TGFβ family ligands and signaling pathways reported in islets. TGFβ family ligands representing members of both TGFβ/activin and BMP subfamilies that have been identified in islets are shown with their receptors, soluble inhibitors, second messengers, and inhibitory Smads. Whereas this family is known for promiscuity in receptor usage owing to the more than 40 members sharing 5 Type II and 7 Type I receptors, there are also differences within the subfamilies that allow some degree of specificity when analyzing genetic alterations in mouse models. For example, Smad7 overexpression inhibits all members of the TGFβ/activin subfamily but usually not members of the BMP subfamily. Follistatin and FSTL3 inhibit members of the activin branch of the TGFβ/activin subfamily but not TGFβ itself. Noggin inhibits BMP ligands but not activin or TGFβ. Despite these mouse models, the actions of individual ligands in regulating β-cell function and glucose homeostasis remain to be elucidated. Adapted from [4] with permission.
Within the TGFβ-activin subfamily, TGFβ signals through a complex of TβRII and ALK5 (TBR1) receptors, whereas activin A signals through ActRIIB and ALK4, and activin B uses ActRIIB and either ALK4 or 7 (Figure 2). Myostatin and GDF11 use ActRIIA or B combined with either ALK4 or 5 [5,9]. These receptors use primarily Smads 2 and 3 for signaling. In contrast, signaling within the BMP subfamily is typically achieved through a complex of BMPRII or ActRIIA and ALK3 (BMPR1A) or ALK6 (BMPR1B). These receptors primarily utilize Smads 1, 5, and 8 as second messengers [7].
Another common feature of these ligands is that they are regulated extracellularly by binding proteins that can neutralize their activities including follistatin and follistatin like-3 (FSTL3) for activin, myostatin, and GDF11 [10] and noggin and chordin for several members of the BMP subfamily [7]. Thus, ligand activity depends on the relative concentrations of the ligands themselves, their receptors, and the binding proteins, all of which exist in a dynamic equilibrium.
Although this review is focused on TGFβ family member actions in the adult pancreas, it is important to note that both activins and BMPs have important roles during specification of the pancreatic buds that give rise to the pancreas and might play additional roles during cellular specification and morphogenesis of the developing ducts and vascular tree [4,11,12]. In addition, a substantial effort has been focused on defining the molecular program regulating differentiation of pancreatic cell types including islet constituents using genetically modified mice as a prominent tool. This research has revealed that pancreatic and duodenal homeobox factor-1 (Pdx1) is expressed at the earliest stage of pancreatic development and then later restricted to differentiating endocrine cells and finally to β-cells [13]. Neurogenein 3 (Ngn3) has been identified as an essential gene for specifying the endocrine fate and has been identified in presumptive progenitors of islet cells along with SRY (sex determining region Y)-box 9 (Sox9) [13]. These Ngn3+ precursor cells then either express predominantly aristaless-related homeobox (Arx) to specify α-cell fate or paired box 4 (Pax4), which specifies β-cell fate [13]. Additional genes characteristic of differentiated β-cells include v-maf musculoaponeurotic fibrosarcoma oncogene homolog A (MafA), NK2 homeobox 2 (Nkx2.2) and NK6 homeobox 1 (Nkx6.1) [4,13,14]. Because members of the TGFβ family influence expression of some of these genes, these ligands might play a role in specification of progenitor cell fate or in proliferation of differentiated islet cells. Moreover, changes in β-cell gene expression while manipulating TGFβ ligand expression or action might provide important clues as to the roles of these ligands in adult islet physiology.
Activin/TGFβ subfamily signaling in regulation of β-cell and islet function
Activin
Activin has been identified in adult rat and human islets, along with its receptors, and the regulatory protein follistatin [4,15-17], suggesting that in addition to its role in embryonic development of pancreatic tissue [18], activin might have important roles in adult islet function. This concept is supported by direct in vitro analysis of activin's actions on human and rat islets in culture, where it enhances glucose stimulated insulin secretion (GSIS) [19-21], an effect due at least in part to enhanced flux of calcium necessary for insulin secretion [20,22]. Activin has also been reported to enhance expression of genes characteristic of mature β-cells, such as insulin, solute carrier family 2 (Slc2a2 or glut2) and proprotein convertase subtilisin/kexin type 2 (Pcsk2 or PC2), that were induced in AR42J cells by ectopic expression of β-cell transcription factors MafA, neurogenic differentiation 1 (NeuroD1 or BETA2) and Pdx1 [23]. This suggests that activin might have a role in promoting differentiation of a β-cell phenotype. In addition, activin treatment induces expression of Pax4, a transcription factor required for β-cell differentiation [24], in cultured rat and human islets [25,26] which then leads to increased β-cell proliferation [25]. This action is mediated through the phosphatidylinositol kinase-3 pathway which also leads to enhanced β-cell survival via increased expression of the anti-apoptotic gene Bcl-xl [25].
In vivo studies also support a role for activin in regulating β-cell mass. Mice with reduced expression of ActRIIA or IIB have hypoplastic islets and glucose intolerance [18]. This effect appears to be mediated through reduced Smad2 signaling because double ActRIIB/Smad2 heterozygotes have an exacerbated phenotype of reduced islet area and insulin content along with impaired glucose tolerance in both embryonic and adult mice [27]. Similarly, blocking activin signaling through transgenic expression of dominant negative activin Type II receptors led to β-cell hypoplasia, loss of glucose control and eventual diabetes in adult mice [17,28]. However, because overexpression of dominant negative receptors can inhibit signaling in multiple ligand pathways, the observed hypoplasia might not be due to inhibition of activin signaling alone. Adult rats that had been administered activin and betacellulin (an EGF like molecule found in the pancreas) as neonates had significantly increased β-cell mass and insulin secretion through enhanced β-cell proliferation, compared to treatment with activin or betacellulin alone [29]. Taken together, these studies support the concept that activin plays a role in regulating β-cell mass and function both in vitro and in vivo, although because the transgenic studies involve genetic modifications active during embryonic development, it is not possible to differentiate activin's effects on β-cell development that is manifested as adults versus a role for activin regulating β-cell mass and/or function in adults.
Most of the in vitro studies exploring activin's actions have been performed with activin A, which usually utilizes the ALK4 Type 1 receptor. Activin B was recently shown to utilize another Type 1 receptor, ALK7, in addition to ALK4 [30]. ALK7 is expressed in both α- and β-cells [22], and its activation induces Smad2/3 phosphorylation and increases transcription of a reporter controlled by the human insulin promoter. This activity involved direct interaction of Smads with Pdx1 [31], a transcription factor for insulin gene transcription [4]. On the other hand, ALK7 KO mice (with presumed loss of activin B signaling) are hyperinsulinemic from an early age, subsequently developing reduced insulin sensitivity, impaired glucose tolerance, and enlarged islets [22]. Moreover, basal and GSIS was enhanced in ALK7 KO islets when tested in vitro, suggesting that altered regulation of insulin release is a primary defect in these mice [22]. Interestingly, in contrast to stimulated calcium influx seen with activin A treatment, activin B treatment inhibited calcium influx in WT islets, suggesting that activins A and B act in opposition to modulate β-cell response to glucose through their opposing actions on calcium flux [22]. As one might predict from these observations, activin B KO mice, which survive to adulthood, are also hyperinsulinemic [22], although the effect of this alteration on glucose homeostasis remains to be established. In addition, it is not yet clear what mechanisms, besides regulation of calcium flux, are involved in activin's modulation of insulin release or whether this is true for all species because it has yet to be demonstrated that GSIS is enhanced in cultured mouse islets in response to activin. Nevertheless, taken together, these in vitro and in vivo studies indicate that activins A and/or B signaling can modulate glucose homeostasis in adults, although the molecular mechanisms involved, and whether the effects are direct or indirect, remain to be elucidated.
Activin A is also expressed in α-cells where it suppresses glucagon gene expression, an effect confirmed in human islets and mediated via the Smad signaling pathway [32,33]. Activin treatment also suppresses proliferation in α-cell lines by reducing cyclin D2 and Arx gene expression, the latter gene being both necessary and sufficient in mice for determining α-cell fate [34]. This suggests that activin influences the relative proportion of α- and β-cells/islet through opposing actions on proliferation and cell fate gene expression (including Pdx1 and Pax4 in β-cells and Arx in α-cells) [11,26,33].
Activin and its receptors are also expressed in epithelial cells lining pancreatic ducts during islet differentiation as well as after injury [35]. Interestingly, this expression is upregulated in models of pancreatic regeneration after injury although the proliferative index of activin or activin receptor-positive cells was not elevated. Moreover, follistatin is expressed in expanding ductal epithelium and localized to proliferating cells, suggesting that inhibition of activin action by local follistatin might lead to increased proliferation during this particular stage [35,36]. Perhaps the increased activin expression and presumably action contributes to differentiation of ductal epithelium into endocrine cells that has been observed after injury [37]. This would be opposite to activin's proposed role in enhancing β-cell proliferation but could still account for an increase in β-cell mass as these new islet precursors from ductal epithelium are incorporated into existing or even new islets. The contribution of ductal cells to islet neogenesis and β-cell mass after injury has been proposed based on carbonic anhydrase II-Cre-mediated lineage tracing in which 42% of islets and 23% of β-cells were derived from ductal lineages after pancreatic duct ligation [37]. However, based on Hnf1β-Cre-mediated lineage tracing, the contribution of ductal cells to acinar or islet lineages was not observed postnatally, even in injury and β-cell ablation models [38]. Moreover, the transcription factor neurogenin-3, a marker of endocrine progenitors that is normally turned off after pancreatic development, is re-expressed in pancreatic cells including ductal cells that subsequently differentiate into β-cells after injury [39]. It is therefore not clear at present whether these seemingly different observations represent unappreciated challenges in the lineage tracing technology or whether different subpopulations of ductal cells co-exist that are differentially labeled by various promoter-driven Cre transgenic mice in these studies. Because activin and follistatin are produced in ductal cells and associated with proliferation, it is possible that activin signaling leads to increased β-cell mass through increased progenitor cell proliferation and differentiation into β-cells. This might be addressed through specifically inhibiting activin signaling in ductal epithelium via transgenic technology. In addition, mouse models combining lineage tracing for β- or ductal cells with activin overexpression might reveal activin's role in regulating adult β-cell mass.
TGFβ
TGFβ 1, 2, and 3 have been localized to both the endocrine and exocrine pancreas [40] along with their receptors and Smad signaling partners [40,41]. Treatment of adult rat islets in vitro with TGFβ1 and 2 (1 pM – 1 nM) in low (2.8 mM) glucose resulted in a dose-dependent increase in insulin secretion whereas insulin release caused by increasing glucose concentrations was enhanced in the presence of 10 pM TGFβ1 [42]. Insulin secretion from fetal rat islets was enhanced by 500 pM TGFβ1 treatment in 11.1 mM glucose, but not at higher or lower glucose concentrations [43]. This enhancement of GSIS might be mediated, at least in part, through increased insulin gene expression that was induced in INS1 cells by TGFβ treatment and involved induction of the PDX-1 transcription factor and its binding to the insulin promoter [44]. On the other hand, TGFβ, acting through Smad3 (but not Smad 2), inhibited insulin mRNA expression and reporter activity in rat islets and in various cell lines after TBRII co-transfection, suggesting that Smad3 signaling inhibits insulin production [45]. It is thus possible that increased insulin secretion in response to TGFβ treatment is not dependent on insulin gene transcription which can be simultaneously suppressed by the same treatment. This raises the question of how insulin stores are replenished in the long term.
Alternatively, whereas Smad3 suppresses insulin gene transcription, Smad2 signaling might enhance it or enhance insulin release, allowing TGFβ signaling to have both actions. Thus, the precise biological actions of TGFβ on islet function and its overall effect on insulin production in adults remain to be defined.
The contribution of TGFβ to islet function and insulin production has also been examined in vivo where both overexpression and signal inhibition appear to produce islet hypoplasia and loss of glucose control. TGFβ1 overexpression under control of the human or rat insulin promoter resulted in vastly reduced exocrine pancreas and islets, although glucose control was maintained [46,47]. Similarly, TGFβ1 overexpression under control of the glucagon promoter resulted in β-cell hypoplasia, reduced insulin secretion, and impaired glucose tolerance [48], suggesting that the developmental stage and location of TGFβ production, as well as the concentration of the TGFβ signal is critical for normal pancreas development with excess TGFβ leading to reduced β-cell number and insulin secretion. However, these studies used transgenic modifications that are typically activated during development so that it is impossible to differentiate defects caused by altered development from those caused by altered TGFβ expression or action in adult β-cells.
One way to address this issue is to use regulatable transgenic expression constructs so that the altered expression or action can be initiated after critical developmental periods have occurred. Conditional transgenic overexpression of Smad 7 inhibits phosphorylation of Smad 2 and 3 (and sometimes the BMP pathway Smads if sufficiently overexpressed), thereby blocking signaling in the TGFβ, activin, GDF11, and/or myostatin pathways. When Smad7 was overexpressed in adult β-cells, islets became hypoplastic, insulin content was significantly reduced, glucose tolerance was impaired, and serum glucose levels increased, indicating that TGFβ-activin subfamily signaling is required for normal islet size and function in adults [49]. Moreover, this condition was completely reversible so that removal of Smad7 overexpression resulted in a return to normal islet size and glucose control. Thus, whereas not specific to the TGFβ pathway, this study demonstrated that blocking the Smad2 and/or 3 signaling pathway in adults resulted in β-cell hypoplasia and elevated glucose eventually producing overt diabetes, consistent with an important role for this subfamily in maintaining adult β-cell function and glucose control.
In contrast to reduced insulin secretion through inhibiting both Smad2 and 3 signaling with Smad7 overexpression, inhibition of Smad3 alone enhances insulin production and release [45]. Smad3 deficient mice exhibit enhanced glucose tolerance and insulin secretion although β-cell mass is not altered, and isolated Smad3 deficient islets secreted more insulin in response to glucose compared to WT islets. Moreover, adenoviral transduction of Smad3 into non-human primate and human islets reduces intracellular and secreted C-peptide, a measure of bioactive insulin production [45]. These results suggest that TGFβ mediated activation of Smad3 inhibits insulin production and secretion, whereas inactivation of Smad3 increases insulin secretion in response to glucose [45]. How do we reconcile the finding that Smad3 signaling inhibits β-cell function with the observation that suppression of both Smad2 and 3 inhibits β-cell function? Perhaps Smad2 signaling mediates the positive effects of activin and TGFβ signaling on β-cell function that were observed in isolated islets [19,21,42] whereas Smad3 transduces the inhibitory actions of TGFβ on islet function. Nevertheless, the net effect of inhibiting both pathways, such as through Smad7 overexpression, is inhibitory to β-cell function, suggesting that the balance between TGFβ ligand, receptor, and Smad concentrations dictates the overall effect of TGFβ on islets. .
GDF11 and Myostatin
Another ligand in the TGFβ-activin subfamily, growth and differentiation factor 11 (GDF11), regulates the number and differentiation of islet progenitor cells during embryonic development [50]. In GDF11 KO mice, the number of Ngn3+ islet precursor cells is substantially increased, but these precursors fail to develop to mature β-cells in large numbers, indicating that GDF11 is somehow critical for final β-cell maturation. Interestingly, it was proposed that GDF11 signals through Smad2; indeed, Smad2 +/− mice (complete Smad2 KO mice fail to form endoderm) have increased numbers of NGN3+ cells and reduced β-cell mass, much like GDF11 KO mice, suggesting that GDF11 acts via Smad2 to regulate β-cell differentiation [50]. It is currently unknown if GDF11 is produced or has important functions in adult islets but this would not be surprising given what is known about activin and TGFβ. Moreover, its signaling through Smad2 would fit with the hypothesis that the Smad2 signaling pathway enhances β-cell function.
One final ligand in this subfamily with important actions in regulating glucose concentrations is myostatin (GDF8), a close structural relative of GDF11. Myostatin is best known for regulating muscle mass, and myostatin KO mice have an overall doubling of skeletal muscle with a decrease in fat mass [51,52]. More recently, myostatin KO mice were analyzed for altered energy metabolism which might account for this loss of fat. These mice show increased glucose utilization as well as insulin sensitivity, due at least in part to increased glucose uptake by muscle [53], suggesting that myostatin itself generally inhibits these processes. It is currently unknown whether myostatin is expressed in adult islets or if it has any direct actions regulating β-cell mass or function in adults. However, because both GDF11 and myostatin signal through activin receptors and Smad second messengers, any effects of these ligands are likely to have similar effects as those of activin and TGFβ. Moreover, because overall inhibition of this pathway via Smad7 overexpression reduces β-cell number and function, it seems that the activity of all these ligands should lead to enhanced β-cell mass and/or function.
Bone Morphogenetic Protein (BMP) subfamily regulation of β-cell function
Both BMP4 and its main receptor ALK3 are expressed in adult mouse and human islets [49]. Reduced BMP signaling, achieved using a mouse model with severely reduced ALK3 expression in β-cells, resulted in glucose intolerance at 2-3 months of age and eventually, overt diabetes [54]. In addition, a blunted first and second phase insulin secretion was observed in response to a glucose challenge. This BMP response was mediated by Smads 1, 5, and 8 because their phosphorylation was reduced in ALK3 KO mice. No change in α- or β-cell number or islet size was observed, suggesting that BMP signaling does not increase β-cell proliferation or precursor incorporation, but rather enhances β-cell function. Indeed, transgenic overexpression of BMP4 in β-cells significantly enhanced glucose tolerance and insulin secretion while overexpression of the BMP antagonists noggin and Smad6 in β-cells resulted in glucose intolerance and diabetes, consistent with BMP's potential role regulating β-cell function [54]. Interestingly, systemic administration of BMP4 also enhanced glucose tolerance and insulin secretion, suggesting that TGFβ family ligands such as BMP4, or synthetic compounds with similar activity, might be useful therapeutic agents for enhancing β-cell function in diabetic patients [54].
Soluble antagonists of TGFβ family ligands
As mentioned, follistatin is a high affinity activin binding and neutralization protein that is expressed in most tissues where activin is found [10]. Follistatin also binds and neutralizes myostatin and GDF11, as well as BMP6 and BMP7, in descending order of affinity [50]. Similar kinetics were observed for the closely related follistatin like-3 (FSTL3) that is expressed in a partially overlapping tissue distribution as follistatin [55-57]. To investigate the potential roles of activin and related TGFβ family ligands in adults, we created an FSTL3 KO mouse that survived to adulthood [58], in contrast to the follistatin KO mouse that was neonatally lethal [59]. FSTL3 KO mice have enlarged islets resulting from β-cell hyperplasia and are more glucose tolerant and slightly hyperinsulinemic compared to wild type littermates. Because antagonism of activin, myostatin, and GDF11 are the only known functions for FSTL3, the FSTL3 KO phenotype suggests an underlying physiological regulatory system important for glucose homeostasis that potentially involves multiple TGFβ family members.
Summary
Both in vitro and in vivo studies support a role for several members of the TGFβ family in regulating β-cell number and/or function. Although the earlier studies were hampered by having the genetic alterations active during embryogenesis and therefore unable to differentiate between developmental alterations and effects on adult β-cell homeostasis, more recent studies in which signaling is blocked after neonatal development suggest that such signaling is important in adults for regulating β-cell mass and/or proper insulin release in response to glucose. Taken together, they suggest that members of the TGFβ family play important roles in maintaining β-cell homeostasis but that their role is not visible until perturbed by increased or decreased activity.
A number of important questions remain. First, it is unknown exactly which TGFβ family members and natural antagonists are produced in islets, in which specific cell types, which ligands have actions on islets and/or β-cells, and whether their biosynthesis is modulated by changing physiological conditions. Another important question is whether these observed actions on β-cells in vivo are direct or dependent on signals from other pancreatic cell types or even non-pancreatic tissues. In other words, are these actions produced by autocrine, paracrine, or endocrine mechanisms of TGFβ family signaling? It is also not clear whether these effects of TGFβ ligands are functional in all mammalian species, or whether there are differences between mice, where most of the in vivo genetic studies have been performed, and rats, which are typically used for in vitro studies. Of clinical importance is the question of whether these ligands are produced in human islets and have roles in regulating β-cell number or function. Finally, it will be important to determine whether alteration in TGFβ family signaling produces a lasting change in β-cell proliferation or survival, or can increase progenitor cell formation and development to mature β-cells. These actions would indicate that pharmacological manipulation of this system might be a useful approach to treat diabetes. The availability of targeted and regulatable expression constructs along with lineage tracing technologies should go a long way toward providing answers to these questions in the near future.
Acknowledgements
The authors are grateful to Dr. Yin Xia for helpful comments on the manuscript. This work was supported by NIH grant R01DK075058 and a CEAR grant award.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Diabetes Genetics Initiative of Broad Institute of Harvard. MIT,L.U.a.N.I.o.B.R. et al. Genome-Wide Association Analysis Identifies Loci for Type 2 Diabetes and Triglyceride Levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 2.Simonis-Bik AM, et al. Gene variants in the novel type 2 diabetes loci CDC123/CAMK1D, THADA, ADAMTS9, BCL11A and MTNR1B affect different aspects of pancreatic beta cell function. Diabetes. 2010;59:293–301. doi: 10.2337/db09-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staiger H, et al. Pathomechanisms of Type 2 Diabetes Genes. Endocr Rev. 2009;30:557–585. doi: 10.1210/er.2009-0017. [DOI] [PubMed] [Google Scholar]
- 4.Oliver-Krasinski JM, Stoffers DA. On the origin of the {beta} cell. Genes and Development. 2008;22:1998–2021. doi: 10.1101/gad.1670808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massague J. TGF[beta] in Cancer. Cell. 2008;134:215–230. [Google Scholar]
- 6.Pangas SA, Woodruff TK. Activin signal transduction pathways. Trends Endocrinol. Metab. 2000;11:309–314. doi: 10.1016/s1043-2760(00)00294-0. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 8.Nickel J, et al. Intricacies of BMP receptor assembly. Cytokine Growth Factor Rev. 2009;20:367–377. doi: 10.1016/j.cytogfr.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Andersson O, et al. Growth differentiation factor 11 signals through the transforming growth factor-beta receptor ALK5 to regionalize the anterior-posterior axis. EMBO Rep. 2006;7:831–837. doi: 10.1038/sj.embor.7400752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welt C, et al. Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium. Exp. Biol. Med. (Mightwood.) 2002;227:724–752. doi: 10.1177/153537020222700905. [DOI] [PubMed] [Google Scholar]
- 11.Kumar M, et al. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev. Biol. 2003;259:109–122. doi: 10.1016/s0012-1606(03)00183-0. [DOI] [PubMed] [Google Scholar]
- 12.Kim SK, MacDonald RJ. Signaling and transcriptional control of pancreatic organogenesis. Curr. Opin. Genet. Dev. 2002;12:540–547. doi: 10.1016/s0959-437x(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 13.Kordowich S, et al. Reprogramming into pancreatic endocrine cells based on developmental cues. Mol Cell Endocrinol. 2010;315:11–8. doi: 10.1016/j.mce.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collombat P, et al. Specifying pancreatic endocrine cell fates. Mech. Dev. 2006;123:501–512. doi: 10.1016/j.mod.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Wada M, et al. Immunohistochemical localization of activin A and follistatin in human tissues. Endocr. J. 1996;43:375–385. doi: 10.1507/endocrj.43.375. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa K, et al. Expression of alpha, beta A and beta B subunits of inhibin or activin and follistatin in rat pancreatic islets. FEBS. Lett. 1993;319:217–220. doi: 10.1016/0014-5793(93)80549-a. [DOI] [PubMed] [Google Scholar]
- 17.Yamaoka T, et al. Hypoplasia of pancreatic islets in transgenic mice expressing activin receptor mutants. J. Clin. Invest. 1998;102:294–301. doi: 10.1172/JCI2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SK, et al. Activin receptor patterning of foregut organogenesis. Genes Dev. 2000;14:1866–1871. [PMC free article] [PubMed] [Google Scholar]
- 19.Florio P, et al. Activin A stimulates insulin secretion in cultured human pancreatic islets. J. Endocrinol. Invest. 2000;23:231–234. doi: 10.1007/BF03343713. [DOI] [PubMed] [Google Scholar]
- 20.Verspohl EJ, et al. Activin A: its effects on rat pancreatic islets and the mechanism of action involved. Life Sci. 1993;53:1069–1078. doi: 10.1016/0024-3205(93)90260-a. [DOI] [PubMed] [Google Scholar]
- 21.Totsuka Y, et al. A novel action of activin A: stimulation of insulin secretion in rat pancreatic islets. Biochem. Biophys. Res. Commun. 1988;156:335–339. doi: 10.1016/s0006-291x(88)80845-3. [DOI] [PubMed] [Google Scholar]
- 22.Bertolino P, et al. Activin B receptor ALK7 is a negative regulator of pancreatic +¦-cell function. Proceedings of the National Academy of Sciences. 2008;105:7246–7251. doi: 10.1073/pnas.0801285105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuoka T.a., et al. MafA Regulates Expression of Genes Important to Islet {beta}-Cell Function. Mol Endo. 2007;21:2764–2774. doi: 10.1210/me.2007-0028. [DOI] [PubMed] [Google Scholar]
- 24.Sosa-Pineda B. The gene Pax4 is an essential regulator of pancreatic beta-cell development. Mol Cells. 2004;18:289–294. [PubMed] [Google Scholar]
- 25.Brun T, et al. The diabetes-linked transcription factor PAX4 promotes {beta}-cell proliferation and survival in rat and human islets. J. Cell Biol. 2004;167:1123–1135. doi: 10.1083/jcb.200405148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brun T, Gauthier BR. A focus on the role of Pax4 in mature pancreatic islet {beta}-cell expansion and survival in health and disease. J Molec Endo. 2008;40:37–45. doi: 10.1677/JME-07-0134. [DOI] [PubMed] [Google Scholar]
- 27.Goto Y, et al. Genetic interactions between activin type IIB receptor and Smad2 genes in asymmetrical patterning of the thoracic organs and the development of pancreas islets. Dev. Dyn. 2007;236:2865–2874. doi: 10.1002/dvdy.21303. [DOI] [PubMed] [Google Scholar]
- 28.Shiozaki S, et al. Impaired differentiation of endocrine and exocrine cells of the pancreas in transgenic mouse expressing the truncated type II activin receptor. Biochim. Biophys. Acta. 1999;1450:1–11. doi: 10.1016/s0167-4889(99)00022-1. [DOI] [PubMed] [Google Scholar]
- 29.Li L, et al. Activin A and betacellulin: effect on regeneration of pancreatic beta-cells in neonatal streptozotocin-treated rats. Diabetes. 2004;53:608–615. doi: 10.2337/diabetes.53.3.608. [DOI] [PubMed] [Google Scholar]
- 30.Bernard DJ, et al. Activin B can signal through both ALK4 and ALK7 in gonadotrope cells. Reprod Biol Endocrinol. 2006;4:52. doi: 10.1186/1477-7827-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe R, et al. Insulin gene is a target in activin receptor-like kinase 7 signaling pathway in pancreatic [beta]-cells. Biochem Biophys Res Comm. 2008;377:867–872. doi: 10.1016/j.bbrc.2008.10.074. [DOI] [PubMed] [Google Scholar]
- 32.Yasuda H, et al. Existence of activin-A in A- and D-cells of rat pancreatic islet. Endocrinology. 1993;133:624–630. doi: 10.1210/endo.133.2.8344202. [DOI] [PubMed] [Google Scholar]
- 33.Mamin A, Philippe J. Activin A decreases glucagon and arx gene expression in {alpha} cell lines. Mol Endo. 2006;21:259–273. doi: 10.1210/me.2005-0530. [DOI] [PubMed] [Google Scholar]
- 34.Collombat P, et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang YQ, et al. Inhibition of activin signaling induces pancreatic epithelial cell expansion and diminishes terminal differentiation of pancreatic beta-cells. Diabetes. 2004;53:2024–2033. doi: 10.2337/diabetes.53.8.2024. [DOI] [PubMed] [Google Scholar]
- 36.Park MK, et al. Effects of activin A on pancreatic ductal cells in streptozotocin-induced diabetic rats. Transplantation. 2007;83:925–930. doi: 10.1097/01.tp.0000259978.62139.9d. [DOI] [PubMed] [Google Scholar]
- 37.Inada A, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc. Natl. Acad. Sci U. S. A. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solar M, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev. Cell. 2009;17:849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Xu X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 40.Yamanaka Y, et al. Synthesis and expression of transforming growth factor beta-1, beta-2, and beta-3 in the endocrine and exocrine pancreas. Diabetes. 1993;42:746–756. doi: 10.2337/diab.42.5.746. [DOI] [PubMed] [Google Scholar]
- 41.Brorson M, et al. Expression of SMAD signal transduction molecules in the pancreas. Histochem. Cell Biol. 2001;116:263–267. doi: 10.1007/s004180100316. [DOI] [PubMed] [Google Scholar]
- 42.Totsuka Y, et al. Stimulation of insulin secretion by transforming growth factor-beta. Biochem. Biophys. Res. Commun. 1989;158:1060–1065. doi: 10.1016/0006-291x(89)92829-5. [DOI] [PubMed] [Google Scholar]
- 43.Sjoholm A, Hellerstrom C. TGF-beta stimulates insulin secretion and blocks mitogenic response of pancreatic beta-cells to glucose. Am J Physiol Cell Physiol. 1991;260:C1046–C1051. doi: 10.1152/ajpcell.1991.260.5.C1046. [DOI] [PubMed] [Google Scholar]
- 44.Sayo Y, et al. Transforming growth factor beta induction of insulin gene expression is mediated by pancreatic and duodenal homeobox gene-1 in rat insulinoma cells. Eur. J Biochem. 2000;267:971–978. doi: 10.1046/j.1432-1327.2000.01080.x. [DOI] [PubMed] [Google Scholar]
- 45.Lin HM, et al. Transforming Growth Factor-+¦/Smad3 Signaling Regulates Insulin Gene Transcription and Pancreatic Islet +¦-Cell Function. J. Biol. Chem. 2009;284:12246–12257. doi: 10.1074/jbc.M805379200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grewal IS, et al. Expression of transgene encoded TGF-beta in islets prevents autoimmune diabetes in NOD mice by a local mechanism. J Autoimmun. 2002;19:9–22. doi: 10.1006/jaut.2002.0599. [DOI] [PubMed] [Google Scholar]
- 47.Lee MS, et al. Accumulation of extracellular matrix and developmental dysregulation in the pancreas by transgenic production of transforming growth factor-beta 1. Am J Pathol. 1995;147:42–52. [PMC free article] [PubMed] [Google Scholar]
- 48.Moritani M, et al. Hypoplasia of endocrine and exocrine pancreas in homozygous transgenic TGF-[beta]1. Mol. Cell. Endocrinol. 2005;229:175–184. doi: 10.1016/j.mce.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 49.Smart NG, et al. Conditional expression of Smad7 in pancreatic beta cells disrupts TGF-beta signaling and induces reversible diabetes mellitus. PLoS. Biol. 2006;4:e39. doi: 10.1371/journal.pbio.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harmon EB, et al. GDF11 modulates NGN3+ islet progenitor cell number and promotes beta-cell differentiation in pancreas development. Development. 2004;131:6163–6174. doi: 10.1242/dev.01535. [DOI] [PubMed] [Google Scholar]
- 51.McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest. 2002;109:595–601. doi: 10.1172/JCI13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McPherron AC, et al. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 53.Guo T, et al. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One. 2009;4:e4937. doi: 10.1371/journal.pone.0004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goulley J, et al. BMP4-BMPR1A signaling in beta cells is required for and augments glucose-stimulated insulin secretion. Cell Metab. 2007;5:207–219. doi: 10.1016/j.cmet.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Tortoriello DV, et al. Human follistatin-related protein: a structural homologue of follistatin with nuclear localization. Endocrinology. 2001;142:3426–3434. doi: 10.1210/endo.142.8.8319. [DOI] [PubMed] [Google Scholar]
- 56.Sidis Y, et al. Biological Activity of Follistatin Isoforms and Follistatin like-3 are Dependent on Differential Cell Surface Binding and Specificity for Activin, Myostatin and BMP's. Endocrinology. 2006;147:3586–3597. doi: 10.1210/en.2006-0089. [DOI] [PubMed] [Google Scholar]
- 57.Schneyer AL, et al. Differential Antagonism of Activin, Myostatin and Growth and Differentiation Factor 11 by Wild-Type and Mutant Follistatin. Endocrinology. 2008;149:4589–4595. doi: 10.1210/en.2008-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mukherjee A, et al. FSTL3 deletion reveals roles for TGF-beta family ligands in glucose and fat homeostasis in adults. Proc. Natl. Acad. Sci. U. S. A. 2007;104:1348–1353. doi: 10.1073/pnas.0607966104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matzuk MM, et al. Multiple defects and perinatal death in mice deficient in follistatin. Nature. 1995;374:360–363. doi: 10.1038/374360a0. [DOI] [PubMed] [Google Scholar]