Abstract
Mononuclear phagocytes (MP) are cells of nonspecific immunity, playing an essential role in defense against bacterial pathogens. Although various MP subpopulations have been described in the pig, relations among these populations in vivo are unknown to date. The present study was aimed at describing porcine MP subpopulations infiltrating inflamed tissue of pigs under in vivo conditions. Actinobacillus pleuropneumoniae (APP) infection was used to induce an inflammatory response. CD172α, CD14, CD163, MHCII and CD203α cell surface molecules were used to identify MP by flow cytometry. Changes in MP subpopulations in the peripheral blood (PB) and bone marrow (BM) compartments along with the analysis of MP appearing in the inflamed lungs were assessed to elucidate the possible origin and maturation stages of the infiltrating MP. The MP population migrating to the inflamed lungs was phenotype CD14+ CD163+ CD203α+/− MHCII+/−. Concomitantly, after APP infection there was an increase in the PB MP CD14+ CD163+ CD203α− MHC II− population, suggesting that these cells give rise to inflammatory monocytes/macrophages. The CD203α and MHCII molecules appear on these cells after leaving the PB. In healthy animals, the BM MP precursors were represented by CD14− CD163− cells maturing directly into CD14+ CD163− that were then released into the PB. After infection, an altered maturation pathway of MP precursors appeared, represented by CD14− CD163− CD203α− MHCII− MP directly switching into CD14+ CD163+ CD203α− MHCII− MP. In conclusion, two different MP maturation pathways were suggested in pigs. The use of these pathways differs under inflammatory and noninflammatory conditions.
Keywords: mononuclear phagocyte, pig, Actinobacillus pleuropneumoniae
1. INTRODUCTION
Mononuclear phagocytes (MP) are cells of nonspecific immunity that play an essential role in the defense against bacterial pathogens.
The MP system is a highly heterogeneous cell population dispersed throughout the organism. Macrophages, the final developmental stage of the MP lineage, originate from a specific progenitor in the bone marrow (BM) that sequentially develops into monoblasts, promonocytes and monocytes [9]. Monocytes enter the blood circulation and from there they migrate to various tissues, thereafter undergoing further differentiation into macrophages.
It was long believed that monocytes comprise a homogeneous population of blood cells. Recent evidence, however, indicates that blood monocytes may consist of several subpopulations of cells differing by size, nuclear morphology, granularity, and functionality (reviewed by [11, 17]). Based on the expression of cell surface molecules, two major subpopulations of blood monocytes have been described to date in humans, mice and rats.
The functional aspects of monocyte heterogeneity have been best described in mice. BM macrophage and dendritic cell progenitors give rise to Ly6Chi BM monocytes, which are sequentially released into the bloodstream [16]. After 3 days of circulation in the bloodstream, the Ly6Chi monocytes switch their phenotype and become Ly6Clow blood monocytes [22]. Under steady state conditions, more mature Ly6Clow blood monocytes migrate to the tissue and renew the population of tissue-specific resident macrophages [10, 13]. Under inflammatory conditions, the less mature Ly6Chi blood monocyte subset is the only one that is able to migrate to the inflamed tissue [10].
Two distinct subpopulations of blood monocytes have been described in pigs similarly to cases in humans, mice and rats. However, information about MP heterogeneity and their role in vivo remains scarce [3, 4]. Differentiation is first based on the expression of the CD163 cell surface molecule [15]. CD163− monocytes were found to be smaller and less granular than CD163+. Moreover, small differences in the expression intensity of many other cell surface markers have been observed [3, 5, 15]. The differing ability of porcine monocyte subsets to produce TNF-α, IL-1β and IL-10 suggest that functional differences between porcine monocyte subsets also exist [5, 15]. The expression of CD163 on monocytes probably identifies a cell population in a more advanced maturation stage than that of CD163− blood monocytes [15]. This is supported by the fact that CD163− monocytes could switch their phenotype to CD163+ after in vitro cultivation [5]. All the in vitro experiments suggest that CD163+ monocytes are a more mature form of blood monocytes and give rise to both tissue macrophages and dendritic cells. However, the in vivo function of less mature forms of porcine monocytes, which are believed to play an important role as precursors of inflammatory macrophages in mice and humans, is completely unknown in pigs at present. The aim of this work was to describe which developmental pathways used by porcine MP subpopulations are involved in infiltrating inflamed tissue under in vivo conditions. Various cell surface molecules that are known to be variably expressed by porcine MP subpopulations in vitro [5] were used for their identification. Moreover, the possible origin and maturation stages of these infiltrating MP were assessed in the PB and BM.
Actinobacillus pleuropneumoniae (APP) was chosen as the causative agent model for inducing an inflammatory response in lungs. APP is a Gram-negative, encapsulated bacterium that colonizes porcine lungs and causes pleuropneumonia. The bacteria bind to cells of the lower respiratory tract [2]. Clinical signs and pathological changes of the disease already appear within a few hours after experimental infection [1]. The infection of non-immunized pigs is followed by destruction of alveolar macrophages and rapid influx of professional phagocytes and lymphocytes to the tissue [1] and bronchoalveolar space [7, 8]. A rapid cellular influx of MP into infected lungs together with a specific localization of the pathogen in the lungs predetermine experimental APP infection to be an appropriate model for observing MP migration under inflammatory conditions in pigs.
2. MATERIALS AND METHODS
2.1. Animals and experimental infection
Ten 8-week-old healthy piglets with low levels of APP-specific antibodies were used in the experiment. The pigs were kept in the accredited barrier-type animal facilities of the Veterinary Research Institute. The animal care protocol for this experiment followed the Czech guidelines for animal experimentation and was approved by the Branch Commission for Animal Welfare of the Ministry of Agriculture of the Czech Republic. The piglets were allowed to acclimatize in the animal facilities for one week, and then the experimental infection was performed.
The infection with APP was performed intranasally during inhalation, and the infectious dose of 2 × 109 bacteria was administered to the second third of each nasal cavity. Health status was monitored during the entire experiment and clinical signs of respiratory disorders were recorded (increased dyspnoea, coughing, anorexia, depression and lethargy).
Seven piglets were infected with APP, while 3 piglets were left as noninfected controls. The three noninfected piglets were euthanized at infection time 0. Infected piglets were euthanized 24 h (3 piglets) and 72 h (4 piglets) after infection.
2.2. Blood and tissue sampling
Immediately after euthanasia, samples of sternum and lung tissue were acquired. Simultaneously, heparinized blood samples from all living piglets were taken at infection time 0 (10 piglets) and then 6 (7 piglets), 24 (7 piglets), 48 (4 piglets) and 72 (4 piglets) h after infection.
2.3. Processing of the samples
Total white blood cell count was ascertained using an auto hematology analyzer (BC-2800Vet, Shenzhen Mindray Bio-Medical Electronics, Shenzhen, People’s Republic of China). Red blood cells were lysed with ammonium chloride solution (154.4 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, all from Sigma-Aldrich, St. Louis, USA), leukocyte suspension was then washed with cell washing solution (CWS, phosphate buffered saline containing 1.84 g/L EDTA, 1 g/L sodium azide and 4 mL/L gelatin, all from Sigma-Aldrich) and the final peripheral blood leukocyte (PBL) count was ascertained.
The last sternebrum was disengaged from the connective tissue and periost and the BM cells were rinsed from the bone tissue with 100 mL of CWS using a 1.7 G needle. Red blood cells were lysed with ammonium chloride solution, leukocyte suspension was washed with CWS, and the final bone marrow leukocyte (BML) count was ascertained.
During the isolation of lung MP, the lung tissue was repeatedly weighed in order to allow calculation of final MP count per gram of tissue (see Sect. 2.6). The caudal part of the cranial lobe of the left lung was separated, weighed, and the vasculature and main bronchus were cannulated with a 1.7 G plastic intravenous catheter. The vasculature was washed with 80 mL of Dulbecco’s phosphate buffered saline (Sigma-Aldrich) containing 0.2% EDTA to remove blood cells from the vasculature. Then, the bronchoalveolar space was lavaged three times with 50 mL of CWS. The alveolar leukocytes (AL) thus obtained were washed two times with CWS and counted. The entire lavaged lobe was weighed. A piece of the lobe tissue was cut, weighed, disintegrated using a fine nylon mesh, and washed with CWS. The remaining red blood cells were then lysed with ammonium chloride solution. Finally, the interstitial leukocytes (ISL) obtained were washed again with CWS and counted.
Since typical APP lesions appeared in the cranial lobe of the right lung 72 h after infection, an analysis of ISL within the lesion (lesion leukocytes, LL) was also performed. A piece of the lesion was excised, disintegrated using a fine nylon mesh, washed with CWS, and LL were counted.
2.4. Detection of cell surface molecules by flow cytometry
Staining of cell surface molecules was performed as described previously [24], with small modifications. Various setups of cell surface molecule combinations, including a list of all primary and secondary antibodies used in the experiment are shown in Tables I and II.
Table I.
Primary mouse anti-porcine cell surface marker antibodies used in the experiment.
| Antibody | Clone | Subisotype | Producer | Catalogue | Expression profile |
|---|---|---|---|---|---|
| CD14 | MIL-2 | IgG2b | Serotec | MCA1218 | mo/mf, neu |
| CD163 | 2A10/11 | IgG1 | Serotec | MCA2311 | subset of mo/mf, DC |
| CD172α (SWC3) | DH59B | IgG1 | VMRD | DH59B | mo/mf, neu, eo, DC |
| CD172α (SWC3) | 74-22-15A | IgG2b | JL | – | mo/mf, neu, eo, DC |
| CD203α (SWC9) | PM19-7 | IgG1 | Serotec | MCA1973 | mf |
| SWC8 | MIL-3 | IgM | JL | – | neu, eo, subset of ly |
| MHCII (SLA-DQ) | K274.3G8 | IgG1 | JL | – | subset of mo, mf, ly |
JL: a generous gift from Dr J.K. Lunney (Animal Parasitology Institute, Beltsville, MO, USA), originally produced by the University of Bristol. DC: dendritic cells; eo: eosinophils; ly: lymphocytes; mo: monocytes; mf: macrophages; neu: neutrophils.
Table II.
Setup of four-color staining with appropriate secondary fluorochrome-conjugated antibodies.
| Setup No. | FL1 | FL2 | FL3 | FL4 |
|---|---|---|---|---|
| 1 | CD14 | SWC8 | Propidium iodide | CD172α (DH59B) |
| 2 | CD172α (74-22-15A) | SWC8 | Propidium iodide | CD163 |
| 3 | CD172α (74-22-15A) | SWC8 | Propidium iodide | MHCII |
| 4 | CD14 | SWC8 | Propidium iodide | CD163 |
| 5 | CD172α (74-22-15A) | SWC8 | Propidium iodide | CD203α |
FL1: goat anti-mouse IgG2b: FITC, SouthernBiotech. FL2: goat anti-mouse IgM: PE, SouthernBiotech. FL4: goat anti-mouse IgG1: AlexaFluor647, Invitrogen.
The 5 × 105 of BML, PBL, AL, ISL or LL were mixed with 20 μL of primary antibody cocktail (see Tab. II, setups nos. 1–5) and 20 μL of heat-inactivated goat serum, then incubated for 20 min at 4 °C. The cells were rinsed twice with CWS. Then, 50 μL of secondary antibody cocktail (anti-IgG2b: FITC, anti-IgM: PE and anti-IgG1: AlexaFluor647) was added and the cells were incubated for another 20 min at 4 °C. Finally, propidium iodide (Sigma-Aldrich) was added to each tube and the samples were measured as soon as possible using FACSCalibur flow cytometer (Becton-Dickinson, New Jersey, USA). At least 50 000 events were acquired.
Since two distinct clones of anti-CD172α antibody (74-22-15 and DH59B) were used for detecting this molecule, it was verified during preliminary experiments by two-color analysis that both clones have identical expression profile.
The post-acquisition analysis of data was performed using the Summit software (DAKO, Glostrup, Denmark).
2.5. Gating strategy
The staining of cell surface molecule combinations listed in Table II was performed in lung (setups nos. 1–3 and 5), PB and BM leukocytes (setups nos. 1–5).
The gating strategy for lung monocytes/macrophages is shown in Figure 1. MP were identified as viable CD172α+ SWC8− mononuclear leukocytes. Monocytes and macrophages were then differentiated according to their light scatter properties. The absolute counts of alveolar, interstitial and lesion CD14+/− CD163+/− CD203α+/− or MHCII+/− monocytes or macrophages before and after APP infection were then calculated per gram of lung tissue.
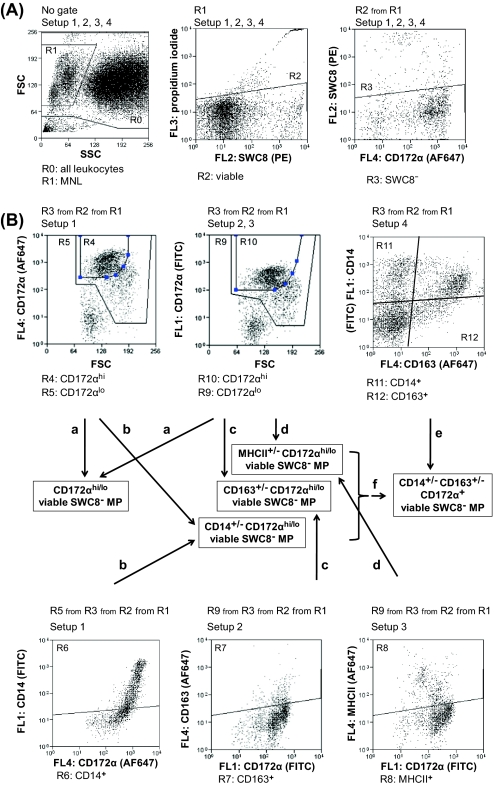
Figure 1.
The general gating strategy for identification of particular monocyte/macrophage (M/M) subpopulations in the lung tissue. Setups nos. 1–3 and 5 from Table II were used for identification of M/M subpopulations. (A) General gating strategy for identification of viable M/M in all setups. (B) Example of identifying CD163+/− M/M in setup no. 2.
The gating strategy for identifying particular PB and BM MP subpopulations is shown in Figure 2. Since CD203α was expressed on neither PB nor BM monocytes, setup no. 5 (Tab. II) was not included in the further analysis.
Figure 2.
The general gating strategy for identification of particular mononuclear phagocyte (MP) subpopulations (bone marrow is shown). Setups nos. 1–4 from Table II were used for identifying MP subpopulations. (A) The counts of all acquired leukocytes (with exclusion of cells debris) were determined (R0). Then the general gating strategy for mononuclear leukocytes (MNL) was applied for all setups. This included gating of mononuclear (R1) viable (R2) leukocytes in which the potentially contaminating neutrophils (SWC8+) were excluded (R3). (B) The CD172α+ (R5, R9) and CD172αhi (R4, R10) populations (a) were gated and CD172αhi/lo MP were calculated. The average from all setups, in which the CD172α staining was performed (setups nos. 1–3) was finally expressed. The counts of CD172αhi/lo CD14+/− (b, setup no. 1, R4, R5, R6) or CD172αhi/lo CD163+/− (c, setup no. 2, R10, R9, R7) or CD172αhi/lo MHCII+/− (d, setup no. 3, R10, R9, R8) MP were ascertained. The CD14+/− CD163+/− MNL (e, setup no. 4, R11, R12) were gated and their count was ascertained. Using the known counts of CD172αhi/lo CD14+/− and CD172αhi/lo CD163+/− (f), the counts of CD172α+ CD14+/− CD163+/− were finally calculated. (A color version of this figure is available online at www.vetres.org.)
2.6. Calculation of the results
Generally, the counts of MP subpopulations in the particular regions and gates were always expressed as proportions of these cells per 1 000 leukocytes (after exclusion of cell debris). Final counts of particular cell subpopulations were then calculated and expressed as a proportion of 1 000 leukocytes (BML) or they were further recalculated to 1 mL of blood (PBL). Since the weight of the whole lobe tissue before and after lavage, as well as the weight of the piece of disintegrated tissue, was ascertained during processing of the lung tissue, it was possible to express the numbers of AL, ISL and LL as absolute counts per 1 g of lung tissue.
2.7. Statistical analysis of the results
The differences between noninfected and infected animals were evaluated by the Mann–Whitney U test (Prisma Software, Graph Pad Software Inc., San Diego, USA).
3. RESULTS
3.1. Lung mononuclear phagocyte subpopulations
The changes in macrophage count and phenotype in the lung compartments (AL and IS including LL) before and after APP infection were monitored. Since the blood monocytes are a source of lung macrophages during inflammation, the monocytes within the lung compartments before and after infection were further evaluated.
3.1.1. Lung macrophages
In noninfected piglets, nearly all lung macrophages were CD14+ CD163+ CD203α+ and MHCII+.
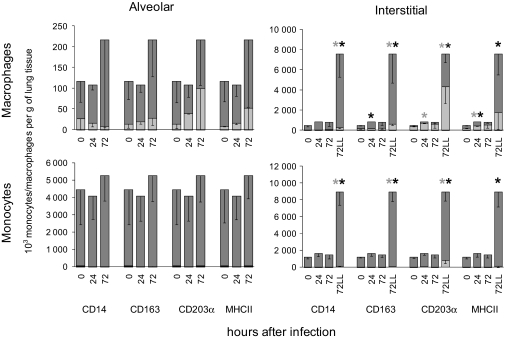
The count and phenotypic profile of alveolar macrophages did not change significantly after infection (Fig. 3). The count of interstitial macrophages increased significantly, however, even though the phenotypic profile of most of these cells was similar after infection to that before infection (CD14+ CD163+ CD203α+ and MHCII+). A small proportion of lesion macrophages were CD203α−. Taken together, the count of lung interstitial macrophages increased during inflammation, and the most so within the lesion. The inflammatory lung macrophages had the same phenotypic profile as resident macrophages. The absence of the CD203α molecule in some lesion macrophages suggests that the lesion macrophages originate from the monocytes, which typically lack this molecule.
Figure 3.
Monocyte/macrophage subpopulations within the lung tissue. Total counts were calculated of CD14+/− CD163+/− CD203α+/− or MHCII+/− monocytes/macrophages (dark bar: CD+, clear bar: CD−) in alveolar and interstitial compartments, including the lesion (LL) compartment, before and 24 and 72 h after Actinobacillus pleuropneumoniae (APP) infection. The total counts of cells ± SEM per gram of lung tissue are shown. The significant differences (p < 0.05) between noninfected (0 h after infection) and infected piglets are marked with asterisks (*). IS, interstitial.
3.1.2. Lung monocytes
In noninfected piglets, most lung monocytes were CD14+ CD163+ CD203α+ and MHCII+.
There appeared to be an increase in the number of AL monocytes and in the proportion of CD203α− and MHC II− subsets at 72 h post-infection, although these changes were not statistically significant (Fig. 3). The expression of CD14 and CD163 molecules did not change, and most of these AL monocytes were CD14+ CD163+.
The count of IS monocytes increased significantly after infection. Similarly to macrophages, the most noticeable changes in monocyte count occurred in the LL monocytes. The newly recruited monocytes were largely CD14+ CD163+ CD203α− MHC II+. At 72 h post-infection, the majority of LL monocytes had the phenotype CD14+ CD163+ CD203α− MHC II+.
This finding suggests that the newly recruited lung monocytes arise from blood monocytes since blood monocytes lack the CD203α molecule, and moreover, most of them also lack the MHCII molecule. Regarding the fact that these recruited monocytes are CD14+ CD163+, it may be assumed that the population of blood monocytes with the phenotype CD14+ CD163+ CD203α− and MHCII+ give rise to these newly recruited lung monocytes.
3.2. Peripheral blood and bone marrow mononuclear phagocyte subpopulations
In order to find the origin of lung inflammatory monocytes/macrophages, PB and BM MP were analyzed before and after infection.
3.2.1. Peripheral blood mononuclear phagocyte subpopulations
In noninfected piglets, two populations of PB MP were identified according to the intensity of CD172α expression: CD172αhi and CD172αlo cells (Fig. 4A). Since CD172αhi cells were exclusively CD14+ and CD172αlo cells were CD14−, they were further divided into monocytes (CD172αhi CD14+) and dendritic cells (CD172αlo CD14−). The count of monocytes increased already 6 h after infection and remained elevated through the 72 h following infection (Fig. 4B). However, the count of dendritic cells did not change significantly during the entire course of the infection.
Figure 4.
The cell surface molecule expression by peripheral blood mononuclear phagocytes (MP) after Actinobacillus pleuropneumoniae (APP) infection. The gating of MP subpopulations was performed as described in Figure 2. Representative dot-plots of CD172α (A), CD14, CD163 or MHCII versus CD172α (C) and CD14 versus CD163 (E) expression by MP are shown. The absolute counts of MP expressing CD172α (B), CD14, CD163 or MHCII versus CD172α (D) and CD14 versus CD163 (F) per mL of peripheral blood were calculated (data are mean ± SEM). In the case of absolute counts of CD14 versus CD163 MP (F) the known counts of CD172αhi/lo CD14+/− and CD172αhi/lo CD163+/− (see Fig. 2F and Sect. 2), were used for calculations. Significant differences (p < 0.05) between noninfected (0 h after infection) and APP-infected piglets are marked with “a”. Significant differences (p < 0.05) between consecutive time-points are marked with “b”. (A color version of this figure is available online at www.vetres.org.)
Then the CD14, CD163 and MHCII molecule expression by CD172αhi (monocytes) and CD172αlo (dendritic cells) PB MP was analyzed (Figs. 4C and 4D). Nearly all (98%) CD172αhi MP in noninfected piglets were CD14+. Fifty percent of them were CD163+ and 31% of them were MHCII+.
The population of CD172αhi MP, which was found to be significantly elevated 6–72 h after infection, was almost exclusively of the CD14+ phenotype, although a small population of CD14− cells was also transiently elevated 6–48 h after infection. As to the CD163 molecule, both CD163+ and CD163− cells were elevated 6 h after infection. The CD163− cells returned to the initial count 24 h after infection, while the CD163+ cell count increased further. Their counts did not change further 48 and 72 h after infection. The elevated population of CD172αhi MP was exclusively of the MHCII− phenotype.
Most CD172αlo MP in the noninfected piglets were CD14− CD163− MHCII+/−. Although some significant changes in these populations were found after infection, these changes appeared to be random and were difficult to associate with the course of infection.
The results stated above suggest that the population of CD172αhi PB MP, which was elevated after infection, was of the CD14+ CD163+ MHCII− phenotype. Moreover, there was a population of CD14+ CD163− MHCII− MP which was transiently increased 6 h after infection.
This was not unambiguous, however, because the CD14, CD163 and MHCII molecules were always each stained separately, in combination with antibodies against the CD172α molecules (setups nos. 1–3, Tab. II). To confirm the hypothesis, the staining of CD14/CD163 (setup no. 4, Tab. II) was performed. Since the CD172α molecule could not be included in this setup, it was impossible to distinguish MP from lymphocytes during gating. However, because neither the CD14 nor CD163 molecule is expressed by lymphocytes, it was possible to calculate the real counts of CD14/CD163 MP populations using the values obtained above in setups nos. 1–3 (Fig. 4F). Thus, the CD14/CD163 expression by all mononuclear leukocytes before and after infection is depicted (Fig. 4E). It was shown that the population of CD172αhi PB MP which was transiently increased 6 h after infection was CD14+ CD163− and the population which was increased during the whole course of infection was CD14+ CD163+.
The population of PB dendritic cells (CD172αlo) remained unchanged during the whole course of infection.
3.2.2. Bone marrow mononuclear phagocyte subpopulations
The exact identification of BM MP subpopulations was more complicated compared to that of PB due to the fact that the population of lymphoblasts can express the CD172α molecule in amounts comparable to CD172αlo MP [19]. Moreover, maturing BM MP show continuous expression of the CD172α molecule (Fig. 5A) in contrast to PB MP where two separated populations of monocytes and dendritic cells were identifiable. Generally, compared to the PB, much more heterogeneous MP populations exist in BM with respect to the expression of these cell surface molecules. Using the display of forward scatter against CD172α, however, it was possible to distinguish two populations of BM MP: CD172αhi FSClo (more mature MP) and CD172αlo FSChi (less mature MP) (Fig. 5A). The counts of both populations increased after infection (Fig. 5B).
Figure 5.
The cell surface molecule expression by bone marrow mononuclear phagocytes (MP) after Actinobacillus pleuropneumoniae (APP) infection. The gating of MP subpopulations was performed as described in Figure 2. Representative dot-plots of CD172α (A), CD14, CD163 or MHCII versus CD172α (C) and CD14 versus CD163 (E) expression by MP are shown. The absolute counts of MP expressing CD172α (B), CD14, CD163 or MHCII versus CD172α (D) and CD14 versus CD163 (F) per mL of bone marrow were calculated (data are mean ± SEM). In the case of absolute counts of CD14 versus CD163 MP (F) the known counts of CD172αhi/lo CD14+/− and CD172αhi/lo CD163+/− (see Fig. 2F and Sect. 2), were used for calculations. Significant differences (p < 0.05) between noninfected (0 h after infection) and APP-infected piglets are marked with “a”. Significant differences (p < 0.05) between adjoining intervals are marked with “b”. (A color version of this figure is available online at www.vetres.org.)
Then, similarly to PB, the expression of the CD14 CD163 and MHCII molecule by CD172αhi (FSClo) (more mature) and CD172αlo FSChi (less mature) BM MP was analyzed (Figs. 5C and 5D).
The CD172αhi (FSClo) MP in noninfected piglets were predominantly CD14+ (70%), CD163− (76%) and MHCII− (70%). The CD14+ MP were increased 24 and 72 h after infection while the CD14− MP transiently decreased 24 h after infection and then returned to the initial counts 72 h after infection. Comparable changes occurred in the counts of CD163-expressing MP. The CD163+ MP were increased 24 and 72 h after infection while the CD163− MP transiently decreased 24 h after infection and then returned to the initial counts 72 h after infection. With respect to MHCII expression, the MHCII− MP were increased after infection only, while MHCII+ MP remained unchanged.
Based on similar counts of the changed CD14+/− CD163+/− MP subpopulations, it could be assumed that those increased MP were CD14+ and CD163+ and those transiently decreased MP were CD14− and CD163−. Similar to the situation for PB, however, this assumption could not be directly verified. The changes in absolute counts of MHCII− MP suggest that both these changed populations (CD14+ CD163+ and CD14− CD163−) were MHCII−.
The CD172αlo (FSChi) MP in noninfected piglets were predominantly CD14− (94%), CD163− (90%) and MHCII+ (59%). The MP that were increased after infection, were CD14−, CD163− and MHCII−. Since no changes occurred in the population of CD14+ or CD163+ or MHCII+ MP, it could be assumed that these increased cells were CD14− CD163− MHCII−.
To confirm the hypothesis that the MP population that changed after infection was CD14+ CD163+ or CD14− CD163−, the CD14/CD163 (setup no. 4, Tab. II) staining and subsequent analysis was performed in the same way as in the PB. In noninfected piglets, two major (CD14− CD163− switching into CD14+ CD163−) MP populations were found (Fig. 5E). A few cells only were CD14+ CD163+. After infection, there was a significant increase in CD14+ CD163+. This population appeared to directly develop from CD14− CD163− BM MP rather than from CD14+ CD163−. Moreover, it was confirmed that the only populations that changed after infection were CD14+ CD163+ or CD14− CD163−, while CD14+ CD163− and CD14− CD163+ cells remained unchanged (Fig. 5F). The CD14+ CD163+ MP were increased 24 as well as 72 h after infection. This represents the increased population of CD172αhi CD14+ CD163+ MHCII− MP. The second population (i.e. CD14− CD163− MP) was increased 72 h after infection only (Fig. 5F). However, this lack of change after 24 h was probably due to the manner in which the data was presented. At this time point, two opposite trends could be detected: the numbers of CD172αlo CD14− CD163− MHCII− were increased whereas the numbers of CD172αhi CD14− CD163− MHCII− decreased (Fig. 5D), with the result that the sum of these two CD14− CD163− MP subpopulations remained unchanged 24 h after infection.
4. DISCUSSION
The aim of this work was to describe which porcine MP subpopulations infiltrate inflamed tissue under in vivo conditions. Moreover, the possible origin and maturation stages of these infiltrating MP were assessed in the PB and BM.
The cell surface molecules CD172α, SWC8 and CD203α were used for the identification of basic MP populations (monocytes, dendritic cells, macrophages). The further discrimination of particular MP subpopulations was based on the presence of CD14, CD163 and MHCII molecules.
The initial identification of all MP was based on the detection of CD172α together with SWC8 molecules according to Haverson et al. [12] and Summerfield et al. [19, 20]. In the PB, two major populations of MP: the CD172αhi CD14+ SWC8− monocytes and CD172αlo CD14− SWC8− dendritic cells [21] were identified. In the BM, typically two continuous subpopulations of MP were identified: less mature CD172αlo SWC8− FSChi and more mature CD172αhi SWC8− FSClo cells.
The CD203α molecule was typically expressed on lung MP but not on PB or BM MP. Since this molecule appears during the development of monocytes into macrophages [14], the lung MP expressing the CD203α molecule were considered to be more mature compared to those lacking this molecule.
After identification of basic MP populations, MP subpopulations were defined, based on the co-expression of other cell surface molecules. The expression profile of the CD14 molecule alone is well documented in the PB as well as in the BM [18–20]. Accordingly, CD14 was expressed by more mature (CD172αhi SWC8− FSClo) but not by less mature (CD172αlo SWC8− FSChi) BM MP and by PB monocytes, but not by dendritic cells. Thus, the use of the CD14 molecule alone for identifying MP subpopulations could yield only little new knowledge. However, the combination of this molecule with two other molecules (CD163 and MHCII) allows the discrimination of up to four distinct MP subpopulations [5, 15]. So far, the heterogeneity of the MP subpopulations has been described in the PB only using these three crucial surface molecules [5]. The present study provides new information about these subpopulations in the BM and lungs, including their possible developmental relationship to PB, under either noninflammatory or inflammatory conditions.
The results of the present study have shown that two different PB and three different BM monocyte subpopulations play roles in the inflammatory response induced by APP. In the PB, the population of (CD172αhi) CD14+ CD163+ MHCII− monocytes was increased even 6 h after infection and probably was a source of CD14+ CD163+ monocytes for the inflamed lung tissue. The (CD172αhi) CD14+ CD163− MHCII− monocytes probably play a role in the initiation of an inflammatory response prior to the CD14+ CD163+ monocytes taking over the role of monocytes infiltrating the inflamed tissue.
In the BM, the more mature BM monocytes (CD172αhi) CD14+ CD163+ MHCII− were increased and probably were a source of (CD172αhi) CD14+ CD163+ MHCII− PB monocytes. The second population of more mature monocytes, (CD172αhi) CD14+ CD163− MHCII−, was transiently decreased 24 h after infection. This was probably caused by a sudden need for monocytes, which are the cells of sequential developmental stages within the BM or PB. The increased count of less matured cells, (CD172αlo) CD14− CD163− MHCII−, probably reflects the accelerated generation of the early monocyte precursors within the BM.
The population of CD14+ CD163+ BM MP that was increased after infection appeared to develop directly from less mature CD14− CD163− rather than from more mature CD14+ CD163− BM MP. This could be caused by the accelerated upregulation of the CD163 molecule on the developing BM MP after receiving the inflammatory stimulus. These CD14+ CD163+ BM MP are then a direct source of PB monocytes during inflammation. However, this type of changed course of cell maturation is relatively unusual in biology. More common during enhanced consumption is the release of less mature cells from the BM to the PB (e.g. shift to the left in neutrophils, release of reticulocytes during regenerative anemia etc.). From this point of view, it is more probable that BM MP are produced by two different maturation pathways and then are released to the bloodstream as two distinct subpopulations. The use of these pathways differs under inflammatory and noninflammatory conditions.
This finding does not, however, exclude the possibility of CD163− PB monocytes switching into CD163+ monocytes, although this has so far been observed under in vitro conditions only [3]. It is likely that for a better understanding of the origin, differentiation pathways and function of MP in pigs, more comprehensive in vivo experiments are necessary in the future.
In the mouse model, two major subpopulations of PB monocytes exist: the Ly6C+ and Ly6C− cells. The phenotypic switch from Ly6C+ into Ly6C− monocytes is a spontaneous process occurring continuously in the bloodstream [23]. The less mature Ly6C+ monocytes could enter the inflamed tissue, while more mature Ly6C− monocytes renew the population of resident macrophages [10, 13]. So far, very similar developmental pathways were suggested in pigs, where CD163− PB monocytes could under in vitro conditions spontaneously switch their phenotype to CD163+ [5]. Since the population of PB MP that was increased under inflammatory conditions in the pig was CD163+ CD14+, it could be suggested that, on the contrary to mice, the subpopulation of these more mature CD163+ PB monocytes plays a role in the infiltration of the inflamed tissue.
The combination of cell surface molecules used in the present study was found to be appropriate for characterizing MP subpopulations in pigs. However, an upgrade of the four-color into seven-color flow cytometry for simultaneous detection of all these molecules together seems to be necessary for future experiments.
From a functional point of view, the porcine CD163+ PB MP are known to express higher amounts of co-stimulatory and adhesion molecules than CD163− MP [3]. Moreover, they are able to produce higher amounts of TNF-α after in vitro cultivation [5]. Taken together, the CD163+ MP exert a higher degree of preparedness to play a role in the defense against pathogenic agents compared to CD163− MP. This closely corresponds to the present data demonstrating an important role for CD163+ MP during the inflammatory response.
An important role of CD163+ PB MP, which produce higher amounts of TNF-α, is finally supported by the fact that TNF-α was found to play a role in the pathophysiology of APP-induced lung inflammation in pigs [6].
In summary, our results show that the CD14+ CD163+ MP subpopulation is involved in infiltrating the inflamed tissue of pigs under in vivo conditions and that the initial response to the inflammatory stimulus appears to occur already in the bone marrow.
Acknowledgments
This study was supported by grants of the Czech Science Foundation (P502/10/P362), Ministry of Agriculture of the Czech Republic (MZE0002716202) and Ministry of Education, Youth and Sports of the Czech Republic (CZ.1.05/2.1.00/01.0006, AdmireVet). The authors wish to thank Mrs. Ludmila Faldikova for critical reading of the manuscript.
References
- 1.Baarsch M.J., Foss D.L., Murtaugh M.P., Pathophysiologic correlates of acute porcine pleuropneumonia, Am. J. Vet. Res. (2000) 61:684–690 [DOI] [PubMed] [Google Scholar]
- 2.Bosse J.T., Janson H., Sheehan B.J., Beddek A.J., Rycroft A.N., Kroll J.S., Langford P.R., Actinobacillus pleuropneumoniae: pathobiology and pathogenesis of infection, Microbes Infect. (2002) 4:225–235 [DOI] [PubMed] [Google Scholar]
- 3.Chamorro S., Revilla C., Alvarez B., Lopez-Fuertes L., Ezquerra A., Dominguez J., Phenotypic characterization of monocyte subpopulations in the pig, Immunobiology (2000) 202:82–93 [DOI] [PubMed] [Google Scholar]
- 4.Chamorro S., Revilla C., Gomez N., Alvarez B., Alonso F., Ezquerra A., Dominguez J., In vitro differentiation of porcine blood CD163− and CD163+ monocytes into functional dendritic cells, Immunobiology (2004) 209:57–65 [DOI] [PubMed] [Google Scholar]
- 5.Chamorro S., Revilla C., Alvarez B., Alonso F., Ezquerra A., Dominguez J., Phenotypic and functional heterogeneity of porcine blood monocytes and its relation with maturation, Immunology (2005) 114:63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho W.S., Chae C., Expression of nitric oxide synthase 2 and tumor necrosis factor α in swine naturally infected with Actinobacillus pleuropneumoniae, Vet. Pathol. (2002) 39:27–32 [DOI] [PubMed] [Google Scholar]
- 7.Delventhal S., Hensel A., Petzoldt K., Pabst R., Cellular changes in the bronchoalveolar lavage (BAL) of pigs, following immunization by the enteral or respiratory route, Clin. Exp. Immunol. (1992) 90:223–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faldyna M., Nechvatalova K., Sinkora J., Knotigova P., Leva L., Krejci J., Toman M., Experimental Actinobacillus pleuropmeumoniae infection in piglets with different types and levels of specific protection: Immunophenotypic analysis of lymphocyte subsets in the circulation and respiratory mucosal lymphoid tissue, Vet. Immunol. Immunopathol. (2005) 107:143–152 [DOI] [PubMed] [Google Scholar]
- 9.Fogg D.K., Sibon C., Miled C., Jung S., Aucouturier P., Littman D.R., et al., A clonogenic bone marrow progenitor specific for macrophages and dendritic cells, Science (2006) 311:83–87 [DOI] [PubMed] [Google Scholar]
- 10.Geissmann F., Jung S., Littman D.R., Blood monocytes consist of two principal subsets with distinct migratory properties, Immunity (2003) 19:71–82 [DOI] [PubMed] [Google Scholar]
- 11.Gordon S., Taylor P.R., Monocyte and macrophage heterogeneity, Nat. Rev. Immunol. (2005) 5:953–964 [DOI] [PubMed] [Google Scholar]
- 12.Haverson K., Bailey M., Higgins V.R., Bland P.W., Stokes C.R., Characterization of monoclonal antibodies specific for monocytes, macrophages and granulocytes from porcine peripheral blood and mucosal tissues, J. Immunol. Methods (1994) 170:233–245 [DOI] [PubMed] [Google Scholar]
- 13.Landsman L., Jung S., Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages, J. Immunol. (2007) 179:3488–3494 [DOI] [PubMed] [Google Scholar]
- 14.McCullough K.C., Schaffner R., Natale V., Kim Y.B., Summerfield A., Phenotype of porcine monocytic cells: modulation of surface molecule expression upon monocyte differentiation into macrophages, Vet. Immunol. Immunopathol. (1997) 58:265–275 [DOI] [PubMed] [Google Scholar]
- 15.Sanchez C., Domenech N., Vazquez J., Alonso F., Ezquerra A., Dominguez J., The Porcine 2A10 antigen is homologous to human CD163 and related to macrophage differentiation, J. Immunol. (1999) 162:5230–5237 [PubMed] [Google Scholar]
- 16.Serbina N.V., Pamer E.G., Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2, Nat. Immunol. (2006) 7:311–317 [DOI] [PubMed] [Google Scholar]
- 17.Strauss-Ayali D., Conrad S.M., Mosser D.M., Monocyte subpopulations and their differentiation patterns during infection, J. Leukoc. Biol. (2007) 82:244–252 [DOI] [PubMed] [Google Scholar]
- 18.Summerfield A., McCullough K., Porcine bone marrow myeloid cells: phenotype and adhesion molecule expression, J. Leukoc. Biol. (1997) 62:176–185 [DOI] [PubMed] [Google Scholar]
- 19.Summerfield A., Haverson K., Thacker E., McCullough K.C., Differentiation of porcine myeloid bone marrow haematopoietic cell populations, Vet. Immunol. Immunopathol. (2001) 80:121–129 [DOI] [PubMed] [Google Scholar]
- 20.Summerfield A., Guzylack-Piriou L., Schaub A., Carrasco C.P., Tache V., Charley B., McCullough K.C., Porcine peripheral blood dendritic cells and natural interferon-producing cells, Immunology (2003) 110:440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Summerfield A., McCullough K.C., The porcine dendritic cell family, Dev. Comp. Immunol. (2009) 33:299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sunderkotter C., Nikolic T., Dillon M.J., Van Rooijen N., Stehling M., Drevets D.A., Leenen P.J., Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response, J. Immunol. (2004) 172:4410–4417 [DOI] [PubMed] [Google Scholar]
- 23.Xu H., Manivannan A., Dawson R., Crane I.J., Mack M., Sharp P., Liversidge J., Differentiation to the CCR2+ inflammatory phenotype in vivo as a constitutive, time-limited property of blood monocytes and is independent of local inflammatory mediators, J. Immunol. (2005) 175:6915–6923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zelnickova P., Faldyna M., Stepanova H., Ondracek J., Kovaru F., Intracellular cytokine detection by flow cytometry in pigs: fixation, permeabilization and cell surface staining, J. Immunol. Methods (2007) 327:18–29 [DOI] [PubMed] [Google Scholar]