Abstract
Exacerbations contribute significantly to the morbidity of COPD, leading to an accelerated decline in lung function, reduced functional status, reduced health status and quality of life, poorer prognosis and increased mortality. Prevention of exacerbations is thus an important goal of COPD management. In patients with COPD, treatment with a combination of the inhaled corticosteroid fluticasone propionate (250 μg) and the long-acting β2-agonist salmeterol (50 μg) in a single inhaler (250/50 μg) is an effective therapy option that has been shown to reduce the frequency of exacerbations, to improve lung function, dyspnea and health status, and to be relatively cost-effective as a COPD maintenance therapy. Importantly, results of various studies suggest that fluticasone propionate and salmeterol have synergistic effects when administered together that improve their efficacy in controlling symptoms and reducing exacerbations. The present non-systematic review summarizes the role of fluticasone propionate/salmeterol combination therapy in the prevention of exacerbations of COPD and its related effects on lung function, survival, health status, and healthcare costs.
Keywords: Advair, COPD, disease exacerbation, fluticasone propionate, salmeterol, combination drug therapy
Introduction
Exacerbations contribute significantly to the morbidity, mortality and cost burden of COPD, a disease characterized by progressive air flow limitation that is not entirely reversible, airway inflammation and mucociliary dysfunction.1,2 In addition to producing short-term increases in breathlessness and other symptoms, exacerbations have sustained effects, including an accelerated decline in lung function,3,4 reduced health status and quality of life,5–9 and increased risk of death.10–11 Although therapeutic interventions to control the clinical symptoms of COPD and reduce airway inflammation are available, most studies indicate available treatments do not slow the long-term progression of the disease.2 However, newer therapies, including combination therapy with an inhaled corticosteroid (ICS) and long-acting beta-agonist (LABA), are able to prevent episodes of exacerbation, thereby enhancing treatment strategies, particularly for moderate-to-severe COPD.
The present non-systematic review summarizes the role of fluticasone propionate and salmeterol combination therapy (FSC) in the prevention of exacerbations of COPD as well as its related effects on lung function, survival, health status and quality of life, and healthcare costs. This review also discusses prescribing considerations of interest to practicing clinicians. Although the intended focus of this review is FSC, where relevant we have referenced studies of budesonide/formoterol (Symbicort®; AstraZeneca), another currently available ICS/LABA combination therapy; however, the majority of the clinical experience, clinical trial data, and other real-world observational outcomes data available at this time concern FSC (Advair®/Seretide®; GlaxoSmithKline), in part because this formulation has been available for a longer period of time.
Impact of exacerbations in COPD
Although there is no consensus on a definition, an exacerbation of COPD is generally defined as an acute worsening of dyspnea, cough, and/or sputum that is beyond normal day-to-day variation and sufficient to warrant a change in medication.1,2 Exacerbations are associated with airway inflammation that is most often triggered by bacterial or viral infection, air pollution or cold weather; no cause is identified about one-third of the time.2,12 A bacterial etiology, related to either pre-existing lower airway colonization or newly acquired bacterial strains, has been identified in approximately 50% of exacerbations.13,14 Bacterial load has been considered the primary factor; however, a recent prospective, longitudinal study utilizing molecular typing found that the concentration of either pre-existing or newly acquired strains had no or only a small impact on exacerbation incidence, a finding which suggests that more complex host-pathogen interactions are involved in exacerbations of bacterial etiology than simply bacterial load.13,14
The precise mechanisms underlying the amplified inflammatory response remain unclear, but exacerbations have been associated with infiltration of neutrophils and eosinophils, markers of oxidative stress, into airway walls along with tumor necrosis factor-α (TNF-α), leukotriene (LT) B4, interleukin (IL)-8, leukotrienes and other pro-inflammatory cytokines.2,12,15
Exacerbations are common in COPD and are recognized more frequently in patients with more severe disease, some of whom are likely to experience three or more exacerbations per year.12 Donaldson and colleagues reported a median of 2.68 exacerbations per year in those with moderate disease (GOLD stage II) and 3.43 exacerbations per year in those with severe disease (GOLD stage III).16 A history of exacerbation in the prior year is predictive of exacerbations in the current year.8 Exacerbations tend to cluster together in time; Hurst et al found that over a quarter of first exacerbations were followed by a second exacerbation within 8 weeks and about one-third of all exacerbations were recurrent ones.17 In a retrospective cohort study of recently hospitalized patients with COPD exacerbations, 13.2% of patients in the reference group were readmitted within 12 months and 24.3% died within the year following a COPD hospitalization.18 Similarly, a study from Canadian hospitals found that 38% of patients admitted for COPD were readmitted within an average of 5 months.19
Even with appropriate treatment during the acute episode, exacerbations often instigate a downward spiral in which the patient never returns to baseline lung or physical functioning levels. In a study of 109 patients with COPD, those with frequent exacerbations (≥2.92 exacerbations/year) were found to experience additional declines of 8.0 mL/year in FEV1 and 2.22 L/min/year in peak expiratory flow (PEF) compared to patients with fewer exacerbations.3 In a longitudinal cohort study, only 75% of COPD patients experiencing an exacerbation had regained their basal pulmonary function by 5 weeks and 7% had not recovered by 3 months.4 Severe exacerbations requiring hospitalization have even poorer outcomes. Inpatient mortality during COPD admission is as high as 4% to 30%.10 The hospitalization itself is associated with functional decline and psychological disorders, including anxiety and depression, which contribute to readmission.20–22 Quality of life, as assessed by the St George’s Respiratory Questionnaire (SGRQ) and other health/functional status instruments, is also severely impacted by acute exacerbation events, which frequently lead to marked restriction in activities of daily living and poorer mental state.5–9 This deterioration in physical functioning and health status occurs even with no significant deterioration in FEV1, but instead correlates with worsened symptoms, particularly dyspnea, following exacerbation.6–8 Thus, preventing exacerbations is important not only from the short-term clinical perspective, but to help patients to function better and feel better in the longer term.
From a societal perspective, exacerbations are costly. Hospitalizations for exacerbations account for nearly 70% of the direct healthcare costs of treating COPD.23 In the United States alone, COPD-related hospital inpatient and emergency department (ED) costs were estimated to be $10 billion in 2003,24 with estimated mean costs in 2001 of $571 for an emergency department visit, $5997 for a standard hospital admission, and $36,743 for an admission requiring intensive care and intubation.24,25 Patients with both asthma and COPD have particularly high respiratory-related and all-cause healthcare costs and use significantly more healthcare services than those with asthma or COPD.26–28 Given the significant clinical, quality of life, and economic consequences of exacerbations, their prevention has become a widely accepted and singularly important goal in the management of COPD.1,2
COPD maintenance therapy goals
The primary goals of COPD treatment are to improve symptoms and health status and reduce the frequency of exacerbations.2 While pharmacologic therapy is needed for most patients with COPD, it is just one part of disease management. Other components of care include regular visits to monitor and assess disease progression (including repeat spirometry) and identify COPD-related complications and co-morbidities, and reducing risk factors such as smoking, occupational exposures, and exposure to indoor and outdoor air pollution.1,2 Pulmonary rehabilitation and patient education can improve health status, help patients cope better with symptoms and activities of daily living, and prompt earlier recognition of exacerbations by the patient and thus timely treatment.2 Pulmonary rehabilitation results in improvements in exercise capacity, endurance, walking distance, dyspnea and quality of life, and is beneficial even in older patients.29,30 Smoking cessation education in particular provides a significant opportunity to influence the course of the disease and reduce age-related decline in FEV1.31 Early identification of exacerbations is an important objective of COPD care, as early treatment is associated with faster recovery, reduced risk for hospitalization, and better quality of life.32
Pharmacologic therapy is based primarily on the patient’s symptoms and disease severity, with additional agents added as the disease progresses and symptoms worsen. According to guidelines, pharmacotherapy in mild COPD (FEV1/FVC < 0.70 and FEV1 ≤ 80% of predicted value) focuses on short-acting bronchodilators to relieve symptoms, including short-acting β2-agonists (eg, albuterol) and short-acting anticholinergics (eg, ipratropium bromide, oxitropium); the latter have a longer duration of effect (6 to 9 hours) than short-acting β2-agonists (4 to 6 hours).1,2 When symptoms are persistent, the addition of one or more long-acting bronchodilators is recommended (FEV1/FVC < 0.70, FEV1 < 80% predicted). Recommended agents include LABAs (eg, formoterol, salmeterol), long-acting anticholinergics (eg, tiotropium), and, rarely, methylxanthines (eg, theophylline). Treatment with a combination of bronchodilators with different durations and mechanisms of action is recommended when single-agent therapy is inadequate, as this can achieve improved bronchodilation while reducing the risk of side effects from a higher dose of a single agent.2 To improve health status and reduce the frequency of exacerbations, the Global Initiative for Chronic Obstructive Long Disease (GOLD) guidelines recommend adding ICS for symptomatic patients with GOLD stage III or IV disease, FEV1 < 50% predicted, and repeated exacerbations2, while the American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines recommend consideration of ICS for patients with FEV1 < 50% predicted and one or more exacerbations in the last year requiring treatment with oral corticosteroids or antibiotics.1 The 50% FEV1 cut-off level is somewhat arbitrary, and many patients with FEV1 > 50% have multiple exacerbations each year. Clinically, combination therapy is often started for patients who are approaching Stage III COPD and have frequent exacerbations. Thus, within the context of a stepped approach to therapy for COPD, FSC is an important option for maintenance therapy and prevention of exacerbations.
Treatment goals are unlikely to be reached if the patient handles the inhaler device incorrectly and the lungs do not receive adequate doses of medication. Teaching the patient correct inhaler technique upon initiation of a new medication and monitoring the patient’s technique at subsequent visits are crucial but often overlooked tasks.33
Pharmacologic effects
Long-acting β2-adrenoceptor agonists have bronchodilator effects. The LABA salmeterol is highly selective for β2-adrenergic receptors and its binding at these sites stimulates production of cyclic adenosine monophosphate (cAMP), leading to relaxation of bronchial smooth muscle and in turn, improved lung emptying during tidal breathing and a decreased perception of dyspnea.34–35 Salmeterol also has non-bronchodilator effects, including improved mucociliary clearance and ciliary beat activity and inhibition of histamine, leukotrienes and other inflammatory mediators produced by mast cells.35,36 Its effects on lung function are apparent within two hours of administration and last for approximately 12 hours.37 A key benefit of salmeterol is thus the symptomatic control of COPD on a day-to-day basis and a decreased need for rescue medication.34,38
Fluticasone propionate, a synthetic trifluorinated corticosteroid, is a glucocorticoid receptor agonist with a receptor binding affinity in vitro greater than that of the glucocorticoid steroids dexamethasone, beclometasone and budesonide.35 Glucocorticoid receptors contain DNA-binding proteins that alter the transcription rates of responsive genes.39 The anti-inflammatory effects of fluticasone propionate derive from its ability to induce conformational changes in cytoplasmic glucocorticoid receptors that subsequently inhibit the expression of inflammatory genes and increase the transcription of anti-inflammatory genes.35,40 An acute decrease in bronchial blood flow occurs following fluticasone propionate administration, and although less well understood, may be due to effects on kinases and phosphatases associated with glucocorticoid receptors that lead to rapid changes in cell function and vasoconstriction.40 The inflammatory processes in COPD primarily involve CD8+ T-lymphocytes (an increase of which is associated with increased airflow obstruction), CD68+ macrophages, and infiltration of neutrophils into airway and lung parenchyma.41,42 Some research indicates that fluticasone propionate does not reduce the number of CD8 cells in bronchial epithelium, but reduces the CD8:CD4 ratio, suggesting that it prevents a rise in CD4 cells.43 It also has been shown to reduce bronchial mucosal mast cells,43,44 neutrophils,45 and systemic markers of inflammation, including C-reactive protein, RP, IL-6, and other inflammatory cytokines.46
Pharmacologic effects of co-administration of fluticasone propionate and salmeterol
There is evidence that the co-administration of fluticasone propionate and salmeterol has synergistic effects at the molecular level whereby fluticasone propionate enhances the β-agonist effect of salmeterol and salmeterol enhances the anti-inflammatory effects of fluticasone propionate.40,41 Several mechanisms appear to be involved. Glucocorticoids prevent the down-regulation of β2-adrenoceptors and increase β2-adrenoceptor gene transcription; this leads to increased β2-adrenoceptor synthesis, providing additional receptors for β2-agonists to activate and increasing intracellular cAMP levels.40,41,47 Long-acting β2-agonists may improve the anti-inflammatory effects of ICS by facilitating translocation of the glucocorticoid receptor/ligand complex into the nucleus.35 Adding salmeterol to fluticasone propionate leads to a greater reduction in TNF-α and macrophage chemokines following rhinovirus infection than is achieved by ICS therapy alone.48,49 These examples of synergistic molecular effects of salmeterol and fluticasone propionate are consistent with results of in vivo studies and clinical trials that have observed greater clinical efficacy with FSC than with its individual components.36,50–54 In contrast, a bronchial biopsy study of steroid-naïve current and former smokers with moderate to severe COPD found that fluticasone propionate 500 μg reduced CD3+, CD4+, CD8+, mast cells and hyperresponsiveness at 6 months compared to placebo, but adding LABA (50 μg) did not lead to greater anti-inflammatory effects.55 Sample size in this study was small (N = 114), however, and a larger follow-up study is needed.
Clinical efficacy
Compared to monotherapy with fluticasone propionate or salmeterol, FSC has been shown in a number of clinical trials to lead to improvements in one or more of the following parameters: lung function, exacerbation rates, dyspnea, chronic bronchitis symptoms, night awakenings, frequency of rescue medication use and health status.36,42,47,51–54 In reviewing clinical efficacy, we have relied primarily on level I evidence from randomized controlled trials (RCTs).
Lung function and symptoms
The comparative effects of FSC on lung function and symptoms versus fluticasone propionate or salmeterol monotherapy have been investigated in several RCTs of COPD (Table 1). In a 24-week trial of 691 patients, Mahler et al reported a significantly greater increase in pre-bronchodilator FEV1 with FSC (500/50 μg) than with salmeterol (50 μg), resulting in an improvement of 14.5% over baseline, and a greater increase in 2-hour post-bronchodilator FEV1 with FSC than with fluticasone propionate (500 μg).52 Patients receiving FSC also had improved morning PEF and less dyspnea than patients who received fluticasone propionate or salmeterol alone. Evidence of a synergistic effect of combination therapy was seen, as the PEF mean change from baseline was greater than the sum of the changes achieved with fluticasone propionate and salmeterol individually. Similarly, a 12-month trial (N = 1465) observed greater improvements in pre- and post-bronchodilator FEV1, health status, cough, and breathlessness with FSC 500/50 μg compared with either fluticasone propionate 500 μg or salmeterol 50 μg, and night-time awakenings were decreased with either FSC or fluticasone propionate compared to salmeterol.50 In these trials, an FSC dosage of 500/50 μg administered twice daily was used. The effects of a lower dose of FSC on lung function were assessed in a randomized controlled trial of 723 patients.36 Hanania and colleagues reported a greater increase in morning predose FEV1 with FSC 250/50 μg than with either fluticasone propionate 250 μg or salmeterol 50 μg, with effects observable within 24 hours of the first dose and sustained over 24 weeks, and an increased 2-hour postdose FEV1 with FSC compared with salmeterol.
Table 1.
Clinical trials of the effect of fluticasone propionate/salmeterol combination therapy on lung function, symptoms and health status
| Study | No. of patients (duration) | Treatment comparisons | Major findings: lung function | Major findings: health status and symptoms |
|---|---|---|---|---|
| Calverley et al 200350 | 1465 (52 weeks) | Twice daily FSC 500/50 μg vs FP 500 μg, SAL 50 μg, or placebo | FEV 1 increased with FSC compared to FP, SAL and placebo | Health status score (SGRQ) was improved with FSC at week 8 (−4.3, SD 10.8) and 52 (−4.5, SD 12.9) compared to placebo and FP Dyspnea and use of relief medication improved with FSC compared to placebo, FP and SAL. Night-time awakenings decreased with FSC compared to placebo and SAL |
| Mahler et al 200252 | 691 (24 weeks) | Twice daily FSC 500/50 μg vs FP 500 μg, SAL 50 μg, or placebo | Pre-dose FEV1 increased with FSC compared to SAL and placebo; 2-hour post-dose FEV1 increased with FSC compared to FP and placebo; dyspnea score improved with FSC compared to FP and placebo | Health status improved with FSC (CDRQ, 10.0) compared to placebo (5.0) and FP (4.8), but not compared to SAL (8.0) Dyspnea and use of relief medication improved with FSC compared to SAL, FP and placebo. Night-time awakenings decreased with FSC compared to SAL and placebo |
| Hanania et al 200336 | 723 (24 weeks) | Twice daily FSC 250/50 μg vs FP 250 μg, SAL 50 μg, or placebo | Morning pre-dose FEV1 increased with FSC compared to SAL and placebo; 2-hour post-dose FEV1increased with FSC compared to FP and placebo | Health status improved with FSC compared to placebo (CDRQ, +5.2) but not compared to FP or SAL Dyspnea improved with FSC and SAL compared to placebo. Use of rescue medication decreased with FSC compared to placebo and FP. Night-time awakenings decreased with FSC compared to placebo and SAL |
| Celli et al 200856 | 5343 (3 years) | Twice daily FSC 500/50 μg vs FP 500 μg, SAL 50 μg, or placebo | The rate of decline in FEV1 was reduced with FSC, FP and SAL compared to placebo; greatest reduction was with FSC compared to placebo; differences between treatment arms were not significant | Not assessed |
Abbreviations: CRDQ, chronic respiratory disease questionnaire; FP, fluticasone propionate; FSC, fluticasone propionate/salmeterol combination; SAL, salmeterol; SGTQ, St George’s respiratory questionnaire; TDI, transition dyspnea index.
The effect of FSC on the rate of decline in lung function (FEV1) was evaluated as a secondary outcome in the Towards a Revolution in COPD Health (TORCH) trial, a 3-year, randomized, controlled trial of 6112 patients with moderate-to-severe COPD.56 Lung function (FEV1) declined more slowly with FSC 500/50 μg treatment (39 mL/year) compared to placebo (55 ml/year); however, this rate of decline was not significantly different from the rates of decline seen in the fluticasone propionate 500 μg (42 mL/year) and salmeterol 50 μg (42 mL/year) treatment arms. Two recent parallel-group trials of FSC 250/50 μg and salmeterol 50 μg evaluated morning predose FEV1 as a secondary endpoint and observed declines in lung function in both groups over the 52-week treatment period, but FSC treatment preserved lung function better than did salmeterol alone.51,53 Moreover, withdrawal of fluticasone propionate in patients receiving FSC (500/50 μg) has been shown to result in deterioration in lung function and dyspnea and an increase in mild exacerbations.57
Exacerbation prevention
There is little evidence that LABA alone is effective in preventing exacerbations, but clinical trials have shown that ICS alone (fluticasone propionate or budesonide) reduces COPD exacerbations in the range of 10% to 20%.50,58–61 Several RCTs also have shown that combination ICS/LABA therapy is superior to LABA or ICS alone in reducing exacerbations (Table 2).42,51,53,54,62 In contrast, a 12-month trial (N = 1465) found no difference in exacerbation rates between FSC 500/50 μg, salmeterol 50 μg or fluticasone propionate 500 μg.50
Table 2.
Clinical trials of efficacy of fluticasone propionate/salmeterol combination therapy in the prevention of COPD exacerbations
| Study | No. of patients (duration) | Treatment comparisons | Primary outcome measure | Major findings |
|---|---|---|---|---|
| Calverley et al 200754 | 6112 (3 years) | Twice daily FSC 500/50 μg vs FP 500 μg, SAL 50 μg, or placebo | All cause mortality; exacerbations | Rate of moderate/severe exacerbations was 12% lower with FSC compared to SAL; 9% lower with FSC compared to FP Rate of exacerbations requiring oral corticosteroids was 13% lower with FSC compared to FP; 29% lower with FSC compared to SAL Differences in all-cause mortality between groups were not significant |
| Kardos et al 200742 | 994 (44 weeks) | Twice daily FSC 500/50 μg vs SAL 50 μg | Exacerbations | Rate of moderate/severe exacerbations was 35% lower with FSC compared to SAL |
| Anzueto et al 200953 | 797 (52 weeks) | Twice daily FSC 250/50 μg vs SAL 50 μg | Exacerbations | Rate of moderate/severe exacerbations was 30.4% lower with FSC compared to SAL Rate of exacerbations requiring systemic corticosteroids was 40% lower with FSC compared to SAL |
| Ferguson et al 200851 | 782 (52 weeks) | Twice daily FSC 250/50 μg vs SAL 50 μg | Exacerbations | Rate of moderate/severe exacerbations was 30.5% lower with FSC compared to SAL Rate of exacerbations requiring systemic corticosteroids was 40% lower with FSC compared to SAL |
Abbreviations: FP, fluticasone propionate; FSC, fluticasone propionate/salmeterol combination; NS, not statistically significant; SAL, salmeterol
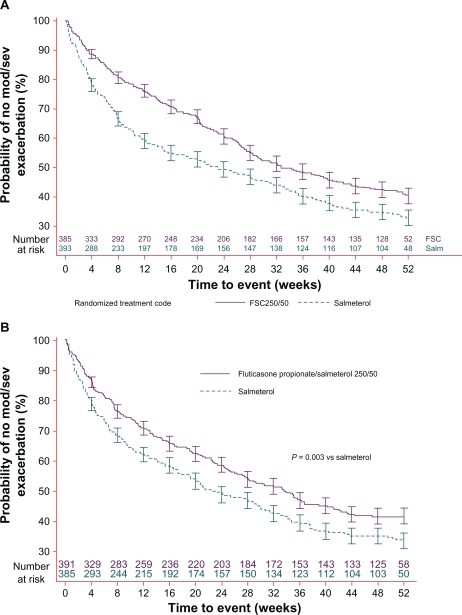
In the 3-year TORCH study, FSC 500/50 μg reduced moderate/severe exacerbations by 12% compared to salmeterol, 9% compared to fluticasone propionate, and 25% compared to placebo.50 A shorter 44-week trial of 994 COPD patients randomized to FSC 500/50 μg or salmeterol 50 μg reported 35% fewer exacerbations in the FSC group.42 Most recently, 2 replicate parallel-group trials evaluated whether the FSC 250/50 μg dose is also associated with reducing annual rates of moderate or severe COPD exacerbations (Figure 1).51,53 These studies observed reductions in moderate/severe exacerbations of 30.4% and 30.5%, respectively, with FSC compared to active treatment with salmeterol 50 μg.
Figure 1.
(A) Reprinted with permission from Anzueto A, Ferguson GT, Feldman G, Chinksy K, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD. 2009;6(5):320–329.53 Copyright © 2009 Taylor & Francis. (B) Reprinted with permission from Ferguson GT, Anzueto A, Fei R, Emmett A, Knobil K, Kalberg C. Effect of fluticasone propionate/salmeterol (250/50 μg) or salmeterol (50 μg) on COPD exacerbations. Respir Med. 2008;102(8): 1099–1108.51 Copyright © 2008 Elsevier.
Similar findings have been observed for budesonide/formoterol (160/4.5 μg). In a 12-month trial, this combination therapy led to a 35% lower rate of mild exacerbations compared to budesonide 200 μg alone.62 The same study observed a 23% lower rate of severe exacerbations with budesonide/formoterol compared to formoterol (4.5 μg) but no difference compared to budesonide.
Improvement in exacerbation rates in patients treated with FSC has been most evident in patients with more severe disease; however, a recent analysis of the TORCH trial data based on disease severity found that FSC treatment decreased exacerbations by 31% relative to placebo in patients with GOLD stage II COPD (moderate; FEV1/FVC < 0.70, 50% ≤ FEV1 ≤ 80% predicted).63
Survival
It is not entirely clear whether or not FSC treatment confers a survival benefit, as clinical studies to date have been underpowered to assess mortality. The TORCH study reported a 17.5% lower risk for death (2.6% absolute risk reduction) in the combination therapy group; however, this finding was just short of statistical significance (P = 0.052).54 Statistical and clinical significance are not always the same and the observed absolute reduction in risk identified in the TORCH trial deserves careful clinical consideration. Practitioners of clinical medicine may feel less concern with a small deviation from a statistically significant finding when the absolute size of the effect is relatively large and clinically relevant, as sample size has a large influence on the P-value.64 In a pooled analysis of seven randomized controlled trials, Sin and colleagues observed a decrease in all-cause mortality of approximately 27% with ICS plus LABA compared to placebo (hazard ratio [HR] 0.73, 95% confidence interval [CI], 0.55 to 0.96).65 In several retrospective observational studies, FSC or ICS have been associated with significantly lower mortality,18,66–69 but one other study found no survival benefit associated with ICS.70
Health status and quality of life
Measures of lung function, such as FEV1, do not necessarily reflect the degree of disability experienced by patients with COPD.6,8 While patients may not experience a decline in FEV1 over a particular period of time, they may find nonetheless that it has become harder to walk to the mailbox, a sign of decreased functional capability. One of the hurdles to gleaning associations between clinical measures and patient-reported outcomes is the lack of congruence between accepted lung function measures and measures important to patient well-being and quality of life. Assessment of health status and quality of life improves our understanding of the effects of treatment and the impact of exacerbations.
Exacerbations produce a short-term deterioration in COPD-specific health status and dyspnea, with significant improvement generally following within 10 days.71 Seemungal followed patients for 1 year and showed that those with more exacerbations had significantly worse health-related quality of life (HRQOL) as measured by the SGRQ.8 In addition, dyspnea was not related to exacerbation frequency but was strongly related to HRQOL, indicating the very real effect of symptoms on health status independent of measurable changes in lung function. In a 2-year study of patients with moderate COPD (GOLD stage II), those with 3 or more exacerbations had SGRQ scores that were approximately 2 points higher (indicating worse quality of life) per year than patients with fewer exacerbations, and the biggest changes were associated with the symptom subscale.6 Hospital admissions were also associated with a higher SGRQ score, and it was noted that patients with frequent exacerbations during follow-up had significantly lower HRQOL at baseline, suggesting that these patients were already at increased risk for exacerbation.
Treatment with FSC has resulted in clinically important improvements in health status (Table 1). In a 24-week study of 691 patients, the FSC group had a significant increase (improvement) from baseline in mean Chronic Respiratory Disease Questionnaire (CRDQ) score compared to the fluticasone propionate and placebo groups (a change of 10.0 points from baseline for FSC versus 4.8 for fluticasone propionate and 5.0 for placebo).52 In 2 parallel 12-month clinical trials, nighttime awakenings were decreased by approximately 1 awakening per week in the FSC groups, while awakenings increased slightly in the salmeterol groups.51,53 Symptoms and health status in COPD patients generally worsen over time, but the FSC groups in both trials had better dyspnea and SGRQ scores compared to baseline than the salmeterol groups, although the changes in SGRQ scores did not reach the threshold of 4 units considered clinically meaningful. Other trials also have shown improvements in symptoms (generally dyspnea or cough) and HRQOL in patients treated with FSC.36,42,47,50,54,72
Pharmacoeconomic evidence
While randomized clinical trials are important for their strong internal validity in interventional research, they are expensive to conduct and lack generalizability due to restrictive patient inclusion criteria. Observational, real-world studies using administrative claims data are an additional way to answer research questions. These have greater external validity and can be designed so as to reduce selection bias in the absence of randomization. In this section, we describe studies that have examined clinical and economic outcomes using observational, retrospective designs.
The direct costs of medical care for COPD patients are approximately double those for patients without COPD, and reducing exacerbations could significantly lower the economic burden of the disease.73 Retrospective analyses of healthcare claims data suggest that sizable cost savings might be achieved with FSC therapy, primarily through prevention of exacerbation-related hospitalizations.
A study using data from the UK General Practice Database found patients prescribed ICS and LABA had a 38% lower risk for a combined endpoint of hospitalization or death than patients prescribed ICS plus a short-acting beta-agonist (HR 0.62; 95% CI, 0.43 to 0.87).74 Similarly Soriano and colleagues reported that patients discharged with ICS and LABA had a 41% lower risk for rehospitalization or death in the following year compared to patients discharged with a short-acting beta-agonist.18 In the TORCH trial, however, annual hospital admission rates for exacerbations were similar in the FSC and salmeterol groups, but were 17% and 18% lower, respectively, than in the placebo group.54
Cost analyses comparing FSC to ipratropium (alone or in combination with albuterol) show cost benefits as well. Among managed care enrollees, those prescribed ICS plus LABA had a 47% lower risk for a COPD hospitalization (HR 0.533, 95% CI, 0.328 to 0.865) and a 36% lower risk for any respiratory admission compared to ipratropium therapy (HR 0.643; 95% CI, 0.512 to 0.808).75 Newly diagnosed COPD patients receiving initial maintenance therapy with FSC had a 32% lower risk for hospitalization/ED visit during the first 6 months of therapy compared with patients receiving ipratropium therapy (HR 0.685, 95% CI, 0.620 to 0.757), and a lower risk than patients receiving either fluticasone propionate or salmeterol.76 Two US studies, one in a Texas Medicaid population and one in a cohort of Medicare-eligible health plan members, reported 27% and 45% reductions in risk, respectively, for a COPD-related hospitalization or ED visit with FSC compared to ipratropium.73,77 In addition, these analyses found lower overall medical costs for FSC therapy even though FSC was associated with higher pharmacy costs than was ipratropium. A third study, however, found no difference in COPD-related costs between FSC and ipratropium.78
Prescribing considerations
In the United States, 250/50 μg FSC is approved for twice-daily maintenance therapy in COPD to improve lung function and reduce exacerbations.79 In Europe, the approved dose is 500/50 μg and its use in patients with milder COPD (FEV1 ≤ 60% of predicted pre-bronchodilator value and a history of exacerbations) has been approved based primarily on the TORCH study, which found that FSC is associated with a reduced rate of exacerbations, improved lung function and HRQOL, and possibly improved survival.34,54,56
Current evidence-based guidelines recommend the addition of inhaled corticosteroid (ICS) for symptomatic patients with moderate-to-severe disease (FEV1 < 50% predicted) and repeated exacerbations.1,2 It is suggested that a dose sufficient in strength to establish control be chosen, followed by a dose reduction, when appropriate, to a level that maintains control and minimizes deleterious effects. Although 3 exacerbations in 3 years is suggested as a threshold for considering more aggressive therapy, as with other illnesses, good judgment must be exercised in the clinical setting. How is one to treat a patient with FEV1 > 50% predicted and 2 exacerbations in the past year, or 1 very severe exacerbation? Another issue clinicians must consider is the prevention of repeat exacerbations. Should one start patients on FSC sooner, given the clinical evidence for its efficacy and safety in COPD? Or add FSC to other long-acting bronchodilators to slow, if not prevent, the inevitable progression of COPD? A weighing of the risks and benefits, including consideration of the patient’s symptoms and functional status, is especially necessary in treating patients with mild or moderate COPD.
Safety
Treatment with ICS/LABA is generally well tolerated. In most clinical trials, there has been good medication adherence and a lower withdrawal rate due to adverse events in the FSC group compared to the placebo and/or salmeterol groups.42,50–53,56 Oral candidiasis is the most frequent adverse event associated with ICS use. In a recent post hoc safety analysis of TORCH trial data, no significant differences in bone density were seen between the groups receiving study drugs and the placebo group.80 An increased risk of pneumonia has been associated with FSC use. In the largest and longest study to date, the three-year TORCH trial, a higher incidence of pneumonia was seen in the FSC (19.6%) and fluticasone propionate (18.3%) groups than in the salmeterol (13.3%) and placebo (12.3%) groups (all P < 0.001).54 However, there was not a corollary increase in pneumonia-related hospitalizations or deaths in the FSC group. The decrease in the exacerbation rate (−0.28 events/100 patients/year) was greater than the increase in pneumonia (+0.03 events/100 patients/year). A more recent post-hoc analysis of TORCH data reported 88 pneumonia events per 1000 treatment-years for FSC and 84 events per 1000 treatment-years for fluticasone propionate, compared with 52 events per 1000 treatment-years with salmeterol or placebo.81 Factors associated with increased risk for pneumonia included age ≥55 years, FEV1 < 50% predicted, COPD exacerbation in the prior year, a worse dyspnea score and body mass index <25 kg/m2. In addition, a 44-week trial comparing FSC to salmeterol reported that a greater incidence of pneumonia was associated with FSC use (4.5% vs 1.8%).42 The INSPIRE study, a trial of the effects of FSC versus tiotropium on exacerbation rates, found equivalent exacerbation outcomes, but a higher incidence of pneumonia in the FSC group (8% vs 4%; P < 0.01); however, overall mortality was significantly lower in the FSC group.82 The pneumonia data from some trials have been difficult to interpret because pneumonia events were not anticipated when the studies began and so the study protocols did not require that diagnosis be confirmed by chest X-ray. Interestingly, a recent meta-analysis of seven large clinical trials found no differences in pneumonia between patients receiving the ICS budesonide (with or without formoterol) and patients receiving other therapy; pneumonia occurred in 3% of patients in each group.83 Nevertheless, the possibility of a slightly increased risk of pneumonia with ICS or FSC therapy, especially in patients at increased risk for respiratory infection, warrants vigilance.
Multiple morbidities
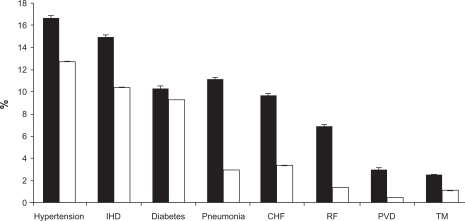
Patients seldom present only with COPD. Many patients have multiple morbidities including asthma, cardiovascular disease, hypercholesterolemia, hypertension, depression, cataracts, osteoporosis and diabetes (Figure 2).84 Many of these conditions are linked by common risk factors, most notably smoking, and COPD and cardiovascular disease are particularly closely related. Comorbid conditions may account for some of the deterioration in lung health seen in COPD and should be assessed and treated.85 Many patients with COPD are nutritionally depleted, and malnutrition is associated with poor prognosis; dietary counseling and nutritional support have been shown to improve airflow and quality of life.86,87 In the presence of multiple morbidities and comorbid conditions, treatment decisions must be based on the various risks and benefits relative to all of the patient’s health problems.80,85,88 Therapy for one condition, however, may be contraindicated in the presence of a second morbidity, complicating treatment decisions.
Figure 2.
Estimated prevalence of hospital discharges with selected comorbidities in patients with and without COPD.
Notes: Bars represent the age-adjusted percentage with SE bars. Black bars show patients with COPD (either as primary or secondary discharge diagnosis). White bars show patients without any mention of a COPD discharge diagnosis. The prevalence of all listed comorbidities is different across COPD categories (P < 0.01).
Abbreviations: IHD, ischemic heart disease; CHF, congestive heart failure; RF, respiratory failure; PVD, pulmonary vascular disease; TM, thoracic malignancy. Reprinted with permission from Holguin F, Folch E, Redd SC, Mannino DM. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest. 2005;128(4):2005–2011.84 Copyright © 2005 American College of Chest Physicians.
Given the high prevalence of osteoporosis in patients with COPD, concern has been raised about the long-term effects of ICS on bone density. A recent analysis of data from the TORCH trial found that 18% of men and 30% of women had osteoporosis and 42% of men and 41% of women had low bone density at baseline.80 However, no significant changes in bone mineral density were observed in any of the treatment arms (FSC, fluticasone propionate, salmeterol, placebo) during 3 years of follow-up. Rates of fractures across treatment groups were also low, with no differences detected between groups. Smoking, lack of calcium and vitamin D, and a sedentary lifestyle contribute significantly to low bone density, and these areas present opportunities for lowering osteoporosis risk in patients with COPD. Therapy for COPD with ICS should not necessarily be ruled out in the case of a patient with low bone density, as more aggressive therapy might improve functional status enough to permit a patient to be more active, not only reducing osteoporosis risk but impacting favorably on the course of COPD and other morbidities, such as hypertension and cardiovascular disease. With appropriate pharmacotherapy, a patient might also be better able to participate in pulmonary rehabilitation, an intervention that may not only improve physical functioning and symptom management, but reduce depression, a common problem in patients with COPD.89–91
There has also been concern that ICS therapy in COPD patients with diabetes may make glycemic control more difficult. However, a small study (N = 12) of the effect of ICS on glucose control in patients with COPD and diabetes found no significant change in hemoglobin A1c over 6 weeks of therapy.92 It appears prudent to carefully monitor patients for changes in glycemic control following ICS or FSC therapy.
Adherence issues
Finally, in making therapy decisions, treatment acceptance and adherence to treatment must be considered, since no medication regimen is effective if the medication is not taken. Increased adherence to COPD inhaled therapy is associated with improved mortality and reduced hospitalization.93 Patient adherence to treatment regimens in COPD is not optimal and treatment persistence is generally low for inhaled medications.94,95 Poor adherence can be related to overuse, underuse, or improper use of a medicine, and any of these problems may be sporadic or systematic, intentional or unintentional.94 Unintentional non-adherence is particularly an issue with inhaled medication; one study reported that only 43% of patients used a metered-dose inhaler (MDI) correctly, 55% used an MDI plus spacer correctly, and 59% used a dry powder inhaler correctly.96 Patients make mistakes even directly following face-to-face instruction, underscoring the importance of physician’s attentiveness to patients’ inhaler technique.95 As a routine part of office visits, adherence needs to be assessed in a neutral manner and addressed collaboratively to improve therapeutic outcomes.
The feasibility of using combination ICS/LABA therapy has improved with the availability of single inhaler devices that contain both medications, and this may also improve patient adherence, in part because salmeterol provides symptom relief, making it more likely that patients will comply with scheduled dosing regimens.97 In a 12-month period, patients with COPD who were prescribed FSC obtained more refills than patients prescribed salmeterol or ICS76 and in a second study of pharmacy claims, medication compliance was 12% higher with FSC than with ipratropium.78 Thus, when both ICS and LABA are needed, FSC delivered via a single inhaler may result in better treatment adherence.94,97
Just as important as optimizing patient adherence to pharmacotherapy is the need to bridge the existing gap between optimal management of COPD and actual practice. An existing hurdle to overcome is the frequent failure to recognize that smoker’s cough and bronchitis are in reality COPD and exacerbations of COPD.98 Simply put, if clinicians do not correctly identify the patient’s condition as COPD, there is little chance the disease will be treated according to existing guidelines for care. Use of a simple questionnaire may be beneficial for screening for COPD in primary care patients.98,99 In addition, increased awareness that the course of COPD and patient’s quality of life can be significantly improved by newer maintenance therapies and patient self-management education is needed. Undertreatment of COPD is common and being recognized.88 A study of Canadian patients with COPD found that 33% had never received a written action plan and 67% had not been told how to prevent an exacerbation.100 Several concerns about potential side effects with ICS have been allayed by new research, and there is a need to educate physicians about the benefits and risks of COPD maintenance therapies, including treatment with FSC, as well as the need for vigilance regarding untoward effects. Other barriers to optimal care, such as formulary decisions that limit the clinician’s ability to do what is best for the patient, will require health policy changes.
Lessons for the future
In appropriate patients, FSC is an effective therapy option that has been shown to reduce the frequency of exacerbations in patients with COPD, modestly improve lung function, and improve dyspnea, health status and quality of life. A range of studies suggests that fluticasone propionate and salmeterol have synergistic effects when administered together that improve their efficacy in controlling symptoms and reducing exacerbations. While clinical trials have clearly shown a benefit of FSC in preventing exacerbations in Stage III and IV COPD, there is also some evidence of benefit in patients with moderate COPD, and this topic warrants further research. Investigations of the optimal doses of FSC and comparative studies of the clinical and pharmacoeconomic benefits of FSC relative to other medications for COPD maintenance therapy are also needed. In particular, additional studies to clarify the potential value of triple therapy with FSC and tiotropium would be beneficial, and research in this area is underway.
For both the clinician and patient, the optimal scenario would be to have available a range of therapeutic options that meet the needs of patients with differing symptoms, lung function profiles, and comorbidities, that improve quality of life, and that minimize adherence issues and deleterious effects. Treatment with FSC within the context of COPD maintenance therapy is a useful, effective option in the clinician’s armamentarium, which based on evidence to date appears to have benefits for disease management, cost, adherence, and quality of life.
Footnotes
Disclosures
BPY has received research funding for COPD studies from GSK, Novartis and NIH. IR and AAD are employees of GlaxoSmithKline (GSK); AAD owns GSK company stock. JSH has received financial compensation from GSK for medical writing services and research funding for COPD studies from Boehringer Ingelheim, GSK, Pfizer Global Pharmaceuticals, and SmithKlineBeecham Pharmaceuticals.
References
- 1.Celli BR, MacNee W, ATS/ERS Task Force Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for Diagnosis, Management and Prevention of COPD. Updated 2009. Gig Harbor, WA: Medical Communications Resources, Inc; 2009. http://www.goldcopd.com [Google Scholar]
- 3.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(5):1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 5.Bourbeau J, Ford G, Zackon H, Pinsky N, Lee J, Ruberto G. Impact on patients’ health status following early identification of a COPD exacerbation. Eur Respir J. 2007;30(5):907–913. doi: 10.1183/09031936.00166606. [DOI] [PubMed] [Google Scholar]
- 6.Miravitlles M, Ferrer M, Pont A, et al. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax. 2004;59(5):387–395. doi: 10.1136/thx.2003.008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimura K, Sato S, Tsukino M, et al. Effect of exacerbations on health status in subjects with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2009;7:69. doi: 10.1186/1477-7525-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 9.Spencer S, Calverley PM, Burge PS, Jones PW. Impact of preventing exacerbations on health status in chronic obstructive pulmonary disease. Eur Respir J. 2004;23(5):698–702. doi: 10.1183/09031936.04.00121404. [DOI] [PubMed] [Google Scholar]
- 10.Donaldson GC, Wedzicha JA. COPD exacerbations: 1: epidemiology. Thorax. 2006;61(2):164–168. doi: 10.1136/thx.2005.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soler-Cataluna JJ, Martinez-Garcia MA, Roman-Sanchez PR, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J. 2003;21(Suppl 41):46s–53s. doi: 10.1183/09031936.03.00078002. [DOI] [PubMed] [Google Scholar]
- 13.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations in chronic obstructive pulmonary disease. N Engl J Med. 2002;347(7):465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 14.Sethi S, Sethi R, Eschberger K, et al. Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease. Am J Crit Clin Care Med. 2007;176(4):356–361. doi: 10.1164/rccm.200703-417OC. [DOI] [PubMed] [Google Scholar]
- 15.Baathorn E, Kerstjens H, Postma D, Timens W, MacNee W. Airways inflammation and treatment during acute exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2008;3(2):217–229. doi: 10.2147/copd.s1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donaldson GC, Seemungal TA, Patel IS, et al. Longitudinal changes in nature, severity and frequency of COPD exacerbations. Eur Respir J. 2003;22(6):931–936. doi: 10.1183/09031936.03.00038303. [DOI] [PubMed] [Google Scholar]
- 17.Hurst JR, Donaldson GC, Quint TK, Boldring JJ, Baghai-Ravary R, Wedzicha JA. Temporal clustering of exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179(5):369–374. doi: 10.1164/rccm.200807-1067OC. [DOI] [PubMed] [Google Scholar]
- 18.Soriano JB, Kiri VA, Pride NB, Vestbo J. Inhaled corticosteroids with/without long-acting beta-agonists reduce the risk of rehospitalization and death in COPD patients. Am J Respir Med. 2003;2(1):67–74. doi: 10.1007/BF03256640. [DOI] [PubMed] [Google Scholar]
- 19.Bahadori K, FitzGerald JM, Levy RD, Fera T, Swiston J. Risk factors and outcomes associated with chronic obstructive pulmonary disease exacerbations requiring hospitalizations. Can Respir J. 2008;16(4):e43–e49. doi: 10.1155/2009/179263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Int Med. 2005;143(11):798–808. doi: 10.7326/0003-4819-143-11-200512060-00008. [DOI] [PubMed] [Google Scholar]
- 21.Cole CS, Williams EB, Williams RD. Assessment and discharge planning for hospitalized older adults with delirium. Medsurg Nurs. 2006;15(2):71–76. [PubMed] [Google Scholar]
- 22.Gruffydd-Jones K. A national strategy for the management of chronic obstructive pulmonary disease in England: aiming to improve the quality of care for patients. Prim Care Respir J. 2008;17(Suppl 1):S1–S8. doi: 10.3132/pcrj.2008.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strassels SA, Smith DH, Sullivan SD, Mahajan PS. The costs of treating COPD in the United States. Chest. 2001;119(2):344–352. doi: 10.1378/chest.119.2.344. [DOI] [PubMed] [Google Scholar]
- 24.Niewoehner DE. The impact of severe exacerbations on quality of life and the clinical course of chronic obstructive pulmonary disease. Am J Med. 2006;119(10 Suppl 1):38–45. doi: 10.1016/j.amjmed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Stanford RH, Shen Y, McLaughlin T. Cost of chronic obstructive pulmonary disease in the emergency department and hospital. Treat Respir Med. 2006;5(5):343–349. doi: 10.2165/00151829-200605050-00005. [DOI] [PubMed] [Google Scholar]
- 26.Blanchette CM, Broder M, Ory C, Change E, Akazawa M, Dalal AA. Cost and utilization of COPD and asthma among insured adults in the US. Curr Med Res Opin. 2009;25:1385–1392. doi: 10.1185/03007990902875927. [DOI] [PubMed] [Google Scholar]
- 27.Shaya FT, Dongyi D, Akazawa MO, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest. 2008;134(1):14–19. doi: 10.1378/chest.07-2317. [DOI] [PubMed] [Google Scholar]
- 28.Simoni-Wastili L, Blanchette CM, Qian J, et al. Burden of chronic obstructive pulmonary disease in Medicare beneficiaries residing in long-term care facilities. Am J Ger Pharmacother. 2009;7(5):262–270. doi: 10.1016/j.amjopharm.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Reis AL, Bauldoff GS, Casaburi R, Mahler DA, Rochester CL, Herrerias C. Pulmonary rehabilitation. Joint ACCP/AACVR evidence-based clinical practice guidelines. Chest. 2007;131(5):4S–42S. doi: 10.1378/chest.06-2418. [DOI] [PubMed] [Google Scholar]
- 30.Sundararajan L, Balami J, Packham S.Effectiveness of outpatient pulmonary rehabilitation in elderly patients with chronic obstructive pulmonary disease J Cardiopulm Rehabil Prev 2009November25, doi: 101097/HCR0b013e3181be7c56 [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 31.Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272(19):1497–1505. [PubMed] [Google Scholar]
- 32.Wilkinson TMA, Donaldson GC, Hurst JR, Seemungal TAR, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169(12):1298–1303. doi: 10.1164/rccm.200310-1443OC. [DOI] [PubMed] [Google Scholar]
- 33.Broeders M, Sanchis J, Levy ML, Crompton GK, Dekhuijzen PN, for the ADMIT Working Group The ADMIT series – issues in inhalation therapy. 2) improving technique and clinical effectiveness. Prim Care Respir J. 2009;18(2):76–82. doi: 10.4104/pcrj.2009.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanania NA. The impact of inhaled corticosteroid and long-acting B-agonist combination therapy on outcomes in COPD. Pulm Pharmacol Ther. 2008;21(3):540–550. doi: 10.1016/j.pupt.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Keating GM, McCormack PL. Salmeterol/fluticasone propionate. A review of its use in the treatment of chronic obstructive pulmonary disease. Drugs. 2007;67(16):2383–2405. doi: 10.2165/00003495-200767160-00006. [DOI] [PubMed] [Google Scholar]
- 36.Hanania NA, Darken P, Horstman D, et al. The efficacy and safety of fluticasone propionate (250 microg)/salmeterol (50 microg) combined in the Diskus Inhaler for the treatment of COPD. Chest. 2003;124(3):834–843. doi: 10.1378/chest.124.3.834. [DOI] [PubMed] [Google Scholar]
- 37.Di Marco F, Milic-Emili J, Boveri B, et al. Effect of inhaled broncho-dilators on inspiratory capacity and dyspnoea at rest in COPD. Eur Respir J. 2003;21(1):86–94. doi: 10.1183/09031936.03.00020102. [DOI] [PubMed] [Google Scholar]
- 38.Calverley PMA. Reducing the frequency and severity of exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1(2):121–124. doi: 10.1513/pats.2306031. [DOI] [PubMed] [Google Scholar]
- 39.Oakley RH, Cidlowski JA. Homologous down regulation of the glucocorticoid receptor: the molecular machinery. Crit Rev Eukaryot Gene Expr. 1993;3(2):63–88. [PubMed] [Google Scholar]
- 40.Chung KF, Caramori G, Adcock IM. Inhaled corticosteroids as combination therapy with β-adrenergic agonists in airways disease: present and future. Eur J Clin Pharmacol. 2009;65(9):853–861. doi: 10.1007/s00228-009-0682-z. [DOI] [PubMed] [Google Scholar]
- 41.Barnes NC, Qiu Y-S, Pavord ID, et al. Anti-inflammatory effects of salmeterol/fluticasone propionate in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(7):736–743. doi: 10.1164/rccm.200508-1321OC. [DOI] [PubMed] [Google Scholar]
- 42.Kardos P, Wenchker M, Glaab T, Vogelmeier C. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(2):144–149. doi: 10.1164/rccm.200602-244OC. [DOI] [PubMed] [Google Scholar]
- 43.Hattotuwa KL, Gizycki MJ, Ansari TW, Jeffery PK, Barnes NC. The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease. Am J Resp Crit Care Med. 2002;165(12):1592–1596. doi: 10.1164/rccm.2105025. [DOI] [PubMed] [Google Scholar]
- 44.Gizycki MJ, Hattotuwa KL, Barnes N, Jeffery PK. Effects of fluticasone propionate on inflammatory cells in COPD: an ultrastructural examination of endobronchial biopsy tissue. Thorax. 2002;57(9):799–803. doi: 10.1136/thorax.57.9.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Confalonieri M, Mainardi E, Della Porta R, et al. Inhaled corticosteroids reduce neutrophilic inflammation in patients with chronic obstructive pulmonary disease. Thorax. 1998;53(7):583–585. doi: 10.1136/thx.53.7.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sin DD, Lacy P, York P, Man SFP. Effects of fluticasone on systemic markers of inflammation. Am J Respir Crit Care Med. 2004;170(7):160–165. doi: 10.1164/rccm.200404-543OC. [DOI] [PubMed] [Google Scholar]
- 47.Dal Negro RW, Pomari C, Tognella S, Micheletto C. Salmeterol and fluticasone 50 μg/250 μg bid in combination provides a better long-term control than salmeterol 50 μg bid alone and placebo in COPD patients already treated with theophylline. Pulm Pharmacol Ther. 2003;16(4):241–246. doi: 10.1016/s1094-5539(03)00065-8. [DOI] [PubMed] [Google Scholar]
- 48.Edwards M, Johnson M, Johnson S. Synergistic effects of salmeterol and fluticasone in human rhinovirus induced pro-inflammatory cytokine production [abstract] Am J Respir Crit Care Med. 2003;167:A399. [Google Scholar]
- 49.Seeto LJ, Burgess JK, Johnson PR, Black JL, Roth M, Lim S. Effect of fluticasone and salmeterol on human alveolar macrophage cytokine production in patients with chronic obstructive pulmonary disease (COPD) [abstract] Am J Respir Crit Care Med. 2003;167:A318. [Google Scholar]
- 50.Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomized clinical trial [Erratum in: Lancet. 2003;351(9369);1660] Lancet. 2003;361(9356):449–456. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 51.Ferguson GT, Anzueto A, Fei R, Emmett A, Knobil K, Kalberg C. Effect of fluticasone propionate/salmeterol (250/50 μg) or salmeterol (50 μg) on COPD exacerbations. Respir Med. 2008;102(8):1099–1108. doi: 10.1016/j.rmed.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 52.Mahler DA, Wire P, Horstman D, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(8):1084–1091. doi: 10.1164/rccm.2112055. [DOI] [PubMed] [Google Scholar]
- 53.Anzueto A, Ferguson GT, Feldman G, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD. 2009;6(5):320–329. doi: 10.1080/15412550903140881. [DOI] [PubMed] [Google Scholar]
- 54.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 55.Lapperre TS, Snoeck-Stroband JB, Gosman MME, et al. the GLU-COLD (Groningen Leiden Universities Corticosteroids in Obstructive Lung Disease) Study Group Effect of fluticasone with and without salmeterol on pulmonary outcomes in chronic obstructive pulmonary disease. Ann Int Med. 2009;151(8):517–527. doi: 10.7326/0003-4819-151-8-200910200-00004. [DOI] [PubMed] [Google Scholar]
- 56.Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in COPD: results from the TORCH Study. Am J Respir Crit Care Med. 2008;178(4):322–323. doi: 10.1164/rccm.200712-1869OC. [DOI] [PubMed] [Google Scholar]
- 57.Wouters EF, Postma DS, Fokkens B, et al. for the COSMIC (COPD and Seretide: a Multi-Center Intervention and Characterization) Study Group Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trial. Thorax. 2005;60(6):480–487. doi: 10.1136/thx.2004.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alsaeedi A, Sin DD, McAlister FA. The effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review of randomized placebo-controlled trials. Am J Med. 2002;113(1):59–65. doi: 10.1016/s0002-9343(02)01143-9. [DOI] [PubMed] [Google Scholar]
- 59.Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320(7245):1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lung Health Study Research Group Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 343(26):1902–1909. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- 61.Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingsworth K, Efthimiou J. Multicenter randomized placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. [Erratum in: Lancet. 1998;351(9120):1968] Lancet. 1998;351(9105):773–780. doi: 10.1016/s0140-6736(97)03471-5. [DOI] [PubMed] [Google Scholar]
- 62.Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Resp J. 2003;21(5):74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- 63.Jenkins CR, Jones PW, Calverley PM, et al. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomized, placebo-controlled TORCH study. Resp Res. 2009;10:59. doi: 10.1186/1465-9921-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Young J. Understanding statistical analysis in the surgical literature: some key concepts. ANZJ Surg. 2009;79(5):398–403. doi: 10.1111/j.1445-2197.2009.04900.x. [DOI] [PubMed] [Google Scholar]
- 65.Sin DD, Wu L, Anderson JA, et al. Inhaled corticosteroids and mortality in chronic obstructive pulmonary disease. Thorax. 2005;60(12):992–997. doi: 10.1136/thx.2005.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mapel DW, Hurley JS, Roblin D, et al. Survival of COPD patients using inhaled corticosteroids and long-acting beta agonists. Respir Med. 2006;100(4):595–609. doi: 10.1016/j.rmed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 67.Mapel DW, Nelson LS, Lydick E, Soriano J, Yood MU, Davis KJ. Survival among COPD patients using fluticasone/salmeterol in combination versus other inhaled steroids and bronchodilators alone. COPD. 2007;4(2):127–134. doi: 10.1080/15412550701341111. [DOI] [PubMed] [Google Scholar]
- 68.Sin DD, Tu JV. Inhaled corticosteroids and the risk of mortality and readmission in elderly patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(4):580–584. doi: 10.1164/ajrccm.164.4.2009033. [DOI] [PubMed] [Google Scholar]
- 69.Soriano JB, Vestbo J, Pride NB, Kiri V, Maden C, Maier WC. Survival in COPD patients after regular use of fluticasone propionate and salmeterol in general practice. Eur Respir J. 2002;20(4):819–825. doi: 10.1183/09031936.02.00301302. [DOI] [PubMed] [Google Scholar]
- 70.Fan VS, Bryson CL, Curtis JR, et al. Inhaled corticosteroids in chronic obstructive pulmonary disease and risk of death and hospitalization: time-dependent analysis. Am J Respir Crit Care Med. 2003;168(12):1488–1494. doi: 10.1164/rccm.200301-019OC. [DOI] [PubMed] [Google Scholar]
- 71.Aaron SD, Vandemheen KL, Clinch JJ, et al. Measurement of short-term changes in dyspnea and disease-specific quality of life following an acute COPD exacerbation. Chest. 2002;121(3):688–696. doi: 10.1378/chest.121.3.688. [DOI] [PubMed] [Google Scholar]
- 72.Zheng JP, Yang L, Wu YM, et al. The efficacy and safety of combination salmeterol (50 μg)/fluticasone propionate (500 μg) inhalation twice daily via Accuhaler in Chinese patients with COPD. Chest. 2007;132(6):1756–1763. doi: 10.1378/chest.06-3009. [DOI] [PubMed] [Google Scholar]
- 73.Rascati KL, Akazawa M, Johnsrud M, Stanford RH, Blanchette CM. Comparison of hospitalizations, emergency department visits, and the associated costs in a historical cohort of Texas Medicaid patients with chronic obstructive pulmonary disease, by initial medication regimen. Clin Ther. 2007;29(6):1203–1213. doi: 10.1016/j.clinthera.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 74.Kiri VA, Bettoncelli G, Testi R, Viegi G. Inhaled corticosteroids are more effective in COPD patients when used with LABA than with SABA. Respir Med. 2005;99(9):1115–1124. doi: 10.1016/j.rmed.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 75.Anzueto A, McLaughlin T, Stanford RH. Initial treatment regimen and risk of hospitalization in patients with chronic obstructive pulmonary disease. COPD. 2004;1(2):205–214. doi: 10.1081/copd-120039808. [DOI] [PubMed] [Google Scholar]
- 76.Akazawa M, Hayflinger DC, Stanford RH, Blanchette CM. Economic assessment of initial maintenance therapy for chronic obstructive pulmonary disease from a managed care perspective. Am J Managed Care. 2008;14(7):21–32. [PubMed] [Google Scholar]
- 77.Blanchette CM, Akazawa M, Dalal A, Simoni-Wastila L. Risk of hopitalizations/emergency department visits and treatment costs associated with initial maintenance therapy using fluticasone propionate 500 microg/salmeterol 50 microg compared with ipratropium for chronic obstructive pulmonary disease in older adults. Am J Geriatr Pharmacother. 2008;6(3):138–146. doi: 10.1016/j.amjopharm.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 78.Delea TE, Hagiwara M, Dalal AA, Stanford RH, Blanchette CM. Healthcare use and costs in patients with chronic bronchitis initiating maintenance therapy with fluticasone/salmeterol vs other inhaled maintenance therapies. Curr Med Res Opin. 2009;35(1):1–13. doi: 10.1185/03007990802534020. [DOI] [PubMed] [Google Scholar]
- 79.Advair DISKUS [package insert] Research Triangle Park, NC: Glaxo-SmithKline; Apr, 2009. [Google Scholar]
- 80.Ferguson GT, Calverley PM, Anderson JA, et al. Prevalence and progression of osteoporosis in patients with COPD. Results from TORCH. Chest. 2009;136(6):1456–1465. doi: 10.1378/chest.08-3016. [DOI] [PubMed] [Google Scholar]
- 81.Crim C, Calverley PM, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination. TORCH study results. Eur Resp J. 2009;34(3):641–647. doi: 10.1183/09031936.00193908. [DOI] [PubMed] [Google Scholar]
- 82.Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA, for the INSPIRE Investigators The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- 83.Sin DD, Tashkin D, Zhang X, et al. Budesonide and the risk of pneumonia: a meta-analysis of individual patient data. Lancet. 2009;374(9691):712–719. doi: 10.1016/S0140-6736(09)61250-2. [DOI] [PubMed] [Google Scholar]
- 84.Holguin F, Folch E, Redd SC, Mannino DM. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest. 2005;128(4):2005–2011. doi: 10.1378/chest.128.4.2005. [DOI] [PubMed] [Google Scholar]
- 85.Tan SL, Wood AM. Chronic obstructive pulmonary disease and comorbidity: a review and consideration of pathophysiology. Panminerva Med. 2009;51(2):81–93. [PubMed] [Google Scholar]
- 86.Weekes CE, Emery PW, Elia M. Dietary counselling and food fortification in stable COPD: a randomised trial. Thorax. 2009;64(4):326–331. doi: 10.1136/thx.2008.097352. [DOI] [PubMed] [Google Scholar]
- 87.Planas M, Alvarez J, Garcia-Peris PA, et al. Nutritional support and quality of life in stable chronic obstructive pulmonary disease (COPD) patients. Clin Nutr. 2005;24(3):433–441. doi: 10.1016/j.clnu.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 88.Barr RG, Celli BR, Mannino DM, et al. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am J Med. 2009;122(4):348–355. doi: 10.1016/j.amjmed.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coventry PA. Does pulmonary rehabilitation reduce anxiety and depression in chronic obstructive pulmonary disease? Curr Opin Pulm Med. 2009;15(2):143–149. doi: 10.1097/MCP.0b013e3283218318. [DOI] [PubMed] [Google Scholar]
- 90.Omachi TA, Katz PP, Yelin EH, et al. Depression and health-related quality of life in chronic obstructive pulmonary disease. Am J Med. 2009;122(8):778.e9–e15. doi: 10.1016/j.amjmed.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schneider C, Jick SS, Bothner U, Meier CR.Chronic obstructive pulmonary disease and the risk of depression Chest 2009October3;doi: 101378/chest09-0614 [Epub ahead of print]. [Google Scholar]
- 92.Faul JL, Wilson SR, Chu JW, Canfield J, Kuschner WG. The effects of an inhaled corticosteroid on glucose control in type 2 diabetes. Clin Med Res. 2009;7(1–2):14–20. doi: 10.3121/cmr.2009.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vestbo J, Anderson JA, Calverley P, et al. Adherence to inhaled therapy, mortality, and hospital admission in COPD. Thorax. 2009;64(11):939–943. doi: 10.1136/thx.2009.113662. [DOI] [PubMed] [Google Scholar]
- 94.Cramer JA, Bradley-Kennedy C, Scalera A. Treatment persistence and compliance with medications for chronic obstructive pulmonary disease. Can Respir J. 2007;14(1):25–29. doi: 10.1155/2007/161652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Restrepo RD, Alvarez MT, Wittnebel LD, et al. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulm Dis. 2008;3(3):371–384. doi: 10.2147/copd.s3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brocklebank D, Ram F. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Tech Assess. 2001;5(26):1–149. doi: 10.3310/hta5260. [DOI] [PubMed] [Google Scholar]
- 97.Cazzola M, Hanania NA. The role of combination therapy with corticosteroids and long-acting beta-2-agonists in the prevention of exacerbations in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(4):345–354. doi: 10.2147/copd.2006.1.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yawn BP, Keenan JM. COPD – the primary care perspective: addressing epidemiology, pathology, diagnosis, treatment of smoking’s multiple morbidities and the patient’s perspective. COPD. 2007;4(1):67–83. doi: 10.1080/15412550601169562. [DOI] [PubMed] [Google Scholar]
- 99.Price DB, Tinkelman DG, Nordyke RJ, Isonaka S, Halbert RJ, COPD Questionnaire Study Group Scoring system and clinical application of COPD diagnostic questionnaires. Chest. 2006;129(6):1531–1539. doi: 10.1378/chest.129.6.1531. [DOI] [PubMed] [Google Scholar]
- 100.Hernandez P, Balter M, Bourbeau J, Hodder R. Living with chronic obstructive pulmonary disease: a survey of patients’ knowledge and attitudes. Respir Med. 2009;103(7):1004–1012. doi: 10.1016/j.rmed.2009.01.018. [DOI] [PubMed] [Google Scholar]