Abstract
Filtrates from crushed Moringa oleifera seeds were tested for their effects on growth and Photosystem II efficiency of the common bloom-forming cyanobacterium Microcystis aeruginosa. M. aeruginosa populations exhibited good growth in controls and treatments with 4- and 8-mg crushed Moringa seeds per liter, having similar growth rates of 0.50 (±0.01) per day. In exposures of 20- to 160-mg crushed Moringa seeds L−1, growth rates were negative and on average −0.23 (±0.05) .day−1. Presumably, in the higher doses of 20- to 160-mg crushed seeds per liter, the cyanobacteria died, which was supported by a rapid drop in the Photosystem II efficiency (ΦPSII), while the ΦPSII was high and unaffected in 0, 4, and 8 mg L−1. High-density populations of M. aeruginosa (chlorophyll-a concentrations of ∼270 µg L−1) were reduced to very low levels within 2 weeks of exposure to ≥80-mg crushed seeds per liter. At the highest dosage of 160 mg L−1, the ΦPSII dropped to zero rapidly and remained nil during the course of the experiment (14 days). Hence, under laboratory conditions, a complete wipeout of the bloom could be achieved. This is the first study that yielded evidence for cyanobactericidal activity of filtrate from crushed Moringa seeds, suggesting that Moringa seed extracts might have a potential as an effect-oriented measure lessening cyanobacterial nuisance.
Keywords: Anti-microbial activity, Cyanobacteria, Microcystis aeruginosa, Lake management, Lake restoration, Water treatment
Introduction
The explosive growth of the world’s human population and subsequent water and energy demand has led to an expansion of standing surface water (Rosenberg et al. 2000). Many of these standing waters, including small watering ponds for cattle, fishing ponds, irrigation impoundments, and drinking water reservoirs, suffer from an over-enrichment of the water with nutrients (Smith and Schindler 2009). Such ongoing eutrophication may lead to dense cyanobacterial blooms and floating scums (Schindler et al. 2008; Smith and Schindler 2009). These blooms may cause high turbidity, anoxia, fish kills, bad smell (Paerl and Huisman 2008), and serious environmental and human health problems because several cyanobacteria can produce a variety of very potent toxins (e.g., Codd et al. 2005; Dittmann and Wiegand 2006).
Climate change is expected to aggravate hazardous blooms (Paerl and Huisman 2008), while safe and aesthetically acceptable water is a growing need in a modern society (Steffensen 2008). Hence, water resource management is challenged worldwide to reduce the vulnerability to the threats of harmful cyanobacterial blooms. Particularly in developing countries where the high costs of chemicals for water and wastewater treatment might limit their application, the development of cost-effective and environmentally acceptable mitigating measures is desirable. In that context, water clarification and disinfection with natural products, such as the seeds of the pan-tropical tree, Moringa oleifera Lam, is of particular interest.
Crushed M. oleifera seeds are highly effective in clarifying very turbid water (Muyibi and Evison 1995; Ndabigengesere and Narasiah 1998). Traditionally, the seeds are used in rural areas of Sudan and Malawi for the clarification of drinking water (Muyibi and Evison 1995; Anwar et al. 2007). Flocculation of raw Nile water revealed that M. oleifera seeds could remove up to 97% of the algae present (Shehata et al. 2002).
Dehusked M. oleifera press cake is efficient in the removal of hydrophobic organic pollutants from water (Boucher et al. 2007), and extracts might remove other pollutants, such as heavy metals and surfactants (Beltrán-Heredia and Sánchez-Martín 2008, 2009). M. oleifera pods are efficient in absorbing organic pollutants and pesticides (Akhtar et al. 2007a, b). High-quality activated carbon can be prepared from the waste husks of M. oleifera (Pollard et al. 1995; Warhurst et al. 1997a), which could effectively remove up to 98% of the cyanobacterial microcystin-LR (Warhurst et al. 1997b). In addition to the strong water-clarifying properties, Moringa seeds have also been reported removing more than 90% of cercariae from the water phase (Olsen 1987). Bacterial numbers can be reduced drastically due to coagulation (Madsen et al. 1987), and a pronounced hygienic effect is caused by a strong antibacterial potential against Gram-negative and Gram-positive bacteria (Suarez et al. 2003, 2005). However, so far, no study has examined direct effects of filtrates from crushed M. oleifera seeds on growth of cyanobacteria and on their ability in terminating cyanobacterial blooms.
The purposes of this research were to (1) examine in the laboratory the effect of filtrates from crushed M. oleifera seeds on the growth and Photosystem II efficiency of the most common bloom-forming cyanobacterium in eutrophic freshwater systems worldwide, Microcystis aeruginosa Kützing and (2) to test the filtrates on their potential to mitigate high densities of M. aeruginosa. Inasmuch as M. oleifera seed extract exerts bactericidal activity in vitro against Gram-positive and Gram-negative bacteria (Cáceres et al. 1991; Ali et al. 2004; Oludoro and Aderiye 2007; Suarez et al. 2003, 2005) and cyanobacteria are Gram-negative (Stanier and Cohen-Bazire 1977), we hypothesize that Moringa seed extracts have an antimicrobial activity against the cyanobacterium M. aeruginosa.
Materials and methods
The cyanobacterium Microcystis aeruginosa NIVA-CYA 43 was obtained from the Norwegian Institute for Water Research (NIVA, Norway). Stock cultures of this strain were grown in our laboratory in 100-mL Erlenmeyer flasks containing 50 mL of slightly modified Woods Hole Chu (WC) medium (Lürling and Beekman 1999) with vitamins added (H (biotin) and B12 (cyanocobalamin) at 50 ng L−1 and B1 (thiamine HCL) at 100 ng L−1). The flasks were placed in a Gallenkamp ORBI-SAFE Netwise Orbital Incubator at 22°C in 40 rpm and in a 18:6-h light/dark cycle. The light/dark cycle was programmed that light intensity increased gradually to a maximum of 130 µmol quanta m−2 s−1 and subsequently decreased again to darkness, which resulted in a daily average light intensity of ∼57 µmol quanta m−2 s−1. This strain of M. aeruginosa is completely uni- and bicellular under the given growth conditions.
Seeds of M. oleifera were obtained from the Miracle Trees Foundation (Rotterdam, the Netherlands). The seed coat was removed and the pulp crushed in a mortar. Aliquots of the powder were transferred into pre-weighed aluminum boats in fivefold. Dry weights were determined after 24 h at 105°C, and ash weights were determined after placing the boats for 3 h at 550°C.
Two grams of the powder was put into 250 mL sterile WC medium, shaken for 5 min, and filtered through a 0.45-µm membrane filter. The filtrate was used in the experiments, had a pH of 7.05–7.10, and was analyzed for dissolved nutrients (ammonium-N, nitrate/nitrite-N, and phosphate-P) using a SKALAR autoanalyzer. Aliquots of the filtrate were analyzed for total dissolved organic carbon content using a TOC analyzer (model 700, OI-Analytical). Turbidity (in NTU) of the filtrate was measured using a Hach 2100P turbidity meter. Absorption was determined over the range 350–750 nm PAR in 1-nm intervals using a 5-cm cuvette that was placed in a Beckman Coulter Du730 Life Sciences UV/VIS spectrophotometer.
Experimental procedure
Two experiments were performed to study the effect of filtrate from crushed M. oleifera seeds on the growth of M. aeruginosa (experiment 1) and on a cultured bloom of M. aeruginosa (experiment 2).
In the first experiment, filtrate from crushed M. oleifera seeds was added to 50 mL autoclaved WC medium in sterile 100-mL Erlenmeyer flasks. To three replicates, the following amounts of filtrate were added: 0, 25, 50, 125, 250, 500, and 1,000 μL, which corresponds to 0, 4, 8, 20, 40, 80, and 160 mg crushed Moringa per liter. An inoculum of M. aeruginosa was added to each Erlenmeyer flask with an initial chlorophyll-a (Chl-a) concentration of 13.4 (±0.1) µg L−1. The flasks were closed with a cellulose plug and placed at random in a Gallenkamp ORBI-SAFE Netwise orbital incubator under the same conditions as outlined above.
Samples were taken initially and after 2, 4, 7, 9, and 11 days and were analyzed on the Chl-a concentration and the Photosystem II efficiency (ΦPSII) using a PhytoPAM phytoplankton analyzer (Heinz Walz GmbH, Germany). Growth rates were determined by linear regression on natural logarithm transformed chlorophyll-a data. Growth rates were statistically evaluated using a one-way ANOVA in the program SPSS version 16.0. Significant differences were determined with a Tukey post hoc comparison test (P < 0.05).
In the second experiment, aliquots of M. aeruginosa cultures that had been grown for 3 weeks reaching chlorophyll-a concentrations of 3,500 (±15) µg L−1 were transferred to sterile 100-mL Erlenmeyer flasks and mixed with filtrate from the crushed Moringa seeds and WC medium such that the final volume was 50 mL. The initial Chl-a concentration was 269 (±4) µg L−1, reflecting a bloom of M. aeruginosa. The amounts of filtrate tested were 0, 10, 100, 500, and 1,000 μL, which corresponds to 0, 1.6, 16, 80, and 160 mg Moringa per liter. Samples were taken initially and after 1, 2, 3, 6, 8, 10, and 14 days and analyzed on the Chl-a concentration and the Photosystem II efficiency. In addition, after 2 days, subsamples were analyzed on the number of particles, biovolume, and mean particle volume using a CASY cell counter (Schärfe System Gmbh., Germany).
Results
Moringa seeds and filtrate
The crushed pulp of Moringa seeds contained 65 (±3) mg water g−1, and the organic content was 96.3% (±0.1%, n = 5). Mixing 2 g of the pulp powder with 250 mL sterile WC medium yielded a turbid white suspension (>1,000 NTU). However, filtration through a 0.45-µm membrane filter removed the turbidity. This filtrate of the 8 g L−1 suspension had a clear appearance and a turbidity of 1.88 (±0.52) NTU (n = 4), while the WC medium had a turbidity of 0.44 (±0.32) NTU (n = 4). Where the absorption coefficients in the PAR waveband were higher in the stock filtrate than in WC medium, absorption in controls (WC medium) and the highest dose of filtrate from the crushed Moringa seeds were similar (Fig. 1).
Fig. 1.
Light absorption (per meter) of algal growth medium (WC medium), algal growth medium with the highest dosage of filtrate from crushed M. oleifera seeds used in the experiments (160 mg L−1; 2.73 mg DOC L−1), and of filtrate from the stock (8,000 mg L−1; 137 mg DOC L−1), over the PAR wave band (350–750 nm)
The filtrate from crushed Moringa seeds (8 g L−1) contained on average 878 (±13) µg PO4-P L−1 (n = 4), 292 (±254) µg NO3/NO2-N L−1 (n = 4), and 810 µg NH4–N L−1 (n = 2). The carbon content of the filtrate was 136.7 (±0.5) mg DOC L−1 (n = 3). Hence, the treatments with filtrates comparable to 0, 4, 8, 20, 40, 80, and 160 mg crushed Moringa L−1 contained about 0, 0.07, 0.14. 0.34, 0.68, 1.37, and 2.73 mg DOC L−1, respectively.
Effect of Moringa seeds on growth of Microcystis
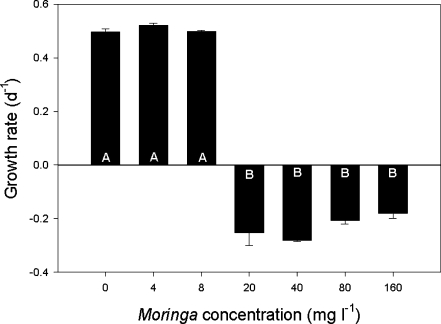
In the absence and lowest dosage of crushed Moringa seeds (4 mg L−1), Chl-a concentrations of M. aeruginosa increased strongly over time, reflecting good growth (Fig. 2a). At 8 mg L−1, this increase was somewhat reduced, while a decrease was observed at Moringa seed concentrations of 20 mg L−1 and more (Fig. 2a). The repeated measure ANOVA revealed that the course of the Chl-a concentrations over time was significantly different between treatments (F 6,14 = 700.2, P < 0.001). Tukey’s test showed three homogeneous groups: (1) 0 and 4 mg L−1, (2) 8 mg L−1, and (3) 20, 40, 80, and 160 mg L−1. Growth rates, however, were similar and on average 0.50 (±0.01) per day in the 0-, 4-, and 8-mg L−1 treatments (Fig. 3). In exposures of 20- to 160-mg Moringa seed per liter, growth rates were not only significantly lower (F 6,14 = 33.2, P < 0.001) but also negative with on average −0.23 (±0.05) per day, indicating that the inoculum did not grow and even declined (Fig. 3).
Fig. 2.
Effects of filtrates from crushed M. oleifera seeds (0–160 mg L−1) on the chlorophyll-a concentrations (a) and Photosystem II efficiency (b) of M. aeruginosa populations. Error bars = SD, n = 3
Fig. 3.
Growth rates (per day) of M. aeruginosa populations that were exposed to different concentrations of filtrate from crushed M. oleifera seeds (0–160 mg L−1). Error bars = SD, n = 3. Similar letters (A, B) indicate homogeneous groups that are not different at the P = 0.05 level (Tukey’s test)
The ΦPSII was high and unaffected in 0, 4, and 8 mg L−1, but dropped to zero rapidly in exposures of 20- to 160-mg Moringa seed per liter (Fig. 2b). Where over the course of the experiment the ΦPSII remained zero in the 40- to 160-mg Moringa seed per liter treatments, it showed recovery in the 20-mg L−1 treatments (Fig. 2b). The repeated measure ANOVA revealed that the course of the ΦPSII over time was significantly different between treatments (F 6,14 = 881.3, P < 0.001). Tukey’s test showed three homogeneous groups: (1) 0, 4, and 8 mg L−1, (2) 20 mg L−1, and (3) 40, 80, and 160 mg L−1.
Effect of Moringa seeds on Microcystis under blooming conditions
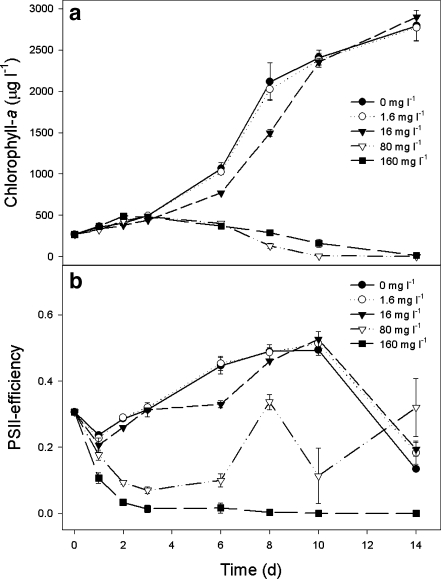
In concentrations of ≤16 mg L−1, filtrate from crushed Moringa seeds had no effect on the high-density M. aeruginosa cultures (Fig. 4). Exposure to concentrations of 0–16 mg L−1 resulted in 2 weeks in more than a tenfold increase in Chl-a concentrations (Fig. 4a). In contrast, in the 80- and 160-mg L−1 treatments, Chl-a concentrations dropped to 1% and 5% of that of the initial bloom, respectively (Fig. 4a). A Tukey’s test, following a repeated measure ANOVA (F 4,10 = 1123, P < 0.001), revealed three homogeneous groups that were all significantly different from each other: (1) 0 and 1.6 mg L−1, (2) 16 mg L−1, and (3) 80 and 160 mg L−1.
Fig. 4.
The course of chlorophyll-a concentrations (a) and Photosystem II efficiency (b) in M. aeruginosa populations in high, blooming densities that were exposed to different concentrations of filtrate from crushed M. oleifera seeds (0–160 mg L−1). Error bars = SD, n = 3
The ΦPSII dropped to and remained zero at the highest dosage of 160 mg L−1. In 80 mg L−1, the ΦPSII also showed and initial decline, but never reached zero and recovered over the course of the experiment (Fig 4b). In the 0- to 16-mg L−1 treatments, the course of the ΦPSII was similar, which was confirmed by a Tukey test indicating that the 0-, 1.6-, and 16-mg L−1 treatments formed one homogeneous group (repeated measure ANOVA, between-subjects effects; F 4,10 = 445.8, P < 0.001). In the 80- and 160-mg L−1 treatments, the ΦPSII was significantly lower than in 0–16 mg L−1; the 80- and 160-mg L−1 treatments also differed significantly from each other (Fig. 4b).
Inasmuch as the Chl-a concentrations increased in the first 2 days while concomitantly the ΦPSII dropped to zero, subsamples were analyzed with a cell counter. The number of particles and the total biovolume were significantly lower in the 80- and 160-mg L−1 treatments (F 4,10 = 45.1, P < 0.001 and F 4,10 = 18.7, P < 0.001, respectively) than in the 0- to 16-mg L−1 treatments (Fig. 4). The mean particle volumes were similar (F 4,10 = 2.13, P = 0.151) among controls and treatments (Fig. 5).
Fig. 5.
Biovolume (black bars, µm3 mL−1), number of particles (white bars, mL−1), and mean particle volume (µm3) in M. aeruginosa populations after 2 days of exposure to different concentrations of filtrate from crushed M. oleifera seeds (0–160 mg L−1). Error bars = SD, n = 3. Similar letters (a, b and α, β, χ) indicate homogeneous groups that are not different at the P = 0.05 level (Tukey’s test)
Discussion
The results of the current study clearly revealed that filtrate from crushed Moringa seeds inhibited the growth of the common cyanobacterium M. aeruginosa. Above 20 mg L−1 (≈0.34 mg DOC per liter), the Chl-a concentrations declined, which was not caused by sedimentation as flasks were shaken. The EC50 here was between 8 and 20 mg L−1, which is considerably lower than the EC50 of 287.5 mg L−1 for the green alga Scenedesmus obliquus (Ali et al. 2004). In that study, the green alga expressed growth in all concentrations tested and reached, after 10 days, even in 300-mg L−1 seed extract Chl-a concentrations of around 1,100 µg L−1 (Ali et al. 2004). In contrast, in the current study , Chl-a concentrations of M. aeruginosa populations became less than the inoculum in Moringa seed extract concentrations of 20 mg L−1 and more. The decline suggests a cyanobactericidal activity, which is corroborated by the ΦPSII measurements that showed a rapid drop to zero. The cyanobacteria died in the treatments where they were exposed to 40 mg L−1 or more, but the ΦPSII showed a recovery in the 20-mg L−1 treatments that was coincided by a slight recovery in chlorophyll-a concentrations (e.g., 1.1 and 3.1 µg L−1 on days 7 and 11, respectively). Similar observations on a transitory inhibition have been made in some bacteria that resumed growth after some period of inhibition (Madsen et al. 1987; Suarez et al. 2003; Oludoro and Aderiye 2007).
The addition of filtered Moringa seed extract to the cyanobacterial cultures implied higher DOC concentrations. DOC can markedly affect the light attenuation in lakes and might be a major light-absorbing component in the blue part of the spectrum, below 500 nm (Markager and Vincent 2000). In most natural freshwaters, DOC concentrations may range between 0.5 and 50 mg L−1, but sometimes go up to a few hundreds of milligrams per liter (Steinberg 2003). In this study, the maximum DOC concentration used was 2.73 mg L−1, which is not sufficient for having an effect on the light attenuation (Jones 1992). The turbidity of the highest amount of filtrate added was slightly higher than the background turbidity, and spectral analysis clearly revealed that absorption over the PAR wave band was similar in controls and treatments with the highest dosage of filtrate (see Fig. 1). Hence, physical effects of the DOC can be excluded as a causal factor in the observed inhibition of the cyanobacterium M. aeruginosa.
Another characteristic of DOC is that it might act as a weak herbicide in a range of 0.025–25 mg DOC per liter by interfering within the photosynthetic electron transport chain and reducing the oxygen release (Steinberg 2003; Prokhotskaya and Steinberg 2007). Also in this study, the filtrate from Moringa seed extract had an effect on the photosynthetic apparatus as reflected in the PAM-derived ΦPSII. Phenolic compounds are asserted as being major players in these algicidal effects in both natural organic matter and leachates from straw and leaf litter (Steinberg 2003). Especially barley straw extracts have received considerable attention: 50% growth inhibition of M. aeruginosa at concentrations between 70 and 230 mg L−1 has been reported (Martin and Ridge 1999). Other studies reported only 5% growth of M. aeruginosa at 2.57 mg L−1 (Newman and Barrett 1993) and toxicity above 35 mg L−1 (Waybright et al. 2009). The EC50 values (8–20 mg L−1) in the current study for Moringa seed extracts might be comparable to those of barley straw extracts, but a striking difference seems the lethal effect of Moringa seed extracts to M. aeruginosa at slightly elevated concentrations (>40 mg L−1), while this is generally not being observed for barley straw extracts.
The concentrations at which growth of M. aeruginosa was inhibited by Moringa are also in the same order of magnitude as those (20–50 mg L−1) that have been reported for bacteria (Oludoro and Aderiye 2007). Although reports on the antimicrobial effects of M. oleifera are still rare (Anwar et al. 2007), several studies have shown that M. oleifera seed extract exerts bactericidal activity in vitro against Gram-positive and Gram-negative bacteria (Cáceres et al. 1991; Ali et al. 2004; Oludoro and Aderiye 2007). This study is the first one reporting a cyanobactericidal activity, which is in line with the findings of others as cyanobacteria are Gram-negative bacteria. Initially, the antimicrobial activity of Moringa seeds was attributed to the presence of 4-α-l-rhamnosyloxy benzyl isothiocyanate, the only known glycosidic mustard oil (Eilert et al. 1981). However, later studies revealed that the Moringa seed-derived polypeptide (Flo), which is responsible for the sedimentation, also mediated bacterial disinfection (Suarez et al. 2003). In a follow-up study, Suarez et al. (2005) showed that the antibacterial activity of Flo did not simply result from a cell aggregation mechanism but that it required additional activities leading to bacterial membrane damage.
The current study also showed that a bloom of M. aeruginosa could be terminated using high concentrations (≥80 mg L−1 or 1.37 mg DOC per liter) of Moringa seed extract. However, at 80 mg L−1, the ΦPSII did not drop to zero as had been observed in the growth experiment. Most likely, it reflects a cyanobacteria biomass-dependent efficacy of the cyanobactericidal compounds where higher concentrations of cyanobacteria, such as under blooming conditions, might inactivate all the compounds by binding to the membranes, thereby leaving a portion of the cells unexposed. Hence, at higher cyanobacteria densities, more seed extract is needed to obtain a complete wipeout of the bloom. High concentrations of Moringa seed extract might, however, come with some serious drawbacks. One drawback of using high amounts of Moringa seed extract is that organic matter from the seeds is released into the water that can cause odor, taste, and color problems (Ndabigengesere and Narasiah 1998) and might also facilitate the development of microorganisms after some time (Madsen et al. 1987; Suarez et al. 2003; Oludoro and Aderiye 2007). Another drawback is that Moringa seed extracts contain nutrients that might promote cyanobacteria. In this study, filtrate from 1-g crushed Moringa seeds per liter yielded approximately 110 µg P L−1, which is lower than values of 1.94 mg P g−1 (Kalogo et al. 2000) and 1.31 mg P g−1 (Ndabigengesere and Narasiah 1998) found in other studies. In the latter study, a dosage of 50 mg L−1 caused an increase in phosphate from 0.4 to 1.6 mg L−1 (Ndabigengesere and Narasiah 1998). In the current study, in the experimental period of 11 to 14 days, no stimulating effect on the cyanobacteria was detected of the Moringa-derived organic carbon and nutrients; however, this aspect warrants further investigation by applying recolonization in the high-dosage Erlenmeyer flasks. The drawbacks of organic carbon and nutrient releases can be overcome using dialysis, delipidation, or ion exchange (Okuda et al. 2001; Ghebremichael 2007). Nonetheless, these methods focused on the coagulant component from M. oleifera seeds that might only express part of the antimicrobial activity. Further research is needed on the cyanobactericidal effect of different fractions from the crushed seeds and other parts of the plant (roots, leaves).
When dense populations of M. aeruginosa were exposed to high concentrations of filtrate from crushed Moringa seeds, the ΦPSII of the suspensions dropped to zero rapidly, but Chl-a concentrations first increased. Cell counter data, however, revealed that total particle numbers and biovolume were significantly lower in the high-dosage treatments. Apparently, cells died and released pigments in the medium that yielded higher fluorescence values due to the package effect (Kirk 1994). Indeed, Flo-derived peptides have been found to decrease strongly cell viability by permeabilizing or disrupting the bacterial membrane (Suarez et al. 2005). As a consequence, cyanotoxins might also be freed from the cells after cell lyses (Berg et al. 1987), concomitantly deteriorating the water quality as cyanotoxins, such as M. aeruginosa hepatotoxin microcystin-LR, may persist for weeks in the water (Lahti et al. 1997). For example, treatment of water infested with the cyanobacterium Cylindrospermospis raciborskii in a drinking water reservoir in 1979 in Palm Island (Australia) with copper sulfate, causing cyanobacterial cell lysis, led to an outbreak of hepatoenteritis among 148 consumers (Hawkins et al. 1985). Such cyanobacterial intoxication of humans may occur not only through consumption of cyanotoxin-contaminated drinking water but also through food and dietary supplements; through recreational and occupational contact, and by hemodialysis (e.g., Carmichael et al. 2001; Dittmann and Wiegand 2006; Stewart et al. 2006). It has been demonstrated that 10 mg L−1 Moringa could remove 93% to 98% of the cyanotoxin microcystin-LR (20 µg L−1) from the aqueous phase, while 50 mg L−1 removed the toxin to levels below the practical detection limit (<0.08 µg L−1) of the HPLC-DAD procedure (Warhurst et al. 1997b). In microcystin-contaminated drinking water, the absorbed material should be removed from the water by coagulation, sedimentation, or filtering prior to consumption, whereas in reservoirs, ponds and irrigation impoundments natural sedimentation might yield sufficient removal. Up to now, the effectiveness of Moringa seeds in removing cyanotoxins other than microcystin-LR from the water is not known. Yet, Moringa seeds have the potential of being cheap biosorbents for cyanotoxins from aqueous media, as they are for the removal of cadmium (Sharma et al. 2006) organic pollutants and methyl parathion (Akhtar et al. 2007a, b).
The pan-tropical tree M. oleifera is a highly nutritive food with medicinal, phytochemical, pharmacological, and potent water-purifying properties (Anwar et al. 2007). The majority of the studies on water purification with M. oleifera seeds have focused on flocculation–coagulation; 97–99% reduction in turbidity can be reached, while 90% of bacteria and 99% of coliforms can be precipitated (Ali et al. 2004; Liew et al. 2006; Oludoro and Aderiye 2007). The results presented here of a clear cyanobactericidal activity of crushed Moringa seeds combined with a growing number of more frequent and longer lasting cyanobacterial blooms (de Figueirdo et al. 2004; Paerl and Huisman 2008) show that Moringa seed extracts might have a potential as an effect-oriented measure lessening cyanobacterial nuisance, especially at household and community level in tropical regions.
Acknowledgments
We owe special thanks to the youngsters of NXT Generation: Jolie, Marte, Thomas, Joris, Chris, Karlijn, Anne, Maxi, and Marene (http://www.dse.nl/∼nxtgeneration) for stimulating us to perform the research and for arranging the Moringa seeds.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Akhtar M, Hasany SM, Bhanger MI, Iqbal S. Low cost sorbents for the removal of methyl parathion pesticide from aqueous solutions. Chemosphere. 2007a;66:1829–1838. doi: 10.1016/j.chemosphere.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Akhtar M, Hasany SM, Bhanger MI, Iqbal S. Sorption potential of Moringa oleifera pods for the removal of organic pollutants from aqueous solutions. J Hazard Mater. 2007;141:546–556. doi: 10.1016/j.jhazmat.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Ali GH, El-Taweel GE, Ali MA. The cytotoxicity and antimicrobial efficiency of Moringa oleifera seeds extracts. Int J Environ Stud. 2004;61:699–708. doi: 10.1080/0020723042000189877. [DOI] [Google Scholar]
- Anwar F, Latif S, Ashraf M, Gilani AH. Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- Beltrán-Heredia J, Sánchez-Martín J. Heavy metals removal from surface water with Moringa oleifera seed extract as flocculant agent. Fres Environ Bull. 2008;17(12 A):2134–2140. [Google Scholar]
- Beltrán-Heredia J, Sánchez-Martín J. Removal of sodium lauryl sulphate by coagulation/flocculation with Moringa oleifera seed extract. J Hazard Mater. 2009;164:713–719. doi: 10.1016/j.jhazmat.2008.08.053. [DOI] [PubMed] [Google Scholar]
- Berg K, Skulberg OM, Skulberg R. Effects of decaying toxic blue-green algae on water quality—a laboratory study. Arch Hydrobiol. 1987;108:549–563. [Google Scholar]
- Boucher J, Steiner L, Marison IW. Bio-sorption of atrazine in the press-cake from oilseeds. Water Res. 2007;41:3209–3216. doi: 10.1016/j.watres.2007.05.038. [DOI] [PubMed] [Google Scholar]
- Cáceres A, Cabrera O, Morales O, Mollinedo P, Mendia P. Pharmacological properties of Moringa oleifera. 1: preliminary screening for antimicrobial activity. J Ethnopharmacol. 1991;33:213–216. doi: 10.1016/0378-8741(91)90078-R. [DOI] [PubMed] [Google Scholar]
- Carmichael WW, Azevedo S, An JS, Molica RJR, Jochimsen EM, Lau S, Rinehart KL, Shaw GL, Eagelsham GK. Human fatalities from cyanobacteria: chemical and biological evidence for cyanotoxins. Environ Health Perspect. 2001;109:663–668. doi: 10.2307/3454781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd GA, Morrison LF, Metcalf JS. Cyanobacterial toxins: risk management for health protection. Toxicol Appl Pharmacol. 2005;203:264–272. doi: 10.1016/j.taap.2004.02.016. [DOI] [PubMed] [Google Scholar]
- de Figueirdo DR, Azeiteiro UM, Esteves SM, Gonçalves FJM, Pereira MJ. Microcystin-producing blooms—a serious global public health issue. Ecotoxicol Environ Saf. 2004;59:151–163. doi: 10.1016/j.ecoenv.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Dittmann E, Wiegand C. Cyanobacterial toxins—occurrence, biosynthesis and impact on human affairs. Mol Nutr Food Res. 2006;50:7–17. doi: 10.1002/mnfr.200500162. [DOI] [PubMed] [Google Scholar]
- Eilert U, Wolters B, Nahrstedt A. The antibiotic principle of seeds of Moringa oleifera and Moringa stenopetala. Planta Med. 1981;42:55–61. doi: 10.1055/s-2007-971546. [DOI] [PubMed] [Google Scholar]
- Ghebremichael K. Overcoming the drawbacks of natural coagulants for drinking water treatment. Wat Sci & Technol: Wat Supply. 2007;7:87–93. doi: 10.2166/ws.2007.144. [DOI] [Google Scholar]
- Hawkins PR, Runnegar MTC, Jackson ARB, Falconer IR. Severe hepatotoxicity caused by the tropical cyanobacterium (blue-green alga) Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju isolated from a domestic supply reservoir. Appl Environ Microbiol. 1985;50:1292–1295. doi: 10.1128/aem.50.5.1292-1295.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RI. The influence of humic substances on lacustrine planktonic food chains. Hydrobiologia. 1992;229:73–91. [Google Scholar]
- Kalogo Y, Rosillon F, Hammes F, Verstraete W. Effect of a water extract of Moringa oleifera seeds on the hydrolytic microbial species diversity of a UASB reactor treating domestic wastewater. Lett Appl Microbiol. 2000;31:259–264. doi: 10.1046/j.1365-2672.2000.00814.x. [DOI] [PubMed] [Google Scholar]
- Kirk JTO. Light and photosynthesis in aquatic systems. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Lahti K, Rapala J, Fardig M, Niemela M, Sivonen K. Persistence of cyanobacterial hepatotoxin, microcystin-LR in particulate material and dissolved in lake water. Water Res. 1997;31:1005–1012. doi: 10.1016/S0043-1354(96)00353-3. [DOI] [Google Scholar]
- Liew AG, Noor MJMM, Muyibi SA, Fugara AMS, Muhammed TA, Iyuke SE. Surface water clarification using M. oleifera seeds. Int J Environ Stud. 2006;63:211–219. doi: 10.1080/00207230500117670. [DOI] [Google Scholar]
- Lürling M, Beekman W. Grazer induced defenses in Scenedesmus (Chlorococcales; Chlorophyceae): coenobium and spine formation. Phycologia. 1999;38:368–376. [Google Scholar]
- Madsen M, Schlundt J, Omer EFE. Effect of water coagulation by seeds of Moringa oleifera on bacterial concentrations. J Trop Med Hyg. 1987;90:101–109. [PubMed] [Google Scholar]
- Markager S, Vincent WF. Spectral light attenuation and the absorption of UV and blue light in natural waters. Limnol Oceanogr. 2000;45:642–650. doi: 10.4319/lo.2000.45.3.0642. [DOI] [Google Scholar]
- Martin D, Ridge I. The relative sensitivity of algae to decomposing barley straw. J Appl Phycol. 1999;11:285–291. doi: 10.1023/A:1008197418074. [DOI] [Google Scholar]
- Muyibi SA, Evison LM. Moringa oleifera seeds for softening hardwater. Water Res. 1995;29:1099–1105. doi: 10.1016/0043-1354(94)00250-B. [DOI] [Google Scholar]
- Ndabigengesere A, Narasiah KD. Quality of water treated by coagulation using Moringa oleifera seeds. Water Res. 1998;32:781–791. doi: 10.1016/S0043-1354(97)00295-9. [DOI] [Google Scholar]
- Newman JR, Barrett PRF. Control of Microcystis aeruginosa by decomposing barley straw. J Aquat Plant Manage. 1993;31:203–206. [Google Scholar]
- Okuda T, Baes AU, Nishijima W, Okada M. Isolation and characterization of coagulant extracted from Moringa oleifera seed by salt solution. Water Res. 2001;35:405–410. doi: 10.1016/S0043-1354(00)00290-6. [DOI] [PubMed] [Google Scholar]
- Olsen A. Low technology water purification by bentonite clay and Moringa oleifera seed flocculation as performed in Sudanese villages: effects on Schistosoma mansoni cercariae. Water Res. 1987;21:517–522. doi: 10.1016/0043-1354(87)90059-5. [DOI] [Google Scholar]
- Oludoro AO, Aderiye BI. Efficacy of Moringa oleifera seed extract on the microflora of surface and underground water. J Plant Sci. 2007;2:453–458. doi: 10.3923/jps.2007.453.458. [DOI] [Google Scholar]
- Paerl HW, Huisman J. Blooms like it hot. Science. 2008;320:57–58. doi: 10.1126/science.1155398. [DOI] [PubMed] [Google Scholar]
- Pollard SJT, Thompson FE, McConnachie GL. Microporous carbons from Moringa oleifera husks for water purification in less developed countries. Water Res. 1995;29:337–347. doi: 10.1016/0043-1354(94)E0103-D. [DOI] [Google Scholar]
- Prokhotskaya VY, Steinberg CEW. Differential sensitivity of a coccal green algal and a cyanobacterial species to dissolved natural organic matter (NOM) Env Sci Pollut Res. 2007;14(Special Issue 1):11–18. doi: 10.1065/espr2007.01.379. [DOI] [PubMed] [Google Scholar]
- Rosenberg DM, McCully P, Pringle CM. Global-scale environmental effects of hydrological alterations: introduction. Bioscience. 2000;50:746–751. doi: 10.1641/0006-3568(2000)050[0746:GSEEOH]2.0.CO;2. [DOI] [Google Scholar]
- Schindler DW, Hecky RE, Findlay DL, Stainton MP, Parker BR, Paterson MJ, Beaty KG, Lyng M, Kasian SEM. Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proc Natl Acad Sci. 2008;105:11254–11258. doi: 10.1073/pnas.0805108105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Kumari P, Srivastava MM, Srivastava S. Removal of cadmium from aqueous system by shelled Moringa oleifera Lam. seed powder. Biores Technol. 2006;97:299–305. doi: 10.1016/j.biortech.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Shehata S, Badr. S, Wahba S. Drinking water treatment options for eliminating freshwater algae. Int J Environ Stud. 2002;59:679–688. doi: 10.1080/00207230214397. [DOI] [Google Scholar]
- Smith VH, Schindler DW. Eutrophication science: where do we go from here? Trends Ecol Evol. 2009;24:201–207. doi: 10.1016/j.tree.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Stanier RY, Cohen-Bazire G. Phototrophic prokaryotes: the cyanobacteria. Ann Rev Microbiol. 1977;31:225–274. doi: 10.1146/annurev.mi.31.100177.001301. [DOI] [PubMed] [Google Scholar]
- Steffensen DA (2008) Chapter 37: economic cost of cyanobacterial blooms. In: Hudnell KE (ed) Cyanobacterial harmful algal blooms: state of the science and research needs. Adv Exp Med Biol, vol 619, pp 855–865 [DOI] [PubMed]
- Steinberg CEW. Ecology of humic substances in freshwaters. Berlin: Springer; 2003. [Google Scholar]
- Stewart I, Webb PM, Schluter PJ, Shaw GR. Recreational and occupational field exposure to freshwater cyanobacteria—a review of anecdotal and case reports, epidemiological studies and the challenges for epidemiologic assessment. Environ Health: A Global Access Science Source. 2006;5:6. doi: 10.1186/1476-069X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez M, Entenza JM, Doerries C, Meyer E, Bourquin L, Sutherland J, Marison I, Moreillon P, Mermod N. Expression of a plant-derived peptide harboring water-cleaning and antimicrobial activities. Biotechnol Bioeng. 2003;81:13–20. doi: 10.1002/bit.10550. [DOI] [PubMed] [Google Scholar]
- Suarez M, Haenni M, Canarelli S, Fisch F, Chodanowski P, Servis C, Michielin O, Moreillon P, Mermod N. Structure–function characterization and optimization of a plant-derived antibacterial peptide. Antimicrob Agents Chemother. 2005;49:3847–3857. doi: 10.1128/AAC.49.9.3847-3857.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warhurst AM, McConnachie GL, Pollard SJT. Characterisation and applications of activated carbon produced from Moringa oleifera seed husks by single-step steam pyrolysis. Water Res. 1997;31:759–766. doi: 10.1016/S0043-1354(97)80989-X. [DOI] [Google Scholar]
- Warhurst AM, Raggett SL, McConnachie GL, Pollard SJT, Chipofya V, Codd GA. Adsorption of the cyanobacterial hepatotoxin microcystin-LR by a low-cost activated carbon from the seed husks of the pan-tropical tree, Moringa oleifera. Sci Total Environ. 1997b;207:207–211. doi: 10.1016/S0048-9697(97)00260-X. [DOI] [PubMed] [Google Scholar]
- Waybright TJ, Terlizzi DE, Ferrier MD. Chemical characterization of the aqueous algistatic fraction of barley straw (Hordeum vulgare) inhibiting Microcystis aeruginosa. J Appl Phycol. 2009;21:333–340. doi: 10.1007/s10811-008-9373-x. [DOI] [Google Scholar]