Abstract
Aging affects every innate immune cell, including changes in cell numbers and function. Defects in the function of some cells are intrinsic, whereas for other cells, defects are extrinsic and possibly the consequence of the complex interactions with other cell types or the environmental milieu that is altered with aging. Abnormal function contributes to worsened outcomes after injury or infection and leads to diseases observed in the elderly. Knowing the mechanisms responsible for the aberrant function of innate immune cells might lead to the development of therapeutic strategies designed to improve innate immunity in aged individuals. Herein, advances in the field of innate immunity and aging with a focus on neutrophils, macrophages and dendritic cells in laboratory animals are discussed.
Introduction
Extensive studies have described the decline in function of the immune system with advanced age (reviewed in Ref. [1]), with age-dependent defects observed in both the innate and the adaptive arms of the immune system [2–4] (also see the other articles in this themed issue). The accumulation of a lifetime exposure to environmental factors triggers many of the changes seen in the function of innate immune cells including the increased production of pro-inflammatorymediators that play a role in the chronic inflammatory state that is known as `inflamm-aging' [5]. As a result, older subjects have impaired immune responses after infectious challenges compared with young adults [6], rendering them more susceptible to viral and bacterial pathogens, opportunistic infections, reactivation of latent viruses, autoimmune diseases and neoplasias [7–10].
Cumulative evidence indicates that aging exerts significant effects on all cells of the innate immune system [2,11–15]. Impairment of multiple neutrophil functions, such as phagocytic capacity, synthesis of reactive oxygen intermediates and intracellular killing efficiency are all observed in the elderly [16,17]. Advanced age also affects macrophage functions, including phagocytic activity, cytokine and chemokine secretion, antibacterial defenses, infiltration and wound repair and antigen presentation [11]. Additionally, recent studies have documented defects in populations of dendritic cells (DCs). In this article, we will describe the effects of advanced age on the functions of these pivotal cells with an emphasis on aberrations in intracellular signaling pathways that lead to altered activation and, in turn, impaired function of the innate immune system.
Neutrophils
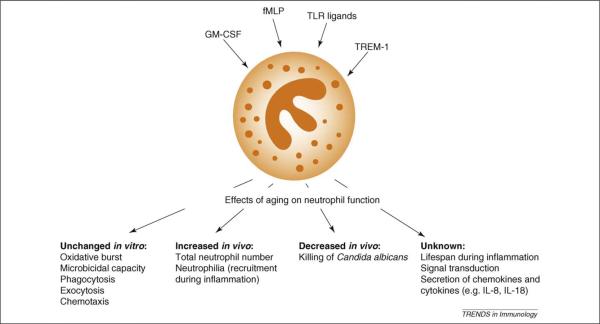
Neutrophil granulocytes are key players in immune reactions and are activated by compounds that bind to receptors recognizing pathogen-associated molecular patterns, such as formyl-methionyl-leucyl-phenylalanine (fMLP), endotoxins and other Toll-like receptor (TLR) ligands; triggering receptor expressed on myeloid cells (TREM-1) ligands or by cytokines such as granulocyte monocyte-colony stimulating factor (GM-CSF), interleukin-15 (IL-15) or IL-18. Consequently, not only are they responsible for the direct elimination of microbial and fungal intruders, but they are also important effector cells in tissue destruction during inflammation and in the regulation of the adaptive immune response [18]. As shown recently, mouse neutrophils produce the pro-inflammatory cytokine IL-18 after Legionella pneumophila infection, leading to the activation of natural killer (NK) cells and resolution of infection [19]. Their importance is highlighted by recurrent bacterial and fungal infections in individuals with neutro-phil dysfunction or neutropenia [20]. There are almost no studies on neutrophil morphologic and functional changes in aged mice, and those that exist date back to the 1980s and 1990s; therefore, most of our knowledge on age-related changes stems from humans.
In murine models, early studies on putative neutrophil alterations showed that the concentration of blood leukocytes, and neutrophils in particular, increased progressively as mice aged. These early observations have been confirmed by a recent study showing that higher numbers of neutrophils were recruited to the peritoneum of aged mice after injection of Candida albicans [21]. Furthermore, aged mice showed a marked inflammation of the liver characterized by elevated levels of cytokines [tumor necrosis factor α (TNFα), interferon γ (IFNγ), transforming growth factor β (TGFβ), and IL-10] accompanied by increased neutrophilic infiltration [22]. The same is true in the lung after intratracheal injection of lipopolysaccharide (LPS), where neutrophilia is accompanied by elevated cytokine levels [23]. However, the functions of neutrophils isolated from aged mice were unaltered, including respiratory burst, exocytosis, chemotaxis and phagocytic activity compared with young mice [21]. This observation suggests the importance of the aged environment itself, which predominates over any intrinsic defects in neutrophils from aged mice. The only circumstance where neutrophil functions have been clearly shown to be altered in aged mice is after a specific organismic alteration (i.e. a combination of a protein-deficient diet and aging), which led to decreased respiratory burst and exocytosis. This is in stark contrast to humans, where even in healthy elderly subjects, neutrophils show impaired function and altered signal transduction [16,24].
Conflicting evidence exists for the ability of agedmice to fight infections. It has been observed that aging does not result in decreased resistance against infection with Streptococcus pneumoniae in C57BL/6 mice. Conversely, recent studies with the same mouse strain have shown a lower ability to kill C. albicans relative to young mice [21]. Although neutrophils from aged mice do show some impairment in killing C. albicans, this can only be seen when killing activity is measured in vivo where there are mixed cell populations [21].In this model, C. albicans is injected into the peritoneum, and the decreased killing activity of neutrophils in aged mice seems to be linked to lower numbers of resident peritonealmacrophages, which secrete significantly less pro-inflammatorymediators (IL-6, IL-1β,TNFα) and chemokines (e.g. MIP-2), but similar amounts of anti-inflammatory IL-10 relative to young mice [21]. It is probable therefore that, in aged mice, neutrophils and other phagocytes are recruited to fight infection; however, as they encounter a net anti-inflammatory milieu in the peritoneum, the overall killing of C. albicans is significantly impaired. This could lead to the observed neutrophilia in the peritoneum of aged mice. Supporting this idea, findings indicate that hematopoietic stem cells from aged mice proliferate in a young micro-environment and have a normal neutrophil-generating capacity [25] (also see the article by Geiger et al. in this themed issue). By contrast, when hematopoietic stem cells from young mice proliferate and differentiate in an aged microenvironment, the functions of these neutrophils were found to be decreased. These results suggest that, in mice, it is the microenvironmental factors that are responsible for decreased neutrophil function under some conditions, such as killing of C. albicans; but not in others, such as S. pneumoniae. Such discrepancies could be explained by intrinsic alterations in the signal transduction of fungi-detecting pattern receptors, such as TLR2 and Dectin-1 in aged murine neutrophils. Similar observations are still lacking in elderly human subjects and could be of importance considering that death among the elderly population is increased in some fungi-associated pathologies such as in sepsis. Collectively, these results indicate that the age-related changes in murine neutrophil function are probably rather subtle and complex; they furthermore suggest that mice might not be the best models for studying neutrophil immunosenescence (Figure 1).
Figure 1.
Aging and murine neutrophils. Neutrophils are activated by a wide array of compounds, such as inflammatory mediators, cytokines or ligands for pattern recognition receptors (e.g. via TLRs). Activation elicits classic neutrophil functions such as chemotaxis, phagocytosis and the production of reactive oxygen species (respiratory burst). Moreover, this activation also results in the synthesis and secretion of peptidic mediators, such as cytokines and chemokines, and lipid mediators, such as prostaglandins and leukotrienes. Therefore, because neutrophils are the first cells recruited to the inflamed tissues, these secretion products make this cell of the innate immune system one of the cornerstones of the induction and shaping of adaptive immunity. fMLP, formyl-methionyl-leucyl-phenylalanine; GM-CSF, granulocyte monocyte-colony stimulating factor; LPS, lipopolysaccharide; TLR, Toll-like receptor; TREM-1, triggering receptor expressed on myeloid cells.
Macrophages
Like neutrophils, macrophages are phagocytic cells and are capable of clearing pathogenic organisms. In addition,macrophages are potent cytokine producers and play a crucial role in a variety of processes ranging from antigen presentation to wound healing. Macrophages isolated from aged subjects exhibit multiple defects in function including decreased chemotactic activity, as well as in various steps of their phagocytic process (e.g. adherence, opsonization, phagocytosis and antibody-dependent cell cytotoxicity).
Macrophages play an important role during inflamma-tory responses, which, depending on the magnitude, can either be beneficial or detrimental to the organism. Cytokine production, as determined using in vitro murine models, is decreased in macrophages from the aged relative to their young counterparts (reviewed in Ref. [2]). For example, extensive studies have established that LPS-induced production of TNFa, IL-1b and IL-6 by macrophages isolated from both the spleen and peritoneum is suppressed compared with macrophages from young mice [25,26–28]. By contrast, the production of these same mediators after in vivo exposure to LPS or other bacterial products is elevated in aged versus young mice [29,30]. Although production of these pro-inflamma-tory cytokines is thought to be in part a macrophage-mediated event, it is clear that a variety of cell types, including stroma (i.e. epithelia and endothelia), as well as other leukocytes, will produce these mediators in vivo. This complex mix of cells is likely to be an important source of the overexuberant production of pro-inflammatory cytokines that characterizes `inflamm-aging'. This overproduction of mediators in vivo might also be compounded by the presence of other stimuli present in the host, including hormones, cytokines, chemokines, adrenergic and cholinergic agonists, fatty acids and immunoglobulins. Because these factors change with advancing age, it follows that the function of macrophages in vivo can be markedly altered in response to this. Thus, the microenvironment in the aged subject probably plays a key role in defining the functionality and activation properties of macrophages in response to LPS [2].
As with neutrophils, defects in various signaling pathways can be found in macrophages from aged subjects. One pathway that was reported to be altered with age is IFNγ-mediated signal transducer and activator of transcription-1 (STAT-1α) activation. [31]. Although no age-related differences in the surface expression of the IFNγ receptor is found in macrophages from aged mice, one report documented a reduction in phosphorylation of the transcription factor, STAT-1α, in peritoneal macrophages from aged (18–24 months old) mice, relative to macrophages from young (2 months old) mice [31]. In addition, total STAT-1α expression was found to be lower after macrophages from aged mice were incubated with IFNγ compared with cells from young mice. The diminished capacity of macrophages from aged animals to produce and activate STAT-1α could affect the ability to initiate the transcription of specific downstream targets of IFNγ stimulation [31]. However, this study did not examine any functional consequences of attenuated STAT-1α activation.
The effect of age on other signaling pathways, including TLR signaling, has been examined in multiple laboratories. Like the IFNγ receptor, age does not affect the expression of TLR4 on macrophages [12,28], nor does it alter basal circulating levels of the LPS binding protein (LBP) [32]. By contrast, there is a report of reduced levels of expression of CD14, a co-receptor for TLR4, in macrophages from aged mice compared with those of young mice [28]. Thus, there are specific, selective effects of age on the expression of proteins involved in the binding of LPS to macrophages, and this might account for the reduced responsiveness described earlier.
The examination of intracellular signaling pathways has identified other specific defects in macrophages from aged mice (reviewed in Ref. [33]). These defects include a reduction in total levels of the mitogen-activated kinases (MAPKs), p38 and Jun N-terminal kinase (JNK), the MAPK-activated protein kinase-2 (APK-2) and the nuclear levels of NF-κB [26,27]. These observations are in agreement with studies on circulating monocytes obtained from aged humans and suggest that the altered cytokine production in macrophages from aged individuals might be dependent on these and other defects in macrophage activation.
Microarray analysis performed on RNA from resting and LPS-stimulated macrophages from young and aged mice has confirmed an age-related reduction in genes involved in the immune response (e.g. pro-inflammatory chemokines, cytokines and their receptors; also see the article by Panda et al. in this themed issue) [25]. In addition, downregulation of signal transduction genes involved in the TLR-signaling pathway leading to NF-κB activation, including the adaptor molecule MyD88, was found. These data have expanded on known age-related defects in cell signaling and suggest that the TLR-dependent pathway has significantly reduced efficiency in macrophages from the aged [25]. Thus, recent studies confirmed that changes in cytokine production with advanced age are primarily associated with reduction in the activation of multiple signal transduction pathways, which are crucial to cell activation. Whether these defects are a function of the cells themselves or a product of how those cells mature in the aged milieu remains to be determined. The age-related differences in macrophage function and phenotype are summarized in Table 1.
Table 1.
Defects in macrophage phenotype and function with advanced age.
| Difference in macrophages from aged versus young mice | Refs | |
|---|---|---|
| Production of IL-6a after LPS in vivo | ↑ | [30,32] |
| Production of IL-6, IL-1β and TNF-α after LPS in vitro | ↓ | [26–28] |
| Production of IL-10 after LPS in vitro | ↑ | [27,28] |
| TLR expression | Unchanged | [26,27] |
| MyD88 expression | ↓ | [25] |
| CD14 expression | ↓ | [28] |
| LBP expression | Unchanged | [32] |
| IL-2 and IFNγ receptors | Unchanged | [27] |
| STAT-1α levels | ↓ | [31] |
| STAT-1α phosphorylation after IFNγ in vitro | ↓ | [31] |
| MAPK levels | ↓ | [27] |
| MAPK phosphorylation after LPS in vitro | ↓ | [27] |
| APK-2 | ↓ | [27] |
| NF-κB activation after LPS in vitro | ↓ | [27] |
| NF-κB activation after IL-2 in vitro | Unchanged | [27] |
IL, interleukin;LPS, lipopolysaccharide; TNF, tumor necrosis factor; TLR, Toll-like receptor; LBP, LPS binding protein; IFN, interferon; STAT-1α, signal transducer and activator of transcription-1; MAPK, mitogen activated protein kinase; APK-2, MAPK-activated protein kinase (APK)-2 activation.
Dendritic cells
DCs serve as the sentinels of the immune response and link innate and adaptive immunity. These cells have specialized antigen-processing capabilities together with co-stimulatory molecules that enable their efficient endocytosis and presentation of endogenous and exogenous antigens to initiate an immune response. Many of the early aging studies examined Langerhans cells or heterogeneous antigen presenting cell (APC) populations and described a decline in cell number, co-stimulatory molecule expression, antigen presentation and the migration to regional lymph nodes (reviewed in Refs. [14,34]). In light of the recent emphasis on the use of DCs and adjuvants that target DCs to enhance vaccine efficacy to infection and cancer, studies of conventional and plasmacytoid DC (pDC) subsets in the aged are becoming increasingly important. Immune responses are initiated by DCs following engagement of pattern recognition receptors, including C-type lectin receptors (CLRs) and TLRs. The DCs of aged mice show decreased expression of some C-type lectins, including CD205 [35] and DC-SIGN [36], although the consequences of this altered expression still need to be ascertained. By contrast, DCs derived from murine bone marrow or spleen have unaffected TLR expression and an intact ability to prime immune responses [37]
Little is known about the age-related changes in antigen processing. Recently, it was shown that the pathway through which peptides are generated for antigen presentation overlaps with autophagy [38,39], a process by which cells remove damaged organelles through the lysosomal compartments. Studies have shown that viral and bacterial antigens enter endosomal compartments after macroautophagy and that knockdown of autophagolysome formation affects viral antigen presentation [38,39]. Importantly, autophagy in general declines with aging [40]. Details of the interrelationship between autophagy and antigen processing/presentation in DCs of the aged remain to be elucidated. One might envision an age-associated reduction in these processes would probably lead to a decline in the T-cell response; however, more subtle differences, such as those in the repertoire of antigens presented by DCs to T cells might also occur.
Although direct sensing of pathogen stimuli seems to be crucial for the elicitation of full DC activation and effective polarization of T helper cell responses [41], a growing body of evidence suggests that cytokines and chemokines in the local microenvironment can also modulate DC function and influence the immune response. The migration of mature DCs that have encountered antigen in peripheral tissues to the secondary lymphoid organs where they can induce an immune response is dependent on chemokine signaling (i.e. CCR7 expression by the DCs and CCL19 and CCL21 by the stromal and endothelial cells in the microenvironment). Although the basal expression of CCR7 on DCs, and CCL19 and CCL21 in the tissue microenvironment is comparable between unprimed young and old mice, the ability to upregulate these molecules after immunization is significantly impaired in aged mice [35,36,42]. These effects would contribute to the reduced accumulation of DCs seen in the draining lymph nodes of aged mice. Although not formally demonstrated, the migration of DC precursor cells that seed the periphery from the bone marrow might likewise be reduced, because migration of immature DC is dependent on the interaction between the adhesion molecule ICAM-2 on endothelial cells and DC-SIGN, which is reduced in bone marrow–derived DCs [42].
pDCs secrete high levels of type I IFNs and IL-12 in response to certain bacteria [43] and viruses [43,44]. In contrast to conventional DCs, pDCs express TLRs 7 and 9 within endosomal compartments [45,46]. The production of type I IFNs and IL-12 occurs via separate pathways because the adaptor protein interferon regulatory factor 7 (IRF-7) is essential for type I IFN production, whereas IL-12 is dependent on NF-κB translocation [47]. Activation of TLR7 and 9 within the endosomes of pDCs is crucial for the recognition of single-stranded RNA via TLR7 [48,49] and unmethylated CpG sequences via TLR9 [46]. The high levels of type I IFNs produced by TLR-activated pDCs [50,51] activate cellular host innate defense mechanisms and initiate adaptive immunity [43,44,52].
Recent research has investigated the effects of aging in response to TLR9 activation by CpG-A or during herpes simplex virus-2 (HSV-2) infection, a pathogen known to activate TLR9 [51]. Aged murine pDCs produced lower IFNa levels than young pDCs in response to either CpG-A or HSV-2 infection. Similar results were obtained when aged pDCs were activated by TLR7 [53]. These data suggest that aging impairs the type I IFN response to downstream of TLR7 or 9 ligation.
Further experiments demonstrated that defective pDC function accounts for the reduced host defense to HSV-2 infection with aging. Young whole bone marrow cells or young bone marrow cells depleted of pDCs were adoptively transferred into virally infected aged mice. Only the transfer of whole bone marrow significantly augmented the systemic IFNα response and led to viral clearance in the infected aged mice [53]. Additionally, transfer of purified young but not aged pDCs into virally infected aged mice significantly increased IFNα responses and viral clearance during HSV-2 infection [53].
Recent work suggests possible mechanisms of crosstalk between distinct DC subsets. The crosstalk between pDCs and conventional DCs in a primary immune response to HSV infection was shown to be necessary for the optimal induction of virus-specific cytotoxic T lymphocytes [54]. As such, it is plausible that the reported decline in the IFNα response by plasmacytoid DCs in aged mice [55] might influence the dynamic interplay between the DC subsets and lead to the observed decline in the T-cell responses in the aged.
Age-related molecular defects within murine pDCs were also found. Aged and young pDCs had comparable IFNαβ receptor and TLR9 receptor expression [53]. However, IFNα treatment or TLR9 ligation via CpG-A either alone or in combination failed to induce IRF-7 gene or protein upregulation in aged pDCs, but induced IRF-7 upregulation in young pDCs [53]. These data suggest that aging impairs IRF-7 upregulation to either TLR9 or IFNαβ receptor activation. A prior descriptive study in humans suggests that IFN-α production is reduced in humans [55] and the defective TLR responses of pDCs might be one of the reasons why the elderly are more susceptible to viral infections.
Future directions
The recent studies reported herein suggest that there are selective defects in the function of neutrophils, macrophages and DCs obtained from aged mice that in many cases parallel those of other species including humans. Interestingly, many of the aberrant responses seem to be dependent on the host environment, the milieu in which the cells reside, and might not be entirely dependent on the innate immune cells themselves. This intriguing observation would suggest that future studies might be best targeted on the extrinsic factors that are present in the host, rather than those that are intrinsic to the individual cell. Hence, future studies designed to improve the function of innate immune cells in aged hosts might need to consider both sides of this biological coin to yield the greatest efficacy from therapeutic interventions.
Acknowledgements
This work was supported by NIH R01 AG018859 (E.J.K.), R01 AG028082 (D.R.G.), R01 AG026727 (P.J.L.), and R21 AG031511 (P.J.L.), the Research Center on Aging, a Canadian Institutes of Health Research (No. 63149) grant (T.F.), the University of Sherbrooke (T.F.), the Canadian Institutes of Health Research (C.C.F.) and the Ralph and Marian C. Falk Medical Research Trust (E.J.K.).
References
- 1.Katz JM, et al. Immunity to influenza: the challenges of protecting an aging population. Immunol. Res. 2004;29:113–124. doi: 10.1385/IR:29:1-3:113. [DOI] [PubMed] [Google Scholar]
- 2.Gomez CR, et al. The aging innate immune system. Curr. Opin. Immunol. 2005;17:457–462. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Allman D, Miller JP. B cell development and receptor diversity during aging. Curr. Opin. Immunol. 2005;17:463–467. doi: 10.1016/j.coi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franceschi C, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 6.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat. Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 7.Pawelec G, et al. Human immunosenescence: is it infectious? Immunol. Rev. 2005;205:257–268. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 8.Effros RB. Genetic alterations in the ageing immune system: impact on infection and cancer. Mech. Ageing Dev. 2003;124:71–77. doi: 10.1016/s0047-6374(02)00171-9. [DOI] [PubMed] [Google Scholar]
- 9.Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmun. Rev. 2006;5:136–139. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Murasko DM, Jiang J. Response of aged mice to primary virus infections. Immunol. Rev. 2005;205:285–296. doi: 10.1111/j.0105-2896.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 11.Sebastian C, et al. MacrophAging: a cellular and molecular review. Immunobiology. 2005;210:121–126. doi: 10.1016/j.imbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Plackett TP, et al. Aging and innate immune cells. J. Leukoc. Biol. 2004;76:291–299. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- 13.Plowden J, et al. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3:161–167. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal A, et al. Biology of dendritic cells in aging. J. Clin. Immunol. 2008;28:14–20. doi: 10.1007/s10875-007-9127-6. [DOI] [PubMed] [Google Scholar]
- 15.Solana R, et al. Aging and innate immunity. Immunity. 2006;24:491–494. doi: 10.1016/j.immuni.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Fulop T, et al. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3:217–226. doi: 10.1111/j.1474-9728.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 17.Tortorella C, et al. Age-related impairment of GM-CSF-induced signalling in neutrophils: role of SHP-1 and SOCS proteins. Ageing Res. Rev. 2007;6:81–93. doi: 10.1016/j.arr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol. Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 19.Sporri R, et al. A novel role for neutrophils as critical activators of NK cells. J. Immunol. 2008;181:7121–7130. doi: 10.4049/jimmunol.181.10.7121. [DOI] [PubMed] [Google Scholar]
- 20.Newburger PE. Disorders of neutrophil number and function. Hematology Am. Soc. Hematol. Educ. Program. 2006:104–110. doi: 10.1182/asheducation-2006.1.104. [DOI] [PubMed] [Google Scholar]
- 21.Murciano C, et al. Influence of aging on murine neutrophil and macrophage function against Candida albicans. FEMS Immunol. Med. Microbiol. 2008;53:214–221. doi: 10.1111/j.1574-695X.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 22.Singh P, et al. Lymphoid neogenesis and immune infiltration in aged liver. Hepatology. 2008;47:1680–1690. doi: 10.1002/hep.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito Y, et al. Lipopolysaccharide-induced neutrophilic inflammation in the lungs differs with age. Exp. Lung Res. 2007;33:375–384. doi: 10.1080/01902140701634843. [DOI] [PubMed] [Google Scholar]
- 24.Fortin CF, et al. Aging and neutrophils: there is still much to do. Rejuvenation Res. 2008;11:873–882. doi: 10.1089/rej.2008.0750. [DOI] [PubMed] [Google Scholar]
- 25.Chelvarajan RL, et al. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J. Leukoc. Biol. 2006;79:1314–1327. doi: 10.1189/jlb.0106024. [DOI] [PubMed] [Google Scholar]
- 26.Boehmer ED, et al. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J. Leukoc. Biol. 2004;75:342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- 27.Boehmer ED, et al. Aging negatively skews macrophage TLR2-and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech. Ageing Dev. 2005;126:1305–1313. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Chelvarajan RL, et al. The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function. J. Leukoc. Biol. 2005;77:503–512. doi: 10.1189/jlb.0804449. [DOI] [PubMed] [Google Scholar]
- 29.Gomez CR, et al. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit. Care Med. 2007;35:246–251. doi: 10.1097/01.CCM.0000251639.05135.E0. [DOI] [PubMed] [Google Scholar]
- 30.Gomez CR, et al. Comparison of the effects of aging and IL-6 on the hepatic inflammatory response in two models of systemic injury: scald injury versus i.p. LPS administration. Shock. 2009;31:178–184. doi: 10.1097/SHK.0b013e318180feb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon P, et al. Macrophage hypo-responsiveness to interferon-gamma in aged mice is associated with impaired signaling through Jak-STAT. Mech. Ageing Dev. 2004;125:137–143. doi: 10.1016/j.mad.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Gomez CR, et al. Aberrant acute-phase response in aged interleukin-6 knockout mice. Shock. 2006;25:581–585. doi: 10.1097/01.shk.000029553.39081.ec. [DOI] [PubMed] [Google Scholar]
- 33.Gomez CR, et al. Signal transduction of the aging innate immune system. Curr. Immunol. Rev. 2007;3:23–30. [Google Scholar]
- 34.Shurin MR, et al. Aging and the dendritic cell system: implications for cancer. Crit. Rev. Oncol. Hematol. 2007;64:90–105. doi: 10.1016/j.critrevonc.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linton PJ, et al. Intrinsic versus environmental influences on T-cell responses in aging. Immunol. Rev. 2005;205:207–219. doi: 10.1111/j.0105-2896.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 36.Grolleau-Julius A, et al. Effect of aging on bone marrow-derived murine CD11c+CD4-CD8alpha- dendritic cell function. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:1039–1047. doi: 10.1093/gerona/61.10.1039. [DOI] [PubMed] [Google Scholar]
- 37.Tesar BM, et al. Murine [corrected] myeloid dendritic cell-dependent toll-like receptor immunity is preserved with aging. Aging Cell. 2006;5:473–486. doi: 10.1111/j.1474-9726.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- 38.Paludan C, et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 39.Vyas JM, et al. The known unknowns of antigen processing and presentation. Nat. Rev. Immunol. 2008;8:607–618. doi: 10.1038/nri2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24:604–612. doi: 10.1016/j.tig.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat. Immunol. 2005;6:163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 42.Grolleau-Julius A, et al. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res. 2008;68:6341–6349. doi: 10.1158/0008-5472.CAN-07-5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 44.Asselin-Paturel C, et al. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J. Exp. Med. 2005;201:1157–1167. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner H. The immunobiology of the TLR9 subfamily. Trends Immunol. 2004;25:381–386. doi: 10.1016/j.it.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Latz E, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 47.Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, et al. The functional effects of physical interactions among Toll-like receptors 7, 8, and 9. J. Biol. Chem. 2006;281:37427–37434. doi: 10.1074/jbc.M605311200. [DOI] [PubMed] [Google Scholar]
- 49.Wang JP, et al. Cutting edge: antibody-mediated TLR7-dependent recognition of viral RNA. J. Immunol. 2007;178:3363–3367. doi: 10.4049/jimmunol.178.6.3363. [DOI] [PubMed] [Google Scholar]
- 50.Guiducci C, et al. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J. Exp. Med. 2006;203:1999–2008. doi: 10.1084/jem.20060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lund J, et al. Toll-like receptor 9-mediated recognition of Herpes Simplex Virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montoya CJ, et al. Activation of plasmacytoid dendritic cells with TLR9 agonists initiates invariant NKT cell-mediated cross-talk with myeloid dendritic cells. J. Immunol. 2006;177:1028–1039. doi: 10.4049/jimmunol.177.2.1028. [DOI] [PubMed] [Google Scholar]
- 53.Stout-Delgado HW, et al. Aging impairs IFN regulatory factor 7 Upregulation in plasmacytoid dendritic cells during TLR9 activation. J. Immunol. 2008;181:6747–6756. doi: 10.4049/jimmunol.181.10.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoneyama H, et al. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J. Exp. Med. 2005;202:425–435. doi: 10.1084/jem.20041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shodell M, Siegal FP. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand. J. Immunol. 2002;56:518–521. doi: 10.1046/j.1365-3083.2002.01148.x. [DOI] [PubMed] [Google Scholar]