Abstract
We assessed the effects of a single episode of maternal alcohol intoxication on fetal brain blood perfusion in three pregnant dam (baboons) at the 24th week of pregnancy, using dynamic susceptibility contrast MRI. Following the oral administration of alcohol there was a four-fold increase in the peak contrast concentrations in the fetal brain. Additionally, we observed a two-to-three fold increase in the contrast uptake and washout rates in fetal brain. The underlying mechanisms of these changes are unknown but we hypothesized these could include the alcohol-mediated changes in placental permeability and fetal cerebral blood flow. Our findings indicate that alcohol intoxication produced profound changes, which may detrimentally influence neurodevelopmental processes in the brain.
Introduction
The detrimental effects of repeated maternal alcohol consumption on fetal brain are well documented [1]. Antenatal exposure to alcohol is the leading cause of preventable birth defects, mental retardation and neurodevelopmental disorders [1]. Exposure to alcohol during gestation can cause extensive central nervous system (CNS) damage, pre- and postnatal brain growth deficiencies, and craniofacial anomalies[1]. Alcohol-induced CNS teratogenicity includes microcephaly, enlarged ventricles, reduced basal ganglia volumes, reduction in gray matter volumes and decreased integrity and metabolism of the cerebral white matter of the frontal and parietal lobes [2–4].
Animal studies clearly demonstrate that alcohol freely crosses the placental barrier and enters the fetal circulation. The magnitudes and rates of CNS aberration due to prenatal alcohol exposure were shown to be dependent on the embryological stage, duration of exposure and absolute levels of blood alcohol [5–7]. Among commonly reported findings are reductions in the pool of neuronal and glial progenitor cells, aberrations in neuronal migrations and neuroglial prolificacy, alterations to synaptogenesis, and glial maturation [8]. Collectively, these data show that ethanol can interfere with many important ontogenetic stages producing widely varying degrees of damage in the affected structures, yet we know comparatively little about the possible negative liability of short-term alcohol exposure, a more common occurrence. Position emission tomography imaging studies in adult humans have shown that acute alcohol intoxication results in regional changes in brain metabolic activity, with increased activity in the temporal cortex and decreased activity in the occipital cortex [9]. Biphasic effects on cerebral blood flow were seen in adults, being depressed in the cerebellum, but elevated in the frontal cortex, following acute alcohol ingestion [10]. In a rodent model of binge drinking, maternal alcohol consumption produced region-specific deficits in brain growth, particularly if consumption occurred early on in gestation [11,12].
The mechanisms by which alcohol-induced CNS aberrations are produced are still elusive. In adults, alcohol abuse is associated with increased rates of vascular-related CNS neuropathies such as stroke, cerebral ischemic injury, sclerosis of small vessels, and perivascular gliosis [13]. These neuropathies are thought to be produced by impairment of the normal vascular function by the vasoactivity of alcohol [14]. Recent studies of alcohol teratogenicity in sheep brain also suggested that the vasoactivity of alcohol could be chief among the factors responsible for abnormal CNS development [15,16]. Alcohol can alter vascular reactivity of immature cerebral blood vessels in a fetal brain, producing cerebral vasodilatation and leading to cerebral hypoxia [15,16]. We undertook this study to investigate changes in vascular reactivity due to acute alcohol ingestion in a non-human primate (baboon). Based on the results in sheep brain, we hypothesized that exposure to alcohol would produce alterations in fetal cerebral blood flow.
The choice of which species to use as an animal model of acute fetal alcohol effects is not straightforward since gestation periods vary, the metabolism of alcohol differs, and any responses are contingent on dose, timing and pattern of exposure. Baboons (Papio hamadryas Anubis) were chosen for this study because both humans and Old World monkeys share a highly orchestrated pattern of cerebral development [17]. Of the non-human primates, baboons and apes are closest to humans from an evolutionary perspective[18]. Additionally, baboons are particularly suitable for neuroimaging-based research compared to other common laboratory monkeys, including rhesus macaques, because they have a larger brain, have a high degree of cerebral gyrification, and express all primary cortical structures[19]. We used a dynamic susceptibility contrast (DSC) magnetic resonance imaging (MRI) approach that is based on tracking a bolus of high molecular weight paramagnetic contrast agent (gadodiamide) in the myometrium of the dam and fetal brain. DSC imaging was performed prior to and following the administration of ethanol alcohol at a dose that approximated alcohol intake during a single binge drinking episode in an adult human.
Methods
Animal subjects
Three pregnant dam (Papio hamadryas Anubis) were imaged at the 24th week of pregnancy. Fetal gestation age was calculated from the date of last ovulation. Imaging was performed using a 3 T Siemens Tim Trio scanner equipped with multi-channel body array coils. Animal handling and anesthesia were described elsewhere[20]. Animals were transported from the Southwest National Primate Research Center (SNPRC) to the animal preparation area at the Research Imaging Institute (RII). Fifteen minutes prior to scanning, each animal was sedated with ketamine (10mg/kg) and intubated with an MR-compatible tracheal tube. Once placed in the scanner, the anesthesia was maintained with an MRI-compatible gas anesthesia machine with 5% isofluorane. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Southwest Foundation for Biomedical Research.
Dynamic Susceptibility Contrast (DSC) Perfusion
DSC is a perfusion technique that employs a bolus of an exogenous agent (gadodiamide) to alter the magnetic relaxation properties of the blood [21]. Gadodiamide (OMNISCAN), an injectable contrast agent for MRI, is a diethylenetriamine pentaacetic acid bismethylamide chelate of gadolinium with a molecular weight of 573.3 Da. In the concentration used for the bolus, gadodiamide is capable of drastically (~ 10 fold) shortening the T1, T2, and T2* relaxation times of blood[21]. The dose of the contrast agent was calculated for the dam’s weight using a human adult dose of 0.2 ml/kg, and the contrast was administered via a catheter placed in the radial vein. A susceptibility (T2*) sensitive imaging sequence was used for DSC studies. The signal intensity is reduced in linear proportion to the concentration of the contrast agent and therefore produced regional reductions in signal that are proportional to the perfusion rates[21].
Sequence details
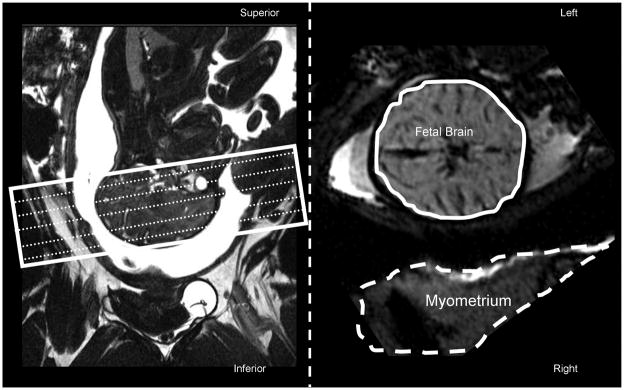
DSC was evaluated in the fetal brain tissue and the myometrium with a gradient echo echo-planar-imaging perfusion sequence. Parameters were adjusted to obtain a high temporal (TR=3sec) and spatial (1.2 × 1.2 × 1.9mm) resolution, acquiring 15 slices for full fetal brain coverage with no slice gaps. Imaging slices were prescribed axially, parallel to the line connecting anterior and posterior commissurals (AC–PC line) (Figure 1, left). Respiratory gating was used to reduce motion artifacts.
Figure 1.
Imaging slices for dynamic susceptibility imaging (white rectangle) were prescribed axially, parallel to the anterior-posterior commissure plane using a sagittal localizer image (Left panel). Right panel shows the axial slice of the fetal brain at the level of mesencephalon and the dam’s uterine muscle, myometrium. Temporal signal intensity trends were analyzed by manually drawing two volumes of interest: one covered the entire fetal brain (solid contour) and the other covered dam’s myometrium (dashed contour).
Protocol
The protocol consisted of two 30 minute DSC studies, one performed before and the other one after the administration of alcohol. During each of the DSC studies, the contrast agent was administered after 5 min of baseline imaging. Alcohol was administered to the dam via a gastro-nasal catheter following the completion of the first DSC study. Alcohol was given at a dose of 3 gm of ethanol per kg of dam’s weight to approximate a blood-alcohol level concentration of ~0.2% at the beginning of the second DSC study. This dose of alcohol is equivalent to the consumption of 6–8 alcoholic drinks during two hours by an adult human to approximate a heavy binge-drinking episode. Blood samples (1ml) were obtained at the end of the first DSC study via radial vein before alcohol administration and 30 min, 50 min and 90 min thereafter. Blood alcohol concentrations were expressed as percent of alcohol in blood, mean± SD for three animals.
Image analysis
EPI images were motion corrected and the signal intensity timelines were analyzed for two volumes of interest manually drawn over the muscles of the uterus, myometrium, (Figure 1, dashed contour) and the fetal brain (Figure 1, solid contour). Signal intensity trends for each of the volumes were normalized to the average baseline value calculated over the first 5 min of acquisition that preceded injection of the gadodiamide.
Data analysis
The DSC trends were averaged for three animals for the pre-and-post alcohol states to identify the minima of signal intensity, which corresponded to the time-of-peak for the contrast concentrations. Linear regression analyses were used to calculate contrast uptake and washout rates (% change from baseline/min) using time-from-peak as the independent variable. The contrast uptake rates were calculated from the time of injection until the TOP. The contrast washout rates were calculated from the time-of-peak until the intensity reached the baseline level.
Results
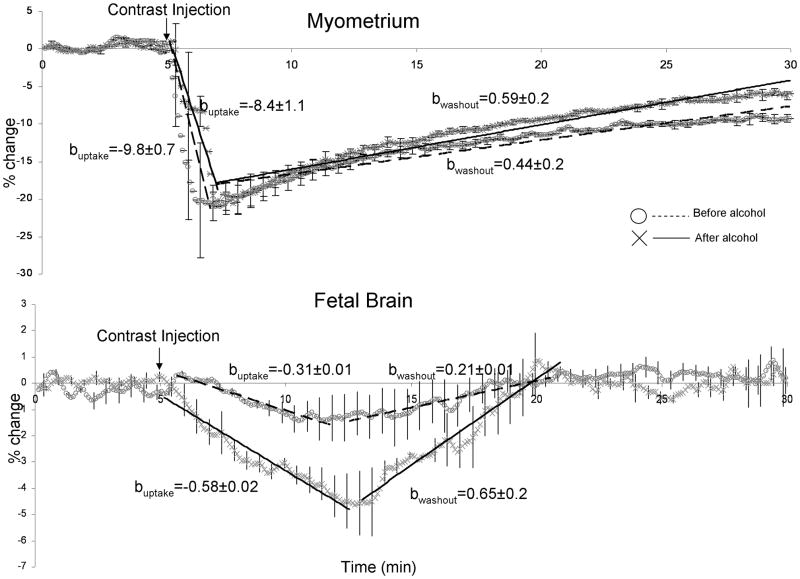
The blood alcohol concentration of the 3 animals was 0.14±0.01% (mean ±SD) at 30min, 0.19±0.02% at 50min and 0.23±0.01% at 90min, following the administration of alcohol. In the myometrium, the peak signal intensity was similar during the baseline and following alcohol administration: −20% vs. −19 %, respectively (Figure 2, top). The times-of-peak for the contrast concentrations were also unaffected by alcohol, with the peak concentrations observed at 147 sec and 150 sec after contrast injection (Figure 2, top). The contrast uptake trends in the uterus also were not significantly different (uptake rates = −9.8±0.7 and −8.4± 1.1 for the pre and post-alcohol states, respectively; t=1.73; p=0.15) (Figure 2 top). However, the rate of the contrast washout was significantly, ~35%, lower for the baseline versus that following the administration of alcohol (washout rates = 0.44±0.2 vs. 0.59±0.02; t=8.6; p<0.01). (Figure 2, top).
Figure 2.
DSC trends in dam’s uterus (top) and fetal brain (bottom). Standard deviation bars are shown for every 10 measurements. Linear regression analysis was used to quantify intake and washout rates before (dotted line) and after (solid line) administration of alcohol. The DSC uptake and washout rates (b-coefficients) are given in % change/min.
In the fetal brain, the peak contrast uptake concentrations and the contrast uptake and washout trends were significantly increased following the administration of alcohol (Figure 2, bottom). The peak signal intensity during the baseline state was about 4 times lower than that following alcohol administration: 1.1% vs. 4.6 %, respectively. Correspondingly, the average signal reduction from the time of the contrast administration until the time of elimination was significantly larger following the administration of alcohol (−0.69±0.54% for baseline and −2.62±1.24% after alcohol administration; t>10.0, p<0.01). The times-of-peak for contrast concentrations were unaffected by alcohol (Figure 2, bottom) but the peak values were observed ~8 min later than in myometrium, indicating that contrast agent continued to increase over multiple passes of maternal circulation. The fetal brain contrast uptake rate was significantly, ~80%, higher, following the administration of alcohol (uptake rates = −0.31±0.01 vs. −0.58± 0.02; t>10.0; p<0.01). The contrast washout rate was also significantly higher, ~300%, following the administration of alcohol (washout rates = 0.21±.01 vs. 0.65±.02; t>10.0, p<0.01) (Figure 2, bottom).
Discussion
To our knowledge, this is the first study to demonstrate the vasoactive effects of alcohol on the fetal cerebral blood flow (CBF) and placental permeability in a non-human primate. It is possible that these findings suggest that the vasoactive effects of alcohol may have increased fetal CBF and/or as altered placental permeability to the contrast agent. Whether these resulted from direct, nitrous-oxide mediated, or the indirect, through vasopressin suppression, mechanism of alcohol action is unclear. The vasoactive effects of the alcohol on the dam’s peripheral circulation were clearly demonstrated by the difference in the DSC trends in the maternal uterus (Figure 2 top). Our data showed that at the blood alcohol concentration levels of ~0.23%, alcohol produced peripheral dilation and increased peripheral blood flow resulting in contrast washout rates that were 35% above baseline. This finding was consistent with other reports of the alcohol-induced vasodilatation, which is thought to be responsible for alcohol-related pathologies in chronic alcohol abusers [14].
We observed several striking differences between the baseline and post-alcohol DSC trends in the fetal brain including the fourfold increase in the peak contrast uptake as well as the two-to-three fold increases in the DSC uptake and washout rates. The baseline DSC trend showed only a small (1%) reduction in the fetal brain signal, compared to ~20% reduction observed in the dam’s uterus (Figure 2). This is an indication that only a small amount of the contrast agent entered fetal cerebral circulation and that the placental barrier was relatively impermeable to the high-molecular weight compounds such as gadodiamide (molecular weight = 573.3Da). Placental membranes serve as an important protective barrier that restrict the passive passage of the higher-molecular weight (greater than 500–600 Da) compounds from the maternal to the fetal blood pools [22,23]. However, following alcohol administration, the signal reduction in the fetal brain was about four times greater (4.5%). If alcohol increases placental permeability, it could result in exposing the fetus to potentially pathogenic substances that normally would not cross the placental barrier. Further studies utilizing gadolinium-labeled compounds with different molecular weights are necessary to map the alcohol-mediated changes placental permeability to even higher-molecular weight compounds.
Additionally, the DSC uptake and washout in the fetal brain were two-to-three times higher following the administration of alcohol. We hypothesize that the increases in the fetal CBF, due to the vasoactive properties of alcohol, are responsible for these differences. The increase in fetal CBF could be explained by several possible mechanisms, including the direct alcohol-mediated vasodilatation of cerebral vasculature such as is observed in adult human brains [9,10]. Similar, vasoactive effects of alcohol and increases in the fetal CBF were previously demonstrated in sheep fetuses [15,16]. There it was concluded that since brain is undergoing a vigorous development during the gestation any perturbation in the normal fetal cerebral physiology should be viewed as potentially detrimental until further studies demonstrate otherwise. The implications of our findings merit further studies, including a study of alcohol-mediated changes of placental permeability to higher molecular weight compounds and a study of alcohol-mediated changes in the fetal CBF using a quantitative, non-invasive perfusion imaging technique, such as the arterial spin labeling[24].
Conclusion
This study used a non-human primate model of antenatal alcohol exposure to demonstrate two potential teratogenic mechanisms associated with maternal alcohol consumption: alcohol-mediated changes in placental permeability and alcohol induced changes in fetal cerebral blood flow. Our findings indicate that even brief alcohol intoxication might detrimentally influence vulnerable neurodevelopmental processes in the brain with potentially long-term consequences.
Acknowledgments
This work was supported, in part, by the NIBIB (K01 #EB006395) grant to PK, the SNPRC (NIH #P51-RR013986) and the NIH/NCRR (#1R01AG029412-01) grants to MDD and NIH (#P01 HD047675 and R24 RR013632) grants to GS.
Footnotes
Author contributions: P.K., C.C, D.M.D., P.T.F., and G.S. designed research;
P.K., C.C., C.S, H.Y.W and D.M.D. performed research and analyzed data;
D.D. D.P. P.T.F. contributed research ideas and tools;
P.K., C.C., D.M.D, D.D., H.Y.W, D.P., P.T.F., C.S., G.S. wrote the paper.
Recent evidence suggests that acute exposure to alcohol (e.g., binge drinking) may also produce untoward effects on fetal CNS.
References
- 1.Guerri C, Bazinet A, Riley EP. Foetal Alcohol Spectrum Disorders and alterations in brain and behaviour. Alcohol Alcohol. 2009;44:108–114. doi: 10.1093/alcalc/agn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fagerlund A, Heikkinen S, Autti-Ramo I, Korkman M, Timonen M, Kuusi T, et al. Brain metabolic alterations in adolescents and young adults with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2006;30:2097–2104. doi: 10.1111/j.1530-0277.2006.00257.x. [DOI] [PubMed] [Google Scholar]
- 3.Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- 4.Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex. 2008;18:136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maier SE, Chen WJ, West JR. Prenatal binge-like alcohol exposure alters neurochemical profiles in fetal rat brain. Pharmacol Biochem Behav. 1996;55:521–529. doi: 10.1016/s0091-3057(96)00282-1. [DOI] [PubMed] [Google Scholar]
- 6.Sulik KK. Genesis of alcohol-induced craniofacial dysmorphism. Exp Biol Med (Maywood) 2005;230:366–375. doi: 10.1177/15353702-0323006-04. [DOI] [PubMed] [Google Scholar]
- 7.Astley SJ, Magnuson SI, Omnell LM, Clarren SK. Fetal alcohol syndrome: changes in craniofacial form with age, cognition, and timing of ethanol exposure in the macaque. Teratology. 1999;59:163–172. doi: 10.1002/(SICI)1096-9926(199903)59:3<163::AID-TERA8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Guerri C, Renau-Piqueras J. Alcohol, astroglia, and brain development. Mol Neurobiol. 1997;15:65–81. doi: 10.1007/BF02740616. [DOI] [PubMed] [Google Scholar]
- 9.Wang GJ, Volkow ND, Franceschi D, Fowler JS, Thanos PK, Scherbaum N, et al. Regional brain metabolism during alcohol intoxication. Alcohol Clin Exp Res. 2000;24:822–829. [PubMed] [Google Scholar]
- 10.Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, et al. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Res. 1988;24:201–209. doi: 10.1016/0165-1781(88)90063-7. [DOI] [PubMed] [Google Scholar]
- 11.Maier SE, Miller JA, West JR. Prenatal binge-like alcohol exposure in the rat results in region-specific deficits in brain growth. Neurotoxicol Teratol. 1999;21:285–291. doi: 10.1016/s0892-0362(98)00056-7. [DOI] [PubMed] [Google Scholar]
- 12.Pierce DR, Goodlett CR, West JR. Differential neuronal loss following early postnatal alcohol exposure. Teratology. 1989;40:113–126. doi: 10.1002/tera.1420400205. [DOI] [PubMed] [Google Scholar]
- 13.Altura BM, Altura BT. Alcohol, the cerebral circulation and strokes. Alcohol. 1984;1:325–331. doi: 10.1016/0741-8329(84)90056-9. [DOI] [PubMed] [Google Scholar]
- 14.Puddey IB, Zilkens RR, Croft KD, Beilin LJ. Alcohol and endothelial function: a brief review. Clin Exp Pharmacol Physiol. 2001;28:1020–1024. doi: 10.1046/j.1440-1681.2001.03572.x. [DOI] [PubMed] [Google Scholar]
- 15.Mayock DE, Ness D, Mondares RL, Gleason CA. Binge alcohol exposure in the second trimester attenuates fetal cerebral blood flow response to hypoxia. J Appl Physiol. 2007;102:972–977. doi: 10.1152/japplphysiol.00956.2006. [DOI] [PubMed] [Google Scholar]
- 16.Ngai AC, Mondares RL, Mayock DE, Gleason CA. Fetal alcohol exposure alters cerebrovascular reactivity to vasoactive intestinal peptide in adult sheep. Neonatology. 2008;93:45–51. doi: 10.1159/000105524. [DOI] [PubMed] [Google Scholar]
- 17.Pillay P, Manger PR. Order-specific quantitative patterns of cortical gyrification. Eur J Neurosci. 2007;25 :2705–2712. doi: 10.1111/j.1460-9568.2007.05524.x. [DOI] [PubMed] [Google Scholar]
- 18.Stewart CB, Disotell TR. Primate evolution - in and out of Africa. Curr Biol. 1998;8:R582–588. doi: 10.1016/s0960-9822(07)00367-3. [DOI] [PubMed] [Google Scholar]
- 19.Kochunov P, Duff Davis M. Development of structural MR brain imaging protocols to study genetics and maturation. Methods. 2009 doi: 10.1016/j.ymeth.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers J, Kochunov P, Lancaster J, Shelledy W, Glahn D, Blangero J, et al. Heritability of brain volume, surface area and shape: An MRI study in an extended pedigree of baboons. Hum Brain Mapp. 2007;28:576–583. doi: 10.1002/hbm.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen BR, Belliveau JW, Vevea JM, Brady TJ. Perfusion imaging with NMR contrast agents. Magn Reson Med. 1990;14:249–265. doi: 10.1002/mrm.1910140211. [DOI] [PubMed] [Google Scholar]
- 22.Dancis J, Dancis J. In: Prenatal and Perinatal Biology and MedicinePhysiology and Growth. Kretchmer N, Quilligan EJ, Johnson JD, editors. Chur: Harwood Academic Publishers; 1987. pp. 1–33. [Google Scholar]
- 23.Audus KL. Controlling drug delivery across the placenta. Eur J Pharm Sci. 1999;8:161–165. doi: 10.1016/s0928-0987(99)00031-7. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Licht DJ. Pediatric perfusion MR imaging using arterial spin labeling. Neuroimaging Clin N Am. 2006;16:149–167. ix. doi: 10.1016/j.nic.2005.10.002. [DOI] [PubMed] [Google Scholar]