Abstract
Current treatments for psychiatric disorders were developed with the aim of providing symptomatic relief rather than reversing underlying abnormalities in neuroplasticity or neurodevelopment that might contribute to psychiatric disorders. This review considers the possibility that psychiatric treatments might be developed that target neuroplasticity deficits or that manipulate neuroplasticity in novel ways. These treatments might not provide direct symptomatic relief. However, they might complement or enhance current pharmacotherapies and psychotherapies aimed at the prevention and treatment of psychiatric disorders. In considering neuroplasticity as a target for the treatment of psychiatric disorders, we build on exciting new findings in the areas of anxiety disorders, mood disorders, and schizophrenia.
Keywords: neuroplasticity, treatment, schizophrenia, depression, anxiety disorders, extinction, fear conditioning, NMDA, glutamate, transcranial magnetic stimulation (TMS), dopamine, neurogenesis, neurotrophin
Neuroplasticity is at the core of all treatments for psychiatric disorders because symptom reduction is presumed to emerge from a change in the function of neural networks. However, a number of recent reviews indicate that psychiatric disorders are, themselves, disorders of neuroplasticity [1–4], suggesting that the efficacy of current treatments may be limited by neuroplasticity abnormalities. This review will consider briefly the hypothesis that new treatments might be developed to enhance neuroplasticity [5]. Medications that enhance neuroplasticity could increase the rapidity of onset or the extent of clinical improvement and mitigate the need for hospitalization in some cases.
This review will highlight a number of examples that illustrate how neuroplasticity might be productively targeted as a strategy for enhancing the treatment of psychiatric disorders. It will begin by considering the use of drugs that enhance the stimulation of the glycineB co-agonist site of N-methyl-D-aspartate (NMDA) glutamate receptors to enhance experience-dependent forms of neuroplasticity, as might be associated with extinction of maladaptive fear in patients with anxiety disorders. The implications of the success of this approach to anxiety disorders for the remediation of cognitive impairments associated with schizophrenia will then be discussed. This review will then present evidence that reductions in cellular resilience and neuroplasticity contribute to mood and stress-related disorders and that these neuroplasticity deficits might be addressed by raising the levels of trophic factors and enhancing related signal transduction mechanisms. Finally, this review will discuss clinical treatments modeled after two well-studied preclinical paradigms for manipulating depression (LTD) [7]. Together, these examples highlight a growing number of novel treatments for psychiatric disorders that are emerging from the effort to design treatments targeting or encompassing neuroplasticity.
Combining a generalized increase in NMDA receptor-dependent neuroplasticity with circuit-specific experience-dependent neuroplasticity: The examples of fear extinction for anxiety disorders and cognitive remediation for schizophrenia
Cognitive-behavioral therapy incorporating graded, prolonged exposure to feared stimuli is among the most promising treatments for anxiety disorders [8]. Exposure interventions include in vivo exposure (direct confrontation of feared and avoided situations or activities), imaginal exposure (prolonged and detailed imagining or remembering of feared and avoided thoughts), and interoceptive exposure (exercises designed to elicit feared physical sensations). The purported mechanism of exposure is extinction, in which repeated presentations of a conditioned stimulus (CS), outside the presence of an unconditioned stimulus (US), eventually leads to reductions in the conditioned response (CR). Extinction does not imply that the organism forgets the original CS-US association; rather, it is thought to represent the learning of new associations (e.g., the CS becomes associated with stimuli other than the US) that eventually inhibits the original association [9]. However, as new adaptive associations are consolidated and reinforced, the reconsolidation of Classical (Pavlovian) fear conditioning, extinction, and reconsolidation are all NMDA receptor-dependent forms of neuroplasticity involving glutamatergic inputs into the basolateral amygdala [10–13]. Drugs that facilitate NMDA receptor function via glycine site have not been shown to have potent direct anxiolytic effects in animals or humans [14–18]. However, drugs that facilitate NMDA receptor function might promote a variety of NMDA receptor-dependent forms of neuroplasticity, including extinction [19]. The addition of D-cycloserine (DCS) to exposure therapy for anxiety disorders provides the clearest example of the capacity of a medication that increases neuroplasticity diffusely in the brain to enhance the efficacy of a behavioral therapy that produces neuroadaptations in particular circuits. DCS, is a partial agonist of the glycineB coagonist site of NMDA receptors with relatively more agonist or antagonist effects at NMDA receptor subtypes [20–22]. When surrounding glycine levels are low, DCS facilitates NMDA receptor function. However, when glycine levels are sufficient to saturate glycineB sites, DCS may reduce NMDA receptor function [23–25]. Therefore, DCS may improve the efficacy of exposure-based psychotherapies by enhancing NMDA receptor functioning, thereby increasing neuroplasticity, or by reducing NMDA receptor function and interfering with the (re) consolidation of fear memories. Both processes are thought to facilitate fear extinction [5,26,27]. DCS promoted the extinction of fear conditioning in animals, regardless of whether it was present during extinction sessions [28] or immediately after extinction training [29]. These data suggest that DCS facilitates extinction by influencing the consolidation of new learning. In the first study of DCS augmentation of exposure therapy in humans [18], acrophobic patients receiving DCS appeared to benefit more from virtual reality exposure therapy than did patients receiving placebo. These results have now been replicated and extended using DCS in combination with exposure therapy for patients with social anxiety disorder [30,31], panic disorder [32], and obsessive-compulsive disorder [33,34].
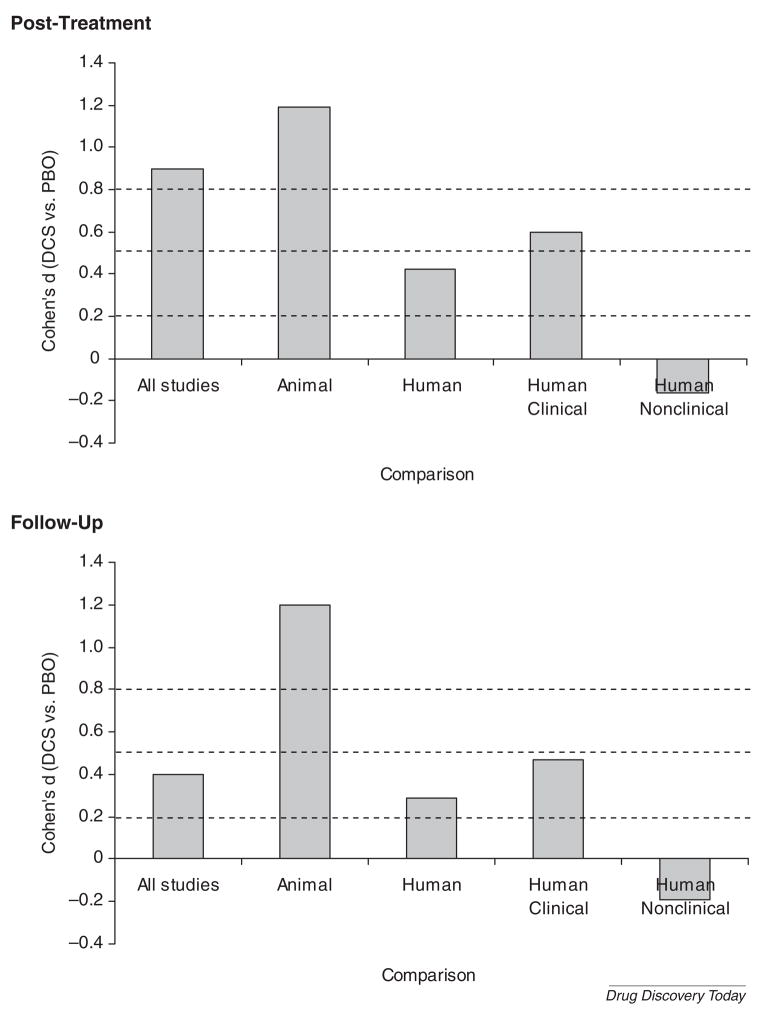
Figure 1 presents the results of a recent quantitative review of 15 placebo-controlled studies (N = 632, 30 independent samples; 5 animal studies, 10 human studies [35]) of DCS augmentation of extinction training/exposure therapy. At post-treatment, DCS augmentation was associated with a significant and large effect, indicating that DCS reliably augments the effects of fear extinction/exposure therapy. Animal studies showed a significantly greater effect than did human studies (perhaps not surprising, given the greater experimental control over genetic variation and extraneous variables in animal studies). A secondary analysis revealed that human nonclinical studies showed no significant DCS effect, while human clinical studies showed that DCS produced significant effects with a moderate effect size. A similar pattern was seen at follow-up, suggesting that the effects of DCS augmentation do not disappear upon treatment discontinuation, a potential improvement over other pharmacotherapy augmentation strategies that may actually increase the risk of relapse after discontinuation [36,37].
Figure 1.
Effect size (Cohen’s d) of controlled trials of d-cycloserine (DCS) vs. placebo (PBO). Note: Adapted from [35].
Examination of moderator variables found no evidence of a DCS dose-response relationship across studies. However, the timing of the DCS dose significantly predicted effect size, with the greatest effects evident among studies in which DCS was administered either immediately before or after exposure, consistent with the preclinical studies. Smaller DCS effects were also seen for those studies in which the combination of DCS and exposure occurred many times. This decrease in efficacy may reflect the development of tolerance to DCS [38] or the high level of efficacy of repeated exposures in the patients studied to date, i.e., a “floor effect” that might reduce the ability to detect the effects of DCS.
Thus, the studies with DCS provide initial support for the hypothesis that a drug that enhanced neuroplasticity by facilitating the activation of NMDA glutamate receptors might promote the efficacy of extinction-based CBT. Future studies with full agonists of the glycineB site of the NMDA receptor complex (glycine, D-serine, D-alanine) as well as glycine or D-serine transporter antagonists will help to better evaluate this treatment modality.
In contrast to the glycine-related agents, β-receptor blockade might be a strategy to preferentially disrupt the reconsolidation of fear-related learning. Noradrenergic systems play a number of roles in the neurobiology of memory in animals and humans [39,40]. Recent animal data suggest that β-receptor blockade preferentially disrupts the reconsolidation of fear learning relative to the initial consolidation of learning [41,42]. This finding may be consistent with the ability of β-receptor blockade to reduce reconsolidation of fear learning associated with post-traumatic stress disorder (PTSD) [43,44], while leaving initial fear learning intact [45]. in contrast preliminary evidence suggests that propranolol may not be effective for the prevention of PTSD [46], despite some intiial promise of this strategy [47].
Drugs, like DCS, that promote neuroplasticity via enhancement of NMDA receptor function might enhance the efficacy of cognitive and behavioral therapies for many psychiatric disorders. It is intriguing to consider the possibility that the efficacy of glycine-related treatments substances for schizophrenia might follow a paradigm similar to the anxiety disorders, i.e., that they may promote neuroplasticity rather than directly suppressing symptoms and cognitive deficits associated with schizophrenia. A large number of studies that have suggested that glycine-related substances (glycine, DCS, D-serine, D-alanine, or sarcosine) modestly improve the efficacy of antipsychotic treatment when added to drugs other than clozapine, a drug that has intrinsic glycine transporter activity [16,48–50]. However, there are a number of negative trials, including the largest study of this mechanism [51]. There may be many reasons for the negative findings. One reason may be that it is not clear how glycine should be optimally dose to produce clinical benefit, i.e., we have only limited information about the central bioavailability of peripherally administered glycine in humans [52]. Based on the data with anxiety disorders [53], one might predict that a limited number of glycine treatments would be more effective than chronic treatment in order to minimize the impact of tolerance to glycine effects. To date, however, all studies of glycine treatments have employed daily or twice-daily administration schedules for several weeks. Also, based on the anxiety studies, glycine might be predicted to augment the efficacy of a rehabilitative treatment while having limited effects on its own. Cognitive remediation therapy, like fear extinction, would seem to be relatively amenable to this strategy. Cognitive remediation involves the repetitive activation of circuits underlying particular aspects of cognition in order to engage use-dependent forms of neuroplasticity to reduce functional impairments within these circuits [54–56]. Reductions in neuroplasticity intrinsic to schizophrenia would be predicted to constrain the benefits of this type of treatment [3]. Currently, there are no published studies evaluating the interactive effects of glycine-related substances and a cognitive remediation strategy or cognitive-behavioral therapy for schizophrenia. However, there are data that might be consistent with a primary glycine effect on neuroplasticity rather than symptom suppression in schizophrenia. When it works, the benefits of glycine persist for several weeks following its termination, despite its short plasma half-life [52,57,58].
Restoring neuroplasticity, neurogenesis, and gliogenesis through neurotrophic mechanisms: The case for depression and stress-related disorders
The concept that depression could be related to decreased cellular resiliency and impaired plasticity emerged from a series of antemortem and postmortem studies of mood disorders describing significant structural abnormalities [59–61] and histopathological changes, including decreased neuronal and glial density and reduced glial cell numbers [62–65] in multiple brain regions. Parallel findings demonstrated that prolonged stress accelerated the age related decreases in the number of hippocampal neurons in rodents [66] and resulted in changes in dendritic branching in hippocampus, amygdala and prefrontal cortex (PFC) [67–69]. More recent data show that these stress-induced morphological changes have functional correlates, resulting in diminished responses to apically targeted excitatory inputs [70], and deficits in attentional control that are commonly associated with stress-related mental illnesses [71]. Additional studies provide strong evidence of stress-related decreases in the rates of cell proliferation and survival [72,73]. Of special interest to the field of drug development, it was noted that many established antidepressant treatments opposed stress effects on dendritic atrophy [74] and glial cell loss [75], as well as cell proliferation and survival [76–79]. The idea that antidepressant-induced effects on cell proliferation mediated the beneficial cognitive and behavioral effects of the drug was bolstered by evidence that hippocampal neurogenesis was required for the expression of the behavioral effects of antidepressants [80].
A neurotrophic hypothesis suggested that the opposing effects of stress and antidepressant drugs are mediated by modulation of kinase activity, resulting in changes in cyclic AMP (cAMP) levels and altered cAMP response element binding protein (CREB) regulated brain-derived neurotrophic factor (BDNF) gene expression [81,82]. This hypothesis is supported by postmortem human studies of the hippocampus and PFC, and serum studies of depressed patients demonstrating decreased levels of BDNF mRNA and protein in non-medicated depressed patients, but either increased or similar BDNF levels in patients taking antidepressant drugs [83–86]. Although much attention remains directed on the specific role of BDNF in the hippocampus and frontal cortex, there is new evidence of the involvement of several additional neurotrophic factors including fibroblast growth factor (FGF) [87], insulin-like growth factor (IGF-1) [88], and vasoendothelial growth factor (VEGF) [89]. In addition, there are regional differences with regard to the effects of stress and antidepressants on neurotrophic factor regulation [90].
Beyond the neurotrophins, other mechanisms critical to the regulation of plasticity may contribute to the pathophysiology of mood disorders and to antidepressant efficacy. For example, there is growing evidence of stress and antidepressant drugs on chromatin remodeling [91], with recent evidence suggesting that early epigenetic processes produce long-standing effects on neuroplasticity and cellular resiliency that may persist into adulthood. Furthermore, the ability of drugs such as valproic acid that modulate histone deacetylase (HDAC) proteins [92,93] and enhance long-term memory for both acquisition and extinction may provide the mechanism of action to some mood stabilizing medications, and suggest a role for the drugs as adjuncts to behavior therapy [94,95].
Recent studies now suggest that activity-dependent plasticity may be impaired in depressed patients and that antidepressant drugs may reverse or attenuate this deficit [96,97]. For example, stimulus induced plasticity is impaired in the visual system of depressed individuals and that chronic administration of sertraline to healthy subjects increased the amplitude and plasticity of the evoked potentials [98]. In addition, fluoxetine administration enhances the plasticity of ocular dominance columns in adult rats [99]. Together these studies provide strong evidence that antidepressant medications promote activity-dependent plasticity in visual cortex, and raise the possibility that similar plasticizing effects can be seen in brain circuits more closely related to mood regulation and cognition. Future studies might target these mechanisms more directly via actions on glutamate and GABA receptors [100,101].
The notion that antidepressants may work by increasing activity-dependent plasticity parallels the prior studies of DCS in anxiety disorders. In this case, again, providing the medication alone may not be sufficient, or at least not the optimal strategy, for reversing the pathophysiological state of depression. Selective activation of specific brain circuits and synapses may synergize with drug therapies to reinforce and strengthen desirable behaviors and cognitive schemata that are useful in reversing and preventing depressive episodes. This may contribute to the interesting finding demonstrating that the combined use of an antidepressant medication with cognitive-behavioral therapy for chronic depression was much more effective than either treatment alone [102]. In an attempt to explore this hypothesis, a recent pilot study found that CBT augmentation of ECT may enhance the antidepressant effectiveness of the treatment and delay the time to relapse [103]. Obviously, this area of investigation now requires much more rigorous studies before any firm conclusions can be made related to clinical practice. Also, it would be interesting to determine whether a drug that enhances neuroplasticity generally, like DCS, promotes the clinical efficacy of traditional antidepressant treatments.
Using a sensitizing administration regimen to achieve lasting benefits from dopamine D1 receptor agonists in schizophrenia
It would be elegant to design a treatment regimen that turned a pathophysiologic process into a treatment mechanism and, in so doing, solved an obstacle in drug development, the problem of the development of tolerance to agonists. There has been substantial interest in the potential value of dopamine D1 receptor agonists for treating cognitive impairments in schizophrenia [104]. The benefits of D1 receptor agonist treatment might, however, be limited by the emergence of tolerance [105–107]. Thus, there has been interest in strategies that might circumvent this limitation.
One potential strategy emerged from studies of the sensitization to the psychotigenic effects of psychostimulants [108]. Although there is recent evidence of psychostimulant sensitization in humans [109], it has been demonstrated more clearly and robustly in animals. In rodents, psychostimulant administration produces glutamate release in multiple brain regions that produces an NMDA receptor-dependent form of synaptic neuroplasticity that contributes to the behavioral features of stimulant sensitization [110–112]. Amphetamine sensitization in the nonhuman primate induces a disorder characterized by long-lasting alterations in behavior, profound working memory impairments, and a deterioration in the integrity of prefrontal neuronal circuitry [113–115].
The process of sensitization, however, also might be exploited for the treatment of cognitive impairments associated with schizophrenia [116]. Thus, long-term administration of neuroleptics down-regulates D1 receptor function, leading to working memory deficits which can be reversed by repeated intermittent treatment with a full D1 agonist [6]. In this case primates received multiple sessions involving the administration of very low doses D1 agonists that were interleaved with washouts. Under these conditions primates began to respond to doses that were previously too low to produce behavioral effects. They also showed progressive improvements in working memory that persisted long after cessation of treatment. Together, these findings were suggestive of an underlying process of sensitization. This hypothesis was tested in another dopamine/D1 deficient state, namely natural aging, and it was found that this same sensitizing regime of D1 agonist treatment profoundly enhanced working memory performance in elderly, but not young-adult, nonhuman primates and again this benefit persisted long after treatment [117]. Thus, by administering low doses of D1 receptor agonists intermittently, it is conceivable that one could surmount the problem of tolerance development to these agents, producing long-lasting or even irreversible improvement of some of the cognitive impairments associated with schizophrenia.
Delivering low frequency transcranial magnetic stimulation to depotentiate cortical synapses: treating antipsychotic-resistant auditory hallucinations
In approximately 25% of patients diagnosed with schizophrenia, auditory hallucinations (AHs) respond poorly or not at all to currently available antipsychotic medication [118]. One important feature of AHs is that they generally are experienced as spoken speech with discernable loudness, timbre and other “percept-like” features. These characteristics suggest direct involvement of speech perception neurocircuitry.
An early O-15 positron emission tomography study found that activation in left temporoparietal regions accompanied AHs [119]. These brain regions are adjacent to the Wernicke’s area and active during speech perception [120]. Numerous studies have found that 1-Hz repetitive transcranial magnetic stimulation (1-Hz rTMS) reduces cortical excitability [121–126]. These effects appear analogous to long-term depression (LTD) elicited by 1-Hz direct electrical stimulation of grey matter in animal studies, which can endure for many weeks [7,127]. We consequently predicted that “suppressive” 1-hertz rTMS delivered to the temporoparietal cortex might reduce AHs. Clinical trials comparing this intervention strategy with sham stimulation in patients experiencing AHs have been undertaken at Yale [128,129] and elsewhere. A meta-analysis considering 10 sham-controlled double-masked studies found robust evidence of efficacy relative to sham stimulation based on a combined total N of 212 (effect size = 0.76 95% CI 0.36–1.17 [130]). Most recently, functional magnetic resonance imaging (fMRI) maps of abnormal activation and functional connectivity have been used to position rTMS in patients with especially severe AHs [131]. Delivering rTMS to temporoparietal sites in Wernicke’s area and the adjacent supramarginal gyrus was accompanied by a greater rate of AH improvement compared to sham stimulation. Repetitive TMS delivered to other sites did not consistently improve AHs. These findings suggest that targeted brain stimulation positive symptoms can produce clinical improvement in patients with schizophrenia.
Commentary
Traditional treatments for psychiatric disorders emerged from the convergence of happy accident and acute clinical observation, i.e., when administration of a substance suppressed symptoms. However, the treatment strategies reviewed above diverge from this approach, emerging from mechanistic foundations in basic research that may translate to novel treatments. As a result, this review identified some promising new treatment approaches as well as emerging conceptual approaches to medications development for psychiatry.
A treatment that works by increasing neuroplasticity may require combination with another treatment, perhaps a cognitive-behavioral therapy, to exhibit efficacy.
Traditional medication development strategies assume that medications, by themselves, produce the necessary adaptations in synaptic function to demonstrate efficacy in animal models. However, a drug that increases neuroplasticity might require testing in animal models involving behavioral change, for example extinction, to exhibit efficacy. Similarly, these drugs may only show clinical efficacy in humans in combination with these cognitive or behavioral manipulations, as was the case for glycine-related treatments for anxiety disorders and perhaps schizophrenia.
Cellular resilience, i.e., neuronal and glial structural integrity may be targeted by treatments for psychiatric disorders.
Treatments for psychiatric disorders have traditionally used behavioral rather than biological endpoints. However, strategies involving raising neurotrophin levels to restore synaptic connectivity or to stimulate neurogenesis might have structural endpoints that precede behavioral change.
Agonist administration schedules may be designed to produce sensitization rather than tolerance.
The model of D1 receptor agonist sensitization suggests that doses that are too low to produce initial behavioral effects might become effective doses with repeated but intermittent administration. Equally intriguing is the possibility that sensitization strategies might produce long-lasting or even irreversible improvement mitigating the need for further drug administration.
TMS, deep brain stimulation [132] and other focal neurostimulation treatments may be administered to shape the function of cortical networks, i.e., to potentiate or depotentiate synaptic function.
TMS may serve to produce specific forms, for example resembling LTD or LTP, in particular circuits. In producing a specific form of use-dependent neuroplasticity in a circumscribed circuitry, TMS shares some features of cognitive and behavioral therapy. From this perspective, future research might explore ways that TMS might be combined with drugs that affect neuroplasticity diffusely in the brain, such as the glycine-related agents.
In considering neuroplasticity as a treatment, many questions emerge. For example, one might expect that new strategies would be needed to identify drugs that would act to modulate neuroplasticity in therapeutic ways, but might be behaviorally inactive by themselves. Major challenges for this field of research may be economic or regulatory rather than scientific. For example, would a pharmacologic treatment for a psychiatric disorder that involved the administration of a limited number of doses be sufficiently profitable to justify the investment of the pharmaceutical industry? If not, what other type of company, research foundation, governmental organization, or academic institution would have the capacity to test these drugs? Also, how does one develop and obtain FDA approval for a medication that must be administered in combination with a specific form of cognitive behavioral therapy? In particular, how important will it be to validate and standardize the psychotherapeutic component of the medication-therapy combination? These and other challenges will accompany the enormous apparent opportunities associated with the development of agents that attempt to facilitate the treatment of psychiatric disorders through the modulation of neuroplasticity.
Acknowledgments
This work was generously supported by Department of Veterans Affairs (via support for the Alcohol Research Center, Merit Review Grant, National Center for PTSD, VA REAP), the National Institute on Alcohol Abuse and Alcoholism (RO1 AA-11321, K05 AA-14906-01, I-P50 AA-12870), the National Institute of Mental Health (MH P50 MH44866, P50 MH068789, K02 MH076222), NARSAD, and the Yale Center for Clinical Investigation (CTSA),
Declaration of financial interests: During the period of 2007–2009, Dr. Krystal has served as a scientific consultant to the following companies: AstraZeneca Pharmaceuticals, Cypress Bioscience, Forest Laboratories, Glaxo SmithKline, Lohocla Research Corporation, HoustonPharma, Eli Lilly and Company, Pfizer Pharmaceuticals, Schering-Plough Research Institute, SK Life Sciences, Takeda Industries, and Transcept Pharmaceuticals. He holds less than $10,000 in exercisable warrant options with Transcept Pharmaceuticals. He is the principal investigator of a multicenter study in which Janssen Research Foundation has provided drug and some support to the Department of Veterans Affairs. He is a co-sponsor for two patents under review for glutamatergic agents targeting the treatment of depression. During the period of 2007–2009, Dr. Tolin received research funding from Organon/Schering-Plough, Pfizer, and Indevus Pharmaceuticals. During the period of 2007–2009, Dr. Sanacora has served as a scientific consultant to or accepted honoraria from the following companies: AstraZeneca Pharmaceuticals, Bristol-Myers Squibb, Cenestra, Eli Lilly and Company, Lundbeck, Pfizer Pharmaceuticals, Hoffmann-La Roche Ltd, Ruxton Inc. and Sepracor. He is or has been the principal investigator of studies funded by AstraZeneca Pharmaceuticals, Bristol-Myers Squibb, Pfizer Pharmaceuticals, Hoffmann-La Roche Ltd, Ruxton Inc, and Sepracor since 2007. He is a cosponsor on a patent under review for glutamatergic agents targeting the treatment of depression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12 (2):120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33 (1):88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 3.Krystal JH, et al. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: Toward a paradigm shift in medication development. Psychopharmacology. 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 4.Sapolsky RM. Stress and plasticity in the limbic system. Neurochem Res. 2003;28 (11):1735–1742. doi: 10.1023/a:1026021307833. [DOI] [PubMed] [Google Scholar]
- 5.Krystal JH. Neuroplasticity as a target for the pharmacotherapy of psychiatric disorders: new opportunities for synergy with psychotherapy. Biol Psychiatry. 2007;62 (8):833–834. doi: 10.1016/j.biopsych.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Castner SA, et al. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287 (5460):2020–2022. doi: 10.1126/science.287.5460.2020. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman RE, Cavus I. Slow transcranial magnetic stimulation, long-term depotentiation, and brain hyperexcitability disorders. American Journal of Psychiatry. 2002;159(7):1093–1102. doi: 10.1176/appi.ajp.159.7.1093. [DOI] [PubMed] [Google Scholar]
- 8.Nathan PE, Gorman JM. A guide to treatments that work. Oxford University Press; 2002. [Google Scholar]
- 9.Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- 10.Miserendino MJ, et al. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345 (6277):716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- 11.Rogan MT, et al. Fear conditioning induces associative long-term potentiation in the amygdal. Nature. 1997;390(6660):604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 12.Royer S, Pare D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115 (2):455–462. doi: 10.1016/s0306-4522(02)00455-4. [DOI] [PubMed] [Google Scholar]
- 13.Goosens KA, Maren S. Long-term potentiation as a substrate for memory: evidence from studies of amygdaloid plasticity and Pavlovian fear conditioning. Hippocampus. 2002;12 (5):592–599. doi: 10.1002/hipo.10099. [DOI] [PubMed] [Google Scholar]
- 14.Krystal JH, et al. NMDA agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv Rev Psychiatry. 1999;7 (3):125–143. [PubMed] [Google Scholar]
- 15.D’Souza DC, et al. Glycine site agonists of the NMDA receptor: a review. CNS Drug Reviews. 1995;1:227–260. [Google Scholar]
- 16.Javitt DC. Is the glycine site half saturated or half unsaturated? Effects of glutamatergic drugs in schizophrenia patients. Curr Opin Psychiatry. 2006;19 (2):151–157. doi: 10.1097/01.yco.0000214340.14131.bd. [DOI] [PubMed] [Google Scholar]
- 17.Linn GS, et al. Behavioral effects of orally administered glycine in socially housed monkeys chronically treated with phencyclidine. Psychopharmacology (Berl) 2007;192 (1):27–38. doi: 10.1007/s00213-007-0771-6. [DOI] [PubMed] [Google Scholar]
- 18.Ressler KJ, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61 (11):1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 19.Davis M. Role of NMDA receptors and MAP kinase in the amygdala in extinction of fear: clinical implications for exposure therapy. European Journal of Neuroscience. 2002;16(3):395–398. doi: 10.1046/j.1460-9568.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 20.Danysz W, Parsons CG. Glycine and N-methyl-D-aspartate receptors: Physiological significance and possible therapeutic applications. Pharmacology Reviews. 1988;50:597–664. [PubMed] [Google Scholar]
- 21.Krueger JM, et al. Glycine site agonists exhibit subunit specific effects on NMDA receptors expressed in Xenopus oocytes. Society of Neuroscience Abstracts. 1997;23:945. [Google Scholar]
- 22.Sheinin A, et al. Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology. 2001;41 (2):151–158. doi: 10.1016/s0028-3908(01)00073-9. [DOI] [PubMed] [Google Scholar]
- 23.Emmett MR, et al. Actions of D-cycloserine at the N-methyl-D-aspartate-associated glycine receptor site in vivo. Neuropharmacology. 1991;30(11):1167–1171. doi: 10.1016/0028-3908(91)90161-4. [DOI] [PubMed] [Google Scholar]
- 24.Hood WF, et al. D-cycloserine: a ligand for the N-methyl-D-aspartate coupled glycine receptor has partial agonist characteristics. Neuroscience Letters. 1989;98(1):91–95. doi: 10.1016/0304-3940(89)90379-0. [DOI] [PubMed] [Google Scholar]
- 25.Watson GB, et al. D-cycloserine acts as a partial agonist at the glycine modulatory site of the NMDA receptor expressed in Xenopus oocytes. Brain Research. 1990;510(1):158–160. doi: 10.1016/0006-8993(90)90745-w. [DOI] [PubMed] [Google Scholar]
- 26.Lee JL. Memory reconsolidation mediates the strengthening of memories by additional learning. Nat Neurosci. 2008;11 (11):1264–1266. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- 27.Nader K. Memory traces unbound. Trend Neurosci. 2003;26 (2):65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- 28.Walker DL, et al. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22 (6):2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ledgerwood L, et al. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117 (2):341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann SG, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63 (3):298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 31.Guastella AJ, et al. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63 (6):544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Otto MW, et al. Efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. 2009 doi: 10.1016/j.biopsych.2009.07.036. Under review. [DOI] [PubMed] [Google Scholar]
- 33.Kushner MG, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62 (8):835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 34.Wilhelm S, et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165(3):335–341. doi: 10.1176/appi.ajp.2007.07050776. quiz 409. [DOI] [PubMed] [Google Scholar]
- 35.Norberg MM, et al. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63 (12):1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Barlow DH, et al. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. Jama. 2000;283 (19):2529–2536. doi: 10.1001/jama.283.19.2529. [DOI] [PubMed] [Google Scholar]
- 37.Marks IM, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry. 1993;162:776–787. doi: 10.1192/bjp.162.6.776. [DOI] [PubMed] [Google Scholar]
- 38.Parnas AS, et al. Effects of multiple exposures to D-cycloserine on extinction of conditioned fear in rats. Neurobiol Learn Mem. 2005;83 (3):224–231. doi: 10.1016/j.nlm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 39.McGaugh JL. Memory: A century of consolidation. Science. 2000;287 (5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 40.Roozendaal B, et al. Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proc Natl Acad Sci U S A. 1999;96 (20):11642–11647. doi: 10.1073/pnas.96.20.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129 (2):267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 42.Debiec J, LeDoux JE. Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: treatment implications for PTSD. Ann N Y Acad Sci. 2006;1071:521–524. doi: 10.1196/annals.1364.056. [DOI] [PubMed] [Google Scholar]
- 43.Taylor F, Cahill L. Propranolol for reemergent posttraumatic stress disorder following an event of retraumatization: a case study. J Trauma Stress. 2002;15 (5):433–437. doi: 10.1023/A:1020145610914. [DOI] [PubMed] [Google Scholar]
- 44.Brunet A, et al. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42 (6):503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Orr SP, et al. Effects of beta blockade, PTSD diagnosis, and explicit threat on the extinction and retention of an aversively conditioned response. Biol Psychol. 2006;73 (3):262–271. doi: 10.1016/j.biopsycho.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Stein MB, et al. Pharmacotherapy to prevent PTSD: Results from a randomized controlled proof-of-concept trial in physically injured patients. J Trauma Stress. 2007;20 (6):923–932. doi: 10.1002/jts.20270. [DOI] [PubMed] [Google Scholar]
- 47.Pitman RK, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51 (2):189–192. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- 48.Yang CR, Svensson KA. Allosteric modulation of NMDA receptor via elevation of brain glycine and d-serine: The therapeutic potentials for schizophrenia. Pharmacol Ther. 2008;120 (3):317–332. doi: 10.1016/j.pharmthera.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Millan MJ. N-Methyl-D-aspartate receptors as a target for improved antipsychotic agents: novel insights and clinical perspectives. Psychopharmacology (Berl) 2005;179 (1):30–53. doi: 10.1007/s00213-005-2199-1. [DOI] [PubMed] [Google Scholar]
- 50.Tuominen HJ, et al. Glutamatergic drugs for schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2005;72 (2–3):225–234. doi: 10.1016/j.schres.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Buchanan RW, et al. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164 (10):1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- 52.D’Souza DC, et al. IV glycine and oral D-cycloserine effects on plasma and CSF amino acids in healthy humans. Biol Psychiatry. 2000;47 (5):450–462. doi: 10.1016/s0006-3223(99)00133-x. [DOI] [PubMed] [Google Scholar]
- 53.Norberg MM, et al. A Meta-Analysis of D-Cycloserine and the Facilitation of Fear Extinction and Exposure Therapy. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 54.Fiszdon JM, et al. Durability of cognitive remediation training in schizophrenia: performance on two memory tasks at 6-month and 12-month follow-up. Psychiatry Res. 2004;125 (1):1–7. doi: 10.1016/j.psychres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Wexler BE, Bell MD. Cognitive remediation and vocational rehabilitation for schizophrenia. Schizophr Bull. 2005;31 (4):931–941. doi: 10.1093/schbul/sbi038. [DOI] [PubMed] [Google Scholar]
- 56.Greig TC, et al. Improved cognitive function in schizophrenia after one year of cognitive training and vocational services. Schizophr Res. 2007;96 (1–3):156–161. doi: 10.1016/j.schres.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heresco-Levy U, et al. Double-blind, placebo-controlled, crossover trial of glycine adjuvant therapy for treatment-resistant schizophrenia. Br J Psychiatry. 1996;169 (5):610–617. doi: 10.1192/bjp.169.5.610. [DOI] [PubMed] [Google Scholar]
- 58.Heresco-Levy U, et al. High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia. Biol Psychiatry. 2004;55 (2):165–171. doi: 10.1016/s0006-3223(03)00707-8. [DOI] [PubMed] [Google Scholar]
- 59.Drevets WC, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386 (6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 60.Sheline YI, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93 (9):3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161 (11):1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 62.Rajkowska G, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biological Psychiatry. 1999;45 (9):1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 63.Ongur D, et al. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences of the United States of America. 1998;95 (22):13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cotter D, et al. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58 (6):545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 65.Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6 (3):219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sapolsky RM, et al. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985;5 (5):1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Magarinos AM, et al. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci U S A. 1997;94 (25):14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Radley JJ, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125 (1):1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 69.McEwen BS, Chattarji S. Molecular mechanisms of neuroplasticity and pharmacological implications: the example of tianeptine. Eur Neuropsychopharmacol. 2004;14(Suppl 5):S497–502. doi: 10.1016/j.euroneuro.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 70.Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A. 2008;105 (1):359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liston C, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26 (30):7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gould E, et al. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. Journal of Neuroscience. 1997;17 (7):2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16 (3):239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 74.McEwen BS, et al. Prevention of stress-induced morphological and cognitive consequences. Eur Neuropsychopharmacol. 1997;7(Suppl 3):S323–328. doi: 10.1016/s0924-977x(97)00064-3. [DOI] [PubMed] [Google Scholar]
- 75.Czeh B, et al. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31 (8):1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- 76.Malberg JE, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. Journal of Neuroscience. 2000;20 (24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Madsen TM, et al. Electroconvulsive seizure treatment increases cell proliferation in rat frontal cortex. Neuropsychopharmacology. 2005;30 (1):27–34. doi: 10.1038/sj.npp.1300565. [DOI] [PubMed] [Google Scholar]
- 78.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18 (5–6):391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 79.Banasr M, et al. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62 (5):496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 80.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301 (5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 81.Duman RS, et al. A molecular and cellular theory of depression. Archives of General Psychiatry. 1997;54 (7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 82.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59 (12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 83.Piccinni A, et al. Plasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatments. J Affect Disord. 2008;105 (1–3):279–283. doi: 10.1016/j.jad.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 84.Lee HY, Kim YK. Plasma brain-derived neurotrophic factor as a peripheral marker for the action mechanism of antidepressants. Neuropsychobiology. 2008;57 (4):194–199. doi: 10.1159/000149817. [DOI] [PubMed] [Google Scholar]
- 85.Sen S, et al. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64 (6):527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Basterzi AD, et al. Effects of fluoxetine and venlafaxine on serum brain derived neurotrophic factor levels in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008 doi: 10.1016/j.pnpbp.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 87.Riva MA, et al. Emerging role of the FGF system in psychiatric disorders. Trends Pharmacol Sci. 2005;26 (5):228–231. doi: 10.1016/j.tips.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 88.Malberg JE, et al. Increasing the levels of insulin-like growth factor-I by an IGF binding protein inhibitor produces anxiolytic and antidepressant-like effects. Neuropsychopharmacology. 2007;32 (11):2360–2368. doi: 10.1038/sj.npp.1301358. [DOI] [PubMed] [Google Scholar]
- 89.Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A. 2007;104 (11):4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eisch AJ, et al. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54 (10):994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 91.Tsankova NM, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9 (4):519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 92.Gottlicher M, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. Embo J. 2001;20 (24):6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Phiel CJ, et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276 (39):36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 94.Bredy TW, Barad M. The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learn Mem. 2008;15 (1):39–45. doi: 10.1101/lm.801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bredy TW, et al. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14 (4):268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Castren E, et al. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7 (1):18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 97.Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry. 2007;12 (12):1079–1088. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- 98.Normann C, et al. Long-term plasticity of visually evoked potentials in humans is altered in major depression. Biol Psychiatry. 2007;62 (5):373–380. doi: 10.1016/j.biopsych.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 99.Maya Vetencourt JF, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320 (5874):385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 100.Hensch TK, Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Prog Brain Res. 2005;147:115–124. doi: 10.1016/S0079-6123(04)47009-5. [DOI] [PubMed] [Google Scholar]
- 101.Ge S, et al. Synaptic integration and plasticity of new neurons in the adult hippocampus. J Physiol. 2008;586 (16):3759–3765. doi: 10.1113/jphysiol.2008.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Keller MB, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med. 2000;342 (20):1462–1470. doi: 10.1056/NEJM200005183422001. [DOI] [PubMed] [Google Scholar]
- 103.Fenton L, et al. Can cognitive behavioral therapy reduce relapse rates of depression after ECT? a preliminary study. J Ect. 2006;22 (3):196–198. doi: 10.1097/01.yct.0000235201.42287.b4. [DOI] [PubMed] [Google Scholar]
- 104.Goldman-Rakic PS, et al. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174 (1):3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- 105.Gulwadi AG, et al. Dinapsoline: characterization of a D1 dopamine receptor agonist in a rat model of Parkinson’s disease. J Pharmacol Exp Ther. 2001;296 (2):338–344. [PubMed] [Google Scholar]
- 106.Lin CW, et al. Persistent activation of the dopamine D1 receptor contributes to prolonged receptor desensitization: studies with A-77636. J Pharmacol Exp Ther. 1996;276 (3):1022–1029. [PubMed] [Google Scholar]
- 107.Wade MR, Nomikos GG. Tolerance to the procholinergic action of the D1 receptor full agonist dihydrexidine. Psychopharmacology (Berl) 2005;182 (3):393–399. doi: 10.1007/s00213-005-0106-4. [DOI] [PubMed] [Google Scholar]
- 108.Lieberman JA, et al. Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology. 1997;17 (4):205–229. doi: 10.1016/S0893-133X(97)00045-6. [DOI] [PubMed] [Google Scholar]
- 109.Boileau I, et al. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63 (12):1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- 110.Wolf ME, Xue CJ. Amphetamine-induced glutamate efflux in the rat ventral tegmental area is prevented by MK-801, SCH 23390, and ibotenic acid lesions of the prefrontal cortex. Journal of Neurochemistry. 1999;73(4):1529–1538. doi: 10.1046/j.1471-4159.1999.0731529.x. [DOI] [PubMed] [Google Scholar]
- 111.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151 (2–3):99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 112.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. Journal of Neuroscience. 1997;17(21):8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Castner SA, Goldman-Rakic PS. Long-lasting psychotomimetic consequences of repeated low-dose amphetamine exposure in rhesus monkeys. Neuropsychopharmacology. 1999;20 (1):10–28. doi: 10.1016/S0893-133X(98)00050-5. [DOI] [PubMed] [Google Scholar]
- 114.Castner SA, et al. Amphetamine sensitization impairs cognition and reduces dopamine turnover in primate prefrontal cortex. Biol Psychiatry. 2005;57 (7):743–751. doi: 10.1016/j.biopsych.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 115.Selemon LD, et al. Amphetamine sensitization alters dendritic morphology in prefrontal cortical pyramidal neurons in the non-human primate. Neuropsychopharmacology. 2007;32 (4):919–931. doi: 10.1038/sj.npp.1301179. [DOI] [PubMed] [Google Scholar]
- 116.Castner SA, Williams GV. From vice to virtue: insights from sensitization in the nonhuman primate. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31 (8):1572–1592. doi: 10.1016/j.pnpbp.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 117.Castner SA, Goldman-Rakic PS. Enhancement of working memory in aged monkeys by a sensitizing regimen of dopamine D1 receptor stimulation. J Neurosci. 2004;24 (6):1446–1450. doi: 10.1523/JNEUROSCI.3987-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shergill SS, et al. Auditory hallucinations: a review of psychological treatments. Schizophr Res. 1998;32 (3):137–150. doi: 10.1016/s0920-9964(98)00052-8. [DOI] [PubMed] [Google Scholar]
- 119.Silbersweig DA, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378 (6553):176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- 120.Benson RR, et al. Parametrically dissociating speech and nonspeech perception in the brain using fMRI. Brain Lang. 2001;78 (3):364–396. doi: 10.1006/brln.2001.2484. [DOI] [PubMed] [Google Scholar]
- 121.Chen R, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48 (5):1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 122.Boroojerdi B, et al. Reduction of human visual cortex excitability using 1-Hz transcranial magnetic stimulation. Neurology. 2000;11:1529–1531. doi: 10.1212/wnl.54.7.1529. [DOI] [PubMed] [Google Scholar]
- 123.Rossi S, et al. Effects of repetitive transcranial magnetic stimulation on movement-related cortical activity in humans. Cereb Cortex. 2000;10 (8):802–808. doi: 10.1093/cercor/10.8.802. [DOI] [PubMed] [Google Scholar]
- 124.Chouinard PA, et al. Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J Neurophysiol. 2003;90 (2):1071–1083. doi: 10.1152/jn.01105.2002. [DOI] [PubMed] [Google Scholar]
- 125.Tergau F, et al. Low-frequency repetitive transcranial magnetic stimulation improves intractable epilepsy. Lancet. 1999;353:2209. doi: 10.1016/S0140-6736(99)01301-X. [DOI] [PubMed] [Google Scholar]
- 126.Wassermann EM, et al. Crossed reduction of human motor cortex excitability by 1-Hz transcranial magnetic stimulation. Neurosci Lett. 1998;250 (3):141–144. doi: 10.1016/s0304-3940(98)00437-6. [DOI] [PubMed] [Google Scholar]
- 127.Post RM, et al. Repetitive transcranial magnetic stimulation as a neuropsychiatric tool: present status and future potential. J Ect. 1999;15 (1):39–59. [PubMed] [Google Scholar]
- 128.Hoffman RE, et al. Transcranial magnetic stimulation of left temporoparietal cortex inpatients reporting auditory hallucinations. Lancet. 2000;355:1074–1076. [Google Scholar]
- 129.Hoffman RE, et al. Temporoparietal transcranial magnetic stimulation for auditory hallucinations: safety, efficacy and moderators in a fifty patient sample. Biol Psychiatry. 2005;58 (2):97–104. doi: 10.1016/j.biopsych.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 130.Sepehry1 AA, et al. Repetitive Transcranial Magnetic Stimulation Treatment for Auditory Hallucinations in Schizophrenia Spectrum Disorders: A meta-analysis. Annual Meeting of the Society of Biological Psychiatry 2006 [Google Scholar]
- 131.Hoffman RE, et al. Probing the Pathophysiology of Auditory/Verbal Hallucinations by Combining Functional Magnetic Resonance Imaging and Transcranial Magnetic Stimulation. Cereb Cortex. 2007;17 (11):2733–2743. doi: 10.1093/cercor/bhl183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]