Abstract
Purpose of review
We discuss studies on a subset of CD4+T-cells, designated Th17, and their role in the pathogenesis of human and simian acquired immune deficiency, caused by infection with HIV and SIV respectively. Most Th17 cells are lost within two weeks from infection at mucosal sites of SIV-infected macaques and are not replenished over time. Comparison of simian pathogenic and non pathogenic models of SIV infection suggests that Th17 cells contribute to the pathogenesis of AIDS.
Recent findings
Th17 cells, a recently identified subset of T helper cells, play a major role in both inducing auto-immune disorders and fencing off extracellular pathogens. Several groups have reported that the number of Th17 cells is decreased in the gut of HIV and SIV infected hosts. The loss of Th17 cells from the mucosal compartment has been associated to the dissemination of Salmonella typhimurium that is normally contained locally by the host immune system. It is believed that microbial translocation sustains immune activation in HIV infection and contributes to AIDS.
Summary
Understanding the mechanisms that lead to disruption of mucosal integrity, viral spread, and chronic immune activation is of crucial importance for the design of efficient vaccines and therapeutic intervention for HIV.
Keywords: Th17, SIV, gut
Introduction
Both HIV infection in humans and SIV infection in macaques are characterized by a dramatic loss of memory CD4+ T cells, which occurs very rapidly and is predominant at mucosal sites [1]. The rapid loss of T helper cells at the major portal of entry for HIV/SIV, likely contributes to the mucosal immune dysfunction that is observed in chronically infected individuals. However, T-cell loss alone is not sufficient to cause disease, since it occurs also in non-pathogenic models of SIV infection [2]. It is now widely accepted that progression to AIDS is due to a combination of both direct killing of CD4+ T cells by the virus as well as to other indirect mechanism of CD4+T cell death. Recent studies have proposed that the disruption of the mucosal integrity leads to translocation of bacterial products from the intestinal lumen to the blood stream, causing chronic immune activation [3]. Interestingly, SIV-infected sooty mangabeys, that have low levels of immune activation and LPS in plasma, do not progress to AIDS [3]. Indeed, the mucosal compartment represents the first line of defense against pathogens and there is increasing interest in investigating how innate and adaptive immune responses which occur locally can influence the course of infection.
In this review, we will discuss the recent finding that Th17 cells are lost very early during the course of pathogenic SIV infection of rhesus macaques, especially at mucosal sites. The loss of Th17 cells correlates with an increased activation of the immune system and is associated with a worsening of the clinical outcome of HIV infection.
Characterization of Th17 cells
Classically, T helper cells have been divided in three main groups, according to the cytokines they produce and the pathogens they fight [4]. Th1 cells are identified as CD4+ IFNγ-producing T cells and are important in clearing intracellular bacterial and viral infections through the activation of macrophages. Th2 cells express IL-4 and mediate protection against extracellular parasites and metazoan; while Treg cells play an immune-regulatory function through the production of IL-10 and TGFβ. Recently, Th17 cells, a new subpopulation of T helper cells, which is considered as the bridge between innate and adaptive immunity, has been described. These cells produce and release IL-17 and IL-22, two cytokines that, acting on surrounding cells, induce the production of chemokines able to recruit neutrophils and dendritic cells to the site of infection and to induce the expression of antimicrobial peptides [5]. This lineage is characterized by the expression of the transcription factor RORγt and by the surface markers CCR6, CCR4, and IL23R [6].
Similarly to humans and mice, Th17 cells in macaques have been shown to be a different lineage from Th1, Th2, and Treg. This population of cells has a memory phenotype [7*;8*] and produces very little IFNγ[7*;9*], no IL-4, IL-10, or TGFβ [7*]. In pigtailed macaques and in humans, a significant fraction of Th17 cells produce IL-2 and TNFα and express CD25 in the absence of FoxP3 [7*;10*].
In contrast, a different group has shown that in rhesus macaques Th17 cells do not express CD25 [9*].
Th17 cells in the intestine
Studies performed in knock-out mice have demonstrated that the lack of IL17 receptor (IL17R) or of IL-23, a key cytokine for the survival of Th17 cells, results in increased susceptibility to bacterial infections [11;12]. On the contrary, Th17 cells contribute to the development of auto-immune diseases such as experimental autoimmune encephalitis and Crohn’s disease [13;14].
Accordingly to their role in fighting bacterial infections, studies performed both in humans and in macaques have shown that Th17 cells are mainly localized in the mucosal compartment of the gastrointestinal tract [9*;10*]. In rhesus macaques, the frequency of Th17 cells in tissues such as colon and jejunum, represents 15-20% of total CD4+ T cells and only 5% in the blood, lymph nodes, and spleen [9*]. The preferential localization of Th17 cells to mucosal compartments could be driven by the expression of CCR6 and α4β7 by this population of cells [6;8*]. Several groups have shown that Th17 cells are found only in the CCR6+ fraction of memory CD4 T cells and that at least in mouse, CCR6 is the homing receptor important for Th17 cell migration to the intestine where its ligand CCL20 is expressed [6;15].
Antigen-specificity studies performed on human PBMCs have revealed that Th17 cells do not respond to viral antigens that can be encountered at mucosal surfaces, such as HIV, adenovirus, CMV, influenza, and EBV; on the contrary they are potently activated by bacterial antigens [10*]. An elegant study performed in rhesus macaque has analyzed the host response to Salmonella typhimurium [16**]. In this set of experiments, animals underwent loop surgery and loops of each macaque were inoculated by injecting either S. typhimurium culture or sterile culture. The authors have shown that S. typhimurium inoculation induces a marked increase in the mRNA levels of many proinflammatory cytokines among which of particular interest is the upregulation of IL-17 expression and the expression of genes induced by IL-17. In particular, the microarray analysis revealed an increase in the mRNA levels for genes involved in epithelial repair and maintenance. These findings support the involvement of Th17 cells in bacterial infections rather than viral infections, and most importantly the role of IL-17 in the maintenance of the epithelial integrity.
Th17 cells in pathogenic and non pathogenic model of SIV infection
HIV/SIV infection results in a progressive loss of intestinal functionality. Indeed, the development of opportunistic infections defines AIDS. Th17 cells represent an attractive subject for several groups because they might be the cause of the mucosal dysfunction that occurs in pathogenic models of SIV infection as well as in humans infected by HIV. Brenchley et al. has shown that Th17 cells isolated from human gut express CCR5 and therefore, they are a conceivable target for HIV [10*]. Studies performed in macaques, have shown that the frequency of Th17 cells declines very rapidly after SIV infection. Lower frequencies of Th17 cells are found both in systemic and mucosal compartments of rhesus macaques (blood, lymph nodes, spleen, jejunum, colon, and rectum) as soon as 14 days after infection [9*]. Moreover, lower expression of IL-17 mRNA and reduction of the frequency of Th17 cells persist also in the chronic phase of infection in several tissues [9*;16**]. These data are further supported by defining that SIVagm-infected pigtailed macacques, also experience a significant reduction of circulating Th17 cells and they develop disease [7*].
In contrast, the decline of Th17 cells is not observed in animals that do not progress to AIDS [7*;10*]. Infected sooty mangabeys and African green monkeys that do not develop disease, maintain normal frequency of Th17 cells during the course of infection. In these two latter experimental models, SIV infection results in high viral replication and in a reduction in the total number of CD4+ T cells at mucosal sites, but only in a transient immune activation with no progression to disease. Th17 cells are not preferentially depleted from the gastrointestinal tracts of SIV-infected sooty mangabeys and in contrast to human, the frequency of memory CD4+ T cells which express the IL23 receptor (IL23R) is maintained to normal levels during infection[10*].
In African green monkeys, the analysis of the acute inflammatory response to the virus has revealed an acute expression of high levels of CXCL1-2 and CCL20, chemokines which specifically recruit neutrophils, immature dendritc cells, and Th17 cells. These chemokines might be responsible for the maintenance of normal levels of Th17 cells both in the blood stream and in tissues [7*].
Importantly, because the total number of CD4+ T cells in the gastrointestinal tract is reduced dramatically during infection, the reduction in the frequency of Th17 cells might be due to the overall depletion of CD4+ T cells in the mucosa. Data from Raffatellu et al. demonstrate that the mRNA levels of IL-17 in the ileal lamina propria directly correlate with the frequency of CD4+ T cells in the same tissue [16**]. These authors suggest that the decrease in IL-17 producing cells is a consequence of the CD4+ T cell depletion. Indeed, it is important to consider that CD4+ cells are not the only IL-17-expressing cells, since this cytokine is produced by other cell types such as CD8+ T cells, γδ T cells, NK cells and neutrophils [5]. Nevertheless, the data on the correlation between IL-17 decrease and total CD4+ T cells depletion are controversial. Favre et al. claims that the depletion of Th17 cells in pigtailed macaques is selective and not just a result of the severe CD4+ T cell depletion that occurs in the mucosa of the colon [7*]. The evidence that the ratios between Th17 cells over other T helper populations decline over time to significantly lower levels in pigtailed macaques, but not in African green monkeys supports this hypothesis.
Many groups have compared the decline in Th17 cells to other T helper population and have shown the reduction in Th17 cells but not in other T helper population. Even though both Th17 and Th1 cells harbor SIV/HIV provirus it is still not clear the mechanism that leads to a higher depletion in Th17 than in Th1 cells. The analysis of the proviral content in the two subsets of cells demonstrates that there is not a preferential infection of one cell subset compared to the other [9*;10*]. A recent study performed by Matapallil et al. demonstrates that α4+β7high CD4+ T cells are preferentially infected by SIV both in vitro and in vivo [8*]. Interestingly, Th17 cells are mainly found in the α4+β7highCD4+ fraction, thus partially explaining why they could be firstly killed by the virus.
Th17 depletion, viral load and immune activation
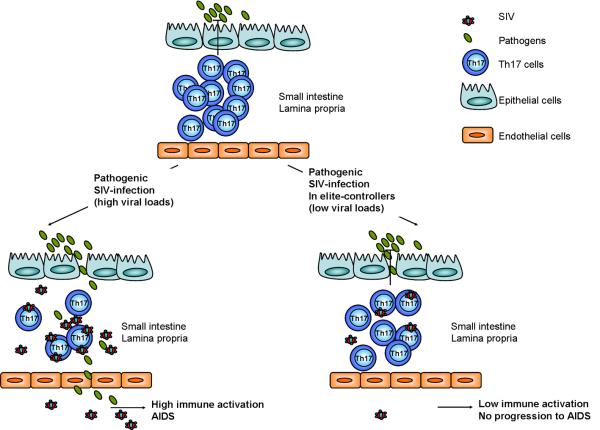
The finding that Th17 cells and not other T helper population are depleted from the intestinal mucosa of SIV-infected animals that progress to AIDS [7*;10*], but not in natural hosts, has led to hypothesize that Th17 cells, by maintaining the epithelial integrity, reduce microbial translocation to systemic compartments and immune activation (Figure 1).
Figure 1. Pathogenic SIV infection in macaques induces depletion of Th17 cells in gut mucosa.
Th17 cells are mainly localized in the intestinal mucosa, where they fight extracellular bacteria. Pathogenic SIV infection in macaques results in a strong depletion of Th17 cells and a consequent spread of bacteria from the intestinal lumen to the systemic compartment. Microbial translocation is believed to be responsible for persistent immune activation and progression to AIDS. Elite-controller animals, which naturally contain viral replication, maintain normal levels of Th17 cells in the intestinal mucosa, thus preventing microbial spreading, immune activation and disease onset.
The importance of Th17 cells during SIV infection is probably best illustrated by Raffatellu et al. While in healthy macaques, S. typhimurium injection in intestinal loops results in the expression of IL-17 and IL-17 induced genes, in SIV chronically infected animals these responses are blunted. More interestingly, in the latter case S. typhimurium is able to escape the immune system and translocate to the mesenteric lymph nodes [16**].
Another piece of evidence that sustains the importance of Th17 cells in AIDS pathogenesis is that both in pathogenic and non-pathogenic SIV infection the frequency of Th17 cells is predictive of T cell immune activation, measured as the frequency of Ki67+CD8+ cells [7*]. Moreover, long-term non progressor macaques and HIV+ individuals under highly active anti-retroviral therapy maintain normal frequency of Th17 cells [9*;17].
Conclusion
The data gathered so far suggests that it is probable that the selective loss of Th17 at mucosal sites and microbial translocation play an important role in the pathogenesis of simian and human AIDS. Luckily, we have excellent animal models of pathogenic and non pathogenic infection by SIVs that can be exploited to address this hypothesis more directly. Both the manipulation of Th17 cells in vivo or blockade of microbial translocation by various treatments will test the hypothesis, and if true, provide novel pharmacological tools to delay disease progression in HIV-I infected individuals.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
(*) of special interest
(**) of outstanding interest
- 1.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 2.Gordon SN, Klatt NR, Bosinger SE, et al. Severe Depletion of Mucosal CD4+ T Cells in AIDS-Free Simian Immunodeficiency Virus-Infected Sooty Mangabeys. J Immunol. 2007;179:3026–3034. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 4.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 6.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- * 7.Favre D, Lederer S, Kanwar B, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. This paper describes the loss of balance between Th17 and Treg cells in pathogenic SIV infection compared to non-pathogenic SIV infection and how Th17 cells frequency is predictive of immune-activation.
- * 8.Kader M, Wang X, Piatak M, et al. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009;2:439–449. doi: 10.1038/mi.2009.90.. This study demonstrates that alpha4(+)beta7(hi)CD4+ memory T cells, which harbor most Th17 cells, are preferentially infected and depleted during acute SIV infection.
- * 9.Cecchinato V, Trindade CJ, Laurence A, et al. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol. 2008;1:279–288. doi: 10.1038/mi.2008.14.. This paper shows that the frequency of Th17 cells is decreased in systemic and mucosal compartments of SIV-infected macaques but normal frequencies are maintained in elite-controller animals.
- *10.Brenchley JM, Paiardini M, Knox KS, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301.. This paper describes the preferential loss of Th17 cells in the gastrointestinal tract of HIV infected individuals and the preservation of healthy frequency in non pathogenic infection of sooty mangabeys.
- 11.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Happel KI, Dubin PJ, Zheng M, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Kang SG, Lee J, et al. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol. 2009;2:173–183. doi: 10.1038/mi.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **16.Raffatellu M, Santos RL, Verhoeven DE, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743.. This paper demonstrates that IL-17 signaling is blunted in SIV infected macaques and that the lack of an adequate IL-17-induced immune response is responsible for S. typhymurium dissemination.
- 17.Macal M, Sankaran S, Chun TW, et al. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 2008;1:475–488. doi: 10.1038/mi.2008.35. [DOI] [PubMed] [Google Scholar]