Abstract
Myocardial contraction is initiated upon the release of calcium into the cytosol from the sarcoplasmic reticulum following membrane depolarization. The fundamental physiological role of the heart is to pump an amount blood that is determined by the prevailing requirements of the body. The physiological control systems employed to accomplish this task include regulation of heart rate, the amount of calcium release, and the response of the cardiac myofilaments to activator calcium ions. Thin filament activation and relaxation dynamics has emerged as a pivotal regulatory system tuning myofilament function to the beat-to-beat regulation of cardiac output. Maladaptation of thin filament dynamics, in addition to dysfunctional calcium cycling, is now recognized as an important cellular mechanism causing reduced cardiac pump function in a variety of cardiac diseases. Here, we review current knowledge regarding protein–protein interactions involved in the dynamics of thin filament activation and relaxation and the regulation of these processes by protein kinase-mediated phosphorylation.
Keywords: Cardiac function, Actin, Myocardial contractility, Muscle mechanics, Troponin, Thin filament
Introduction and scope
Myocardial force generation is the result of cyclic interactions between actin and myosin (a.k.a. cross-bridges) that are initiated upon the release of calcium ions from the sarcoplasmic reticulum [7, 24]. The principal proteins involved in this process are illustrated schematically in Fig. 1a and structural estimates are shown in Fig. 1b. Actin monomers (globular actin) are polymerized into a double helical structure to form filamentous actin (F-actin). F-actin, together with two tropomyosin (Tm) strands, each binding a troponin (Tn) complex, forms the thin filament. Tm and the Tn complex regulate the affinity of F-actin towards myosin. Tn is composed of three subunits: troponin C (TnC), troponin I (TnI), and troponin T (TnT) with 1:1:1 molar ratio. Two alpha-helix Tm monomers dimerize to form a coiled-coil structure that overlaps partially end to end with neighboring Tm dimers (by about eight amino acid residues) to form a continuous Tm strand that lies in the two grooves of actin. Each Tm dimer binds one Tn complex such that the tail region of the Tn complex extends to the C-terminus of the Tm molecule at the Tm–Tm overlap region [83]. One Tm covers seven actin molecules, thereby forming one regulatory unit of the thin filament.
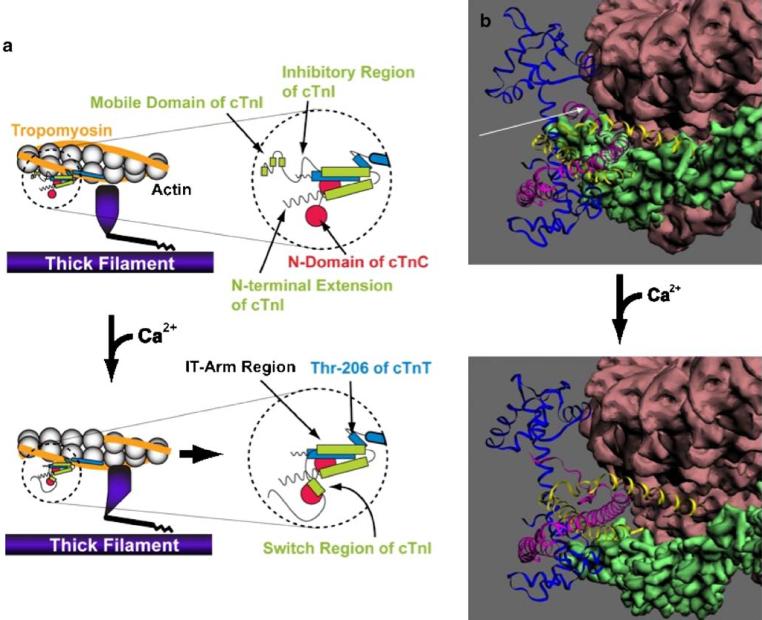
Fig. 1.
a Schematic diagram illustrating protein components of the cardiac sarcomere with a specific emphasis on the thin filament. The top panels illustrate the sarcomere in the resting condition in the absence of calcium bound to troponin C (cTnC, red). In this condition, troponin I (cTnI, green) acts to inhibit myosin cross-bridge formation (thick filament, purple) to actin (gray) via troponin T (cTnT, blue) and tropomyosin (orange). Binding of Ca2+ to cTnC (bottom panels) relieves this inhibition and allows movement of tropomyosin to the outer domain of the actin filament, thus exposing myosin-binding sites on actin. The right panels illustrate in greater detail the molecular protein–protein interactions in the cardiac troponin complex upon Ca2+ activation. b Estimated structure of the troponin complex (TnC, blue; TnI, pink; TnT, yellow), the tropomyosin strand (green), and actin filament (brown) in the Ca2+-free diastolic state (top panel) and Ca2+-bound systolic state (bottom panel). The approximate position of the inhibitory region of TnI is indicated in the top panel by the white arrow. The structure and relative positions employed to construct these projections are based on data obtained for rabbit skeletal muscle (actin) and bovine cardiac muscle (troponin and tropomyosin) by the Lehman group using electron microscopy data and helical and single particle reconstruction [55, 56]
Due to the spatial constraints within the sarcomere lattice, it may be that the interaction between actin and myosin during physiological muscle contraction is limited to only a single cross-bridge for each functional unit of the thin filament [34, 60]. In addition, recent evidence suggests that the structural states of the seven actin monomers in the regulatory unit are not identical, supporting the notion of a preferential binding site for myosin halfway between the Tm–Tm overlap region close to the location of Tn on the actin filament [55, 77, 78]. Finally, whether simultaneous independent cross-bridge formation can take place on opposite sides of the F-actin filament is not known.
Unlike smooth muscle where formation of cross-bridges is regulated via Ca2+-dependent phosphorylation of one of the lights chains associated with the myosin molecule, Ca2+ activation in striated muscle is mostly regulated at the level of the thin filament [24]. Thus, Ca2+ binding to the regulatory troponin complex troponin induces a structural change in troponin that causes relocation of tropomyosin away from the actin groove towards a more peripheral location, thereby exposing the myosin binding site(s) on actin to allow for cross-bridge formation [12]. Further fine control of striated muscle contraction dynamics is exerted by pathways involving other contractile proteins such as the myosin light chains, myosin-binding protein C, and possibly, titin. In skeletal muscle, individual myocytes are fully activated via motor nerve activity and, thus, whole muscle force is principally regulated via motor unit recruitment. In contrast, in the heart, electrical stimulation readily spreads via low resistance gap junctions such that all myocytes contract each beat, and the strength of the heart is regulated by variation of the contractile strength of the cardiac myocytes. This is accomplished by two main cellular excitation–contractions mechanisms: (a) variation of the amount of Ca2+ released from the sarcoplasmic reticulum and (b) variation in the response of the myofilaments to activator Ca2+ [7, 16, 37, 72]. For a discussion of factors that regulate myocardial Ca2+ fluxes, the reader is referred to [7]; it is important to note that physiologically, the level of Ca2+ activation in the heart is always less than saturated. The main modulators of myofilament Ca2+ sensitivity are sarcomere length (Frank–Starling relation) [40] and post-translational modification of sarcomere proteins, most notably by kinase-mediated phosphorylation [37, 72].
In general, skeletal muscle contractile proteins lack the functional phosphorylation targets found in the cardiac contractile protein isoform counterparts, a finding that is consistent with the fundamentally different cellular mechanisms underlying regulation of contractile force between cardiac and skeletal muscle. Recent investigations have provided new insights into the molecular mechanisms that underlie thin filament activation and the role of contractile protein phosphorylation in modulating myofilament function in the heart [37, 67]. Moreover, contractile protein phosphorylation has emerged as an important determinant of depressed myofilament function in cardiac diseases such as the syndrome of congestive heart failure, cardiac hypertrophy, and diabetic cardiomyopathy [5, 6, 27, 67]. Accordingly, this review is focused on the cardiac thin filament and its role in regulating cardiac myofilament dynamics both in health and disease.
Thin filament structure and function
Biochemical and structural studies strongly indicate that the thin filament as allosteric regulator of muscle contraction can exist in multiple states [24, 37]. Binding of the globular domain of myosin (S1) to reconstituted thin filaments has been found to be highly cooperative in the absence of Ca2+ [25]. In the presence of Ca2+, the binding of S1 becomes less cooperative. This observation has been interpreted in a model where the thin filament can exist in two functional states, “on” or “off”. Thin filaments in the on state bind S1 strongly, whereas those in the off state bind S1 weakly. Strong S1 binding to a structural unit of the thin filament induces other structural units along the filament to be more permissive to enter the on state via a nearest neighbor effect, possibly communicated by Tm [31]. On the other hand, based on both equilibrium and transient kinetic studies of S1 binding to isolated thin filaments, Geeves's group concluded that the binding of myosin S1 to actin occurs in two distinct molecular steps, leading to a three-state molecular model of thin filament activation [21, 47]. These three states of the thin filament include a blocked state (B-state) reflecting steric block of cross-bridge formation, a closed state (C-state) reflecting cross-bridges that are only weakly bound and do not produce force, and an open or strong myosin binding state (M-state) in which force is generated. In this alternative model, the regulatory role of Ca2+ is to affect the equilibrium between these three thin filaments states. In cardiac muscle, it is estimated that in the absence of Ca2+, 50% of thin filament regulatory units are present in the B-state and 40% in the C-state. Conversely, in the presence of Ca2+, 75% of thin filaments are present in the C-state [46]. Although the thin filaments states were defined based on biochemical studies in terms of the ability of the thin filaments to interact with myosin heads (S1) in solution, recent electron microscopy analysis of thin filaments has indicated that these three states may indeed correspond to different physical positions of tropomyosin on the thin filament [57]. It should be mentioned that although Geeves' model predicts that S1 binding can fully activate the thin filaments irrespective of [Ca2+], it has been shown that in order for the thin filament to be fully activated, both Ca2+ and S1 binding are required [29].
Actin–tropomyosin
Actin forms two intertwined right-handed helical arrays with an approximate 770-Å repeat. Tm is composed of two polypeptide chains that form an alpha-coiled-coil structure 400 Å in length, which wraps around the actin filament. Head-to-tail interaction between Tm molecules is considered to play an important role in cooperative activation properties of the thin filament. For example, removal of the Tm overlap region by carboxypeptidase treatment was shown to induce partial loss of cooperativity of S1 binding to the thin filament [54]. Ser-283, which is the second residue from the C-terminus of Tm, can be phosphorylated by tropomyosin kinase and/or an as of yet unidentified protein kinase(s). Phosphorylation of Ser-283, as well as a charge mutation (Ser283Glu) believed to mimic the phosphorylated state of this residue, increases the viscosity of Tm in solution by increasing head-to-tail interactions between Tm molecules [30, 64]. The NMR solution structure of the head-to-tail region, reported by Greenfield et al. [26], indicates that Ser283 is close to Lys7 or Lys12 of the next Tm molecule, although there are no specific NOEs defining such interactions. Phosphorylation of Ser283 may introduce an additional salt bridge between the phosphate group and the e-amino group of Lys7 and/or Lys12 to stabilize the head-to-tail interaction. Recently, our group showed depression of both tension and ATPase activity in skinned cardiac fiber bundles isolated from a transgenic mouse model in which p38 MAPK was constitutively activated by overexpression of the upstream activator MKK6bE, and this was associated with a significant reduction in the phosphorylation level of Tm [79]. Together, these results suggest that the strength of Tm head-to-tail interaction is an important determinant of myofilament functional activity and that this mechanism is modulated by Tm phosphorylation at Ser238.
Troponin C
TnC is a dumbbell-shape molecule with two globular domains (the N-domain and the C-domain) connected with a central linker. TnC is a member of the broad class of E–F hand-type Ca2+-binding proteins. It has four E–F hand motifs, two in each N- and C-domain. The Ca2+-binding sites in the C-domain (sites III and IV) have a strong affinity for Ca2+ such that these sites do not control Ca2+-dependent muscle contraction. Because of this, the C-domain is considered a structural domain. That is, it interacts with TnI and TnT irrespective of the prevailing cytosolic [Ca2+]. The Ca2+-binding site I in the N-domain of cardiac TnC (cTnC) cannot bind Ca2+ under physiological conditions due amino acid replacements in the key Ca2+-coordinating position of this site. Thus, the Ca2+-binding site II in the N-domain of cTnC is the only domain that is directly involved in Ca2+ regulation of cardiac muscle contraction, in contrast to the two functional regulatory Ca2+-binding sites in fast skeletal muscle TnC. Ca2+ binding to the N-terminal regulatory domain of TnC induces a series of structural transitions both inthe Tn complexand in actin–Tm to induce activation of the thin filaments [24, 37].
It is of note that Ca2+ binding to isolated cardiac troponin complex in solution is not cooperative, as would be expected based on the presence of a single-regulatory low-affinity Ca2+-binding site on cTnC [13, 38]. Furthermore, although the head-to-tail interactions between Tm molecules is considered to be important in cooperative S1 binding, this does not extend to Ca2+ binding to the Tn–Tm complex. Only when the Tn complex is incorporated into the thin filament does the single Ca2+-binding site on TnC bind Ca2+ in a cooperative manner, indicating the presence of a positive cooperative feedback mechanism that is transmitted along the thin filament to neighboring regulatory units, a process that likely involves Tm–actin–Tn interaction [13, 38]. Finally, we have found that the relationship between activator [Ca2+] and myofilament force is highly cooperative in both cardiac and fast skeletal muscle but not in slow skeletal muscle despite the fact that the slow skeletal sarcomere contains cTnC [41].
Troponin I
TnI is the inhibitory subunit of the Tn complex; it has extensively been studied both by others and by us. For a detailed description of the structure/domain organization of cTnI, the reader is referred to a recent overview [70]. There are at least two actin-interacting sites along TnI sequence: the inhibitory region and a second actin–Tm binding site.
The inhibitory region is highly rich in basic amino acid residues, and its amino acid sequence is highly conserved among species. The minimum inhibitory region, which was originally identified by Talbot and Hodges for fsTnI [24], itself is capable of interacting with actin to inhibit actin-activated myosin ATPase activity. However, its inhibitory function is more pronounced in the presence of Tm. Several hypertrophic/restrictive cardiomyopathy-linked mutations have been reported within the inhibitory region [22, 49]. As might be expected, these amino acid replacement(s) in the inhibitory region result in impaired inhibitory function at diastole. Furthermore, the weakened interaction between mutated TnI and actin–Tm causes sensitization of the thin filaments to Ca2+ [38] and associated slowed relaxation of force kinetics [44]. Consistent with this notion, thin filaments that contain cTnI with a HCM/RCM-linked mutation in the inhibitory region bind Ca2+ stronger than their wild-type counterpart [38, 61]. Finally, the inhibitory region includes the PKC-specific phosphorylation site, Thr-144. The functional consequence of phosphorylation of this residue has not been fully resolved. On one hand, Burkart et al. found that by replacing Thr-144 by Glu to mimic phosphorylation, detergent-skinned cardiac fiber bundles containing mutant cTnI showed no significant difference in Ca2+ sensitivity and maximum force from those containing wild-type cTnI, whereas in the in vitro motility assay, cTnI with a Thr144Glu mutation displayed desensitization to Ca2+ [11]. On the other hand, Wang et al. showed that when cardiac myocytes expressing cTnI (Ser23Ala/Ser24Ala) were exposed to PKC-bII, phosphorylation of PKC-specific sites induced increased myofilament Ca2+ sensitization [81]. These investigators additionally found that the Thr-144 residue is the most permissive site for PKC-mediated phosphorylation, consistent with previous in vitro findings [39, 53]. Finally, TnI, and in particular the inhibitory region of TnI, is emerging as an important molecule in relation to modulation of myofilament function by sarcomere length [40]. That is, cardiac sarcomeres containing the slow skeletal isoform of TnI (ssTnI) display a markedly blunted impact of sarcomere length on myofilament function [4, 42]. The inhibitory region of ssTnI differs from cTnI by the presence of a proline in the equivalent position of Thr144 in cTnI. We recently found that TnI-Thr144 per se is able to impart length-dependent properties onto the cardiac sarcomere, while a proline at that position virtually eliminates myofilament length dependency [75]. These results suggest that myofilament-length-dependent activation has, at its basis, a signal transduction pathway that includes Tn–Tm–actin signaling rather than a more direct mechanism involving myosin–actin affinity modulated by inter-filament spacing [19, 20, 40]. The underlying molecular mechanisms, however, are as of yet still largely unknown.
The second actin–Tm binding site is located in the C-terminal mobile domain of TnI. A structure of the mobile domain in solution has been proposed based on NMR NOE measurements [50]. However, the chemical shifts of amino acid residues in the mobile domain strongly indicate that this domain is intrinsically unfolded [10]. Furthermore, Hoffman et al. suggested that the mobile domain undergoes a disordered–ordered structural transition when it interacts with actin in the absence of Ca2+ [32], indicative of an association of this region with actin–Tm (termed “fly-casting mechanism”). It has been shown, at least in rodents, that moderate ischemia/reperfusion induces proteolytic cleavage of 17 residues from the C-terminal of TnI that includes the second actin-interacting sites [80]. Human myofibrils containing truncated cTnI display impaired force relaxation kinetics upon rapid solution switching Ca2+ deactivation [51]. In addition, presence of truncated cTnI induces a marked increase in myofilament Ca2+ sensitivity concomitant with a reduction in cooperativity in Ca2+ regulation of force. We recently showed that these phenomena require interaction between Tn and actin–Tm, consistent with the fly-casting mechanism [74]. In addition, we found a significant increase in cross-bridge cycling kinetics, suggestive of an inhibitory role for the C-terminus of cTnI [74]. However, the question as to whether this domain by itself inhibits myofilament function has not yet been resolved. Finally, hypertrophic/restrictive cardiomyopathy-linked mutations have also been found in this region and, as is the case with mutations found in the inhibitory region, mutations in the C-terminal mobile domain are associated with increased myofilament Ca2+ sensitivity [49].
At the distal end of the C-terminal mobile domain of TnI towards the N-terminal side of the molecule, cTnI has an alanine residue at position 164, while both in fsTnI and ssTnI this residue is substituted by a histidine. Neonatal myocardium is relatively insensitive to the decreased myofilament Ca2+ responsiveness that is normally seen under acidic conditions in adult myocardium [68]. This phenomenon is due to the presence of ssTnI that is predominantly expressed in the neonatal heart, being replaced by the adult cTnI isoform shortly after birth [43]. Recently, it has been demonstrated that the presence of the valine residue at the 164 position is largely responsible for this phenomenon such that transgenic mice that express A164V cTnI in the heart are resistant to depression of twitch force under conditions of cellular acidosis [14]. This “proof of concept” experiment indicates that alteration of cardiac contractile proteins, in this case cTnI, via gene therapy techniques may be a viable therapeutic approach in various cardiac diseases.
In between the inhibitory region and the second actin–Tm binding domain is a segment termed the “switch region”. It is a domain that interacts with a hydrophobic patch of the N-terminal regulatory domain of TnC only when Ca2+ occupies the regulatory site on TnC. The Ca2+-induced interaction between the switch region of TnI and TnC pulls both the inhibitory region and the second actin–Tm site from the actin-Tm surface so as to release the inhibitory action of TnI on the thin filament. In addition, Hoffman et al. suggested that the switch region also undergoes a disordered–ordered structural transition when it binds to TnC in the presence of Ca2+ [32]. TnI also interacts with TnC near the N-terminal part in the IT–arm domain. This region forms an alpha-helix and strongly interacts with the C-domain of TnC. The very N-terminal end of this C-domain-interacting site of cTnI contains the other PKC-specific phosphorylation sites, Ser-43 and Ser-45. Despite the recent finding that these sites are poor substrates for PKC, phosphorylation of these sites has a strong impact on myofilament activity, in particular Ser-45 [72]. For example, substitution of Ser-43/Ser-45 with the charged residue Glu induces a marked depression of actin-activated S1 ATPase activity in solution and a reduction in myofilament Ca2+ sensitivity [11, 48]. This depression of myofilament activity by phosphorylation at these PKC-specific sites appears to be due to stabilization of the thin filament in the off-state [45]. Consistent with this notion, replacing Ser43/Ser45 with non-phosphorylatable Ala43/Ala45 residues in a transgenic murine model resulted in both increased cardiac contractility [62] and increased cross-bridge cycling kinetics in isolated skinned myocardium [58]. Finally, a TnT-interacting site follows the C-domain interacting site of TnI (termed the “IT-arm”). Until recently, this part of the Tn complex was considered structural rather than functional. However, recent data from our group suggest that the IT-arm region, in particular residues 33–80, is important for cross-bridge-dependent cooperative myofilament activation and its sensitivity to acidic pH [18].
Unlike the fast and slow skeletal isoforms of TnI, cTnI contains a unique N-terminal extension about 30 amino acids in length. This region contains the PKA-phosphorylation sites, Ser-23/Ser-24. However, other kinases such as PKC and PKD may also mediate phosphorylation at these sites [28, 37, 72]. The N-terminal extension is thought to be too flexible to be resolved by crystallography. Nevertheless, a partial structure of this segment has been proposed [33, 36]. It has been shown that the cardiac-specific N-terminal extension interacts with the N-domain of cTnC near the non-functional Ca2+-binding site 1 in a phosphorylation-dependent manner. That is, phosphorylation of Ser23/Ser24 results in a weakening of the interaction between the N-terminal extension of cTnI and the N-terminal domain of cTnC [1, 82], concomitant with the characteristic reduction that is seen in myofilament Ca2+ sensitivity [69]. The underlying molecular mechanism of this phenomenon is the destabilization of the Ca2+-bound state of cTnC. In addition, we recently found that phosphorylation of Ser23/ Ser24, in isolation, induces an increase in cross-bridge cycling kinetics [9]. This result is in contrast to earlier findings indicating that PKA treatment of isolated skinned myocardium did not affect cross-bridge cycling rate [17, 35], albeit that this has not been a universal observation [63, 71]. Interestingly, phosphorylation of Ser-23/Ser-24 does not affect the maximum Ca2+-dependent association rate of S1 to reconstituted thin filaments [59], indicating that the enhanced cross-bridge cycling kinetics must be the result of modulation of acto-myosin reaction steps subsequent to initial cross-bridge formation, most likely the rate of cross-bridge detachment. Thus, Ser-23/Ser-24 phosphorylation leads to decreased myofilament Ca2+ sensitivity and increased cross-bridge cycling rate. This notion is supported by a recent study of Biesiadecki et al. [9] investigating a dilated cardiomyopathy-linked mutation in cTnC (G159D). Using recombinant Tn exchange techniques in skinned myocardium, these investigators found no effect on myofilament function when this mutation was introduced in isolation. However, the Ser-23/Ser-24 phosphorylation-induced myofilament desensitization was virtually eliminated in the presence of the cTnC mutation. In contrast, the Ser-23/Ser-24 phosphorylation-induced accelerated cross-bridge cycling kinetics was not affected by the cTnC mutation. Thus, the G159D mutation of cTnC blunts only one part of the two separate functional effects of Ser23/Ser24 phosphorylation. A similar result has been reported for another cTnC mutation linked to hypertrophic cardiomyopathy (L29Q) [66]. How these two cTnC mutants that blunt the impact of PKA mediated phosphorylation of cTnI can lead to two entirely different disease states is yet to be resolved. Nevertheless, it is apparent that mishandling of the beta-adrenergic stimulus by the cardiac myocytes may play a key role in the development of cardiac disease.
Troponin T
TnT is a markedly polar molecule containing 30% acidic and 20% basic residues in its amino acid composition. TnT can be readily cleaved into two soluble fragments, T1 and T2, by mild chymotryptic treatment. The T1 region, which is N-terminal part of TnT, binds to tropomyosin strongly and is located closest to the Z-disk extending towards the head–tail interaction site of Tm. This region of TnT may play an important role in the cooperative activation of the thin filament by modulating the Tm–Tm interaction in the head-to-tail junction of the Tm strand. In the human heart, alternative splicing of exons in the near N-terminal part of TnT produces at least four isoforms of TnT that are developmentally regulated [2]. In addition, it has been reported that human heart failure is associated with reexpression of the fetal TnT isoform profile [3]. Based on studies in the developing rabbit heart, it was originally thought that cardiac sarcomeres containing different isoforms of cTnT differ in terms of myofilament Ca2+ sensitivity [52]. More recent studies employing recombinant troponin exchange techniques, however, have demonstrated that the fetal TnT isoform profile only affects myofilament Ca2+ sensitivity in the presence of ssTnI [23], the fetal isoform of TnI expressed early after birth [43]. Similar to the proteolytic cleavage of C-terminus of cTnI, it is now apparent that the N-terminal hyper-variable region [8], a region that includes the exons that produce the splice variants mentioned above, is also cleaved by m-calpain during ischemia-reperfusion [84]. Of note and in contrast, m-calpain treatment of isolated cTnT in solution only results in non-specific protein degradation. The impact of cTnT N-terminal cleavage on myofilament function has not been studied in detail. Finally, the T2 region of cTnT, consisting of the C-terminal ~100 residues of TnT, binds both TnI and TnC and forms the globular domain of the Tn complex. The T2–TnI–TnC complex is sufficient to render S1 binding to actin–Tm Ca2+-sensitive, albeit it not with a high level of cooperativity [65].
TnT is not a substrate for PKA. However, it does contain up to four potential PKC-specific phosphorylation sites [73]. Sumandea et al., employing recombinant Tn exchange techniques in skinned myocardium, demonstrated that Thr-206 is the functionally important site [73]. That is, either phosphorylation or a charge mutation phospho-mimic (Thr206Glu) at this residue significantly reduces maximum Ca2+-saturated force, myofilament Ca2+ sensitivity, and cross-bridge cycling rate. Although the underlying molecular mechanisms are still largely unknown, it was noted that Thr-206 is located close to the end of a N-cap residue of an alpha-helix. Phosphorylation or Glu-substitution of this residue, therefore, could conceivably extend the alpha-helix up to Gly-200, causing either local or global conformational alterations of the Tn complex [73]. Regardless of the underlying mechanisms, it is clear that PKC-mediated phosphorylation of cTnT may play a significant role in the regulation of the cardiac thin filament.
Cardiac diseases
There are several factors that modulate cardiac myofilament activity, especially the activation state of the thin filament. One important factor that affects myofilament function that is not discussed in detail in this review is the genetic alteration of one more of the proteins that comprise the sarcomere. Over the past decade, a large number of such mutations (well over 50) have been reported that are causal to cardiac diseases such as familial hypertrophic cardiomyopathy, genetic dilated cardiomyopathy, and electrical abnormalities that lead to rhythm disturbances and sudden cardiac death [49, 76]. As such, these diseases have now emerged as specific sarcomeric diseases of genetic origin. The clinical importance in comparison to that of other non-genetic cardiac diseases such as ischemic-dilated cardiomyopathy and ventricular hypertrophy is modest. Nevertheless, these genetic sarcomeric diseases provide important clues to identify regions of the contractile proteins that are pivotal to sarcomeric function in vivo.
Heart failure is characterized by a general decline in pump function of the heart that has, at its basis, a decline in contractile properties of the cardiac myocyte. Although it is well recognized that disturbances in calcium regulation constitute an important mechanism in this process [7], it is now clear that heart failure is also associated with a decline in myofilament response to activator Ca2+ [15, 16, 27, 37, 67, 72]. Although isoform distribution and proteolytic cleavage of contractile proteins may play a role, (inappropriate) contractile protein phosphorylation has emerged as an important determinant of depressed myofilament function in various etiologies of heart failure [5, 6, 27, 67]. For example, in experimental heart failure in the rat, we found a depression in myofilament function that was causally linked to alterations in Tn; a follow-up study demonstrated that this was due to, most likely, up-regulation of PKC-α activity and the subsequent phosphorylation of cardiac contractile proteins [5, 6]. Likewise, studies by van der Velden et al. indicate that depressed myofilament function in human heart failure is associated with alterations in Tn phosphorylation, as well as other contractile proteins such as myosin light chain and myosin-binding protein C [27]. Thus, maladaptive contractile protein function, and in particular contractile protein phosphorylation, may be a worthy signaling mechanism to target so as to restore contractile function of the ventricular myocyte in end-stage CHF.
Future perspectives
The coupling between excitation and contraction in the heart involves processes leading from membrane depolarization and subsequent release of calcium from the sarcoplasmic reticulum to the activation of the cardiac sarcomere. Although each of these physiological processes is subject to regulation, dynamics of thin filament activation has emerged as both a central and integral component of this control system. Accordingly, this review has focused on the molecular mechanisms and pathways that govern thin filament dynamics. The heart is an amazing biological mechanical pump capable of delivering cardiac output appropriate for a vast variety of loading conditions ranging from very low demand during sleep to very high demand during vigorous exercise. How the cardiac cell is able to tune its performance to such a vast range of physiological conditions and how the same signals may become maladaptive in cardiac diseases is only partly understood. Unraveling of this mystery will undoubtedly pave the way for the development of new therapeutic strategies to combat cardiac diseases.
Acknowledgments
Much of our own data discussed in this review were derived from experiments that would not have been possible without the strong collaborative environment that exist within the center for cardiovascular research at UIC. Furthermore, we apologize that were not able, due to space limitations, to include many references to excellent works published by our colleagues in the field. Supported, in part, by grants from the American Heart Association and NIH grants HL62426, HL75494, HL77195, HL082923, HL22231, HL73828, HL07692, and HL072742.
References
- 1.Abbott MB, Dong WJ, Dvoretsky A, DaGue B, Caprioli RM, Cheung HC, Rosevear PR. Modulation of cardiac troponin C-cardiac troponin I regulatory interactions by the amino-terminus of cardiac troponin I. Biochemistry. 2001;40:5992–6001. doi: 10.1021/bi0100642. [DOI] [PubMed] [Google Scholar]
- 2.Anderson PA, Moore GE, Nassar RN. Developmental changes in the expression of rabbit left ventricular troponin T. Circ Res. 1988;63:742–747. doi: 10.1161/01.res.63.4.742. [DOI] [PubMed] [Google Scholar]
- 3.Anderson PAW, Greig A, Mark TM, Malouf NN, Oakeley AE, Ungerleider RM, Allen PD, Kay BK. Molecular basis of human cardiac troponin T isoforms expressed in the developing, adult, and failing heart. Circ Res. 1995;76:681–686. doi: 10.1161/01.res.76.4.681. [DOI] [PubMed] [Google Scholar]
- 4.Arteaga GM, Palmiter KA, Leiden JM, Solaro RJ. Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin I. J Physiol. 2000;526(Pt 3):541–549. doi: 10.1111/j.1469-7793.2000.t01-1-00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belin RJ, Sumandea MP, Allen EJ, Schoenfelt K, Wang H, Solaro RJ, de Tombe PP. Augmented protein kinase C-alpha-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res. 2007;101:195–204. doi: 10.1161/CIRCRESAHA.107.148288. [DOI] [PubMed] [Google Scholar]
- 6.Belin RJ, Sumandea MP, Kobayashi T, Walker LA, Rundell VL, Urboniene D, Yuzhakova M, Ruch SH, Geenen DL, Solaro RJ, de Tombe PP. Left ventricular myofilament dysfunction in rat experimental hypertrophy and congestive heart failure. Am J Physiol. 2006;291:H2344–H2353. doi: 10.1152/ajpheart.00541.2006. [DOI] [PubMed] [Google Scholar]
- 7.Bers DM. Excitation–contraction coupling and cardiac contractile force. Kluwer Academic; Dordrecht, The Netherlands: 2001. [Google Scholar]
- 8.Biesiadecki BJ, Chong SM, Nosek TM, Jin JP. Troponin T core structure and the regulatory NH2-terminal variable region. Biochemistry. 2007;46:1368–1379. doi: 10.1021/bi061949m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biesiadecki BJ, Kobayashi T, Walker JS, John Solaro R, de Tombe PP. The troponin C G159D mutation blunts myofilament desensitization induced by troponin I Ser23/24 phosphorylation. Circ Res. 2007;100:1486–1493. doi: 10.1161/01.RES.0000267744.92677.7f. [DOI] [PubMed] [Google Scholar]
- 10.Blumenschein TM, Stone DB, Fletterick RJ, Mendelson RA, Sykes BD. Dynamics of the C-terminal region of TnI in the troponin complex in solution. Biophys J. 2006;90:2436–2444. doi: 10.1529/biophysj.105.076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burkart EM, Sumandea MP, Kobayashi T, Nili M, Martin AF, Homsher E, Solaro RJ. Phosphorylation or glutamic acid substitution at protein kinase C sites on cardiac troponin I differentially depress myofilament tension and shortening velocity. J Biol Chem. 2003;278:11265–11272. doi: 10.1074/jbc.M210712200. [DOI] [PubMed] [Google Scholar]
- 12.Craig R, Lehman W. Crossbridge and tropomyosin positions observed in native, interacting thick and thin filaments. J Mol Biol. 2001;311:1027–1036. doi: 10.1006/jmbi.2001.4897. [DOI] [PubMed] [Google Scholar]
- 13.Davis JP, Norman C, Kobayashi T, Solaro RJ, Swartz DR, Tikunova SB. Effects of thin and thick filament proteins on calcium binding and exchange with cardiac troponin C. Biophys J. 2007;92:3195–3206. doi: 10.1529/biophysj.106.095406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Day SM, Westfall MV, Fomicheva EV, Hoyer K, Yasuda S, La Cross NC, D'Alecy LG, Ingwall JS, Metzger JM. Histidine button engineered into cardiac troponin I protects the ischemic and failing heart. Nat Med. 2006;12:181–189. doi: 10.1038/nm1346. [DOI] [PubMed] [Google Scholar]
- 15.de Tombe PP. Altered contractile function in heart failure. Cardiovasc Res. 1998;37:367–380. doi: 10.1016/s0008-6363(97)00275-7. Review. [DOI] [PubMed] [Google Scholar]
- 16.de Tombe PP, Solaro RJ. Integration of cardiac myofilament activity and regulation with pathways signaling hypertrophy and failure. Ann Biomed Eng. 2000;28:991–1001. doi: 10.1114/1.1312189. [DOI] [PubMed] [Google Scholar]
- 17.de Tombe PP, Stienen GJ. Protein kinase A does not alter economy of force maintenance in skinned rat cardiac trabeculae. Circ Res. 1995;76:734–741. doi: 10.1161/01.res.76.5.734. [DOI] [PubMed] [Google Scholar]
- 18.Engel PL, Kobayashi T, Biesiadecki B, Davis J, Tikunova S, Wu S, Solaro RJ. Identification of a region of troponin I important in signaling cross-bridge-dependent activation of cardiac myofilaments. J Biol Chem. 2007;282:183–193. doi: 10.1074/jbc.M512337200. [DOI] [PubMed] [Google Scholar]
- 19.Farman GP, Allen EJ, Gore D, Irving TC, de Tombe PP. Interfilament spacing is preserved during sarcomere length isometric contractions in rat cardiac trabeculae. Biophys J. 2007;92:L73–L75. doi: 10.1529/biophysj.107.104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farman GP, Walker JS, de Tombe PP, Irving TC. Impact of osmotic compression on sarcomere structure and myofilament calcium sensitivity of isolated rat myocardium. Am J Physiol. 2006;291:H1847–H1855. doi: 10.1152/ajpheart.01237.2005. [DOI] [PubMed] [Google Scholar]
- 21.Geeves MA, Holmes KC. The molecular mechanism of muscle contraction. Adv Protein Chem. 2005;71:161–193. doi: 10.1016/S0065-3233(04)71005-0. [DOI] [PubMed] [Google Scholar]
- 22.Gomes AV, Potter JD. Cellular and molecular aspects of familial hypertrophic cardiomyopathy caused by mutations in the cardiac troponin I gene. Mol Cell Biochem. 2004;263:99–114. doi: 10.1023/B:MCBI.0000041852.42291.aa. [DOI] [PubMed] [Google Scholar]
- 23.Gomes AV, Venkatraman G, Davis JP, Tikunova SB, Engel P, Solaro RJ, Potter JD. Cardiac troponin T isoforms affect the Ca2 sensitivity of force development in the presence of slow skeletal troponin I: insights into the role of troponin T isoforms in the fetal heart. J Biol Chem. 2004;279:49579–49587. doi: 10.1074/jbc.M407340200. [DOI] [PubMed] [Google Scholar]
- 24.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 25.Greene LE, Eisenberg E. Cooperative binding of myosin subfragment-1 to the actin–troponin–tropomyosin complex. Proc Natl Acad Sci USA. 1980;77:2616–2620. doi: 10.1073/pnas.77.5.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenfield NJ, Huang YJ, Swapna GV, Bhattacharya A, Rapp B, Singh A, Montelione GT, Hitchcock-DeGregori SE. Solution NMR structure of the junction between tropomyosin molecules: implications for actin binding and regulation. J Mol Biol. 2006;364:80–96. doi: 10.1016/j.jmb.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 27.Hamdani N, Kooij V, van Dijk S, Merkus D, Paulus WJ, Dos Remedios C, Duncker DJ, Stienen GJ, van der Velden J. Sarcomeric dysfunction in heart failure. Cardiovasc Res. 2008;77:649–658. doi: 10.1093/cvr/cvm079. [DOI] [PubMed] [Google Scholar]
- 28.Haworth RS, Cuello F, Herron TJ, Franzen G, Kentish JC, Gautel M, Avkiran M. Protein kinase D is a novel mediator of cardiac troponin I phosphorylation and regulates myofilament function. Circ Res. 2004;95:1091–1099. doi: 10.1161/01.RES.0000149299.34793.3c. [DOI] [PubMed] [Google Scholar]
- 29.Heeley DH, Belknap B, White HD. Maximal activation of skeletal muscle thin filaments requires both rigor myosin S1 and calcium. J Biol Chem. 2006;281:668–676. doi: 10.1074/jbc.M505549200. [DOI] [PubMed] [Google Scholar]
- 30.Heeley DH, Watson MH, Mak AS, Dubord P, Smillie LB. Effect of phosphorylation on the interaction and functional properties of rabbit striated muscle alpha alpha-tropomyosin. J Biol Chem. 1989;264:2424–2430. [PubMed] [Google Scholar]
- 31.Hill TL, Eisenberg E, Greene L. Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin–troponin–tropomyosin complex. Proc Natl Acad Sci USA. 1980;77:3186–3190. doi: 10.1073/pnas.77.6.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman RM, Blumenschein TM, Sykes BD. An interplay between protein disorder and structure confers the Ca2 regulation of striated muscle. J Mol Biol. 2006;361:625–633. doi: 10.1016/j.jmb.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 33.Howarth JW, Meller J, Solaro RJ, Trewhella J, Rosevear PR. Phosphorylation-dependent conformational transition of the cardiac specific N-extension of troponin I in cardiac troponin. J Mol Biol. 2007;373:706–722. doi: 10.1016/j.jmb.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 34.Hussan J, de Tombe PP, Rice JJ. A spatially detailed myofilament model as a basis for large-scale biological simulations. IBM J Res Develop. 2006;50:583–600. [Google Scholar]
- 35.Janssen PM, de Tombe PP. Protein kinase A does not alter unloaded velocity of sarcomere shortening in skinned rat cardiac trabeculae. Am J Physiol. 1997;273:H2415–H2422. doi: 10.1152/ajpheart.1997.273.5.H2415. [DOI] [PubMed] [Google Scholar]
- 36.Jaquet K, Lohmann K, Czisch M, Holak T, Gulati J, Jaquet R. A model for the function of the bisphosphorylated heart-specific troponin-I N-terminus. J Muscle Res Cell Motil. 1998;19:647–659. doi: 10.1023/a:1005381131102. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi T, Solaro RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol. 2005;67:39–67. doi: 10.1146/annurev.physiol.67.040403.114025. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi T, Solaro RJ. Increased Ca2 affinity of cardiac thin filaments reconstituted with cardiomyopathy-related mutant cardiac troponin I. J Biol Chem. 2006;281:13471–13477. doi: 10.1074/jbc.M509561200. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi T, Yang X, Walker LA, Van Breemen RB, Solaro RJ. A non-equilibrium isoelectric focusing method to determine states of phosphorylation of cardiac troponin I: identification of Ser-23 and Ser-24 as significant sites of phosphorylation by protein kinase C. J Mol Cell Cardiol. 2005;38:213–218. doi: 10.1016/j.yjmcc.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 40.Konhilas JP, Irving TC, De Tombe PP. Frank–Starling law of the heart and the cellular mechanisms of length-dependent activation. Pflugers Arch. 2002;445:305–310. doi: 10.1007/s00424-002-0902-1. [DOI] [PubMed] [Google Scholar]
- 41.Konhilas JP, Irving TC, de Tombe PP. Length-dependent activation in three striated muscle types of the rat. J Physiol. 2002;544:225–236. doi: 10.1113/jphysiol.2002.024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konhilas JP, Irving TC, Wolska BM, Jweied EE, Martin AF, Solaro RJ, de Tombe PP. Troponin I in the murine myocardium: influence on length-dependent activation and inter-filament spacing. J Physiol. 2003;547:951–961. doi: 10.1113/jphysiol.2002.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kruger M, Kohl T, Linke WA. Developmental changes in passive stiffness and myofilament Ca2 sensitivity due to titin and troponin-I isoform switching are not critically triggered by birth. Am J Physiol. 2006;291:H496–H506. doi: 10.1152/ajpheart.00114.2006. [DOI] [PubMed] [Google Scholar]
- 44.Kruger M, Zittrich S, Redwood C, Blaudeck N, James J, Robbins J, Pfitzer G, Stehle R. Effects of the mutation R145G in human cardiac troponin I on the kinetics of the contraction-relaxation cycle in isolated cardiac myofibrils. J Physiol. 2005;564:347–357. doi: 10.1113/jphysiol.2004.079095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathur MC, Kobayashi T, Chalovich JM. Negative charges at protein kinase C sites of troponin I stabilize the inactive state of actin. Biophys J. 2008;94:542–549. doi: 10.1529/biophysj.107.113944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maytum R, Westerdorf B, Jaquet K, Geeves MA. Differential regulation of the actomyosin interaction by skeletal and cardiac troponin isoforms. J Biol Chem. 2003;278:6696–6701. doi: 10.1074/jbc.M210690200. [DOI] [PubMed] [Google Scholar]
- 47.McKillop DF, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montgomery DE, Wolska BM, Pyle WG, Roman BB, Dowell JC, Buttrick PM, Koretsky AP, Del Nido P, Solaro RJ. Alpha-adrenergic response and myofilament activity in mouse hearts lacking PKC phosphorylation sites on cardiac TnI. Am J Physiol. 2002;282:H2397–2405. doi: 10.1152/ajpheart.00714.2001. [DOI] [PubMed] [Google Scholar]
- 49.Morimoto S. Sarcomeric proteins and inherited cardiomyopathies. Cardiovasc Res. 2008;77:659–666. doi: 10.1093/cvr/cvm084. [DOI] [PubMed] [Google Scholar]
- 50.Murakami K, Yumoto F, Ohki SY, Yasunaga T, Tanokura M, Wakabayashi T. Structural basis for Ca2+regulated muscle relaxation at interaction sites of troponin with actin and tropomyosin. J Mol Biol. 2005;352:178–201. doi: 10.1016/j.jmb.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 51.Narolska NA, Piroddi N, Belus A, Boontje NM, Scellini B, Deppermann S, Zaremba R, Musters RJ, dos Remedios C, Jaquet K, Foster DB, Murphy AM, van Eyk JE, Tesi C, Poggesi C, van der Velden J, Stienen GJ. Impaired diastolic function after exchange of endogenous troponin I with C-terminal truncated troponin I in human cardiac muscle. Circ Res. 2006;99:1012–1020. doi: 10.1161/01.RES.0000248753.30340.af. [DOI] [PubMed] [Google Scholar]
- 52.Nassar R, Malouf NN, Kelly MB, Oakeley AE, Anderson PA. Force-pCa relation and troponin T isoforms of rabbit myocardium. Circ Res. 1991;69:1470–1475. doi: 10.1161/01.res.69.6.1470. [DOI] [PubMed] [Google Scholar]
- 53.Noland TA Jr, Kuo JF. Protein kinase C phosphorylation of cardiac troponin I or troponin T inhibits Ca2()-stimulated actomyosin MgATPase activity. J Biol Chem. 1991;266:4974–4978. [PubMed] [Google Scholar]
- 54.Pan BS, Gordon AM, Luo ZX. Removal of tropomyosin overlap modifies cooperative binding of myosin S-1 to reconstituted thin filaments of rabbit striated muscle. J Biol Chem. 1989;264:8495–8498. [PubMed] [Google Scholar]
- 55.Pirani A, Vinogradova MV, Curmi PM, King WA, Fletterick RJ, Craig R, Tobacman LS, Xu C, Hatch V, Lehman W. An atomic model of the thin filament in the relaxed and Ca2+ activated states. J Mol Biol. 2006;357:707–717. doi: 10.1016/j.jmb.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 56.Pirani A, Xu C, Hatch V, Craig R, Tobacman LS, Lehman W. Single particle analysis of relaxed and activated muscle thin filaments. J Mol Biol. 2005;346:761–772. doi: 10.1016/j.jmb.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 57.Poole KJ, Lorenz M, Evans G, Rosenbaum G, Pirani A, Craig R, Tobacman LS, Lehman W, Holmes KC. A comparison of muscle thin filament models obtained from electron microscopy reconstructions and low-angle X-ray fibre diagrams from non-overlap muscle. J Struct Biol. 2006;155:273–284. doi: 10.1016/j.jsb.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 58.Pyle WG, Sumandea MP, Solaro RJ, De Tombe PP. Troponin I serines 43/45 and regulation of cardiac myofilament function. Am J Physiol. 2002;283:H1215–H1224. doi: 10.1152/ajpheart.00128.2002. [DOI] [PubMed] [Google Scholar]
- 59.Reiffert SU, Jaquet K, Heilmeyer LM, Jr, Ritchie MD, Geeves MA. Bisphosphorylation of cardiac troponin I modulates the Ca (2)-dependent binding of myosin subfragment S1 to reconstituted thin filaments. FEBS Lett. 1996;384:43–47. doi: 10.1016/0014-5793(96)00274-8. [DOI] [PubMed] [Google Scholar]
- 60.Rice JJ, Wang F, Bers DM, de Tombe PP. Approximate model of cooperative activation and crossbridge cycling in cardiac muscle using ordinary differential equations. Biophys J. 2008 doi: 10.1529/biophysj.107.119487. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robinson P, Griffiths PJ, Watkins H, Redwood CS. Dilated and hypertrophic cardiomyopathy mutations in troponin and alpha-tropomyosin have opposing effects on the calcium affinity of cardiac thin filaments. Circ Res. 2007;101:1266–1273. doi: 10.1161/CIRCRESAHA.107.156380. [DOI] [PubMed] [Google Scholar]
- 62.Roman BB, Goldspink PH, Spaite E, Urboniene D, McKinney R, Geenen DL, Solaro RJ, Buttrick PM. Inhibition of PKC phosphorylation of cTnI improves cardiac performance in vivo. Am J Physiol. 2004;286:H2089–H2095. doi: 10.1152/ajpheart.00582.2003. [DOI] [PubMed] [Google Scholar]
- 63.Saeki Y, Kobayashi T, Minamisawa S, Sugi H. Protein kinase A increases the tension cost and unloaded shortening velocity in skinned rat cardiac muscle. J Mol Cell Cardiol. 1997;29:1655–1663. doi: 10.1006/jmcc.1997.0401. [DOI] [PubMed] [Google Scholar]
- 64.Sano K, Maeda K, Oda T, Maeda Y. The effect of single residue substitutions of serine-283 on the strength of head-to-tail interaction and actin binding properties of rabbit skeletal muscle alpha-tropomyosin. J Biochem. 2000;127:1095–1102. doi: 10.1093/oxfordjournals.jbchem.a022703. [DOI] [PubMed] [Google Scholar]
- 65.Schaertl S, Lehrer SS, Geeves MA. Separation and characterization of the two functional regions of troponin involved in muscle thin filament regulation. Biochemistry. 1995;34:15890–15894. doi: 10.1021/bi00049a003. [DOI] [PubMed] [Google Scholar]
- 66.Schmidtmann A, Lindow C, Villard S, Heuser A, Mugge A, Gessner R, Granier C, Jaquet K. Cardiac troponin C-L29Q, related to hypertrophic cardiomyopathy, hinders the transduction of the protein kinase A dependent phosphorylation signal from cardiac troponin I to C. FEBS J. 2005;272:6087–6097. doi: 10.1111/j.1742-4658.2005.05001.x. [DOI] [PubMed] [Google Scholar]
- 67.Solaro RJ, de Tombe PP. Review focus series: sarcomeric proteins as key elements in integrated control of cardiac function. Cardiovasc Res. 2008;77:616–618. doi: 10.1093/cvr/cvn004. [DOI] [PubMed] [Google Scholar]
- 68.Solaro RJ, Lee JA, Kentish JC, Allen DG. Effects of acidosis on ventricular muscle from adult and neonatal rats. Circ Res. 1988;63:779–787. doi: 10.1161/01.res.63.4.779. [DOI] [PubMed] [Google Scholar]
- 69.Solaro RJ, Moir AJ, Perry SV. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature. 1976;262:615–617. doi: 10.1038/262615a0. [DOI] [PubMed] [Google Scholar]
- 70.Solaro RJ, Rosevear PR, Kobayashi T. The unique functions of cardiac troponin I in the control of cardiac muscle contraction and relaxation. Biochemical Biophysical Research Communications. 2008;369:82–87. doi: 10.1016/j.bbrc.2007.12.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Strang KT, Sweitzer NK, Greaser ML, Moss RL. b-Adrenergic receptor stimulation increases unloaded shortening velocity of skinned single ventricular myocytes from rats. Circ Res. 1994;74:542–549. doi: 10.1161/01.res.74.3.542. [DOI] [PubMed] [Google Scholar]
- 72.Sumandea MP, Burkart EM, Kobayashi T, De Tombe PP, Solaro RJ. Molecular and integrated biology of thin filament protein phosphorylation in heart muscle. Ann N Y Acad Sci. 2004;1015:39–52. doi: 10.1196/annals.1302.004. [DOI] [PubMed] [Google Scholar]
- 73.Sumandea MP, Pyle WG, Kobayashi T, de Tombe PP, Solaro RJ. Identification of a functionally critical protein kinase C phosphorylation residue of cardiac troponin T. J Biol Chem. 2003;278:35135–35144. doi: 10.1074/jbc.M306325200. [DOI] [PubMed] [Google Scholar]
- 74.Tachampa K, Kobayashi T, Wang H, Martin AF, Biesiadecki BJ, Solaro RJ, de Tombe PP. Increased crossbridge cycling kinetics after exchange of C-terminal truncated troponin-I in skinned rat cardiac muscle. J Biol Chem. 2008 doi: 10.1074/jbc.M801636200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tachampa K, Wang H, Farman GP, de Tombe PP. Cardiac troponin I threonine 144: role in myofilament length dependent activation. Circ Res. 2007;101:1081–1083. doi: 10.1161/CIRCRESAHA.107.165258. [DOI] [PubMed] [Google Scholar]
- 76.Tardiff JC. Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes. Heart Fail Rev. 2005;10:237–248. doi: 10.1007/s10741-005-5253-5. [DOI] [PubMed] [Google Scholar]
- 77.Tobacman LS, Butters CA. A new model of cooperative myosin-thin filament binding. J Biol Chem. 2000;275:27587–27593. doi: 10.1074/jbc.M003648200. [DOI] [PubMed] [Google Scholar]
- 78.Tregear RT, Reedy MC, Goldman YE, Taylor KA, Winkler H, Franzini-Armstrong C, Sasaki H, Lucaveche C, Reedy MK. Cross-bridge number, position, and angle in target zones of cryofixed isometrically active insect flight muscle. Biophys J. 2004;86:3009–3019. doi: 10.1016/S0006-3495(04)74350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vahebi S, Ota A, Li M, Warren CM, de Tombe PP, Wang Y, Solaro RJ. p38-MAPK induced dephosphorylation of alpha-tropomyosin is associated with depression of myocardial sarcomeric tension and ATPase activity. Circ Res. 2007;100:408–415. doi: 10.1161/01.RES.0000258116.60404.ad. [DOI] [PubMed] [Google Scholar]
- 80.Van Eyk JE, Powers F, Law W, Larue C, Hodges RS, Solaro RJ. Breakdown and release of myofilament proteins during ischemia and ischemia/reperfusion in rat hearts: identification of degradation products and effects on the pCa–force relation. Circ Res. 1998;82:261–271. doi: 10.1161/01.res.82.2.261. [DOI] [PubMed] [Google Scholar]
- 81.Wang H, Grant JE, Doede CM, Sadayappan S, Robbins J, Walker JW. PKC-betaII sensitizes cardiac myofilaments to Ca2 by phosphorylating troponin I on threonine-144. J Mol Cell Cardiol. 2006;41:823–833. doi: 10.1016/j.yjmcc.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 82.Ward DG, Brewer SM, Gallon CE, Gao Y, Levine BA, Trayer IP. NMR and mutagenesis studies on the phosphorylation region of human cardiac troponin I. Biochemistry. 2004;43:5772–5781. doi: 10.1021/bi036310m. [DOI] [PubMed] [Google Scholar]
- 83.White SP, Cohen C, Phillips GN., Jr Structure of co-crystals of tropomyosin and troponin. Nature. 1987;325:826–828. doi: 10.1038/325826a0. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Z, Biesiadecki BJ, Jin JP. Selective deletion of the NH2-terminal variable region of cardiac troponin T in ischemia reperfusion by myofibril-associated mu-calpain cleavage. Biochemistry. 2006;45:11681–11694. doi: 10.1021/bi060273s. [DOI] [PMC free article] [PubMed] [Google Scholar]