Abstract
Eicosanoids, including prostaglandins and leukotrienes, are biologically active lipids that have been implicated in various pathological processes, such as inflammation and cancer. This Review highlights our understanding of the intricate roles of eicosanoids in epithelial-derived tumours and their microenvironment. The knowledge of how these lipids orchestrate the complex interactions between transformed epithelial cells and the surrounding stromal cells is crucial for understanding tumour evolution, progression and metastasis. Understanding the molecular mechanisms underlying the role of prostaglandins and other eicosanoids in cancer progression will help to develop more effective cancer chemopreventive and/or therapeutic agents.
A large body of evidence indicates that genetic mutations, epigenetic changes, chronic inflammation, diet and lifestyle are risk factors for cancer1-3. Epidemiological and animal studies provide evidence that a high-fat diet can be associated with an increased risk for cancer, in particular colorectal, breast, pancreatic and prostate cancer3. Arachidonic acid is one major ingredient of animal fats and the biologically active lipids derived from this substrate have crucial roles in chronic inflammation and cancer. The metabolism of arachidonic acid by cyclooxygenase (COX), lipoxygenase (LOX) and P450 epoxygenase pathways generates eicosanoids, including prostanoids, leukotrienes, hydroxyeicosatetraenoic acids (HETEs), epoxyeicosatrienoic acids (EETs) and hydroperoxyeicosatetraenoic acids (HPETEs) (FIG. 1). Epidemiological, clinical and animal studies provide evidence that activation of COX and LOX pathways during chronic inflammation and carcinogenesis results in aberrant metabolism of arachidonic acid, which may be one mechanism for the contribution of dietary fats to carcinogenesis.
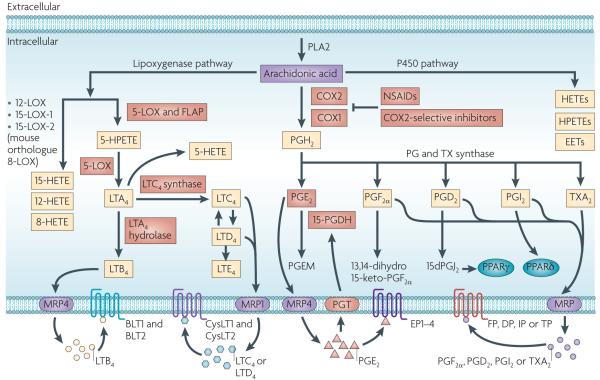
Figure 1. An overview of eicosanoid synthesis pathways.
Arachidonic acid is a polyunsaturated fatty acid that constitutes the phospholipid domain of most cell membranes and is liberated from the cellular membranes by cytoplasmic phospholipase A2 (PLA2). Free arachidonic acid can be metabolized to eicosanoids through three major pathways: the cyclooxygenase (COX), the lipoxygenase (LOX) and the cytochrome P450 monooxygenase pathways. In the COX pathway, the key step is the enzymatic conversion of arachidonic acid to the intermediate prostaglandin G2 (PGG2), which is then reduced to an intermediate PGH2 by the peroxidase activity of COX. PGH2 is sequentially metabolized to prostanoids, including prostaglandins (PGs) and thromboxanes (TXs) by specific prostaglandin and thromboxane synthases. LOXs convert arachidonic acid into biologically active metabolites such as leukotrienes and hydroxyeicosatetraenoic acids (HETEs); P450 metabolizes arachidonic acid into epoxyeicosatrienoic acids (EETs), HETEs and hydroperoxyeicosatetraenoic acids (HPETEs). In the 5-LOX pathway, arachidonic acid is converted to an intermediary 5-HPETE, which is further metabolized to form the unstable leukotriene A4 (LTA4). LTA4 is subsequently converted to 5-HETE, LTB4, LTC4, LTD4 and LTE4. Each of the prostaglandins and leukotrienes exerts its biological effects by binding to its cognate G protein-coupled receptor. PGI2 can transactivate the nuclear peroxisome proliferator-activated receptor-δ (PPARδ), and a PGD2 dehydration product, 15dPGJ2, is a natural ligand for PPARγ. The multidrug resistance-associated protein (MRP) gene family is comprised of efflux transporters for both prostaglandins and leukotrienes, and PGT is an influx transporter for prostaglandins. Hydroxyprostaglandin dehydrogenase 15-(NAD) (15-PGDH) mainly metabolizes intracellular PGE2 and PGF2α to a stable 13,14-dihydro-15-keto-PGE2 and 13,14-dihydro-15-keto-PGF2α. The red boxes indicate the signalling pathways that are discussed in this Review. CysLT, cysteinyl leukotriene; NSAID, non-steroidal anti-inflammatory drug.
Non-steroidal anti-inflammatory drugs (NSAIDs) have been reported to have beneficial effects on reducing the risk of developing some solid tumours, including the four most prevalent cancers worldwide: breast, colon, lung and prostate cancer4. NSAIDs exert some of their anti-inflammatory and anti-tumour effects by reducing prostanoid production through the inhibition of COX enzyme activity. In addition, emerging evidence suggests that LOX pathways are also involved in carcinogenesis. In general, 5-LOX (also known as ALOX5) and 12-LOX (also known as ALOX12) have potential procarcinogenic roles, whereas 15-LOX-2 (also known as ALOX15B) is thought to have an anti-carcinogenic effect, and the role of 15-LOX-1 (also known as ALOX15) remains controversial5. As 5-LOX has been shown to have a major role in carcinogenesis, understanding the contribution of each COX-derived prostanoid and 5-LOX-derived leukotriene in the pathogenesis of cancer could enable identification of new and safer therapeutic and chemopreventive agents with reasonable benefit and fewer side effects.
In this Review, we focus on recent insights into the roles of prostanoids and leukotrienes in several epithelial-derived malignancies, from their involvement in governing tumour epithelial cell proliferation, survival, and migration and invasion to their involvement in adapting the tumour microenvironment by influencing angiogenesis, inflammation and immunosuppression.
Prostanoid and leukotriene biosynthesis
The importance of the prostanoid and leukotriene biosynthetic pathway in carcinogenesis and chronic inflammation is supported by population studies, clinical trials and animal experiments. COX enzymes (correctly referred to as prostaglandin G/H synthases) exist in two isoforms: COX1 (also known as PTGS1) and COX2 (also known as PTGS2). COX2 is an immediate-early response gene that is normally absent from most cells but is highly induced at sites of inflammation and during tumour progression6. Our laboratory was the first to report that COX2 expression is upregulated in colorectal cancer7. Multiple follow-up studies have revealed that COX2 levels are increased in other premalignant and malignant solid tumours, including those of the stomach, oesophagus, liver, pancreas, head and neck, lung, breast, prostate and bladder, and increased COX2 expression is associated with decreased survival among these cancer patients8. By contrast, COX1 was thought to be a housekeeping enzyme responsible for maintaining basal prostanoid levels that are important for tissue homeostasis. However, upregulation of COX1 expression has been observed in ovarian cancer9. Although most attention has been focused on the cyclooxygenase pathway, a few reports have indicated that 5-LOX is generally absent in normal epithelia but is induced by pro-inflammatory stimuli and is often constitutively expressed in various epithelial cancers including those of the colon, oesophagus, lung, prostate and breast5. As other LOX isoforms are not involved in leukotriene synthesis, the relevance of their expression and function is not included in this Review.
At a glance.
The altered metabolism of arachidonic acid by cyclooxygenase (COX) and lipoxygenase (LOX) is a common feature of several epithelial-derived malignancies and has been shown to have crucial roles in cancer progression.
The production of arachidonic acid-derived prostanoids and leukotrienes occurs in single cells or takes place in a complex manner in which these biologically active lipids, specifically leukotrienes, are generated by transcellular biosynthesis through the cooperation of multiple different types of cells in the tumour and inflamed tissues.
Pro-inflammatory prostaglandins and leukotrienes promote tumour growth by regulating tumour epithelial cells themselves and orchestrating the complex interactions between transformed epithelial cells and surrounding stromal cells to establish the tumour microenvironment that facilitates tumour-associated angiogenesis and evades attack by the immune system.
Prostaglandins and leukotrienes can modulate tumour epithelial cell proliferation, apoptosis, and migration and invasion through multiple signalling pathways in both an autocrine and paracrine fashion.
Prostaglandins and leukotrienes are central molecules in the regulation of stem cell homeostasis.
Pro-inflammatory prostaglandins and leukotrienes are key mediators in the crosstalk between tumour epithelial cells and their surrounding stromal cells in establishing a tumour microenvironment with chronic inflammation and immunosuppression.
Although non-steroidal anti-inflammatory drugs (NSAIDs), which target COX enzymes, are still among the most promising chemopreventive agents for cancer, cardiovascular and gastrointestinal side effects have dampened enthusiasm for their use as chemopreventive agents. Understanding the roles of prostaglandins and leukotrienes in epithelial-derived tumours and their microenvironment may help to develop cancer biomarkers and chemopreventive and/or therapeutic agents with a greater benefit and fewer side effects than NSAIDs.
Prostanoid biosynthesis
Cyclooxygenase enzymes catalyse the conversion of arachidonic acid to prostanoids, including prostaglandins and thromboxane A2 (TXA2) (FIG. 1). The prostaglandins exert their biological effects in an autocrine or paracrine manner by binding to their cognate cell surface receptors, which belong to the G protein-coupled receptor (GPCR) family. These receptors are designated DP (also known as PTGDR) and GPR44 for prostaglandin D2 (PGD2); EP1, EP2, EP3 and EP4 (also known as PTGER1, PTGER2, PTGER2 and PTGER4, respectively) for PGE2; FP (also known as PTGFR) for PGF2α; IP (also known as PTGIR) for PGI2; and TP (also known as TBXA2R) for TXA2. In some cases, however, certain prostaglandins and their metabolites bind nuclear receptors such as peroxisome proliferator-activated receptors (PPARs). For example, PGI2 can transactivate PPARδ, and a PGD2 dehydration product, 15-deoxy-Δ12,14-PGJ2 (15dPGJ2), is a natural ligand for PPARγ. PGE2 can also indirectly activate PPARδ in certain contexts10. The specific action of the different prostaglandins in a particular type of tissue predominantly depends on the cell type-specific expression of their cognate receptors as well as prostaglandin production. In addition to their synthesis, the steady-state extracellular levels of prostaglandins also depend on a carrier-mediated transport process, as well as inactivation in the cytoplasm. These processes are regulated by prostaglandin transporter (PGT; an influx transporter), multidrug resistance-associated protein 4 (MRP4; an efflux transporter) and hydroxyprostaglandin dehydrogenase 15-(NAD) (HPGD; also known 15-PGDH). For example, PGE2 and PGF2α are rapidly metabolized in vivo by 15-PGDH to a stable 13,14-dihydro-15-keto-PGE2 (PGEM) and 13,14-dihydro-15-keto-PGF2α, respectively. Other prostaglandins are mainly metabolized in a non-enzymatic manner11.
Leukotriene biosynthesis
The 5-LOX enzyme interacts with a 5-LOX-activating protein (FLAP) and converts arachidonic acid to the unstable leukotriene A4 (LTA4) through an HPETE. FLAP enhances the activity of 5-LOX by binding to arachidonic acid and presenting it to 5-LOX. LTA4 is subsequently converted to 5-HETE, or hydrolysed into biologically active LTB4 by LTA4 hydrolase, or to the cysteinyl leukotriene (CysLT), LTC4, by LTC4 synthase. LTC4 is then converted to another CysLT, LTD4, which is sequentially metabolized to LTE4 (FIG. 1). LTB4 and LTD4 are the most potent leukotrienes. They exert their biological effects through the activation of GPCRs. LTB4 can bind to two receptors, BLT1 (also known as LTB4R) with high affinity and BLT2 (also known as LTB4R2) with low affinity; CysLTs bind to at least two distinct receptors, CysLT1 (also known as CYSLTR1) and CysLT2 (also known as CYSLTR2). Like LTB4 receptors, CysLT1 has a high affinity for CysLTs — which is higher for LTD4 than LTC4 — whereas CysLT2 has a lower overall affinity for CysLTs that is equal for LTD4 and LTC4. BLT1 and CysLT1 are exclusively expressed in leukocytes, whereas BLT2 and CysLT2 are expressed in a wide variety of cells.
Transcellular biosynthesis
Eicosanoid biosynthesis can occur in a single cell that contains the complete complement of enzymes, but the production of eicosanoids in a tissue, in particular in tumour tissues and sites of inflammation, takes place in a more complex manner in which some of these biologically active lipids, specifically leukotrienes, are generated by transcellular biosynthesis through the cooperation of multiple different cell types.
The leukotrienes are primarily produced by stimulated leukocytes that express the enzymes required for their synthesis. Epithelial and endothelial cells can also generate LTB4, LTC4 and LTD4 at inflammatory sites through transcellular metabolism by which epithelial or endothelial cells use LTA4 that is released from immune cells, in particular neutrophils, as epithelial and endothelial cells express LTA4 hydrolase12. However, leukocytes can also use arachidonic acid that is secreted from epithelial cells as a substrate to generate leukotrienes. Transcellular leukotriene biosynthesis has been observed during inflammation in vivo13. Therefore, the transcellular biosynthesis between epithelial or endothelial and immune cells can generate the overproduction of leukotrienes, which in turn further amplifies the inflammatory response. Additional research is needed to determine the extent of transcellular biosynthesis in the tumour microenvironment. To date, there is no published evidence demonstrating transcellular biosynthesis of prostaglandins between immune and epithelial cells, although this is reported to occur between platelets and endothelial cells12.
Eicosanoids in cancer
Prostanoids and cancer
Among prostanoids, proinflammatory PGE2 has a predominant role in promoting tumour growth. PGE2 is the most abundant prostaglandin that is found in various human malignancies, including colon, lung, breast, and head and neck cancer, and is often associated with a poor prognosis14-17. By contrast, 15-PGDH is highly expressed in normal tissues but is ubiquitously lacking in human colon, gastric, lung and breast cancer18-21. Lack of 15-PGDH expression in these tumours results in increased endogenous PGE2 levels. Multiple lines of evidence from mouse models of colorectal cancer (CRC) demonstrate that COX2-derived PGE2 promotes tumour growth. PGE2 treatment blocks NSAID-induced regression of small intestinal adenomas in ApcMin/+ mice22 and increased endogenous PGE2 levels through the loss of 15-PGDH inhibit the anti-tumour effects of celecoxib in the azoxymethane (AOM) mouse model23. Direct evidence that PGE2 promotes tumour growth comes from recent studies showing that PGE2 treatment dramatically increased both small and large intestinal adenoma burden in ApcMin/+ mice and significantly enhanced AOM-induced colon tumour incidence and multiplicity10,24. Furthermore, increased endogenous PGE2 through the genetic deletion of 15-Pgdh promotes colon tumour growth in ApcMin/+ and AOM mouse models25. By contrast, inhibition of endogenous PGE2 through the genetic deletion of prostaglandin E synthase (Ptges) suppresses intestinal tumorigenesis in ApcMin/+ and AOM models26. The central role of PGE2 in colorectal tumorigenesis has been further confirmed by evaluating mice with a homozygous deletion of individual PGE2 receptors27-29. Limited information is available regarding the role of PGE2 signalling in animal models of other cancers. Increased PGE2 levels through the overexpression of COX2 and PTGES cause gastric tumorigenesis in Wnt1-transgenic mice driven by the keratin 19 (Krt19) promoter30. Deletion of the EP2 receptor inhibits murine lung tumorigenesis that is induced by a chemical carcinogen31 and significantly suppresses COX2-induced mammary hyperplasia in mice32. Similarly, an EP1 antagonist inhibits chemically induced breast cancer development in rats33. Collectively, these studies demonstrate that PGE2 plays an important part in cancer progression.
The role of PGD2 in carcinogenesis remains ambiguous. Disruption or overexpression of PGD2 synthase (PTGDS) in ApcMin/+ mice accelerates or reduces intestinal tumour growth34, suggesting that PGD2 has anti-tumour effects. However, the evidence that genetic disruption of its receptor (DP) has no effect on colon tumour formation in the AOM mouse model does not support the hypothesis that PGD2 has anti-tumour effects28. One possible explanation for the differences in phenotype caused by PTGDS compared with DP in mouse models is that the PGD2-derived product 15dPGJ2 inhibits tumour growth by binding to PPARγ. It is well established that the activation of PPARγ inhibits tumour growth through anti-proliferative, pro-apoptotic, pro-differentiation and anti-angiogenic effects in cell lines and animal models35. Alternatively, overexpression of PTGDS might shift the conversion of PGH2 away from PGE2, which in turn would suppress tumour growth. Further investigation is required to assess the role of PGD2 and its receptors in cancer progression.
Little information regarding the role of other prostaglandins in animal models of cancer is available; however, disruption of FP, IP or TBXA2R receptors does not affect colon tumour formation in the AOM mouse model28, suggesting that these receptors are not involved in CRC progression. The question is now whether these receptors modulate tumour growth in other models of CRC or in other types of cancer. A role for PGF2α in tumorigenesis has been suggested as it can enhance carcinogen-induced transformation of fibroblasts in vitro through the induction of COX2 (REF. 36). Activation of PPARδ accelerates intestinal tumour growth in ApcMin/+ mice37, supporting the idea that PGI2 might promote colon tumour progression through this receptor. Further work is required to explore the role of PGI2 in colon carcinogenesis and other cancers.
Leukotrienes and cancer
Compared with prostaglandins, much less is known about the pro-inflammatory leukotrienes in cancer. However, emerging data suggest that leukotrienes can have an important role in carcinogenesis.
LTB4 levels are increased in human colon and prostate cancer38,39, and the expression of LTB4 receptors is increased in human pancreatic cancer40. LTB4 expression is also increased in HRAS-v12-transformed cells and the receptor BLT2 is required for Ras-induced transformation in vivo41. Furthermore, inhibition of LTB4 synthesis by treatment with an LTA4 hydrolase inhibitor, bestatin, reduced the burden of oesophageal adenocarcinoma in a rat model42.
The CysLT1 receptor is highly expressed in human colon and prostate cancers and negatively correlates with patient survival43,44. Increased CysLT1 expression in CRC correlates with the ability of LTD4 to induce proliferation and inhibit apoptosis. By contrast, reduced expression of the CysLT2 receptor is associated with a poor prognosis in patients with CRC, and CysLT2 signalling is involved in inducing apoptosis and terminal differentiation45. However, comparatively little is known about the effects of CysLT2 on signalling and biological function.
Mechanisms of eicosanoids in carcinogenesis
To understand the mechanism(s) underlying the effects of prostaglandins and leukotrienes on cancer progression, researchers have been investigating precisely how these lipids affect cancer biology. As mentioned earlier, pro-inflammatory eicosanoids are abundantly produced by various types of cancer cells and their surrounding cells. These biologically active lipids can modulate tumour progression through several mechanisms, such as by directly activating their receptors on tumour epithelial cells to regulate cell proliferation, apoptosis, migration and invasion; directly inducing epithelial cells to secrete growth factors, pro-inflammatory mediators and angiogenic factors that switch a normal microenvironment to one that supports tumour growth and spread; and directly binding receptors on stromal cells to promote a tumour-supportive microenvironment by inducing angiogenesis and evading attack by the immune system (FIG. 2).
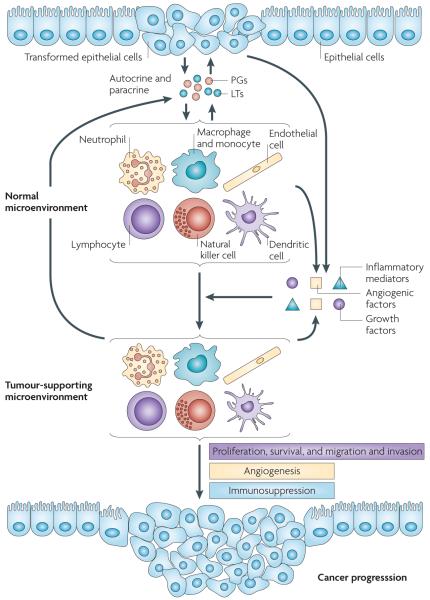
Figure 2. Models of pro-inflammatory prostaglandins and leukotrienes in promoting cancer progression.
Following the initiation of epithelial tumours, the reciprocal interactions between transformed epithelial and stromal cells have a key role in facilitating cancer progression. Pro-inflammatory prostaglandins and leukotrienes produced by tumour epithelial cells and their surrounding stromal cells are key mediators in this crosstalk and can accelerate tumour growth and metastasis through several methods. First, the pro-inflammatory prostaglandins (PGs) and leukotrienes (LTs) can directly induce epithelial tumour cell proliferation, survival, and migration and invasion in autocrine and paracrine manners. These pro-inflammatory lipids also stimulate epithelial cells and their surrounding stromal cells to produce growth factors, pro-inflammatory mediators and angiogenic factors, which switch the microenvironment from normal to tumour supporting. The tumour microenvironment, in turn, recruits immune cells and endothelial cells (tumour-infiltrating cells), which produce more pro-inflammatory mediators including eicosanoids, growth factors and angiogenic factors. These factors accelerate tumour growth and stimulate metastatsis through an autocrine loop by inducing angiogenesis and evading attack by the immune system.
The role of prostaglandins and leukotrienes in tumour epithelial cells
Prostaglandins and leukotrienes can modulate tumour cell proliferation, differentiation and apoptosis through multiple signalling pathways in both an autocrine and paracrine fashion (FIG. 3; TABLE 1). PGE2 induces proliferation by activating at least two signalling pathways: Ras–Erk and glycogen synthase kinase-3β (GSK3β)–β-catenin in colon and lung cancer cells46-48. In breast cancer, PGE2 can upregulate aromatase production in stromal fat cells and concomitantly oestrogen production, which stimulates tumour cell proliferation49. In addition, PGE2 promotes colon tumour cell survival by activating a PI3K–Akt–PPARδ cascade in ApcMin/+ mice10. PGE2 upregulation of BCL-2, an anti-apoptotic protein, and induction of nuclear factor-κB (NF-κB) transcriptional activity might also be involved in PGE2-induced inhibition of apoptosis50,51. Similarly, PGF2α induces cell proliferation through an FP–Erk–fibroblast growth factor 2 (FGF2)–FGF receptor 1 (FGFR1)–Erk cascade in endometrial tumour cell lines52. By contrast, PGD2 secreted by stromal cells inhibits prostate tumour cell growth in vitro through a PPARγ-dependent mechanism53.
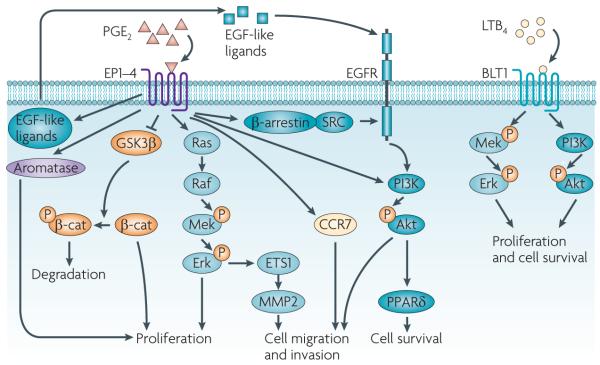
Figure 3. PGE2 and LTB4 promote cancer progression through the induction of tumour epithelial cell proliferation, survival, and migration and invasion.
Multiple cellular signalling pathways mediate the effects of prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) on the regulation of epithelial tumour cell proliferation, survival, and migration and invasion. PGE2 stimulates cell proliferation through multiple cascades in both colorectal cancer (CRC) and non-small-cell lung cancer cells. PGE2 also induces cell proliferation through a glycogen synthase kinase-3β (GSK3β)–β-catenin (β-cat) pathway in CRC cells or by the upregulation of aromatase in breast cancer cells. PGE2 inhibition of GSK3β reduces β-catenin phosphorylation and thereby prevents its degradation, leading to accumulation, nuclear translocation and functional activity of β-catenin. PGE2 promotes CRC cell survival by activating a PI3K–Akt– peroxisome proliferator-activated receptor-δ (PPARδ) cascade in vitro and in vivo. In addition, PGE2 induces CRC cell migration and invasion through β-arrestin-1–SRC–epidermal growth factor receptor (EGFR)–PI3K–Akt signalling in vitro and in vivo. PGE2 transactivation of EGFR also depends on the extracellular release of an EGF-like ligand in CRC cell lines and a normal gastric epithelial cell line. PGE2 also induces cell migration and invasion through an Erk–ETS1–matrix metalloproteinase 2 (MMP2) cascade in pancreatic cancer cell lines or through the upregulation of C-C chemokine receptor 7 (CCR7) in breast cancer cell lines. LTB4 stimulates cell proliferation and promotes cell survival through a BLT1–Erk pathway in CRC cell lines or through both Mek–Erk and PI3K–Akt pathways in human pancreatic cancer cell lines. P, phosphorylation.
Table 1. Signals that mediate the effects of eicosanoids on carcinoma cell proliferation, survival, and migration and invasion.
| Lipids | Receptors | Pathways | Functions | Tumour type | In vitro | In vivo* | refs |
|---|---|---|---|---|---|---|---|
| PGE2 | EP1–4‡ | Ras–Erk | Proliferation | Colorectal | + | + | 46 |
| EP1–4‡ | Ras–Erk | Proliferation | Non-small-cell lung | + | 48 | ||
| EP2 | GSK3β–β-catenin | Proliferation | Colorectal | + | 47 | ||
| EP1–4‡ | PI3K–Akt–PPARδ | Survival | Colorectal | + | + | 10 | |
| EP1–4‡ | BCL-2 | Survival | Colorectal | + | 50 | ||
| EP1–4‡ | NF-κB | Survival | Colorectal | + | 51 | ||
| EP4 | SRC–EGFR–PI3K–Akt | Migration and invasion | Colorectal | + | + | 61,62 | |
| EP1 | SRC–EGFR | Migration and invasion | Hepatocellular | + | 63 | ||
| EP1–4‡ | Erk–ETS1 | Migration and invasion | Pancreatic | + | 65 | ||
| EP2 and EP4 | CCR7 | Migration and invasion | Breast | + | 66 | ||
| EP4 | PI3K–Akt | Migration and invasion | Lung and colorectal | + | + | 67 | |
| PGF2α | FP | Erk–FGF2–FGFR1–Erk | Proliferation | Endometrial | + | 52 | |
| FP | ? | Migration and invasion | Colorectal and endometrial |
+ | 68, 69 | ||
| PGD2 | PPARδ | ? | Proliferation inhibition | Prostate | + | 53 | |
| TXA2 | TP | RHOA | Migration | Prostate | + | 70 | |
| LTB4 | BLT1 | Mek–Erk and PI3K–Akt | Proliferation | Pancreatic | + | 56 | |
| BLT1 | Erk | Survival | Colorectal | + | 55 | ||
| ? | ? | Migration and invasion | Pancreatic | + | 71 | ||
| LTD4 | CysLT1–2‡ | GSK3β–β-catenin | Survival | Intestinal | + | 57 | |
| CysLT1 | PKC–Raf–Erk | Proliferation and survival | Intestinal | + | 58 | ||
| CysLT1–2‡ | COX2 and BCL-2 | Survival | Intestinal | + | 59 | ||
| CysLT1 | ? | Proliferation | Colorectal | + | 60 | ||
| CysLT1 | ? | Survival | Prostate | + | 44 | ||
| CysLT1–2‡ | PI3K–Akt–Rac | Migration | Intestinal | + | 72 |
BLT1, leukotriene B4 receptor (also known as LTB4R); CCR7, C-C chemokine receptor 7; COX2, cyclooxygenase 2; CysLT1, cysteinyl leukotriene receptor 1; EGFR, epidermal growth factor receptor; EP1, prostaglandin E receptor 1; FGF2, fibroblast growth factor 2; FGFR1, FGF receptor 1; FP, prostaglandin F receptor; GSK3β, glycogen synthase kinase 3β; LTB4, leukotriene B4; NF-κB, nuclear factor-κB; PGE2, prostaglandin E2; PKC, protein kinase C; PPARδ, peroxisome proliferator-activated receptor-δ; TXA2, thromboxane A2; TP, TXA2 receptor.
In vivo indicates animal studies.
No data available on which receptor mediates the effects of the lipid.
Knock down of LTA4 hydrolase inhibits anchorage-independent growth of HCT-116 colon cancer cells, suggesting that leukotrienes are involved in cell growth regulation54. Indeed, LTA4 hydrolase-derived LTB4 stimulates cell proliferation and promotes cell survival through a BLT1-Erk pathway in colon tumour cell lines55, and induces cell proliferation through both Mek–Erk and PI3K–Akt pathways in human pancreatic cancer cell lines56. Activation of the LTD4–CysLT1 axis promotes cell proliferation and survival through multiple parallel pathways such as GSK3β–β-catenin, protein kinase C (PKC)–Raf–ERK1 and ERK2, BCL-2 and COX2 in non-transformed human intestinal epithelial cell lines57-59 and induces cell proliferation but not survival in CRC cell lines60. By contrast, inhibition of LTD4 signalling by a CysLT1 antagonist can induce apoptosis in prostate cancer cell lines44. The LTD4 induction of COX2, as well as PGE2 production, implies that crosstalk exists between the 5-LOX and COX2 pathways. Consistent with the negative correlation of CysLT2 expression with a poor prognosis in patients with CRC, CysLT2 signalling leads to terminal differentiation and growth inhibition in colon carcinoma cell lines45.
In addition, prostaglandins and leukotrienes also affect the migration and invasion of carcinoma cells. Our group has demonstrated that PGE2 induces CRC cell migration and invasion through epidermal growth factor receptor (EGFR)–PI3K–Akt signalling in vitro61. Subsequently, we found that PGE2 induction of an EP4–β-arrestin 1–SRC complex was crucial in transactivating EGFR to induce downstream Akt signalling and stimulate CRC cell migration in vitro, as well as the metastatic spread of disease to the liver in vivo62. The SRC–EGFR pathway also mediates PGE2-induced human hepatocellular carcinoma cell invasion in vitro63. These studies revealed that activation of PGE2 receptors transactivates EGFR through an intracellular mechanism. It has been reported that PGE2 transactivation of EGFR depends on the extracellular release of an EGF-like ligand in vitro64. PGE2 can also induce cell migration and invasion through other signalling pathways, including the induction of matrix metalloproteinase 2 (MMP2) through an Erk–ETS1 cascade in pancreatic cancer cell lines65 and the upregulation of C-C chemokine receptor 7 (CCR7) through EP2 and EP4 in breast cancer cell lines66. In addition, treatment with an EP4 antagonist inhibits lung carcinoma cell migration and invasion in vitro and in vivo67. Similar to PGE2, PGF2α also stimulates motility and invasion of colon68 and endometrial69 carcinoma cell lines and TXA2 of prostate carcinoma cell lines70. Relatively little is known about the ability of leukotrienes to regulate tumour cell migration and invasion. In an in vivo study, an LTB4 antagonist was shown to inhibit the metastatic spread of pancreatic cancer cells to the liver and other organs71. In an in vitro study, LTD4 induced non-transformed intestinal cell migration through the activation of a PI3K–Rac cascade72.
Solid tumours are thought to originate from a single replication-competent cell (stem cell or proliferative progenitor cell)1. Recent studies have established that both prostaglandins and leukotrienes are central molecules in the regulation of stem cell homeostasis. PGE2 protects mouse embryonic stem cells from undergoing apoptosis through an EP2–PI3K–Akt cascade73 and regulates growth and development of both embryonic haematopoietic stem cells and adult stem cells in several different organisms74,75. Similarly, LTB4 and LTD4 stimulate the proliferation of several types of stem and progenitor cells76-79. Collectively, the novel functions of these biologically active lipids on stem cell growth and maturation might suggest their ability to regulate cancer stem cell growth. This deserves considerable attention, and future research must be directed towards obtaining a better understanding of the role of prostaglandins and leukotrienes in regulating progenitor cells in solid tumours.
The role of eicosanoids in the inflammatory microenvironment
Chronic inflammation causes stromal cells to produce pro-inflammatory mediators, including eicosanoids, cytokines and chemokines, that shift the tissue microenvironment from normal to aberrant. In general, the inflammatory microenvironment, which is associated with changes in leukocyte profiles as well as their functionality, can initiate epithelial cell transformation and promote tumour growth, angiogenesis and metastasis80. A growing body of evidence demonstrates that prostaglandins and leukotrienes are key immunomodulators mediating the crosstalk between epithelial cells and their surrounding stromal cells in the tumour microenvironment (FIG. 4).
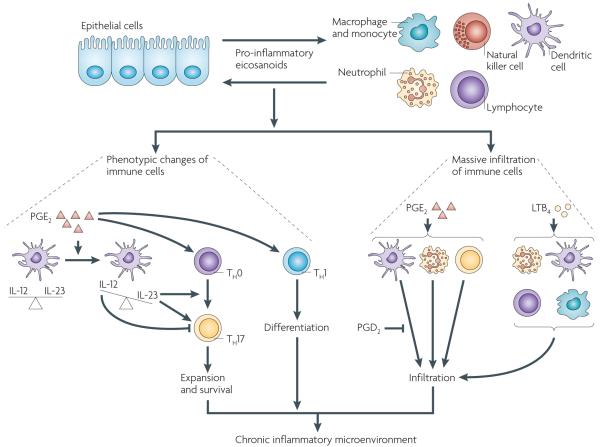
Figure 4. Prostaglandins and leukotrienes are key pro-inflammatory mediators in orchestrating crosstalk between tumour epithelial cells and immune cells.
During the initiation of epithelial tumours or chronic inflammation, transformed or normal epithelial cells and tissue-resident immune cells locally secrete pro-inflammatory prostaglandins and leukotrienes such as prostaglandin E2 (PGE2) and leukotriene B4 (LTB4), which recruit large numbers of immune cells from the circulation into the mucosa and reprogramme the immune cells into pro-inflammatory leukocytes. For example, PGE2 induces expansion of inflammatory T helper 17 (TH17) cells by regulating interactions between T cells and dendritic cells and facilitates TH1 differentiation. PGE2 shifts the interleukin-12 (IL-12)/IL-23 balance in favour of IL-23 through the induction of IL-23 and inhibition of IL-12 expression in dendritic cells. IL-23 is essential for TH17 expansion and survival, whereas IL-12 suppresses TH17 development and function. In recruitment of immune cells, PGE2 induces infiltration of neutrophils and TH17 cells and enhances dendritic cell migration whereas PGD2 inhibits dendritic cell migration. LTB4 has a major role in attracting neutrophils, T cells, dendritic cells and macrophages from the circulation into inflammatory sites. Collectively, PGE2 and LTB4 induce the massive infiltration of immune cells and change their functionality, which in turn results in the establishment of a chronic inflammatory microenvironment.
The normal presence and trafficking of immune cells into the mucosal compartment has been termed physiological inflammation. In response to tumour-associated or chronic inflammation, transformed or normal epithelial cells and tissue-resident immune cells locally secrete cytokines, chemokines and pro-inflammatory eicosanoids that recruit additional leukocytes from the circulation into the tissue. The common pathological changes associated with chronic inflammation include increased infiltration of dysregulated immune cells, production of pro-inflammatory mediators, diminished epithelial integrity and deficient feedback systems that normally downregulate the mucosal response to antigens. Furthermore, the immune cells recruited to the tumour microenvironment are phenotypically different from the normal immune cells and can maintain inflammation and induce angiogenesis80. For example, the massively recruited macrophages in the mucosa of active inflammatory bowel disease (IBD) are phenotypically different from normal macrophages and have a major role in chronic mucosal inflammation by secreting many pro-inflammatory cytokines81.
Although epidemiological and experimental evidence strongly implicates chronic inflammation as a risk factor for cancer, the mechanisms by which inflammation and inflammatory mediators result in neoplastic transformation and progression have not been completely resolved. A strong association between chronic inflammation and malignant diseases occurs in CRC that arises in patients with IBD. The role of PGE2 in IBD is the best characterized example of the role of prostaglandins in chronic inflammation. PGE2 exacerbates inflammation and disease severity through increasing the infiltration of neutrophils and T helper 17 (TH17) cells to the colonic tissue in a murine model of IBD82. Several lines of evidence further suggest that PGE2-induced expansion of inflammatory TH17 cells depends on the involvement of T cells and dendritic cells. PGE2 shifts the interleukin-12 (IL-12)/IL-23 balance in dendritic cells through EP2 and EP4 receptors in favour of IL-23, which in turn increases the number of TH17 cells in vitro82. IL-12 promotes T helper 1 (TH1) responses and suppresses TH17 development and function, whereas IL-23 is essential for TH17 expansion and survival. PGE2 also facilitates IL-23-induced TH17 expansion from peripheral blood mononuclear cells and naive T cells in vitro83,84, induces TH1 cell differentiation in vitro and promotes inflammation through TH1 and TH17 cells in an animal model of chronic inflammation through the EP4 receptor85. In addition, PGE2 induces dendritic cell migration in vitro by upregulation of CCR7 (REF. 86). By contrast, PGD2 inhibits the migration of dendritic cells to the lymph nodes in vivo87. During chronic inflammation, dendritic cells secrete large amounts of inflammatory cytokines that further recruit monocytes and immature dendritic cells into inflamed tissues.
Leukotrienes are powerful lipid mediators of inflammation in various acute and chronic inflammatory and allergic diseases, including IBD88. In fact, urinary LTE4 can serve as a biomarker for IBD89. Similar to chemokines, LTB4 is another potent chemoattractant and activator of neutrophils, eosinophils, basophils, T cells, dendritic cells and macrophages in inflammatory sites90-93; a Rac–Erk signalling cascade might also be responsible for LTB4-induced chemotaxis94. Similar to PGE2, LTB4 promotes the migration of dendritic cells though the upregulation of CCR7 in vitro and in vivo95. However, it is not clear whether Rac–Erk signalling mediates LTB4-induced CCR7 expression. Collectively, these studies show that pro-inflammatory prostaglandins and leukotrienes could stimulate tumour growth through establishing an inflammatory microenvironment.
The role of eicosanoids in tumour immunosuppression
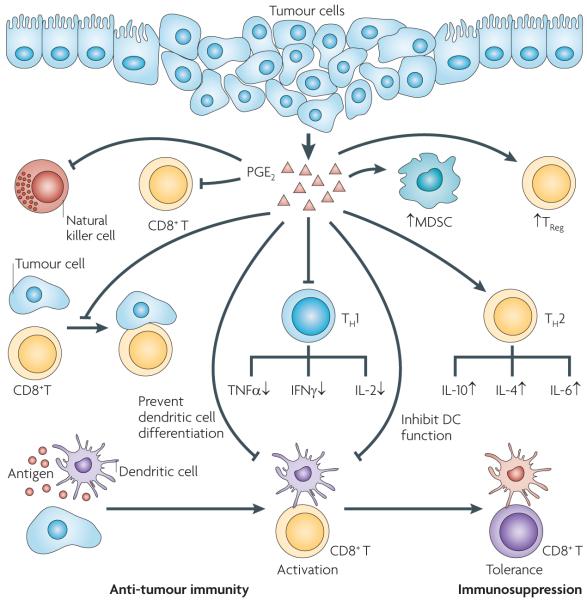
It is well accepted that cooperative interactions between carcinoma cells and other cells in the tumour microenvironment contribute to cancer progression. Tumour epithelial cells secrete cytokines, chemokines and pro-inflammatory eicosanoids that recruit and reprogramme various pro-inflammatory leukocytes to establish an immunosuppressive tumour microenvironment. Of prostaglandins and leukotrienes, only PGE2 has been shown to have a clear role in the regulation of tumour immunosuppression through T cells, CD8+ cytotoxic T cells, regulatory T cells, dendritic cells and myeloid-derived suppressor cells (MDSCs) (FIG. 5). PGE2 helps to shift the tumour microenvironment from anti-tumour TH1 responses to immunosuppressive T helper 2 (TH2) responses by downregulation of TH1 cytokines (tumour necrosis factor-α (TNFα), interferon-γ (IFNγ) and IL-2) and upregulation of TH2 cytokines (IL-4, IL-10 and IL-6) in immune cells96-98. Moreover, PGE2 directly inhibits the activity of cytotoxic T cells through the upregulation of a CD94 and NKG2A complex and induces regulatory T cell function in vitro99,100. PGE2 produced by tumour cells can also indirectly abolish the antitumour effects of cytotoxic T cells in vivo and in vitro through the downregulation of both direct antigen presentation by tumour cells and cross-presentation by dendritic cells101. For example, an in vitro study showed that PGE2 switches the function of dendritic cells from induction of immunity to T cell tolerance through the upregulation of CD25 and indoleamine 2,3-dioxygenase102. In addition to modulating T cell and dendritic cell function, PGE2 modulates cancer-associated immunosuppression through the inhibition of dendritic cell differentiation and T cell proliferation in vivo103,104. Furthermore, PGE2 promoted mammary tumour progression through the induction of MDSCs in vivo105. Collectively, the effects of PGE2 on the immune system may allow neoplastic cells to evade attack. To our knowledge, there is nothing further reported in the literature indicating the participation of other prostaglandins and leukotrienes in cancer-associated immunosuppression.
Figure 5. PGE2 provides coordinated regulation of tumour immunosuppression.
Pro-inflammatory prostaglandin E2 (PGE2) produced by tumour epithelial cells and/or their surrounding stromal cells induces immunosuppression through several ways, including: downregulating anti-tumour T helper 1 (TH1) cytokines and upregulating immunosuppressive TH2 cytokines; inhibiting CD8+ T cell proliferation and activity, suppressing the anti-tumour activity of natural killer cells and stimulating the expansion of regulatory T cells (TReg cells) and myeloid-derived suppressor cells (MDSCs); and inhibiting CD8+ T cell anti-tumour functions by impairing the ability of tumour cells to directly present tumour antigen, inhibiting dendritic cell differentiation and switching the function of dendritic cells from induction of immunity to T cell tolerance. The yellow CD8+ T cells have anti-tumour activity and the purple CD8+ T cell does not have anti-tumour activity. The purple dendritic cells have the ability to present tumour antigens from tumour cells with major histocompatibility complex (MHC) class I molecules to activate naive CD8+ T cells. The orange dendritic cell does not have the ability to activate CD8+ T cells (T cell tolerance). IFNγ, interferon-γ; IL, interleukin; TNFα, tumour necrosis factor-α.
Angiogenesis
It is widely recognized that angiogenesis is required for tumours to grow beyond a certain size and to metastasize. To develop a stable blood supply for tumour growth, many cells in the tumour microenvironment, including tumour epithelial cells, stromal cells and immune cells, secrete various proangiogenic factors that stimulate endothelial cell recruitment, proliferation, migration and tubule formation. Numerous in vitro and in vivo studies indicate that prostaglandins and leukotrienes modulate angiogenesis at different levels (FIG. 6).
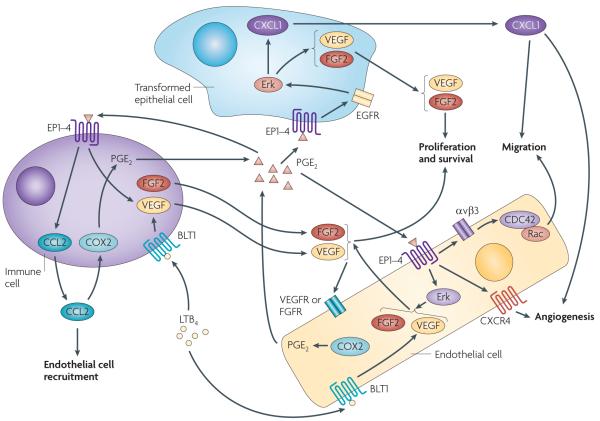
Figure 6. A model of PGE2 and LTB4 coordinately regulating angiogenesis in the tumour microenvironment.
Pro-inflammatory prostaglandin E2 (PGE2) and/or B4 LTB4 promote angiogenesis on at least two levels. First, PGE2 and/or LTB4 can directly act on epithelial, endothelial and/or immune cells to induce angiogenic factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor 2 (FGF2) and the chemokines CXCL1 and CCL2. In transformed epithelial cells, PGE2 induces VEGF and CXCL1 secretion through an EP2 or EP4–epidermal growth factor (EGFR)–Erk cascade. In endothelial cells, PGE2 induces VEGF and FGF2 secretion through a MAPK pathway and LTB4 also stimulates VEGF expression. Moreover, PGE2 not only binds to endothelial cells to stimulate cell migration through an αVβ3 integrin–CDC42 and Rac pathway, but also mediates VEGF-and FGF2-induced CXCR4-dependent neovessel assembly in vivo. In immune cells, PGE2 promotes mast cells to release VEGF and CCL2, and LTB4 stimulates VEGF expression. Secretion of VEGF and FGF2 from tumour epithelial, endothelial and immune cells promotes endothelial cell proliferation and survival, and the chemokine CXCL1 released from tumour epithelial cells stimulates endothelial cell migration and tube formation in vitro and angiogenesis in vivo. CCL2 can attract endothelial cells to the tumour microenvironment. Interestingly, VEGF and FGF2 induce COX2 and subsequently PGE2 in endothelial cells, and CCL2 also induces COX2 and PGE2 in macrophages. Therefore, the effects of PGE2 on the regulation of VEGF, FGF2 and CCL2 are probably amplified through this positive feedback loop.
Homozygous deletion of Ep2 completely abrogates the induction of vascular endothelial growth factor (VEGF) in the intestinal polyp stroma of ApcΔ716 mice and inhibits intestinal polyp growth, demonstrating that PGE2 induction of VEGF is important for tumour growth in this mouse model29. Moreover, PGE2 induced expression of CXCL1, a pro-angiogenic chemokine, in human CRC cells, and the release of CXCL1 from tumour epithelial cells in turn stimulated microvascular endothelial cell migration and tube formation in vitro and in vivo106. Activation of EP2 receptors by PGE2 induces FGF2 expression through potential multiple protein kinase A (PKA), SRC, EGFR, and ERK1 and ERK2 signalling pathways in an endometrial adenocarcinoma cell line and in endometrial adenocarcinoma tissue explants107. PGE2 released from mammary tumour epithelial cells induces angiogenesis through the induction of pro-angiogenic factors through a cyclic AMP-dependent PKA pathway in vivo and in vitro108. PGE2 can also induce VEGF secretion in ovarian, prostate and gastric cancer cell lines through the activation of EGFR downstream of EP2 and EP4 (REFS 109-111). Similar to PGE2, PGF2α induces VEGF expression through an FP–EGFR–Ras–Erk pathway in an endometrial adenocarcinoma cell line112. Moreover, PGF2α-FP signalling can stimulate the secretion of CXCL1 in endometrial tumour cells to promote neutrophil infiltration in mouse xenograft tumours113; neutrophil infiltration has also been shown to be essential for angiogenesis in other tumour models114. In tumour implantation models, increased TXA2 levels as a result of overexpression of TXA2 synthase in colon-26 adenocarcinoma cells accelerated tumour growth and tumour-associated angiogenesis in syngeneic BALB/c mice115.
In addition to inducing the production of angiogenic factors in epithelial cells, prostaglandin signalling in stromal cells also contributes to angiogenesis. A host PGE2–EP2 signal is required for tumour angio genesis by enhancing endothelial cell motility and vascular hyperpermeability in a mouse model of breast cancer116. Similarly, the host PGE2–EP3 signal is a prerequisite for vEGF expression in the stroma and tumour angiogenesis in a mouse model of lung carcinoma117. In endothelial cells, PGE2 not only upregulates VEGF and FGF2 expression through the stimulation of an ERK2-JUN N-terminal kinase 1 (JNK1) signalling pathway, which is important for inducing cell growth and survival118, but also promotes αVβ3 integrin-dependent migration and spreading of endothelial cells119 and mediates VEGFand FGF2-induced CXCR4-dependent neovessel assembly in vivo120. In addition, TXA2 mediates the effects of COX2 on endothelial cell migration and angiogenesis in vitro and in vivo121. Intriguingly, VEGF and FGF2 induce COX2, and subsequently PGE2 and PGI2, in endothelial cells, suggesting that the effects of PGE2 on regulation of VEGF and FGF2 are probably amplified through a positive feedback loop120. In contrast to PGE2 signalling, deficiency of the DP receptor in the host accelerates tumour growth of implanted lung carcinomas by stimulating angiogenesis122, suggesting that the host PGD2–DP signal inhibits tumour growth by inhibiting angiogenesis.
The physiological significance of leukotrienes in angiogenesis is much less well characterized than that of the prostaglandins. LTB4 induced endothelial cell migration and tube formation in vitro and stimulated VEGF-induced angiogenesis through the BLT2 receptor in vivo123. Similarly, LTC4 induced endothelial cell proliferation in vitro124 and LTC4 and LTD4 promoted angiogenesis in the chick chorioallantoic membrane system125. In addition, LTB4 enhances hypoxia-induced microvascular alterations in vivo126.
In addition to the involvement of PGE2 and leukotrienes in signalling crosstalk between tumour epithelial and endothelial cells, PGE2 also stimulates immune cells to produce pro-angiogenic factors. For example, PGE2 promotes mast cells to release VEGF and the chemokine CCL2 in vitro127,128. CCL2 can induce tumour-associated angiogenesis129 by directly recruiting endothelial cells that express its receptor CCR2 in vitro and in vivo130, by inducing VEGF release from macrophages through a COX2-PGE2 pathway in vitro131, and/or by indirectly attracting more tumour-associated monocytes and macrophages, which are phenotypically distinct from normal macrophages in their ability to promote angiogenesis in mice132. LTB4 induces neutrophil-mediated vascular permeability in vitro and in vivo133. Moreover, inhibition of CysLT production by the deletion of LTC4 synthase reduces vascular permeability in zymosan A-induced, monocyte and macrophage-mediated peritoneal inflammation and in immunoglobulin E-dependent, mast cell-mediated passive cutaneous anaphylaxis134, suggesting that CysLTs promote inflammation-mediated vascular permeability.
Therapeutic and chemopreventive agents
A retrospective cohort study shows that aspirin, a nonselective NSAID, specifically reduces cancer risk in the subgroup of patients whose colon tumours expressed higher levels of COX2 (REF. 135). In addition to prevention, regular aspirin use after the diagnosis of CRC at stages I, II and III improves overall survival, especially among individuals whose tumours overexpress COX2 (REF. 136). However, the prolonged use of non-selective NSAIDs is associated with adverse gastrointestinal side effects. Long-term use of high doses of selective COX2 inhibitors is currently not recommended because of the unacceptable cardiovascular side effects in certain patients, especially those with a history of atherosclerotic heart disease137. However, a selective COX2 inhibitor (celecoxib) is still approved by the US Food and Drug Administration for use as an adjuvant treatment in patients at a high risk for developing CRC, such as those with familial adenomatous polyposis. It has been proposed that some of the adverse effects related to NSAID use are associated with a global reduction in prostanoid production138. For instance, COX2 deficiency in mice contributes to the pro-atherogenic properties of high-density lipoprotein cholesterol, with increased lipid deposition in the aorta and the dramatic imbalance in circulating prostanoids139. One hypothetical method to avoid the cardiovascular side effects of COX2 inhibition would be to target only COX-derived PGE2 signalling that mediates the tumour-promoting effects of COX2. For example, antagonists of PGE2 receptors have been developed and show promising effects for preventing and/or inhibiting growth of different types of tumours, including colon, oesophageal, lung and breast in preclinical animal models27-29,33,67,140.
Inhibition of the 5-LOX pathway may also be useful for cancer therapy. For example, a 5-LOX inhibitor, zileuton, has been shown to prevent lung tumorigenesis in carcinogen-treated mice141. A combined treatment regimen using a selective COX2 inhibitor and a 5-LOX inhibitor not only had additive effects on reducing tumour growth in mouse xenograft models of human colon, oesophageal, breast and skin cancer142-145, but also reduced liver metastases in a hamster model of chemically induced ductal pancreatic adenocarcinoma146. Moreover, blockage of leukotriene production by the LTA4 hydrolase inhibitor bestatin reduced tumour burden in rats, as described above42. The LTB4 receptor antagonist, LY293111, significantly inhibits tumour growth and metastases in a fluorescent orthotopic model of pancreatic cancer71 and a mouse xenograft model of human CRC147. Unfortunately, the results from two Phase II trials showed that LY293111 did not improve progressionfree survival in patients with non-small-cell lung or pancreatic cancer148. In addition, a CysLT1 receptor antagonist, zafirlukast, reduced tumour burden in a mouse model of carcinogen-induced lung tumours149. Although NSAIDs are still among the most promising chemopreventive agents for cancer, cardiovascular and gastrointestinal side effects have dampened enthusiasm for their use as chemopreventive agents. Therefore, it is now crucial to evaluate whether the antagonists of PGE2 and leukotriene receptors have better specificity for human cancer prevention and treatment.
Summary
Many strategies exist to develop more effective cancer prevention and treatment options. Chemoprevention and targeted therapies are being carefully evaluated on several fronts as effective measures to achieve better cancer control. A big challenge remains to develop chemopreventive agents that are both effective and safe. An entirely new approach, referred to as personalized cancer prevention and treatment, is likely to have an important role in achieving these goals. This idea is supported by the observations that patients whose colon tumours express higher levels of COX2 benefit from regular aspirin use in cancer prevention and risk reduction135,136, and that patients whose tumours express a mutant form of KRAS do not benefit from EGFR-specific therapy with cetuximab or panitumumab150. However, this approach relies on a foundation of knowledge derived from the molecular basis of carcinogenesis to the molecular characterization of individual cancers. Understanding the role of eicosanoids in tumorigenesis and profiling these biologically active lipids in each patient are two crucial steps towards this personalized approach as they may be biomarkers for predicting which patients respond to NSAIDs in prevention and/or as intermediate markers for assessing response during treatment.
In addition to biomarkers, certain eicosanoids are also potential drug targets for personalizing cancer prevention and/or treatment. The evidence that proinflammatory eicosanoids such as PGE2, LTB4 and LTD4 promote tumour growth and metastasis, whereas anti-inflammatory PGD2 suppresses tumour growth will provide a rationale to develop the next generation of personalized cancer preventive agents. Therefore, it is now crucial to evaluate whether these prostaglandin and leukotriene receptor antagonists and agonists, as well as agents that lower cellular levels of pro-inflammatory prostaglandins and leukotrienes, have better efficacy with fewer adverse effects than NSAIDs. For example, the activators of 15-PGDH and inhibitors of PGE2 synthases, LTA4 hydrolase and LTC4 synthases may be useful agents for future studies.
Acknowledgements
This work is partly supportedby the US National Institutes of Health Grants RO1DK 62112, P01-CA-77839, R37-DK47297 and P30 DK-58404 (R.N.D.). R.N.D. (R37-DK47297) is recipient of an NIH MERIT award. We also thank the National Colorectal Cancer Research Alliance (NCCRA) for generous support (R.N.D.). We also kindly thank D. Menter for his assistance in the preparation of figures.
Glossary
- Cysteinyl leukotriene (CysLT)
Leukotriene that contains the amino acid cysteine conjugated to the lipid backbone.
- ApcMin/+ mice
Carry a point mutation in one Apc allele and spontaneously develop intestinal adenomas. Used as a model for human familial adenomatous polyposis and for human sporadic colorectal cancer.
- Azoxymethane (AOM) mouse model
One of the chemically induced colorectal cancer models in which mice are exposed to a chemical carcinogen, AOM.
- T helper 17 (TH17) cells
A functional subset of CD4+ T helper cells that secrete particular inflammatory cytokines, including interleukin-17, which mediate pathogenic responses in autoimmune diseases.
- Dendritic cells
Bone marrow-derived immune accessory cells that function as antigen-presenting cells for naive T cells and that lead to the initiation of adaptive immune responses to protein antigens.
- Cytotoxic T cells
A subgroup of T cells (also known as CD8+ T cells or killer T cells) that are capable of recognizing and inducing the death of infected somatic or tumour cells. CD8+ T cells are recognized as cytotoxic T cells once they become activated.
- Regulatory T cells
A T cell subpopulation that suppresses activation of other T cells and maintains immune system homeostasis and peripheral tolerance to self-antigens.
- Myeloid-derived suppressor cells (MDSCs)
Immature myeloid cells with potent immunosuppressive functions.
- Cross-presentation
A mechanism by which a professional antigen-presenting cell takes up, processes and presents extracellular antigens from a third cell (for example, a virus-infected or tumour cell) with major histocompatibility complex class I molecules to activate a naive CD8+ T cell.
- T cell tolerance
Unresponsiveness of the adaptive immune system to antigens, as a result of the inactivation or death of antigen-specific T cells, which is induced by exposure to the antigens.
- ApcΔ716 mice
Generated by inserting neomycin into exon 15 of Apc, which results in truncated APC at codon 716. Spontaneously develop intestinal adenomas and are another model for human familial adenomatous polyposis and sporadic colorectal cancer.
- Chick chorioallantoic membrane system
A biological assay using the well-vascularized chorioallantoic membrane of the chicken egg to evaluate the biological activity of pro-angiogenic and anti-angiogenic factors.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/genePtges
National Cancer Institute Drug Dictionary: http://www.cancer.gov/drugdictionary/celecoxib
UniProtKB: http://www.uniprot.org
5-LOX | 12-LOX | 15-LOX-1 | 15-LOX-2 | BLT1 | BLT2 | CCR7 | COX1 | COX2 | CysLT1 | CysLT2 | DP | EGFR | EP1 | EP2 | EP3 |EP4 | FLAP | FP | GPR44 | IP | PPARδ | PPARγ | PTGDS | TP
FURTHER INFORMATION
Raymond N. DuBois’ homepage: http://faculty.mdanderson.org/Raymond_DuBois/Default.asp?SNID=310943223
References
- 1.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nature Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 2.Ting AH, McGarvey KM, Baylin SB. The cancer epigenome — components and functional correlates. Genes Dev. 2006;20:3215–3231. doi: 10.1101/gad.1464906. [DOI] [PubMed] [Google Scholar]
- 3.Woutersen RA, Appel MJ, van Garderen-Hoetmer A, Wijnands MV. Dietary fat and carcinogenesis. Mutat. Res. 1999;443:111–127. doi: 10.1016/s1383-5742(99)00014-9. [DOI] [PubMed] [Google Scholar]
- 4.Harris RE. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. 2009;17:55–67. doi: 10.1007/s10787-009-8049-8. [DOI] [PubMed] [Google Scholar]
- 5.Pidgeon GP, et al. Lipoxygenase metabolism: roles in tumor progression and survival. Cancer Metastasis Rev. 2007;26:503–524. doi: 10.1007/s10555-007-9098-3. [DOI] [PubMed] [Google Scholar]
- 6.Dubois RN, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 7.Eberhart CE, et al. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. This study was the first to report that COX2 expression is increased in human CRC.
- 8.de Groot DJ, de Vries EG, Groen HJ, de Jong S. Non-steroidal anti-inflammatory drugs to potentiate chemotherapy effects: from lab to clinic. Crit. Rev. Oncol. Hematol. 2007;61:52–69. doi: 10.1016/j.critrevonc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Gupta RA, et al. Cyclooxygenase-1 is overexpressed and promotes angiogenic growth factor production in ovarian cancer. Cancer Res. 2003;63:906–911. [PubMed] [Google Scholar]
- 10.Wang D, et al. Prostaglandin E2 promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell. 2004;6:285–295. doi: 10.1016/j.ccr.2004.08.011. This report was the first to indicate crosstalk between PGE2 signalling and Wnt signalling in promoting colon tumour growth.
- 11.Wang D, DuBois RN. Measurement of eicosanoids in cancer tissues. Methods Enzymol. 2007;433:27–50. doi: 10.1016/S0076-6879(07)33002-4. [DOI] [PubMed] [Google Scholar]
- 12.Folco G, Murphy RC. Eicosanoid transcellular biosynthesis: from cell-cell interactions to in vivo tissue responses. Pharmacol. Rev. 2006;58:375–388. doi: 10.1124/pr.58.3.8. [DOI] [PubMed] [Google Scholar]
- 13.Zarini S, Gijon MA, Ransome AE, Murphy RC, Sala A. Transcellular biosynthesis of cysteinyl leukotrienes in vivo during mouse peritoneal inflammation. Proc. Natl Acad. Sci. USA. 2009;106:8296–8301. doi: 10.1073/pnas.0903851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rigas B, Goldman IS, Levine L. Altered eicosanoid levels in human colon cancer. J. Lab. Clin. Med. 1993;122:518–523. [PubMed] [Google Scholar]
- 15.Wang D, Dubois RN. Cyclooxygenase-2: a potential target in breast cancer. Semin. Oncol. 2004;31:64–73. doi: 10.1053/j.seminoncol.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 16.McLemore TL, et al. Profiles of prostaglandin biosynthesis in normal lung and tumor tissue from lung cancer patients. Cancer Res. 1988;48:3140–3147. [PubMed] [Google Scholar]
- 17.Hambek M, et al. Inverse correlation between serum PGE2 and T classification in head and neck cancer. Head Neck. 2007;29:244–248. doi: 10.1002/hed.20503. [DOI] [PubMed] [Google Scholar]
- 18.Backlund MG, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J. Biol. Chem. 2005;280:3217–3223. doi: 10.1074/jbc.M411221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf I, et al. 15-hydroxyprostaglandin dehydrogenase is a tumor suppressor of human breast cancer. Cancer Res. 2006;66:7818–7823. doi: 10.1158/0008-5472.CAN-05-4368. [DOI] [PubMed] [Google Scholar]
- 20.Hughes D, et al. NAD+-dependent 15-hydroxyprostaglandin dehydrogenase regulates levels of bioactive lipids in non-small cell lung cancer. Cancer Prev. Res. (Phila Pa) 2008;1:241–249. doi: 10.1158/1940-6207.CAPR-08-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiel A, et al. 15-hydroxyprostaglandin dehydrogenase is down-regulated in gastric cancer. Clin. Cancer Res. 2009;15:4572–4580. doi: 10.1158/1078-0432.CCR-08-2518. [DOI] [PubMed] [Google Scholar]
- 22.Hansen-Petrik MB, et al. Prostaglandin E2 protects intestinal tumors from nonsteroidal anti-inflammatory drug-induced regression in ApcMin/+ mice. Cancer Res. 2002;62:403–408. [PubMed] [Google Scholar]
- 23.Yan M, et al. 15-Hydroxyprostaglandin dehydrogenase inactivation as a mechanism of resistance to celecoxib chemoprevention of colon tumors. Proc. Natl Acad. Sci. USA. 2009;106:9409–9413. doi: 10.1073/pnas.0902367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawamori T, Uchiya N, Sugimura T, Wakabayashi K. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis. 2003;24:985–990. doi: 10.1093/carcin/bgg033. [DOI] [PubMed] [Google Scholar]
- 25.Myung SJ, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc. Natl Acad. Sci. USA. 2006;103:12098–12102. doi: 10.1073/pnas.0603235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakanishi M, et al. Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res. 2008;68:3251–3259. doi: 10.1158/0008-5472.CAN-07-6100. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe K, et al. Role of the prostaglandin E receptor subtype EP1 in colon carcinogenesis. Cancer Res. 1999;59:5093–5096. [PubMed] [Google Scholar]
- 28.Mutoh M, et al. Involvement of prostaglandin E receptor subtype EP4 in colon carcinogenesis. Cancer Res. 2002;62:28–32. [PubMed] [Google Scholar]
- 29.Sonoshita M, et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in ApcΔ 716 knockout mice. Nature Med. 2001;7:1048–1051. doi: 10.1038/nm0901-1048. This report was first to indicate that PGE2 signalling promotes tumour-associated angiogenesis in vivo.
- 30.Oshima H, et al. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology. 2006;131:1086–1095. doi: 10.1053/j.gastro.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Keith RL, et al. Prostaglandin E2 receptor subtype 2 (EP2) null mice are protected against murine lung tumorigenesis. Anticancer Res. 2006;26:2857–2861. [PubMed] [Google Scholar]
- 32.Chang SH, Ai Y, Breyer RM, Lane TF, Hla T. The prostaglandin E2 receptor EP2 is required for cyclooxygenase 2-mediated mammary hyperplasia. Cancer Res. 2005;65:4496–4499. doi: 10.1158/0008-5472.CAN-05-0129. [DOI] [PubMed] [Google Scholar]
- 33.Kawamori T, et al. Chemopreventive effects of ONO-8711, a selective prostaglandin E receptor EP1 antagonist, on breast cancer development. Carcinogenesis. 2001;22:2001–2004. doi: 10.1093/carcin/22.12.2001. [DOI] [PubMed] [Google Scholar]
- 34.Park JM, et al. Hematopoietic prostaglandin D synthase suppresses intestinal adenomas in ApcMin/+ mice. Cancer Res. 2007;67:881–889. doi: 10.1158/0008-5472.CAN-05-3767. [DOI] [PubMed] [Google Scholar]
- 35.Sertznig P, Seifert M, Tilgen W, Reichrath J. Present concepts and future outlook: function of peroxisome proliferator-activated receptors (PPARs) for pathogenesis, progression, and therapy of cancer. J. Cell Physiol. 2007;212:1–12. doi: 10.1002/jcp.20998. [DOI] [PubMed] [Google Scholar]
- 36.Wolfle D. Enhancement of carcinogen-induced malignant cell transformation by prostaglandin F2α. Toxicology. 2003;188:139–147. doi: 10.1016/s0300-483x(03)00077-5. [DOI] [PubMed] [Google Scholar]
- 37.Wang D, et al. Crosstalk between peroxisome proliferator-activated receptor δand VEGF stimulates cancer progression. Proc. Natl Acad. Sci. USA. 2006;103:19069–19074. doi: 10.1073/pnas.0607948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larre S, et al. PGE2 and LTB4 tissue levels in benign and cancerous prostates. Prostaglandins Other Lipid Mediat. 2008;87:14–19. doi: 10.1016/j.prostaglandins.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Dreyling KW, et al. Leukotriene synthesis by human gastrointestinal tissues. Biochim. Biophys. Acta. 1986;878:184–193. doi: 10.1016/0005-2760(86)90145-1. [DOI] [PubMed] [Google Scholar]
- 40.Hennig R, et al. 5-Lipoxygenase and leukotriene B4 receptor are expressed in human pancreatic cancers but not in pancreatic ducts in normal tissue. Am. J. Pathol. 2002;161:421–428. doi: 10.1016/S0002-9440(10)64198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoo MH, Song H, Woo CH, Kim H, Kim JH. Role of the BLT2, a leukotriene B4 receptor, in Ras transformation. Oncogene. 2004;23:9259–9268. doi: 10.1038/sj.onc.1208151. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, et al. Leukotriene A4 hydrolase in rat and human esophageal adenocarcinomas and inhibitory effects of bestatin. J. Natl Cancer Inst. 2003;95:1053–1061. doi: 10.1093/jnci/95.14.1053. [DOI] [PubMed] [Google Scholar]
- 43.Ohd JF, et al. Expression of the leukotriene D4 receptor CysLT1, COX-2, and other cell survival factors in colorectal adenocarcinomas. Gastroenterology. 2003;124:57–70. doi: 10.1053/gast.2003.50011. [DOI] [PubMed] [Google Scholar]
- 44.Matsuyama M, et al. Overexpression of cysteinyl LT1 receptor in prostate cancer and CysLT1R antagonist inhibits prostate cancer cell growth through apoptosis. Oncol. Rep. 2007;18:99–104. [PubMed] [Google Scholar]
- 45.Magnusson C, Ehrnstrom R, Olsen J, Sjolander A. An increased expression of cysteinyl leukotriene 2 receptor in colorectal adenocarcinomas correlates with high differentiation. Cancer Res. 2007;67:9190–9198. doi: 10.1158/0008-5472.CAN-07-0771. [DOI] [PubMed] [Google Scholar]
- 46.Wang D, Buchanan FG, Wang H, Dey SK, DuBois RN. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res. 2005;65:1822–1829. doi: 10.1158/0008-5472.CAN-04-3671. [DOI] [PubMed] [Google Scholar]
- 47.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-β-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. This report was the first to show that PGE2 activates Wnt signalling by the disruption of a β-catenin inhibitory complex.
- 48.Krysan K, et al. Prostaglandin E2 activates mitogen-activated protein kinase/Erk pathway signaling and cell proliferation in non-small cell lung cancer cells in an epidermal growth factor receptor-independent manner. Cancer Res. 2005;65:6275–6281. doi: 10.1158/0008-5472.CAN-05-0216. [DOI] [PubMed] [Google Scholar]
- 49.Zhou J, et al. Interactions between prostaglandin E2, liver receptor homologue-1, and aromatase in breast cancer. Cancer Res. 2005;65:657–663. [PubMed] [Google Scholar]
- 50.Sheng H, Shao J, Morrow J, Beauchamp RD, DuBois RN. Modulation of apoptosis by prostaglandin treatment in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 51.Poligone B, Baldwin AS. Positive and negative regulation of NF-κB by COX-2: roles of different prostaglandins. J. Biol. Chem. 2001;276:38658–38664. doi: 10.1074/jbc.M106599200. [DOI] [PubMed] [Google Scholar]
- 52.Sales KJ, Boddy SC, Williams AR, Anderson RA, Jabbour HN. F-prostanoid receptor regulation of fibroblast growth factor 2 signaling in endometrial adenocarcinoma cells. Endocrinology. 2007;148:3635–3644. doi: 10.1210/en.2006-1517. [DOI] [PubMed] [Google Scholar]
- 53.Kim J, et al. Suppression of prostate tumor cell growth by stromal cell prostaglandin D synthase-derived products. Cancer Res. 2005;65:6189–6198. doi: 10.1158/0008-5472.CAN-04-4439. [DOI] [PubMed] [Google Scholar]
- 54.Jeong CH, et al. [6]-gingerol suppresses colon cancer growth by targeting leukotriene A4 hydrolase. Cancer Res. 2009;69:5584–5591. doi: 10.1158/0008-5472.CAN-09-0491. [DOI] [PubMed] [Google Scholar]
- 55.Ihara A, et al. Blockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancer. J. Pharmacol. Sci. 2007;103:24–32. doi: 10.1254/jphs.fp0060651. [DOI] [PubMed] [Google Scholar]
- 56.Tong WG, Ding XZ, Talamonti MS, Bell RH, Adrian TE. LTB4 stimulates growth of human pancreatic cancer cells via MAPK and PI-3 kinase pathways. Biochem. Biophys. Res. Commun. 2005;335:949–956. doi: 10.1016/j.bbrc.2005.07.166. [DOI] [PubMed] [Google Scholar]
- 57.Mezhybovska M, Wikstrom K, Ohd JF, Sjolander A. The inflammatory mediator leukotriene D4 induces β-catenin signaling and its association with antiapoptotic Bcl-2 in intestinal epithelial cells. J. Biol. Chem. 2006;281:6776–6784. doi: 10.1074/jbc.M509999200. [DOI] [PubMed] [Google Scholar]
- 58.Paruchuri S, Hallberg B, Juhas M, Larsson C, Sjolander A. Leukotriene D4 activates MAPK through a Ras-independent but PKCε-dependent pathway in intestinal epithelial cells. J. Cell Sci. 2002;115:1883–1893. doi: 10.1242/jcs.115.9.1883. [DOI] [PubMed] [Google Scholar]
- 59.Ohd JF, Wikstrom K, Sjolander A. Leukotrienes induce cell-survival signaling in intestinal epithelial cells. Gastroenterology. 2000;119:1007–1018. doi: 10.1053/gast.2000.18141. [DOI] [PubMed] [Google Scholar]
- 60.Paruchuri S, Mezhybovska M, Juhas M, Sjolander A. Endogenous production of leukotriene D4 mediates autocrine survival and proliferation via CysLT1 receptor signalling in intestinal epithelial cells. Oncogene. 2006;25:6660–6665. doi: 10.1038/sj.onc.1209666. [DOI] [PubMed] [Google Scholar]
- 61.Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J. Biol. Chem. 2003;278:35451–35457. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- 62.Buchanan FG, et al. Role of beta-arrestin 1 in the metastatic progression of colorectal cancer. Proc. Natl Acad. Sci. USA. 2006;103:1492–1497. doi: 10.1073/pnas.0510562103. This study was the first to report a link between PGE signalling and the metastatic spread of colon tumour cells to the liver in vivo.
- 63.Han C, Michalopoulos GK, Wu T. Prostaglandin E2 receptor EP1 transactivates EGFR/MET receptor tyrosine kinases and enhances invasiveness in human hepatocellular carcinoma cells. J. Cell Physiol. 2006;207:261–270. doi: 10.1002/jcp.20560. [DOI] [PubMed] [Google Scholar]
- 64.Pai R, et al. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nature Med. 2002;8:289–293. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- 65.Ito H, et al. Prostaglandin E2 enhances pancreatic cancer invasiveness through an Ets-1-dependent induction of matrix metalloproteinase-2. Cancer Res. 2004;64:7439–7446. doi: 10.1158/0008-5472.CAN-04-1177. [DOI] [PubMed] [Google Scholar]
- 66.Pan MR, Hou MF, Chang HC, Hung WC. Cyclooxygenase-2 up-regulates CCR7 via EP2/EP4 receptor signaling pathways to enhance lymphatic invasion of breast cancer cells. J. Biol. Chem. 2008;283:11155–11163. doi: 10.1074/jbc.M710038200. [DOI] [PubMed] [Google Scholar]
- 67.Yang L, et al. Host and direct antitumor effects and profound reduction in tumor metastasis with selective EP4 receptor antagonism. Cancer Res. 2006;66:9665–9672. doi: 10.1158/0008-5472.CAN-06-1271. [DOI] [PubMed] [Google Scholar]
- 68.Qualtrough D, et al. Prostaglandin F2α stimulates motility and invasion in colorectal tumor cells. Int. J. Cancer. 2007;121:734–740. doi: 10.1002/ijc.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sales KJ, Boddy SC, Jabbour HN. F-prostanoid receptor alters adhesion, morphology and migration of endometrial adenocarcinoma cells. Oncogene. 2008;27:2466–2477. doi: 10.1038/sj.onc.1210883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nie D, et al. Thromboxane A2 receptors in prostate carcinoma: expression and its role in regulating cell motility via small GTPase Rho. Cancer Res. 2008;68:115–121. doi: 10.1158/0008-5472.CAN-07-1018. [DOI] [PubMed] [Google Scholar]
- 71.Hennig R, et al. LY293111 improves efficacy of gemcitabine therapy on pancreatic cancer in a fluorescent orthotopic model in athymic mice. Neoplasia. 2005;7:417–425. doi: 10.1593/neo.04559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paruchuri S, Broom O, Dib K, Sjolander A. The pro-inflammatory mediator leukotriene D4 induces phosphatidylinositol 3-kinase and Rac-dependent migration of intestinal epithelial cells. J. Biol. Chem. 2005;280:13538–13544. doi: 10.1074/jbc.M409811200. [DOI] [PubMed] [Google Scholar]
- 73.Liou JY, et al. Cyclooxygenase-2-derived prostaglandin e2 protects mouse embryonic stem cells from apoptosis. Stem Cells. 2007;25:1096–1103. doi: 10.1634/stemcells.2006-0505. [DOI] [PubMed] [Google Scholar]
- 74.North TE, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. This paper describes the first evidence that PGE2 can regulate stem and progenitor cell homeostasis.
- 75.Goessling W, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wada K, et al. Leukotriene B4 and lipoxin A4 are regulatory signals for neural stem cell proliferation and differentiation. FASEB J. 2006;20:1785–1792. doi: 10.1096/fj.06-5809com. [DOI] [PubMed] [Google Scholar]
- 77.Chung JW, et al. Leukotriene B4 pathway regulates the fate of the hematopoietic stem cells. Exp. Mol. Med. 2005;37:45–50. doi: 10.1038/emm.2005.6. [DOI] [PubMed] [Google Scholar]
- 78.Boehmler AM, et al. The CysLT1 ligand leukotriene D4 supports α4β1- and α5β1-mediated adhesion and proliferation of CD34+ hematopoietic progenitor cells. J. Immunol. 2009;182:6789–6798. doi: 10.4049/jimmunol.0801525. [DOI] [PubMed] [Google Scholar]
- 79.Braccioni F, et al. The effect of cysteinyl leukotrienes on growth of eosinophil progenitors from peripheral blood and bone marrow of atopic subjects. J. Allergy Clin. Immunol. 2002;110:96–101. doi: 10.1067/mai.2002.125000. [DOI] [PubMed] [Google Scholar]
- 80.Noonan DM, De Lerma Barbaro A, Vannini N, Mortara L, Albini A. Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev. 2008;27:31–40. doi: 10.1007/s10555-007-9108-5. [DOI] [PubMed] [Google Scholar]
- 81.Mahida YR. The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflamm. Bowel Dis. 2000;6:21–33. doi: 10.1097/00054725-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 82.Sheibanie AF, et al. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23-IL-17 axis. J. Immunol. 2007;178:8138–8147. doi: 10.4049/jimmunol.178.12.8138. This was the first study that offered direct evidence that PGE2 treatment exacerbates inflammation and disease severity in a mouse model of IBD.
- 83.Chizzolini C, et al. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 2008;112:3696–3703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boniface K, et al. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J. Exp. Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yao C, et al. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nature Med. 2009;15:633–640. doi: 10.1038/nm.1968. This paper showed the first in vivo evidence that EP4 mediates the effects of PGE2 on promoting chronic inflammation.
- 86.Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–1361. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 87.Angeli V, et al. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J. Exp. Med. 2001;193:1135–1147. doi: 10.1084/jem.193.10.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Henderson WR., Jr. The role of leukotrienes in inflammation. Ann. Intern. Med. 1994;121:684–697. doi: 10.7326/0003-4819-121-9-199411010-00010. [DOI] [PubMed] [Google Scholar]
- 89.Stanke-Labesque F, Pofelski J, Moreau-Gaudry A, Bessard G, Bonaz B. Urinary leukotriene E4 excretion: a biomarker of inflammatory bowel disease activity. Inflamm Bowel Dis. 2008;14:769–774. doi: 10.1002/ibd.20403. [DOI] [PubMed] [Google Scholar]
- 90.Bomalaski JS, Dundee D, Brophy L, Clark MA. Leukotriene B4 modulates phospholipid methylation and chemotaxis in human polymorphonuclear leukocytes. J. Leukoc. Biol. 1990;47:1–12. doi: 10.1002/jlb.47.1.1. [DOI] [PubMed] [Google Scholar]
- 91.Islam SA, et al. The leukotriene B4 lipid chemoattractant receptor BLT1 defines antigen-primed T cells in humans. Blood. 2006;107:444–453. doi: 10.1182/blood-2005-06-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shin EH, Lee HY, Bae YS. Leukotriene B4 stimulates human monocyte-derived dendritic cell chemotaxis. Biochem. Biophys. Res. Commun. 2006;348:606–611. doi: 10.1016/j.bbrc.2006.07.084. [DOI] [PubMed] [Google Scholar]
- 93.Haribabu B, et al. Targeted disruption of the leukotriene B4 receptor in mice reveals its role in inflammation and platelet-activating factor-induced anaphylaxis. J. Exp. Med. 2000;192:433–438. doi: 10.1084/jem.192.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woo CH, et al. Leukotriene B4 stimulates Rac-ERK cascade to generate reactive oxygen species that mediates chemotaxis. J. Biol. Chem. 2002;277:8572–8578. doi: 10.1074/jbc.M104766200. [DOI] [PubMed] [Google Scholar]
- 95.Del Prete A, et al. Regulation of dendritic cell migration and adaptive immune response by leukotriene B4 receptors: a role for LTB4 in up-regulation of CCR7 expression and function. Blood. 2007;109:626–631. doi: 10.1182/blood-2006-02-003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Snijdewint FG, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J. Immunol. 1993;150:5321–5329. [PubMed] [Google Scholar]
- 97.Huang M, et al. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 1998;58:1208–1216. [PubMed] [Google Scholar]
- 98.Stolina M, et al. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J. Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 99.Zeddou M, et al. Prostaglandin E2 induces the expression of functional inhibitory CD94/NKG2A receptors in human CD8+ T lymphocytes by a cAMP-dependent protein kinase A type I pathway. Biochem. Pharmacol. 2005;70:714–724. doi: 10.1016/j.bcp.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 100.Baratelli F, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J. Immunol. 2005;175:1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 101.Ahmadi M, Emery DC, Morgan DJ. Prevention of both direct and cross-priming of antitumor CD8+ T-cell responses following overproduction of prostaglandin E2 by tumor cells in vivo. Cancer Res. 2008;68:7520–7529. doi: 10.1158/0008-5472.CAN-08-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.von Bergwelt-Baildon MS, et al. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood. 2006;108:228–237. doi: 10.1182/blood-2005-08-3507. [DOI] [PubMed] [Google Scholar]
- 103.Yang L, et al. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J. Clin. Invest. 2003;111:727–735. doi: 10.1172/JCI16492. This study reported the first in vivo evidence that PGE2 is a mediator of cancer-associated immunodeficiency.
- 104.Goodwin JS, Ceuppens J. Regulation of the immune response by prostaglandins. J. Clin. Immunol. 1983;3:295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- 105.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 106.Wang D, et al. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J. Exp. Med. 2006;203:941–951. doi: 10.1084/jem.20052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Battersby S, et al. Seminal plasma and prostaglandin E2 up-regulate fibroblast growth factor 2 expression in endometrial adenocarcinoma cells via E-series prostanoid-2 receptor-mediated transactivation of the epidermal growth factor receptor and extracellular signal-regulated kinase pathway. Hum. Reprod. 2007;22:36–44. doi: 10.1093/humrep/del328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chang SH, et al. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc. Natl Acad. Sci. USA. 2004;101:591–596. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Spinella F, Rosano L, Di Castro V, Natali PG, Bagnato A. Endothelin-1-induced prostaglandin E2-EP2, EP4 signaling regulates vascular endothelial growth factor production and ovarian carcinoma cell invasion. J. Biol. Chem. 2004;279:46700–46705. doi: 10.1074/jbc.M408584200. [DOI] [PubMed] [Google Scholar]
- 110.Jain S, Chakraborty G, Raja R, Kale S, Kundu GC. Prostaglandin E2 regulates tumor angiogenesis in prostate cancer. Cancer Res. 2008;68:7750–7759. doi: 10.1158/0008-5472.CAN-07-6689. [DOI] [PubMed] [Google Scholar]
- 111.Ding YB, et al. PGE2 up-regulates vascular endothelial growth factor expression in MKN28 gastric cancer cells via epidermal growth factor receptor signaling system. Exp. Oncol. 2005;27:108–113. [PubMed] [Google Scholar]
- 112.Sales KJ, et al. A novel angiogenic role for prostaglandin F2α-FP receptor interaction in human endometrial adenocarcinomas. Cancer Res. 2005;65:7707–7716. doi: 10.1158/0008-5472.CAN-05-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wallace AE, et al. Prostaglandin F2α-F-prostanoid receptor signaling promotes neutrophil chemotaxis via chemokine (C-X-C motif) ligand 1 in endometrial adenocarcinoma. Cancer Res. 2009;69:5726–5733. doi: 10.1158/0008-5472.CAN-09-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc. Natl Acad. Sci. USA. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pradono P, et al. Gene transfer of thromboxane A2 synthase and prostaglandin I2 synthase antithetically altered tumor angiogenesis and tumor growth. Cancer Res. 2002;62:63–66. [PubMed] [Google Scholar]
- 116.Kamiyama M, et al. EP2, a receptor for PGE2, regulates tumor angiogenesis through direct effects on endothelial cell motility and survival. Oncogene. 2006;25:7019–7028. doi: 10.1038/sj.onc.1209694. [DOI] [PubMed] [Google Scholar]
- 117.Amano H, et al. Host prostaglandin E2-EP3 signaling regulates tumor-associated angiogenesis and tumor growth. J. Exp. Med. 2003;197:221–232. doi: 10.1084/jem.20021408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pai R, et al. PGE2 stimulates VEGF expression in endothelial cells via ERK2/JNK1 signaling pathways. Biochem. Biophys. Res. Commun. 2001;286:923–928. doi: 10.1006/bbrc.2001.5494. [DOI] [PubMed] [Google Scholar]
- 119.Dormond O, Bezzi M, Mariotti A, Ruegg C. Prostaglandin E2 promotes integrin alpha Vbeta 3-dependent endothelial cell adhesion, rac-activation, and spreading through cAMP/PKA-dependent signaling. J. Biol. Chem. 2002;277:45838–45846. doi: 10.1074/jbc.M209213200. [DOI] [PubMed] [Google Scholar]
- 120.Salcedo R, et al. Angiogenic effects of prostaglandin E2 are mediated by up-regulation of CXCR4 on human microvascular endothelial cells. Blood. 2003;102:1966–1977. doi: 10.1182/blood-2002-11-3400. [DOI] [PubMed] [Google Scholar]
- 121.Daniel TO, Liu H, Morrow JD, Crews BC, Marnett LJ. Thromboxane A2 is a mediator of cyclooxygenase-2-dependent endothelial migration and angiogenesis. Cancer Res. 1999;59:4574–4577. [PubMed] [Google Scholar]
- 122.Murata T, et al. Role of prostaglandin D2 receptor DP as a suppressor of tumor hyperpermeability and angiogenesis in vivo. Proc. Natl Acad. Sci. USA. 2008;105:20009–20014. doi: 10.1073/pnas.0805171105. This was the first report to show that PGD2–DP signalling inhibits tumour-associated angiogenesis and tumour growth in vivo.
- 123.Kim GY, Lee JW, Cho SH, Seo JM, Kim JH. Role of the low-affinity leukotriene B4 receptor BLT2 in VEGF-induced angiogenesis. Arterioscler Thromb. Vasc. Biol. 2009;29:915–920. doi: 10.1161/ATVBAHA.109.185793. [DOI] [PubMed] [Google Scholar]
- 124.Modat G, Muller A, Mary A, Gregoire C, Bonne C. Differential effects of leukotrienes B4 and C4 on bovine aortic endothelial cell proliferation in vitro. Prostaglandins. 1987;33:531–538. doi: 10.1016/0090-6980(87)90276-0. [DOI] [PubMed] [Google Scholar]
- 125.Tsopanoglou NE, Pipili-Synetos E, Maragoudakis ME. Leukotrienes C4 and D4 promote angiogenesis via a receptor-mediated interaction. Eur. J. Pharmacol. 1994;258:151–154. doi: 10.1016/0014-2999(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 126.Steiner DR, Gonzalez NC, Wood JG. Leukotriene B4 promotes reactive oxidant generation and leukocyte adherence during acute hypoxia. J. Appl. Physiol. 2001;91:1160–1167. doi: 10.1152/jappl.2001.91.3.1160. [DOI] [PubMed] [Google Scholar]
- 127.Abdel-Majid RM, Marshall JS. Prostaglandin E2 induces degranulation-independent production of vascular endothelial growth factor by human mast cells. J. Immunol. 2004;172:1227–1236. doi: 10.4049/jimmunol.172.2.1227. [DOI] [PubMed] [Google Scholar]
- 128.Nakayama T, Mutsuga N, Yao L, Tosato G. Prostaglandin E2 promotes degranulation-independent release of MCP-1 from mast cells. J. Leukoc. Biol. 2006;79:95–104. doi: 10.1189/jlb.0405226. [DOI] [PubMed] [Google Scholar]
- 129.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 130.Salcedo R, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- 131.Tanaka S, et al. Monocyte chemoattractant protein 1 and macrophage cyclooxygenase 2 expression in colonic adenoma. Gut. 2006;55:54–61. doi: 10.1136/gut.2004.059824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–5066. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- 133.Rosengren S, Olofsson AM, von Andrian UH, Lundgren-Akerlund E, Arfors KE. Leukotriene B4-induced neutrophil-mediated endothelial leakage in vitro and in vivo. J. Appl. Physiol. 1991;71:1322–1330. doi: 10.1152/jappl.1991.71.4.1322. [DOI] [PubMed] [Google Scholar]
- 134.Kanaoka Y, Maekawa A, Penrose JF, Austen KF, Lam BK. Attenuated zymosan-induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J. Biol. Chem. 2001;276:22608–22613. doi: 10.1074/jbc.M103562200. [DOI] [PubMed] [Google Scholar]
- 135.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N. Engl. J. Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. This paper reported the first clinical evidence that aspirin seems to reduce the risk of CRCs that overexpress COX2 but not the risk of CRCs with weak or absent expression of COX2.
- 136.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–658. doi: 10.1001/jama.2009.1112. This paper was the first to provide clinical evidence that aspirin can be used to treat advanced CRC.
- 137.Cuzick J, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 138.Fitzgerald GA. Coxibs and cardiovascular disease. N. Engl. J. Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 139.Narasimha A, et al. A novel anti-atherogenic role for COX-2 — potential mechanism for the cardiovascular side effects of COX-2 inhibitors. Prostaglandins Other Lipid Mediat. 2007;84:24–33. doi: 10.1016/j.prostaglandins.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Piazuelo E, et al. Effects of selective PGE2 receptor antagonists in esophageal adenocarcinoma cells derived from Barrett’s esophagus. Prostaglandins Other Lipid Mediat. 2006;81:150–161. doi: 10.1016/j.prostaglandins.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 141.Rioux N, Castonguay A. Inhibitors of lipoxygenase: a new class of cancer chemopreventive agents. Carcinogenesis. 1998;19:1393–1400. doi: 10.1093/carcin/19.8.1393. [DOI] [PubMed] [Google Scholar]
- 142.Barry M, et al. Neoplasms escape selective COX-2 inhibition in an animal model of breast cancer. Ir. J. Med. Sci. 2009;178:201–208. doi: 10.1007/s11845-009-0335-3. [DOI] [PubMed] [Google Scholar]
- 143.Fegn L, Wang Z. Topical chemoprevention of skin cancer in mice, using combined inhibitors of 5-lipoxygenase and cyclo-oxygenase-2. J. Laryngol. Otol. 2009;123:880–884. doi: 10.1017/S0022215109004617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ye YN, et al. Dual inhibition of 5-LOX and COX-2 suppresses colon cancer formation promoted by cigarette smoke. Carcinogenesis. 2005;26:827–834. doi: 10.1093/carcin/bgi012. [DOI] [PubMed] [Google Scholar]
- 145.Chen X, et al. Overexpression of 5-lipoxygenase in rat and human esophageal adenocarcinoma and inhibitory effects of zileuton and celecoxib on carcinogenesis. Clin. Cancer Res. 2004;10:6703–6709. doi: 10.1158/1078-0432.CCR-04-0838. [DOI] [PubMed] [Google Scholar]
- 146.Wenger FA, et al. Effects of Celebrex and Zyflo on liver metastasis and lipidperoxidation in pancreatic cancer in Syrian hamsters. Clin. Exp. Metastasis. 2002;19:681–687. doi: 10.1023/a:1021387826867. [DOI] [PubMed] [Google Scholar]
- 147.Hennig R, et al. Effect of LY293111 in combination with gemcitabine in colonic cancer. Cancer Lett. 2004;210:41–46. doi: 10.1016/j.canlet.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 148.Adrian TE, Hennig R, Friess H, Ding X. The role of PPARγ receptors and leukotriene B4 receptors in mediating the effects of LY293111 in pancreatic cancer. PPAR Res. 2009 Jan 27; doi: 10.1155/2008/827096. doi:10.1155/2008/827096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gunning WT, Kramer PM, Steele VE, Pereira MA. Chemoprevention by lipoxygenase and leukotriene pathway inhibitors of vinyl carbamate-induced lung tumors in mice. Cancer Res. 2002;62:4199–4201. [PubMed] [Google Scholar]
- 150.Monzon FA, et al. The role of KRAS mutation testing in the management of patients with metastatic colorectal cancer. Arch. Pathol. Lab. Med. 2009;133:1600–1606. doi: 10.5858/133.10.1600. [DOI] [PubMed] [Google Scholar]