Summary
Hormones are trophic factors that integrate central and peripheral nervous system functions, and can influence social, cognitive, emotional and physical (SCEP) processes. Greater understanding of behavioral and neurobiological underpinnings of mental, cognitive, and/or physical changes with maturation is becoming increasingly important as the world’s population ages. There are individual differences in how people age, but the factors that influence these differences are not well understood. Social supports are one factor that may influence the trajectory of age-related processes. The loss of close relationships, especially among older persons, is one of the greatest risk factors for mental and physical decline. Progesterone, secreted by the ovaries, or produced de novo in the brain, is readily converted centrally to 5α-pregnan-3α-ol-20-one (3α,5α-THP), and can influence SCEP, through rapid, non-classical steroid-mediated actions. Our hypothesis is that 3α,5α-THP is a key trophic factor in SCEP and development. Our research has demonstrated that 3α,5α-THP facilitates social and sexual behavior of rodents, which evokes further increases in 3α,5α-THP in midbrain and hippocampus, brain areas involved in SCEP. The role of 3α,5α-THP to influence social and/or sexual experience, and thereby SCEP, is discussed in this review. Further understanding of these neurobiological and/or behavioral factors may lead to findings that ultimately can promote health and prevent disease.
Keywords: Neurosteroids, non-genomic, allopregnanolone, lordosis, THP
1. Introduction
Steroid hormones, such as progesterone (P), play a fundamental role in trophic actions that influence the development and function of the central nervous system (CNS) throughout the lifespan. In adult rodents, P facilitates social and sexual interactions. Progesterone influences the onset and duration of sexual behavior of rodents in part through its actions in the midbrain Ventral Tegmental Area (VTA). To elucidate functionally-relevant mechanisms of P, we have manipulated P’s actions in the VTA of naturally, sexually-receptive or ovariectomized (OVX), estrogen (E)-primed rodents, and examined changes in social and sexual (socio-sexual) behavior. Progesterone’s actions in the VTA occur independent of traditional actions at cognate intracellular progestin receptors (PRs). Rather, P’s actions in the VTA involve its conversion to 3α-hydroxy-5α-pregnan-20-one (3α,5α-THP), which is devoid of affinity for intracellular PRs and has actions via neurotransmitter substrates (Frye 2001a,b, 2007; Frye et al., 2006; Frye & Vongher, 1999). In addition to P and 3α,5α-THP facilitating mating, sex further increases levels of neurosteroids.
Mating has dynamic effects upon levels of 3α,5α-THP in the midbrain VTA. 3α,5α-THP is readily formed in the midbrain from ovarian P, and is also a neurosteroid that is rapidly produced de novo in the midbrain following mating (Frye, 2007). Mating induces neurosteroidogenesis of 3α,5α-THP in the midbrain, as well as the hippocampus, an important area of the brain for cognitive and emotional processes (Frye & Rhodes, 2006; Frye et al., 2006, 2007). We, and others, have demonstrated in separate lines of research that P and/or 3α,5α-THP can influence social, cognitive, emotional and physical (SCEP) performance (Engel & Grant, 2001; Frye, 2007, 2008; Herbison, 2001; Miczek et al., 2002; Pinna et al., 2008; Sherwin, 1999).
In young adulthood and/or middle-age, alterations in 3α,5α-THP may play a fundamental role to enhance reproduction and social bonds, thereby influencing SCEP. Social relationships have a substantial effect on health. Grief and bereavement increases morbidity and mortality. The loss of close relationships, especially among older persons, is one of the greatest risk factors for mental and physical decline. However, the neurobiological and behavioral mechanisms underlying these effects are not well understood. A greater understanding of the neurobiological and behavioral factors associated with social responding is essential in order to elucidate interventions that will promote health and/or prevent disease among individuals. The neurobiological and behavioral mechanisms that underlie mating-induced neurosteroidogenesis, and the subsequent impact on social, cognitive, emotional and physical (SCEP) function among young and/or mid-aged rats is the subject of this review.
2. Significance
The understanding of mental, cognitive, and/or physical decline with aging is becoming increasingly important as the world’s population ages. There are individual differences in how people age. For example, about 60% of aging individuals will experience some cognitive decline. Among these, about half will experience benign senescent forgetfulness, or mild cognitive impairment (MCI) associated with cell loss in the subiculum region of the hippocampus. Others will experience Alzheimer’s Disease (AD). In the early stages of AD, behavior impairments are virtually indistinguishable from those with MCI. However, the neural deficits associated with early AD are in the entorhinal cortex. With time, those with AD will experience profound cognitive and emotional impairment and neural deficits in the subiculum and entorhinal cortex. Indeed, some individuals will age well (“super” aging), others will experience normal aging, and many will experience cognitive decline. The neurobiological and/or behavioral underpinnings associated with these types of differences in aging between individuals are not well understood. It is likely that life experience factors, such as those related to social factors, may have an effect, but this needs to be investigated.
One factor that may have a profound influence on the trajectory of age-related processes is social supports. Loss of a social relationship can contribute to decline in mental, cognitive, and/or physical functioning. The risk of bereavement (loss of a close personal relationship) increases from ~4% before adulthood (children loosing parents) to ~15% and 45% among men and women, respectively, over 65 who are widowed (Stroebe et al., 2001, 2007). Those who are bereaved have an increased risk of mortality due to suicide and natural causes, independent of baseline health status, and other major risk factors (i.e. body-mass index, cardiovascular health, tobacco and/or alcohol use; Berkman, 2005; Murberg & Bru, 2001; Seeman et al., 1987). Bereaved persons have greater morbidity, as demonstrated by increases in new illnesses, exacerbation of existing conditions, greater use of medications, and poorer self-ratings of wellness (Thompson et al., 1984). Severe grief early in bereavement increases risk of heart attack and cancer (Chen et al., 1999). Dysregulation of autonomic, endocrine, and immune systems occur following loss of a loved one, or other forms of social isolation, which may contribute to heart disease and/or cancer (Berkman, 2000; Berkman et al., 2004; Cacioppo & Hawkley, 2003). Given that hormones are mediating factors between the central and peripheral nervous system, and can influence social, affective, and cognitive processes, their role underlying neurobiological and/or behavioral mechanisms that influence aging, are of interest.
It may be that quality social relationships improve health and/or well-being, but these effects can often be confounded with trophic actions of steroids. An example of this is parity, in which women experience robust fluctuations in hormones (progestins, oxytocin, etc) and their trophic actions during pregnancy and post-parturition, and then intense social bonding with their offspring. Parity is typically associated with a reduction in breast cancer risk. In this example, and others, the extent to which these differences are due to hormonal experience or social factors is not clear. Oxytocin has clear effects to enhance affiliative behaviors, but its role for affective and cognitive responses is less clear than that observed with ovarian steroids and neurosteroids (Carter, 2007; Frye, 2008). A question is whether social and/or sexual experiences may have effects throughout the lifespan on social, affective, cognitive, and/or physical function. To address this, we have developed an animal model in which the neurosteroid, 3α,5α-THP’s, trophic effects have been characterized. Background about this model follows.
3. Background
Hormones are pleiotropic and may have diverse actions that underlie their organizational and/or activational effects to influence normal cellular functions and/or behavioral processes. Emerging findings regarding the sources, mechanisms, and effects of steroids have challenged traditional dogma and revealed non-traditional actions of hormones to influence these processes. First, hormones are typically thought to be secreted from peripheral endocrine glands and get into circulation whereupon they can exert effects at distant target sites. The brain, like the gonads, adrenals, and placenta, is also an endocrine organ that requires coordinated actions of steroidogenic enzymes in neurons and glia in different parts of the CNS to metabolize peripheral steroids to neuroactive products (neuroactive steroids) or to produce steroids de novo independent of peripheral gland secretion (neurosteroids; Table 1). As such, hormones exert intracrine effects to mediate intracellular events, and paracrine, or neurotransmitter-like, effects to induce biological response in adjacent cells. Second, the classic mechanisms of steroids are considered to involve their binding to cognate, intracellular steroid receptors, which are present throughout the brain (Osterlund et al., 2000; Shughrue et al., 1997), and modulate transcription and translation (Pfaff et al., 1976). This process may take days, hours, or at least 10 minutes. Steroid hormones can also act in the CNS via membrane receptors or rapid-signaling actions, which occur within seconds to minutes. Neuro(active)steroids produce rapid effects on neuronal excitability and synaptic function that involve direct or indirect modulation of neurotransmitter-gated ion channels, or other neurotransmitter receptors and transporters, rather than classic, nuclear hormone receptors (Baulieu, 1980; Gee et al., 1995; Herd et al., 2007; Rupprecht & Holsboer, 1999; Schlichter et al., 2006). Third, some of the most salient effects of hormones are to change the threshold for a biological or behavioral response to appropriate stimuli. We now know that hormone levels can change in response to extreme environmental and/or behavioral situations and, thereby, influence the likelihood of subsequent hormone-mediated processes (Barbaccia et al., 1996; Drugan et al., 1995; Patchev et al., 1994, 1996). There is now a greater understanding of more subtle and dynamic changes in hormones to mediate homeostatic processes, such as HPA function, which may exert proximate and discrete effects on physiological and/or behavioral processes. Details of the non-traditional sources, mechanisms, and effects of steroids relevant for SCEP are discussed below.
Table 1.
Provides a break down of the timing, ligands, sources, and targets of steroid actions
| Parameter | Classic Steroid Action | Neurosteroid Action |
|---|---|---|
| Timing | Minutes, days, weeks | Seconds to minutes |
| Ligands | Progesterone (P), DHP | 3α,5α-THP |
| Sources | Ovaries, Adrenals | Brain |
| Targets | Intracellular PRs | NT receptors, HPA |
The brain as a source of 3α,5α-THP
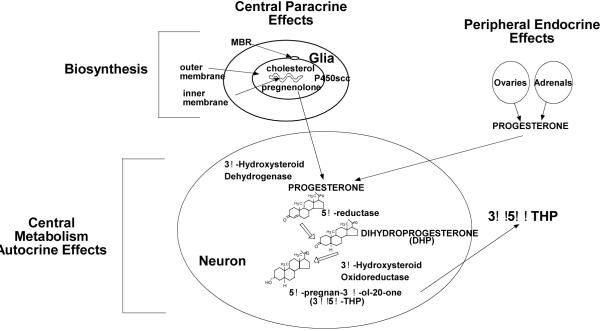
It is now generally understood that the brain, like peripheral glands (gonads, adrenals, placenta), is an endocrine organ (Diagram 1). The brain requires coordinated actions of steroidogenic enzymes in neurons and glia in different parts of the CNS to metabolize peripheral steroids to neuroactive products (neuroactive steroids) or to produce steroids de novo in the brain independent of peripheral gland secretion (neurosteroids). Baulieu and colleagues, discovered in the early eighties, that steroids could be synthesized within the brain and peripheral nerves and they termed these brain-derived steroids, neurosteroids (Baulieu, 1980, 1991). The initial discovery that steroids were synthesized in the brain came from observations that levels of steroids, such as pregnenolone, dehydroepiandrosterone, and their sulfate and lipoidal esters, were greater in the CNS and PNS, than in circulation. The same enzymes involved in steroidogenesis in peripheral glands were identified in the nervous system and found to be responsible for biosynthesis; albeit, they are typically expressed in brain at levels 2 to 5 orders of magnitude lower than in adrenal or gonadal tissues (Compagnone & Mellon, 2000; Furukawa et al., 1998). Central steroid concentrations also can be much greater than in the circulation and these levels can persist after extirpation of peripheral glands (gonadectomy-GDX, adrenalectomy-ADX). Together, these findings demonstrate that steroids are synthesized in the nervous system and their localization in the CNS/PNS was neither due to peripheral synthesis, nor sequestration and/or accumulation in neural tissues (Baulieu, 1980, 1991; Majewska, 1992; Mellon, 1994; Paul & Purdy, 1992). Summarized below is some of the key information regarding the production of neurosteroids.
Diagram 1.
Sources of 3α,5α-THP in the CNS include de novo biosynthesis in glial cells and metabolism of peripheral or central P.
Metabolic Pathways for Neurosteroidogenesis
The nervous system expresses all of the enzymes required for steroid biosynthesis to produce a variety of neurosteroids. However, it is beyond the scope of this review to address effects and mechanisms of all neurosteroids. Our focus is on 3α,5α-THP.
The peripheral-type benzodiazepine receptor (PBR), which is essential for neurosteroidogenesis, binds cholesterol in nanomolar affinities (Diagram 2). The PBR helps import cholesterol into the mitochrondria, where it can be oxidized to pregnenolone by two proteins that initiate steroidogenesis - the steroidogenic acute regulatory (StAR) protein and cytochrome P450-dependent C27 side chain cleavage enzymes (P450scc), rate-limiting steps in steroid biosynthesis (King et al., 2004; Mellon & Deschepper, 1993; Papadopoulos et al., 2006). Once pregnenolone is synthesized from cholesterol by StAR and P450scc, several 3α-hydroxysteroid dehydrogenase (3α-HSD) enzymes can convert the prohormones to neuroactive steroids. Key examples of neurosteroids/neuroactive steroids, which are derived from cholesterol and/or blood-borne precursors, respectively, are pregnenolone, which is converted to P, and its product, 5α-pregnane-3,20-dione-dihydroprogesterone, DHP), which can be converted to 3α,5α-THP and its 5β-stereoisomer (3α,5β-THP).
Diagram 2.
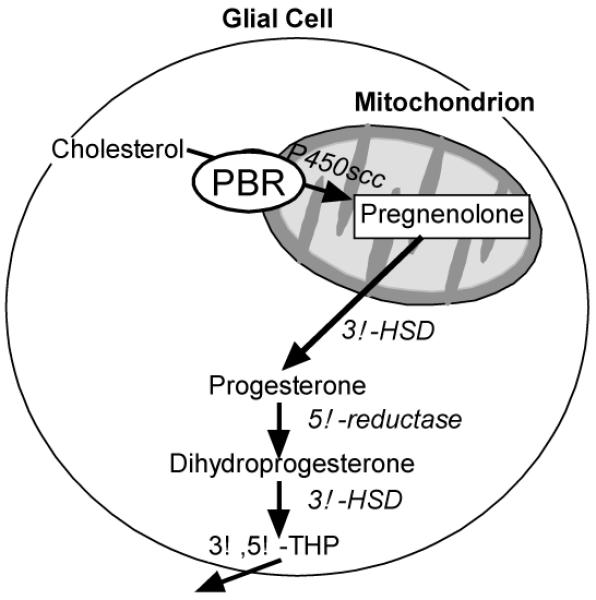
Depicts cholesterol transport into mitochondrion via PBR and enzymatic processes involved in neurosteroidogenesis.
Whether estrogens, androgens, and/or progesterone and its metabolites are synthesized in the CNS/PNS depends upon many factors including timing during development, expression of metabolism enzymes, and their substrates in particular tissues (brain regions), cell types (i.e. neurons vs. glia), and/or cellular regions (cell bodies, fibers etc). Neurosteroids are formed from biosynthesis of cholesterol by P450 and non-P450 enzymes (17β-HSD, aromatase, 3α-HSD, 5α-reductase). Gene expression of P450c17, is developmentally regulated with early expression being different from that observed in the mature brain (Mellon, 2007). This implies that certain metabolites may play key roles that are relegated throughout development without continuous expression in adulthood. Further, there are developmental differences in the substrates that two 3α-HSDs in human fetal brain utilize, with one using DHP and another using DHT (Reviewed in Frye 2008). Conservation in the localization of steroidogenic enzymes, which are involved in synthesizing neurosteroids, to specific brain regions within a variety of species, indicates that their function may be similar throughout evolution. Some investigations have attempted to colocalize steroidogenic enzymes in whole brain, neuronal, and/or glial cell cultures. Indeed, some steroidogenic enzymes are expressed only in neurons, or only in glia (type 1 astrocytes, Schwann cells), while other enzymes are expressed in both cell types (Martini et al., 2003; Mellon and Griffin, 2002). Investigations are still needed to elucidate how specific brain regions synthesize or metabolize steroids, which are essential to reveal the function of neurosteroids. Ascertaining the relative concentrations of peripheral versus central levels of 3α,5α-THP is of great interest to understanding the functional significance of neurosteroids.
Non-classical actions of 3α,5α-THP
Neurosteroids can have more immediate, rapid-signaling effects than steroids secreted by peripheral glands that act through classic nuclear steroid receptors. Neurosteroids can act through ion channel-associated membrane receptors to elicit rapid changes in signaling that can occur within milliseconds to seconds. The most extensively investigated actions of neurosteroids are those at synaptic and extrasynaptic γ-aminobutyric acid type A (GABAA) receptors. In the presence of GABA, 3α,5α-THP directly potentiates GABAA receptors in nanomolar concentrations (Concas et al., 1998; Fodor et al., 2005; Puia et al., 1990; Schmid et al., 1998). 3α,5α-THP increases chloride channel currents and lowers neuronal excitability with 20 and 200-fold higher efficacy than benzodiazepines or barbiturates, respectively (Belelli & Lambert, 2005; Brot et al., 1997; Gee et al., 1995; Lambert et al., 2003; Morrow et al., 1987; Reddy, 2004; Weir et al., 2004). Like other neurosteroids, 3α,5α-THP can also have actions through other non-steroidal, ligand-gated, ion channel and/or G protein-coupled receptors and glutamate (among others; Rupprecht & Holsboer, 1999). P and/or 3α,5α-THP have negative modulatory actions via norepinephrine, dopamine, serotonin, acetylcholine, oxytocin, and nicotinic/muscarinic receptors (Table 2) and may alter neuronal function through membrane E receptors (Chaban et al., 2004) and G protein-coupled membrane PRs (Zhu et al., 2003). Thus, 3α,5α-THP has actions through non-traditional steroid targets that may play a role in SCEP.
Table 2.
Provides the steroid sources and receptor targets of non-classical actions of neurosteroids
| Receptor | Neurosteroid (Modulatory effect: + positive, - negative) |
|---|---|
| GABAA | 3α,5α-THP (+), THDOC (+), 3α-diol (+), pregnenolone sulfate (-), DHEA-S (-) |
| Glycine | P (-), 3α,5α-THP (-), pregnanolone (-), Pregnenolone sulfate (-) DHEA-S (-) |
| Sigma type 1 | DHEA (+), DHEA-S (+), pregnenolone sulfate (+), P (-) |
| NMDA | DHEA (+), pregnenolone sulfate (+), E2 (-) |
| AMPA | Pregnenolone sulfate (-) |
| Kainate | P (+), Pregnenolone sulfate (-) |
| Serotonin type 3 | 3α,5α-THP (-), P (-), E2 (-), testosterone (-) |
| Neuronal nicotinic acetylcholine | 3α,5α -THP (-), P (-), pregnenolone sulfate (-) |
Activational effects of progesterone and its metabolites
Activational effects are typically considered temporary changes in brain function and/or behavior that occur with the presence of hormones in the already developed CNS. In people, variations in P and 3α,5α-THP levels have been examined. During the menstrual cycle, patterns in plasma levels of pregnenolone and 3α,5α-THP are similar to that of P. During the luteal phase, circulating concentrations of pregnenolone and 3α,5α-THP are 2 to 4 fold higher (2 - 4 nmol/L) than they are during the follicular phase (~1 nmol/L; Genazzani et al., 1998; Purdy et al., 1990; Sundström and Bäckström, 1998a,b; Wang et al., 1996; Table 3). Throughout pregnancy, serum levels of pregnenolone, P and 3α,5α-THP increase with gestation (Luisi et al., 2000) and peak late in the 3rd trimester (50-100nmol/L; Herbison, 2001; Luisi et al., 2000). The levels achieved during late pregnancy are within the range that can produce sedation (80-160 nmol/L; Sundström et al., 1999). Notably, within 1 hour after delivery, maternal serum 3α,5α-THP levels are decreased significantly, albeit pregnenolone levels in serum do not decline as substantially until 1 day later. There is evidence that P and 3α,5α-THP bioaccumulate in brain. Investigation of levels of P and 3α,5α-THP in the post-mortem brain of pre- and post-menopausal women show the expected patterns of menstrual variations and lower levels post-climacteric (Bixo et al., 1997; Purdy et al., 1991). Notably, 3α,5α-THP levels varied by region with the highest concentrations being seen in the midbrain and hypothalamus (14-21ng/g; Bixo et al., 1997). These variations with reproductive experience imply that central neurosteroidogenesis may influence, or be influenced by, circulating steroid concentrations. Indeed, one of the rate-limiting factors in understanding more about the functional significance of neurosteroids lies is the challenge of being able to parse out the relative contributions of central versus peripheral endocrine glands. There is evidence that local biosynthesis and paracrine/autocrine effects of neurosteroids can precisely and rapidly alter neuronal function in a manner not achievable by neuroactive steroids derived from circulation. Some of these diverse activational effects are discussed below.
Table 3.
Plasma progestin measures in men and women in follicular or luteal phases of the menstrual cycle, or who are pregnant
| Women | ||||
|---|---|---|---|---|
| Progestins (nmol/l) | Men | Folicular | Luteal | Pregnant |
| Pregnenolone | 2 | 2 | 4 | 60 |
| Progesterone | 1-2 | 1-2 | 25 | 650 |
| 3α,5α-THP | 0.3 | 0.3 | 2 | 14 |
3α,5α-THP & Sexual Behavior of Female Rodents
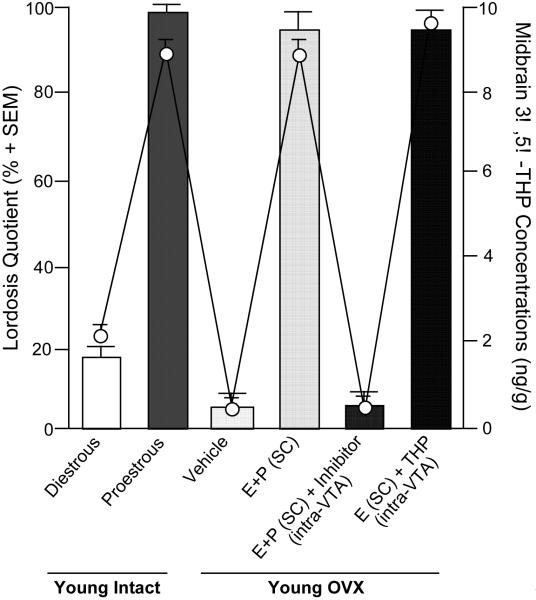
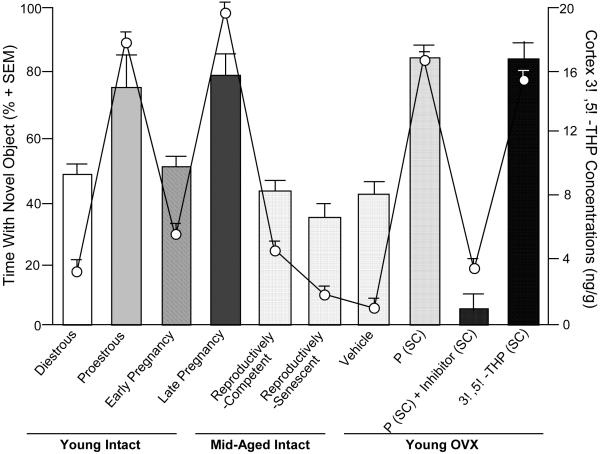
Given the potential involvement of neurosteroids in the etiology and/or therapeutic treatment of anxiety, stress and/or mood dysregulation, how neurosteroids influence sexual behavior, another robust sexually-dimorphic and hormonally-mediated function, has been investigated as a means to elucidate their physiological and ethological role. 3α,5α-THP may be an important neuroendocrine factor that helps an organism respond to various environmental challenges, such as can occur with mating. In order for female rodents to be sexually receptive, actions of estradiol (E2) and P are required. Notably, the effects and mechanisms of these steroids on sexual receptivity are diverse and site-specific. For example, in rodents, E and P initiate the facilitation of lordosis in part through actions to increase PR expression in the ventral portion of the ventromedial nucleus (VMN) (Schwartz-Giblin & Pfaff, 1986); however, in the midbrain VTA, which is devoid of PRs in adulthood (Blaustein, 2003; Frye & Vongher, 1999), 3α,5α-THP mediates the duration and intensity of P-facilitated lordosis. In support, enhancing 3α,5α-THP levels in the midbrain VTA facilitates sexual receptivity of rodents. 3α,5α-THP levels in the midbrain are particularly dynamic and change in response to ovarian secretion of E2 and P (and with mating, discussed below). In order for rodents to be sexually receptive, ovarian secretion or exogenous administration of E2 is necessary. E2 increases the formation of central 3α,5α-THP, the activity of the 5α-reductase enzyme (Cheng & Karavolas, 1973; Sinchak et al., 2003). Further increases in receptive behaviors are produced by ovarian secretion of, or exogenous administration of, progesterone and its metabolites, which increases midbrain levels of 3α,5α-THP over that of diestrous, OVX, or OVX, E2-primed rodents (reviewed in Frye, 2001a,b). The frequency of lordosis displayed by proestrous rats, which have high endogenous 3α,5α-THP in the midbrain VTA, are increased, compared to that of diestrous rats (Frye, 2001a,b; Figure 1). These effects are reversed with OVX. Administration of E2 and P or 3α,5α-THP to the midbrain, but not co-administration of P and an inhibitor of 3α,5α-THP formation, increases lordosis (Frye et al., 2006). These effects of 3α,5α-THP in the midbrain VTA to enhance lordosis occur through 3α,5α-THP’s actions at GABAA, D1 receptors, and their downstream signal transduction targets, as blocking 3α,5α-THP’s actions at these substrates attenuate P- or 3α,5α-THP-facilitated sexual receptivity (Frye & Walf, 2008b).
Figure 1.
Sexual behavior correlates with 3α,5α-THP levels in the midbrain of young (diestrous or proestrous) or ovx (estradiol (E) and progesterone (P) with or without inhibition of 3α,5α-THP formation, or 3α,5α-THP) rats. Bars represent lordosis quotients (mean±SEM) and dots represent 3α,5α-THP levels (ng/g±SEM) in midbrain.
It is important to understand the role of 3α,5α-THP because of its potential effects to mediate sexual and other, related behavioral processes and also because of its responsiveness to social factors. For example, following mating, midbrain 3α,5α-THP levels are increased over those of non-mated naturally-receptive or hormone-primed rodents (Frye, 2001a,b). The rapidity of the mating-induced increase in midbrain 3α,5α-THP, and independence of secretion from the ovaries and/or adrenals, suggest that biosynthesis and subsequent metabolism of central, rather than peripheral, progestogens underlie mating-induced increases in midbrain 3α,5α-THP that have been observed for rats, mice, and hamsters (reviewed in Frye, 2001a,b; Frye & Rhodes, 2008a). The successive increases in midbrain 3α,5α-THP produced by E2 and P-priming, and mating have led us to consider whether 3α,5α-THP in varying concentrations, or when derived from peripheral versus central precursors, may influence sexual behavior and/or related processes, such as affect, which may influence reproduction. Indeed, one well-recognized behavioral phenomenon about 3α,5α-THP is its bimodal effect on mood and aggression (Beauchamp et al., 2000; Fish et al., 2001; Miczek et al., 2003). Indeed, mating increases 3α,5α-THP levels in the midbrain and in the hippocampus, an important brain region that mediates affective and cognitive processes. We have found that mating increases anti-anxiety behaviors in the elevated plus maze, the open field and in social interactions tasks, concomitant with biosynthesis of 3α,5α-THP in the hippocampus and midbrain (Frye et al., 2007; Frye & Rhodes, 2008a). This has led us to consider whether 3α,5α-THP biosynthesis, due to social factors, may influence SCEP.
3α,5α-THP & Homeostasis
One role of 3α,5α-THP may be to serve as a neuroendocrine factor that mediates parasympathetic tone and activity of the hypothalamic-pituitary-adrenal axis (HPA). 3α,5α-THP may be a homeostatic modulator that protects an animal from stressors and enables appropriate behavioral responses to occur (Engel & Grant, 2001). In support 3α,5α-THP is present during prenatal development and increases in response to stressors, such as maternal separation, as early as postnatal day 6 (Kellogg & Frye, 1999; Kehoe et al., 2000; McCormick et al., 2002). Stress manipulations during the critical time during hippocampal formation in the rodent brain (when there is much neuronal specification and cell body migration) can produce some deficits in long-term potentiation and long-term depression in hippocampus, spatial memory, increase anxiety and depressive behavior, and enhance susceptibility to addiction (Deminiere et al., 1992; Kippin et al., 2008; Walf & Frye, 2006a,b; Weinstock, 2001, 2005, 2007). In adults, increases in 3α,5α-THP in response to acute cold-water swim, shock, and/or carbon dioxide exposure can be seen in intact, GDX, and/or ADX animals (Barbaccia et al., 1997; Paul & Purdy, 1992; Purdy et al., 1991; Valee et al., 2000), albeit, this is not always observed in ADX rats (Barbaccia et al., 1997). 3α,5α-THP has been demonstrated to attenuate enhancement of hypothalamic stress factor (corticotrophin-releasing hormone and vasopressin) mRNA expression in response to stress and its replacement to ADX rats attenuates anxiety associated with administration of corticotrophin-releasing hormone (Patchev et al., 1994, 1996). More subtle stimuli, such as social challenge and/or mating, also alter neurosteroidogenesis (Frye, 2001a,b; Frye & Rhodes, 2008a; Miczek et al., 2003). Increases in 3α,5α-THP produced by such experiences are conserved across species, enhance GBR function, produce anxiolysis, and attenuate activation of sympathetic/HPA responses, which may help individuals return to a state of homeostasis following challenge (Barbaccia et al., 2001; Frye, 2001a,b, 2008b; Patchev & Almeida, 1996; Paul & Purdy, 1992). As stress can alter neuroendocrine, cognitive and/or affective responding, we will investigate the role of 3α,5α-THP on SCEP of young adult and mid-aged rats.
Sex differences in 3α,5α-THP & stress responses
There are gender/sex differences in stress responding. Women have higher cortisol levels and respond with greater HPA reactivity to lower levels of stress stimuli than do men, particularly during perimenstrual or post-partum 3α,5α-THP withdrawal (Ellermeier & Westphal, 1995; Ferrini et al., 1997; Hinojosa-Laborde et al., 1999; Jezova et al., 1996; Rhodes & Rubin, 1999). Basal and stress-induced corticosterone levels are higher for female compared to male rats during 3α,5α-THP decline (Carey et al., 1995; Neumann, 2001; Oglivie & Rivier, 1997). Further, 3α,5α-THP administration to female or male rats attenuates the elevation of plasma ACTH and serum corticosterone secretion produced by emotional stress (Frye, 2007; Patchev et al., 1996). There are also gender differences in the social aspects of the response to stress. Males are thought to more likely respond to stress with agonistic/“fight-or-flight” approach, whereas females may use a more pro-social approach (i.e. “tend and befriend”; Taylor, 2008). The relative adaptive responses to stress, and the extent to which these differences in neurobiological and behavioral responses to stressors, are mediated by 3α,5α-THP, are of interest. Indeed, we have found that 3α,5α-THP levels among those with post-traumatic stress disorder are higher and negatively correlated with state anxiety following exposure to PTSD-relevant stimuli (Frye & Rhodes, 2008b). Throughout life, chronic stress can have neurodegenerative effects. 3α,5α-THP has been demonstrated to be neuroprotective, but this has mainly been investigated in in vitro models (Wang et al., 2007). However, we have data of the neuroprotective effects of 3α,5α-THP in in vivo systems, which are described in more detail below (Frye, 2008). Thus, we have investigates 3α,5α-THP’s role to promote and ameliorate stress- and age-induced decrements in socio-cognitive and/or stress responding among young adult and middle-aged rats.
3α,5α-THP & affect of people
P and its metabolites may have effects upon depressive and anxiety behaviors in both humans and animals. Throughout the life-span, women experience varied and occasionally dramatic changes in their hormonal and reproductive cycles, and sensitivity in these periods of some women may contribute to greater incidence of anxiety and mood disorders of women compared to men. Among some women, hormonal and/or reproductive events may influence the onset or expression of depression and/or anxiety disorders, such as premenstrual syndrome, premenstrual dysphoric disorder (PMDD), and post-partum depression, syndromes which occur when endogenous levels of P and its metabolites are low (Backstrom et al., 2003; Endicott et al., 1999; Glick and Bennett, 1981; Markou et al., 2005; Pearlstein et al., 2005; Rapkin et al., 2002). For example, women with PMDD may be more sensitive to fluctuations in steroids, such that they report improved mood with ovarian suppression, but negative mood is increased by administration of E2 and/or P (Schmidt et al., 1998). Furthermore, mood is more negative when hormone levels are higher during the luteal phase in PMDD patients (Hammarbäck et al., 1989; Seipel & Backstrom, 1998). Indeed, in collaboration with Dr. Ellen Freeman, we have also found that among some PMDD patients treated with selective serotonin reuptake inhibitors (SSRI), that had clinical improvement induced by placebo or SSRI, had lower levels of 3α,5α-THP (Freeman et al., 2002, 2004). Differences in affect are also observed with aging and the changes in steroid secretion at this time. Among some women at menopause, reduced levels of 3α,5α-THP and other neurosteroids has been associated with depression and other mood disorders (Barbaccia et al., 2000; Freeman et al., 2002; Girdler et al., 2001; Pearlstein, 1995). Moreover, changes in 3α,5α-THP levels are observed in people with affective disorders. For example, baseline plasma levels of 3α,5α-THP are normal in patients with generalized anxiety disorders and social phobia (Le Melledo & Baker, 2002). Moreover, levels are within normal limits following administration of pentagastrin, a panic-inducing agent (Tait et al., 2002). In people with panic disorder, 3α,5α-THP levels are higher than normal (Brambilla et al., 2003; Strohle et al., 2003), but were decreased by infusions of sodium lactate or cholecystokinintetrapeptide (CCK4) to induce panic attacks (Strohle et al., 2003). Notably, in people without a history of panic attacks, levels of neurosteroids are not affected or are increased following CCK4 treatment (Eser et al., 2005; Zwanzger et al., 2004). In women with panic disorder and agoraphobia, perimenstrual, but not midluteal, 3α,5α-THP levels, were significantly higher than among normal controls, and they correlated with their panic-phobic symptoms (Brambilla et al., 2003). Some of the most compelling evidence that neurosteroids are involved in depression comes from clinical use of finasteride, a 5α-reductase inhibitor used for treatment of alopecia, which decreases production of 3α,5α-THP (Altomare & Capella, 2002; Townsend & Marlowe, 2004). As well, men with benign prostate hyperplasia are much more likely to develop depressive disorders when their treatment includes finasteride (Clifford and Farmer, 2002). That decreasing 3α,5α-THP production with finasteride can precipitate depressive symptoms in some individuals supports its role in the etiology of affective disorders.
The efficacy of some therapeutics to treat depressive and/or anxiety disorders are associated with their capacity to increase products of P metabolism (Griffin and Mellon, 1999; Uzunov et al., 1996; Uzunova et al., 1998). Interestingly, clinical findings indicate that P’s anti-depressant effects may involve actions of 3α,5α-THP. Some patients who have depressive disorders have reduced plasma and/or cerebrospinal fluid levels of 3α,5α-THP (Romeo et al., 1998; Stahl, 1997; Uzunova et al., 1998). Administration of antidepressants, such as fluoxetine or fluvoxamine, normalize low 3α,5α-THP levels concomitant with reducing symptoms of depression (Uzunova et al., 2004, 2006). This implies that 3α,5α-THP may contribute to the antidepressant effects of some therapeutics.
3α,5α-THP & Affective Behavior in Animal Models
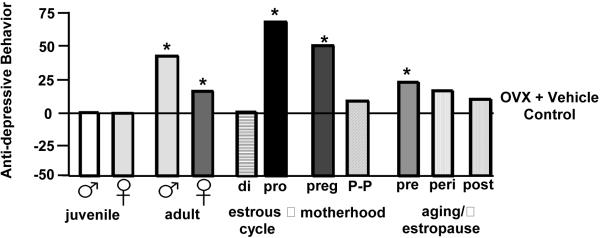
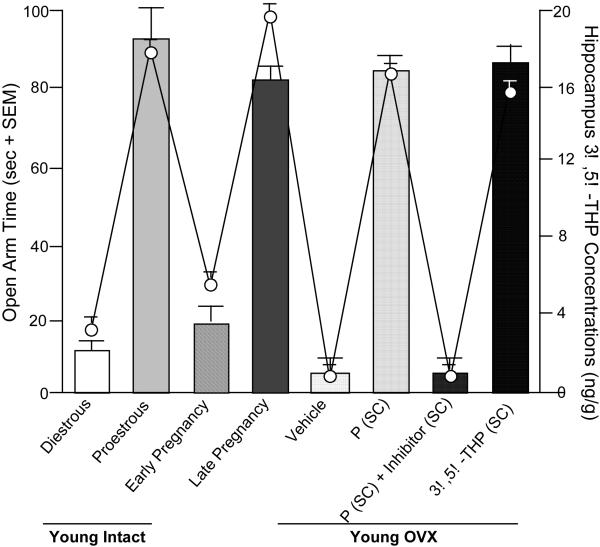
Findings from animal models also support a role for P and its metabolites in depressive and anxiety behaviors. Figure 2 depicts a summary of depression behavior (as measured by time spent immobile in the forced swim test, a typical task utilized to measure depression-like behavior of rats) of rats across endogenous steroid milieu, in comparison to rats that have had their main endogenous source of circulating E2 and P surgically removed (OVX) and were administered placebo vehicle. First, we have found little evidence for sex differences between male and female rats for depression behavior if they are tested as juveniles (i.e. before the pubertal onset in steroid secretion from the gonads in a sex-specific manner). Second, there are changes across the estrous cycle in depression behavior of rats. Rats during the late proestrous phase of the estrous cycle (i.e. behavioral estrus), when E2 and P levels are higher than in other stages of the estrous cycle, have decreased depressive behavior in the forced swim test, compared to that observed in diestrous or male rats (Contreras et al., 2000; Frye & Walf, 2002; Frye & Wawrzycki, 2003; Marcondes et al., 2001; Marvan et al., 1996). Animal models also support a role of P and 3α,5α-THP in anxiety behavior, as measured by activity on the open arms of the elevated plus maze (Bitran et al., 2000; Guidotti & Costa, 1998; Jain et al., 2005). When P and 3α,5α-THP levels are high, such as during proestrus and pregnancy, anxiety behavior is reduced compared to when levels are declining or low (Becker & Cha, 1989; Bitran et al., 1991; Frye et al., 2000; Gulinello et al., 2003; Walf & Frye, 2006). OVX increases anxiety behavior (i.e. decreases open arm time) of female rodents and P or 3α,5α-THP reverses this (Frye & Walf, 2002; 2004; Frye et al., 2004a,b; Walf et al., 2006b), unless 3α,5α-THP formation is compromised or withdrawal occurs (Frye & Walf, 2002; Frye & Walf, 2004b; Rhodes & Frye, 2001; Figure 3). Given that intractable depression is a significant problem among the elderly, we have examined, the role of P and 3α,5α-THP to alter anxiety behavior of young adult and middle-aged rodents.
Figure 2.
Anti-depression behavior in the Porsolt forced swim task is greater among intact male and female adult rats compared to juvenile male and females rats. It is greater among proestrous or pregnant rats, compared to diestrous or post-partum rats. As well, it is greater among middle-aged reproductively competent rats compared to middle-aged rats that are reproductively senescent or transition to senescence. * indicates different from respective juvenile, diestrous, post-partum, or reproductively-senescent control, p < 0.05.
Figure 3.
Anti-anxiety behavior on the elevated plus maze correlates with 3α,5α-THP in hippocampus of young (diestrous or proestrous), mid-aged (reproductively-competent or reproductively-senescent), or OVX (progesterone (P) with/without 3α,5α-THP inhibition, or 3α,5α-THP administration) rats. Bars represent open arm time (seconds±SEM) and dots represent 3α,5α-THP levels (ng/g±SEM) in hippocampus.
3α,5α-THP & Cognition
In addition to changes in mood, anxiety, and social functioning that characterize affective disorders of people, there are changes in cognitive function. Endogenous changes in progestins may influence cognitive performance mediated by the hippocampus. In young adult women, spatial performance either does not vary or is better among those tested in the menstrual and follicular, compared to the luteal (high P), phases (Maki et al, 2002; Rosenberg & Park, 2002). Among women, performance in the Wisconsin card sorting task and visual memory is better in the P-dominant luteal phase than during the ovulatory and/or menstrual phases (Berman et al, 1997; Hampson, 1990; Solis-Ortiz et al, 2004; Phillips & Sherwin, 1992). P administration may have beneficial effects on PFC-mediated tasks. Transvaginal P (400 mg daily) to young women on lupron, a gonadotropin-releasing hormone agonist that suppresses endogenous gonadal steroid hormones, enhances cognition-related neural activity in the PFC (Berman et al, 1997). Furthermore, aging is associated with decline in cognitive performance of some women, and these changes occur at the same time that there are reductions in ovarian secretion of steroids. Thus, 3α,5α-THP may play a role in cognition of people.
3α,5α-THP & Cognitive Behavior in Animal Models
There are activational effects of P and its metabolites for cognitive performance of rats. Young, rats, which have higher 3α-5α, THP levels in the cortex (proestrus and late pregnancy), have better performance in the object recognition task than do diestrous rats or rats in early pregnancy (Figure 4). Among mid-aged rats, performance declines, particularly in reproductively-senescent rats compared to non-reproductively senescent rats. OVX impairs performance in this task and this can be reversed with administration of P or 3α,5α-THP; however, administration of P to OVX rats administered a 5α-reductase inhibitor produces similar effects as vehicle administration to OVX rats. Other studies have shown that administration of P, DHP, or 3α,5α-THP to ovx rats enhances performance in the object recognition and Y-maze tasks, both of which involve prefrontal and hippocampal processes, as well as conditioned and passive avoidance, tasks (Diaz-Veliz et al., 1994; Ebner et al., 1981; van Wimersma Greidanus, 1977, Walf et al., 2006a). Indeed, regression analyses revealed significant positive correlations between E2 and 3α,5α-THP levels in the hippocampus and 3α,5α-THP levels in the prefrontal cortex for performance in the object recognition task (Walf et al., 2006a). It must be noted that there are reports of disorganizing effects of progestins that need to be taken into consideration. For example, among pregnant professional women, symptoms such as forgetfulness, disorientation, confusion, and reading difficulties are commonly reported (Poser et al, 1986). Studies in which ovx improved performance or progestin administration failed to enhance performance in ovx and/or aged rats may be due in part to disruptive effects of high progestin levels (Bimonte-Nelson et al, 2003; 2004; Johansson et al, 2002; Zou et al, 2000), which can produce sedative/anesthetic effects (Holzbauer, 1975, 1976; Selye, 1942) and decrease optimal levels of arousal for learning/memory performance. Thus, physiological levels of 3α,5α-THP may improve cognitive performance of rodents and decline in 3α,5α-THP with aging may contribute to cognitive decline.
Figure 4.
Cognitive performance on the object recognition task correlates with 3α,5α-THP levels in the cortex of young (diestrous or proestrous), mid-aged (reproductively-competent or reproductively-senescent), or OVX (progesterone (P) with/without 3α,5α-THP inhibition, or 3α,5α-THP administration) rats. Bars represent time with the novel object (seconds±SEM) and dots represent 3α,5α-THP levels (ng/g±SEM) in cortex.
Steroids & AD
Female-typical steroids may play a role in AD. Ovarian steroids, such as P and E decline with reproductive senescence and aging. Women are twice as likely as are men to develop AD (Gao et al., 1998; Payami et al., 1994). Potential beneficial effects of hormone therapy (HT) decline with delay in initiating HT (Resnick et al., 1997; Shumaker et al., 2003), age (Csizmadi et al., 2005), neurodegeneration (Henderson, 2000; Sherwin, 2006; Troncoso et al., 1996), and perhaps low levels of the P metabolite, and neurosteroid, 3α,5α-THP, are related to pathophysiology (Genazzani et al., 2001; Smith et al., 2006; Weill-Engerer et al., 2002). For example, we have shown that circulating 3α,5α-THP levels of those with AD or non-AD dementia are 33% lower than age-, gender-, and HT-matched, non-demented controls (Smith et al., 2006). We have recently investigated the role of P in a mouse model of AD, i.e. mice that overexpress the APPswe and presenilin Δ exon 9 mutation (APPswe+PSEN1Δe9 mice; obtained from Jackson Laboratory) (Frye & Walf, 2008a). Deficits in hippocampus-dependent tasks coincide with decrements in 3α,5α-THP in the hippocampus and P to ovx, wildtype (WT), but not APPswe+PSEN1Δe9, mice improves performance in hippocampal tasks and reinstates hippocampal 3α,5α-THP levels (Frye & Walf, 2008a). P and/or 3α,5α-THP can have protective effects against seizures (Ciriza et al., 2006; Herzog & Frye, 2003; Reddy & Rogawski, 2001), glutamate toxicity in cultured hippocampal neurons (Nilsen & Brinton, 2002), and in animal models of neurodegeneration (Griffin et al., 2004; He et al., 2004a-c; Roof et al., 1994; Vongher & Frye, 1999). It may be that 3α,5α-THP deficiencies, particularly in the hippocampus, may contribute, or be due, to age-related pathologies.
There is clear evidence that neurosteroids can play a role in hormonally- and developmentally-mediated behavioral processes, and that they may serve clinically-relevant functions across the lifespan. In order to further elucidate the role of neurosteroids, it may be necessary to think beyond heretofore defined clinical syndromes and consider common underlying symptoms that may be explained, or influenced in part by, neurosteroids. A truly productive translational approach will require careful elucidation and consideration of common features of neurosteroids’ role in basic animal research and in clinical syndromes. As such, continued evaluation of the relationship between neurosteroidogenic capacity, neuroprotection, neurogenesis and therapeutic effects on basic biobehavioral processes across a number of paradigms may provide insight to their role in neurobiological and behavioral processes involved in SCEP.
4. Mating increases 3α,5α-THP in midbrain, cortex, hippocampus, and striatum
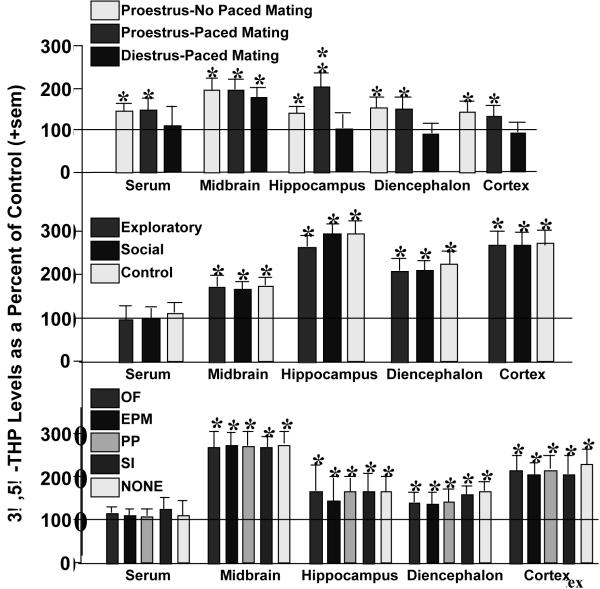
3α,5α-THP levels in the midbrain are particularly dynamic and increase with mating. Notably, following standard mating (10 minutes or 10 sexual contacts with a male in an aquarium), midbrain 3α,5α-THP levels are increased over those of non-mated naturally-receptive or hormone-primed rodents (Frye, 2001a,b; Fig. 5, top). In follow-up experiments, the effects of paced mating, in which proestrous rats are mated in a larger chamber wherein they can control the access to the male and thereby sexual contacts, rats were allowed to engage in an exploratory/anxiety tasks, social task, or no tasks, with or without paced mating. Irrespective of portion (Fig. 5, middle) or individual task engaged in (Fig. 5, bottom), paced mating was associated with 3α,5α-THP enhancement in brain but not serum (Frye et al., 2007). Engaging in paced mating uniquely increased biosynthesis of P metabolites, DHP and 3α,5α-THP, in midbrain > cortex > hippocampus > diencephalon (Frye & Rhodes, 2006). Thus, social interactions, such as paced mating, dynamically alter synthesis of P metabolites in brain areas involved in SCEP function. However, differences between standard and paced mating have not been compared and are of interest. As well, the acute and chronic consequences of such social interactions on SCEP will be investigated.
Figure 5.
Depicts 3α,5α-THP levels in serum, midbrain, hippocampus, diencephalon, and cortex of proestrous or diestrous rats that engaged in paced mating or not (top), proestrous rats that engaged in exploratory (open field and elevated plus maze) or social (partner preference and social interaction) or no tasks with paced mating (middle), and proestrous rats that engage in only one task from above (open field, elevated plus maze, partner preference, social interaction, or no task) with paced mating (bottom). Line indicates performance of non-mated diestrous (top) or proestrous (middle, bottom) control. * indicates different from control, p < 0.05.
5. Mating increases gene expression in the midbrain
We examined differences in gene expression in the midbrain of naturally-receptive rats that underwent paced mating compared to naturally-receptive rats that did not mate. Midbrain tissues were sent to two core microarray facilities for Affymetrix GeneChip Rat 230 2.0 arrays per Affymetrix protocol. First, among mated rats, genes that were upregulated in the midbrain were primarily related to those substrates that our past studies have elucidated as targets for P and/or 3α,5α-THP actions to influence lordosis (i.e. GABA, glutamate, dopamine, signal transduction targets; Frye & Walf, 2008b). Second, mating induces 3α,5α-THP biosynthesis and many genes that were upregulated were those involved in steroid metabolism. Third, genes involved in cell proliferation and cell death were upregulated in the midbrain VTA of mated vs. non-mated rats. These data suggest that mating alters expression of genes associated with non-traditional steroid actions, steroid biosynthesis, and trophic actions in the midbrain of rats.
6. Parity enhances cognitive and anti-anxiety performance and 3α,5α-THP levels
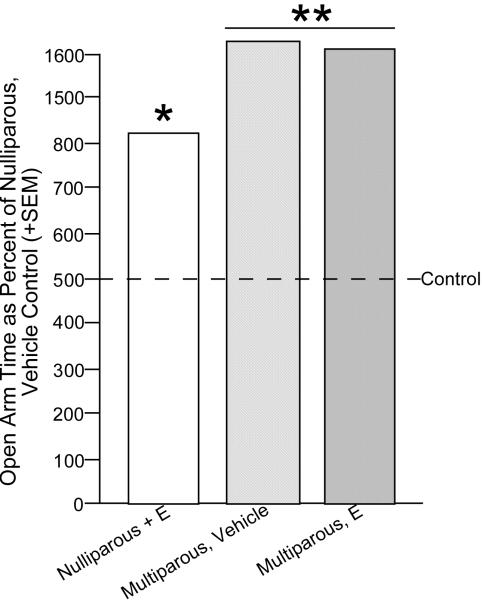
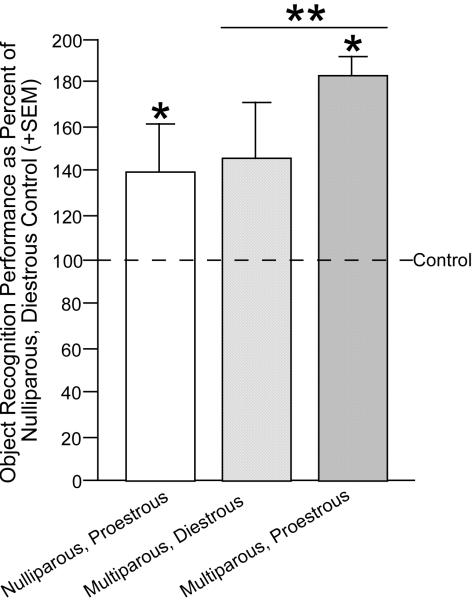
One intriguing possibility that may underlie some of the common actions of 3α,5α-THP is its effects on neuroplasticity. For example, naked mole rats that are breeders have more favorable neural morphology than do non-breeders (Holmes et al., 2007). Given that 3α,5α-THP is elevated in brain during pregnancy and that 3α,5α-THP has demonstrated trophic effects, the extent to which 3α,5α-THP may influence behavior associated with parity is of interest. Parity enhances cognitive function and affective behavior (Kinsley & Lambert, 2008; Paris & Frye, 2008; Pawluski et al., 2006; Walf & Frye, 2007). Furthermore, following mating and pregnancy, there is enhancement of neurogenesis in the hippocampus (Leuner et al., 2007; Pawluski & Galea, 2007). Indeed, rats with greater parity have more neurogenesis in the hippocampus. In our hands, we see that multiparous rats perform better on hippocampally-mediated tasks (elevated plus maze Figure 6, object recognition-Figure 7), than do nulliparous and/or primiparous rats and enhancements in cognitive performance occur concomitant with enhancements in circulating 3α,5α-THP among primiparous dams, albeit causal effects have not been established (Paris & Frye, 2008). For this reason, we will examine acute and long-lasting effects of mating and/or parity on social experience on SCEP.
Figure 6.
Ovariectomized, vehicle or estradiol (E)-primed multiparous rats have greater anti-anxiety behavior in the elevated plus maze than do nulliparous rats. Line indicates performance of nulliparous, vehicle-administered control. * indicates different from control, p < 0.05.
Figure 7.
Naturally-cycling, proestrous or multiparous rats outperform diestrous nulliparous rats in the object recognition task. Line indicates performance of diestrous, nulliparous control. * indicates different from control, p < 0.05.
7. Reproductive senescence influences social, cognitive, and affective performance
Social, cognitive and affective deficits can occur with aging in people and rodents. In females, these deficits may be initiated and/or coincide with the transition to reproductive senescence. Whether individual differences in the natural transition to reproductive senescence is associated with alterations in sexual, cognitive, and affective performance was investigated among age-matched, 12-month-old, Long-Evans female rats that were still reproductively-competent, transitioning to reproductive-senescence, or were reproductively-senescent based upon their estrous cyclicity, fertility, and fecundity. Rats were assessed for sexual behavior, object recognition and water maze performance, and behavior in the plus maze on separate testing days. Reproductively-competent rats had normal 4-5 day estrous cycles, were readily able to become pregnant, and had normal litter sizes. These rats performed significantly better than did reproductively-senescent rats (↓cyclicity, fertility, fecundity) on sexual responding in the paced mating task (Figure 8), object recognition and water maze (Figure 9), and anti-anxiety behavior in the elevated plus maze (Figure 10). Rats that were transitioning to reproductive-senescence performed intermediate to other groups on these tasks. These data suggest that there are individual differences in sexual, cognition, and/or anti-anxiety behavior that coincide with changes in reproductive status in aging. However, these rats were all retired breeders and the nature of these effects in virgin rats needs to be assessed.
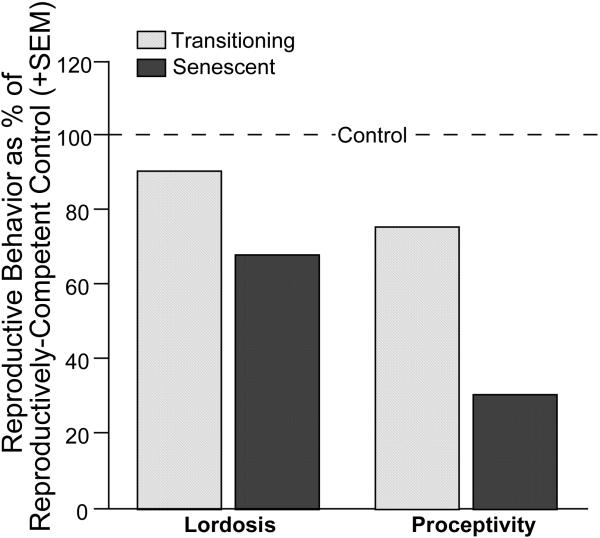
Figure 8.
Among middle-aged rats, reproductively-competent rats had greater lordosis and proceptivity in a paced-mating task than did rats that were reproductively-senescent or transitioning to reproductive-senescence. Line indicates performance of reproductively-competent rats.
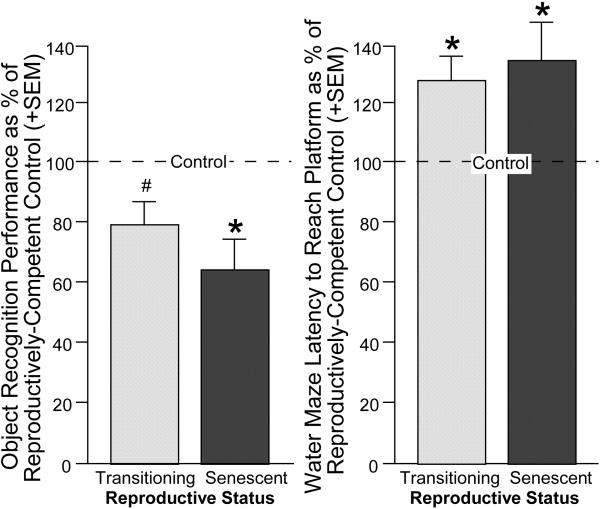
Figure 9.
Among middle-aged rats, reproductively-competent rats had greater cognitive performance in the object recognition task (left) and were faster to locate a hidden platform in the Morris water maze (right) compared to rats that were reproductively-senescent or transitioning to reproductive-senescence. Line indicates performance of reproductively-competent rats.* indicates different from control (p < 0.05). # indicates tendency to be different from control (p < 0.10).
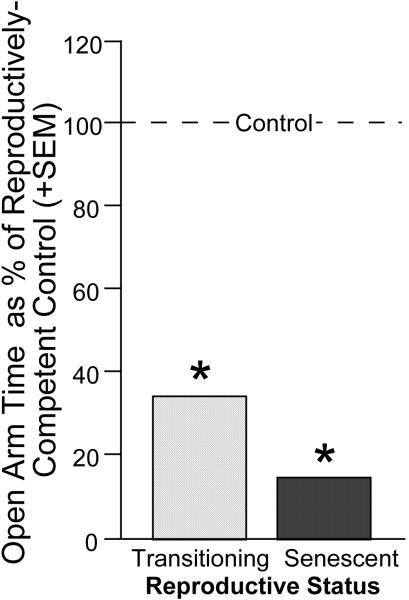
Figure 10.
Among middle-aged rats, reproductively-competent rats had greater anti-anxiety behavior in an elevated plus maze than did rats that were reproductively-senescent or transitioning to reproductive-senescence. Line indicates performance of reproductively-competent rats. * indicates different from control, p < 0.05.
8. Conclusion
One factor that may have a profound influence on the trajectory of age-related processes are social supports. Loss of a social relationship can contribute to decline in mental, cognitive, and/or physical functioning. Given that hormones are mediating factors between the central and peripheral nervous system, and can influence social, affective, and cognitive processes, their role underlying neurobiological and/or behavioral mechanisms that influence aging, are of interest. The loss of close relationships, especially among older persons, is one of the greatest risk factors for mental and physical decline. However, the neurobiological and behavioral mechanisms underlying these effects are not well understood. The role of hormonal versus life experience factors, especially those related to social factors, have the potential to greatly alter social, affective, cognitive and physical functioning. How alterations in 3α,5α-THP may play a fundamental role to enhance reproduction and social bonds, and, thereby, influence SCEP is of interest throughout the lifespan. To summarize, our findings to date indicate indicate the following. Mating increases levels of 3α,5α-THP in the midbrain, cortex, hippocampus and striatum. Mating increases expression in midbrain of genes involved in GABA, dopamine, and glutamate function, as well as steroid metabolism, neurogenesis, and apoptosis. Parity enhances levels of circulating 3α,5α-THP, cognitive, anti-anxiety, and sexual behavior. Reproductive senescence is associated with decrements in paced mating, cognitive and anti-anxiety behavior. Questions to be addressed are whether: (1) There are specific types of sexual interactions that most readily evoke 3α,5α-THP biosynthesis in the midbrain and/or hippocampus. (2) There are causal effects of sexual experiences on SCEP (coincident with increases in 3α,5α-THP levels in the midbrain and/or hippocampus). (3) 3α,5α-THP biosynthesis or metabolism in the midbrain and/or hippocampus is necessary for sexual experience to alter SCEP. (4) Mating-induced 3α,5α-THP biosynthesis is due to neurogenesis/trophic factors in the midbrain and/or hippocampus.
9. Future Directions
The effects, sites of action, and neural mechanisms of mating-relevant interactions for social, cognitive, and affective behavior are of interest. Levels of P and its metabolites and effects on metabolism enzymes need to be examined in different regions of the brain. 3α,5α-THP can delay progression of neurodegenerative processes (Brinton & Wang, 2006; Langmade et al., 2006; Mellon et al., 2008) perhaps in part through activation of metabolism enzymes, including those involved in conversion of cholesterol to pregnenolone, the rate limiting step in neurosteroidogenesis. The neurotransmitter receptor targets are also of interest. 3α,5α-THP has agonist-like effects at GBRs and may have indirect antagonist-like effects via NMDARs (Frye et al., 2006; Leśkiewicz et al., 1998; Wang et al., 2007). Actions at these receptors, their downstream signal transduction pathways, and/or other substrates, such as membrane progestin receptors, will be investigated in the future (Kirkness et al., 1989; Lin et al., 1996; Marshall, 1994; McDonald & Moss, 1994; Nilsen & Brinton, 2003; Singh, 2001; 2005; Singer et al., 1999). Whether manipulations of 3α,5α-THP or its targets directly in specific brain regions, such as the hippocampus, influence social, cognitive, and/or affective function will be elucidated.
Acknowledgements
The efforts of Dr. Alicia Walf, Jason Paris, and other members of my laboratory, present and past, are greatly appreciated. Danielle Osborne provided technical assistance in formatting the manuscript.
Role of Funding Source
This research was supported by grants from The National Institutes of Health (MH06769801, MD003373, NS06323301A1) and National Science Foundation (03-16083).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altomare G, Capella GL. Depression circumstantially related to the administration of finasteride for androgenetic alopecia. J Dermatol. 2002;29:665–9. doi: 10.1111/j.1346-8138.2002.tb00200.x. [DOI] [PubMed] [Google Scholar]
- Bäckström T, Andersson A, Andreé L, Birzniece V, Bixo M, Björn I, Haage D, Isaksson M, Johansson IM, Lindblad C, Lundgren P, Nyberg S, Odmark IS, Strömberg J, Sundström-Poromaa I, Turkmen S, Wahlström G, Wang M, Wihlbäck AC, Zhu D, Zingmark E. Pathogenesis in menstrual cycle-linked CNS disorders. Ann N Y Acad Sci. 2003;1007:42–53. doi: 10.1196/annals.1286.005. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Lello S, Sidiropoulou T, Cocco T, Sorge RP, Cocchiarale A, Piermarini V, Sabato AF, Trabucchi M, Romanini C. Plasma 5alpha-androstane-3alpha,17betadiol, an endogenous steroid that positively modulates GABA(A) receptor function, and anxiety: a study in menopausal women. Psychoneuroendocrinology. 2000;25:659–75. doi: 10.1016/s0306-4530(00)00017-2. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63:166–72. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Purdy RH, Mostallino MC, Concas A, Biggio G. The effects of inhibitors of GABAergic transmission and stress on brain and plasma allopregnanolone concentrations. Br J Pharmacol. 1997;120:1582–8. doi: 10.1038/sj.bjp.0701046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. Int Rev Neurobiol. 2001;46:243–72. doi: 10.1016/s0074-7742(01)46065-x. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Steroid hormone receptors. Expos Annu Biochim Med. 1980;34:1–25. [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: a new function in the brain. Biol Cell. 1991;71:3–10. doi: 10.1016/0248-4900(91)90045-o. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Ormerod BK, Jhamandas K, Boegman RJ, Beninger RJ. Neurosteroids and reward: allopregnanolone produces a conditioned place aversion in rats. Pharmacol Biochem Behav. 2000;67:29–35. doi: 10.1016/s0091-3057(00)00299-9. [DOI] [PubMed] [Google Scholar]
- Becker JB, Cha JH. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 1989;35:117–25. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–75. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Berkman LF. Tracking social and biological experiences: the social etiology of cardiovascular disease. Circulation. 2005;111:3022–4. doi: 10.1161/CIRCULATIONAHA.104.509810. [DOI] [PubMed] [Google Scholar]
- Berkman LF. Social support, social networks, social cohesion and health. Soc Work Health Care. 2000;31:3–14. doi: 10.1300/J010v31n02_02. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Melchior M, Chastang JF, Niedhammer I, Leclerc A, Goldberg M. Social integration and mortality: a prospective study of French employees of Electricity of France-Gas of France: the GAZEL Cohort. Am J Epidemiol. 2004;159:167–74. doi: 10.1093/aje/kwh020. [DOI] [PubMed] [Google Scholar]
- Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, Ostrem JL, Weinberger DR. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci U S A. 1997;94:8836–41. doi: 10.1073/pnas.94.16.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Hunter CL, Price KL, Moore AB, Granholm AC. Ovarian hormones and cognition in the aged female rat: I. Long-term, but not short-term, ovariectomy enhances spatial performance. Behav Neurosci. 2003;117:1395–406. doi: 10.1037/0735-7044.117.6.1395. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm AC. Ovarian hormones and cognition in the aged female rat: II. Progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behav Neurosci. 2004;118:707–14. doi: 10.1037/0735-7044.118.4.707. [DOI] [PubMed] [Google Scholar]
- Bitran D, Foley M, Audette D, Leslie N, Frye CA. Activation of peripheral mitochondrial benzodiazepine receptors in the hippocampus stimulates allopregnanolone synthesis and produces anxiolytic-like effects in the rat. Psychopharmacology. 2000;151:64–71. doi: 10.1007/s002130000471. [DOI] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3!-hydroxy-5![!]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–61. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Bixo M, Andersson A, Winblad B, Purdy RH, Bäckström T. Progesterone, 5!-pregnane-3,20-dione and 3!-hydroxy-5!-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 1997;764:173–178. doi: 10.1016/s0006-8993(97)00455-1. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Progestin receptors: neuronal integrators of hormonal and environmental stimulation. Ann N Y Acad Sci. 2003;1007:238–50. doi: 10.1196/annals.1286.023. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Biggio G, Pisu MG, Bellodi L, Perna G, Bogdanovich-Djukic V, Purdy RH, Serra M. Neurosteroid secretion in panic disorder. Psychiatry Res. 2003;118:107–16. doi: 10.1016/s0165-1781(03)00077-5. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer’s disease: allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res. 2006;3:185–90. doi: 10.2174/156720506777632817. [DOI] [PubMed] [Google Scholar]
- Brot MD, Akwa Y, Purdy RH, Koob GF, Britton KT. The anxiolytic-like effects of the neurosteroid allopregnanolone: interactions with GABA(A) receptors. Eur J Pharmacol. 1997;325:1–7. doi: 10.1016/s0014-2999(97)00096-4. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC. Social isolation and health, with an emphasis on underlying mechanisms. Perspect Biol Med. 2003;46:839–52. [PubMed] [Google Scholar]
- Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on HPA regulation in the female rat. J Endocrinol. 1995;144:311–21. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res. 2007;176:170–86. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–95. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- Chen JH, Bierhals AJ, Prigerson HG, Kasl SV, Mazure CM, Jacobs S. Gender differences in the effects of bereavement-related psychological distress in health outcomes. Psychol Med. 1999;29:367–80. doi: 10.1017/s0033291798008137. [DOI] [PubMed] [Google Scholar]
- Cheng J, Karavolas HJ. Conversion of progesterone to 5!-pregnane-3,20-dione and 3!-hydroxy-5!-pregnan-20-one by rat medical basal hypothalami and the effects of estradiol and stage of estrous cycle on the conversion. Endocrinology. 1973;93:1157–1162. doi: 10.1210/endo-93-5-1157. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Carrero P, Frye CA, Garcia-Segura LM. Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J Neurobiol. 2006;66:916–28. doi: 10.1002/neu.20293. [DOI] [PubMed] [Google Scholar]
- Clifford GM, Farmer RD. Drug or symptom-induced depression in men treated with! 1-blockers for benign prostatic hyperplasia? A nested case-control study. Pharmacoepidemiol Drug Saf. 2002;11:55–61. doi: 10.1002/pds.671. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Concas A, Pierobon P, Mostallino MC, Porcu P, Marino G, Minei R, Biggio G. Modulation of gamma-aminobutyric acid (GABA) receptors and the feeding response by neurosteroids in Hydra vulgaris. Neuroscience. 1998;85:979–88. doi: 10.1016/s0306-4522(97)00515-0. [DOI] [PubMed] [Google Scholar]
- Contreras CM, Molina M, Saavedra M, Martinez-Mota L. Lateral septal neuronal firing rate increases during proestrus-estrus in the rat. Physiol Behav. 2000;68:279–84. doi: 10.1016/s0031-9384(99)00169-9. [DOI] [PubMed] [Google Scholar]
- Csizmadi I, Friedenreich CM, Bryant HE, Courneya KS. An analysis of the effect of selection bias on the association of hormone replacement therapy and breast cancer risk. Chronic Dis Canada. 2005;26:73–9. [PubMed] [Google Scholar]
- Deminière JM, Piazza PV, Guegan G, Abrous N, Maccari S, Le Moal M, Simon H. Increased locomotor response to novelty and propensity to intravenous amphetamine self-administration in adult offspring of stressed mothers. Brain Res. 1992;586:135–9. doi: 10.1016/0006-8993(92)91383-p. [DOI] [PubMed] [Google Scholar]
- Díaz-Véliz G, Urresta F, Dussaubat N, Mora S. Progesterone effects on the acquisition of conditioned avoidance responses an other motoric behaviors in intact and ovariectomized rats. Psychoneuroendocrinology. 1994;19:387–94. doi: 10.1016/0306-4530(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Drugan RC, Holmes PV, Scher DM, Luczak S, Oh H, Ferland RJ. Environmentally induced changes in peripheral benzodiazepine receptors are stressor and tissue specific. Pharmacol Biochem Behav. 1995;50:551–62. doi: 10.1016/0091-3057(94)00341-6. [DOI] [PubMed] [Google Scholar]
- Ebner DL, Richardson R, Riccio DC. Ovarian hormones and retention of learned fear in rats. Behav Neural Biol. 1981;33:45–58. doi: 10.1016/s0163-1047(81)92215-9. [DOI] [PubMed] [Google Scholar]
- Ellermeier W, Westphal W. Gender differences in pain ratings and pupil reactions to painful pressure stimuli. Pain. 1995;61:435–9. doi: 10.1016/0304-3959(94)00203-Q. [DOI] [PubMed] [Google Scholar]
- Endicott J, Amsterdam J, Eriksson E, Frank E, Freeman E, Hirschfeld R, Ling F, Parry B, Pearlstein T, Rosenbaum J, Rubinow D, Schmidt P, Severino S, Steiner M, Stewart DE, Thys-Jacobs S. Is premenstrual dysphoric disorder a distinct clinical entity? J Woman’s Health Gend-Based Med. 1999;8:663–679. doi: 10.1089/jwh.1.1999.8.663. [DOI] [PubMed] [Google Scholar]
- Engel SR, Grant KA. Neurosteroids and behavior. Int Rev Neurobiol. 2001;46:321–48. doi: 10.1016/s0074-7742(01)46067-3. [DOI] [PubMed] [Google Scholar]
- Eser D, di Michele F, Zwanzger P, Pasini A, Baghai TC, Schüle C, Rupprecht R, Romeo E. Panic induction with cholecystokinin-tetrapeptide (CCK-4) Increases plasma concentrations of the neuroactive steroid 3!,5!-tetrahydrodeoxycorticosterone (3!,5!-THDOC) in healthy volunteers. Neuropsychopharmacology. 2005;30:192–5. doi: 10.1038/sj.npp.1300572. [DOI] [PubMed] [Google Scholar]
- Ferrini MG, Grillo CA, Piroli G, de Kloet ER, De Nicola AF. Sex difference in glucocorticoid regulation of vasopressin mRNA in the paraventricular hypothalamic nucleus. Cell Mol Neurobiol. 1997;17:671–86. doi: 10.1023/A:1022538120627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, DeBold JF, Miczek KA. Alcohol, allopregnanolone and aggression in mice. Psychopharmacology (Berl) 2001;153:473–83. doi: 10.1007/s002130000587. [DOI] [PubMed] [Google Scholar]
- Fodor L, Bíró T, Maksay G. Nanomolar allopregnanolone potentiates rat cerebellar GABAA receptors. Neurosci Lett. 2005;383:127–30. doi: 10.1016/j.neulet.2005.03.064. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Frye CA, Rickels K, Martin PA, Smith SS. Allopregnanolone levels and symptom improvement in severe premenstrual syndrome. J Clin Psychopharmacol. 2002;22:516–20. doi: 10.1097/00004714-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61:62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res Rev. 2001a;37:201–22. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- Frye CA. The role of neurosteroids and nongenomic effects of progestins in the ventral tegmental area in mediating sexual receptivity of rodents. Horm Behav. 2001b;40:226–33. doi: 10.1006/hbeh.2001.1674. [DOI] [PubMed] [Google Scholar]
- Frye CA. Progestins influence motivation, reward, conditioning, stress, and/or response to drugs of abuse. Pharmacol Biochem Behav. 2007;86:209–19. doi: 10.1016/j.pbb.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA. In: Neurosteroids-From Basic Research to Clinical Perspectives. Rubin Robert T., Pfaff Donald W., editors. 2008. [Google Scholar]
- Frye CA, Paris JJ, Rhodes ME. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5!-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 2007;133:663–74. doi: 10.1530/rep.1.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav. 2000;67:587–96. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Progestin concentrations are increased following paced mating in midbrain, hippocampus, diencephalon, and cortex of rats in behavioral estrus, but only in midbrain of diestrous rats. Neuroendocrinology. 2006;83:336–47. doi: 10.1159/000096051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. The role of midbrain 3!,5!-THP in mediating exploration, anxiety, social and reproductive behavior. In: Ritsner Michael S., Weizman Abraham., editors. Neuroactive Steroids in Brain: From Experiments to Psychopathology and Treatment. 2008a. [Google Scholar]
- Frye CA, Rhodes ME. The role and mechanisms of steroid hormones to enhance approach behavior. In: Elliot A, editor. Handbook of approach and avoidance motivation. LEA; Mahwah, NJ: 2008b. [Google Scholar]
- Frye CA, Rhodes ME, Petralia SM, Walf AA, Sumida K, Edinger KL. 3α-hydroxy-5α-pregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience. 2006c;138:1007–14. doi: 10.1016/j.neuroscience.2005.06.015. PMCID: PMC2527995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Vongher JM. 3α,5α-THP in the midbrain ventral tegmental area of rats and hamsters is increased in exogenous hormonal states associated with estrous cyclicity and sexual receptivity. J Endocrinol Invest. 1999;22:455–64. doi: 10.1007/BF03343590. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–15. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Hippocampal 3α,5α-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav. 2004a;78:531–40. doi: 10.1016/j.pbb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and pain-reducing effects in ovariectomized rats. Behav Neurosci. 2004b;118:306–13. doi: 10.1037/0735-7044.118.2.306. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Effects of progesterone administration and APPswe+PSEN1Δe9 mutation for cognitive performance of mid-aged mice. Neurobiol Learn Mem. 2008a;89:17–26. doi: 10.1016/j.nlm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Membrane actions of progestins at dopamine type 1-like and GABAA receptors involve downstream signal transduction pathways. Steroids. 2008b;73:906–13. doi: 10.1016/j.steroids.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5 alpha-reductase. Brain Res. 2004;1004:116–24. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Frye CA, Wawrzycki J. Effect of prenatal stress and gonadal hormone condition on depressive behaviors of female and male rats. Horm Behav. 2003;44:319–26. doi: 10.1016/s0018-506x(03)00159-4. [DOI] [PubMed] [Google Scholar]
- Furukawa A, Miyatake A, Ohnishi T, Ichikawa Y. Steroidogenic acute regulatory protein (StAR) transcripts constitutively expressed in the adult rat central nervous system: colocalization of StAR, cytochrome P-450SCC (CYP XIA1), and 3!-hydroxysteroid dehydrogenase in the rat brain. J Neurochem. 1998;71:2231–8. doi: 10.1046/j.1471-4159.1998.71062231.x. [DOI] [PubMed] [Google Scholar]
- Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psych. 1998;55:809–15. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- Gee KW, McCauley LD, Lan NC. A putative receptor for neurosteroids on the GABAA receptor complex: the pharmacological properties and therapeutic potential of epalons. Crit Rev Neurobiol. 1995;9:207–27. [PubMed] [Google Scholar]
- Genazzani AR, Monteleone P, Stomati M, et al. Clinical implications of circulating neurosteroids. Int Rev Neurobiol. 2001;46:399–419. doi: 10.1016/s0074-7742(01)46070-3. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83:2099–103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Glick ID, Bennett SE. Psychiatric complications of progesterone and oral contraceptives. J Clin Psychopharmacol. 1981;1:350–67. doi: 10.1097/00004714-198111000-00003. [DOI] [PubMed] [Google Scholar]
- Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10:704–11. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96:13512–7. doi: 10.1073/pnas.96.23.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Costa E. Can the antidysphoric and anxiolytic profiles of selective serotonin reuptake inhibitors be related to their ability to increase brain 3α,5α-tetrahydroprogesterone (allopregnanolone) availability? Biol Psychiatry. 1998;44:865–873. doi: 10.1016/s0006-3223(98)00070-5. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Orman R, Smith SS. Sex differences in anxiety, sensorimotor gating and withdrawal. Eur J Neurosci. 2003;17:641–8. doi: 10.1046/j.1460-9568.2003.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarbäck S, Damber JE, Bäckström T. Relationship between symptom severity and hormone changes in women with premenstrual syndrome. J Clin Endocrinol Metab. 1989;68:125–30. doi: 10.1210/jcem-68-1-125. [DOI] [PubMed] [Google Scholar]
- Hampson E. Variations in sex-related cognitive abilities across the menstrual cycle. Brain Cogn. 1990;14:26–43. doi: 10.1016/0278-2626(90)90058-v. [DOI] [PubMed] [Google Scholar]
- He J, Evans CO, Hoffman SW, Oyesiku NM. Stein DG. Progesterone and 3α,5α-THP reduce inflammatory cytokines after traumatic brain injury. Exp Neurol. 2004a;189:404–12. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- He J, Hoffman SW, Stein DG. 3α,5α-THP, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor Neurol Neurosci. 2004b;22:19–31. [PubMed] [Google Scholar]
- He J, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor Neurol Neurosci. 2004c;22:19–31. [PubMed] [Google Scholar]
- Henderson VW. Oestrogens and dementia. Novartis Foundation Symposium. 2000;230:254–65. doi: 10.1002/0470870818.ch18. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Physiological roles for the neurosteroid allopregnanolone in the modulation of brain function during pregnancy and parturition. Prog Brain Res. 2001;133:39–47. doi: 10.1016/s0079-6123(01)33003-0. [DOI] [PubMed] [Google Scholar]
- Herd MB, Belelli D, Lambert JJ. Neurosteroid modulation of synaptic and extrasynaptic GABA(A) receptors. Pharmacol Ther. 2007;116:20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Frye CA. Seizure exacerbation associated with inhibition of progesterone metabolism. Annals of Neurology. 2003;53:390–1. doi: 10.1002/ana.10508. [DOI] [PubMed] [Google Scholar]
- Hinojosa-Laborde C, Chapa I, Lange D, Haywood JR. Gender differences in sympathetic nervous system regulation. Clin Exp Pharmacol Physiol. 1999;26:122–6. doi: 10.1046/j.1440-1681.1999.02995.x. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Rosen GJ, Jordan CL, de Vries GJ, Goldman BD, Forger NG. Social control of brain morphology in a eusocial mammal. Proc Natl Acad Sci U S A. 2007;104:10548–52. doi: 10.1073/pnas.0610344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbauer M. Physiological variations in the ovarian production of 5α-pregnane derivatives with sedative properties in the rat. J Steroid Biochem. 1975;6:1307–10. doi: 10.1016/0022-4731(75)90357-x. [DOI] [PubMed] [Google Scholar]
- Holzbauer M. Proceedings: Physiological aspects of the hypnotic properties of steriod hormones. Br J Pharmacol. 1976;56:382P. [PMC free article] [PubMed] [Google Scholar]
- Jain NS, Hirani K, Chopde CT. Reversal of caffeine-induced anxiety by neurosteroid 3-!-hydroxy-5-!-pregnane-20-one in rats. Neuropharmacology. 2005;48:627–38. doi: 10.1016/j.neuropharm.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Jezová D, Juránková E, Mosnárová A, Kriska M, Skultétyová I. Neuroendocrine response during stress with relation to gender differences. Acta Neurobiol Exp (Wars) 1996;56:779–85. doi: 10.55782/ane-1996-1183. [DOI] [PubMed] [Google Scholar]
- Johansson IM, Birzniece V, Lindblad C, Olsson T, Bäckström T. Allopregnanolone inhibits learning in the Morris water maze. Brain Res. 2002;934:125–31. doi: 10.1016/s0006-8993(02)02414-9. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Mallinson K, McCormick CM, Frye CA. Central allopregnanolone is increased in rat pups in response to repeated, short episodes of neonatal isolation. Brain Res Dev Brain Res. 2000;124:133–6. doi: 10.1016/s0165-3806(00)00106-1. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Frye CA. Endogenous levels of 5 alpha-reduced progestins and androgens in fetal vs. adult rat brains. Brain Res Dev Brain Res. 1999;115:17–24. doi: 10.1016/s0165-3806(99)00041-3. [DOI] [PubMed] [Google Scholar]
- King SR, Matassa AA, White EK, Walsh LP, Jo Y, Rao RM, Stocco DM, Reyland ME. Oxysterols regulate expression of the steroidogenic acute regulatory protein. J Mol Endocrinol. 2004;32:507–17. doi: 10.1677/jme.0.0320507. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Lambert KG. Reproduction-induced neuroplasticity: natural behavioural and neuronal alterations associated with the production and care of offspring. J Neuroendocrinol. 2008;4:515–25. doi: 10.1111/j.1365-2826.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Szumlinski KK, Kapasova Z, Rezner B, See RE. Prenatal stress enhances responsiveness to cocaine. Neuropsychopharmacology. 2008;33:769–82. doi: 10.1038/sj.npp.1301447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkness EF, Bovenkerk CF, Ueda T, Turner AJ. Phosphorylation of γ-aminobutyrate (GABA)/benzodiazepine receptors by cyclic AMP-dependent protein kinase. Biochem J. 1989;259:613–6. doi: 10.1042/bj2590613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Langmade SJ, Gale SE, Frolov A, Mohri I, Suzuki K, Mellon SH, Walkley SU, Covey DF, Schaffer JE, Ory DS. Pregnane X receptor (PXR) activation: a mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proc Natl Acad Sci U S A. 2006;103:13807–12. doi: 10.1073/pnas.0606218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Mellédo JM, Baker GB. Neuroactive steroids and anxiety disorders. J Psychiatry Neurosci. 2002;27:161–5. [PMC free article] [PubMed] [Google Scholar]
- Le!kiewicz M, Budziszewska B, Jaworska-Feil L, Kajta M, Laso! W. Effect of neurosteroids on glutamate binding sites and glutamate uptake in rat hippocampus. Pol J Pharmacol. 1998;50:355–60. [PubMed] [Google Scholar]
- Leuner B, Mirescu C, Noiman L, Gould E. Maternal experience inhibits the production of immature neurons in the hippocampus during the postpartum period through elevations in adrenal steroids. Hippocampus. 2007;17:434–42. doi: 10.1002/hipo.20278. [DOI] [PubMed] [Google Scholar]
- Lin YF, Angelotti TP, Dudek EM, Browning MD, Macdonald RL. Enhancement of recombinant α1 β1 γ2L γ-aminobutyric acidA receptor whole-cell currents by protein kinase C is mediated through phosphorylation of both β1 and γ2L subunits. Mol Pharmacol. 1996;50:185–95. [PubMed] [Google Scholar]
- Luisi S, Petraglia F, Benedetto C, Nappi RE, Bernardi F, Fadalti M, Reis FM, Luisi M, Genazzani AR. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85:2429–33. doi: 10.1210/jcem.85.7.6675. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38:379–95. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Maki PM, Rich JB, Rosenbaum RS. Implicit memory varies across the menstrual cycle: estrogen effects in young women. Neuropsychologia. 2002;40:518–29. doi: 10.1016/s0028-3932(01)00126-9. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 2001;74:435–40. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- Markou A, Duka T, Prelevic GM. Estrogens and brain function. Hormones (Athens) 2005;4:9–17. doi: 10.14310/horm.2002.11138. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994;4:82–9. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Martini L, Magnaghi V, Melcangi RC. Actions of progesterone and its 5alpha-reduced metabolites on the major proteins of the myelin of the peripheral nervous system. Steroids. 2003;68:825–9. doi: 10.1016/s0039-128x(03)00134-x. [DOI] [PubMed] [Google Scholar]
- Marvan ML, Chavez-Chavez L, Santana S. Clomipramine modifies fluctuations of forced swimming immobility in different phases of the rat estrous cycle. Arch Med Res. 1996;27:83–6. [PubMed] [Google Scholar]
- McCormick CM, Kehoe P, Mallinson K, Cecchi L, Frye CA. Neonatal isolation alters stress hormone and mesolimbic dopamine release in juvenile rats. Pharmacol Biochem Behav. 2002;73:77–85. doi: 10.1016/s0091-3057(02)00758-x. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Moss SJ. Differential phosphorylation of intracellular domains of γ-aminobutyric acid type A receptor subunits by calcium/calmodulin type 2-dependent protein kinase and cGMP-dependent protein kinase. J Biol Chem. 1994;269:18111–7. [PubMed] [Google Scholar]
- Mellon SH. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78:1003–8. doi: 10.1210/jcem.78.5.8175951. [DOI] [PubMed] [Google Scholar]
- Mellon SH. Neurosteroid regulation of central nervous system development. Pharmacol Ther. 2007;6:107–24. doi: 10.1016/j.pharmthera.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;3:283–92. doi: 10.1016/0006-8993(93)91332-m. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Gong W, Schonemann MD. Endogenous and synthetic neurosteroids in treatment of Niemann-Pick Type C disease. Brain Res Rev. 2008;57:410–20. doi: 10.1016/j.brainresrev.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, DeBold JF. Neurosteroids, GABAA receptors, and escalated aggressive behavior. Horm Behav. 2003;44:242–57. doi: 10.1016/j.yhbeh.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, De Bold JF, De Almeida RM. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology (Berl) 2002;163:434–58. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–5. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Murberg TA, Bru E. Social relationships and mortality in patients with congestive heart failure. J Psychosom Res. 2001;51:521–7. doi: 10.1016/s0022-3999(01)00226-4. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Alterations in behavioral and neuroendocrine stress coping strategies in pregnant, parturient and lactating rats. Prog Brain Res. 2001;133:143–52. doi: 10.1016/s0079-6123(01)33011-x. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci U S A. 2003;100:10506–11. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinol. 2002;143:205–12. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- Ogilvie KM, Rivier C. Gender difference in hypothalamic-pituitary-adrenal axis response to alcohol in the rat: activational role of gonadal steroids. Brain Res. 1997;766:19–28. doi: 10.1016/s0006-8993(97)00525-8. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor ! (ER!) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ER! mRNA. J Clin Endocrinol Metab. 2000;85:3840–6. doi: 10.1210/jcem.85.10.6913. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Lecanu L, Brown RC, Han Z, Yao ZX. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience. 2006;138:749–56. doi: 10.1016/j.neuroscience.2005.05.063. [DOI] [PubMed] [Google Scholar]
- Paris JJ, Frye CA. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 2008;136:105–15. doi: 10.1530/REP-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev VK, Almeida OF. Corticosteroid regulation of gene expression and binding characteristics of vasopressin receptors in the rat brain. Eur J Neurosci. 1995;7:1579–83. doi: 10.1111/j.1460-9568.1995.tb01153.x. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Hassan AH, Holsboer DF, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–40. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Shoaib M, Holsboer F, Almeida OF. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62:265–71. doi: 10.1016/0306-4522(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–22. [PubMed] [Google Scholar]
- Pawluski JL, Galea LA. Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience. 2007;149:53–67. doi: 10.1016/j.neuroscience.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Vanderbyl BL, Ragan K, Galea LA. First reproductive experience persistently affects spatial reference and working memory in the mother and these effects are not due to pregnancy or ‘mothering’ alone. Behav Brain Res. 2006;175:157–65. doi: 10.1016/j.bbr.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Payami H, Montee K, Grimslid H, Shattuc S, Kaye J. Increased risk of familial late-onset Alzheimer’s disease in women. Neurology. 1996;46:126–9. doi: 10.1212/wnl.46.1.126. [DOI] [PubMed] [Google Scholar]
- Pearlstein TB. Hormones and depression: what are the facts about premenstrual syndrome, menopause, and hormone replacement therapy? Am J Obstet Gynecol. 1995;173:646–53. doi: 10.1016/0002-9378(95)90297-x. [DOI] [PubMed] [Google Scholar]
- Pearlstein TB, Bachmann GA, Zacur HA, Yonkers KA. Treatment of premenstrual dysphoric disorder with a new drospirenone-containing oral contraceptive formulation. Contraception. 2005;72:414–21. doi: 10.1016/j.contraception.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Gerlach JL, McEwen BS, Ferin M, Carmel P, Zimmerman EA. Autoradiographic localization of hormone-concentrating cells in the brain of the female rhesus monkey. J Comp Neurol. 1976;170:279–93. doi: 10.1002/cne.901700302. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Variations in memory function and sex steroid hormones across the menstrual cycle. Psychoneuroendocrinology. 1992;17:497–506. doi: 10.1016/0306-4530(92)90008-u. [DOI] [PubMed] [Google Scholar]
- Pinna G, Agis-Balboa RC, Pibiri F, Nelson M, Guidotti A, Costa E. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochem Res. 2008;33:1990–2007. doi: 10.1007/s11064-008-9718-5. [DOI] [PubMed] [Google Scholar]
- Poser CM, Kassirer MR, Peyser JM. Benign encephalopathy of pregnancy. Preliminary clinical observations. Acta Neurol Scand. 1986;73:39–43. doi: 10.1111/j.1600-0404.1986.tb03239.x. [DOI] [PubMed] [Google Scholar]
- Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4:759–65. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Moore PH, Jr, Rao PN, Hagino N, Yamaguchi T, Schmidt P, Rubinow DR, Morrow AL, Paul SM. Radioimmunoassay of 3α-hydroxy-5α-pregnan-20-one in rat and human plasma. Steroids. 1990;55:290–6. doi: 10.1016/0039-128x(90)90031-6. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of γaminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci U S A. 1991;88:4553–7. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapkin AJ, Mikacich JA, Moatakef-Imani B, Rasgon N. The clinical nature and formal diagnosis of premenstrual, postpartum, and perimenopausal affective disorders. Curr Psychiatry Rep. 2002;4:419–28. doi: 10.1007/s11920-002-0069-7. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Pharmacology of catamenial epilepsy. Methods Find Exp Clin Pharmacol. 2004;26:547–61. doi: 10.1358/mf.2004.26.7.863737. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:337–44. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Metter EJ, Zonderman AB. Estrogen replacement therapy and longitudinal decline in visual memory. A possible protective effect? Neurology. 1997;49:1491–7. doi: 10.1212/wnl.49.6.1491. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Inhibiting progesterone metabolism in the hippocampus of rats in behavioral estrus decreases anxiolytic behaviors and enhances exploratory and antinociceptive behaviors. Cogn Affect Behav Neurosci. 2001;1:287–96. doi: 10.3758/cabn.1.3.287. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Rubin RT. Functional sex differences (‘sexual diergism’) of central nervous system cholinergic systems, vasopressin, and hypothalamic-pituitary-adrenal axis activity in mammals: a selective review. Brain Res Brain Res Rev. 1999;30:135–52. doi: 10.1016/s0165-0173(99)00011-9. [DOI] [PubMed] [Google Scholar]
- Romeo E, Ströhle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–3. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Braswell L, Stein DG. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. 1994;129:64–9. doi: 10.1006/exnr.1994.1147. [DOI] [PubMed] [Google Scholar]
- Rosenberg L, Park S. Verbal and spatial functions across the menstrual cycle in healthy young women. Psychoneuroendocrinology. 2002;27:835–41. doi: 10.1016/s0306-4530(01)00083-x. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–6. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- Schlichter R, Keller AF, De Roo M, Breton JD, Inquimbert P, Poisbeau P. Fast nongenomic effects of steroids on synaptic transmission and role of endogenous neurosteroids in spinal pain pathways. J Mol Neurosci. 2006;28:33–51. doi: 10.1385/jmn:28:1:33. [DOI] [PubMed] [Google Scholar]
- Schmid G, Sala R, Bonanno G, Raiteri M. Neurosteroids may differentially affect the function of two native GABA(A) receptor subtypes in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:401–7. doi: 10.1007/pl00005185. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;33:209–16. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Schwartz-Giblin S, Pfaff DW. Hypothalamic output controlling reticulospinal and vestibulospinal systems important for emotional behavior. Int J Neurol. 1986;19:89–110. [PubMed] [Google Scholar]
- Seeman TE, Kaplan GA, Knudsen L, Cohen R, Guralnik J. Social network ties and mortality among the elderly in the Alameda County Study. Am J Epidemiol. 1987;126:714–23. doi: 10.1093/oxfordjournals.aje.a114711. [DOI] [PubMed] [Google Scholar]
- Seippel L, Bäckström T. Luteal-phase estradiol relates to symptom severity in patients with premenstrual syndrome. J Clin Endocrinol Metab. 1998;83:1988–92. doi: 10.1210/jcem.83.6.4899. [DOI] [PubMed] [Google Scholar]
- Selye H. Anesthetic effects of steroid hormones. Proceed Soc Experiment Biolog Med. 1941;46:116–121. [Google Scholar]
- Sherwin BB. Progestogens used in menopause. Side effects, mood and quality of life. J Reprod Med. 1999;44:227–32. [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–6. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Regulation of progesterone receptor messenger ribonucleic acid in the rat medial preoptic nucleus by estrogenic and antiestrogenic compounds: an in situ hybridization study. Endocrinology. 1997;138:5476–84. doi: 10.1210/endo.138.12.5595. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK, Micevych P. Estrogen induces de novo progesterone synthesis in astrocytes. Dev Neurosci. 2003;5:343–348. doi: 10.1159/000073511. [DOI] [PubMed] [Google Scholar]
- Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci. 1999;19:2455–63. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine. 2001;14:407–15. doi: 10.1385/ENDO:14:3:407. [DOI] [PubMed] [Google Scholar]
- Singh M. Mechanisms of progesterone-induced neuroprotection. Ann N Y Acad Sci. 2005;1052:145–51. doi: 10.1196/annals.1347.010. [DOI] [PubMed] [Google Scholar]
- Smith CD, Wekstein DR, Markesbery WR, Frye CA. 3α,5α-THP: a potential plasma neurosteroid biomarker in Alzheimer’s disease and perhaps non-Alzheimer’s dementia. Psychopharmacol. 2006;18:481–5. doi: 10.1007/s00213-005-0186-1. [DOI] [PubMed] [Google Scholar]
- Solis-Ortiz S, Guevara MA, Corsi-Cabrera M. Performance in a test demanding prefrontal functions is favored by early luteal phase progesterone: an electroencephalographic study. Psychoneuroendocrinology. 2004;29:1047–57. doi: 10.1016/j.psyneuen.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Sex therapy in psychiatric treatment has a new partner: reproductive hormones. J Clin Psychiatry. 1997;58:468–9. doi: 10.4088/jcp.v58n1101. [DOI] [PubMed] [Google Scholar]
- Stroebe MS, Hansson RO, Stroebe W, Schut HAW. Handbook of bereavement research, consequences, coping, and care. 2001. [Google Scholar]
- Stroebe M, Schut H, Stroebe W. Health outcomes of bereavement. Lancet. 2007;370:1960–73. doi: 10.1016/S0140-6736(07)61816-9. [DOI] [PubMed] [Google Scholar]
- Ströhle A, Romeo E, di Michele F, Pasini A, Hermann B, Gajewsky G, Holsboer F, Rupprecht R. Induced panic attacks shift γ-aminobutyric acid type A receptor modulatory neuroactive steroid composition in patients with panic disorder: preliminary results. Arch Gen Psychiatry. 2003;60:161–8. doi: 10.1001/archpsyc.60.2.161. [DOI] [PubMed] [Google Scholar]
- Sundström I, Bäckström T. Citalopram increases pregnanolone sensitivity in patients with premenstrual syndrome: an open trial. Psychoneuroendocrinology. 1998a;23:73–88. doi: 10.1016/s0306-4530(97)00064-4. [DOI] [PubMed] [Google Scholar]
- Sundström I, Bäckström T. Patients with premenstrual syndrome have decreased saccadic eye velocity compared to control subjects. Biol Psychiatry. 1998b;44:755–64. doi: 10.1016/s0006-3223(98)00012-2. [DOI] [PubMed] [Google Scholar]
- Sundström I, Bäckström T, Wang M, Olsson T, Seippel L, Bixo M. Premenstrual syndrome, neuroactive steroids and the brain. Gynecol Endocrinol. 1999;13:206–20. doi: 10.3109/09513599909167557. [DOI] [PubMed] [Google Scholar]
- Tait GR, McManus K, Bellavance F, Lara N, Chrapko W, Le Mellédo JM. Neuroactive steroid changes in response to challenge with the paγnicogenic agent pentagastrin. Psychoneuroendocrinology. 2002;27:417–29. doi: 10.1016/s0306-4530(01)00051-8. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Tend and Befriend: Biobehavioral Bases of Affiliation Under Stress. Curr Dir Psychol Sci. 2008;15:273–277. [Google Scholar]
- Thompson LW, Breckenridge JN, Gallagher D, Peterson J. Effects of bereavement on self-perceptions of physical health in elderly widows and widowers. J Gerontol. 1984;39:309–14. doi: 10.1093/geronj/39.3.309. [DOI] [PubMed] [Google Scholar]
- Townsend KA, Marlowe KF. Relative safety and efficacy of finasteride for treatment of hirsutism. Ann Pharmacother. 2004;38:1070–3. doi: 10.1345/aph.1D461. [DOI] [PubMed] [Google Scholar]
- Troncoso JC, Martin LJ, Dal Forno G, et al. Neuropathology in controls and demented subjects from the Baltimore Longitudinal Study of Aging. Neurobiol Aging. 1996;17:365–71. doi: 10.1016/0197-4580(96)00028-0. [DOI] [PubMed] [Google Scholar]
- Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci USA. 1996;93:12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Sampson L, Uzunov DP. Relevance of endogenous 3!-reduced neurosteroids to depression and antidepressant action. Psychopharmacology. 2006;186:351–61. doi: 10.1007/s00213-005-0201-6. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A. 1998;95:3239–44. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Wrynn AS, Kinnunen A, Ceci M, Kohler C, Uzunov DP. Chronic antidepressants reverse cerebrocortical allopregnanolone decline in the olfactory-bulbectomized rat. Eur J Pharmacol. 2004;486:31–4. doi: 10.1016/j.ejphar.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Vallée M, Rivera JD, Koob GF, Purdy RH, Fitzgerald RL. Quantification of neurosteroids in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/mass spectrometry. Anal Biochem. 2000;287:153–66. doi: 10.1006/abio.2000.4841. [DOI] [PubMed] [Google Scholar]
- Van Wimersma Greidanus TB. Pregnene-type steroids and impairment of passive avoidance behavior in rats. Horm Behav. 1977;9:49–56. doi: 10.1016/0018-506x(77)90049-6. [DOI] [PubMed] [Google Scholar]
- Vongher JM, Frye CA. Progesterone in conjunction with estradiol has neuroprotective effects in an animal model of neurodegeneration. Pharmacol Biochem Behav. 1999;64:777–785. doi: 10.1016/s0091-3057(99)00140-9. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Estradiol decreases anxiety behavior and enhances inhibitory avoidance and gestational stress produces opposite effects. Stress. 2007;10:251–60. doi: 10.1080/00958970701220416. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006a;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Sumida K, Frye CA. Inhibiting 5α-reductase in the amygdala attenuates antianxiety and antidepressive behavior of naturally receptive and hormone-primed ovariectomized rats. Psychopharmacology (Berl) 2006b;186:302–11. doi: 10.1007/s00213-005-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Irwin RW, Liu L, Chen S, Brinton RD. Regeneration in a degenerating brain: potential of allopregnanolone as a neuroregenerative agent. Curr Alzheimer Res. 2007;4:510–7. doi: 10.2174/156720507783018262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Marx CE, Morrow AL, Wilson WA, Moore SD. Neurosteroid modulation of GABAergic neurotransmission in the central amygdala: a role for NMDA receptors. Neurosci Lett. 2007;415:118–23. doi: 10.1016/j.neulet.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Seippel L, Purdy RH, Bäckström T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5!-pregnane-3,20-dione and 3!-hydroxy-5!-pregnan-20-one. J Clin Endocrinol Metab. 1996;81:1076–1082. doi: 10.1210/jcem.81.3.8772579. [DOI] [PubMed] [Google Scholar]
- Weill-Engerer S, David JP, et al. Neurosteroid quantification in human brain regions: comparison between Alzheimer’s and nondemented patients. J Clinical Endocrinol Metabol. 2002;87:5138–43. doi: 10.1210/jc.2002-020878. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65:427–51. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem Res. 2007;32:1730–40. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- Weir CJ, Ling AT, Belelli D, Wildsmith JA, Peters JA, Lambert JJ. The interaction of anaesthetic steroids with recombinant glycine and GABAA receptors. Br J Anaesth. 2004;92:704–11. doi: 10.1093/bja/aeh125. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci U S A. 2003;100:2237–42. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou LB, Yamada K, Sasa M, Nakata Y, Nabeshima T. Effects of σ1 receptor agonist SA4503 and neuroactive steroids on performance in a radial arm maze task in rats. Neuropharmacology. 2000;39:1617–27. doi: 10.1016/s0028-3908(99)00228-2. [DOI] [PubMed] [Google Scholar]
- Zwanzger P, Eser D, Padberg F, Baghai TC, Schüle C, Rupprecht R, di Michele F, Romeo E, Pasini A, Ströhle A. Neuroactive steroids are not affected by panic induction with 50 microg cholecystokinin-tetrapeptide (CCK-4) in healthy volunteers. J Psychiatr Res. 2004;38:215–7. doi: 10.1016/s0022-3956(03)00109-2. [DOI] [PubMed] [Google Scholar]