Abstract
(+)-Germacrene A, an important intermediate in sesquiterpene biosynthesis, was isolated in pure form from a genetically engineered yeast and was characterized by chromatographic properties (TLC, GC), MS, optical rotation, UV, IR, 1H NMR and 13C NMR data. Variable-temperature 500 MHz 1H NMR spectra in CDCl3 showed that this flexible cyclodecadiene ring exists as three NMR-distinguishable conformational isomers in a ratio of about 5:3:2 at or below ordinary probe temperature (25° C). The conformer structures were assigned by 1H NMR data comparisons, NOE experiments, and vicinal couplings as follows: 1a (52%, UU), 1b (29% UD), and 1c (19%, DU).
1. Introduction
The germacrene sesquiterpenes, (+)-germacrene A (1) and (+)-hedycaryol (2), are structurally characterized by the flexible trans, trans-cyclodeca-1(10), 4(5)-diene ring system, and are believed to be important intermediates in the biosynthesis of several classes of sesquiterpenes including the germacranolides, eudesmanes, eremophilanes, spirovetivanes, and guaianes (Scheme 1).1 The biosynthesis of the bicyclic sesquiterpenes can be easily rationalized by enzymatic protonation of either the Δ1(10) or Δ4 double bond of 1 and 2 followed by cyclization (intramolecular C5-C10 or C1-C5 bond formation), rearrangements and final quenching of the carbocations by deprotonation or nucleophilic capture of a water molecule as the final step.2
Scheme 1.
Sesquiterpene families derived from (EE)-farnesyl diphosphate through (7R)-germacrene A (1) and (7R)-hedycaryol (2).
(+)-Germacrene A (1) is considered to be the biogenetic precursor of (+)-costunolide (3) in chicory,3 the simplest member of the large group of naturally occurring germacranolides,4 and an important natural product itself owing to its role as a common precursor of all gemacranolide-derived lactones (ie., guaianolides and eudesmanolides).5 In addition, germacrene A has often been postulated as an intermediate in the biosynthesis of phytoalexins6 including capsidiol,7 debneyol,7a,b,c costunolide,3a 8 vetispiradienes (lubimin and rishitin),9 solavetivone10 and lettucenin A.8 11
Germacrene A (1) has also been proposed as an enzyme-bound intermediate in the biosynthesis of the sesquiterpene alcohol patchoulol,12 and the bicyclic hydrocarbons aristolochene13 and 5-epi-aristolochene.7c, 14 A small amount of germacrene A (7.5%) is in fact released by aristolochene synthase.15a Further evidence for the intermediacy of germacrene A in the production of bicyclic sesquiterpenes has been obtained through site-directed mutagenesis of aristocholene and tobacco epi-aristolochene synthases and isolation of major amounts of the monocyclic precursor,14d, 15 and by formation of dihydrogermacrene A using the substrate analogue 6,7-dihydrofarnesyl diphosphate.16
Both (7R)- and (7S)-germacrene A enantiomers are known and presumed to be precursors of the stereochemically related sesquiterpenes through cyclizations and oxidative metabolism. Both enantiomers are found in many essential oils,17 indicating the widespread occurrence of the germacrene synthases that produce and release the enantiomerically pure 10-membered ring sesquiterpenes. To date, germacrene A synthases have been isolated, purified, and characterized from chicory roots,3a, 18 and Ixeris dentata,19 and the cyclases from lettuce,20 goldenrod (Solidago canadensis),21 Artemisia annua,22 and Crepidiastrum sonchifolium23 have been cloned and expressed in Escherichia coli.
The isolation of pure (E, E)-germacrenes from natural sources is often complicated by the sensitivity of these sesquiterpenes to acidic conditions and elevated temperatures. During distillation and GC analysis germacrene A is known to undergo facile Cope rearrangement to β-elemene.24 In some instances, the absolute configuration of both (+)-1 and (−)-1 has been established by means of Cope rearrangement on chiral GC columns able to distinguish the β-elemene enantiomers.3a, 25 Adsorption on silica gel induces transannular cyclization of (+)-germacreme A to a mixture of eudesmanes: (−)-selin-11-en-4-ol, (−)-α-selinene, (+)-selinene and (+)-selina-4,11-diene.26 The instability of germacrene A upon storage at freezer temperatures has also been reported.24a, 24c, 27
(−)-Germacrene A was first isolated in pure form from the gorgonian Eunicea mammosa24a and subsequently from a soft coral of the genus Lobophytum.28 (−)-Germacrene A of terrestrial origin was extracted from the aphid Therioaphis maculala and identified as an alarm pheromone.24c, 29 The major sesquiterpene component (10 µg/inset) of the defensive secretions of soldiers termites proved to be (−)-1,30 and in some instances, this fact has been used as a chemotaxonomic marker.31 To our knowledge, the occurrence of (+)-germacrene A seems to be limited to higher plants, and few reports describe its isolation.26,32
The cyclic sesquiterpene was first characterized by Weinheimer et al.24a by means of IR and MS data, 60 MHz proton NMR (CCl4) spectra, and an optical rotation [α]D −3.2° (c 14.4, CCl4).* Nishino et al.24c published the first 13C NMR data (25 MHz, CDCl3) for terrestrial (−)-1 and reported that the 1 MHz 1H NMR spectrum (CDCl3, 30° C) of germacrene A showed broad signals pointing to the possibility of a mixture of conformers. High field 1H and 13C NMR data were recently published by Adio et al.26 using an impure sample of (+)-1 isolated from S. canadensis.33
In solution, most simple (E, E)-germacrene sesquiterpenes (eg. germacrene B and hedycaryol) behave as several interconvertible conformational isomers in equilibrium,24b, 34 which usually results in broadened NMR signals or even multiple sets of NMR signals.24a, 24b, 35 The conformation of these flexible 10- membered ring sesquiterpenes played a fundamental role in the proposals concerning the biogenesis of other types of sesquiterpenes by Ruzicka,36 Barton and de Mayo,37 Hendrickson38 and Parker et al.39 and is a critical element in the current mechanisms of action of sesquiterpene cyclases.2
The present work was initiated by the isolation of considerable amounts of (+)-germacrene A (1) from an engineered yeast, the lack of reliable characterization data in the literature, and the inability to obtain an authentic sample for direct comparisons. GC analysis showed a major, sharp peak, preceded by a significantly smaller, hump-shaped peak having a retention time similar to that of germacrene A.3a This GC behavior is typically attributed to the occurrence of Cope rearrangement on the column.3a, 20, 24, 26 Although the MS* and 13C NMR data were very similar to the limited literature data, the presence of broad 1H NMR signals prevented assessment of purity and confident structure and absolute configurational assignments.
In view of the prominent position of germacrene A in sesquiterpene biosynthesis and the conflicting data in the literature, we decided to investigate the behavior of this C15 cyclodecadiene sesquiterpene by means of variable temperature NMR spectroscopy.
2. Results and discussion
2.1 Engineering yeast for germacrene A biosynthesis
As part of our continuing effort to dissect the catalytic features of terpene synthases by molecular comparisons between distinct synthases,40 we carried out a bioinformatic screen of the lettuce genomic database (http://compositdb.ucdavis.edu/database) and uncovered a putative, but unique terpene synthase gene. While the lettuce gene showed sequence homology to 5-epi-aristolochene synthase, it also showed similarity to several other well-characterized terpene synthases including germacrene synthases isolated from chicory (Cichorium intybus),18 lettuce (Lattuca sativa),20 and goldenrod (Solidago canadensis).21 To functionally characterize this putative terpene synthase, we isolated a full-length cDNA copy of the mRNA using an RT-PCR method,41 inserted it into a modified Yep352-URA3 yeast expression vector under the transcriptional control of a modified ADH promoter, and transformed the yeast strain Cali7-1 according to Takahashi et al.42 Cali7-1 is a yeast line genetically engineered for high-level production of farnesyl diphosphate, the substrate for sesquiterpene synthases. Cali7-1 also requires an exogenous supply of uracil for growth owing to a genetic mutation in the endogenous URA3 gene that is essential for uracil biosynthesis.
2.2. Isolation and characterization of germacrene A
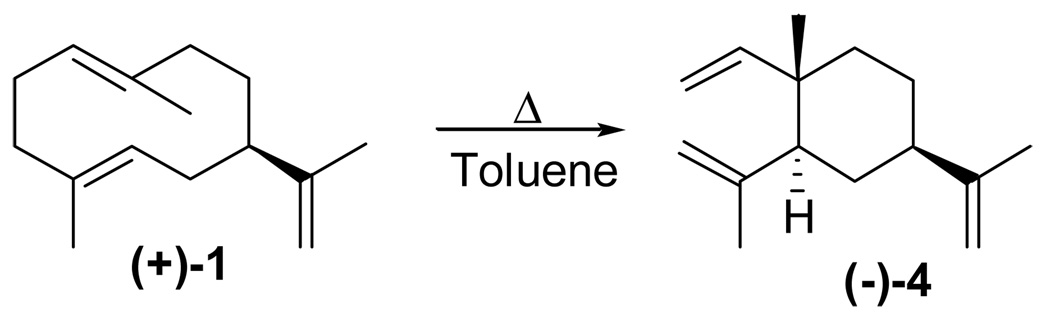
The hexane extract from an incubation of the engineered yeast was evaporated, and the resulting residue was re-dissolved in pentane and filtered through a small column of silica gel at room temp. Further purification by preparative TLC (silica gel) at room temp. afforded what proved to be pure (+)-germacrene A (1) as a clear oil, [α]D + 42.1° (CCl4). GC analysis (method A, see Experimental Section) showed a major peak at Rt 38 min and a small, ill-defined peak with a variable Rt of 29–37 min, both accounting for 97 % of the integration, and another small peak at Rt 28 min (3 %). The hump at 29–37 min in the chromatogram is characteristic of cyclodeca-1,5-dienes and indicates the occurrence of heat-induced Cope rearrangement to the corresponding β-elemenes (peak at Rt 28 min) during GC analysis. When the injection port temp. was increased to 180° C (method B), the composition was β-elemene (4, 49 %) and germacrene A (1, 50 %). The structure and absolute configuration were confirmed by Cope rearrangement to (−)-β-elemene (4, [α]D −15.8°; Lit.43 −11.8°; Lit.44 +15.4° for the enantiomer) brought about by heating a solution of (+)-germacrene A in toluene at reflux (Scheme 2). The MS of the β-elemene showed the molecular ion at m/z 204 (M+, 3%, C15H24) and a fragmentation pattern very similar to that recently described for (−)-β-elemene.32 Furthermore, the 1H and 13C NMR data of the rearrangement product thus obtained are identical to those previously reported by Brauchli and Thomas.45
Scheme 2.
Cope rearrangement of germacrene A to β-elemene.
The measured optical rotation of + 42.1° for germacrene A in the present work differs notably from the alues (−3.2° and −26.8°) published earlier for the (−)-enantiomer,24a,c perhaps indicating that the germacrene A obtained previously was impure or was a mixture of both enantiomers. The IR spectrum was very similar to that described by Nishino et al.24c except for a doublet absorption (1385 and 1375 cm−1) characteristic of the isopropenyl group observed at 1275 and 1261 cm,−1 respectively, in the present study. Although the absence of UV absorption above 200 nm for the (−)-enantiomer was noted by Weinheimer et al.,24a a weak absorption at λmax 214 nm (log ε = 2.50) was observed for (+)- germacrene A suggesting the possibility of a transannular interaction between the double bonds,46 similar to those postulated for germacrol (λmax 210 nm), germacrone (λmax 213 nm) and costunolide (λmax 213 nm).47
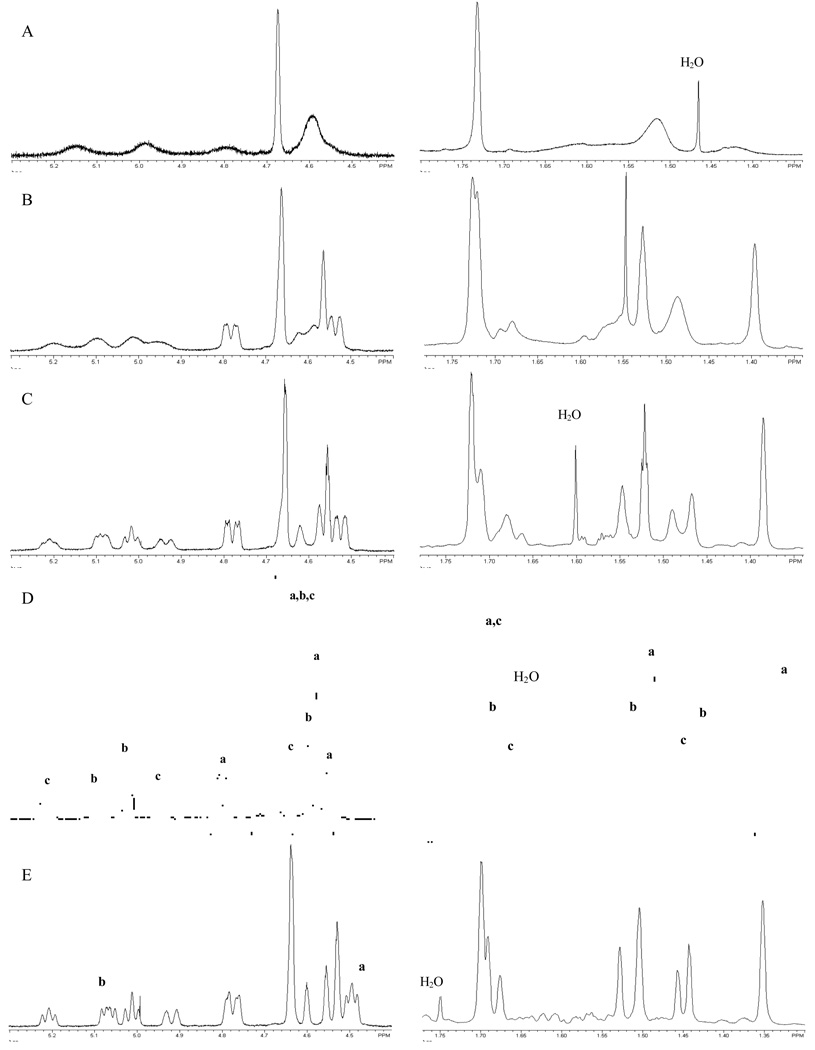
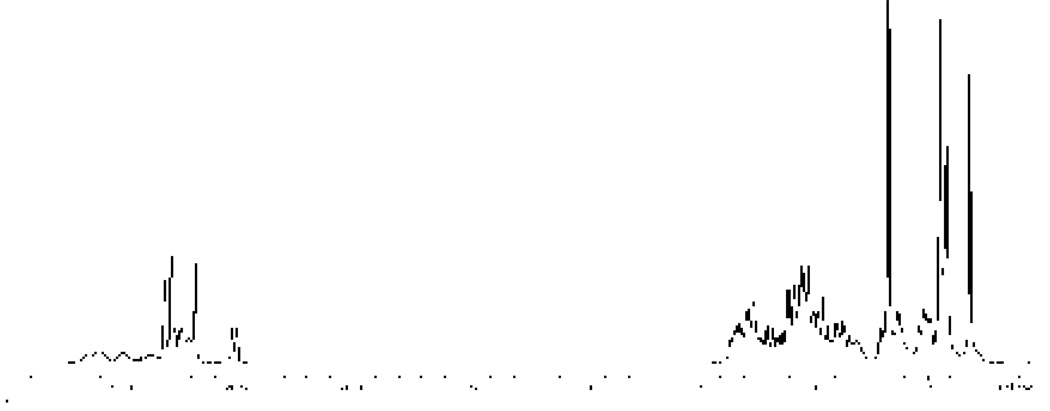
The 500 MHz 1H NMR spectrum of (+)-germacrene A was recorded at room temp. in both CDCl3 (Figure 1B) and C6D6 (Figure 2) for direct comparison of the data (see Table 1) with the published values. In CDCl3, the downfield olefinic region showed broad peaks for the vinyl protons at δH 5.26-5.15 (s, 1H, 19%), 5.15-5.05 (s, 1H, 28%), 5.05-4.90 (m, 2H, 47%), 4.78 (d, J = 11.5 Hz, 1H, 53%), 4.66 (s, 3H, 100%), 4.62 (s, 1H, 19%), 4.58 (s, 1H, 28%), 4.56 (s, 1H, 53%) and 4.53 (d, J = 10.0 Hz, 1H, 53%) ppm.48 Broad CH3 singlets were observed at 1.73, 1.71, 1.55, 1.48 and 1.39 ppm. The large number of individual NMR signals (three sets) led us to consider the possibility that in solution this sesquiterpene (1) in fact exists as a mixture of three interconvertible conformers in ca 5:3:2 (52:29:19) ratio at room temp. This behavior was previously suggested for (−)-germacrene A24c and later demonstrated for hedycaryol.35b
Figure 1.
1H NMR (500 MHz) spectra of (+)-germacrene A at different temperatures: A) 50° C, B) 25° C, C) 0° C, D) −20° C, and E) −50° C. The letters a, b and c designate peaks for three conformers a, b and c.
Figure 2.
1H NMR spectrum (500 MHz, C6D6) of (+)-germacrene A at room temperature.
Table 1.
500 MHz 1H NMR spectral data and assignments for (+)-germacrene A (1) at 25° C.
| protona | CDC13 δHb | C6D6 δHb |
|---|---|---|
| 5.26-5.15 (m) | 5.22-5.05 (m) | |
| 5.15-5.05 (m) | ||
| 5.05-4.98 (m) | 5.04-4.94 (m) | |
| 4.98-4.90 (m) | 4.94-4.84 (m) | |
| 4.81 (s) | ||
| 4.78 (brd, 11.5) | ||
| 12 | 4.67 (s) | 4.79 (s) |
| 4.65-4.58 (m) | ||
| 1 | 4.78-4.69 (m) | |
| 12’ | 4.57 (s) | 4.68 (s) |
| 5 | 4.54 (brd, 10.0) | 4.52 (brd, 10.5) |
| 2,3,6,7,9 | 2.44-1.78 (m)c | 2.48-1.75 (m)c |
| 8α | 1.73-1.59 (m) | |
| 13 | 1.73 (d, 2.5) | 1.67 (s) |
| 1.53 (brs) | ||
| 8β | 1.55-1.57(m) | |
| 15 | 1.49 (brs) | 1.45 (brs) |
| 1.42 (brs) | ||
| 14 | 1.40 (brs) | 1.31 (brs) |
Assignments based on ref 26.
Multiplicity and J values in Hertz are given in parenthesis
Nine allylic protons
When the 500 MHz 1H NMR spectrum of 1 was recorded at 50° C (Figure 1A), the olefinic region showed broad peaks at 5.21-5.07 (m, 1H), 5.06-4.90 (m, 1H), 4.86-4.73 (m, 1H), 4.68 (s, 2H), and 4.65-4.50 (s, 3H) revealing an equilibrium mixture of two conformers in ca 4:3 ratio. In addition, two broad CH3 singlets at high field 1.73 and 1.52 ppm (6H and 12H) were observed. These NMR values match very well those previously described by Nishino et al.24c at the same temp.: ie 5.2-4.75 (2H), 4.65 (1H), 4.58 (1H), 1.72 (3H), and 1.52 (6H) ppm.
The 500 MHz 1H NMR data (25° C) of germacrene A in C6D6 (Figure 2, Table 1) agree with the limited set of data recently reported by Adio et al.26 However, in addition to the peaks described by these authors, broad signals were also observed downfield in the 5.16-4.76 ppm region (Figure 2), and one extra methyl singlet at higher field (1.38) was also noticeable.
The chemical shifts for the more intense signals in the 13C NMR spectrum (125 MHz, 25° C) in C6D6 (see Experimental Section) are almost identical to those previously recorded and assigned.26 However, eighteen other peaks now attributed to the minor conformers were not reported, presumably because they might have arisen from impurities known to have been present in the sample.33 Relatively strong resonances for three methyl groups at 15.4, 19.9, and 24.2 ppm observed in the 13C NMR spectrum of compound 1 in the present work would then belong to one or both of the minor conformers.
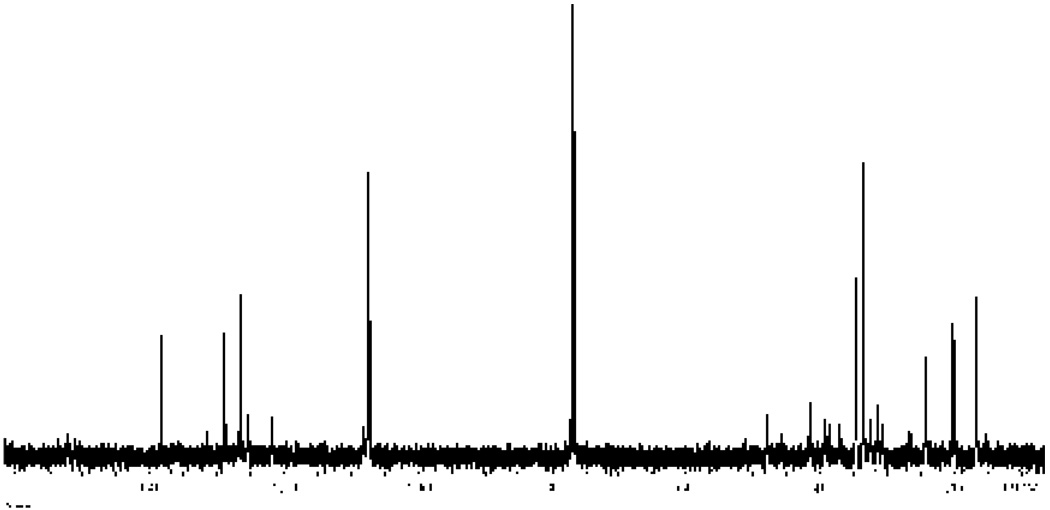
The 13C NMR spectrum (125 MHz, 25° C) of (+)-germacrene A (1) in CDCl3 (see Figure 3 and Experimental Section) is very similar to that recorded in C6D6. The 36 resonances observed contrast with the limited 13C NMR data reported in CDCl3 (25 MHz, 25° C) by Nishino et al,24c although the shifts previously reported for the trigonal carbons are in good agreement with our data. Interestingly, the peak at 37.0 ppm assigned to C9 by these authors appeared as a minor peak in both CDCl3 and C6D6. In addition, the peak at 41.4 ppm first assigned to C7 has been recently reassigned to C9 by 2D NMR experiments in benzene.26
Figure 3.
13C NMR spectrum (125 MHz, CDCl3) of germacrene A at room temperature. A total of 36 separate signals are attributed to germacrene A, 15 of which are ascribed to the major conformer based on previous 2-dimensional correlations with the 1H NMR spectrum in benzene.26
2.3. Conformational analysis
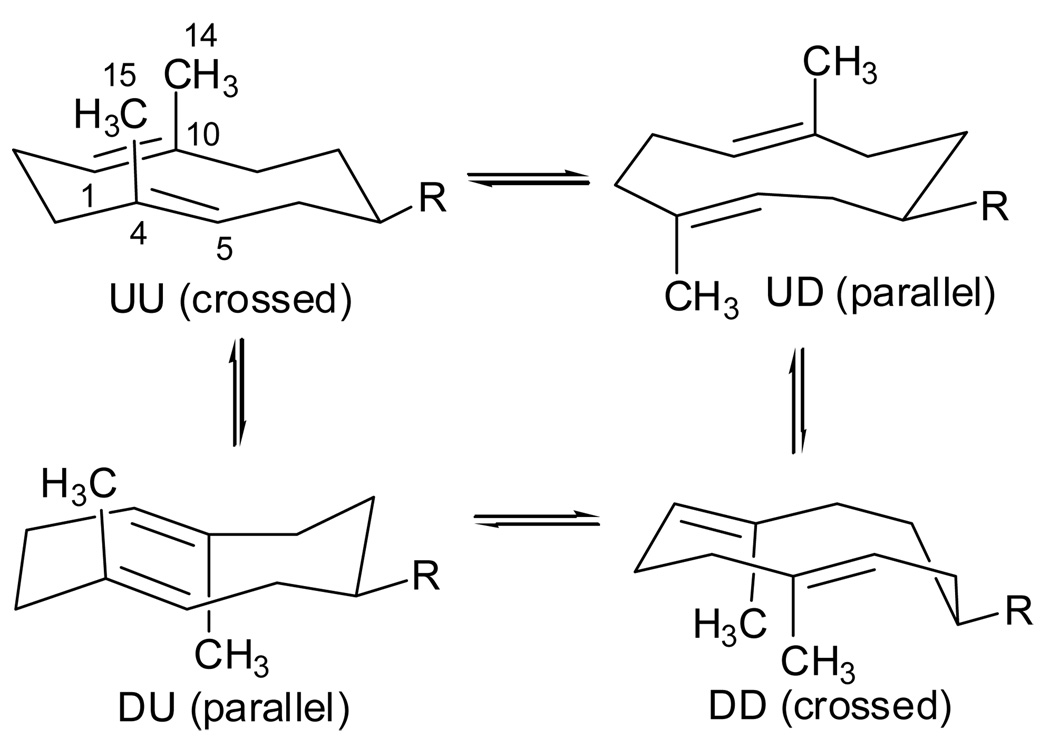
The 1H NMR spectrum (CDCl3) of the (E, E)-configured sesquiterpene exhibited broad signals at room temp. (Figure 1B) and suggested a conformational equilibrium in solution (Figure 4). If the isopropenyl group of 1 is large enough to ensure an equatorial or pseudo-equatorial position on the cyclodecadiene ring, germacrene A (1) can, therefore, adopt any of four distinct conformations, namely UU, UD, DU and DD (Figure 4).34, 35b
Figure 4.
Possible conformations of germacradiene sesquiterpenes such as germacrene A (1) and hedycaryol (2) fixed by the pseudo-equatorial position of the relatively large substituents (R = isopropenyl and hydroxypropyl). The conformers are denoted as UU, UD, DU and DD in reference to the U (up) and D (down) orientations of C10 and C4 methyl groups on the ten-membered ring.34, 35b
These conformations have either a parallel (UD and DU) or crossed (UU and DD) relationship of the double bonds, and all four conformers are interconvertible by rotations of the 1,10 and 4,5 double bonds through the ring, and by rotations of the C6-C7-C8 segment. In the ground state, Allinger MM2 calculations49 predict that conformers UD and DD should be almost isoenergetic and about 1.4–1.5 kcal/mol higher in strain energy than the UU isomer (Table 2), and the DU conformer is predicted to be the least stable with a relative strain energy of 2.65 kcal/mol. These values agree with previous MM1 calculations for germacrene A, which also predicted the UU and UD conformations to be more stable and more populated (62 % UU and 36 % UD) at room temp.50 In addition, our MM2 calculations for the similar (E, E)-cyclodecadiene sesquiterpenoids (Table 2) are in good agreement with those previously reported for germacrene B,34b 51 hedycaryol,50, 52 11,12-dehydrogermacrene A,53 and costunolide (3).34b,53
Table 2.
Relative steric energies (kcal/mol) of the four conformers (see Fig. 4) of germacrene A and related germacrene-type sesquiterpenes calculated by the Allinger MM2 force field method in the present work. Populations (%) at 25 °C are given in parenthesis for germacrene A.a
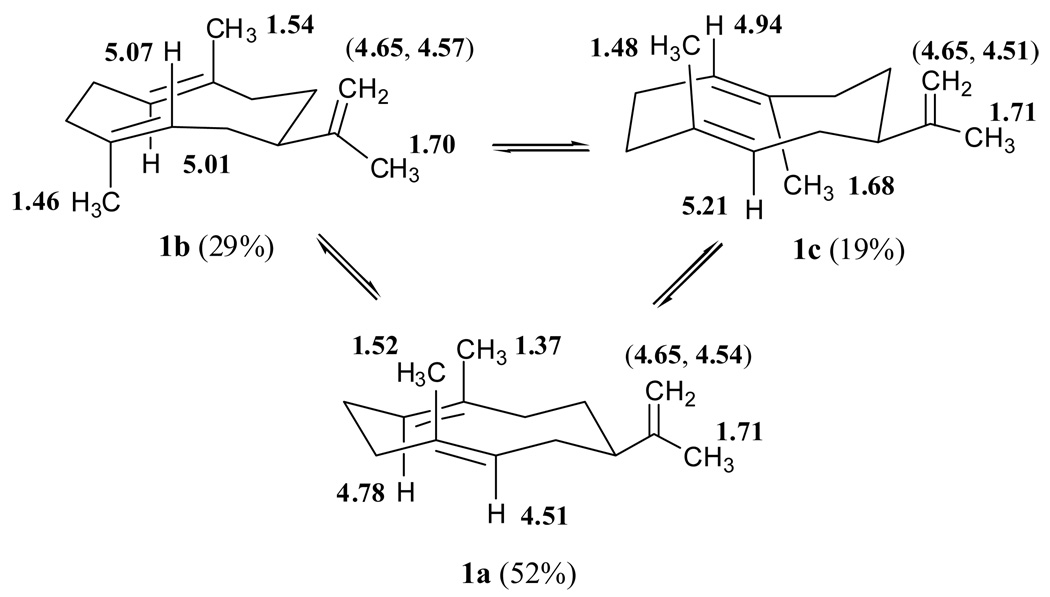
A variable-temperature 500 MHz 1H-NMR study of (+)-germacrene A (1) was carried out in order to verify the co-existence of more than one conformer on the NMR time scale. At low temperatures, the rate of rotation around the 1,10 and 4,5 double bonds would be slowed down, and in fact less broadening and well separated multiple signals corresponding to a mixture of three conformers were observed (Figure 1, C, D and E). At −20° C, the vinyl protons (H1, H5 and H12) appear as six downfield resonances indicating the presence of at least three NMR-distinguishable conformational isomers of similar energy in a ratio of 52:29:19 (Figure 1D, Table 3).48 In addition, the 1H NMR spectrum at −20° C showed eight high field singlets for the vinyl methyls, which were fully separated at temperatures lower than 0° C (Figure 1D and E). Interestingly, the multiplicity of the vinyl proton centered at 4.51 ppm (broad d) seems to be temperature-dependent, becoming a triplet (J = 6.5 Hz) at temperatures below −30° C (Figure 1E, a). Similarly, the unsymmetrical triplet centered at 5.07 ppm starts to develop as a doublet of doublets with approximate J values of 9.5 and 6.6 Hz at temperatures lower than −20° C (Figure 1E, b).
Table 3.
Selected 500 MHz 1H NMR data (CDCl3, −20° C) for the (+)-germacrene A (1) conformers a (52%), b (29%), and c (19%), (δH, m, (J in Hz)).
| H-12 | H-12’ | H-13 | H-14 | H-15 | H-1 | H-5 | |
|---|---|---|---|---|---|---|---|
| a | 4.65 s | 4.54 s | 1.71 s | 1.37 s | 1.52 s | 4.78 dd (11.2, 4.0) | 4.51 d (10.0) |
| b | 4.65 s | 4.57 s | 1.70 s | 1.54 s | 1.46 s | 5.01 t (8.1) | 5.07 dd (9.5, 6.6)a |
| c | 4.65 s | 4.51 s | 1.71 s | 1.68 s | 1.48 s | 4.94 d (12.1) | 5.21 t (7.9) |
Multiplicity and J values at −30° C.
A previous variable-temperature 1H NMR study of hedycaryol (2) at 60 MHz by Wharton and co-workers35b showed that this related sesquiterpene alcohol exists in solution mainly as one crossed and two parallel conformers (DU and UD). The latter were favored at −30 °C to the extent of 75%. The crossed and parallel conformers were distinguished by the relative high field absorption of the H5 vinyl hydrogen at 4.4 ppm (d, J = 10 Hz) in the crossed conformer due to the shielding effect of the opposing double bond. The assignments of the methyl groups for germacrene sesquiterpenes were established by Sathe et al.54 and corroborated by Wharton et al.35b using deuterated dihydropregeijerenes to identify the olefinic methyl singlets (1.32 (H14) and 1.48 (H15)) for the predominant crossed (UU) conformer. However, evidently the lower chemical shift dispersion at 60 MHz was insufficient for more precise determination of the populations of the three hedycaryol conformers.
Based on these considerations, we assigned the most stable conformer (52% at −20°, Fig. 1 panel D) of germacrene A (1), which exhibits a relatively high field 1H NMR signal at 4.51 (d, J = 10.0 Hz, H5) and a methyl singlet at 1.37 (H14) ppm, to the UU conformation (a in Fig. 1D and 1a in Table 3), as previously suggested for one conformer of hedycaryol and dihydropregeijerene.35b Accordingly, the other two downfield sets of 1H NMR signals observed in the spectra at low temperatures (Figure 1, Table 3) correspond to the less populated b (29%) and c (19%) conformers of germacrene A, which are predicted to have the parallel orientation of double bonds as demonstrated earlier for hedycaryol.
NOE measurements have been used to determine the conformation of germacrene-type sesquiterpenes in solution.24b NOE data have proven effective in ascertaining the preferred conformation of the relatively rigid germacranolides,55 in which not all the operations (inversion of double bonds and flip of the C6-C7-C8 segment) leading to all conformers of a given structure are energetically possible. In a few instances NOE experiments, in combination with variable-temperature 1H NMR spectra and/or molecular mechanic calculations have been applied to establish the preferred conformation of simpler and flexible germacrenes.56, 57
In the present work, NOE measurements were complicated by the fact that, even at low temperatures (-20° C), the flexible sesquiterpene (dynamic system) was in the slow exchange regime on the chemical shift time scale (separate signals from each contributing form are observable), and consequently transfer of saturation took place.58 Thus, saturation of the well-separated frequency corresponding to H1 (4.78 ppm) of the major conformer a was transferred to the signals at δH 5.01 (conformer b) and 4.94 (conformer c) ppm by the exchange process (Table 3). This unexpected phenomenon turned out to be extremely useful by verifying the H1 chemical shift position of the less populated conformers b and c. In addition, a small NOE enhancement (1%) was observed at 4.51 (H5, conformer a) ppm, thus corroborating the syn relationship of the vinylic hydrogens in the predominant chair-chair (UU) conformation of germacrene A, ie 1a (Figure 5).
Figure 5.
The three conformers observed in the proton NMR spectra of germacrene A, 1a (52%), and 1b (29%) and 1c (19%), are assigned to one major crossed form (UU) and two minor parallel arrangements (UD and DU. The characteristic 1H NMR chemical shifts and assignments of hydrogens 1, 5, 12, 13, 14 and 15 of each conformer are shown by the placement of the bold numbers (see also Table 3).
Saturation transfer was again observed when the downfield resonance at δH 5.21 (H5) corresponding to the least populated conformer (c) was irradiated. In this case the saturation was transferred to the frequencies at δH 5.07 (H5, conformer b) and 4.51 (H5 conformer a) ppm (Table 3). This phenomenon was used again to corroborate chemical shift assignments for the olefinic methyl singlets of a, b, and c conformers (Table 3) by selective saturation of the signals at δH 1.37 (H14, conformer a) and 1.54 (H14 conformer b) and simultaneous saturation of δH 1.48 and 1.46 (H15, conformers c and b, respectively). In addition, six small NOE enhancements (2–3%) were observed as follows: H5/H14 (b), H5/H14 (c), H15/H1 (b), H15/H1 (c), H14/H5 (b) and H14/H5 (c). These results clearly verify that the two minor conformers (b and c) of germacrene A adopt the parallel configurations (UD and DU) of double bonds, with anti orientations of their vinylic protons H1 and H5. These two minor parallel conformers account for almost 50% of the total mixture of conformers in solution and therefore, germacrene A exists as a 1:1 mixture of one crossed (52%) and two parallel forms (48%). Furthermore, the absence of an observable NOE between H1 and H5 rules out the crossed DD conformer as one of the observable minor conformational isomers. These experimental results (in solution) contrast with the theoretical MM2 calculations, which predict that germacrene A (1) would exist as an equilibrium mixture of two crossed (UU and DD) and one parallel (UD) set of conformers in the gas phase (Table 2).
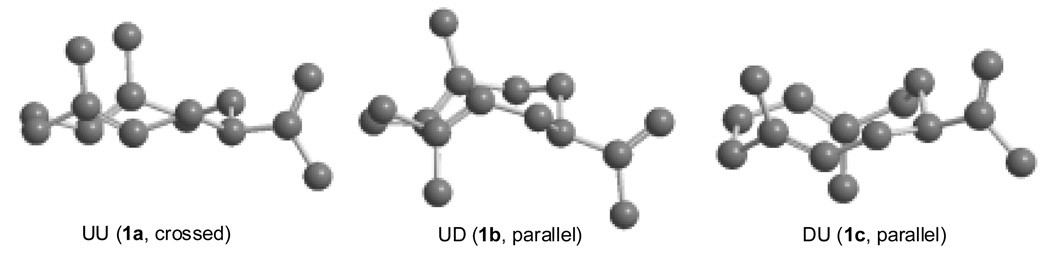
The experimental J values for the olefinic hydrogens (H1 and H5) of conformer b (Table 3) were compared with those predicted for the UD conformation using the Karplus equation59 and the dihedral angles (Ф) of −22.9° (H1H2β, J = 8.9 Hz), −138.2° (H1H2α, J = 7.7 Hz), 33.1° (H5H6β, J = 7.6 Hz), 150.2° (H5H6α, J = 9.6 Hz), which were calculated by the MM2 program. The calculated J values for H1 and H5 seem to be in good agreement with the observed coupling constants for conformer b (Table 3). This agreement provided the basis for a tentative assignment of the second most populated conformational isomer (30%, 1b) as the parallel (UD) conformation. The conformational equilibria and NMR assignments are summarized in Fig. 5. The energy-minimized Chem3D figures of the three distinguishable conformers (1a, 1b and 1c) of germacrene A are displayed in Figure 6. It is interesting to note that the recently isolated isogermacrene A (isopropenyl moiety at C6) exists as one predominant UU conformation at temperatures lower than room temp. (eg. 10° C) 60
Figure 6.
Chem3D representations of the three observable conformers of germacrene A in solution. The structures were minimized according to the MM2 force field method.
X-ray crystallographic structures of silver nitrate adducts of (E, E)-configured germacrenes such as germacrene B,61 germacrone,62 pregeijerene,47b, 63 and costunolide64 have shown that the most stable (UU) conformation is also the preferred one in the solid state. Unfortunately attempts to prepare X-ray quality crystals of the AgNO3 adduct of (+)-germacrene A (1) following literature procedures47b, 65 were unsuccessful.
3. Conclusion
Cultures of a yeast strain harboring a terpene synthase gene from lettuce produced large quantities of a pure C15 hydrocarbon identified as (R)-(+)-germacrene A. This widely occurring but sensitive sesquiterpene was thoroughly characterized for the first time by its TLC and GC behavior, by MS, optical rotation, and spectral properties, and by thermal rearrangement to (−)-β-elemene. Analysis of the complex variable-temperature NMR spectra indicated that germacrene A exists as a mixture of three conformational isomers in a ratio of about 5:3:2 at temperatures at or below ordinary probe temperatures (25° C). The most stable conformer (52%) was assigned as the UU (up - up) form (1a) in which the two methyl groups and isopropenyl substituent adopt positions on the top face of the crossed cyclodecadiene ring. The less populated conformers observed (29 % and 19 %) are attributed to the parallel UD and DU (up-down and down-up) orientations having the C4 and C10 vinyl methyl groups on opposite faces of the cyclodecadiene core (1b and 1c). These conclusions are similar to those reached for hedycaryol (2), except that the UD and DU conformers predominate over the UU alternative, 35b a difference attributable to the larger steric size of the hydroxypropyl substituent. The conformer populations of germacrene A in the sesquiterpene cyclase active sites are likely to be very different than those in solution, and that is certainly a major factor in the product specificities of these enzymes. The proximity and orientations of the C=C double bonds and the positions of the ring substituents set the stage for the course of the ensuing cyclizations and rearrangements.
4. Experimental
4.1 General methods
Optical rotations were measured on a JASCO DIP-370 digital polarimeter at 25ºC. The UV spectrum was obtained on a Shimadzu UV-2401 PC spectrophotometer. IR spectra were measured on a Perkin Elmer Spectrum BX, FT-IR spectrophotometer. GC analyses were conducted on a Rtx-5 30-m fused silica capillary column (split ration ca 100:1). The following programs were used: Method A = initial temp. 50° C for 1 min, ramp 5° C/min to 130° C at an injection temp. of 110° C. Method B = initial temp. 60° C for 3 min, ramp 4° C/min to 150° C at an injection temp. of 180° C.
1H and 13C-NMR spectra were recorded on a Varian 500 spectrometer (500 MHz for 1H and 125 MHz for 13C). Chemical shifts are given in ppm using TMS as internal standard. CDCl3 from Aldrich Chemical Co. was purified by filtering through basic alumina (Brockmann I, standard grade, 150 mesh, 58 Å) and dried overnight over molecular sieves (4 Å) in the dark. Benzene-d6 was used without purification. The silica gel used for column chromatography (Merck 60 230–400 mesh) was purified as follows: a suspension of 3.0 g of silica gel in 15 mL of pentane (15 % Et3N) was stirred at room temp. for 30 min, filtered under vacuum, washed with n-pentane, and dried in an oven (110° C). TLC was carried out with Merck 60F-254 plates with 0.25 mm thickness. Visualizations of the TLC spots were performed by spraying with an 0.1% solution of berberine hydrochloride in EtOH, and/or UV light. n-Pentane (HPLC grade) was purchased from Fisher Scientific and was used without further purification
Molecular mechanics calculations (employing the MM2 force field)49 were performed with the program CS Chem 3D Ultra® (version 8.0) from Cambridge Soft. All calculations were carried out on a standard PC equipped with a Pentium[R] 4 CPU 2.40 GHz processor and 512 MB RAM. A total of 24 start geometries were modeled using this method with relative steric energies ranging from 0.0 to 13.0 kcal/mol. After the minimization process, the four conformations (UU, UD, DU and DD) shown in Figure 4 were judged to be the most likely to be populated in the gas phase. The UU (20.83 kcal/mol) conformation was found to correspond to the global minimum, and the conformations UD, DD and DU were interpreted as local minima with steric energies of 22.20, 22.32 and 23.47 kcal/mol respectively.
4.2. Production of (+)-germacrene A (1) with the engineered yeast
Yeast transformation, culturing, and terpene production were performed according to Takahashi et al.42 In brief yeast transformed with the recombinant Yep352 vector harboring the lettuce terpene synthase gene and a wild type copy of the URA3 gene were selected for prototrophic growth on minimal media without uracil supplementation, and three independent colonies were selected by PCR screening for the lettuce gene. Each colony was grown in 10 mL of minimal media at 28°C for 2–4 days before transferring the entire culture into the nutrient rich YPDE medium (150 mL) for terpene production.42 After 7 days, the yeast cultures were lysed by vigorous mixing (30 sec) with an equal amount of acetone (160 mL), followed by a second 30-sec mixing with an equal volume of hexane (160 mL). The upper hexane layer was carefully transferred to a collection vessel, and the lower aqueous layer was extracted with additional hexane. The combined organic extracts were concentrated to a final volume of 20 mL using a stream of nitrogen. Large-scale production of the sesquiterpene product was achieved by inoculating the initial 10-mL yeast cultures into each of ten 200-mL aliquots of the YPDE medium and by allowing the cultures to grow for an additional 7 days. The 2 L of combined yeast culture was extracted as described above and concentrated under a stream of nitrogen to a final volume of 20–30 mL.
4.2.1 (+)-Germacrene A (1)
Cold (dry ice) hexane solutions containing germacrene A were shipped from the University of Kentucky to the University of Illinois, and immediately evaporated with a stream of nitrogen. The residue was re-dissolved in pentane and filtered through silica gel with additional pentane as eluent. Fractions containing sesquiterpene 1 (TLC Rf 0.71 (pentane)) were combined and evaporated with a stream of nitrogen to afford a total of 363 mg (two batches) of pure germacrene A as a clear oil. The purity of this material was estimated to be > 95% by 1H NMR spectroscopy. For characterization purposes, small quantities (10–15 mg) were further purified by preparative TLC on silica gel using n-pentane as developing solvent. Data for germacrene A: TLC Rf 0.71 (pentane); [α]D25 +42.1° (c 1.0, CCl4), lit.24c −26.8° (c 1.0 , CCl4), lit.24a −3.2° (c 14.4 , CCl4)); UV (CH3OH) λmax nm (log ε) 214 (2.49); IR νmax (liquid film, CH2Cl2) 3061, 1643, 1275, 1261, 886, 841 cm−1; 1H NMR (see Table 1 and Table 3); 13C NMR (125 MHz, CDCl3, 25° C) 153.7 (C11), 152.3* , 138.2 (C10), 131.7 (C5), 128.9 (C4), 128.6*, 126.9*, 126.4 (C1), 125.3*, 121.8*, 108.2*, 107.5*, 107.3 (C13), 51.3 (C7), 47.9*, 45.7*, 41.7 (C9), 41.5*, 39.5 (C2 or C3), 38.8*, 37.0*, 34.8 (C6), 34.6*, 33.6 (C8), 33.4*, 32.6*, 31.5*, 30.7*, 26.7 (C2 or C3), 24.2, 21.9* , 20.3 (C12), 19.9, 16.7 (C15), 16.2 (C14), 15.4. 13C NMR (125 MHz, C6D6, 25° C) 153.6 (C11), 152.2*, 137.8 (C10), 131.9 (C5), 128.8 (C4), 126.8 (C1), 125.6*, 122.2*, 108.8*, 108.0*, 107.8 (C13), 51.8 (C7), 48.4*, 46.1*, 41.9 (C9), 41.8*, 39.8 (C3 or C2), 39.1*, 37.4*, 35.2 (C6), 34.9*, 33.9 (C8), 33.7*, 33.0*, 31.7*, 31.1*, 27.0 (C2 or C3)*, 24.6, 22.0*, 20.3 (C12), 19.9, 16.7 (C15), 16.2 (C14), 15.4. The asterisks designate less intense resonances. Assignments were made based on those in references 24c and 26 (benzene-d6), and correspond to the major conformer at room temp.
4.2.2 (−)-β-Elemene (4)
A solution of germacrene A (1) (10.1 mg, 0.05 mmol) in 1 mL of toluene was heated at reflux for 2 h in a flame-stretched 10-mL test tube as the reaction vessel. After cooling, the solution was applied to a preparative TLC plate (see general methods). The toluene was allowed to evaporate under a nitrogen stream, and the plate was developed using n-pentane to give (−)-β-elemene (9.9 mg, quant.) as a clear oil: TLC Rf0.81 (pentane); [α]D25 −15.8° (c 0.50, CHCl3), lit.43 −11.8° (c 4.63 CHCl3), lit.44 +15.4° enantiomer (c 0.60, CHCl3); UV (CH3OH) λmax nm (log ε) 210 (2.00); 1H and 13C-NMR data in total agreement with Brauchli and Thomas.45 EIMS m/z (%) 204 (M+, 3), 189 (42), 175 (13), 161 (65), 147 (100), 133 (69), 121 (76), 119 (62), 107 (84), 105 (53), 93 (77), 81 (41), 79 (25), 67 (22), 55 (37).
Acknowledgements
We are very grateful to Dr. A. M. Adio for exchanges of information and for 1-D and 2-D NMR spectra of an impure sample of germacrene A isolated from S. Canadensis,26 to Dr. Vera Mainz of the School of Chemical Sciences NMR Facility at the UI for assistance with the VT and NOE NMR experiments, and to Dr. Jerome Baudry in the School of Chemical Sciences’ Computer Applications Facility at the UI for consultations and advice concerning the MM2 calculations. This work was supported by grants from the National Institutes of Health (GM 13956 to RMC and GM 054029 to JC), and from the Kentucky Agriculture Experiment Station (JC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
It should be noted that germacrene A undergoes Cope rearrangement to β-elemene under the conditions of the MS determination. Hence the MS data for germacrene A are actually those of β-elemene.32
References and notes
- 1.Banthorpe DV. Natural Products: Their Chemistry and Biological Significance. Harlow: Longman Scientific & Technical; 1994. Chapter 5. [Google Scholar]
- 2.(a) Cane DE. Acc. Chem. Res. 1985;18:220–226. [Google Scholar]; (b) Cane DE. Chem. Rev. 1990;90:1089–1103. [Google Scholar]; (c) Cane DE. Chap. 6. In: Barton D, Nakanishi K, Meth-Cohn O, editors. Comprehensive Natural Products Chemistry. Vol 2. 1999. pp. 155–200. [Google Scholar]
- 3.(a) de Kraker JW, Franssen MCR, de Groot A, Koning WA, Bouwmeester H. J. Plant Physiol. 1998;117:1381–1392. doi: 10.1104/pp.117.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) De Kraker JW, Franssen MCR, Dalm M, de Groot A, Bouwmeester H. J. Plant Physiol. 2001;125:1930–1940. doi: 10.1104/pp.125.4.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) de Kraker JW, Franssen MCR, Joerink M, de Groot A, Bouwmeester H. Plant Physiol. 2002;129:257–268. doi: 10.1104/pp.010957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorm FJ. Agric. Food Chem. 1971;19:1081–1087. [Google Scholar]
- 5.Fischer NH. In: Terpenoids. Charlwood BV, Banthorpe DV, editors. New York: Academic; 1991. pp. 187–211. [Google Scholar]
- 6.Whitehead IM, Threlfall DR. J. Biotech. 1992;26:63–81. [Google Scholar]
- 7.(a) Threlfall DR, Whitehead IM. Phytochemistry. 1988;27:2567–2580. [Google Scholar]; (b) Whitehead IM, Ewing DF, Threlfall DR. Phytochemistry. 1988;27:1365–1370. [Google Scholar]; (c) Whitehead IM, Threlfall DR, Ewing DF. Phytochemistry. 1989;28:775–779. [Google Scholar]; (d) Chappell J. Annu. Rev. Plant. Physiol. Plant Mol. Biol. 1995;46:521–547. [Google Scholar]; (e) Egea C, Alcazar MD, Candela ME. Physiol. Plant. 1996;98:737–742. [Google Scholar]
- 8.Takasugi M, Okinaka S, Katsui N, Masamune T, Shirata A, Ohuchi M. Chem. Commun. 1985:621–622. [Google Scholar]
- 9.(a) Whitehead IM, Atkinson AL, Threlfall DR. Planta. 1990;182:81–88. doi: 10.1007/BF00239988. [DOI] [PubMed] [Google Scholar]; (b) Back K, Chappell J. J. Biol. Chem. 1995;270:7375–7381. doi: 10.1074/jbc.270.13.7375. [DOI] [PubMed] [Google Scholar]
- 10.(a) Afzal M, Al-Oriqut G. Heterocycles. 1986;24:2947–2948. and references cited therein. [Google Scholar]; (b) Hwu JR, Wetzel JM. J. Org. Chem. 1992;57:922–928. and references cited therein. [Google Scholar]
- 11.(a) Tahara S, Hanawa F, Harada Y, Mizutani J. Agric. Biol. Chem. 1988;52:1947–2948. [Google Scholar]; (b) Bennett MH, Gallagher MDS, Bestwick CS, Rossiter JT, Mansfield JW. Physiol. Mol. Plant Pathol. 1994;44:321–333. [Google Scholar]; (c) Bestwick L, Bennett MH, Mansfield JW, Rossiter JT. Phytochemistry. 1995;39:775–777. [Google Scholar]; (d) Bennett L, Mansfield MH. J.W. Plant Physiol. 1995;108:503–516. doi: 10.1104/pp.108.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Sessa RA, Bennett MH, Lewis MJ, Mansfield JW, Beale MH. J. Biol. Chem. 2000;275:26877–26884. doi: 10.1074/jbc.M000244200. [DOI] [PubMed] [Google Scholar]
- 12.(a) Croteau R, Munck SL, Akoh CC, Fisk HJ, Satterwhite DM. Arch. Biochem. Biophys. 1987;256:56–68. doi: 10.1016/0003-9861(87)90425-5. [DOI] [PubMed] [Google Scholar]; (b) Munck SL, Croteau R. Arch. Biochem. Biophys. 1990;282:58–64. doi: 10.1016/0003-9861(90)90086-e. [DOI] [PubMed] [Google Scholar]
- 13.(a) Hohn TM, Plattner RD. Arch. Biochem. Biophys. 1989;272:137–143. doi: 10.1016/0003-9861(89)90204-x. [DOI] [PubMed] [Google Scholar]; (b) Cane DE, Prabhakaran PC, Oliver JS, McIlwaine DB. J. Am. Chem. Soc. 1990;112:3209–3210. [Google Scholar]; (c) Cane DE, Wu Z, Proctor RH, Hohn TM. Arch. Biochem. Biophys. 1993;304:415–419. doi: 10.1006/abbi.1993.1369. [DOI] [PubMed] [Google Scholar]; (d) Proctor RH, Hohn TM. J. Biol. Chem. 1993;268:4543–4548. [PubMed] [Google Scholar]
- 14.(a) Whitehead IM, Ewing DF, Threlfall DR, Cane DE, Prabhakaran PC. Phytochemistry. 1990;29:479–482. [Google Scholar]; (b) Facchini PJ, Chappell J. Proc. Natl. Acad. Sci. USA. 1992;89:11088–11092. doi: 10.1073/pnas.89.22.11088. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Back K, Yin S, Chappell J. Arch. Biochem. Biophys. 1994;315:527–532. doi: 10.1006/abbi.1994.1533. [DOI] [PubMed] [Google Scholar]; (d) Rising KA, Starks CM, Noel JP, Chappell J. J. Am. Chem. Soc. 2000;122:1861–1866. [Google Scholar]
- 15.(a) Calvert MJ, Ashton PR, Allemann RK. J. Am. Chem. Soc. 2002;124:11636–11641. doi: 10.1021/ja020762p. [DOI] [PubMed] [Google Scholar]; (b) Felicetti B, Cane DE. J. Am. Chem. Soc. 2004;126:7212–7221. doi: 10.1021/ja0499593. [DOI] [PubMed] [Google Scholar]
- 16.Cane DE, Tsantrizos YS. J. Am. Chem. Soc. 1996;118:10037–10040. [Google Scholar]
- 17.Wichtmann EM, Stahl-Biskup E. Flavour Fragrance J. 1987;2:83–89. See for example. [Google Scholar]
- 18.Bouwmeester HJ, Kodde J, Verstappen FWA, Altug IG, de Kraker J-W, Wallaart TE. Plant Physiol. 2002;129:134–144. doi: 10.1104/pp.001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(a) Kim M-Y, Chang Y-J, Bang M-H, Baek N-I, Jin J, Lee C-H, Kim S-UJ. Plant Biol. 2005;48:178–186. [Google Scholar]; (b) Chang Y-J, Jin J, Nam H-Y, Kim S-U. Biotech. Lett. 2005;27:285–288. doi: 10.1007/s10529-005-0681-9. [DOI] [PubMed] [Google Scholar]
- 20.Bennett MH, Mansfield JW, Lewis MJ, Beale MH. Phytochemistry. 2002;60:255–261. doi: 10.1016/s0031-9422(02)00103-6. [DOI] [PubMed] [Google Scholar]
- 21.Prosser I, Phillips AL, Gittings S, Lewis MJ, Hooper AM, Pickett JA, Beale MH. Phytochemistry. 2002;60:691–702. doi: 10.1016/s0031-9422(02)00165-6. [DOI] [PubMed] [Google Scholar]
- 22.Bertea CM, Voster A, Verstappen FWA, Maffei M, Beekwilder J, Bouwmeester HJ. Arch. Biochem. Biophys. 2006;448:3–12. doi: 10.1016/j.abb.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Ren J, Liu Y, Yang L, Ni R, You S. Chemical Research in Chinese Universities. 2006;22:606–611. [Google Scholar]
- 24.(a) Weinheimer AJ, Youngblood WW, Washecheck PH, Karns TKB, Ciereszko LS. Tetrahedron Lett. 1970;11:497–500. [Google Scholar]; (b) Takeda K. Tetrahedron. 1974;30:1525–1534. [Google Scholar]; (c) Nishino C, Bowers WS, Montgomery ME, Nault LR, Nielson MW. J. Chem. Ecol. 1977;3:349–357. [Google Scholar]
- 25.Forcat S, Allemann RK. Org. Biomol. Chem. 2006;4:2563–2567. doi: 10.1039/b604147g. [DOI] [PubMed] [Google Scholar]
- 26.Adio AM, Paul C, Tesso H, Kloth P, Konig WA. Tetrahedron: Asymmetry. 2004;15:1631–1635. [Google Scholar]
- 27.Teisserie PJ. Chemistry of Fragrant Substances. New York: VCH Publishers Inc; 1994. pp. 193–289. [Google Scholar]
- 28.Dunlop RW, Wells RJ. Aust. J. Chem. 1979;32:1345–1351. [Google Scholar]
- 29.Bowers WS, Nishino C, Montgomery ME, Nault LR, Nielson MW. Science. 1977;196:680–681. doi: 10.1126/science.558651. [DOI] [PubMed] [Google Scholar]
- 30.Baker R, Parton AH, Howse PE. Experientia. 1982;38:297–298. [Google Scholar]
- 31.(a) Nelson LJ, Cool LG, Forschler BT, Haverty MI. J. Chem. Ecol. 2001;27:639–652. doi: 10.1023/a:1010325511844. [DOI] [PubMed] [Google Scholar]; (b) Quintana A, Reinhard J, Faure R, Uva P, Bagneres A-G, Massiot G, Clement J-L. J. Chem. Ecol. 2003;29:349–357. doi: 10.1023/a:1022868603108. [DOI] [PubMed] [Google Scholar]
- 32.de Kraker J-W, Franssen MCR, de Groot A, Shibata T, Bouwmeester HJ. Phytochemistry. 2001;58:481–487. doi: 10.1016/s0031-9422(01)00291-6. [DOI] [PubMed] [Google Scholar]
- 33.We thank Dr. Adio for providing original 1H NMR and 2D NMR spectra of (+)-germacrene A (see reference 26).
- 34.(a) Sutherland JK. Tetrahedron. 1974;30:1651–1660. [Google Scholar]; (b) Watson WH, Kashyap RP. J. Org. Chem. 1986;51:2521–2524. [Google Scholar]
- 35.(a) Kulkarni GH, Kelkar GR, Bhattacharyya SC. Tetrahedron. 1964;20:1301–1315. [Google Scholar]; (b) Wharton PS, Poon Y-C, Kluender HC. J. Org. Chem. 1973;38:735–740. [Google Scholar]
- 36.Ruzicka L. Experientia. 1953;9:357–367. doi: 10.1007/BF02167631. [DOI] [PubMed] [Google Scholar]
- 37.Barton DHR, de Mayo P. Quart. Revs. (London) 1957;11:189–211. [Google Scholar]
- 38.Hendrickson JB. Tetrahedron. 1959;7:82–89. [Google Scholar]
- 39.Parker W, Roberts JS, Ramage R. Quart. Rev., Chem. Soc. 1967;21:331–363. [Google Scholar]
- 40.Greenhagen BT, O’Maille PE, Noel JP, Chappell J. Proc. Natl. Acad. Sci USA. 2006;103:9826–9831. doi: 10.1073/pnas.0601605103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deguerry F, Laurence P, Wu S, Clark A, Chappell J, Schalk M. Arch. Biochem. Biophys. 2006;454:123–136. doi: 10.1016/j.abb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi S, Yeo Y, Greenhagen BT, McMullin T, Song L, Maurina-Brunker J, Rosson R, Noel JP, Chappell J. Biotech. Bioeng. 2006:21216. doi: 10.1002/bit.21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganter C, Keller-Wojtkiewcz FB. Helv. Chim. Acta. 1971;54:183–206. [Google Scholar]
- 44.Corey EJ, Roberts BE, Dixon BR. J. Am. Chem. Soc. 1995;117:193–196. [Google Scholar]
- 45.Brauchli R, Thomas AF. J. Agric. Food. Chem. 1991;39:431–431. [Google Scholar]
- 46.Paldus J, Koutecky J. Coll. Czech. Chem. Commun. 1962;27:2139–2151. [Google Scholar]
- 47.(a) Sorm F. Pure Appl. Chem. 1961;2:533–549. [Google Scholar]; (b) Jones RVH, Sutherland MD. Aust. J. Chem. 1968;21:therein. [Google Scholar]
- 48.The % in parenthesis refers to the relative populations of each conformer which were calculated by integration of the individual. 1H NMR signals [Google Scholar]
- 49.Allinger NL. J. Am. Chem. Soc. 1977;99:8127–8134. [Google Scholar]
- 50.Terada Y, Yamamura S. Tetrahedron Lett. 1979;20:3303–3306. [Google Scholar]
- 51.Shirahama H, Osawa E, Matsumoto T. Tetrahedron Lett. 1979;20:2245–2246. [Google Scholar]
- 52.Osawa E, Shimada K, Kodama M, Ito S. Tetrahedron Lett. 1979;20:2353–2354. [Google Scholar]
- 53.Tashkhodzhaev B, Makhmudov MK. Chem. Nat. Prod. 1997;33:289–292. [Google Scholar]; English translation of Khimiya Prirodnykh Soedinenii. 1997:379–382. [Google Scholar]
- 54.Sathe RN, Kulkarni GH, Kelkar GR. Chem. Ind. (London) 1968:448–449. [Google Scholar]
- 55.Bhacca NS, Fischer NH. Chem. Commun. 1969:68–69. [Google Scholar]
- 56.(a) Horibe I, Tori K, Takeda K. Tetrahedron Lett. Chem. Pharm. Bull. 1973;14:735–738. [Google Scholar]; (b) Inayama S, Gao JF, Harimaya K, Iitaka Y, Guo YT, Kawamata T. Chem. Pharm. Bull. 1985;33:1323–1326. doi: 10.1248/cpb.32.3783. [DOI] [PubMed] [Google Scholar]; (c) Ohkura T, Gao J, Harimaya K, Hikichi M, Iitaka Y, Kawamata T, Kuroyanagi M, Fukushima S, Inayama S. Chem. Pharm. Bull. 1986;34:4435–4438. [Google Scholar]; (d) Mori M, Okada K, Shimazaki K, Chuman T, Kuwahara S, Kitahara T, Mori KJ. Chem. Soc. Perkin Trans. 1990;1:1769–1777. [Google Scholar]
- 57.J. Am. Chem. Soc. 1996;118:12821–12825. Variable temperature 13C and 1H NMR spectra indicate that trans-cyclodecene exists in five populated (3–38%) conformations; see. [Google Scholar]
- 58.Neuhaus D, Williamson MP. The Nuclear Overhauser Effect in Structural and Conformational Analysis. New York: Wiley-VCH; Chap. 5; pp. 129–190. 200. [Google Scholar]
- 59.Haasnoot CAG, de Leeuw FAAM, Altona C. Tetrahedron. 1980;36:2783–2792. [Google Scholar]
- 60.Hackl T, König WA, Muhle H. Phytochemistry. 2004;65:2261–2275. doi: 10.1016/j.phytochem.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 61.Allen FH, Rogers D. J. Chem. Soc. (B) 1971:257–262. [Google Scholar]
- 62.Hikino H, Konno C, Nagashima T, Takemoto T. Tetrahedron Lett. 1971;12:337–340. [Google Scholar]
- 63.Coggon P, McPhail AT, Sim GA. Chem. Soc. (B) 1970:1024–1028. [Google Scholar]
- 64.Sorm F, Suchy M, Holub M, Linek A, Hadinec I, Novak C. Terahedron Lett. 1970;11:1893–1896. [Google Scholar]
- 65.Brown ED, Sam TW, Sutherland JK, Torre A. J. Chem. Soc. Perkin Trans. 1975;1:2326–2332. [Google Scholar]