Abstract
Silica-based nanomaterials show promise for biomedical applications such as cell-selective drug delivery and bioimaging. They are easily functionalized, which allows for the conjugation or encapsulation of important biomolecules. Although recent in vitro studies suggested that silica-derived nanomaterials are nontoxic, in vivo studies of silica nanomaterial toxicity have not been performed. Using the embryonic zebrafish as a model system, we show that silica nanomaterials with aspect ratios greater than 1 are highly toxic (LD50 = 110 pg/g embryo) and cause embryo deformities, whereas silica nanomaterials with an aspect ratio of 1 are neither toxic nor teratogenic at the same concentrations. Silica nanowires also interfere with neurulation and disrupt expression of sonic hedgehog, which encodes a key midline signaling factor. Our results demonstrate the need for further testing of nanomaterials before they can be used as platforms for drug delivery.
From the Clinical Editor: Silica-based nanomaterials show promise for biomedical applications such as cell-selective drug delivery and bioimaging. Using an embryonic zebrafish model system silica nanomaterials with aspect ratios greater than one were found to be highly toxic; whereas silica nanomaterials with an aspect ratio of one are neither toxic nor teratogenic. These results demonstrate the need for testing “nanomaterials” before they can be used as platforms for drug delivery.
Keywords: Nanowires, Silica, Teratogenic, Toxicity, Zebrafish
Nanomaterials show promise for use in biomedical applications including drug delivery and diagnostics due to their size-dependent properties. Many nanomaterials can be functionalized to increase or modify access to specific cells, to deliver molecular payloads to their targets, and/or to report cellular activities.1–3 Silica-based materials are particularly attractive for biomedical applications because functionalization is readily accomplished,4–9 and a wide range of sizes and aspect ratios can be fabricated, allowing for control of nanomaterial surface area and therefore the number of sites for the conjugation of biomolecules.4,10 In addition, fluorescently modified silica nanoparticles are easily tracked in vivo6,7,11,12 and can be targeted to select regions of the body.13 Silica nanoparticles (those having an aspect ratio of 1) have a tendency to form clumps, whereas silica nanowires (those having an aspect ratio much greater than 1) do not14,15 suggesting that silica nanowires may offer a higher degree of functional control for biomedical applications.
With the great interest in developing nanomaterial-based drug delivery platforms comes a greater responsibility for testing new nanomaterials for biocompatibility. Several silica-derived nano-materials have been tested for toxicity in cell culture.16–22 In these studies, silica nanoparticles were benign, even at very high concentrations. Similarly, silica nanoparticles delivered to the lateral ventricles of the mouse brain were nontoxic and efficiently carried their payload to neurons within the region of the ventral midbrain.23 Nevertheless, the toxicity of silica nanoparticles is the subject of debate. One study has demonstrated the size-dependent toxicity of silica nanoparticles in the rat lung. In this study, particles on the nanoscale showed little if any toxicity, whereas those on the micrometer scale were significantly toxic10 suggesting that a corresponding increase in surface area may lead to detrimental effects in vivo. Despite several promising studies, silica nanoparticles have been shown to stimulate the immune system response when injected into the peritoneal cavity of mice and cause cells to undergo an oxidative stress response in vitro and in vivo.24 Silica nanowires were nontoxic at concentrations less than 190 µg/mL in one in vitro study but at higher concentrations caused cell necrosis.18 Although these concentrations exceed nanowire doses that successfully deliver biomolecular payloads,5 these studies suggest caution in the development of applications for silica nanowires, despite their greater versatility in functionalization. These studies also suggest that aspect ratio may be an important physicochemical property of silica nanomaterials with respect to toxicity.
Thorough testing prior to or in parallel with the development of biomedical applications must include the use of in vivo systems.25 An outstanding choice for an in vivo toxicity testing model is the embryonic zebrafish, as this vertebrate is suited for rapid and high-throughput evaluation of toxicity as well as teratogenicity (the propensity to cause birth defects).26 The effects of potential toxins or teratogens can be quantified by simple visual assessment, and a host of genetic and molecular tools are available for testing mechanistic hypotheses related to toxicity. The use of the zebrafish model has already provided valuable information regarding the toxicity and teratogenicity of carbon-based nanomaterials.27–29
In the current study, we selected two silica-derived nanomaterials that are being developed as drug delivery platforms, one having an aspect ratio of 1 (silica nanoparticles) and one having an aspect ratio much greater than 1 (silica nanowires; Table 1). We tested nanomaterial toxicity by injecting materials directly into the yolk to expose the developing embryos to defined quantities of nanomaterial. We find strikingly selective and robust toxic and teratogenic effects of silica nanowires compared with their more benign nanoparticle counterparts. These differences in toxicity are not related to differential access to the developing embryo and are developmental stage specific. The silica nanowires have their major toxic effects at the time of embryonic gastrulation and neurulation and influence the expression of a gene involved in these processes, sonic hedgehog.
Table 1.
Silica nanomaterials and their associated toxicities in zebrafish embryos
| Nanomaterial | Size | Conjugate | Toxicity of 1 µg/mL nanomateriala | |
|---|---|---|---|---|
| Significance (P value) | Time (stage) of significance measurea | |||
| Silica nanowires | 55 nm × 2.1 µm | None | 0.0013 | 12 hpf (neurulation) |
| FITC | 0.013 | 12 hpf (neurulation) | ||
| Silica nanoparticles | 50 nm | None | 0.14 | 132 hpf |
| Amine | 0.08 | 132 hpf | ||
| FITC | 0.08 | 132 hpf | ||
| Rhodamine | 0.65 | 132 hpf | ||
| 200 nm | Rhodamine | 0.10 | 132 hpf | |
Wilcoxon rank sum test.
Methods
Silica nanowires were fabricated using Fe as a catalyst and were then suspended in HPLC-grade methanol and converted to shorter segments (Table 1) by mechanical breakage. Five hundred microliters of sterile RNase-free water was added to the nanowire suspension and incubated at 65°C to allow the methanol to evaporate. The nanowire-in-water suspension was autoclaved.5 Silica nanoparticles were prepared using a cyclohexane, Triton X-100, n-hexanol, water microemulsion system.2,30
Fluorescein isothiocyanate (FITC) was conjugated to the surface of the nanowires using 8-Aminopyrene-1,3,6-trisulfonic acid trisodium salt (APTS) (Sigma Aldrich Allentown, Pennsylvannia; ≥ 95% purity) as a linker.6,31 The fluorescence of the modified wires was measured using two different methods. Fluorescence was measured as photon flux on an in vivo imaging system (IVIS) and was linear with respect to material concentration (data not shown). Alternatively, silica nanowires were briefly sonicated to prevent aggregation, and fluorescence was observed directly by confocal microscopy. Elimination of APTS from the procedure resulted in loss of the fluorophore with washing and centrifugation, indicating that the linkage via APTS was covalent.
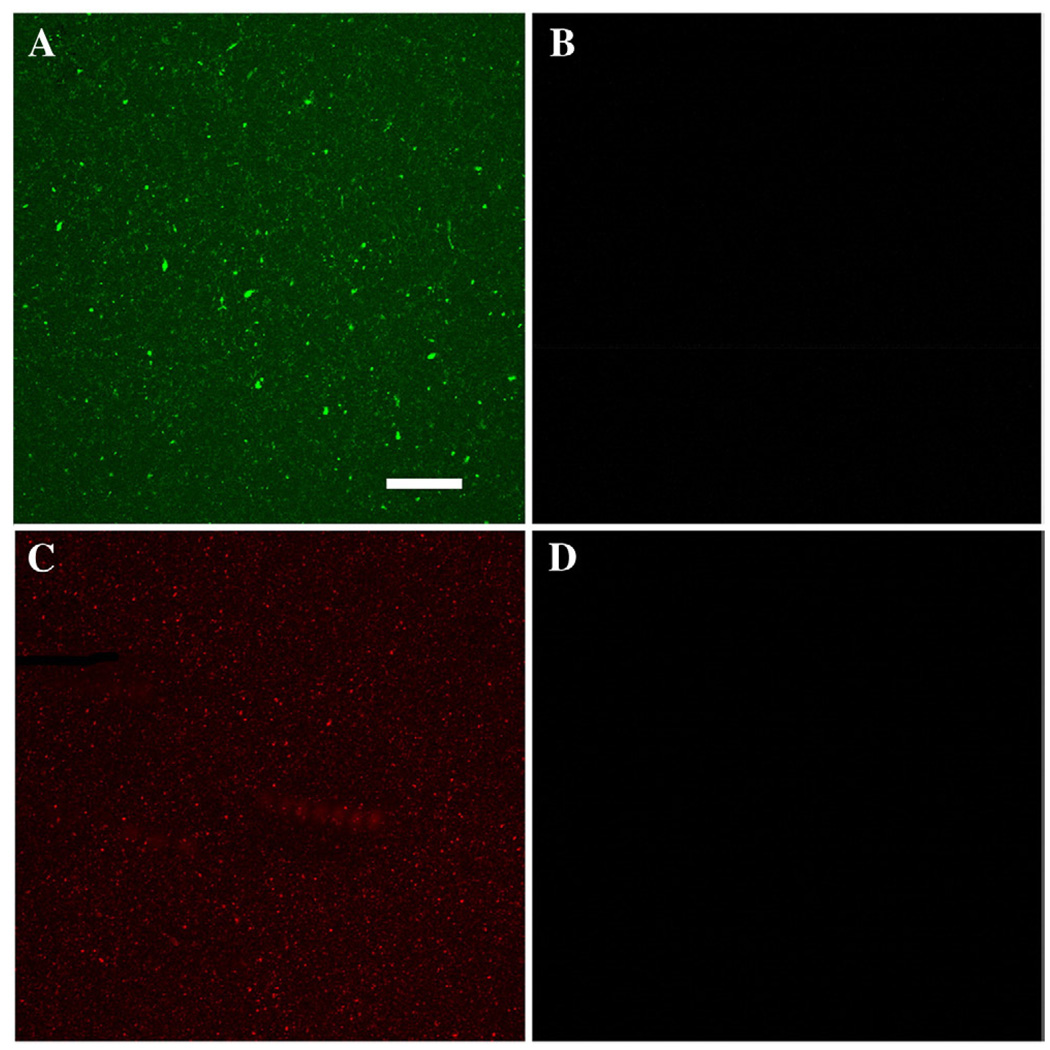
Large-diameter (200 nm; 24-hour emulsion) and smalldiameter (50 nm; 2-hour emulsion) silica nanoparticles were functionalized during particle preparation. The hydrolysis and co-condensation reaction of tetraethyl ortho silicate (TEOS; Sigma Aldrich; 99.99% purity), fluor/APTS conjugate, and a water-dispersible silane agent, 3-(trihydroxy silyl) propyl methyl phosphonate (THPMP), in the presence of ammonium hydroxide, produced dye-doped amine-functionalized silica nanoparticles.2,32 Nonfluorescent amine-functionalized silica nanoparticles were synthesized as previously described without the inclusion of fluor/ APTS conjugate. Thus, all fluorescent nanoparticles used in this study contained external amine functional groups. Bare silica nanoparticles were synthesized following the Stöber method, according to Rossi et al.33 Size and fluorescence of the modified nanoparticles were verified by scanning electron microscopy (SEM) and confocal microscopy (Figure 1, A, C).
Figure 1.
Silica nanomaterials retain fuorophore after sonication and incubation in cell-free solutions. (A) FITC-conjugated silica nanowires retain external fuorophore conjugate after sonication and incubation in a solution designed to mimic intracellular conditions. (B) Supernatant showing the lack of FITC departure from the nanowire substrate. (C) Rhodamine-doped silica nanoparticles do not leach fluorescent material after incubation in a solution designed to mimic intracellular conditions. (D) Supernatant showing the lack of fluorescent material. Scale bar in (A) (applies to all) = 20 µm.
Zebrafish were maintained and bred according to Westerfield34 in accordance with an approved animal care and use protocol. Zebrafish of a wild-type strain originally obtained from Scientific Hatcheries (now Aquatica Habitats; Plant City, Florida) were used for most of the experiments. The 2.2shh:GFP line35 was used to visualize the effects of nanomaterial exposure on expression of the sonic hedgehog (shh) gene and was the gift of Carl Neumann. Each nanomaterial preparation was suspended in sterile, nuclease-free water at the concentrations indicated. Clumping of nanomaterials was minimized prior to injection by sonicating the nanomaterial solution three times at low frequency in 1-minute durations to avoid excess thermal buildup. Nanomaterials were then pressure-injected into the yolk of embryos at the 1- to 2-cell stage (=0 to 1 hours postfertilization; hpf) or at 6 hpf, directly underneath the animal pole, in a volume of 3 nL. In separate experiments at a later developmental time, 36 hpf embryos received a 3-nL volume pressure-injected near the developing heart.36 Individual embryos were placed in wells of a 96-well plate and incubated at 28.5°C until 132 hpf.28 Embryo mortality and teratogenesis were visually assessed at the times indicated using a Nikon (Meridian Instruments; Freeland, Washington) stereomicroscope. Each experiment consisted of five treatment groups (a noninjected control, water-injected control, and nanomaterial treatments at 10 ng/mL, 100 ng/mL, and 1 µg/mL) and was performed on embryos of the same clutch. Each experiment was performed three times (N = 12 to 32 embryos per treatment per clutch) yielding a total of 36 to 96 embryos examined for each treatment. Higher concentrations of nanomaterials were used to generate more complete dose-response curves.
To determine if the fluorophore remained associated with nanowires or nanoparticles in physiologic solutions, we sonicated a 1 mg/mL stock and then incubated each nanomaterial in a 1:1 ratio of solutions designed to mimic the extracellular environment (125 mM NaCl, 2.4 mM KCl, 0.28 mM MgSO4, 0.89 mM MgCl2, 2.4 mM CaCl2, 2 mM 4-(2-hydroxyethyl)-l-pipera-zineethanesulfonic acid (HEPES), 5.6 mM glucose, pH 7.5 and 290 mOsm; Goldfish Ringer’s37), and the intracellular environment (105 mM d-gluconic acid, 16 mM KCl, 2 mM MgCl2, 10 mM HEPES, 10 mM ethylene glycol tetraacetic acid (EGTA), 10 mM sodium ATP, pH 7.2 and 290 mOsm38). In addition, we incubated materials in homogenized whole embryos obtained immediately after fertilization and therefore consisting predominately of yolk. Materials were incubated for 12 hours followed by a brief centrifugation at 850g. The resulting pellets and supernatants were analyzed for the presence of fluorophore using confocal microscopy. Our results indicate that neither the sonication of FITC-conjugated silica nanowires or rhodamine dye–doped silica nanoparticles nor their incubation in physiologically relevant solutions caused a loss of the fluorophore, and therefore these fluorescent nanomaterials can be used to accurately track the fate of the silica nanomaterials (Figure 1, A–D).
To visualize nanomaterial distribution and to view expression of the 2.2shh:GFP transgene, live embryos were embedded in low-melting-point agarose and imaged using bright-field and epifluorescence optics on a Leica DMR (Bartels and Stout; Issaquah, Washington) compound microscope using a SPOT (Diagnostic Instruments; Sterling Heights, Michigan) digital camera and associated software. Statistical evaluations were performed in the R statistical environment (R Core Development Team, 2000; Freeware at http://www.r-project.org/) with P values equal to or less than 0.05 considered statistically significant for all experiments performed.
Results
Silica nanomaterials with high aspect ratios (=nanowires) were toxic to zebrafish embryos when supplied via microinjection into the yolk at the 1- to 2-cell stage (Figure 2). Survival curves indicated that embryos treated with unmodified silica nanowires (Figure 2, A) or FITC-conjugated silica nanowires (Figure 3, A) died sooner and in greater numbers than did those injected with water or saline. At 1 µg/mL (corresponding with 3 pg of material per embryo), silica nanowires caused a statistically significant impact on embryo survival beginning at 12 hpf (Table 1). The LD50 for silica nanowires was approximately 110 pg/g embryo. In contrast, silica nanoparticles were remarkably nontoxic (Figure 2, B), including those containing fluorescent material (rhodamine or FITC) or those modified with an external amine group (Figure 3, B–D). Mortality rates in response to silica nanoparticles below a concentration of 1 µg/mL were never significantly different from control treatments for the duration of the experiment; 50-nm-diameter particles as well as 200-nm-diameter particles did not show appreciable toxicity (Table 1). To establish the LD50 for silica nanoparticles, we performed experiments using much higher concentrations; the LD50 for silica nanoparticles was approximately 20 ng/g embryo, three orders of magnitude greater than that for silica nanowires (Figure 2, C). These data suggest that aspect ratio is a key property of silica nanomaterials for predicting biocompatibility.
Figure 2.
Silica nanomaterials show material-specific effects on survival of zebrafish embryos. (A) Unmodified silica nanowires show dose-dependent toxicity to embryos, predominately between 8 and 20 hours postfertilization (hpf). (B) Unmodified silica nanoparticles have negligible effects on embryo survival. Standard deviations (from clutch to clutch) for these experiments ranged from 0.004 to 0.013 and were omitted for figure clarity. (C) Dose-response curves for silica nanowires and silica nanoparticles. The LD50s were 110 pg/g embryo for silica nanowires and 10 ng/g embryo for silica nanoparticles.
Figure 3.
Functionally modified silica nanomaterials show material-specific effects on survival of zebrafish embryos. (A) FITC-conjugated nanowires show dose-dependent toxicity to embryos, predominately between 8 and 20 hpf. (B–D) Silica nanoparticles modified with (B) amine groups, (C) FITC, or (D) rhodamine have negligible effects on embryo survival. Standard deviations (from clutch to clutch) for these experiments ranged from 0.006 to 0.09 and were omitted for figure clarity.
When embryos were exposed at 36 hpf, a time that corresponds with a major period of organogenesis in the zebrafish,34 none of the tested silica nanomaterials were toxic at the concentrations tested (Table 1; Figure 3) considering P = 0.05 as statistically significant. However, when exposed at 6 hpf, which corresponds with the time of gastrulation,34 silica nanowires generated mortality rates and survival curves indistinguishable from those corresponding with the 1- to 2-cell stage injections (Figure 4). Collectively, these data suggest peak embryonic sensitivity to silica nanowires beginning at the time of gastrulation and lasting through the time of neurulation (10 to 14 hpf34).
Figure 4.
Toxicity of silica nanomaterials is material- and exposure time-dependent. (A–F) Embryos were exposed at 36 hpf via microinjection into the yolk to (A) unmodified silica nanowires, (B) FITC-modified silica nanowires, (B) unmodified silica nanoparticles, (D) amine-modified silica nanoparticles, (E) FITC-modified silica nanoparticles, or (F) rhodamine-modified silica nanoparticles, and mortality was assessed at 132 hpf. No tested material was appreciably toxic using this exposure regime. (G, H) Embryos were exposed at 6 hpf via microinjection into the yolk to (G) FITC-modified silica nanowires or (H) rhodamine-modified silica nanoparticles, and mortality was assessed at 132 hpf. Nanowires but not nanoparticles are toxic to developing zebrafish embryos using this exposure regime. Standard deviations (from clutch to clutch) for these experiments ranged from 0.035 to 0.071 and were omitted for figure clarity.
We also noted an increased incidence of embryo deformities after exposure to silica nanowires at the 1- to 2-cell stage or at 6 hpf but not after exposure beginning at 36 hpf or after exposure to other silica nanomaterials (Figure 5). We regularly observed a number of profound deformities including holoprosencephaly (incomplete separation of the forebrain into hemispheres) with cyclopia (Figure 5, A) or with anophthalmia (Figure 5, B), and rarely observed two-headed embryos (Figure 5, C) indicative of anterior body axis duplication. The natural occurrence of holoprosencephaly in the zebrafish population has not been documented; however, the incidence of holoprosencephaly in humans is better documented. The frequencies of deformities we observed after nanowire exposure significantly exceeded the occurrence of holoprosencephaly reported in the human population.26 We did not observe embryonic deformity in any of the control (untreated or sham injected) or silica nanoparticle exposure categories (data not shown). Silica nanowires are therefore selectively teratogenic as well as developmentally toxic.
Figure 5.
Exposure to silica nanowires has teratogenic effects in zebrafish embryos. (A–C) Nanowire-exposed (at 0 hpf or 6 hpf) embryos with gross deformities (assessed at 60 hpf), such as (A) holoprosencephaly with cyclopia, (B) holoprosencephaly with anophthalmia, and (C) anterior axis duplication. (D) Control (water-injected) embryo assessed at 60 hpf. Scale bar in (D) (applies to all) = 50 µm. (e) eye, (h) heart, (y) yolk.
We sought to determine whether silica nanomaterials injected into the yolk accessed the embryo proper or if they were retained in the yolk and perhaps simply impeded nutrient flow to the embryo. Therefore, we generated fluorescently modified silica nanowires and nanoparticles to track their fate microscopically. The presence of an encapsulated fluorophore or an external functional group (amine) did not appreciably change the toxicity profile of any of the silica-derived nanomaterials; silica nanoparticles remained nontoxic and non-teratogenic, whereas silica nanowires remained toxic and teratogenic at relatively low doses (Figure 3; Table 1). FITC-modified nanowires were injected into the yolk of embryos at 6 hpf, and the embryos were imaged at 8 hpf. Labeled nanowires rapidly accumulated into the developing embryo, which at this developmental stage is referred to as the embryonic shield,34 and no appreciable fluorescence appeared in the yolk (Figure 6). Fluorescently modified silica nanoparticles also rapidly accumulated within the embryo after injection at 6 hpf (Figure 7, A–C, and data not shown), indicating that differential access to the embryo is not related to the differential toxicity of nanowires versus nanoparticles. Similarly, fluorescently modified silica nanowires and nanoparticles accumulated within tissues and organs of the developing embryo, most notably the eye and head, after injection at 36 hpf (Figure 7, D–F, and data not shown). The period of embryonic sensitivity to nanomaterials is therefore also not related to lack of nanomaterial access to the embryo.
Figure 6.
Modified silica nanowires are distributed throughout the embryo after exposure via microinjection into the yolk. (A) Bright-field image of embryo exposed at 6 hpf and imaged at 8 hpf(Y, yolk; ES, embryonic shield). (B) Same embryo imaged with epifluorescence optics showing the distribution of FITC-conjugated nanowires. (C) Merged image. Scale bar in (A) (applies to all) = 50 µm.
Figure 7.
Modified silica nanoparticles are distributed throughout the embryo after exposure via microinjection into the yolk. (A–C) Embryo exposed to rhodamine-conjugated silica nanoparticles at 6 hpf and imaged at 8 hpf (Y, yolk; ES, embryonic shield); (A) bright-field, (B) epifluorescence, and (C) merged images show nanomaterial distribution. (D–F) Embryo exposed to rhodamine-conjugated silica nanoparticles at 36 hpf and imaged at 38 hpf; (D) bright-field, (E) epifluorescence, and (F) merged images show distribution of nanomaterials within the head and eye (arrow in F). Scale bars in (A) (applies to A–C) and (D) (applies to D–F) = 100 µm.
The period of embryonic sensitivity to silica nanowires, together with the types of teratogenic defects observed, suggested that the nanowires may interfere with the morphogenetic processes of gastrulation and/or neurulation. To determine whether these processes were disrupted after silica nanomaterial exposure, we used a transgenic line that reports the expression pattern of the sonic hedgehog (shh) gene, 2.2shh:GFP.35 Native shh (and the 2.2shh:GFP transgene) is first expressed in zebrafish at 8 hpf at the anterior embryonic midline, in mesodermal tissues that form as a consequence of gastrulation. Expression then expands posteriorly, and the secreted shh protein regulates morphogenesis of the neural tube39 and the proximodistal patterning of the head and eyes.40,41 Defective SHH signaling during human development results in severe teratogenic effects including holoprosencephaly.42 Transgenic embryos injected with water or with unmodified silica nanoparticles and observed during neurulation (at 14 hpf) showed an expression domain of the transgene that was compact and that directly followed the midline (Figure 8, A; n = 24), indicating that gastrulation had taken place and neurulation was proceeding normally. In contrast, transgenic embryos injected with unmodified silica nanowires showed a diffuse expression domain of the 2.2shh: GFP transgene, and the level of fluorescence due to transgene expression was reduced in >60% of the embryos (Figure 8, B, n = 21), suggesting a defect in shh expression or in the formation of the shh-expressing structures during gastrulation. In addition, 12 of the 21 nanowire-treated embryos displayed a curiously raised, yet flattened neural keel (Figure 8, B); this was observed in two independent sets of experiments. This feature constitutes an unusual developmental morphology and represents clear defects in the process of neurulation.
Figure 8.
Silica nanowire exposure disrupts sonic hedgehog (shh) expression and neurulation. (A) Control, shh2.2:GFP transgenic embryo injected with water at 0 hpf and imaged at 14 hpf. (B) Transgenic embryo exposed to unmodified silica nanowires at 0 hpf and imaged at 14 hpf, showing abnormal neural keel morphology (arrows) and reduced expression of sonic hedgehog (arrowhead). Scale bar in (B) (applies to both) = 50 µm. Embryos were imaged using a combination of bright-field and epifluorescence microscopy.
Discussion
We have demonstrated that silica nanowires are highly and selectively toxic and teratogenic to developing zebrafish embryos. These and other silica nanomaterials enter the developing embryo from the yolk, but only those with high aspect ratios cause abnormalities and embryonic death. The results of our cell-free studies suggest that the visualization of fluorophore-conjugated nanomaterials is a relevant method for determining nanomaterial location within the developing embryo. Toxic doses of silica nanowires were extremely low, with an LD50 of 110 pg/g embryo. This degree of toxicity was not anticipated, as relatively high concentrations (190 µg/mL) of silica nanowires are required to achieve appreciable cytotoxicity in immortalized cell lines.18 Clearly, the impact of nanomaterial exposure for a developing, multicellular organism is greater than that for cultured cells.
The toxicity of silica nanowires in developing zebrafish embryos (the current study; LD50 = 110 pg/g embryo) is greater than that measured for carbon (C60) fullerenes (LD50 = 79 ng/g embryo29). However, we note that, in the current study, silica nanowire exposure and toxicity were achieved by microinjection of the material at embryonic stages prior to gastrulation, whereas fullerene toxicity was achieved by external administration to zebrafish at 36 hpf.29 A preliminary experiment administering fullerenes at the 1- to 2-cell stage by microinjection suggested an LD50 in the range of 10 pg/g embryo (data not shown). Embryos were sensitive to nanowire exposure over the period of gastrulation and neurulation and show defects in neurulation itself. Furthermore, embryos showed defects in gene expression related to gastrulation and neurulation after nanowire treatment. Some of the surviving embryos displayed developmental defects such as holoprosencephaly, consistent with an impact of nanowire exposure on these morphogenetic processes. In humans gastrulation and neurulation take place after implantation into the uterine wall, but prior to the establishment of a blood-placental barrier, and in many cases before maternal awareness of pregnancy. It is possible that nanomaterials present in maternal tissues or the maternal circulation would therefore have access to the developing embryo. Nanomaterial exposure to human embryos at this time would therefore result in an apparent outcome of reduced fertility and/or the generation of severe birth defects that are incompatible with postnatal life. Intraperitoneal injection of C60 fullerenes into pregnant mice results in abnormal embryonic development and ultimately embryonic death, corroborating that maternal exposure to nanomaterials has an impact on developing embryos in mammals.43 Future biomedical applications of silica nanowires must take this rather serious issue into consideration.
It is noteworthy that 1 µg/mL concentrations of silica nanomaterials—nanowires as well as nanoparticles—were nontoxic when delivered to embryos during organogenesis rather than prior to gastrulation. In vivo toxicity at a particular developmental time is evidently not predictive of toxicity at another. Therefore, although the development of nanowire-based drug delivery and imaging applications must still be approached with caution, the current studies suggest that general, in vivo toxicity of silica nanomaterials is low. Further work in other animal models will be needed to determine whether this is a broadly applicable finding. Our studies highlight the need for further in vivo testing of the effects of nanomaterials proposed for internal, biomedical use or heavy industrial use and the importance of considering sensitive periods of embryonic development when assessing toxicity.
The strikingly different effects of two silica-based nanomaterials are consistent with the hypothesis that aspect ratio may be an excellent predictor for the relative toxicities of nanomaterials having otherwise similar chemical properties.44 Poland and colleagues reported profound, toxic effects of carbon nanotubes within the abdominal cavities of mice that were directly related to nanotube aspect ratio.44 It is possible that materials with large surface areas and elongated shapes may generate mechanical disturbances in animal tissues that spherical materials do not. In the case of carbon nanotubes in adult mice, the consequences included a pathogenic inflammatory response,44 whereas in the current study, silica nanowires interfered with morphogenetic processes of early embryogenesis. The mechanism of that interference is currently under investigation.
Acknowledgments
We would like to thank Ann Norton for providing technical assistance with IVIS and confocal imaging.
This work was supported by the University of Idaho-BANTech Initiative and by NIH R01 EY012146 (D.L.S.).
Footnotes
Dr. McIlroy is the Vice President of Research of GoNano Technologies, Inc. The current article has no relationship to GoNano. No conflict of interest was reported by the authors of this article.
References
- 1.Rossin R, Muro S, Welch MJ, Muzykantov VR, Schuster DP. In vivo imaging of 64Cu-labeled polymer nanoparticles targeted to the lung endothelium. J Nucl Med. 2008;49(1):103–111. doi: 10.2967/jnumed.107.045302. [DOI] [PubMed] [Google Scholar]
- 2.Santra S, Dutta D, Moudgil B. Functional dye-doped silica nanoparticles for bioimaging, diagnostics and therapeutics. Food Bioprod Process. 2005;83(2):136–140. [Google Scholar]
- 3.Goldberg M, Langer R, Jia X. Nanostructured materials for applications in drug delivery and tissue engineering. J Biomater Sci. 2007;18(3):241–268. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaux IIM, Wang L, Zhang D, Gangadean D, McIlroy D, Kwon N, et al. Fibronectin bonding to nanowires and their internalization by epithelial cells. J Biomed Nanotech. 2006;2(1):1–6. [Google Scholar]
- 5.Kwon N, Beaux IIM, Ebert C, Wang L, Lassiter B, Park Y, et al. Nanowire-based delivery of Escherichia coli O157 Shiga toxin 1 A subunit into human and bovine cells. Nano Lett. 2007;7(9):2718–2723. doi: 10.1021/nl071179f. [DOI] [PubMed] [Google Scholar]
- 6.Santra S, Yang H, Dutta D, Stanley JT, Holloway PH, Tan W, et al. TAT conjugated, FITC doped silica nanoparticles for bioimaging applications. Chem Commun. 2004:2810–2811. doi: 10.1039/b411916a. [DOI] [PubMed] [Google Scholar]
- 7.Kumar M, Sameti M, Mohapatra S, Kong X, Lockey R, Bakowsky U, et al. Cationic silica nanoparticles as gene carriers: synthesis, characterization and transfection efficiency in vitro and in vivo. J Nanosci Nanotech. 2004;4(7):876–881. doi: 10.1166/jnn.2004.120. [DOI] [PubMed] [Google Scholar]
- 8.Lee CH, Cheng SH, Wang YJ, Chen YC, Chen NT, Souris J, et al. Near-infrared mesoporous silica nanoparticles for optical imaging: characterization and in vivo biodistribution. Adv Funct Mater. 2008;19(2):215–222. [Google Scholar]
- 9.Luo D, Saltzman W. Nonviral gene delivery: thinking of silica. Gene Ther. 2006;13(7):585–586. doi: 10.1038/sj.gt.3302662. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Chen J, Dong J, Jin Y. Comparing study of the effect of nanosized silicon dioxide and microsized silicon dioxide on fibrogenesis in rats. Toxicol Ind Health. 2004;20:21–27. doi: 10.1191/0748233704th190oa. [DOI] [PubMed] [Google Scholar]
- 11.He X, Nie H, Wang K, Tan W, Wu X, Zhang P. In vivo study of biodistribution and urinary excretion of surface-modified silica nano-particles. Anal Chem. 2008;80(24):9597–9603. doi: 10.1021/ac801882g. [DOI] [PubMed] [Google Scholar]
- 12.Ping W, He X, Wang K, Tan W, Ma D, Yang W, et al. Imaging breast cancer cells and tissues using peptide-labeled fluorescent silica nanoparticles. J Nanosci Nanotechnol. 2008;8(5):2483–2487. doi: 10.1166/jnn.2008.362. [DOI] [PubMed] [Google Scholar]
- 13.Tan K, Cheang P, Ho I, Lam P, Hui K. Nanosized bioceramic particles could function as efficient gene delivery vehicles with target specificity for the spleen. Gene Ther. 2007;14(10):828–835. doi: 10.1038/sj.gt.3302937. [DOI] [PubMed] [Google Scholar]
- 14.Chen M, von Mikecz A. Formation of nucleoplasmic protein aggregates impairs nuclear function in response to SiO2 nanoparticles. Exp Cell Res. 2005;305(1):51–62. doi: 10.1016/j.yexcr.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Choi D, McIlroy D, Nagler J, Aston E, Hrdlicka P, Gustin K, et al. One-dimensional silica structures and their application to the biological sciences. In: Choi D, Kumar C, editors. Nanomaterials for the life sciences, Volume 2, Nanostructured oxides. Hoboken (NJ): John Wiley & Sons; 2009. pp. 83–108. [Google Scholar]
- 16.Kneuer C, Sameti M, Bakowsky U, Schiestel T, Schirra H, Schmidt H, et al. A nonviral DNA delivery system based on surface modified silica-nanoparticles can efficiently transfect cells in vitro. Bioconjug Chem. 2000;11(6):926–932. doi: 10.1021/bc0000637. [DOI] [PubMed] [Google Scholar]
- 17.Luo D, Han E, Belcheva N, Saltzman W. A self-assembled, modular DNA delivery system mediated by silica nanoparticles. J Control Release. 2004;95(2):333–341. doi: 10.1016/j.jconrel.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Adili A, Crowe S, Beaux MF, Cantrell T, Shapiro PJ, McIlroy DN, et al. Differential cytotoxicity exhibited by silica nanowires and nanoparticles. Nanotoxicology. 2008;2(1):1–8. [Google Scholar]
- 19.Lin W, Huang YW, Zhou XD, Ma Y. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol Appl Pharmacol. 2006;217(3):252–259. doi: 10.1016/j.taap.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Jin Y, Kannan S, Wu M, Zhao JX. Toxicity of luminescent silica nanoparticles to living cells. Chem Res Toxicol. 2007;20(8):1126–1133. doi: 10.1021/tx7001959. [DOI] [PubMed] [Google Scholar]
- 21.Yang H, Liu C, Yang D, Zhang H, Xi Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol. 2008;29(1):69–78. doi: 10.1002/jat.1385. [DOI] [PubMed] [Google Scholar]
- 22.Qi S, Yi C, Chen W, Fong CC, Lee ST, Yang M. Effects of silicon nanowires on HepG2 cell adhesion and spreading. Chem Biochem. 2007;8(10):1115–1118. doi: 10.1002/cbic.200700119. [DOI] [PubMed] [Google Scholar]
- 23.Bharali DJ, Klejbor I, Stachowiak EK, Dutta P, Roy I, Kaur N, et al. Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Proc Natl Acad Sci USA. 2005;102(32):11539–11544. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park E, Park K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol Lett. 2009;184(1):18–25. doi: 10.1016/j.toxlet.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Borm PJA, Robbins D, Haubold S, Kuhlbusch T, Fissan H, Donaldson K, et al. The potential risks of nanomaterials: a review carried out for ECETOC. Particle Fibre Toxicol. 2006;3(11):1–35. doi: 10.1186/1743-8977-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsunaga E, Shiota K. Holoprosencephaly in human embryos: epidemiologic studies of 150 cases. Teratology. 1977;16(3):261–272. doi: 10.1002/tera.1420160304. [DOI] [PubMed] [Google Scholar]
- 27.Usenko CY, Harper SL, Tanguay RL. Fullerene C60 exposure elicits an oxidative stress response in embryonic zebrafish. Toxicol Appl Pharmacol. 2007;229(1):44–55. doi: 10.1016/j.taap.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harper S, Maddux B, Hutchison J, Tanguay R. Biodistribution and toxicity of nanomaterials in vivo: effects of composition, size, surface functionalization and route of exposure. NSTI-Nanotech. 2007;2:666–669. [Google Scholar]
- 29.Isaacson CW, Usenko CY, Tanguay RL, Field JA. Quantification of fullerenes by LC/ESI-MS and its application to in vivo toxicity assays. Anal Chem. 2007;79(23):9091–9097. doi: 10.1021/ac0712289. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Qin W, Zhang J, Cao C, Zhang J, Jin Y, et al. Europium(III) complexes/silica hybrid nanospheres synthesized in microemulsion. J Nanosci Nanotechnol. 2008;8(3):1218–1220. doi: 10.1166/jnn.2008.367. [DOI] [PubMed] [Google Scholar]
- 31.Yun H, Bang H, Lee WG, Lim H, Park J, Lee J, et al. Fluorescent intensity-based differential counting of FITC-doped silica nanoparticles: applications of CD4+ T-cell detection in microchip-type flowcytometers. Proc SPIE. 2006;6416:641–605. [Google Scholar]
- 32.Santra S, Wang K, Tapec R, Tan W. Development of novel dye-doped silica nanoparticles for biomarker application. J Biomed Opt. 2001;6(2):160–166. doi: 10.1117/1.1353590. [DOI] [PubMed] [Google Scholar]
- 33.Rossi L, Shi L, Quina F, Rosenzweig Z. Stöber synthesis of monodispersed luminescent silica nanoparticles for bioanalytical assays. Langmuir. 2005;21(10):4277–4280. doi: 10.1021/la0504098. [DOI] [PubMed] [Google Scholar]
- 34.Westerfield M. The zebrafish book: A guide for the laboratory use of the zebrafish (Danio rerio) 4th ed. Eugene (Ore): Oregon University of Oregon Press; 2000. [Google Scholar]
- 35.Shkumatava A, Fischer S, Muller F, Strahle U, Neumann CJ. Sonic hedgehog, secreted by amacrine cells, acts as a short-range signal to direct differentiation and lamination in the zebrafish retina. Development (Cambridge, England) 2004;131(16):3849–3858. doi: 10.1242/dev.01247. [DOI] [PubMed] [Google Scholar]
- 36.Nasevicius A, Ekker SC. Effective targeted gene ’knockdown’ in zebrafish. Nat Genet. 2000;26(2):216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 37.Tokumoto T, Tokumoto M, Horiguchi R, Ishikawa K, Nagahama Y. Diethylstilbestrol induces fish oocyte maturation. Proc Natl Acad Sci USA. 2004;101(10):3686–3690. doi: 10.1073/pnas.0400072101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chong M, Liao M, Drapeau P. The vesicular integral protein-like gene is essential for development of a mechanosensory system in zebrafish. Dev Neurobiol. 2008;68(12):1391–1405. doi: 10.1002/dneu.20671. [DOI] [PubMed] [Google Scholar]
- 39.Takamiya M, Campos-Ortega JA. Hedgehog signalling controls zebrafish neural keel morphogenesis via its level-dependent effects on neurogenesis. Dev Dyn. 2006;235(4):978–997. doi: 10.1002/dvdy.20720. [DOI] [PubMed] [Google Scholar]
- 40.Ekker SC, Ungar AR, Greenstein P, von Kessler DP, Porter JA, Moon RT, et al. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr Biol. 1995;5(8):944–955. doi: 10.1016/s0960-9822(95)00185-0. [DOI] [PubMed] [Google Scholar]
- 41.Stenkamp DL, Frey RA. Extraretinal and retinal hedgehog signaling sequentially regulate retinal differentiation in zebrafish. Dev Biol. 2003;258(2):349–363. doi: 10.1016/s0012-1606(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 42.Cohen MM, Jr, Shiota K. Teratogenesis of holoprosencephaly. Am J Med Genet. 2002;109(1):1–15. doi: 10.1002/ajmg.10258. [DOI] [PubMed] [Google Scholar]
- 43.Tsuchiya T, Oguri I, Yamakoshi YN, Miyata N. Novel harmful effects of [60]fullerene on mouse embryos in vitro and in vivo. FEBS Lett. 1996;393(1):139–145. doi: 10.1016/0014-5793(96)00812-5. [DOI] [PubMed] [Google Scholar]
- 44.Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3(7):423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]