Abstract
Enzyme replacement therapy has been successful in alleviating morbidity and improving endurance in Mucopolysaccharidosis (MPS) type I, II, and VI, however little attention has been paid to the effects on bone mineralization. Brief case reports in MPS type III and IV suggest that bone mineral density (BMD) is diminished, but did not account for patient size. In this report, BMD was evaluated by quantitative computed tomography and by dual-energy x-ray absorptiometry (DXA) in separate studies involving 10 patients with MPS type VI (7 Female; 7.0 to 21.0 y) and 4 male patients with MPS II (8.1 to 35.5 y). Vitamin D intake met the current RDA (200 IU) for most, though 25-OH vitamin D was insufficient (< 30 ng/mL) in 87.5% of patients tested. Ht Z-score was low −5.8 ± 3.6, with height deficits greatest in MPS VI. Spine and whole body BMD Z-scores by DXA were considered normal for chronological age in all MPS II, and after correction for Ht Z-score, in all but one subject with MPS VI. These results suggest that vitamin D insufficiency is quite common in MPS. BMD by DXA is within normal range for most, particularly after correction for short stature. A review of bone health assessment is provided as well as a discussion of these results.
Keywords: DXA, Bone mineral density, low bone mass, vitamin D, MPS II, MPS VI
1. Introduction
Mucopolysaccharidoses are rare inherited lysosomal storage disorders. Twelve primary metabolic disorders have been identified with resulting morbidities originating from an inadequate breakdown of glycosaminoglycans (GAGs). GAGs accumulate in the lysosomes of the effected individuals leading to profound growth deficits, facial dysmorphia, organomegaly and poor joint mobility. Though bone growth and mineralization are affected by GAG accumulation in the tissues of animal models [30,31], there is a paucity of research focused on the assessment of bone mineralization in patients with MPS. This report is focused on the assessment of bone density in 2 types of MPS patients, those with Hunters syndrome or MPS type II, and those with Maroteaux-Lamy Syndrome or MPS type VI. A brief overview of the assessment of bone in healthy populations is provided as a framework from which details of the pilot studies are provided.
1.1. Low bone mass and fracture risk
Osteoporosis is one of the most common chronic conditions in the U.S., one in every two Americans over 50 years of age will be diagnosed with osteopenia or osteoporosis in the next 10 years [33]. However osteoporosis is not just a disease of the elderly. It is now understood that the development of osteoporosis occurs over a lifetime and many children with chronic illnesses are at risk for low bone mass. The acquisition of bone mass proceeds steadily until peak bone mass is achieved, between 16 to 25 years of age for most individuals. A plateau in bone mineralization occurs over the third and forth decades of life. As individuals enter the later years, bone resorption is greater than formation and there are gradual withdrawals from the ‘bone bank’ due to aging, menopause and exposure to illness or medications. The greater the peak bone mass attained, the more bone losses an individual can tolerate without becoming vulnerable to fractures and subsequent pain and disability.
It has been estimated that 60 to 80% of the variability in peak bone mass is attributed to heritable factors [14]. There are, however, a number of modifiable factors that may positively influence the development of peak bone mass including: weight bearing physical activity [32], calcium, vitamin D and protein intake [16,25]. Therefore, patients with MPS and a particularly small frame, abnormal gait or limited mobility and decreased physical activity, or post-surgical non-weight bearing are at increased risk for poor bone mineralization. Additionally, the presence of nutritional deficiencies, in particular vitamin D deficiency, in this population may also negatively impact bone accumulation.
Ultimately, low bone mass places an individual at increased risk for fracture. Fragility type fractures are most commonly reported in post-menopausal women, however, there is a second peak in fracture incidence which occurs during adolescence [23]. Fracture is more common in males than females during adolescence with the peak age of fracture around 14 years and for females around 12 years, similar to the age of peak height velocity. It has been theorized that fracture incidence is elevated during this period of rapid growth due to the lag in mineralization of bone following skeletal growth [2]. In adolescents, the most common site of fracture is the forearm, resulting from high impact, sports-related injuries and increased overall physical activity. Fragility fractures of the spine or hip which commonly occur in the elderly are less likely to be seen in children, with a few exceptions for some children with low bone mass as a result of a chronic illness [10,21,35].
There are two published case reports of atraumatic fragility type fracture in patients with Mucopolysaccharidosis (MPS). One was a report from Spain published in 1992, of a 20-year-old female with MPS type VI who presented with a stress fracture of the right femoral neck [11]. A screw was surgically fixed into the femoral neck, however, the patient returned after 12 months complaining of persistent pain and decreased range of motion. A second surgical intervention with bone graft was required for successful healing. A second case report was published in 1999 from Japan of a 12-year-old male with MPS type II with a transverse fracture of the left femoral neck from minimal trauma, followed by resorption of the femoral head. Then, at 16, he had a right femoral neck stress fracture [18]. Stress fractures, such as these, may occur in MPS due to abnormal length and diameter of the femoral neck compounded by hip dysplasia and/or osteonecrosis of the femoral head.
Bone strength, elasticity, architecture and geometry are all bone-related factors important in the prediction of fracture. However bone-independent factors are sometimes overlooked, especially in children, for example the propensity to fall, as well as the patient’s vision, balance and/or gait. Muscle strength and coordination are also quite important as well as the impact of the load placed on the skeleton during a fall, or the overall weight of the patient.
1.2. Assessment of bone strength and fracture risk
There are two main determinants of bone strength: bone density and quality. The areal density of bone is determined by the size of the bone as well as the content or grams of mineral per unit area. The quality is determined by its material properties, the microarchitecture of the bone, or its shape and geometry as well as the presence of microfractures. As these parameters are inaccessible in vivo, bone mineral density (aBMD) is used as a proxy measure for bone strength, with the clinical outcome of interest being fracture. Approximately 70 percent of bone strength is predicted via BMD by DXA; there is no better single measure for predicting fracture risk than BMD in adults. The use of DXA in pediatrics is a developing field, though it remains the most frequently used tool given the rapid scan time, low radiation exposure and availability of robust pediatric reference data [7]. Whole body, lumbar spine and proximal femur bone mineral content (BMC) and BMD are acquired by DXA. Following analysis, standard deviation scores or Z-scores based on age and gender specific reference data are used to categorize BMD values into normal or low density for chronological age (Z-score < −2.0) [27].
1.3. Challenges to the assessment of bone health in MPS
One of the main challenges to the assessment of bone health in patients with MPS is accurate interpretation of the results obtained. Patients with MPS have considerable height deficits, often with disproportionately large heads (macrocephaly) with relatively dense skulls. BMD assessed by DXA is a 2-dimensional image or an “areal” (g/cm2) density, denoted aBMD, and not a true volumetric density [3], vBMD. In general a larger diameter bone will have greater aBMD than a smaller diameter bone, even if both have the same vBMD. Therefore, size considerations are very important when interpreting aBMD measures [8]. Additionally, there are assumptions made regarding the composition of the head region in the analysis software for the whole body scan. If the head is relatively large, or the bones have abnormal thickness in relationship to the rest of the body, those assumptions are overestimated for the whole body. Skeletal contractures and stiff shoulder, hip, and elbow joints, poor rotation of the hip and hip dysplasia also create challenges towards accurate and reproducible positioning for a DXA scan. Finally, the presence of orthopedic hardware, tracheostomies, and feeding tubes will artificially increase bone mineral content.
Brief case reports in MPS type III and IV suggest that aBMD is diminished [28,29], but do not account for patient size, specific treatment effects [Bone marrow transplant (BMT) or enzyme replacement therapy (ERT)], or other endocrine or nutritional factors that might impact bone health. It remains unknown as to which tools are most informative in predicting fracture risk or most reproducible for monitoring bone mineralization in patients on ERT. The purpose of these pilot studies was: 1) to characterize bone density in a small cohort of patients before and after ERT, and 2) to explore associations between bone density, growth and vitamin D levels in patients maintained on ERT.
2. Methods
Two separate studies were performed with patients from Children’s Hospital & Research Center at Oakland: Study A: a longitudinal assessment of growth and bone mineral density by Quantitative Computed Tomography (QCT) in patients with MPS type VI naïve to enzyme replacement therapy before and after treatment [BioMarin-sponsored Phase 1/2 clinical trial of recombinant human n-acetylgalactosamine 4-sulfatase (rhASB)] and, Study B: A cross-sectional study of bone density and strength in relation to nutritional intake and vitamin D status in subjects with MPS type II or VI on enzyme replacement therapy. Methods pertaining to these 2 studies are detailed separately.
2.1. Study A
Six subjects with confirmed MPS VI were recruited and informed consent obtained as part of the Phase 1/2 study of rhASB [12]. Standing height was measured and volumetric bone mineral density (vBMD) was determined by quantitative computed tomography (QCT) in coordination with liver volume assessment at 4 time points: baseline then at 24, 96 and 144 weeks after initiation of rhASB therapy. Trabecular vBMD (mg/cm3) of lumbar spine vertebrae L2-L4 was assessed using Mindways software (San Francisco, CA) according to published methods, and Z-scores calculated [5]. QCT is an established technique for measuring vBMD, with an effective radiation dose to the patient of 60 μSv and a precision of 1 to 3% [1]. Patients were randomized to receive either 0.2 (n = 3) or 1.0 mg/kg (n = 3) of rhASB as weekly intravenous infusions in a double blind design. Subjects remained on the assigned dose until after the week 48 evaluation, at which time the dose was escalated to 1.0 mg/kg/wk according to protocol [13], Two subjects who were on the low dose switched to the higher dose at weeks 59 (#45) and weeks 69 (#41).
2.2. Study B
Subjects with either confirmed MPS II or VI who received ERT on a regular basis at CHRCO were invited to participate in a cross-sectional assessment of bone health and informed consent was obtained. Lumbar spine (L1–L4), proximal femur (hip) and whole body bone mineral content and “areal” bone mineral density (aBMD) were assessed using DXA (Discovery A, Hologic, Bedford MA, software version 12.6). Additionally, blood samples were drawn pre-ERT infusion in the fall months, anthropometry assessed and pubertal stage determined by physician examination. A brief calcium and vitamin D focused food frequency was completed by interview, and compared to the U.S. Dietary Reference Intake for age and gender (% of Adequate Intake). Height was calculated from knee height [6]. Age and gender specific aBMD Z-scores were calculated from manufacturer reference databases and BMC Z-scores calculated from the published BMDCS reference data [20]. aBMD Z-scores were then adjusted for height Z-score according to the method of Zemel [36]. This novel method provides an adjustment for growth deficits on aBMD by DXA, while avoiding possible confounding of pubertal maturation. A group of 30 healthy controls without MPS (8 to 30 years) were recruited locally for a separate study, consented and similar assessments made.
For both studies, height Z-scores were calculated as the difference between the observed value and the age and gender-specific median value for the CDC U.S. reference population divided by standard deviation of the reference population [22,9]. For patients > 20 years, height Z-score was calculated using the oldest age in the reference database. Consistent with the ISCD guidelines, a cut off aBMD Z-score value of −2.0 is used to characterize patients with normal or low bone mass for chronological age [26]. Statistical analyses were conducted using Stata 9.0 (Stata, Inc., College Station, TX), statistical significance was defined as p <0.05.
3. Results
3.1. Study A
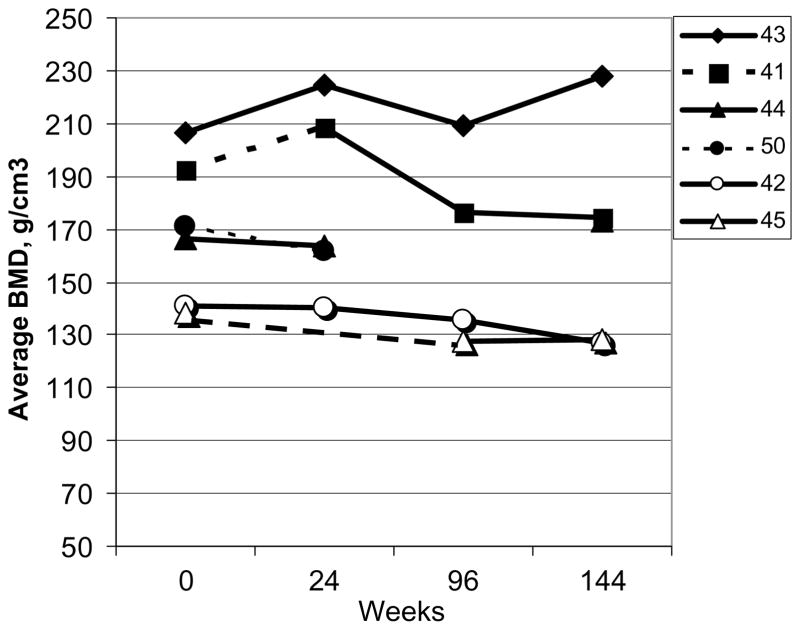
Six subjects were included in this study, 4 female, 7 to 16 years of age with the majority pre-pubertal (Table 1). Initial GAG levels, an indication of disease severity, were quite variable, as well as the distance traveled during the 6 minute standardized walk test. Lumbar spine vBMD is presented for the six subjects with MPS VI over time (Fig. 1). An increase in absolute vBMD from baseline was observed in two patients during Phase I, one each on the starting rhASB dose 0.2 and 1.0 mg/kg/wk. There were no other distinguishing characteristics of these 2 subjects. Considerable within subject variability was observed with time. Change in absolute vBMD varied from 8.0% to −10.2% depending upon the time point; all were considered greater than instrumentation error of 3%.
Table 1.
Demographics for subjects with MPS VI enrolled in study A
| Patient ID | Age, yrs | Sex | Height cm | HAZ | Tanner Stage | Initial GAG ug/mg Cr | Initial 6 min walk distance | Initial Dose of rhASB |
|---|---|---|---|---|---|---|---|---|
| 43 | 16 | F | 96.5 | −9.9 | 2 | 500 | 53 m | 1.0 |
| 44 | 12 | F | 93.5 | −8.5 | 3 | 416 | 88 m | 1.0 |
| 42 | 7 | M | 110 | −2.3 | 1 | 218 | 388 m | 1.0 |
| 41 | 7 | F | 93.5 | −4.9 | 1 | 520 | 197 m | 0.2 |
| 50 | 13 | M | 86.0 | −8.5 | 1 | 379 | 133 m | 0.2 |
| 45 | 11 | F | 119 | −3.7 | 1 | 158 | 283 m | 0.2 |
HAZ: height-for-age Z-score.
GAG: glycosaminoglycan dermatan sulphate, ug/mg creatinine.
6 minute walk distance: m = meters.
rhASB dose: mg/kg/week.
Fig. 1.
Individual data for volumetric bone mineral density (vBMD, g/cm3) of the spine by QCT in 6 subjects with MPS VI: Phase I/II clinical trial for rhASB (Study A). Solid line: 1.0 gm/kg/wk dose of rhASB; Dashed Line 0.2 gm/kg/wk dose. Patient #43: patient with vitamin D deficiency prior to study initiation Patient #50 withdrew from the study at week 32.
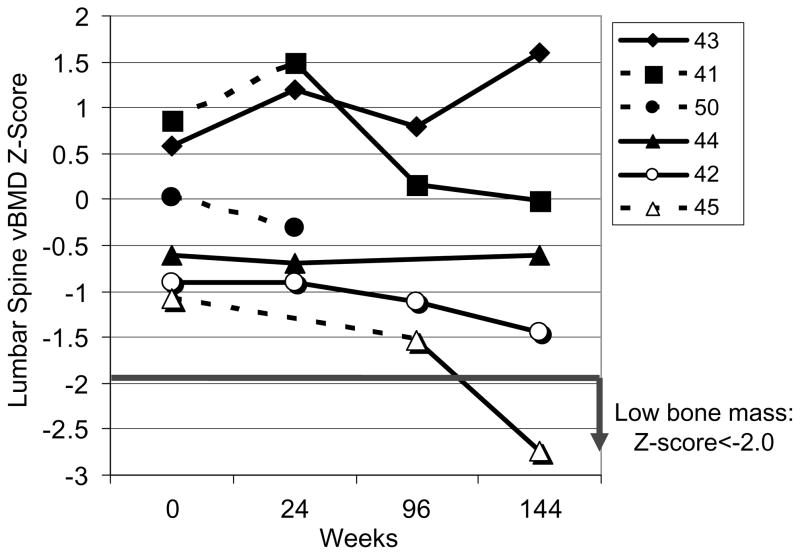
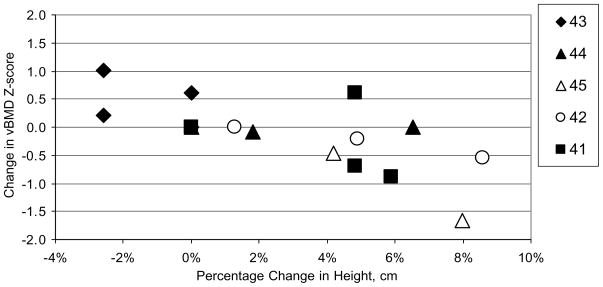
vBMD Z-scores by time of study are presented in Fig. 2. One patient with the greatest improvement in spine Z-score (#43) was vitamin D deficient prior to the start of the study, was receiving vitamin D replacement during the study and had a dramatic improvement in her walk test. This patient was also on the higher dose of rhASB (1.0 mg/kg/wk) throughout the study. All patients had vBMD Z-scores above −2.0 at study initiation, and all but one (#45) remained above −2.0 throughout the study. The subject with the greatest improvement in vBMD Z-score, had no change in stature during the study, whereas the subject with the greatest drop in vBMD Z-score had an 8% increase in height during the 144 week trial (Fig. 3). For the group as a whole, there was a negative correlation observed between change in vBMD Z-score and change in height (r2 = 0.55), suggesting that as growth improved during enzyme replacement therapy, volumetric bone mineral density decreased.
Fig. 2.
Individual data for volumetric bone mineral density (vBMD) Z-score of the spine by QCT in 6 subjects with MPS VI on Enzyme replacement therapy at either 1.0 mg/kg/wk (Solid line) or 0.2 mg/kg/wk (Dashed line). Low bone mass, defined as Z-score < −2.0, observed in only one subject at 144 weeks. Patient #50 withdrew from the study at week 32.
Fig. 3.
Change in Spine vBMD Z-score relative to Percentage Change in Height (cm) with time of study (24, 96, 144 weeks) in 5 subjects with MPS VI on Enzyme replacement therapy.
3.2. Study B
Eight subjects (8 to 35 years) with MPS type II or VI were included in this cross-sectional, observational pilot study. All had been on enzyme replacement therapy [Elaprase® (iduronate-2-sulfase) or Naglazyme® (galsulfase)] for a minimum of 20 months. Two subjects with MPS II were also diagnosed with von Wilde-brand’s syndrome (#102 and #103). More detailed subject demographics are included in Table 2. In this small sample there was considerable variability in disease severity and ambulation. Two subjects had tracheostomies and hearing aids (#101, 107), one was measured one month status post spinal decompression surgery (#101). Only one of eight subjects (#106, 12.5%) had a history of fracture, this was the oldest subject with MPS II, with the mildest disease who was quite active in a variety of sports. He sustained a shoulder fracture at age 11 subsequent to falling from a bunk bed.
Table 2.
Demographics for subjects enrolled in study B
| Age, yrs | Sex | HAZ | Yrs ERT | Ambulation | PTH pg/mL | 25OH D ng/mL | Diet Ca %AI | Diet Vit D %AI | Baseline GAG ug/mg cr | |
|---|---|---|---|---|---|---|---|---|---|---|
| MPS II | ||||||||||
| ID-102 | 11.4 | M | −2.4 | 4.7 | Independent | 50 | 16 | 92% | 134% | 316 |
| ID-103 | 8.1 | M | 1.4 | 1.8 | Toe walker | 25 | 23 | 143% | 134% | 818 |
| ID-104 | 10.1 | M | 0.3 | 3.8 | Independent | 38 | 11 | 29% | 2% | 591 |
| ID-106 | 35.5 | M | −2.6 | 4.5 | Active | 42 | 24 | 161% | 174% | 88 |
| MPS VI | ||||||||||
| ID-101 | 21.0 | M | −9.9 | 5.0 | Scooter | 34 | 148 | 143% | 218% | 384 |
| ID-105 | 16.8 | F | −6.3 | 4.6 | Independent | 40 | 24 | 37% | 28% | 243 |
| ID-107 | 18.5 | F | −9.3 | 4.5 | Scooter | . | 14 | 14% | 74% | 432 |
| ID-108 | 13.2 | F | −7.4 | 4.5 | Independent | 54 | 19 | 66% | 145% | 383 |
| Controls | ||||||||||
| n = 34 | 8–30 | 15 M 19 F | 0.0 ± 1.1 | Active | 15–75 | 32 ± 8 (18–54) | 98 ± 44 | 110 ± 71 | n/a | |
Mean ± SD (range).
HAZ: Height-for-age Z-score.
PTH: parathyroid hormone, reference range for laboratory = 15–75 pg/mL.
25-OH vitamin D: insufficient range defined as < 30 ng/mL.
Dietary Calcium and Vitamin D, % of Adequate Intake for age and gender.
Baseline GAG: glycosaminoglycan levels prior to start of enzyme replacement therapy.
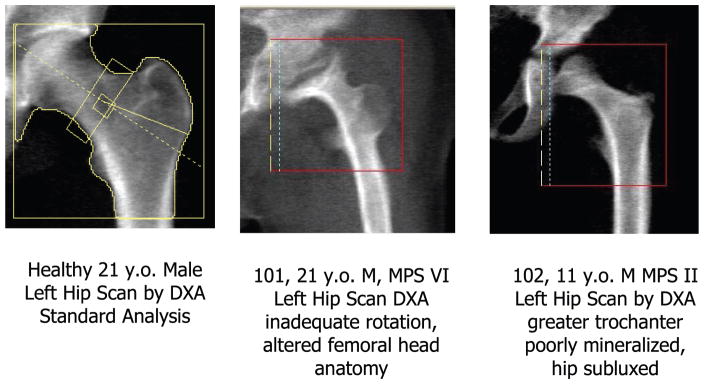
As expected there were some difficulties with obtaining accurate scans by DXA. Some subjects had non-removable artifacts for the whole body scans (e.g. tracheostomies), difficulty in optimal positioning for the whole body and hip scans due to contractures and stiff joints. Additionally, as is shown in Fig. 4, many of the hip scans were poor or non-analyzable, due either to inadequate mineralization or substantially altered anatomy. As a result, the hip scans did not contribute to the remainder of the analyses and discussion.
Fig. 4.
Diagnosis of Low Bone Mass in MPS using proximal femur (hip) scans by DXA is complicated due to poor hip rotation, altered femoral head anatomy and therefore poor analytic reproducibility.
On average spine aBMD Z-scores were within the normal range for chronological age for all subjects with MPS II (Fig. 5a). Spine aBMD Z-score was low in 3 of 4 subjects with MPS VI, but normalized in all but one subject (#101), after correction for height-for-age Z-score. Whole body aBMD Z-scores were also within normal range for chronological age for all subjects with MPS II (Fig. 5b) and remained so after correction for their mild short stature. Whereas whole body aBMD Z-scores were notably reduced in two subjects with MPS VI (#107: Z = −2.7; #108: Z = −3.7). Subject #101, one of the more severely effected patients with MPS VI, had a particularly dense and large head, which appeared to compensate for reduced bone mineral content for reduced skeletal size (HAZ = −9.9). When these whole body aBMD Z-scores were corrected for height-for-age Z-score, their Z-scores normalized. Given the size and density of the head region for some with MPS VI, the height Z-score adjustment method appears to over estimate the corrected whole body aBMD Z-score, as observed in patients #105, #107 and #108; adjusted Z-scores were> + 2.0 for some. The more moderate adjustments for subjects #101 and #106 were likely the result of their mature age and pubertal status.
Fig. 5.
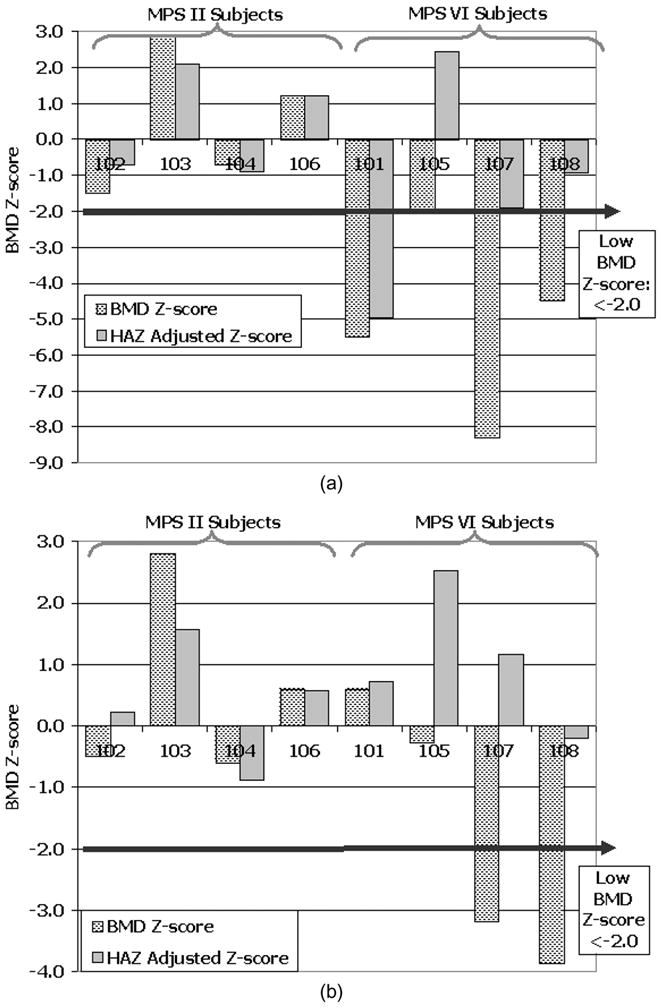
(a) PA lumbar spine (L1-L4) aBMD Z-score by DXA for chronological age (hashed bars) and corrected for Height-for-age Z-score (grey bars) in subjects with MPS II and VI. (b) Whole body aBMD Z-score by DXA for chronological age (hashed bars) and corrected for Height-for-age Z-score (grey bars) in subjects with MPS II and VI.
Circulating 25-OH vitamin D levels were in the in-sufficient range (20 to 30 ng/mL) in 3 of 8 subjects, and deficient in four subjects with levels < 20 ng/mL. 25-OH vitamin D was only above 30, the level considered sufficient for bone health, in one subject who was on high-dose supplementation. Interestingly, this is the only MPS VI patient (#101) with a normal whole body scan Z-score which is quite surprising given his notable short stature, height Z-score −9.9. Overall, 25-OH vitamin D values were low despite the majority of subjects with dietary vitamin D intakes consistent with current Institute of Medicine recommendations for adequate intake of vitamin D for healthy populations of 200 IU/day [19]. Additionally, all samples were drawn between August and October, a season in which the highest level of 25-OH vitamin D is typically observed in North America. Despite these low circulating values of vitamin D, PTH was not elevated in any subject.
4. Discussion
4.1. Study A
This pilot study is the first to assess volumetric bone mineral density in patients with MPS before and after enzyme replacement therapy. In this small sample, we observed that spine vBMD Z-score was within the normal range for chronological age for all 6 patients prior to receiving enzyme replacement therapy. Two subjects had slight improvements in Z-score during the first six months of treatment, one possibly related to vitamin D supplementation.
An interesting finding in this pilot study was the inverse relationship between the change in spine vBMD Z-score and the change in height (Fig. 3). These preliminary findings suggest that as a patient’s growth improves on enzyme replacement, there may be a decrease in bone mineral density Z-score. A similar scenario is commonly reported in healthy children during the adolescent growth spurt when bone growth precedes mineralization and bone density decreases [9]. Unfortunately, due to instrumentation and software upgrades, we were unable to follow these patients after week 144, and therefore, unable to confirm the recovery of vBMD. Additionally, measurement error, or an increase in joint stiffness cannot be ruled out regarding the decrease in linear growth observed for subject 43. In order to better inform clinical care and the potential transitory nature of the BMD deficits, this finding should be reproduced in larger studies.
Bone densitometry by QCT can be extremely precise and is able to differentiate trabecular from cortical bone changes, therefore is often more sensitive to small changes subsequent to an intervention, such as bisphosphonate administration. Additionally, QCT images are less influenced than DXA by the effects of overlying tissue or abnormal skeletal anatomy such as spine osteophytes. Central QCT is however, limited to the assessment of only the spine or hip. In this study, data are available for the lumbar spine only, therefore, we are unable to make generalizations to whole body bone homeostasis. Another limitation of the study was the higher radiation exposure a patient receives from an abdominal CT scan for bone densitometry assessment, approximately 60 μSv for the new scanners in comparison with a DXA scan of 2 μSv. At our institution, this technique is not recommended as a routine tool for assessment and monitoring bone density unless the patient was to undergo a CT scan for another purpose, such as in this study. Alternative tools with lower radiation exposure and cost, such as DXA or peripheral QCT, are preferable for routine clinical assessment.
4.2. Study B
To our knowledge, this is the first study to assess bone mineralization by DXA in young patients with MPS II and VI. Somewhat to our surprise, we found that patients with MPS II treated with ERT for at least 20 months, had normal aBMD Z-scores (> −2.0) for chronological age at the spine and whole body. Patients with MPS VI on ERT had more sizeable deficits in vBMD Z-score at the spine and whole body. Unadjusted aBMD appears to be most depleted in older patients who started ERT perhaps later in life, particularly those with the greatest height deficits. However, when new techniques are utilized to adjust for the extensive height deficits observed, aBMD Z-scores improved to within the normal range in all but one subject.
Proximal femur scans proved to be un-analyzable for most of the MPS VI patients and the younger patients with MPS II. Inadequate hip rotation due to joint contractures and altered anatomy and hip subluxation due to limited weight bearing contributed to the difficulty of the manufacturer software to accurately analyze these scans. For the few subjects with relatively normal anatomical scans, Z-scores were all within the normal range. The lateral distal femur scan is now considered an alternative scan for many contracted patients that are non-weight bearing and difficult to position such as those with cerebral palsy. Robust pediatric reference data are now available for this site and, though it was not assessed in the present study, it may prove to be a useful alternative site for patients with MPS as well [37].
Others have used the same technology (DXA) to assess aBMD in patients with MPS III and IV. Rigante et al. published a case report of three patients with MPS III between 11 and 24 years of age [19]. Spine and hip Z-scores were reported in the normal range in the youngest ambulatory subject. However, the older 2 non-weight bearing subjects had greater reductions in Z-scores. These Z-scores were not corrected for height deficits. All three subjects had deficient 25(OH) vitamin D values, though PTH was in the normal range. Two years later, the same group assessed spine and hip aBMD by DXA in 2 pre-pubertal subjects with MPS IV [20]. Spine aBMD Z-scores for these children were within the normal range. Hip Z-scores were more affected than spine. No history of fracture was reported in either case report. They summarized that immobilization and vitamin D deficiency are likely contributing factors to decreased aBMD.
Vitamin D deficiency was quite common in our population despite blood sampling at the end of the summer (87.5% of MPS vs. 31% of healthy local controls). Vitamin D deficiency has been observed in many other chronic pediatric disorders such as sickle cell disease [4], thalassemia [34], and cerebral palsy [15]. The relationship between vitamin D sufficiency and bone mineralization is robust [17], therefore it is interesting that all 4 patients with MPS II had normal aBMD Z-scores by DXA, regardless of vitamin D insufficiency. What is clear is that vitamin D insufficiency is common in patients with MPS, despite residence in northern California. Identification, treatment and monitoring of vitamin D insufficiency according to current protocols is suggested, not only for bone health but also for extraskeletal effects such as optimal immune and cardiac function and cancer reduction [24,25].
One limitation of this study is that all subjects were currently prescribed ERT, and bone density scans were not obtained prior to initiation of enzyme replacement therapy. Given this lack of a true baseline value, we are unable to determine if the normative values obtained in this population of subjects existed prior to ERT or resulted from ERT. Additional useful tools to characterize bone health in this population will be biochemical markers of bone turnover and inflammation. Those samples have been collected and will be analyzed as part of the ongoing longitudinal assessments in this cohort of subjects. Future studies should expand upon these findings and include tools to assess bone strength and fracture risk in vivo, such as peripheral quantitative computed tomography.
5. Recommendations for assessment of bone density in MPS
Spine and whole-body aBMD by DXA are reasonable modalities for monitoring bone mineral density in patients with MPS on enzyme replacement therapy.
The relationship between the change in aBMD and growth should be closely monitored and considered in the interpretation of DXA scans.
aBMD Z-score should be interpreted with relation to height deficits and/or pubertal delay, particularly in patients with height Z-score < −2.0 or height < 5th percentile.
When available, whole body aBMD scan ‘less head’ is preferable to total whole body aBMD scan, given the relative size of the head and thickness of skull bones in proportion to the whole body. This is particularly important for patients with MPS VI.
Proximal femur scans should not be performed in subjects who are non-weightbearing, with notable contractures due to incomplete hip rotation and altered anatomy. These scans should not be performed in patients < 10 years of age due to inadequate mineralization. These factors limit the predictability and reproducibility of the scan.
Acknowledgments
The authors express their gratitude and appreciation to the subjects and their families who participated in these studies, and to the expert assistance of all the study site coordinators and site personnel who participated in the Phase I/II Trials of rhASB for MPS VI. We also acknowledge Annie Higa for her assistance with the blood collection, storage and sample processing, and the contributions of the BioMarin employees and consultants who performed important roles during the Phase I/II studies of MPS VI. The bone studies reported herein were supported in part by the following grants: UL1RR024131 from the National Center for Research Resources, the 2008 Student Research program funded by the American Pediatric Society & Society for Pediatric Research, the National Institute of Child Health and Human Development (T35-HD007446). BioMarin Pharmaceutical Inc. provided study data and baseline urine GAG results for subjects who participated in the ASB-03-05 clinical study and Shire Human Genetic Therapies, Inc. provided baseline urine GAG results for subjects who participated in TKT 024 clinical study. BioMarin Pharmaceutical Inc. reviewed this manuscript for accuracy of dates and discussion of Phase I/II MPS VI trial of rhASB. This manuscript was developed as the result of a meeting of experts entitled “Promoting Bone Health in MPS VI: Framing New Therapies” held in Oakland, California in October, 2008. This meeting was supported by an educational grant from BioMarin Pharmaceutical, Inc., Novato, CA. BioMarin had no role in the content presented and discussed at the meeting. This work was also supported in part by the following NIH grant: K23HL076468. All authors participated in the development and writing of the manuscript and are fully responsible for its content.
Footnotes
Conflict of interest
Dr. Harmatz has provided consulting support to BioMarin Pharmaceutical Inc, Novato, CA, and Shire HGT Inc. Cambridge, MA. Dr. Harmatz has also received speaker’s honorarium and travel support from BioMarin and Shire. Dr. Fung has received speaker’s honorarium from BioMarin Pharmaceutical Inc. Novato, CA. Tiffany Kim has received travel support from BioMarin.
References
- 1.Adams JE. Non-invasive bone mass measurement techniques and applications. Advanced Hospital Technology. 1992:1. [Google Scholar]
- 2.Bailey DA, McKay HA, Minwald RL, Corcker PRE, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: The University of Saskatchewan bone mineral accrual study. J Bone Miner Res. 1999;14:1672–1679. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 3.Binkovitz LA, Henwood MJ. Pediatric DXA: Technique and interpretation. Pediatric Radiol. 2006 doi: 10.1007/s00247-006-0153-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buison AM, Kawchak DA, Schall J, Ohene-Frempong K, Stallings VA, Zemel BS. Low vitamin D status in children with sickle cell disease. J Pediatr. 2004;145(5):622–627. doi: 10.1016/j.jpeds.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 5.Cann CE, Genant HK, Kolb FO, Ettinger B. Quantitative computed tomography for prediction of vertebral fracture risk. Bone. 1985;6:1–7. doi: 10.1016/8756-3282(85)90399-0. [DOI] [PubMed] [Google Scholar]
- 6.Chumlea WC, Guo SS, Steinbaugh ML. Prediction of stature from knee height for black and white adults and children with application to mobility-impaired or handicapped persons. J Am Diet Assoc. 1994;94:1385–1388. doi: 10.1016/0002-8223(94)92540-2. [DOI] [PubMed] [Google Scholar]
- 7.Crabtree NJ, Leonard MB, Zemel BS. Dual-Energy X-ray Absorptiometry. In: Sawyer AJ, Bachrach LK, Fung EB, editors. Bone Densitometry in Growing Patients: Guidelines for Clinical Practice. Humana Press; Totowa NJ: 2007. [Google Scholar]
- 8.Fewtrell MS, Gordon I, Biassoni L, Cole TJ. Dual X-ray absorptiometry (DXA) of the lumbar spine in a clinical paediatric setting: does the method of size-adjustment matter? Bone. 2005;37:413–419. doi: 10.1016/j.bone.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 9.Frisancho AR. Anthropometric Standard for the Assessment of Growth and Nutritional Status. Ann Arbor, MI: University of Michigan Press; 1990. [Google Scholar]
- 10.Fung EB, Harmatz P, Milet M, Coates T, Thompson A, Ranalli M, Mignaca R, Scher C, Giardina P, Robertson S, Neumayr L, Vichinsky E. Fracture Prevalence and Relationship to Endocrinopathy in Iron Overloaded Patients with Sickle Cell Disease and Thalassemia. Bone. 2008 March 15; doi: 10.1016/j.bone.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guiral J, Sanchez JM, Gonzalez MA. Stress fracture of the femoral neck in a young adult with Maroteaux–Lamy Syndrome. Acta Orthopaedica Belgica. 1992;58(1):91–92. [PubMed] [Google Scholar]
- 12.Harmatz P, Kramer WG, Hopwood JJ, Simon J, Butensky E, Swiedler SJ. Pharmacokinetic profile of recombinant human N-acetylgalactosamine 4-sulphatase enzyme replacement therapy in patients with mucopolysaccarharidosis VI (Maroteaux-Lamy syndrome) Acta Paediatrica. 2005;94(Suppl 447):61–68. doi: 10.1111/j.1651-2227.2005.tb02115.x. [DOI] [PubMed] [Google Scholar]
- 13.Harmatz P, Whitley CB, Waber L, Rais R, Steiner R, Plecko B, Kaplan P, Simon J, Butensky E, Hopwood JJ. Enzyme replacement therapy in mucopolysaccharidosis VI (Marteaux-Lamy Syndrome) J Pediatric. 2004;144:574–580. doi: 10.1016/j.jpeds.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Heaney RP, Abrams S, Dawson-Hughes B, et al. Peak bone mass. Osteoporosis Inter. 2000;11:985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 15.Henderson RC. Vitamin D levels in non-institutionalized children with cerebral palsy. J Child Neurol. 1997;12:443–447. doi: 10.1177/088307389701200706. [DOI] [PubMed] [Google Scholar]
- 16.Holick MF, Dawson-Hughes B. Nutrition and Bone Health. Humana Press; Totowa NJ: 2004. [Google Scholar]
- 17.Holick MF. The role of vitamin D for bone health and fracture prevention. Curr Osteoporos Rep. 2006;4(3):96–102. doi: 10.1007/s11914-996-0028-z. [DOI] [PubMed] [Google Scholar]
- 18.Ichikawa T, Nishimura G, Tsukune Y, Dezawa A, Miki H. Progressive bone resorption after pathological fracture of the femoral neck in Hunter’s syndrome. Pediatr Radiol. 1999;12:914–916. doi: 10.1007/s002470050725. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Medicine. Dietary Reference Intakes for calcium, phosphorus, magnesium, vitamin D and Fluoride. National Academy Press; Washington DC: 1997. [PubMed] [Google Scholar]
- 20.Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, Mahboubi S, Fan B, Frederick MM, Winer K, Shepherd JA. The bone mineral density in childhood study: bone mineral content and density according to age, sex and race. J Clin Endo Metab. 2007;92:2087–2099. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 21.Krassas GE. Idiopathic juvenile osteoporosis. Ann NY Acad Sci. 2000;900:409–412. doi: 10.1111/j.1749-6632.2000.tb06253.x. [DOI] [PubMed] [Google Scholar]
- 22.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC Growth Charts: United States. Advance Data. 2000;314:1–28. [PubMed] [Google Scholar]
- 23.Landin LA. Fracture patterns in children. Acta Orthop Scan. 1983;54(suppl 202):1–109. [PubMed] [Google Scholar]
- 24.Lee JH, OKeefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52(24):1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 25.Misra M, Pacaud D, Petryk A, et al. Vitamin D deficiency in children and its management: review of the current knowledge and recommendations. Pediatrics. 2008;122:398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 26.Official Positions of the International Society for Clinical Densitometry. 2007 October; www.ISCD.org.
- 27.Petit M, Kent K, Leonard MB, McKay H, Zemel BS. Analysis. In: Sawyer AJ, Bachrach LK, Fung EB, editors. Bone Densitometry in Growing Patients: Guidelines for Clinical Practice. Humana Press; Totowa NJ: 2007. [Google Scholar]
- 28.Rigante D, Buonuomo PS, Caradonna P. Early-onset osteoporosis with high bone turnover in children with Morquio-Brailsford syndrome. Rheumatol Int. 2006;26:1163–1164. doi: 10.1007/s00296-006-0150-3. [DOI] [PubMed] [Google Scholar]
- 29.Rigante D, Caradonna P. Secondary skeletal involvement in Sanfilippo syndrome. Q J Med. 2004;97:205–209. doi: 10.1093/qjmed/hch041. [DOI] [PubMed] [Google Scholar]
- 30.Simonaro CM, D’Angelo M, Haskins ME, Schuchman EH. Bone and Joint Disease in Mucopolysaccharidoses VI and VII. Pediatric Research. 2005;57(5):701–707. doi: 10.1203/01.PDR.0000156510.96253.5A. [DOI] [PubMed] [Google Scholar]
- 31.Simonaro CM, D’Angelo M, He X, Eliyahu E, Shtraizent N, Haskins ME, Schuchman EH. Mechanism of Glycosaminoglycan-mediated bone and joint disease. Am J Pathol. 2008;172:112–122. doi: 10.2353/ajpath.2008.070564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slemenda CW, Miller JZ, Hui SL, et al. Role of physical activity in the development of skeletal mass in children. J Bone Mineral Res. 1991;6:1227–1233. doi: 10.1002/jbmr.5650061113. [DOI] [PubMed] [Google Scholar]
- 33.U.S. Department of Health and Human Services. Bone Health and Osteoporosis: A report of the surgeon general. Office of the Surgeon General; Rockville MD: 2004. [Google Scholar]
- 34.Vogiatzi M, Macklin EA, Fung EB, et al. Bone disease in thalassemia: a frequent and still unresolved problem. Journal Bone Mineral Research. 2008 May 27; doi: 10.1359/jbmr.080505. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whyte MP. Osteogenesis Imperfecta. In: Favus M, editor. Primer on Metabolic Diseases and Disorders of Mineral Metabolism. Washington DC: American Society for Bone and Mineral Research; 2003. pp. 470–473. [Google Scholar]
- 36.Zemel BS, Leonard MB, Kelly A, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010 Jan 26; doi: 10.1210/jc.2009-2057. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zemel BS, Stallings VA, Leonard MB, et al. Revised reference data for the lateral distal femur measured by Hologic Discovery/Delphi Dual energy x-ray absorptiometry. J Clin Densitometry. 2009;12(2):207–218. doi: 10.1016/j.jocd.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]