Abstract
Purpose
Idiopathic pulmonary fibrosis (IPF) has not been shown to respond to corticosteroid therapy; however, many patients receive these drugs at the time of diagnosis. The factors that are associated with the decision to prescribe corticosteroids have not been examined.
Methods
We conducted a retrospective cohort study of 1126 patients with a new diagnosis of IPF using The Health Improvement Network database from the United Kingdom. We used generalized estimating equation (GEE) regression models to test the association of patient characteristics, co-morbid diseases, and disease characteristics with the use of corticosteroids within 30 days of IPF diagnosis.
Results
Bivariable analyses demonstrated an association between female sex, the presence of dyspnea, the need for oxygen, past steroid use, and the use of corticosteroids immediately prior to diagnosis with the use of corticosteroids at the time of diagnosis. After adjustment with multivariable GEE regression, only the use of oxygen at the time of diagnosis (OR 1.69, CI 1.14-2.49), the past use of corticosteroids (OR 1.50, CI 1.04-2.15), and use of corticosteroids immediately prior to diagnosis (OR 5.72, CI 3.80-8.60) remained significantly associated with increased use of corticosteroids. No association was found between prior diabetes, osteoporosis, glaucoma, hypertension, congestive heart failure, obesity or peptic ulcer disease and use of corticosteroids at diagnosis.
Conclusions
The decision to prescribe corticosteroids is associated with oxygen use and past corticosteroid use but is not influenced by factors such as age, gender, or common co-morbid conditions that may pre-dispose patients to adverse events of therapy.
Keywords: Idiopathic pulmonary fibrosis, prescribing, drug therapy, corticosteroids, adverse events
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most common form of idiopathic interstitial pneumonia with an incidence in the US estimated to be between 6.8 and 16.3 cases per 100,000 person years.1, 2 IPF is associated with a significantly shortened survival3 and to date, no therapeutic agents have been shown in well-controlled prospective trials to be effective in reducing disease mortality. Limited evidence has suggested a small subgroup of patients may derive a partial or transient treatment response when prescribed corticosteroids,4-6 however these agents have also been associated with a variety of adverse events when used in this population.4
Despite the significant risk of toxicity and the absence of data demonstrating a survival benefit, corticosteroids are often prescribed either alone or in combination with cytotoxic agents in the management of IPF.7-10 Existing guidelines from the American Thoracic Society/European Respiratory Society recommend that the decision to treat should be made after weighing the potential for benefit against the likelihood of toxicity.11 Unfortunately, the factors that predict a response to anti-inflammatory drugs including corticosteroids and the factors that predict significant toxicity have not been definitively characterized. This makes it difficult in practice to assess the balance between the potential for benefit and the potential for harm. The evidence that does exist suggests that patients who are younger or who have less established fibrosis and more active inflammation may be more likely to respond to therapy compared to patients who are older and have more advanced fibrotic disease.4, 5, 11 It is also generally accepted, although not clearly established, that patients with other medical conditions including diabetes, osteoporosis and obesity may be at higher risk for toxicity that those without significant co-morbidities.11
It is not known how physicians use these limited data in clinical practice and whether patient characteristics such as disease severity or co-morbid conditions influence treatment decisions at the time of diagnosis with IPF. This study was designed to examine whether the use of corticosteroids in the initial management of IPF is influenced by patient demographics including age and gender, a history of co-morbid conditions such as diabetes or osteoporosis, or disease characteristics including dyspnea and oxygen use. We hypothesized that patient symptoms and oxygen use would be associated with the use of corticosteroids at diagnosis while factors including advanced age and pre-existing co-morbid conditions such as diabetes or osteoporosis would be associated with a decision to withhold or postpone corticosteroid therapy.
Methods
Study design
We conducted a retrospective cohort study of patients with an incident diagnosis of IPF using The Health Improvement Network (THIN) Database. THIN is a medical records database containing more than 5 million primary care records from participating general practices in the United Kingdom (UK). This database contains demographic, diagnostic, and complete prescription information for each patient, and has been used in several pharmacoepidemiology studies.12-15 This study was reviewed by the Institutional Review Board at the University of Pennsylvania and exempted from further review.
Patients with a new diagnosis of IPF were identified using diagnostic codes for either “idiopathic fibrosing alveolitis” (H563.00) or “cryptogenic fibrosing alveolitis” (H563.12). These codes have been used in prior research in THIN,16 and their accuracy in identifying patients with IPF has been established in a similar database that uses the same computer software for data entry.17 In order to ensure that prevalent cases were not misclassified as incident cases, patients had to have at least 12 months of existing medical records in the database prior to the first entry of a diagnostic code for IPF to be included in the cohort.18
We excluded patients with a previous diagnosis of an autoimmune disease that might also be treated with corticosteroids such as vasculitis or polymyalgia rheumatica. We also excluded patients who were under the age of 40 at the time of diagnosis and patients whose records were identified by THIN as failing to meet quality standards.
The primary outcome was a new prescription for an oral corticosteroid either alone or in combination with a cytotoxic agent written within 30 days of the first diagnosis of IPF. Potential risk factors for the use of corticosteroids were divided into three broad categories. Patient and practice characteristics included age at diagnosis, gender, and the number of patients with IPF seen by the patient's practice location each year. Co-morbid conditions were identified using diagnostic codes that predated the initial diagnosis of IPF and included diabetes; osteoporosis and fracture; glaucoma and cataracts; obesity; hypertension or congestive heart failure; peptic ulcer disease or gastrointestinal bleeding; asthma or COPD; a form of ILD other than IPF; and smoking status. Factors related to IPF itself included a history of cough or dyspnea prior to the diagnosis of IPF, and oxygen use either prior to or at the time of diagnosis with IPF. Prior treatment with corticosteroids was also included as a risk factor.
Statistical analysis
All statistical analyses were conducted using STATA version 9 (StataCorp, College Station, TX) and used a p-value of 0.05 as the threshold for statistical significance. To measure the effect of risk factors on the use of corticosteroids at the time of diagnosis, we used generalized estimating equation (GEE) regression models clustered on the practice in which the patient was treated.19 These models provide robust estimates of variances by accounting for the non-independence of observations made among patients treated by the same group of physicians. We first analyzed each risk factor in separate GEE models with corticosteroid use as the outcome to arrive at unadjusted OR's for each exposure variable. Age, practice experience with IPF, and BMI were analyzed first as continuous variables then as categorical variables divided in quintiles for practice experience and BMI and 10 year increments for age. To control for potential confounding among risk factors, all pre-specified risk factors were then included in a single GEE regression model to provide fully adjusted OR's for each factor independent of the effects of other variables.
Because the publication of treatment guidelines may have altered physician prescribing behavior over the observed study period, we then conducted a sensitivity analysis in which the cohort was restricted to patients diagnosed after January 1, 2000. Also, because some of the co-morbid conditions considered as risk factors could be the result of corticosteroid use among patients treated empirically before diagnosis, we conducted a separate sensitivity analysis in which those who received corticosteroids in the 90 days prior to diagnosis were excluded. Finally, to account for the possibility that a patient's first prescription may have been written by a specialty physician and not captured in the database, we conducted an analysis in which the outcome definition was expanded to include patients who received corticosteroids within 90 days after diagnosis.
Results
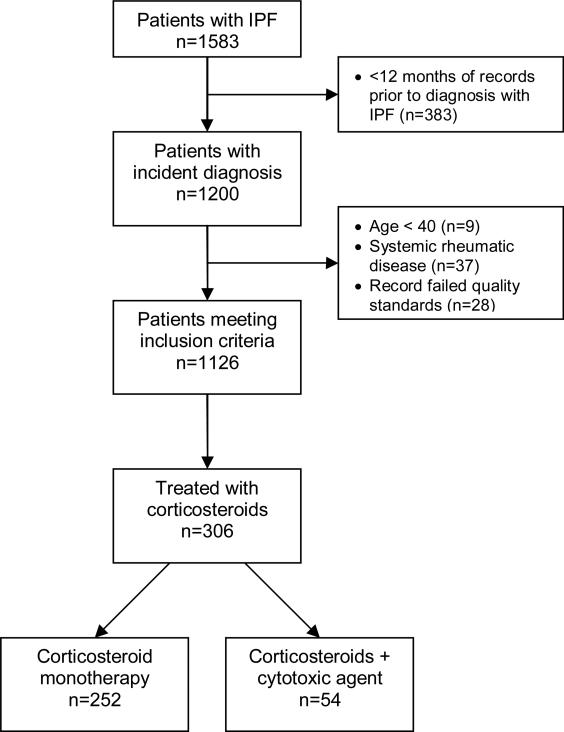
We identified a total of 1583 patients with an incident diagnosis of IPF diagnosed between 1989 and 2006. Of these, 1126 met our inclusion criteria (Figure 1). The average age at the date of diagnosis was 71.4 years (SD 10.4 years) and 61.5% of the cohort was male. The characteristics of included patients as well as the prevalence of co-morbid conditions and symptoms prior to the date of diagnosis are summarized in Table 1. Nearly 15% of patients required oxygen at the time of diagnosis with IPF, 6.8% had been diagnosed with an alternative form of ILD prior to their diagnosis of IPF, and 15.4% had received corticosteroids in the 90 days preceding their incident diagnosis.
Figure 1.
Definition of study cohort and initial treatment
Table 1.
Patient characteristics and prevalence of risk factors prior to the incident diagnosis of IPF
| Risk factor | Mean or prevalence |
|---|---|
| Age (mean in yrs) | 71.4 |
| Standard deviation | 10.4 |
| Sex (%male) | 61.5% |
| Diabetes | 13.6% |
| Obesity† | 17.9% |
| HTN or CHF* | 31.5% |
| PUD or GIB** | 7.3% |
| Glaucoma or cataracts | 15.5% |
| Osteoporosis | 16.0% |
| Smoking† | 28.6% |
| Alternative ILD‡ diagnosis | 6.8% |
| Asthma or COPD*** | 19.2% |
| Dyspnea | 39.0% |
| Cough | 48.9% |
| Oxygen use | 14.9% |
| Past steroid use | 26.5% |
| >90 days prior to IPF diagnosis | |
| Recent steroid use | 15.4% |
| ≤90 days prior to IPF diagnosis |
calculated with missing values excluded
Hypertension or congestive heart failure
Peptic ulcer disease or gastrointestinal bleeding
Chronic obstructive pulmonary disease
Interstitial lung disease other than IPF
A total of 306 patients (27.2%) received a prescription for corticosteroids within 30 days of diagnosis with IPF. Of these, 252 were treated with corticosteroids alone and 54 received corticosteroids in combination with either cyclophosphamide or azathioprine. The results of unadjusted analyses for each exposure variable are presented in Table 2. Bivariable analysis of patient demographics and practice characteristics demonstrated a statistically significant relationship between female gender and corticosteroid use (unadjusted OR 1.40, CI 1.07-1.83, p=0.013). Age was found to have a non-linear relationship with corticosteroid use with patients under the age of 50 and over the age of 70 having the lowest OR's for corticosteroid use; however, none of the unadjusted OR's for age categories was statistically significant. Practice experience was not associated with corticosteroid use.
Table 2.
Unadjusted association of risk factor variables with corticosteroid therapy at diagnosis
| Risk factor | OR (95% CI) | P-value |
|---|---|---|
| Patient and practice characteristics | ||
| Age (continuous) | 0.99 (0.98-1.01) | 0.39 |
| Age by 10 years | 0.65 | |
| 40-49 | 0.75 (0.33-1.72) | 0.50 |
| 50-59 | 1.22 (0.77-1.95) | 0.39 |
| 60-69† | 1.0 | -- |
| 70-79 | 0.89 (0.64-1.25) | 0.51 |
| >=80 | 0.91 (0.63-1.33) | 0.64 |
| Sex (female) | 1.40 (1.07-1.83) | 0.013 |
| Practice experience (IPF patients/year) | 1.00 (0.97-1.03) | 0.98 |
| Practice experience (quintiles) | 0.97 | |
| 1† | 1.0 | -- |
| 2 | 0.93 (0.62-1.42) | 0.75 |
| 3 | 1.01 (0.66-1.53) | 0.97 |
| 4 | 0.89 (0.58-1.36) | 0.58 |
| 5 | 0.92 (0.60-1.42) | 0.71 |
| Co-morbid conditions | ||
| Diabetes | 0.77 (0.51-1.15) | 0.20 |
| Obesity | ||
| Yes | 1.13 (0.77-1.66) | 0.54 |
| missing | 0.85 (0.62-1.17) | 0.33 |
| BMI | 1.00 (0.98-1.02) | 0.96 |
| BMI quintiles | 0.36 | |
| 1 | 1.27 (0.80-2.00) | 0.32 |
| 2 | 0.79 (0.49-1.29) | 0.36 |
| 3† | 1.0 | -- |
| 4 | 0.93 (0.58-1.49) | 0.76 |
| 5 | 1.14 (0.71-1.83) | 0.59 |
| HTN or CHF* | 0.93 (0.70-1.24) | 0.63 |
| PUD or GIB** | 0.85 (0.50-1.43) | 0.53 |
| Glaucoma/cataract | 0.98 (0.68-1.42) | 0.93 |
| Osteoporosis | 1.18 (0.83-1.68) | 0.35 |
| Smoking | ||
| Yes | 0.87 (0.64-1.19) | 0.39 |
| missing | 0.97 (0.69-1.37) | 0.87 |
| Past ILD‡ | 1.44 (0.88-2.36) | 0.15 |
| Asthma or COPD*** | 1.16 (0.84-1.62) | 0.36 |
| Disease characteristics | ||
| Dyspnea | 1.44 (1.10-1.88) | 0.007 |
| Cough | 1.18 (0.91-1.53) | 0.22 |
| Oxygen use | 2.71 (1.93-3.79) | <0.001 |
| Past steroid use | 3.24 (2.44-4.30) | <0.001 |
| Recent steroid use | 7.90 (5.55-11.24) | <0.001 |
Reference category
Hypertension or congestive heart failure
Peptic ulcer disease or gastrointestinal bleeding
Chronic obstructive pulmonary disease
Interstitial lung disease other than IPF
None of the tested co-morbid conditions had a statistically significant association, either positive or negative, with corticosteroid use. Diabetes (unadjusted OR 0.77, CI 0.51-1.15) and a history of peptic ulcer disease or GI bleed (unadjusted OR 0.85, CI 0.50-1.43) had the strongest inverse associations, however in both cases, the 95% CI crossed unity.
The unadjusted analyses of disease characteristics and past therapy showed that both a history of dyspnea prior to diagnosis (OR 1.44, CI 1.10-1.88, p=0.007) and treatment with oxygen at the time of diagnosis (OR 2.71, CI 1.93-3.79, p<0.001) were associated with use of corticosteroids. Additionally, having received corticosteroids more than 3 months prior to diagnosis (OR 3.24, CI 2.44-4.30, p<0.001) and in the 90 days leading up to the initial diagnosis (OR 7.90, CI 5.55-11.24, p<0.001) were strongly associated with receiving corticosteroids at the time of initial diagnosis.
The results of multivariable regression are presented in Table 3. Only oxygen use (OR 1.68, CI 1.14-2.49, p=0.009), use of corticosteroids more than 90 days before diagnosis (OR 1.64, CI 1.13-2.38, p=0.010) and recent corticosteroid use in the 90 days before diagnosis (OR 5.79, CI 3.84-8.74, p<0.001) were associated with the use of corticosteroids at the time of the initial diagnosis with IPF. The association of female gender (OR 1.29 CI 0.95-1.74, p=0.11) with corticosteroid use was still present, however it was attenuated in the fully adjusted model and was no longer statistically significant. Similarly, the presence of dyspnea (adjusted OR 1.33, CI 0.97-1.80, p=0.07) had a smaller adjusted OR and now failed to meet pre-specified criteria for statistical significance. In the fully adjusted model, a history of asthma and COPD had an inverse relationship with corticosteroid use (OR 0.71, CI 0.48-1.05, p=0.09) that had not been observed in the unadjusted bivariable analysis. On subsequent analyses controlling for the effects of potential confounders individually, the unadjusted OR for asthma and COPD (OR 1.16) was found to be the result of confounding by past corticosteroid use.
Table 3.
Results of multivariable analysis
| Risk factor | OR (95% CI) | P-value |
|---|---|---|
| Age by 10 years | ||
| 40-49 | 0.58 (0.23-1.48) | 0.26 |
| 50-59 | 1.13 (0.68-1.91) | 0.63 |
| 60-69† | 1.0 | -- |
| 70-79 | 0.81 (0.55-1.18) | 0.26 |
| >=80 | 0.92 (0.59-1.43) | 0.71 |
| Sex (female) | 1.29 (0.95-1.74) | 0.11 |
| Practice experience (quintiles) | ||
| 1† | 1.0 | -- |
| 2 | 0.91 (0.58-1.45) | 0.71 |
| 3 | 1.12 (0.70-1.79) | 0.63 |
| 4 | 0.86 (0.53-1.38) | 0.53 |
| 5 | 0.89 (0.55-1.45) | 0.65 |
| Diabetes | 0.73 (0.46-1.18) | 0.20 |
| Obesity | 1.05 (0.68-1.63) | 0.82 |
| HTN or CHF* | 0.79 (0.56-1.11) | 0.17 |
| PUD or GIB** | 0.86 (0.48-1.53) | 0.61 |
| Glaucoma/cataract | 0.89 (0.58-1.37) | 0.60 |
| Osteoporosis | 0.97 (0.63-1.49) | 0.90 |
| Smoking | 0.81 (0.57-1.15) | 0.24 |
| Past ILD‡ | 0.97 (0.54-1.72) | 0.91 |
| Asthma or COPD*** | 0.71 (0.48-1.05) | 0.09 |
| Dyspnea | 1.33 (0.97-1.80) | 0.07 |
| Cough | 1.00 (0.74-1.35) | 0.99 |
| Oxygen use | 1.68 (1.14-2.49) | 0.009 |
| Past steroid use | 1.64 (1.13-2.38) | 0.010 |
| Recent steroid use | 5.79 (3.84-8.74) | <0.001 |
Reference category
Hypertension or congestive heart failure
Peptic ulcer disease or gastrointestinal bleeding
Chronic obstructive pulmonary disease
Interstitial lung disease other than IPF
We compared those patients that were receiving corticosteroids in the weeks before diagnosis to those with no recent steroid use (see Table 4) and found that recent steroid users were more likely to have a history of a different form of ILD (11% vs 6%, p=0.02), a history of asthma or COPD (29% vs. 17%, p=0.0004), and a history of dyspnea prior to diagnosis (47% vs. 38%, p=0.02). They were also much more likely to require oxygen at the time of diagnosis with IPF (34% vs. 11%, p<0.0001). However, with respect to demographic variables and co-morbid illnesses tested as risk factors, the only difference was that recent corticosteroid users were more likely to have a diagnosis of osteoporosis prior to diagnosis with IPF (22% vs. 15%, p=0.02).
Table 4.
Characteristics of patients treated and not treated with corticosteroids in the 3 months prior to diagnosis
| Variable | Corticosteroid users | Treatment naive | p-value |
|---|---|---|---|
| Age (yrs) | 70.2 | 71.6 | 0.11 |
| Male sex | 57% | 62% | 0.16 |
| Obesity | 18% | 18% | 0.96 |
| Smoking | 38% | 35% | 0.54 |
| Diabetes | 12% | 14% | 0.54 |
| Osteoporosis | 22% | 15% | 0.02 |
| HTN/CHF* | 32% | 31% | 0.54 |
| PUD/GIB** | 6% | 7% | 0.61 |
| Glaucoma/cataracts | 17% | 15% | 0.46 |
| Cough | 54% | 48% | 0.12 |
| Dyspnea | 47% | 38% | 0.02 |
| Oxygen use | 34% | 11% | <0.0001 |
| Hx of asthma/COPD *** | 29% | 17% | 0.0004 |
| Hx of different ILD ‡ | 11% | 6% | 0.02 |
| Hx of biopsy | 10% | 9% | 0.53 |
Hypertension or congestive heart failure
Peptic ulcer disease or gastrointestinal bleeding
Chronic obstructive pulmonary disease
Interstitial lung disease other than IPF
When patients who used corticosteroids in the 90 days prior to diagnosis with IPF were excluded, the presence of dyspnea at diagnosis was now associated with the use of corticosteroids (OR 1.63, CI 1.16-2.30, p=0.005) where an association of similar magnitude had not been statistically significant in the primary analysis. The remainder of the tested exposure-outcome associations was unchanged from the primary analysis. An additional sensitivity analysis in which the outcome definition was expanded to include patients treated within 90 days did not change the results. Finally, in order to account for the possibility that the use of corticosteroids may have been altered by the publication of treatment guidelines in 2000, a sensitivity analysis was conducted in which the cohort was restricted to patients with a new diagnosis made after January 1, 2000. The results of this analysis were not substantially different from the primary findings (data not shown).
Discussion
The results of our study provide important insights into the decision-making process of physicians confronted with a patient newly-diagnosed with IPF. We found that patients who required oxygen and those that had a past history of corticosteroid use were more likely to receive corticosteroids within 30 days of their first diagnosis with IPF. Importantly, we also describe for the first time that factors such as gender and a past history of co-morbid conditions that might increase the risk of corticosteroid complications did not appear to influence the decision to prescribe corticosteroids.
The observed association between the use of oxygen and the decision to treat with corticosteroids at diagnosis is consistent with the work of Rudd and colleagues who found that patients with IPF treated within 3 months of diagnosis had more severe disease as measured by a decreased DLCO and FVC than those in whom treatment was prescribed later in their disease course.10 The decision to offer corticosteroids to those requiring oxygen may be based on limited data that suggest worsened disease severity at presentation is associated with a positive short term treatment response.5, 10 There is no evidence to suggest that this is predictive of a sustained treatment effect,4, 5 however, and in fact oxygen use may be indicative of more advanced fibrosis that is unlikely to respond to long-term corticosteroid therapy. It is also likely that oxygen use identifies a population of patients with very little pulmonary reserve in whom the possibility of any treatment effect, however unlikely, may be perceived to outweigh the risk of even significant toxicities.
Perhaps the most important finding of this study is that physicians do not avoid prescribing corticosteroids to patients with co-morbidities that might increase the risk of steroid toxicity such as diabetes, osteoporosis, peptic ulcer disease, or glaucoma. This is in contrast to our hypothesis that the presence of these diseases would discourage the use of corticosteroids at the time of diagnosis and appears to conflict with both the ATS/ERS guidelines on the treatment of IPF and the more recent British Thoracic Society guidelines on diffuse parenchymal lung diseases each of which recommend that significant co-morbidities should be considered in the decision to initiate treatment with corticosteroids.11, 20
There are several potential explanations for our findings. First, physicians and patients may believe that any potential for benefit in a relentless and debilitating disease outweighs the risk of even significant toxicity. Second, physicians may consider many of the most severe toxicities such as hip fractures, GI hemorrhage or severe infection to be sufficiently rare as to be worth the risk for any potential benefit. A third possibility is that patients offered corticosteroids at diagnosis, particularly those with more severe disease, are estimated to have a considerably shortened life-span during which adverse events are unlikely to occur. To control for this latter possibility, we restricted our analysis to those prescriptions that were written within 30 days of the initial diagnosis when the anticipated survival should be adequate in most cases to make adverse events a relevant consideration.
The strongest statistical association we found was between the use of corticosteroids in the 3 months prior to diagnosis with IPF and the use of corticosteroids at the time of diagnosis. It is possible that these patients may have had IPF that was not “classic” in appearance, leading their physicians to prescribe corticosteroids empirically either as a trial of treatment for a suspected alternative form of ILD or as therapy for an alternative and more common cause of breathlessness before arriving at the diagnosis of IPF. When ultimately diagnosed, a subset of these patients may have demonstrated either that they can take the drug without significant toxicity or that their disease stabilized while on empiric treatment, thus favoring continued corticosteroid use.
It is also possible that those patients who received corticosteroids prior to diagnosis and at the time of diagnosis were misclassified as starting therapy for IPF when in fact they were tapering an existing empiric therapy either for a past diagnosis of IPF that was not recorded or for an alternative cause of their symptoms. Importantly, excluding these patients from the analysis did not change the remainder of the results.
There are important limitations to this study. Although this is one of the largest studies examining patients with IPF, the size of the cohort provides limited power to detect small differences. However, the observed effect estimates do not suggest that a clinically meaningful difference was missed. It is possible that the co-morbid conditions considered may have been misclassified and are in fact the result of corticosteroid use. This is unlikely for three reasons: we considered only those co-morbid conditions recorded prior to the diagnosis with IPF; there was no change in the observed associations after restricting the cohort to corticosteroid-naïve patients; and the direction of the observed associations is the opposite of what should be observed if these conditions were the result of corticosteroid use.
Although our results demonstrate that the use of corticosteroids is not influenced by common co-morbid conditions, we did not query physicians directly regarding the rationale for their treatment decisions. Additionally, the use of cytotoxic agents and N-acetylcysteine was not sufficiently common to determine if these agents were used preferentially among those with significant co-morbidities. We also did not examine prescriptions for corticosteroids written more than 30 days after diagnosis for our primary analysis. This was done in an effort to exclude prescriptions written in the terminal phase of the disease and to minimize the influence of variables such as symptomatic progression that may not be well quantified in the database. Finally, we do not have pathologic or radiographic confirmation of the diagnosis of IPF; however, the diagnostic codes we used have been validated in a similar database.17 Additionally, the primary focus of this study was to examine prescribing behavior among physicians who believed they were treating IPF even if that was not ultimately the correct diagnosis.
Conclusion
The decision to offer patients treatment with corticosteroids at the time of diagnosis with IPF is complex. Our findings suggest that physicians are influenced in their prescribing practices primarily by disease characteristics such as oxygen use rather than co-morbid conditions that may pre-dispose to drug toxicity. Additionally, patients who have been started on corticosteroids prior to diagnosis with IPF are likely to continue therapy after diagnosis despite the absence of conclusive efficacy data. These practices may place patients at risk for potentially avoidable and significant adverse events.
Funding
This study was supported by the National Institutes of Health in the form of an institutional training grant (T32-GM075766-02) and a Ruth Kirchstein National Research Service Award (1F32-HL092741-01). This study was also supported in part by a Clinical and Translational Science Award from the National Institutes of Health (UL1-RR024134).
Footnotes
- Corticosteroids are often used to treat idiopathic pulmonary fibrosis (IPF) despite the absence of data demonstrating a positive treatment effect
- Many patients with IPF have co-morbid conditions that may increase the risk of adverse events associated with corticosteroid therapy
- The use corticosteroids in the initial management of IPF is more common among patients who require supplemental oxygen at the time of diagnosis and among those who have received corticosteroids in the past
- The decision to prescribe corticosteroids is not influenced by the presence of pre-existing co-morbidities such as diabetes, osteoporosis, or advanced age
Competing Interests: JM, ZC, and JC have no competing interests to declare. MK is the PI on a clinical trial sponsored by Gilead Sciences Inc, Seattle WA. SK has performed research and done consulting work funded by various pharmaceutical companies, none directly related to the topic of this manuscript.
References Cited
- 1.Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med. 1994 Oct;150(4):967–972. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and Prevalence of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2006 October 1;174(7):810–816. doi: 10.1164/rccm.200602-163OC. 2006. [DOI] [PubMed] [Google Scholar]
- 3.Gribbin J, Hubbard RB, Le Jeune I, Smith CJ, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006 Nov;61(11):980–985. doi: 10.1136/thx.2006.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaherty KR, Toews GB, Lynch JP, 3rd, et al. Steroids in idiopathic pulmonary fibrosis: a prospective assessment of adverse reactions, response to therapy, and survival. Am J Med. 2001 Mar;110(4):278–282. doi: 10.1016/s0002-9343(00)00711-7. [DOI] [PubMed] [Google Scholar]
- 5.Gay SE, Kazerooni EA, Toews GB, et al. Idiopathic pulmonary fibrosis: predicting response to therapy and survival. Am J Respir Crit Care Med. 1998 Apr;157(4 Pt 1):1063–1072. doi: 10.1164/ajrccm.157.4.9703022. [DOI] [PubMed] [Google Scholar]
- 6.Johnson MA, Kwan S, Snell NJ, Nunn AJ, Darbyshire JH, Turner-Warwick M. Randomised controlled trial comparing prednisolone alone with cyclophosphamide and low dose prednisolone in combination in cryptogenic fibrosing alveolitis. Thorax. 1989 Apr;44(4):280–288. doi: 10.1136/thx.44.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collard HR, Loyd JE, King TE, Jr, Lancaster LH. Current diagnosis and management of idiopathic pulmonary fibrosis: A survey of academic physicians. Respiratory Medicine. 2007;101(9):2011–2016. doi: 10.1016/j.rmed.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hubbard R, Venn A. The impact of coexisting connective tissue disease on survival in patients with fibrosing alveolitis. Rheumatology (Oxford) 2002 Jun;41(6):676–679. doi: 10.1093/rheumatology/41.6.676. [DOI] [PubMed] [Google Scholar]
- 9.Johnston ID, Prescott RJ, Chalmers JC, Rudd RM. British Thoracic Society study of cryptogenic fibrosing alveolitis: current presentation and initial management. Fibrosing Alveolitis Subcommittee of the Research Committee of the British Thoracic Society. Thorax. 1997 Jan;52(1):38–44. doi: 10.1136/thx.52.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudd RM, Prescott RJ, Chalmers JC, Johnston ID. British Thoracic Society Study on cryptogenic fibrosing alveolitis: Response to treatment and survival. Thorax. 2007 Jan;62(1):62–66. doi: 10.1136/thx.2005.045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000 Feb;161(2 Pt 1):646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 12.Hubbard R, Lewis S, Smith C, et al. Use of nicotine replacement therapy and the risk of acute myocardial infarction, stroke, and death. Tob Control. 2005 Dec;14(6):416–421. doi: 10.1136/tc.2005.011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kell P, Hvidsten K, Morant S, Harnett J, Bridge S. Factors that predict changing the type of phosphodiesterase type 5 inhibitor medication among men in the UK. British Journal of Urology. 2007 April;99(4):860–863. doi: 10.1111/j.1464-410X.2006.06668.x. [DOI] [PubMed] [Google Scholar]
- 14.Mortimer KJ, Tata LJ, Smith CJ, et al. Oral and inhaled corticosteroids and adrenal insufficiency: a case-control study. Thorax. 2006 May;61(5):405–408. doi: 10.1136/thx.2005.052456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006 Apr 1;367(9516):1075–1079. doi: 10.1016/S0140-6736(06)68474-2. [DOI] [PubMed] [Google Scholar]
- 16.Gribbin J, Hubbard R, Smith C. Role of diabetes mellitus and gastrooesophageal reflux in the aetiology of idiopathic pulmonary fibrosis. Respir Med. 2008 Dec 4; doi: 10.1016/j.rmed.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med. 2000 Jan;161(1):5–8. doi: 10.1164/ajrccm.161.1.9906062. [DOI] [PubMed] [Google Scholar]
- 18.Lewis JD, Bilker WB, Weinstein RB, Strom BL. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf. 2005 Jul;14(7):443–451. doi: 10.1002/pds.1115. [DOI] [PubMed] [Google Scholar]
- 19.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986 Mar;42(1):121–130. [PubMed] [Google Scholar]
- 20.Wells AU, Hirani N. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008 September 1;63(Suppl_V):v1–58. doi: 10.1136/thx.2008.101691. al e. 2008. [DOI] [PubMed] [Google Scholar]