Abstract
PURPOSE OF REVIEW
The following is a review of the most recent research concerning the potential role of immune system dysfunction in autism. This body of literature has expanded dramatically over the past few years as researchers continue to identify immune anomalies in individuals with autism.
RECENT FINDINGS
The most exciting of these recent findings is the discovery of autoantibodies targeting brain proteins in both children with autism and their mothers. In particular, circulating maternal autoantibodies directed towards fetal brain proteins are highly specific for autism. This finding has great potential as a biomarker for disease risk, and may provide an avenue for future therapeutics and prevention. Additionally, data concerning the cellular immune system in children with autism suggest there may be a defect in signaling pathways that are shared by the immune and central nervous systems. While studies to explore this hypothesis are ongoing, there is great interest in the commonalities between the neural and immune systems and their extensive interactions.
SUMMARY
In summary, there is exciting research regarding the role of the immune system in autism spectrum disorders that may have profound implications for diagnosis and treatment of this devastating disease.
Keywords: Immune system, autoantibodies, immunoglobulin, cytokines, autism
1. Introduction
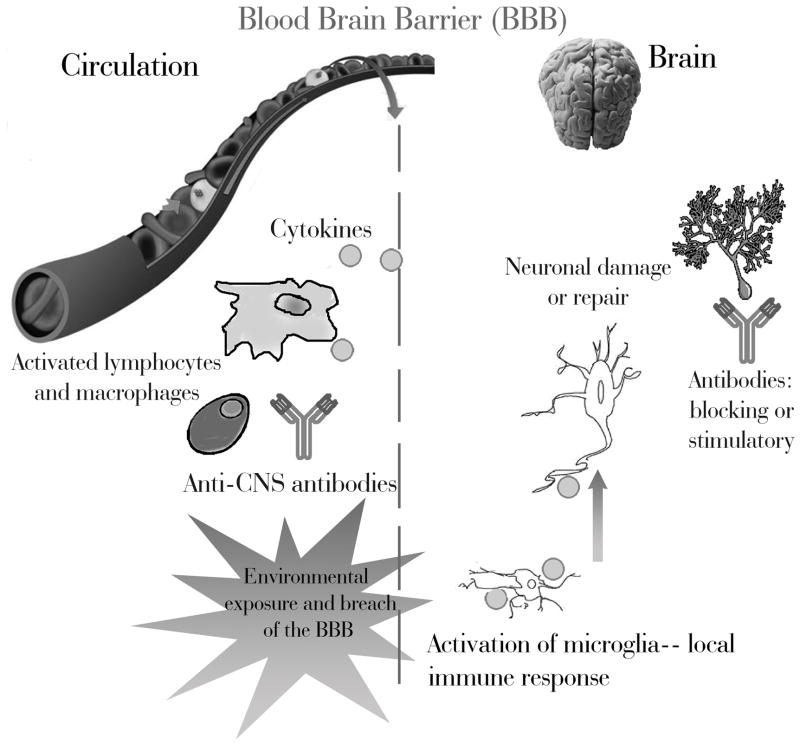
Autism Spectrum Disorders (ASD) are neurodevelopmental diseases characterized by restricted interests, repetitive behaviors, and deficient language and social skills [1]. While there are no concrete biological markers for the disorder, immune anomalies are frequently described among individuals with ASD and their family members [2, 3]. Historically, studies chronicling an immunological component in ASD have been inconsistent and controversial; which is due in part to small sample sizes, inappropriate controls, and no consideration for ASDs phenotypic heterogeneity. Recent studies have readily addressed these concerns, and the immunological connection for ASD is becoming widely accepted. Figure 1 illustrates the potential interactions between the immune and neural systems in ASD. The following presents the most recent findings linking immunity to autism.
Figure 1. Interactions between the Immune and Central Nervous Systems (CNS) in Autism Spectrum Disorders.
During postnatal life, an intact blood brain barrier limits the entry of immune species into the brain. Lymphocytes, macrophages, various cytokines, and antibodies are generally maintained in the periphery. However, the blood brain barrier is permeable during fetal development and can be compromised by infections and environmental exposures throughout life. The absence of a complete barrier allows immune components access to the brain. Individuals with autism show increased pro-inflammatory cytokines in the brain, as well as activation of resident immune cells known as microglia. Additionally, antibodies that target brain tissues have been described in both children with autism and their mothers. These immunological phenomena may interfere with normal brain development and function; potentially contributing to the development and/or symptoms of ASD.
2. Altered Immunity in individuals with ASD
Immunological anomalies involving cytokines, immunoglobulins, inflammation, and cellular activation have been noted in individuals with autism.
2.1 Altered Cytokine Profiles
Skewed cytokine profiles have been repeatedly linked to ASD [4–8]. Cytokines are secreted proteins that control the intensity, duration, and character of an immune response. Cytokines also interact with neural systems, and are involved in neural development and maintenance [9].
Transforming Growth Factor Beta (TGF-β) has been linked to ASD in multiple studies [10–12]. TGF-β is involved in diverse aspects of development, cell migration, apoptosis, and regulation in both the immune system and central nervous system (CNS) [13, 14]. Independent studies have described decreased levels of TGF-β in blood samples from individuals with ASD. Okada et al reported their findings among a group of adults with ASD compared to age-matched controls [10]. Similar data were reported by Ashwood et al. in a large, thoroughly-characterized group of children; and found that lower TGF-β correlated with more severe behavioral scores in ASD children [11]. In contrast to the observation of lower TGF-β in peripheral blood, TGF-β levels in postmortem brain and cerebrospinal fluid samples were higher in persons with ASD than controls [12]. While the relationship between CNS and peripheral TGF-β is unclear, these studies collectively suggest that TGF-β dysregulation may have a lifelong role in ASD.
Another cytokine recently linked recently to ASD is macrophage inhibitory factor (MIF) [15]. MIF is a pro-inflammatory immune regulator that is constitutively expressed in brain tissues, [16] and has important influences on neural and endocrine systems [17]. Genotyping studies on more than one thousand families uncovered two polymorphisms in the promoter region of MIF that associated with autism. Additionally, plasma levels of MIF were higher in individuals with autism than in typically developing controls [15]. Finally, individuals with autism with the highest levels of plasma MIF were found to have the most severe behavioral symptoms [15].
Variations in plasma levels of the cytokine/hormone leptin have been noted among individuals with ASD [18]. Leptin is produced primarily by adipocytes, though recent evidence shows that it is also produced by lymphocytes [19]. Leptin shares functional similarities with inflammatory cytokines such as IL-6 and IL-12 [20], and is capable of crossing the blood brain barrier [21]. Ashwood et al. recently demonstrated that plasma leptin levels were elevated in a large well characterized population of children with ASD compared to typically developing controls [18]. This was especially dramatic among children with early onset autism as opposed to those with clinical regression (i.e. normal development followed by loss of skills). Increased levels of leptin were also noted by Vargas et al in postmortem brain samples from persons with autism [12].
Collectively, the most exciting revelation in the above studies is the relationship between particular cytokine levels and behavioral variations within the autism spectrum. With continued research, cytokines may become an easily measured biomarker for phenotypic variations within ASD. It is unclear whether altered cytokine levels are harmful or beneficial, or if immune modulation therapy would benefit certain individuals with ASD.
2.2 Immunoglobulin Levels
Immunoglobulins (Ig) are proteins produced by B cells that specifically target entities for destruction and removal. There are several classes of Ig, each with a specific role in immunological processes. Recently, decreased levels of total plasma IgG and IgM were described in a large group of individuals with autism compared to age-matched individuals without autism [22*]. The reduced levels also correlated with behavior, such that individuals with autism with the most severe behavioral symptom scores had the lowest IgG and IgM levels [22**]. Further characterization of IgG subclasses demonstrated that young children with autism have significantly higher levels of IgG4 compared with age matched typically-developing children [23]. While the relationship between reduced total Ig and behavior is unclear, it is possible that a defect in a shared signaling pathway leads to both altered neurodevelopment and immune function. Studies are currently underway to examine this hypothesis.
2.3 Altered cellular immunity
Alterations in various immune cells including Natural Killer Cells and Macrophages have been noted in individuals with autism.
2.3.1 Functional Differences in Natural Killer (NK) cell activity
Natural killer (NK) cells are unique members of the immune system with roles in the viral response, pregnancy maintenance, tumor cytotoxicity, and autoimmunity [24]. Examination of peripheral blood cells in individuals with ASD revealed differences in the expression of several genes related to NK cell activity [25]. Further analysis of NK cells confirmed that the genetic alterations showed functional significance [26, 27]. Resting cells from children with ASD had increased expression of several NK cell receptors and effector molecules. Interestingly, upon stimulation, NK cells from individuals with ASD showed diminished cytotoxic activity compared to controls [27]. Similar findings were reported by Vojdani et al, where analysis of blood from over 1,000 individuals showed reduced NK cell activity in ASD compared to persons with ASD. [26]. The nature of the link between NK cells and ASD is not clear, though NK cells are known to produce cytokines and cytotoxic substances that could impact the CNS [28–30].
2.3.2 Monocyte Response to TLR stimulation
Monocytes are innate immune cells found in the peripheral blood that identify pathogens and direct the subsequent immune response. Monocytes express Toll-Like Receptors (TLR), which recognize molecular patterns associated with viruses and bacteria. A recent study examined monocyte cytokine responses to TLR stimulation in a well-characterized group of individuals with ASD and matched individuals without ASD. Dramatic differences were observed between the groups following stimulation of various TLRs. TLR-2 and TLR-4 stimulation of monocytes caused a marked production of pro-inflammatory cytokines in individuals with ASD that was not observed in the comparison group. Conversely, TLR 9 stimulation showed a decreased production of pro-inflammatory cytokines in the ASD compared to the group without ASD. This suggests that monocytes from children with ASD respond differently to innate immune stimulation compared to controls. Given the integral role of monocytes in the direction of an immune response, altered response to TLR stimulation can have wide-ranging impacts [*31].
2.4 Neuroinflammation
Some individuals with ASD demonstrate active inflammation in the CNS (reviewed in [32]). Post-mortem brain and spinal cord samples from 11 individuals with ASD showed increased activation of astroglia and microglia; and increased levels of cytokines MCP-1 and TGF-β compared to control specimens [12]. A more recent study of post-mortem brain tissues by Li et al further demonstrated CNS inflammation in persons with ASD. Cytokine levels were measured in homogenized brain samples from individuals with ASD and compared to levels in specimens from age- and gender-matched individuals without ASD. Specimens from persons with ASD demonstrated a significant increase in pro-inflammatory and Th1 cytokines [*33]. Post mortem studies are often limited by the availability of quality specimens from appropriately characterized individuals. However, these studies give valuable insight into the immune status of the CNS in some persons with ASD. It is unclear whether CNS immune activation contributes to the pathology of autism, or if it is an epiphenomenon.
3. Altered Sensitivity to Environmental Toxicants-PBDEs, immunity, and autism
A complex interplay between immunological and environmental factors may have a role in autism. Polybrominated diphenyl ethers (PBDEs) are environmental toxicants that impact neurodevelopment and immunity [34–37]. A 2009 study by Ashwood et al. explored the interaction between PBDEs and cellular immunity in children with ASD [**38]. Peripheral blood mononuclear cells from ASD and typically developing children were pretreated with a PBDE, stimulated with the bacterial derivative LPS, and compared to non-PBDE-treated cell cultures. PBDE-treatment of control cultures led to reduced production of inflammatory cytokines and chemokines. This suggests that PBDEs suppress immune responses in neurotypical populations. In contrast, PBDE-treated cultures from persons with ASD showed dramatically increased production of pro-inflammatory cytokines and chemokines. These results suggest that individuals with ASD have different immune sensitivity to the environmental toxicant than neurotypical children [**38]. This may be indicative of differential genetic susceptibility to PBDEs and/or a breakdown of proper immune regulation in ASD.
4. Allergy and ASD
Some have postulated that allergic disease may be associated with ASD. However, many early studies linking allergy to ASD lacked appropriate control groups [39, 40]. Recent properly-controlled research suggests no difference in allergic phenomena between persons with ASD and neurotypical individuals. In a study of allergic sensitization, individuals with ASD showed no difference in the frequency of allergic disorders, the number of positive skin prick tests, or in serum IgE levels (which are high in allergic individuals) [41]. An independent study also showed no difference in total plasma IgE between individuals with ASD and a group without ASD matched for age and geographic location [22].
5. Autoimmunity in ASD
Autoimmunity occurs when the immune system mistakenly targets the body’s own tissues. Various autoimmune phenomena have been described for decades among individuals with autism [3].
5.1 Antibodies Directed towards Neural Antigen(s) in individuals with ASD
Many studies have described circulating antibodies directed toward brain proteins in persons with ASD [3, 44, 45]. A recent study characterized the targets of these anti-brain antibodies using immunohistochemical staining of cerebellum sections from rhesus macaques [46]. Individuals with ASD showed a higher rate of plasma IgG directed towards golgi cells compared to age-matched persons without ASD [46]. The pathologic significance of these autoantibodies remains unclear, and they may be an epiphenomenon.
While anti-brain antibodies are found significantly more often in persons with ASD, they are also found occasionally in neurotypical persons and those who are developmentally delayed without ASD[45, 46]. The presence of these autoantibodies in other populations suggests that they may be a susceptibility factor that requires another exposure, such as a xenobiotic, to abrogate the blood brain barrier and facilitate access of the autoantibodies to their target antigens. Further studies are needed to resolve this important issue.
5.2 Antinuclear antibodies in children with ASD
A recent study examined the occurrence of antibodies directed towards nuclear proteins in a large group of children with autism and age-matched children with typical development [47]. Anti-nuclear antibodies are observed in autoimmune diseases like systemic lupus erythematosus (SLE). Children with autism had a significantly higher frequency of anti-nuclear antibodies (20%) than the neurotypical children (2.5%). As discussed below, individuals with SLE often demonstrate neurological abnormalities; which suggests a link between autoimmunity and behavior [48].
5.3 Behavior and autoimmunity
Certain autoimmune disorders impact behavior. This has been demonstrated among individuals with SLE accompanied by neuropsychiatric symptoms [49]. Serum anti-nuclear antibodies isolated from neuropsychiatric SLE patients cross react with the N-methyl-D-aspartate (NMDA) receptor for the neurotransmitter glutamate [49, 50]. Further, exposure of mice to serum from patients with SLE led to cognitive impairments and neuronal death in the hippocampus [51].
Autoantibodies specific to nervous system components have been reported in psychiatric disorders besides ASD, including schizophrenia, obsessive-compulsive disorder, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS), and Gilles de la Tourette’s Syndrome [50, 52–57]. Antibodies directed towards the brain might interfere with development and function, or cause immune-mediated destruction.
6. Immune findings in Families of individuals with ASD
Family members of individuals with autism also have various immune abnormalities. Examination of familial immunity in autism provides important insight into the disorder.
6.1 Siblings of Individuals with Autism
Historically, unaffected siblings of children with ASD have served as healthy controls in various studies. However, unaffected siblings are known to exhibit a higher prevalence of traits that distinguish them from neurotypical populations without qualifying them for an autism diagnosis. For example, neuroimaging studies demonstrated differences in brain function between unaffected siblings and neurotypical populations [58, 59]. Additionally, immune parameters differ between siblings of children with autism and typically-developing children [*60]. A small study of children with autism, their unaffected siblings, and healthy age and gender-matched children without ASD demonstrated that lymphocyte populations in unaffected siblings were more similar to those of children with autism than typically-developing children [*60]. Therefore, unaffected siblings should not be used as healthy neurotypical controls in autism research. Additionally, evaluating immunity in healthy siblings may provide important information regarding the relationship between immune dysfunction and autism.
6.2 Epidemiological Associations for Familial Non Infectious Diseases
Several immunological diseases occur at an increased rate among primary family members of individuals with autism. There is a higher incidence of Type 1 diabetes in fathers and ulcerative colitis in mothers of children with ASD [61]. Independent studies have also suggested that persons with autism have a greater family history of autoimmune disease compared to controls [47]. In children with autism, 47.5% were shown to have a family member with an autoimmune disease, compared to only 8.8% among healthy controls. This is consistent with previous work examining the occurrence of familial autoimmune disorders in autism [62].
6.3 Antibodies to fetal tissue in mothers of children with ASD
Exciting immune-related findings in autism come from research involving mothers of children with autism. Independent studies showed that a subset of mothers of children with autism have circulating antibodies that target the fetal brain [44, **63–65]. Certain patterns of these antibodies are only found in mothers of children with autism, and are not found in controls [63]. The targets of these antibodies in the fetal brain are the topic of current research.
Maternal antibodies (IgG) are transferred across the placenta to the fetus throughout pregnancy [66]. These antibodies serve a protective role until the child’s immune system matures, and persist in the child’s circulation for 6 months after birth [67]. Maternal antibodies are passed without regard to their specificity, meaning that both protective and autoantibodies have equal access to the developing fetus. Maternal autoantibodies can cause pathology in the neonate, which may be transient (in the case of myasthenia gravis) [68] or permanent, as in the case of SLE [69].
Unlike mothers of children with autism, children with autism, themselves, do not appear to harbor antibodies specific to the fetal brain [70]. Rather, antibodies found in children with autism appear to react with the fully developed brain [45, 46, 71, 72].
6.3.1 Animal Models: gestational exposure to maternal antibodies and offspring behavior
Animal models have demonstrated that maternal anti-brain antibodies can impact offspring brain development and behavior. In one study, antibodies that bind the neural NMDA receptor were induced in female mice throughout gestation. The resulting offspring demonstrated histological abnormalities in the brain, and had cognitive impairments in adulthood [**73]. Animal models have similarly demonstrated the pathologic significance of brain-directed IgG from mothers of children with autism. In 2008, IgG purified from mothers of children with autism was injected into pregnant rhesus macaque monkeys. A control group of monkeys received IgG from mothers of neurotypical children, and the offspring from each group were observed for behavioral differences. Unique stereotypic behaviors were observed in monkeys exposed prenatally to IgG from mothers of children with ASD [**74]. A murine model has also demonstrated the impacts of maternal IgG on offspring behavior [*75]. Prenatal exposure to antibodies from mothers of children with autism led to neurobehavioral alterations that were not observed in mice prenatally exposed to antibodies from control mothers. Collectively, these studies suggest that brain-directed antibodies in mothers have the potential to impact the child’s behavioral outcome.
7. Conclusion
Decades of research links immunological abnormalities to ASD. In light of the extensive crosstalk between the immune and neural systems, which includes shared signaling and developmental pathways, this line of research can yield important insights in atypical brain development. The nature of the connection between immunity and autism is the focus of ongoing research. To examine the immunobiology of autism, it is important to (1) use healthy, age-matched, comparison groups that do not have a family history of ASD, and (2) take into account the heterogeneity and subtypes within the ASD population. Proper exploration of immunological features in autism presents an exciting opportunity to tease apart the biology of disorder, and may lead to therapeutic interventions.
Acknowledgments
Grant support: NIEHS 1 P01 ES11269-01, the U.S. Environmental Protection Agency (U.S. EPA) through the Science to Achieve Results (STAR) program (Grant R829388), NIEHS 1 R01-ES015359, the UC Davis M.I.N.D. Institute, and Autism Speaks.
References
- 1.Association AP. Diagnostic and Statistical Manual of Mental Disorders. Washington, D.C: 1994. [Google Scholar]
- 2.Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J Leukoc Biol. 2006;80(1):1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- 3.Enstrom AM, Van de Water JA, Ashwood P. Autoimmunity in autism. Curr Opin Investig Drugs. 2009;10(5):463–73. [PMC free article] [PubMed] [Google Scholar]
- 4.Jyonouchi H, Sun S, Itokazu N. Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with autism spectrum disorder. Neuropsychobiology. 2002;46(2):76–84. doi: 10.1159/000065416. [DOI] [PubMed] [Google Scholar]
- 5.Jyonouchi H, Sun S, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol. 2001;120(1–2):170–9. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Aggarwal S, Rashanravan B, Lee T. Th1- and Th2-like cytokines in CD4+ and CD8+ T cells in autism. J Neuroimmunol. 1998;85(1):106–9. doi: 10.1016/s0165-5728(98)00021-6. [DOI] [PubMed] [Google Scholar]
- 7.Croonenberghs J, Bosmans E, Deboutte D, Kenis G, Maes M. Activation of the inflammatory response system in autism. Neuropsychobiology. 2002;45(1):1–6. doi: 10.1159/000048665. [DOI] [PubMed] [Google Scholar]
- 8.Molloy CA, Morrow AL, Meinzen-Derr J, Schleifer K, Dienger K, Manning-Courtney P, et al. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006;172(1–2):198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Bauer S, Kerr BJ, Patterson PH. The neuropoietic cytokine family in development, plasticity, disease and injury. Nat Rev Neurosci. 2007;8(3):221–32. doi: 10.1038/nrn2054. [DOI] [PubMed] [Google Scholar]
- 10.Okada K, Hashimoto K, Iwata Y, Nakamura K, Tsujii M, Tsuchiya KJ, et al. Decreased serum levels of transforming growth factor-beta1 in patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):187–90. doi: 10.1016/j.pnpbp.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Ashwood P, Enstrom A, Krakowiak P, Hertz-Picciotto I, Hansen RL, Croen LA, et al. Decreased transforming growth factor beta1 in autism: A potential link between immune dysregulation and impairment in clinical behavioral outcomes. J Neuroimmunol. 2008 doi: 10.1016/j.jneuroim.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 13.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 14.Gomes FC, Sousa Vde O, Romao L. Emerging roles for TGF-beta1 in nervous system development. Int J Dev Neurosci. 2005;23(5):413–24. doi: 10.1016/j.ijdevneu.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Grigorenko EL, Han SS, Yrigollen CM, Leng L, Mizue Y, Anderson GM, et al. Macrophage migration inhibitory factor and autism spectrum disorders. Pediatrics. 2008;122(2):e438–45. doi: 10.1542/peds.2007-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacher M, Meinhardt A, Lan HY, Dhabhar FS, Mu W, Metz CN, et al. MIF expression in the rat brain: implications for neuronal function. Mol Med. 1998;4(4):217–30. [PMC free article] [PubMed] [Google Scholar]
- 17.Fingerle-Rowson GR, Bucala R. Neuroendocrine properties of macrophage migration inhibitory factor (MIF) Immunol Cell Biol. 2001;79(4):368–75. doi: 10.1046/j.1440-1711.2001.01024.x. [DOI] [PubMed] [Google Scholar]
- 18.Ashwood P, Kwong C, Hansen R, Hertz-Picciotto I, Croen L, Krakowiak P, et al. Brief report: plasma leptin levels are elevated in autism: association with early onset phenotype? J Autism Dev Disord. 2008;38(1):169–75. doi: 10.1007/s10803-006-0353-1. [DOI] [PubMed] [Google Scholar]
- 19.Sanna V, Di Giacomo A, La Cava A, Lechler RI, Fontana S, Zappacosta S, et al. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J Clin Invest. 2003;111(2):241–50. doi: 10.1172/JCI16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 21.Banks WA. Leptin transport across the blood-brain barrier: implications for the cause and treatment of obesity. Curr Pharm Des. 2001;7(2):125–33. doi: 10.2174/1381612013398310. [DOI] [PubMed] [Google Scholar]
- 22**.Heuer L, Ashwood Paul, Schauer Joseph, Goines Paula, Krakowiak Paula, Hertz-Picciotto Irva, Hansen Robin, Croen Lisa A, Pessah Isaac N, Van de Water Judy. Reduced Levels of Immunoglobulin in Children With Autism Correlates With Behavioral Symptoms. Autism Research. 2008;1(5):275–283. doi: 10.1002/aur.42. This study showed variations in the level of immunoglobulin can be correlated with severity in behavior for individuals with autism. This is first study demonstrate a correlation with the level immune function and behavioral outcome. This finding may aid in determining biological markers for prognosis in autism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enstrom A, Krakowiak P, Onore C, Pessah IN, Hertz-Picciotto I, Hansen RL, et al. Increased IgG4 levels in children with autism disorder. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perricone R, Perricone C, De Carolis C, Shoenfeld Y. NK cells in autoimmunity: a two-edg’d weapon of the immune system. Autoimmun Rev. 2008;7(5):384–90. doi: 10.1016/j.autrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Gregg JP, Lit L, Baron CA, Hertz-Picciotto I, Walker W, Davis RA, et al. Gene expression changes in children with autism. Genomics. 2008;91(1):22–9. doi: 10.1016/j.ygeno.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Vojdani A, Mumper E, Granpeesheh D, Mielke L, Traver D, Bock K, et al. Low natural killer cell cytotoxic activity in autism: The role of glutathione, IL-2 and IL-15. J Neuroimmunol. 2008;205(1–2):148–54. doi: 10.1016/j.jneuroim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Enstrom AM, Lit L, Onore CE, Gregg JP, Hansen R, Pessah IN, et al. Altered gene expression and function of peripheral blood natural killer cells in children with autism. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czlonkowska A, Ciesielska A, Gromadzka G, Kurkowska-Jastrzebska I. Estrogen and cytokines production - the possible cause of gender differences in neurological diseases. Curr Pharm Des. 2005;11(8):1017–30. doi: 10.2174/1381612053381693. [DOI] [PubMed] [Google Scholar]
- 29.Lambertsen KL, Gregersen R, Meldgaard M, Clausen BH, Heibol EK, Ladeby R, et al. A role for interferon-gamma in focal cerebral ischemia in mice. J Neuropathol Exp Neurol. 2004;63(9):942–55. doi: 10.1093/jnen/63.9.942. [DOI] [PubMed] [Google Scholar]
- 30.Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol. 2005;159(1–2):106–12. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 31*.Enstrom AM, Onore CE, Van de Water JA, Ashwood P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.08.001. This study showed altered innate immune responses in a well-characterized population of children with autism and neurotypical controls. This has wide-ranging implications for immune reactions and neural function in children with autism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pardo CA, Vargas DL, Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005;17(6):485–95. doi: 10.1080/02646830500381930. [DOI] [PubMed] [Google Scholar]
- 33*.Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, et al. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207(1–2):111–6. doi: 10.1016/j.jneuroim.2008.12.002. This study reinforced the seminal 2005 findings of Vargas, et al. that first described inflammation in post-mortem brain samples from individuals with autism. The Vargas study marked a major shift in the acknowledgement of the immune component to ASD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO WHO. Environmental Health Criteria, No 162. World Health Organization; 1994. Brominated diphenyl ethers. [Google Scholar]
- 35.Johnson-Restrepo B, Kannan K. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere. 2009 doi: 10.1016/j.chemosphere.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 36.Costa LG, Giordano G, Tagliaferri S, Caglieri A, Mutti A. Polybrominated diphenyl ether (PBDE) flame retardants: environmental contamination, human body burden and potential adverse health effects. Acta Biomed. 2008;79(3):172–83. [PubMed] [Google Scholar]
- 37.Lundgren M, Darnerud PO, Blomberg J, Friman G, Ilback NG. Polybrominated diphenyl ether exposure suppresses cytokines important in the defence to coxsackievirus B3 infection in mice. Toxicol Lett. 2009;184(2):107–13. doi: 10.1016/j.toxlet.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 38**.Ashwood P, Schauer J, Pessah IN, de Water JV. Preliminary evidence of the in vitro effects of BDE-47 on innate immune responses in children with autism spectrum disorders. J Neuroimmunol. 2009;208(1–2):130–5. doi: 10.1016/j.jneuroim.2008.12.012. This is the first study to examine the impact of environmental toxicants on immune function in children with autism. The differential response to BDEs suggests a susceptibility in the autism population for this toxicant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menage P, Thibault G, Martineau J, Herault J, Muh JP, Barthelemy C, et al. An IgE mechanism in autistic hypersensitivity? Biol Psychiatry. 1992;31(2):210–2. doi: 10.1016/0006-3223(92)90208-h. [DOI] [PubMed] [Google Scholar]
- 40.Renzoni E, Beltrami V, Sestini P, Pompella A, Menchetti G, Zappella M. Brief report: allergological evaluation of children with autism. J Autism Dev Disord. 1995;25(3):327–33. doi: 10.1007/BF02179294. [DOI] [PubMed] [Google Scholar]
- 41.Bakkaloglu B, Anlar B, Anlar FY, Oktem F, Pehlivanturk B, Unal F, et al. Atopic features in early childhood autism. Eur J Paediatr Neurol. 2008 doi: 10.1016/j.ejpn.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Cormier E, Elder JH. Diet and child behavior problems: fact or fiction? Pediatr Nurs. 2007;33(2):138–43. [PubMed] [Google Scholar]
- 43.Millward C, Ferriter M, Calver S, Connell-Jones G. Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database Syst Rev. 2008;(2):CD003498. doi: 10.1002/14651858.CD003498.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmerman AW, Connors SL, Matteson KJ, Lee LC, Singer HS, Castaneda JA, et al. Maternal antibrain antibodies in autism. Brain Behav Immun. 2007;21(3):351–7. doi: 10.1016/j.bbi.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Cabanlit M, Wills S, Goines P, Ashwood P, Van de Water J. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann N Y Acad Sci. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- 46.Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral DG, Van de Water J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mostafa GA, Kitchener N. Serum anti-nuclear antibodies as a marker of autoimmunity in Egyptian autistic children. Pediatr Neurol. 2009;40(2):107–12. doi: 10.1016/j.pediatrneurol.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 48.Colasanti T, Delunardo F, Margutti P, Vacirca D, Piro E, Siracusano A, et al. Autoantibodies involved in neuropsychiatric manifestations associated with systemic lupus erythematosus. J Neuroimmunol. 2009;212(1–2):3–9. doi: 10.1016/j.jneuroim.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Diamond B, Kowal C, Huerta PT, Aranow C, Mackay M, DeGiorgio LA, et al. Immunity and acquired alterations in cognition and emotion: lessons from SLE. Adv Immunol. 2006;89:289–320. doi: 10.1016/S0065-2776(05)89007-8. [DOI] [PubMed] [Google Scholar]
- 50.Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci U S A. 2006;103(3):678–83. doi: 10.1073/pnas.0510055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kowal C, DeGiorgio LA, Nakaoka T, Hetherington H, Huerta PT, Diamond B, et al. Cognition and immunity; antibody impairs memory. Immunity. 2004;21(2):179–88. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Jones AL, Mowry BJ, Pender MP, Greer JM. Immune dysregulation and self-reactivity in schizophrenia: do some cases of schizophrenia have an autoimmune basis? Immunol Cell Biol. 2005;83(1):9–17. doi: 10.1111/j.1440-1711.2005.01305.x. [DOI] [PubMed] [Google Scholar]
- 53.Kiessling LS, Marcotte AC, Culpepper L. Antineuronal antibodies: tics and obsessive-compulsive symptoms. J Dev Behav Pediatr. 1994;15(6):421–5. [PubMed] [Google Scholar]
- 54.Pandey RS, Gupta AK, Chaturvedi UC. Autoimmune model of schizophrenia with special reference to antibrain antibodies. Biol Psychiatry. 1981;16(12):1123–36. [PubMed] [Google Scholar]
- 55.Rothermundt M, Arolt V, Bayer TA. Review of immunological and immunopathological findings in schizophrenia. Brain Behav Immun. 2001;15(4):319–39. doi: 10.1006/brbi.2001.0648. [DOI] [PubMed] [Google Scholar]
- 56.Perrin EM, Murphy ML, Casey JR, Pichichero ME, Runyan DK, Miller WC, et al. Does group A beta-hemolytic streptococcal infection increase risk for behavioral and neuropsychiatric symptoms in children? Arch Pediatr Adolesc Med. 2004;158(9):848–56. doi: 10.1001/archpedi.158.9.848. [DOI] [PubMed] [Google Scholar]
- 57.Yeh CB, Wu CH, Tsung HC, Chen CW, Shyu JF, Leckman JF. Antineural antibody in patients with Tourette’s syndrome and their family members. J Biomed Sci. 2006;13(1):101–12. doi: 10.1007/s11373-005-9033-y. [DOI] [PubMed] [Google Scholar]
- 58.Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol Psychiatry. 2007;61(4):512–20. doi: 10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 59.Kawakubo Y, Kuwabara H, Watanabe K, Minowa M, Someya T, Minowa I, et al. Impaired prefrontal hemodynamic maturation in autism and unaffected siblings. PLoS One. 2009;4(9):e6881. doi: 10.1371/journal.pone.0006881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60*.Saresella M, Marventano I, Guerini FR, Mancuso R, Ceresa L, Zanzottera M, et al. An Autistic Endophenotype Results in Complex Immune Dysfunction in Healthy Siblings of Autistic Children. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.06.020. This study confirmed that siblings of children with autism should not be used as healthy controls in biological studies. With regard to immune parameters, it appears that siblings have more in common with autism subjects than with healthy neurotypical controls. [DOI] [PubMed] [Google Scholar]
- 61.Mouridsen SE, Rich B, Isager T, Nedergaard NJ. Autoimmune diseases in parents of children with infantile autism: a case-control study. Dev Med Child Neurol. 2007;49(6):429–32. doi: 10.1111/j.1469-8749.2007.00429.x. [DOI] [PubMed] [Google Scholar]
- 62.Sweeten TL, Bowyer SL, Posey DJ, Halberstadt GM, McDougle CJ. Increased prevalence of familial autoimmunity in probands with pervasive developmental disorders. Pediatrics. 2003;112(5):e420. doi: 10.1542/peds.112.5.e420. [DOI] [PubMed] [Google Scholar]
- 63**.Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, et al. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29(2):226–31. doi: 10.1016/j.neuro.2007.10.010. This was the first report of highly specific antibodies directed towards human fetal brain proteins in a subset of mothers of children with autism. This paper preceded the 2008 study by Martin et al (ref 74) which demonstrated the pathological significance of these maternal antibodies in a primate model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J Neuroimmunol. 2008;194(1–2):165–72. doi: 10.1016/j.jneuroim.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 65.Croen LA, Braunschweig D, Haapanen L, Yoshida CK, Fireman B, Grether JK, et al. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol Psychiatry. 2008;64(7):583–8. doi: 10.1016/j.biopsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21(24):3365–9. doi: 10.1016/s0264-410x(03)00334-7. [DOI] [PubMed] [Google Scholar]
- 67.Heininger U, Desgrandchamps D, Schaad UB. Seroprevalence of Varicella-Zoster virus IgG antibodies in Swiss children during the first 16 months of age. Vaccine. 2006;24(16):3258–60. doi: 10.1016/j.vaccine.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 68.Djelmis J, Sostarko M, Mayer D, Ivanisevic M. Myasthenia gravis in pregnancy: report on 69 cases. Eur J Obstet Gynecol Reprod Biol. 2002;104(1):21–5. doi: 10.1016/s0301-2115(02)00051-9. [DOI] [PubMed] [Google Scholar]
- 69.Motta M, Rodriguez-Perez C, Tincani A, Lojacono A, Chirico G. Outcome of infants from mothers with anti-SSA/Ro antibodies. J Perinatol. 2007;27(5):278–83. doi: 10.1038/sj.jp.7211688. [DOI] [PubMed] [Google Scholar]
- 70.Morris CM, Zimmerman AW, Singer HS. Childhood serum anti-fetal brain antibodies do not predict autism. Pediatr Neurol. 2009;41(4):288–90. doi: 10.1016/j.pediatrneurol.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 71.Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol. 2006;178(1–2):149–55. doi: 10.1016/j.jneuroim.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 72.Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral D, Van de Water J. Autoantibodies in autism spectrum disorders (ASD) Ann N Y Acad Sci. 2007;1107:79–91. doi: 10.1196/annals.1381.009. [DOI] [PubMed] [Google Scholar]
- 73**.Lee JY, Huerta PT, Zhang J, Kowal C, Bertini E, Volpe BT, et al. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat Med. 2009;15(1):91–6. doi: 10.1038/nm.1892. This elegant study showed that brain-directed antibodies produced by mothers during pregnancy can enter the fetal brain and cause alterations in behavior and brain structure. This was especially intriguing because antibody production was induced in female mice; and antibodies were not passively transferred an external source. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74**.Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2007.12.007. This novel study used a primate model to demonstrate the pathogenic significance of the anti-brain antibodies found in mothers of children with autism, which were initially described by Braunschwieg et al in 2008 (ref 63) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75*.Singer HS, Morris C, Gause C, Pollard M, Zimmerman AW, Pletnikov M. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. J Neuroimmunol. 2009;211(1–2):39–48. doi: 10.1016/j.jneuroim.2009.03.011. This was the first demonstration in a murine model that maternal anti-brain antibodies can mediate behavioral changes in prenatally-exposed offspring. This study corroborated findings in the Marin et al study (ref 74) [DOI] [PubMed] [Google Scholar]