Abstract
A neonate with congestive heart failure at birth due to a nearly holohemispheric pial arteriovenous malformation is described. This occurred despite a normal second trimester prenatal sonogram. Successful treatment of heart failure was achieved by embolization alone. This case demonstrates that hemodynamically significant lesions may arise later or enlarge more rapidly in utero than previously thought.
Congestive heart failure (CHF) in a neonate due to an intracerebral arteriovenous shunt is a rare event, most commonly caused by a vein of Galen malformation. Giant pial arteriovenous malformations (AVMs) are an even less frequent cause of CHF in neonates as the malformation must be unusually large. It is unclear whether pial AVMs form early or late in prenatal development. We report a case of a nearly holohemispheric AVM resulting in neonatal CHF, requiring extensive therapeutic embolization, which was undetected on second trimester fetal sonography. This case suggests hemodynamically significant AVMs can arise or grow significantly during the second and third trimesters of gestation.
CASE REPORT
A 39-week, 2930-g boy was born via spontaneous vaginal delivery to a healthy 29-year-old woman. Prenatal screening sonograms performed at 20 and 26 weeks’ gestational age were normal (as confirmed at our institution), including the brain (Figure 1). Apgar scores were 3 and 4 at 1 and 5 min, respectively, but the patient developed respiratory distress. Echocardiography demonstrated pulmonary hypertension and retrograde flow in the descending aorta, prompting concern for an intracranial arteriovenous shunt. CT angiography confirmed a right frontotemporoparietal AVM with multiple large arteriovenous fistulae involving the right anterior, middle and posterior cerebral arteries, and massive dilatation of the dural venous sinuses (Figure 2A).
Figure 1.

(A) Sonogram of the fetal head at 20 weeks’ gestation at the level of the lateral ventricles is normal. (B) Sonogram of the fetal head at 26 weeks’ gestation at the level of the lateral ventricles with the fetus in opposite lie is also normal.
Figure 2.
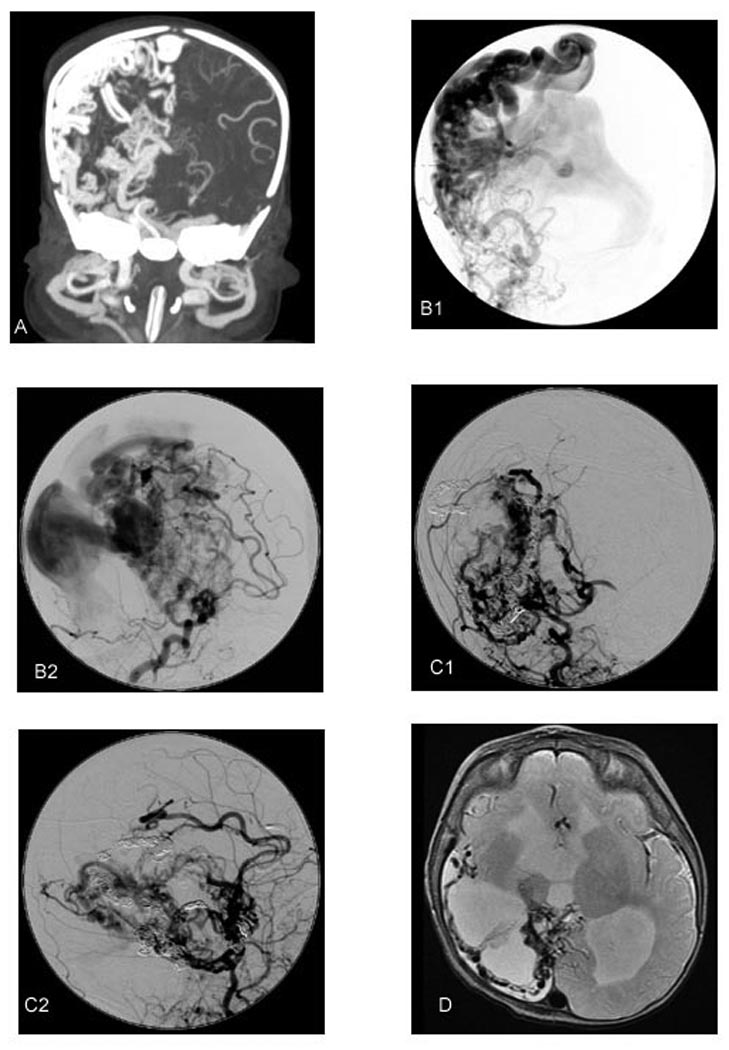
(A) Coronal maximal intensity projection of CT angiogram at postnatal day 1 demonstrates dilated middle cerebral, anterior cerebral, anterior choroidal, and lenticulostriate arteries, arteriovenous fistulas, and dilated draining veins and venous sinuses. (B) Catheter angiogram: anteroposterior (B1) and lateral (B2) early arterial phase right common carotid artery injection at postnatal day 5 prior to initial embolization. Dilated middle cerebral, anterior cerebral, posterior cerebral, anterior choroidal, and lenticulostriate arteries, arteriovenous fistulas, dilated draining cortical veins and massively dilated venous sinuses are present. (C) Catheter angiogram: anteroposterior (C1) and lateral (C2) mid arterial phase right common carotid artery injection at 6 weeks postnatal following final embolization. Marked reduction in arteriovenous shunting and presence of embolic materials in branches of the right middle cerebral, anterior cerebral, posterior cerebral, and anterior choroidal arteries are evident. (D) Axial T2 weighted MR at the level of the foramen of Monro at postnatal age 20 months demonstrates right hemispheric encephalomalacia sparing a portion of the frontal lobe, ventriculomegaly, and a rim of flow voids compatible with residual arteriovenous malformation.
The patient was transferred to our hospital on day 3 of life in severe cardiac failure with anasarca, encephalopathy, diffuse hypotonia and minimal withdrawal to pain. After extensive discussions with the patient’s family and the hospital ethics committee, we planned endovascular treatment to reduce CHF. On day 6 of life, a catheter angiogram (Figure 2B) and transcatheter embolization of three large right middle cerebral artery and three large right posterior cerebral artery shunts were performed using Guglielmi detachable coil and n-butylcyanoacrylate glue. Due to persistent pulmonary hypertension, the patient underwent two additional embolizations at 9 days and 6 weeks of age, with n-butylcyanoacrylate and Guglielmi detachable coil occlusion of additional right middle, posterior and anterior cerebral arteries, and anterior choroidal artery fistulae. The patient gradually became arousable and his pulmonary hypertension improved. He was discharged home on no cardiac medications.
Six months after discharge, supplemental oxygen therapy was discontinued. At 20 months of age, the patient was speaking several words, had a left hemiparesis and no detectable visual field deficit, heart failure, seizures or headaches. MRI demonstrated right hemispheric atrophy and a residual rim of AVM (Figure 2D).
DISCUSSION
Giant pial AVMs are rare and may present neonatally with high output CHF, acting as a circuit of low resistance and high capacity. A key question highlighted by this case is whether we observed third trimester lesion genesis or merely a rapid enlargement of an already extant vascular lesion. AVMs of significant size could remain clinically occult in utero while the fetus is supplied from the placental circulation. With the first breath, pulmonary vascular resistance decreases and fetal shunts close, resulting in decreased central venous pressure and increased aortic pressure. The marked increase in the trans-nidal pressure gradient may augment shunt flow, thus allowing a previously undetected lesion to become evident by an increase in the caliber of AVM vessels. In contrast with pial AVMs, vein of Galen malformations, which are often successfully imaged sonographically in the second trimester, may have structural aspects such as dural attachments that facilitate early imaging.
The pathogenesis of AVMs remains uncertain. Although often presumed to be congenital lesions as a result of embryonic maldevelopment during the 4th–8th week,1 there is a conspicuously low number of brain AVMs detected in the prenatal cerebral circulation, given the large number of prenatal ultrasounds performed annually. Furthermore, AVMs are reported to grow, regress, recur and—rarely—arise de novo after angiographically confirmed complete resections.2, 3 Even if these instances are uncommon, they demonstrate that the capacity for postnatal AVM growth exists.
There is probably no single genetic influence that results in AVM formation and growth. For those associated with the hereditary hemorrhagic telangectasias, endoglin or ALK1 haploinsufficiency is an overwhelming risk factor.4 Based on knowledge of the genetic alterations in hereditary hemorrhagic telangectasias, genetic (or occult environmental) causal factors probably include a signaling pathway that is related to the participation of endoglin and ALK1 in TGF-b signaling.4
In terms of the molecular phenotype of resected AVM tissue, the emerging picture is one of active endothelial proliferation and ongoing angiogenesis, akin to a slowly growing vascular neoplasm. Furthermore, there is prominent expression of inflammatory cells, along with high levels of proteases (e.g., matrix metalloproteinase 9) and cytokines (e.g., interleukin 6).5–7 These phenotypic observations have led to exploratory efforts to slow or arrest growth or progression of AVMs with antiangiogenic or anti-inflammatory therapy.8
The prognosis for neonates who present with CHF due to an AVM has historically been poor. Endovascular treatment for pediatric AVMs was first described in the 1960s.9 Current treatment for pediatric brain AVMs is multimodal with endovascular embolization often preceding surgical excision or gamma knife radiotherapy.10 Due to the logistical challenges of neonatal surgery, AVMs are especially difficult to treat in this age group. In cases of severe CHF, embolization alone may be the best treatment modality to temporize CHF until the child reaches an age at which definitive surgical resection is more manageable.
Our case demonstrates that development of a hemodynamically significant lesion can occur rapidly during late in utero development and present immediately after birth. It seems likely that high flow and endothelial shear rates in the nidus are not only an effect of shunts forming but also a stimulus for further vascular remodeling and lesion progression in the highly angiogenic milieu in utero. The severity of this case suggests to us that the same angiogenic pathways seen in the less aggressive setting of adult AVMs became activated in the rapidly developing fetus. We also speculate that, if an AV shunt forms during rapid prenatal growth and is associated with high levels of growth factors, the resulting lesion should be large and rapidly progressive, as seen in our case.
Contributor Information
Christopher A. Potter, Department of Radiology and Biomedical Imaging, UCSF Medical Center, San Francisco, CA
Jennifer Armstrong-Wells, Department of Neurology, UCSF Medical Center, San Francisco, CA.
Heather J. Fullerton, Department of Neurology, UCSF Medical Center, San Francisco, CA.
William L. Young, Department of Anesthesia and Perioperative Care, UCSF Medical Center, San Francisco, CA.
Randall T. Higashida, Department of Radiology and Biomedical Imaging, UCSF Medical Center, San Francisco, CA.
Christopher F. Dowd, Department of Radiology and Biomedical Imaging, UCSF Medical Center, San Francisco, CA.
Van V. Halbach, Department of Radiology and Biomedical Imaging, UCSF Medical Center, San Francisco, CA.
Steven W. Hetts, Department of Radiology and Biomedical Imaging, UCSF Medical Center, San Francisco, CA.
REFERENCES
- 1.Mullan S, Mojtahedi S, Johnson DL, et al. Embryological basis of some aspects of cerebral vascular fistulas and malformations. J Neurosurg. 1966;85:1–8. doi: 10.3171/jns.1996.85.1.0001. [DOI] [PubMed] [Google Scholar]
- 2.Du R, Hashimoto T, Tihan T, et al. Growth and regression of an arteriovenous malformation in a patient with hereditary hemorrhagic telangiectasia: case report. J Neurosurg. 2007;106:470–477. doi: 10.3171/jns.2007.106.3.470. [DOI] [PubMed] [Google Scholar]
- 3.Stevens J, Leach JL, Abruzzo T, et al. De novo cerebral arteriovenous malformation: case report and literature review. AJNR Am J Neuroradiol. 2009;30:111–112. doi: 10.3174/ajnr.A1255. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim H, Pawlikowska L, Chen Y, et al. Brain arteriovenous malformation biology relevant to hemorrhage and implication for therapeutic development. Stroke. 2009;40:S95–S97. doi: 10.1161/STROKEAHA.108.533216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CZ, Xue Z, Hao Q, et al. Nitric oxide in vascular endothelial growth factor-induced focal angiogenesis and matrix metalloproteinase-9 activity in the mouse brain. Stroke. 2009;40:2879–2881. doi: 10.1161/STROKEAHA.109.552059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herzig R, Burval S, Vladyka V, et al. Familial occurrence of cerebral arteriovenous malformation in sisters: case report and review of the literature. Eur J Neurol. 2000;7:95–100. doi: 10.1046/j.1468-1331.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto T, Wu Y, Lawton MT, et al. Co-expression of angiogenic factors in brain arteriovenous malformations. Neurosurgery. 2005;56:1058–1065. [PubMed] [Google Scholar]
- 8.Frenzel T, Lee CZ, Kim H, et al. Feasibility of minocycline and doxycyline use as potential vasculostatic therapy for brain vascular malformations: pilot study of adverse events and tolerance. Cerebrovasc Dis. 2008;25:157–163. doi: 10.1159/000113733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upchurch K, Feng L, Duckwiler GR, et al. Nongalenic arteriovenous fistulas: history of treatment and technology. Neurosurg Focus. 2006;20:E8. doi: 10.3171/foc.2006.20.6.8. [DOI] [PubMed] [Google Scholar]
- 10.Chang SD, Marcellus ML, Marks MP, et al. Multimodality treatment of giant intracranial arteriovenous malformations. Neurosurgery. 2003;53:1–13. doi: 10.1227/01.neu.0000068700.68238.84. [DOI] [PubMed] [Google Scholar]


