Abstract
Regulatory T cells (Treg cells) that express the transcription factor Foxp3 suppress the activity of other cells. Here we show that interleukin 10 (IL-10) produced by CD11b+ myeloid cells in recombination-activating gene 1–deficient (Rag1−/−) recipient mice was needed to prevent the colitis induced by transferred CD4+ CD45RBhi T cells. In Il10 −/− Rag1−/− mice, Treg cells failed to maintain Foxp3 expression and regulatory activity. The loss of Foxp3 expression occurred only in recipients with colitis, which indicates that the requirement for IL-10 is manifested in the presence of inflammation. IL-10 receptor–deficient (Il10rb−/−) Treg cells also failed to maintain Foxp3 expression, which suggested that host IL-10 acted directly on the Treg cells. Our data indicate that IL-10 released from myeloid cells acts in a paracrine manner on Treg cells to maintain Foxp3 expression.
CD4+ regulatory T cells (Treg cells) express the transcription factor Foxp3 (A002750), which is required for their suppressive function. A T cell–transfer model of colitis has been widely used to study the function of Treg cells in vivo. When CD4+CD45RBhi T cells are transferred into immunodeficient mice, some of the transferred T cells secrete proinflammatory cytokines and induce an inflammatory bowel disease–like syndrome1,2. Cotransfer of sufficient numbers of Treg cells can prevent or even cure this disease3,4. The transferred Treg cell populations expand considerably in vivo, and most maintain Foxp3 expression5,6.
Mice deficient in interleukin 10 (IL-10 (A001243); Il10−/− mice) or the IL-10 receptor β-chain (IL-10Rβ (A001245); Il10rb−/− mice) develop spontaneous inflammation of the large intestine, a process dominated by a T helper type 1 immune response7,8. Many cell types can produce IL-10, however, and therefore the IL-10 source(s) needed to prevent inflammation must be identified. Much emphasis has been placed on the role of IL-10 released by CD4+ T cells, and in fact mice with conditional deletion of IL-10 in the CD4+ subset develop spontaneous inflammation of the intestine9. Mice with deletion of IL-10 solely in Foxp3+ cells also develop inflammation in the intestine and elsewhere, although the pathogenesis is less intense than that in mice completely lacking IL-10 (ref. 10). Transgenic mice that overexpress IL-10 in intestinal epithelial cells are protected from colitis11, which suggests that IL-10 from nonlymphoid sources can be beneficial, although altered expression in the transgenic mice may not be physiologically relevant.
To further elucidate the cellular and molecular basis of the function of IL-10 in regulating colitis, we used the T cell–transfer model described above. We found that IL-10 from nonlymphoid cells, particularly CD11b+CD11c+ cells, had an unexpectedly important influence on the development of colitis. Furthermore, we provide evidence that this IL-10 acted in part on Treg cells to maintain their expression of Foxp3, which was otherwise lost in inflammatory conditions after transfer.
RESULTS
Colitis prevention by Il10−/− Treg cells
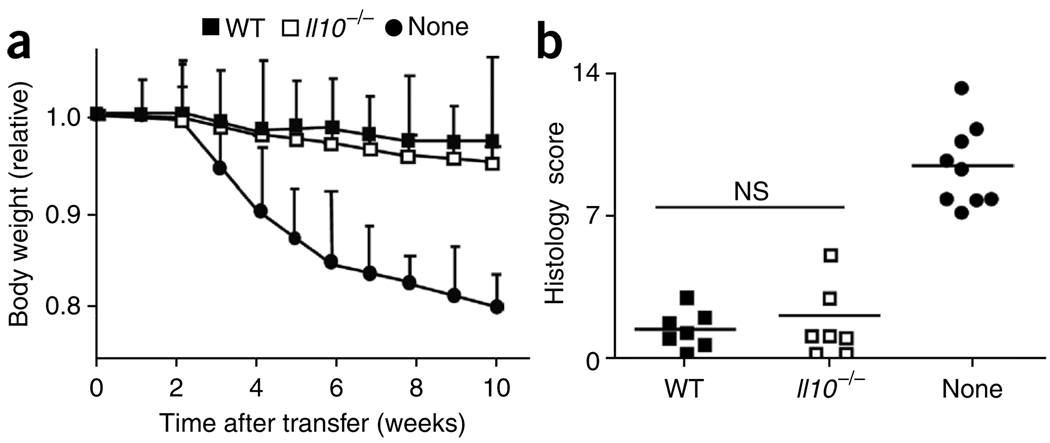
Several studies have suggested that IL-10 production by Treg cells is required for the prevention of colitis10,12,13. However, some studies suggest that Treg cell–derived IL-10 is less important than other studies do14,15. To assess the suppressive function of Il10−/−CD4+CD25+ Treg cells, we did both in vitro and in vivo experiments. Il10−/− CD4+CD25+ Treg cells were as capable as wild-type cells of inhibiting the proliferation of CD4+CD45RBhi T cells in vitro (data not shown). For in vivo analysis, we transferred sorted CD4+CD45RBhi T cells together with sorted CD4+CD45RBloCD25+ Treg cells from wild-type or Il10−/− mice into recombination-activation gene 1–deficient (Rag1−/−) mice. As a positive control for colitis induction, we injected CD4+CD45RBhi T cells without Treg cells. Host mice given either wild-type or Il10−/− Treg cells remained healthy without losing body weight (Fig. 1a) and survived for more than 4 months after transfer (data not shown). The average histology scores of recipients of wild-type or Il10−/− Treg cells were similar and were indicative of a low degree of inflammation compared with that of mice that did not receive Treg cells (Fig. 1b). These findings show that IL-10 derived from Treg cells was dispensable for the prevention of colitis in our mice.
Figure 1.
IL-10-deficient Treg cells prevent colitis. (a) Body weight of Rag1−/− mice given sorted Il10−/− or wild-type (WT) CD4+CD25+ Treg cells, together with CD4+CD45RBhi T cells, or of Rag1−/− mice given CD4+CD45RBhi T cells alone (control; None), presented relative to initial body weight. Data are pooled from two independent experiments with six mice each (error bars, s.d.). (b) Histology scores of sections of the large intestine at 6 weeks after the cell transfer described in a. Each symbol represents an individual mouse; small horizontal lines indicate the mean. NS, not significant. Data are pooled from at least two independent experiments.
Host IL-10 is required for colitis suppression
The negative results of the analysis of Treg cell–derived IL-10 raised the issue of the possible involvement of host-derived IL-10 in the inhibition of colonic inflammation. To address this possibility, we used Il10−/−Rag1−/− mice as recipients in transfer experiments. These mice did not spontaneously develop colitis (data not shown); however, both Rag1−/− and Il10−/−Rag1−/− recipients of CD4+CD45RBhi T cells had lost approximately 20% of their initial weight at 8 weeks after donor cell injection (Supplementary Fig. 1a). The severity of colitis in Rag1−/− mice was similar to that in the Il10−/−Rag1−/− hosts (Supplementary Fig. 1b), which suggested that host-derived IL-10 did not have a major effect on the colitis pathogenesis induced by the transferred T cell population.
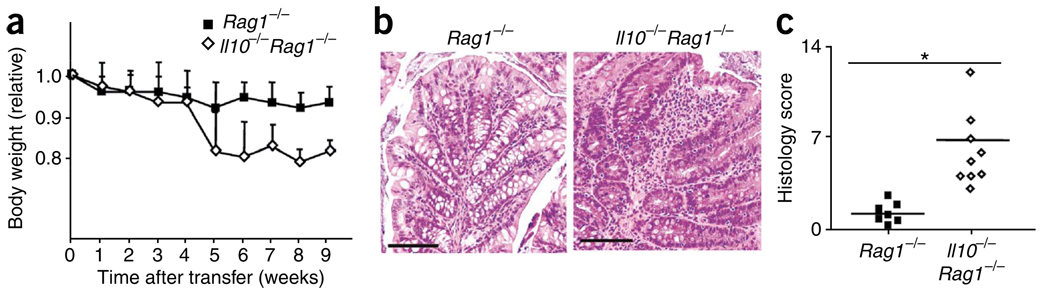
Next we determined if host-derived IL-10 was essential for Treg cell–mediated prevention of colitis. To more precisely define the transferred Treg cell population, we used reporter mice with sequence encoding green fluorescent protein (GFP) inserted inframe into the Foxp3 gene (Foxp3gfp)16. We selected the Treg cell population from these mice on the basis of green fluorescence, as well as expression of CD25, CD4 and small amounts of CD45RB. The selected cells were up to 99% Foxp3+ (Supplementary Fig. 2). We transferred CD45.1+ CD4+CD45RBhi T cells together with CD45.2+ Foxp3gfp Treg cells into either Rag1−/− or Il10−/−Rag1−/− hosts so that the two donor T cell populations could be distinguished on the basis of their CD45 alleles. As expected, Rag1−/− hosts that received CD4+CD45RBhi T lymphocytes and Treg cells did not lose weight (Fig. 2a). Unexpectedly, at 5 weeks after transfer, Il10−/−Rag1−/− hosts injected with CD4+CD45RBhi T lymphocytes and Treg cells showed a weight loss of nearly 20% (Fig. 2a), similar to that induced by transfer of CD4+CD45RBhi T cells alone. Furthermore, Il10−/−Rag1−/− recipients developed colonic inflammation characterized by the infiltration of mononuclear cells, loss of goblet cells and epithelial cell hyperplasia in both the distal colon (data not shown) and proximal colon (Fig. 2b,c). We obtained similar results with Treg cells isolated from wild-type mice and sorted on the basis of CD25, CD4 and CD45RBlo expression alone (data not shown), which indicated that the results presented above were not related to the insertion of the gene encoding GFP into the Foxp3 locus. These findings suggest that host-derived IL-10 is required for Treg cell–mediated prevention of colitis.
Figure 2.
Rag1−/− host IL-10 is required for Treg cell function. (a) Body weight of Rag1−/− or Il10−/−Rag1−/− hosts given CD4+CD45RBhi T cells plus sorted Foxp3gfp Treg cells, presented relative to initial body weight. Data are pooled from two independent experiments with ten mice each (error bars, s.d.). (b) Proximal colon of Rag1−/− and Il10−/−Rag1−/− mice at 6 weeks after the donor cell transfer described in a; sections are stained with hematoxylin and eosin. Original magnification, ×100; scale bars, 100 µm. Data are representative of one of three independent experiments. (c) Histology scores of sections of the large intestine at 6 weeks after the cell transfer described in a. Each symbol represents an individual mouse; small horizontal lines indicate the mean. *P < 0.001 (two-tailed Student’s t-test). Data are pooled from three independent experiments with a total of nine mice.
Lower Foxp3 expression in Il10−/−Rag1−/− recipients
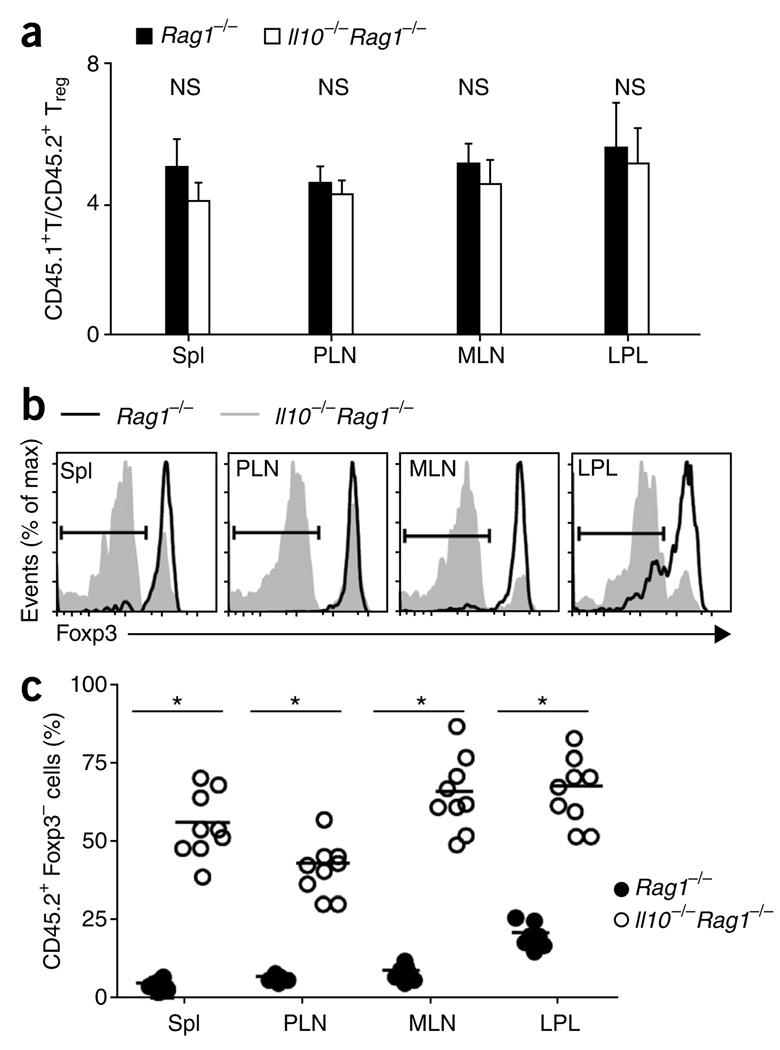
To determine if the Treg cells underwent population expansion and homed to different tissues in the absence of host IL-10, we collected lymphocytes from the lamina propria of the large intestine (LPL), spleen, peripheral lymph nodes (PLNs; inguinal and axillary) and mesenteric lymph nodes (MLNs); we separately analyzed by flow cytometry the CD45.1+ progeny of CD4+CD45RBhi T cells and the CD45.2+ progeny of Treg cells sorted from Foxp3gfp mice. By 6 weeks after donor cell injection, the CD45.1+ cell/CD45.2+ cell ratios in Rag1−/− and Il10−/−Rag1−/− hosts were maintained at approximately the starting ratio of 4:1 in each of the organs analyzed (Fig. 3a). This suggested that the population expansion of Foxp3gfp Treg cells in vivo was similar to that of the CD4+CD45RBhi T cells in Rag1−/− and Il10−/−Rag1−/− hosts.
Figure 3.
Foxp3 is downregulated in Il10−/−Rag1−/− recipients. (a) Composite ratios of CD45.1+ to CD45.2+ TCRβ+CD4+ cells in the spleen (Spl), PLNs, MLNs and LPL of Rag1−/− or Il10−/−Rag1−/− recipient mice at 6 weeks after injection of 4 × 105 CD4+CD45RBhi T cells derived from C57BL/6 (CD45.1+) mice, plus 1 × 105 Foxp3gfp (CD45.2+) Treg cells. (b) Foxp3 expression in the cells in a, gated on TCRβ+CD4+ CD45.2+ cells. Bracketed lines indicate the Foxp3− population. max, maximum. (c) Foxp3− cells in the gated TCRβ+CD4+ CD45.2+ populations in b. Each symbol represents an individual mouse; small horizontal lines indicate the mean. *P < 0.001 (two-tailed Student’s t-test). Data are pooled from three independent experiments with a total of nine mice (a (mean and s.d.) and c) or are representative of one of three independent experiments (b).
Unexpectedly, however, Treg cells in Il10−/−Rag1−/− hosts showed profound downregulation of the expression of Foxp3 protein (Fig. 3b,c) and Foxp3 mRNA (data not shown). In contrast, Treg cells transferred into Rag1−/− hosts maintained Foxp3 expression, although the percentage of Foxp3-expressing progeny of sorted Treg cells in the lamina propria was less than that in the lymphoid organs (Fig. 3b,c). The Treg cells obtained from Il10−/−Rag1−/− and Rag1−/− hosts expressed similar amounts of the immunomodulatory receptor GITR and cytokine receptor CD25 (data not shown). We detected some loss of Foxp3 expression, especially in LPL, as early as 2–3 weeks after transfer (Supplementary Fig. 3), the earliest times at which transferred Treg cells were readily detectable.
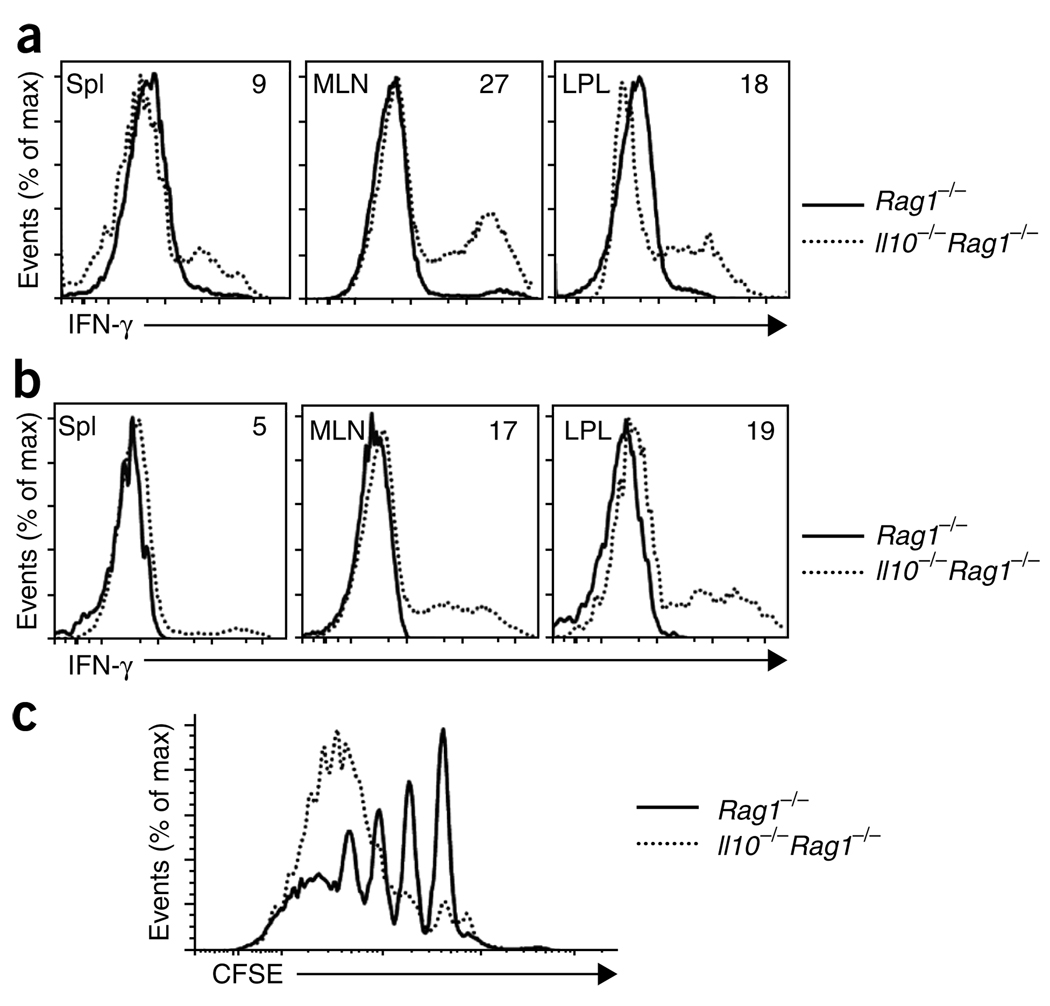
To determine if Treg cells from Il10−/−Rag1−/− hosts had altered function, we assessed cytokine production. At 6 weeks after donor cell injection, CD4+CD45RBhi (CD45.1+) T cells obtained from Il10−/−Rag1−/− hosts produced interferon-γ (IFN-γ) when stimulated in vitro with phorbol 12-myristate 13-acetate (PMA) and ionomycin (Fig. 4a). In addition, some of these cells produced IL-17 (Supplementary Fig. 4) and tumor necrosis factor (data not shown). In contrast, CD4+CD45RBhi T cells obtained from Rag−/− hosts did not produce IFN-γ or other proinflammatory cytokines after restimulation (Fig. 4a), which probably reflected the suppressive activity of the cotransferred Treg cells.
Figure 4.
Loss of function by Treg cells from Il10−/−Rag1−/− recipients. (a,b) Flow cytometry of intracellular IFN-γ in CD4+CD45RBhi T cells derived from C57BL/6 (CD45.1+) mice transferred with Foxp3gfp (CD45.2+) Treg cells into Rag1−/− or Il10−/−Rag1−/− hosts; plots are gated on the TCRβ+CD4+ CD45.2− progeny of donor CD4+CD45RBhi T cells (a) and the TCRβ+CD4+ CD45.2+ progeny from donor Foxp3gfp + Treg cells (b), isolated from spleen (Spl), MLNs and LPL in mice at 6 weeks after donor cell injection and then stimulated with PMA and ionomycin. Numbers in plots indicate percent IFN-γ-producing cells. (c) Suppressive function in vitro of sorted TCRβ+CD4+ CD45.2+ cells from MLNs of Rag1−/− or Il10−/−Rag1−/− recipients of CD45.1+ CD4+CD45RBhi and CD45.2+ CD4+CD25+CD45RBlo Treg cell populations, cultured for 4 d together with CFSE-labeled CD45.1+ naive T cells; after stimulation of cultures, CFSE dilution was assessed by flow cytometry. Data are representative of one of three (a,b) or two (c) independent experiments.
Notably, CD45.2+ progeny of Treg cells obtained from all organs of Il10−/−Rag1−/− hosts also produced IFN-γ after restimulation, although this was less evident in the spleen than in the mucosal immune system (Fig. 4b). These cells, however, did not produce IL-17 (Supplementary Fig. 4) or tumor necrosis factor (data not shown), which distinguishes their cytokine profile from that of the CD45.1+ CD4+CD45RBhi T cells obtained from the same recipients. In agreement with their ability to produce IFN-γ, the CD45.2+ progeny of Treg cells transferred into Il10−/−Rag1−/− recipients also expressed the transcription factor T-box 21 (also called T-bet), as detected by flow cytometry and RT-PCR (data not shown). Consistent with their maintenance of Foxp3 expression, the progeny of sorted Treg cells obtained from Rag1−/− hosts did not produce IFN-γ (Fig. 4b), IL-17 or tumor necrosis factor and they did not express T-bet (data not shown).
The distinct cytokine profile of the cells that previously expressed Foxp3, compared with that of their CD4+CD45RBhi counterparts, suggested that these cells were not derived from the progeny of a small number of contaminating CD4+CD45RBhi To determine if contaminating CD4+ T lymphocytes with an activated memory phenotype could have outgrown the transferred Treg cells in IL-10-deficient conditions, we transferred CD90.1+ CD4+CD45RBhi cells together with CD45.2+ Treg cells and CD45.1+ CD44hiCD62L−Foxp3− cells with an activated memory phenotype into Il10−/−Rag1−/− hosts. The number of memory cells transferred was 3% the number of Treg cells, a degree of impurity greater than that in the transferred Treg cell populations that were enriched by flow cytometry. These memory cells did not outgrow the transferred Treg cells in MLNs, perhaps outgrew them only slightly in spleen and were under-represented in lamina propria when analyzed 2 weeks after transfer (Supplementary Fig. 5). At this time, however, up to 50% of the original transferred Treg cell population had lost Foxp3 expression.
To further define the function of the transferred Treg cells, we assessed their suppressive activity in vitro. We sorted Treg cells on the basis of CD45.2 expression from the MLNs of recipients at 6 weeks after donor cell injection. We cultured Treg cells obtained from Rag1−/− or Il10−/−Rag1−/− recipients with carboxyfluorescein succinimidyl ester (CFSE)-labeled splenic CD45.1+CD4+CD25− T cells obtained from wild-type mice. Whereas Treg cells recovered from Rag1−/− hosts suppressed the proliferation of the CFSE-labeled naive CD4+ T cells, Treg cells isolated from Il10−/−Rag1−/− recipients failed to suppress the proliferation of naive T cells (Fig. 4c). Collectively these findings show that IL-10 produced by cells in Rag1−/− mice is required for the maintenance of Foxp3 expression and the suppressive activity of transferred Treg cells.
Il10rb−/− Treg cells do not prevent colitis
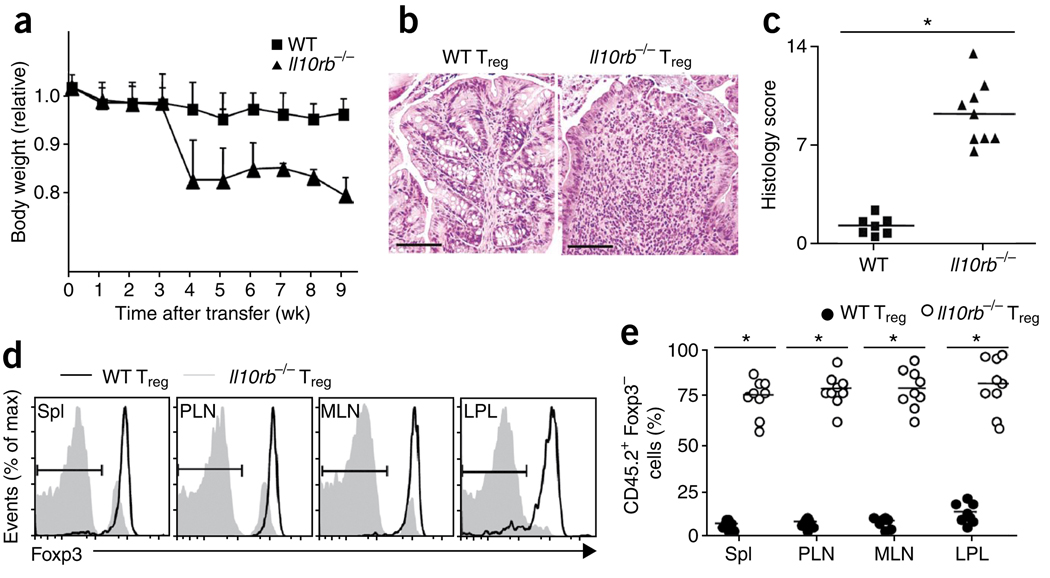
IL-10 produced by cells in the Rag1−/− hosts may act directly on the transferred Treg cells or may act on some other cell type, such as dendritic cells, which might be involved in stabilizing Foxp3 expression. To test for a direct effect of IL-10 on transferred Treg cells, we compared the ability of Treg cells from wild-type and Il10rb−/− mice to suppress colitis in Rag1−/− hosts, with Foxp3gfp mice crossed to Il10rb−/− mice as a source of ‘marked’ Treg cells. The percentage of Foxp3gfp Treg cells in 6- to 7-week-old Il10rb−/− mice, which were generally healthy, was similar to that in wild-type mice (Supplementary Fig. 6a). Furthermore, Foxp3gfp T cells from Il10rb−/− mice were as capable as wild-type cells of inhibiting the proliferation of CD4+CD45RBhi T cells in vitro (Supplementary Fig. 6b). However, Rag1−/− recipients of CD4+CD45RBhi T cells and Il10rb−/− T cells had only approximately 20% of their initial starting weight by 4 weeks after donor cell injection (Fig. 5a). Moreover, these mice had severe colonic inflammation in both the distal colon (data not shown) and proximal colon (Fig. 5b,c). Similar to the outcome produced by transfer of wild-type Treg cells into Il10−/−Rag1−/− hosts, transferred Il10rb−/− T cells showed profound downregulation of Foxp3 expression (Fig. 5d,e). These findings suggest that IL-10 signaling is required, at least in part in a Treg cell–intrinsic manner, for the maintenance of Foxp3 expression and suppressive function.
Figure 5.
Il10rb−/− Treg cells fail to prevent colitis. (a) Body weight of Rag1−/− recipients of C57BL/6 (CD45.1+) CD4+CD45RBhi T cells transferred together with wild-type or Il10rb−/− (CD45.2+) Treg cells, presented relative to initial body weight. Data are pooled from two independent experiments with a total of ten mice (error bars, s.d.). (b) Proximal colon of recipient mice at 6 weeks after injection of cells as described in a; sections are stained with hematoxylin and eosin. Original magnification, ×100; scale bars, 100 µm. Data are representative of one of three independent experiments. (c) Histology scores of sections of the large intestine at 6 weeks after the cell transfer described in a. Each symbol represents an individual mouse; small horizontal lines indicate the mean. *P < 0.001 (two-tailed Student’s t-test). Data are pooled from three independent experiments with a total of nine mice. (d) Foxp3 expression by cells isolated from the spleen, PLNs, MLNs and LPL of the recipient mice in a, with gating on TCRβ+CD4+CD45.2+ cells. Bracketed lines indicate the Foxp3− population. Data are representative of one of three independent experiments with a total of nine mice. (e) Foxp3− cells in the TCRβ+CD4+ CD45.2+ T lymphocyte populations described in d. Each symbol represents an individual mouse; small horizontal bars indicate the mean. *P < 0.001 (two-tailed Student’s t-test). Data are pooled from three independent experiments with a total of nine mice.
Loss of Foxp3 expression requires inflammation
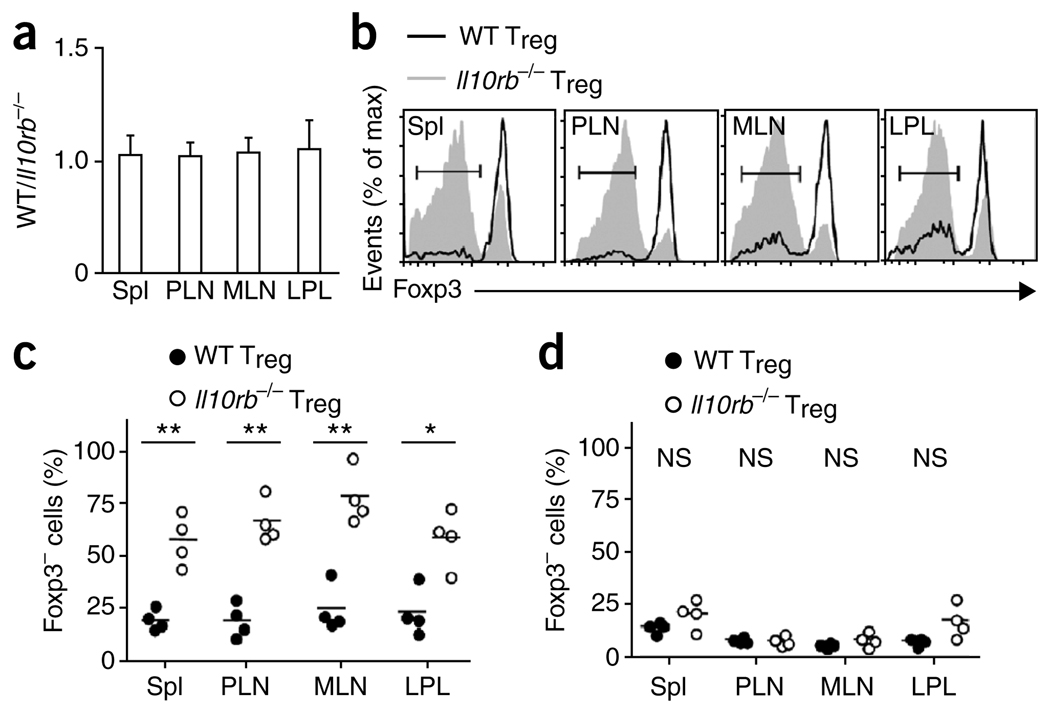
It may be problematic to compare Foxp3 expression in Treg cells in Rag1−/− and Il10−/−Rag1−/− recipients, because only the latter recipients developed intestinal inflammation. It is possible that without IL-10 signaling, transferred Treg cells are only marginally less effective, but that inflammation may amplify the loss of Foxp3 expression and Treg cell function. Therefore, we did cotransfer experiments in which we injected equal numbers of allelically distinguishable wild-type (CD45.1+) and Il10rb−/− (CD45.2+) Treg cells together with CD4+CD45RBhi (CD90.1+) T cells into Rag1−/− hosts and monitored each subset over time (Supplementary Fig. 7a). At a ratio of 20:1 (CD4+CD45RBhi T cells/Treg cells), as anticipated, all mice that received either type of Treg cells or the mixture developed colitis (Supplementary Fig. 7b,c). At 6 weeks after transfer, wild-type and Il10rb−/− Treg cells recovered from these mice were present at approximately the starting ratio of 1:1 (Fig. 6a). Notably, however, Il10rb−/− Treg cells tended to lose Foxp3 expression more than the wild-type Treg cells in the same hosts did (Fig. 6b,c). Also, there was a somewhat greater tendency for the wild-type Treg cells to lose Foxp3 expression in these recipient mice than in recipient mice in which sufficient numbers of Treg cells were present to prevent inflammation (Figs. 3b and 5d). These results show that inflammation itself was not the sole factor that caused the loss of Foxp3 expression, although it may have contributed to this. Furthermore, our results suggest that there is a cell-intrinsic effect of the interaction of IL-10 with its receptor in the maintenance of Foxp3 expression in inflammatory conditions.
Figure 6.
Foxp3 is lost ‘preferentially’ by Il10rb−/− Treg cells in mice with colitis. (a) Ratio of CD45.1+ to CD45.2+ CD90.1−CD4+TCRβ+ cells isolated from spleen, PLNs, MLNs and LPL of Rag1−/− recipients at 6 weeks after injection of 8 × 105 C57BL/6 (CD90.1+) CD4+CD45RBhi T cells, transferred with 2 × 104 wild-type (CD45.1+) Treg cells and 2 × 104 Il10rb−/− (CD45.2+) Treg cells. Data are pooled from two independent experiments with a total of four mice (mean and s.d.). (b) Flow cytometry of Foxp3 expression by cells isolated from a Rag1−/− recipient mouse as described in a, with gating on CD90.1−CD4+TCRβ+CD45.2− cells (wild-type Treg cells) or CD90.1−CD4+TCRβ+CD45.2+ cells (Il10rb−/− Treg cells). Bracketed lines indicate the Foxp3− population. Data are representative of one of two independent experiments. (c) Foxp3− cells in the CD90.1−CD4+TCRβ+CD45.2− (wild-type Treg) and CD90.1−CD4+TCRβ+CD45.2+ (Il10rb−/− Treg) populations isolated from Rag1−/− mice as described in a. Each symbol represents an individual mouse; small horizontal bars indicate the mean. Data are pooled from two independent experiments with a total of four mice. (d) Foxp3− cells in CD90.1−CD4+TCRβ+CD45.2− (wild-type Treg) or CD90.1−CD4+TCRβ+CD45.2+ (Il10rb−/− Treg) populations isolated from Rag1−/− recipients of 4 × 105 C57BL/6 (CD90.1+) CD4+CD45RBhi T cells, transferred together with 1 × 105 wild-type (CD45.1+) and 1 × 105 Il10rb−/− (CD45.2+) Treg cells. Each symbol represents an individual mouse; small horizontal bars indicate the mean. * P < 0.01; ** P < 0.001 (two-tailed Student’s t-test). Data are pooled from two independent experiments with a total of four mice.
We also investigated whether the lack of IL-10 signaling in Treg cells caused the loss of Foxp3 expression when inflammation was not present. We transferred wild-type Treg cells and Il10rb−/− Treg cells (at a ratio of 1:1), along with CD4+CD45RBhi T cells, similar to the experiments described above but at a final ratio of 2:1 for CD4+CD45RBhi T cells/Treg cells. In this case, the mice were completely protected from colitis (data not shown). In the absence of inflammation, the loss of Foxp3 expression by the transferred Il10rb−/− Treg cells was blunted, and the maintenance of Foxp3 expression by cells in this population was similar to that of wild-type Treg cells (Fig. 6d). In addition, when we transferred Treg cells in the absence of CD4+CD45RBhi T cells, Il10rb−/− Treg cells tended to not lose Foxp3 expression any more than wild-type Treg cells did (data not shown). Collectively these data suggest that the loss of Foxp3 expression could be attributed to both the absence of IL-10 signaling in the Treg cells and the presence of inflammatory signals and colitis in the hosts but that neither factor alone caused substantial loss of Foxp3. Therefore, unlike transforming growth factor-β, which is important for the homeostasis of natural Treg cells17–22, IL-10 is important for maintaining Foxp3 expression once the Treg cells are differentiated and exposed to inflammation.
Myeloid cells in the mucosa produce IL-10
We next evaluated which cell types produced IL-10 in the Rag1−/− recipients. We used IL-10 reporter mice in which an internal ribosome entry site upstream and the gene encoding GFP were inserted in Il10 (Il10gfp; ‘Vert-X’ mice). There was good concordance between GFP fluorescence and IL-10 production when we activated T lymphocytes from these mice in vivo (Supplementary Fig. 8). We used cells from these mice as a source of CD4+CD45RBhi T lymphocytes and Treg cells and also crossed these mice with Rag1−/− mice to track IL-10-producing nonlymphoid cells in the recipient mice.
As expected, before transfer, some CD4+CD45RBloCD25+ Treg cells from the spleen were GFP+ (an average of 4.0% ± 1.2%; Fig. 7a), as were some Treg cells in MLNs (data not shown). In the CD4+CD45RBhi T cell population, in contrast, fewer cells expressed GFP (Fig. 7a). When we isolated lymphocytes from wild-type mice, stimulated them with PMA and ionomycin and analyzed IL-10 protein by intracellular cytokine staining, we found that IL-10+ cells were confined to the Treg cell population and constituted a minority of this group (data not shown).
Figure 7.
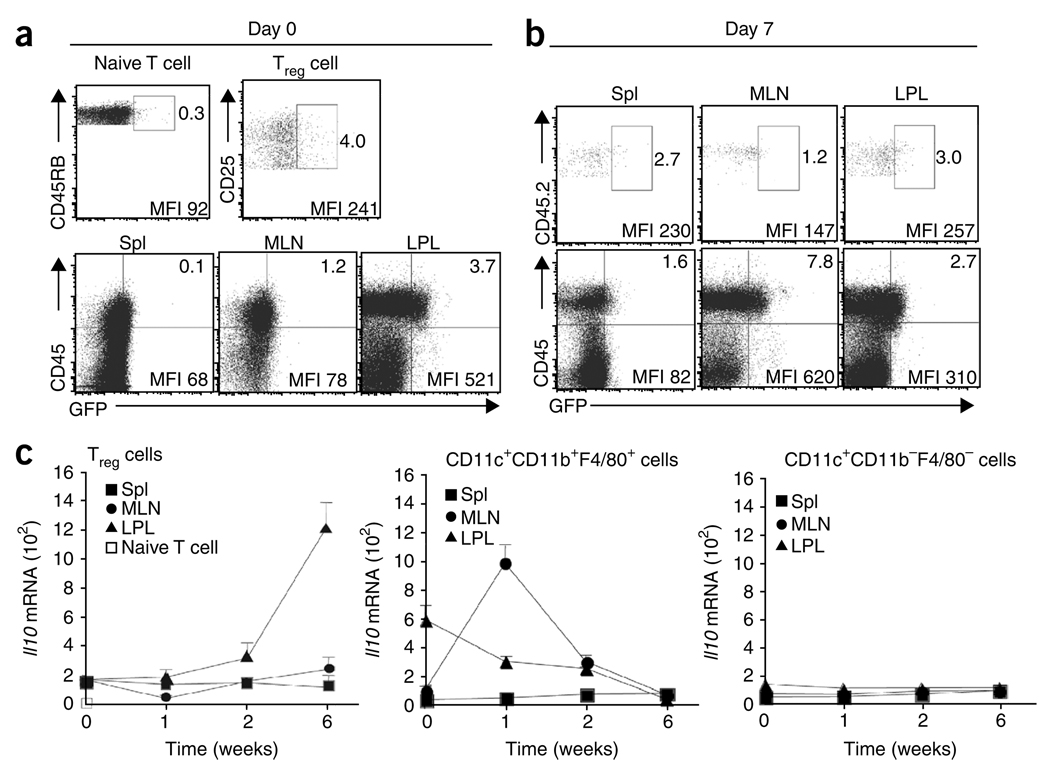
Kinetics of IL-10 expression by Treg cells and host cells. (a) GFP+ cells in IL-10 reporter mice. Numbers adjacent to outlined areas (top row) indicate percent GFP+ cells among gated naive splenocytes (TCRβ+CD4+CD45RBhi) and Treg splenocytes (TCRβ+CD4+CD45RBloCD25+) from Il10gfp mice; numbers in top right quadrants (bottom row) indicate percent CD45+GFP+ cells in tissues from Il10gfpRag1−/− mice. MFI, mean fluorescence intensity. (b) Flow cytometry of GFP+ cells in tissues 7 d after transfer of a mixture of CD45.1+ CD4+CD45RBhi T cells and CD45.2+ Il10gfp Treg cells (ratio, 4:1). Top row, Treg cells gated as CD45.2+ TCRβ+CD4+ cells; bottom row, gated TCRβ−CD4− nonlymphoid cells. Numbers adjacent to outlined areas and in top right quadrants indicate percent GFP+ cells. (c) Real-time PCR analysis of Il10 mRNA in sorted Treg cells (left), CD11b+ cells (middle) and CD11c+ dendritic cells (right) from various sites (keys) before transfer (0) or at 1, 2 and 6 weeks after transfer as in b. Data are from representative one of two independent experiments with six mice (a,b) or are pooled from two independent experiments with six mice (c; mean and s.d.).
Analysis of nonlymphoid cells in the Rag1−/− recipients indicated that before T cell transfer, 3.7% ± 0.9% of CD45+ cells from LPL of Il10gfpRag1−/− mice expressed GFP (Fig. 7a); we detected a lower percentage of GFP+CD45+ cells in the MLNs and spleen, and GFP+ cells were essentially undetectable in the CD45− population in LPL and MLNs (Fig. 7a). We also analyzed GFP expression in Il10gfpRag1−/− recipients 1 week after cotransfer of CD45.1+ CD4+CD45RBhi T cells and CD45.2+ Il10gfp Treg cells. We noted a much higher percentage of nonlymphoid cells in MLNs that were GFP+ (an average of 7.8% ± 1.8% of the CD45+ cells; Fig. 7b). Most of these GFP+ cells (82.1% ± 5.2%) were CD11b+, 62.1% ± 9.6% expressed both CD11b and CD11c, and most expressed the macrophage marker F4/80 (Supplementary Fig. 9). In contrast to cells in the MLNs, in the LPL, the percentage of GFP+CD45+ nonlymphoid cells was slightly lower by 1 week after transfer (an average of 2.7% ± 0.8%; Fig. 7a,b). The source of the greater number of GFP+CD11b+ cells in MLN after transfer could have been due in part to migration from the LPL, although we cannot exclude the possibility of cell division or recruitment from other sources. At 1 week after transfer, in the LPL there were very few GFP+ cells in the CD45− population, and there was no higher GFP expression by gated CD45.2+ Il10gfp Treg cells (an average of 3.0% ± 0.6%) than by the donor population of splenic Treg cells (Fig. 7a,b).
To further define the kinetics of IL-10 production, we analyzed Il10 mRNA by real-time PCR. Before donor cell injection, Treg cells contained 40 times more Il10 transcripts than did CD4+CD45RBhi T cells (Fig. 7c). These data are consistent with the greater percentage of GFP+ cells in Treg cells in the IL-10 reporter mice (Fig. 7a). Before donor cell injection, CD11c+CD11b+ cells of the LPL contained almost four times more Il10 transcripts than did Treg cells (Fig. 7c), whereas CD11c+CD11b+ cells of the MLNs had less Il10 mRNA than did either Treg cells or their counterparts in the LPL (Fig. 7c). At 1 week after injection of CD45.1+ CD4+CD45RBhi T cells and CD45.2+ Il10gfp Treg cells, however, there were substantial changes in the expression of Il10 mRNA in the nonlymphoid cell populations in the intestine. Il10 transcripts in the CD11c+CD11b+F4/80+ cells from MLNs were 6.6-fold more abundant, whereas Il10 mRNA in the same population from the LPL was slightly less abundant (Fig. 7c). These alterations in mRNA are in agreement with the changes in GFP+ cells in the IL-10 reporter mice (Fig. 7b). The greater abundance of Il10 mRNA in CD11c+CD11b+F4/80+ cells in MLNs was transient, however, and peaked at 1 week after donor cell injection. Il10 mRNA in Treg cells increased much later, and the increase occurred selectively in the LPL (Fig. 7c). Those sorted CD11c+ cells that did not express macrophage markers (CD11b and F4/80) and were probably dendritic cells did not induce Il10 mRNA at any time after T cell transfer (Fig. 7c).
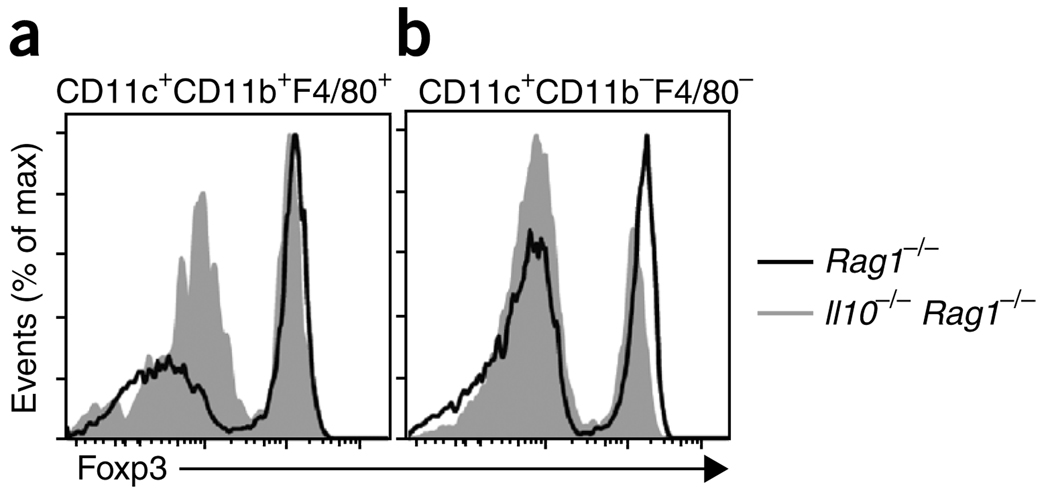
To determine if IL-10 production by myeloid cells was important for the maintenance of Foxp3 expression by Treg cells, we transferred CD11c+CD11b+F4/80+ cells from the LPL of Rag1−/− or Il10−/−Rag1−/− mice into Il10−/−Rag1−/− recipients. When the myeloid cells were derived from Rag1−/− donors, the loss of Foxp3 expression by Treg cells was less than that produced by transfer of the same population from Il10−/−Rag1−/− donors (Fig. 8a). Furthermore, this effect was specific to the CD11b+ cells, as transfer of the same number of CD11c+CD11b−F4/80− cells was not effective in stabilizing Foxp3 expression (Fig. 8b). We conclude that CD11b+ myeloid cells, perhaps mostly macrophages, are key producers of IL-10 in the intestine at early times after T cell transfer and that this IL-10 is necessary for maintenance of Foxp3 expression in Treg cells.
Figure 8.
IL-10-producing CD11b+ myeloid cells prevent the downregulation of Foxp3. Flow cytometry of Foxp3 expression 3 weeks after the injection of 5 × 106 Rag1−/− or Il10−/−Rag1−/− intestinal CD11c+CD11b+F4/80+ cells (a) or CD11c+CD11bF4/80− cells (b) into Il10−/−Rag1−/− recipients, transferred intravenously on days 0 and 7 (where ‘day 0’ is the day of T cell transfer) with 4 × 105 CD4+ CD45RBhi (CD45.1+) cells in the presence of 1 × 105 (CD45.2+) Treg cells from Foxp3gfp mice. Plots are gated on TCRβ+CD4+CD45.2+ splenocytes. Data are representative of one of two independent experiments with a total of three mice.
DISCUSSION
The results from several studies have indicated that T cell–derived IL-10 is important for the prevention of colitis9,10,12,23. However, here we did not identify a major role for Treg cell–derived IL-10 in the transfer model of colitis. The reasons for our discordant findings could reflect aspects of the endogenous flora in different colonies and/or the intensity of the pathogenesis. Regardless of the source of the discrepancy, our results have demonstrated that in some circumstances, Treg cells can prevent colitis by means other than IL-10 secretion. Most notably, we made the unexpected observation that IL-10 produced by cells other than T lymphocytes was required for Treg cell function, despite the ability of the donor Treg cells to secrete IL-10. Treg cells transferred into Il10−/−Rag1−/− recipient mice expanded in number in vivo and homed to various tissues, including the intestine. However, these cells failed to maintain Foxp3 expression and suppressive activity in the absence of IL-10 signaling.
Despite rigorous sorting of Treg cells on the basis of Foxp3 expression, it was possible that a small population (<1%) of contaminating activated effector T cells outgrew the transferred Foxp3+ Treg cells. However, the results from an experiment in which we deliberately transferred congenic activated memory cells together with Treg cells demonstrated that these cells did not outgrow the Treg cells in Il10−/−Rag1−/− recipients. Therefore, loss of Foxp3 expression is the most plausible explanation for the ineffectiveness of Treg cells in the absence of IL-10 signaling.
Studies have indicated that the phenotype and function of Treg cells can be unstable. For example, a minority of Treg cells lose Foxp3 expression in vitro when IL-6 is added24. Also, antibody ligation of T cell immunoglobulin mucin 1 causes loss of Foxp3 mRNA expression and Treg cell function in vitro25. The effect of engagement of T cell immunoglobulin mucin 1 on Foxp3+ cells by its ligand Tim-4 in vivo remains to be analyzed. In addition, a sub-population of CD25−Foxp3+ Treg cells has a tendency to lose Foxp3 expression26. However, we excluded that subset from our analysis, as our Treg cell populations were selected for high expression of CD25. Moreover, transferred Treg cells can generate follicular helper T cells in the Peyer’s patches under the influence of CD40 expression by B lymphocytes27. A study using a Foxp3 reporter lineage marker system has shown that there is some spontaneous loss of Foxp3 expression in vivo, which is enhanced in nonobese diabetic mice with autoimmune disease28. As in our experiments, these formerly Treg cells secrete proinflammatory cytokines.
Many cell types, including mast cells, epithelial cells and dendritic cells, synthesize IL-10 (refs. 29–32). Our data have indicated that a population of CD11b+ myeloid cells in the LPL constitutively produced IL-10 and that a phenotypically similar population of IL-10-producing cells was greater in abundance in the MLNs early after T cell transfer. Many of the IL-10-producing myeloid cells were probably macrophages, given their expression of F4/80, although we did not exclude the possibility that other cell types were involved. Our findings are in agreement with the results of a study showing that IL-10 from lamina propria macrophages is important for the induction of Foxp3 expression33. However, that investigation focused on cells from the small intestine rather than the large intestine and studied induction rather than the maintenance of Foxp3 expression. Despite that, the concept is emerging that intestinal macrophages are important for supporting natural and induced Treg cell function.
Why is Treg cell–derived IL-10 not sufficient for the maintenance of Foxp3 expression in mice developing colitis? We suggest that the requirement for host cell–derived IL-10 is a matter of kinetics. At early time points, few Treg cells were producing IL-10, although at 6 weeks after transfer, Treg cells in the intestine were the main IL-10 producers. Our data therefore suggest that Treg cell–derived IL-10 cannot sustain Foxp3 expression because it is induced relatively late.
The outcome of the transfer of Il10rb−/− Treg cells suggested that the IL-10 produced by cells in the Rag1−/− hosts acted in part directly on the Treg cells to maintain Foxp3 expression, although these findings do not exclude the possibility that IL-10 serves additional functions by acting on other cell types that might influence Treg cell function. A caveat to our conclusion is that IL-10Rβ can participate in the signals delivered by several other cytokines34. However, the concordance with the results obtained from transfer to Il10−/−Rag1−/− recipients suggests that the ineffectiveness of the Il10rb−/− Treg cells was due to the absence of Treg cell IL-10 signaling.
Our data have indicated that colitis in the Rag1−/− recipients also contributed to the loss of Treg cell Foxp3 expression. At relatively early times after transfer, when inflammation was less severe, the loss of Foxp3 was less pronounced and more localized to the intestine. However, Foxp3+ T lymphocytes developed in essentially normal numbers in Il10rb−/− mice. The Il10rb−/− mice we used were mainly on the colitis-resistant C57BL/6 background and were only 8–10 weeks old, and therefore they did not have signs of colitis (data not shown). The results from the transfer of mixed populations of Treg cells into recipients that developed colitis indicated that colitis alone could not explain the loss of Foxp3. Furthermore, Foxp3 expression was not much lower in Il10rb−/− Treg cells in recipients of mixed Treg cell populations that did not develop colitis. We conclude, therefore, that the combined effects of the absence of IL-10 signaling and the inflammatory milieu in mice with severe colitis were responsible for loss of Foxp3 expression and Treg cell function.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/natureimmunology/.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Steinberg and D. Mucida for critical reading of this manuscript, members of the Kronenberg and Cheroutre laboratories for discussions; Y. Wang-Zhu for genotyping mice; L. Fernandez, C. Kim and B. Sears for assistance with cell sorting; P. Allen (Washington University) for Il10rb−/− mice; and A. Rudensky (Memorial Sloan Kettering Cancer Center) for Foxp3gfp mice. Supported by US National Institutes of Health (PO1 DK46763 to M.K., RO1 AI057992 to C.L.K. and RO1 AI50265 to H.C.) and the Crohn’s & Colitis Foundation of America (M.K. and M.M.).
Footnotes
Accession codes. UCSD-Nature Signaling Gateway (http://www.signaling-gateway.org): A002750, A001243 and A001245.
Note: Supplementary information is available on the Nature Immunology website.
AUTHOR CONTRIBUTIONS
M.M. and M.K. designed experiments; M.M. did experiments; O.T. did histology and helped with cell preparation; R.M. and C.L.K. generated and provided IL-10 reporter mice; G.K. and H.C. helped with critical advice and discussions throughout; and M.M. and M.K. wrote the manuscript.
References
- 1.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 2.Powrie F, et al. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 3.Powrie F, Correa-Oliveira R, Mauze S, Coffman RL. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J. Exp. Med. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 5.Annacker O, et al. CD25+CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J. Immunol. 2001;166:3008–3018. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 6.Gavin MA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 8.Spencer SD, et al. The orphan receptor CRF2–4 is an essential subunit of the interleukin 10 receptor. J. Exp. Med. 1998;187:571–578. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roers A, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J. Exp. Med. 2004;200:1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Hagenbaugh A, et al. Altered immune responses in interleukin 10 transgenic mice. J. Exp. Med. 1997;185:2101–2110. doi: 10.1084/jem.185.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groux H, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 13.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asseman C, Read S, Powrie F. Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: control by CD4+ regulatory T cells and IL-10. J. Immunol. 2003;171:971–978. doi: 10.4049/jimmunol.171.2.971. [DOI] [PubMed] [Google Scholar]
- 15.Uhlig HH, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J. Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25v regulatory T cells. J. Exp. Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fahlen L, et al. T cells that cannot respond to TGF-β escape control by CD4+CD25+ regulatory T cells. J. Exp. Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, et al. A critical function for TGF-β signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat. Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 23.Roncarolo MG, et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-β. J. Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 25.Degauque N, et al. Immunostimulatory Tim-1-specific antibody deprograms Tregs and prevents transplant tolerance in mice. J. Clin. Invest. 2008;118:735–741. doi: 10.1172/JCI32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komatsu N, et al. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc. Natl. Acad. Sci. USA. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuji M, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 30.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat. Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 31.De Winter H, et al. Regulation of mucosal immune responses by recombinant interleukin 10 produced by intestinal epithelial cells in mice. Gastroenterology. 2002;122:1829–1841. doi: 10.1053/gast.2002.33655. [DOI] [PubMed] [Google Scholar]
- 32.Brooks DG, et al. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 34.Donnelly RP, Sheikh F, Kotenko SV, Dickensheets H. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J. Leukoc. Biol. 2004;76:314–321. doi: 10.1189/jlb.0204117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.