Abstract
Background
The predictors for developing varices in patients with primary sclerosing cholangitis (PSC) have not been well studied prospectively. We sought to define the predictors for the presence of varices at baseline and for newly developing varices in patients with PSC.
Methods
We used prospectively collected data from a multicenter randomized trial of high dose ursodeoxycholic acid for PSC. For the first aim, all 150 patients enrolled were reviewed and the second aim, we excluded 26 patients who had esophageal varices at baseline. Clinical examination, blood tests and upper endoscopy were done before randomization, at two years and after 5 years. Liver biopsy was performed at entry and at 5 years.
Results
The median age (interquartile range; IQR) of patients was 45.9 years (35.8, 54.9). In a multivariable logistic regression, a higher Mayo risk score (>0.87) or a higher AST/ALT ratio (>1.12) were significantly associated with the presence of varices at initial endoscopy (OR=1.9 and 3.9). By the end of the study, 25 patients had new varices (20.2%). In a Cox model, after adjustment for baseline variables lower platelet count and higher total bilirubin at 2 years were significantly associated with the presence of new varices. The platelet count of 205 (× 109/l) and the total bilirubin level of 1.7 mg/dL were the best cut off values for the detection of new varices.
Conclusions
A higher Mayo risk score and higher AST/ALT ratio were significantly associated with the presence of varices at initial endoscopy. Lower platelet count and higher total bilirubin at 2 years were significantly associated with an increased risk of developing new varices in patients with PSC.
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease of unknown etiology characterized by fibrosing inflammation and destruction of the extrahepatic and/or intrahepatic bile ducts. No effective medical therapy exists for patients with PSC (1). The diseases progresses slowly and usually leads to biliary cirrhosis, portal hypertension and liver failure over 10-15 years with significantly shorter survival than people of similar age and gender (2). A previous study showed that 36% of PSC patients had esophageal varices (EV) at the time of their first visit and half of them had moderate or large EV. Noninvasive markers including platelet count, albumin level, and advanced histologic disease were independent predictors of EV(3). The limitation of that study is that 51% of patients had advanced histologic stage at baseline and the Mayo PSC risk score was not shown. Another study showed that clinical predictors including thrombocytopenia and splenomegaly could be used to stratify cirrhotic patients for the risk of large EV (4, 5). A platelet count of less than 88,000/mm3 carried a risk of large EV of 28% whereas a platelet count of less than 68,000/mm3 was used as a cut-off value for large EV with a sensitivity of 71% and a specificity of 73% (4, 5). However, the causes of liver diseases in most patients in these studies were alcohol abuse and viral hepatitis and the associations were usually made at one point in time.
Liver biopsy may not routinely be necessary for diagnosis of PSC in the absence of atypical findings and is also hampered by sampling variability (6, 7). Moreover, liver biopsy also has several limitations for clinical practice including the expense and the invasive nature of the procedure which is associated with a number of complications (8, 9). A revised natural history model for PSC risk score from the Mayo Clinic based on patient age, bilirubin levels, albumin, AST levels, and history of variceal bleeding was developed to assess 1-year to 4-year probability of survival of patients with PSC (10). An advantage of the Mayo PSC risk score is that there is no requirement for liver histology (10).
The predictors for developing varices varices in patients with PSC have not been well studied prospectively. Our aims were to evaluate predictors for the presence of varices at baseline and also for developing new varices in patients with PSC.
METHODS
Inclusion
Patients were entered based on the following criteria with exceptions approved by our Institutional Review Boards.
Inclusion Criteria
Primary sclerosing cholangitis was defined as present when all the following criteria were met: 1) chronic cholestatic disease of at least six months' duration; 2) serum alkaline phosphatase (ALP) at least 1 ½ times the upper limits of normal; 3) retrograde, operative, percutaneous or magnetic resonance cholangiography demonstrating intrahepatic and/or extrahepatic biliary duct obstruction, beading or narrowing consistent with PSC within one year of the study entry; 4) liver biopsy in the previous one year which was available for review and compatible with the diagnosis of PSC (7 patients did not have entry liver biopsy due to low platelet count and/or presence of cirrhosis). Compatible biopsy features included fibrous cholangitis, ductopenia with periportal inflammation and biliary fibrosis.
Exclusion Criteria
Patients were excluded if they had any of the following: 1) coexistent conditions such as pre-existing advanced malignancies or severe cardiopulmonary disease which would limit their life expectancy to less than two years; 2) inability to provide consent; 3) treatment with ursodeoxycholic acid (UDCA), pentoxifylline, corticosteroids, cyclosporin, colchicine, azathioprine, methotrexate, D-penicillamine, budesonide, nicotine, pirfenidone or tacrolimus in the three months prior to study entry; 4) inflammatory bowel disease (IBD) patients requiring specific treatment in the preceding three months except for maintenance therapy with a 5-ASA compound; 5) anticipated need for liver transplantation within two years (expected survival of <80% at two years based on Mayo risk score) ; 6) history of recurrent variceal bleeds, spontaneous uncontrolled encephalopathy, INR > 1.5 uncorrected by vitamin K or resistant ascites that suggested an anticipated survival of less than one year; 7) pregnancy or lactation (patients who became pregnant during the study were discontinued and referred to their physicians); 8) age less than 18 years or greater than 75 years; 9) findings highly suggestive of liver disease of other etiology such as chronic alcoholic liver disease, chronic hepatitis B or C infection, autoimmune hepatitis, primary biliary cirrhosis, hemochromatosis, Wilson's disease, congenital biliary disease or cholangiocarcinoma; 10) previous intraductal stones or operations on the biliary tree, other than cholecystectomy, such as biliary drainage procedures preceding the diagnosis of PSC; 11) recurrent ascending cholangitis requiring hospitalization occurring more than two times per year.
Patient enrollment
From the 455 patients assessed, one-hundred fifty patients were entered over a three-year period from seven sites. Of the 305 patients with PSC (67%) who were screened but not enrolled, 11 were eligible but declined, 141 were not eligible, and 153 had unknown eligibility. The majority of the 141 patients were not eligible due to inadequate ALP elevation and exclusionary medication use; other reasons included advanced liver disease, age, and complicating medical conditions. Eligibility was not known in 153 as these patients declined further testing for reasons including cost, the randomized nature of the trial, concern about side effects, and in the majority, unknown reasons.
Randomization and Stratification
All screened patients found to be eligible and who provided written informed consent to participate in the study were randomized to one of two groups: 1) UDCA at a dose of 28-30 mg/kg/day, or 2) an identical-appearing placebo.
The randomization was stratified by histologic stage I or II vs. III or IV, presence or absence of varices, and Mayo risk score. Computer-based dynamic allocation was used to assign patients to study groups via the coordinating center in Rochester, Minnesota. Further details regarding the drug administration, patient compliance and termination were reported elsewhere (11).
Monitoring
Mayo risk score at baseline was calculated to obtain survival estimates up to 4 years of follow-up (10) and it can be accessed from the web site, http://www.mayoclinic.org/gi-rst/mayomodel3.html. Liver enzymes were assessed by mailed containers or patient visit every three months to monitor for possible toxicity and to assess biochemical response. Patients were examined annually. Complete blood counts; prothrombin time and ultrasound were assessed at baseline and annually. Rate of total bilirubin (TB) or platelet counts change per year (%) were calculated by the difference of total bilirubin or platelet counts at the 2nd year minus by those at baseline and divided by the total bilirubin or platelet counts at baseline by the following formula:
Upper endoscopy to assess for varices was done at two years. The standard classification for EV grading was reported as no varices (F0), small and non-tortuous (F1), medium or tortuous but less than 50% radius of esophagus (F2) and large (F3) (12). Endoscopic retrograde cholangiography and liver biopsy were scheduled to be repeated at five years after entry. Treatment was stopped if liver transplantation was required.
Data Collection
Data was collected prospectively at each clinical center and forwarded to Mayo Clinic Rochester, which served as the coordinating center. The data were entered into computers at the coordinating center once the initial quality assurance audits were completed at the originating study site. Laboratory values such as serum liver biochemistries were normalized by dividing the actual value by the upper limits of normal for the clinical laboratory in which the test was performed.
Statistical Analyses
For the first aim, 150 patients with PSC were analyzed and for the second aim, we excluded 26 patients who had esophageal or gastric varices at baseline, thus one-hundred twenty four patients were included for analysis. Baseline characteristics were calculated as the median (interquartile range; IQR) for continuous variables, and the number and percent in each group were tabulated for categorical variables.
The PSC patients with a fibrosis stage of 1-2 were classified as “mild liver fibrosis” and those with a fibrosis stage of 3-4 were classified as “advanced liver fibrosis”.
We assessed the effect of Mayo risk score for predicting the presence of EV at baseline in patients with PSC adjusting for a variety of baseline characteristics. Logistic regression analysis was used to identify the factors significantly associated with advanced liver fibrosis or presence of varices in 150 PSC patients. Only those variables with a P value < 0.2 by univariate analysis were included in multivariate analysis. In order to avoid overestimation of the model, we excluded those variables used as a part of Mayo Risk Scores calculation. We used a receiver operating characteristic (ROC) of related variables for detection of patients with development of varices with the best area under the curve (AUC). Time to event was time from randomization to first development of varices in 124 patients who had no EV at baseline and was assessed using a Cox regression model. Patients were censored at 5-years of follow-up or the day they went off the protocol. This model was adjusted for the stratification variables (Mayo risk score and histological staging I or II vs III or IV).
Sample Size
Sample size calculations were made assuming that UDCA would halve the risk of a primary endpoint which was based on projections from our pilot study (13). Based on our previous study, we expected 35% of patients to reach a primary endpoint in 5 years (14). With α = 0.05 and power = 80 % we estimated a need to recruit at least 149 patients.
All analyses used a 5% two-sided type I error rate. Analyses were performed with the SPSS statistical software package (SPSS Version 15.0.1.1, Windows VISTA, July3, 2007). After a planned analysis once 75% of expected endpoints had been reached, the Data Safety and Monitoring Board reviewed the data and terminated the study due to futility.
RESULTS
Characteristic data of 150 patients with PSC
One-hundred fifty patients were enrolled over a five-year period from seven sites and their baseline characteristics based on stage of liver fibrosis were shown in Table 1. The median age (IQR) of patients was 45.9 (35.8, 54.9) years with a male predominance (57.3%). Advanced liver fibrosis was present in 62 patients (41.3%) with average (med; IQR) Mayo risk score of 0.73 (0.21, 1.35). Colitis was present in 115 patients (76.7%). Forty-seven (53.4%) of the patients with mild liver fibrosis were treated with UDCA, as compared to 29 (46.8%) of the patients with advanced liver fibrosis.
Table 1.
Characteristic data of 150 patients with PSC categorized by baseline stage of liver fibrosis
| Variables Median (Interquartile range; IQR) or Number (%) |
Mild liver fibrosis (N=88) |
Advanced liver fibrosis (N= 62) |
P value* |
|---|---|---|---|
| Age (years) | 44.6 (35.4, 53.3) | 48.7 (40, 58.1) | 0.09 |
| Gender, % male | 52 (59.1) | 34 (54.8) | 0.60 |
| Patients with history of IBD | 69 (78.4) | 46 (74.2) | 0.55 |
| Mayo risk score at baseline | −0.004 (−0.50, 0.64) | 0.73 (0.21, 1.35) | <0.0001* |
| Treatment with Ursodeoxycholic acid | 47 (53.4) | 29 (46.8) | 0.42 |
| Presence of varices at baseline | 6 (6.8) | 20 (32.3) | <0.0001* |
Note:
P value < 0.05 for mild liver fibrosis vs. advanced liver fibrosis, and those variables with a P value < 0.2 by univariate analysis and were not used for Mayo risk score calculation were included in multivariate analysis. The Wilcoxon rank sum test was used for continuous variables and the Chi-square test was used to determine statistical significance for categorical data.
Patients with advanced liver fibrosis had significantly higher Mayo risk score, higher total bilirubin, higher AST level and higher AST/ALT ratio at baseline, lower platelet count, lower albumin, and had varices at baseline more often than those with mild liver fibrosis. Table 2 shows the laboratory tests at baseline and at the 2nd year of follow up. Patients with advanced liver fibrosis had significantly higher AST, ALT, total bilirubin, AST/ALT ratio and ALP, with a lower platelet count, hemoglobin, albumin level than those with mild liver fibrosis. In a multivariable logistic regression model, higher Mayo risk score and higher AST/ALT ratio remained significantly associated with the presence of advanced liver fibrosis (OR=2.4, P=0.002, 95%CI 1.4-4.2 and OR=8.7, P =0.003, 95%CI 2.1-37.1) as shown in Table 3.
Table 2.
Laboratory tests at baseline and at the 2nd year of follow up of 150 patients with PSC categorized by baseline stage of liver fibrosis
| Laboratory tests, Median (IQR) or Number (%) |
Mild liver fibrosis (N=88) |
Advanced liver fibrosis (N= 62) |
P value* |
|---|---|---|---|
| A. At baseline | |||
| ALT (U/L) | 110 (63.5, 193) | 104.5 (70.5, 147.3) | 0.59 |
| AST (U/L) | 72 (42, 112) | 87 (69, 138.8) | 0.004* |
| AST/ALT ratio | 0.61 (0.51, 0.83) | 0.88 (0.68, 1.26) | <0.0001* |
| Albumin (g/dL) | 4.1 (3.9, 4.3) | 3.8 (3.5, 4.2) | <0.0001* |
| Total bilirubin (mg/dL) | 0.8 (0.6, 1.2) | 1.2 (0.8, 1.8) | <0.0001* |
| ALP (U/L) | 433 (246, 694) | 525 (340.5, 797.5) | 0.08 |
| Hemoglobin (g/dL) | 13.8 (12.7, 15.1) | 13.2 (12.2, 14.7) | 0.13 |
| White cell count (X 103/l) | 6.4 (5.4, 8.1) | 6.3 (4.5, 7.7) | 0.14 |
| Platelet (X 109/l) | 265 (210, 332) | 216 (152, 289) | 0.0009* |
| B. At the 2nd year of follow up | |||
| HDL-cholesterol (mg/dL) | 62.5 (49.8, 76.3) | 54 (40, 74) | 0.10 |
| ALT (U/L) | 51.5 (34.3, 83.8) | 71 (39, 133.8) | 0.05* |
| AST (U/L) | 42 (30, 71) | 71.5 (47.8, 102.3) | 0.0001* |
| AST/ALT ratio | 0.80 (0.65, 1.02) | 1.10 (0.73, 1.31) | 0.01* |
| Total bilirubin (mg/dL) | 0.8 (0.6, 1.2) | 1.3 (0.9, 2.7) | 0.0002* |
| Albumin (g/dL) | 4.2 (3.9, 4.4) | 3.8 (3.5, 4.1) | <0.0001* |
| ALP (U/L) | 232 (141, 342) | 286 (175.5, 415.3) | 0.03* |
| Hemoglobin (g/dL) | 14.2 (12.9, 15.3) | 13.3 (11.9, 14.5) | 0.01* |
| White cell count (X 103/l) | 6.4 (5.2, 7.4) | 4.8 (3.5, 7.8) | 0.02* |
| Platelet (X 109/l) | 256 (204, 331) | 194 (107, 275) | 0.001* |
Note:
P value < 0.05 for mild liver fibrosis vs. advanced liver fibrosis
Table 3.
Multivariate Logistic Regression Model showing OR (95% CI) of advanced liver fibrosis in 150 patients with PSC.
| Multivariate analysis | B | SE | P value | Odd ratio | 95% CI (OR) |
|---|---|---|---|---|---|
| Mayo risk score at baseline | 0.87 | 0.29 | 0.002* | 2.4 | 1.4-4.2 |
| AST/ALT ratio at baseline | 2.17 | 0.74 | 0.003* | 8.7 | 2.1-37.1 |
Note:
P value < 0.05
Predicting varices at baseline
Table 4 shows that patients with varices at baseline had significantly higher Mayo risk score, higher total bilirubin, higher AST/ALT ratio at baseline, a high proportion of patients with advanced liver fibrosis at baseline, lower hemoglobin, lower white blood cell and platelet count, lower albumin, and lower triglyceride than those without varices. In a multivariable logistic regression model with removal of the presence of advanced liver fibrosis (model 2), the higher Mayo risk score and higher AST/ALT ratio at baseline were significantly associated with the presence of varices at baseline (OR=1.9, P =0.04, 95%CI 1.0-3.6 and OR=3.9, P =0.02, 95%CI 1.3-11.8) as shown in Table 5. The ROC curve for the presence of varices at baseline was derived and showed that the Mayo risk score of 0.87 was the best cut off value based on the sensitivity (65.4%), specificity (78.2%), PPV (38.6%), and NPV (91.5%) with area under the curve (AUC) of 0.75. The best cut off value of AST/ALT ratio at baseline for predicting varices at baseline was 1.12 with a sensitivity of 47.8%, specificity of 87%, PPV of 42.3%, NPV of 89.2% and AUC of 0.69.
Table 4.
Comparison of 150 PSC patients with and without esophageal or gastric varices at baseline
| Clinical features Median (IQR) or Number (%) |
Patients without varices at baseline (N=124) |
Patients with varices at baseline (N=26) |
P value* |
|---|---|---|---|
| Gender, % male | 72 (58.1) | 14 (53.9) | 0.69 |
| Age (years) | 45.4 (35.6, 53.9) | 48.6 (42.4, 58.3) | 0.26 |
| Proportion of patients with advanced liver fibrosis at baseline |
42 (33.9) | 20 (76.9) | <0.001* |
| AST at baseline (U/L) | 77 (50, 115) | 95 (69.8, 142.5) | 0.06 |
| AST/ALT ratio at baseline | 0.68 (0.54, 0.93) | 0.88 (0.74, 1.47) | 0.004* |
| Proportion of patients with high AST/ALT ratio (>1.12) at baseline |
15 (12.1) | 11 (42.3) | <0.001* |
| Albumin at baseline (g/dL) | 4.1 (3.8, 4.3) | 3.8 (3.5, 4.0) | <0.001* |
| Total bilirubin at baseline (mg/dL) | 0.9 (0.6, 1.2) | 1.3 (0.98, 2.03) | <0.001* |
| Triglyceride at baseline (mg/dL) | 103 (73, 139) | 77.5 (60.8, 112.5) | 0.01* |
| Hemoglobin at baseline (g/dL) | 13.8 (12.7, 14.9) | 12.9 (12.2, 14.3) | 0.16 |
| White cell counts at baseline (X 103/l) | 6.4 (5.4, 8.1) | 5.3 (4.2, 6.8) | 0.03* |
| Platelet at baseline (X 109/l) | 253.5 (203.3, 322) | 170 (111.8, 267.8) | 0.001* |
| Mayo risk score at baseline | 0.15 (−0.38, 0.77) | 0.92 (0.35, 1.43) | <0.001* |
Note:
P value < 0.05 for patients with varices vs. those without varices at baseline.
Table 5.
Multivariate Logistic Regression Model showing OR (95% CI) of the presence of varices at baseline in 150 patients with PSC.
| Multivariate analysis | B | SE | P value | OR | 95% CI (OR) |
|---|---|---|---|---|---|
| Model 1; | |||||
| - Presence of advance liver fibrosis |
1.58 | 0.57 | 0.005* | 4.9 | 1.6-14.9 |
| - High AST/ALT ratio (>1.12) at baseline |
1.41 | 0.55 | 0.01* | 4.1 | 1.4-11.9 |
|
| |||||
| Model 2**; | |||||
| - High AST/ALT ratio (>1.12) at baseline |
1.36 | 0.57 | 0.02* | 3.9 | 1.3-11.8 |
| - Mayo risk score at baseline | 0.65 | 0.32 | 0.04* | 1.9 | 1.0-3.6 |
Note:
P value < 0.05 for patients with varices vs. those without varices at baseline and those variables with a P value < 0.2 by univariate analysis and were not used for Mayo risk score calculation were included in multivariate analysis (model1).
Model 2; presence of advanced liver fibrosis at baseline was removed.
Predicting new varices
From 124 patients with PSC who had no varices at initial endoscopy, 25 patients had new varices (20.2%) by the end of the study. The size of new esophageal varices were graded as small in 20 patients (80%), moderate in 3 patients (12%) and large in 2 patients (8%). Portal hypertensive gastropathy (PHG) was present in 21 of 124 patients (16.9%) with the fundus or body the predominant area. Other endoscopic findings were esophageal ulcer or erosion (n=10, 8.1%), gastric ulcer or erosion (n=9, 7.3%) and duodenal ulcer or erosion (n=4, 3.2%). Six patients (4.8%) died during the follow up period. The causes of deaths were liver related (3 patients), unknown cause (2 patients) and coronary heart disease (1 patient).
Table 6 shows the univariate analysis of predictors for developing new varices in 124 patients with PSC who had no varices at initial endoscopy which found that patients with new varices had significantly more frequent treatment with UDCA, higher AST/ALT ratio, lower albumin, and lower hemoglobin and platelet count at baseline than those without varices. At the 2nd year of follow-up, patients with new varices had significantly lower albumin, lower hemoglobin, white blood cell, and platelet count with higher AST/ALT ratio, higher total bilirubin and higher %rate change of total bilirubin and platelet counts per year than those without varices.
Table 6.
Comparison of 124 PSC patients who had no varices at baseline with and without the presence of new varices
| Clinical features Median (IQR) or Number (%) |
Patients without varices (N=99) |
Patients with varices (N=25) |
P value* |
|---|---|---|---|
| Treatment with Ursodeoxycholic acid | 45 (45.5) | 18 (72.0) | 0.02* |
| Proportion of patients with advanced liver fibrosis at baseline |
30 (30.3) | 12 (48.0) | 0.09 |
| ALT at baseline (U/L) | 110 (73, 214) | 80 (55, 174) | 0.13 |
| AST/ALT ratio at baseline | 0.6 (0.5, 0.9) | 0.9 (0.6, 1.2) | 0.01* |
| ALP (U/L) | 431 (259, 710) | 576 (383, 774) | 0.16 |
| Total bilirubin (mg/dL) | 0.8 (0.6, 1.2) | 1.1 (0.7, 1.6) | 0.08 |
| Albumin at baseline (g/dL) | 4.1 (3.9, 4.3) | 3.9 (3.6, 4.2) | 0.05* |
| Total protein at baseline (g/dL) | 9.9 (9.2, 12.4) | 11.4 (9.5, 12.7) | 0.15 |
| Triglyceride at baseline (mg/dL) | 108 (76, 144.8) | 83 (70, 121) | 0.14 |
| HDL-cholesterol at baseline (mg/dL) | 62.5 (48.3, 75) | 70 (59, 77) | 0.08 |
| Hemoglobin at baseline (g/dL) | 14.0 (12.8, 14.9) | 13.2 (11.5, 14.3) | 0.05* |
| Platelet at baseline (X 109/l) | 263 (211, 327) | 225 (155, 283.5) | 0.02* |
| Mayo risk score at baseline | 0.13 (−0.43, 0.75) | 0.53 (−0.21, 1.29) | 0.10 |
| Albumin at the 2nd year (g/dL) | 4.2 (3.9, 4.4) | 3.8 (3.5, 4.1) | 0.0003* |
| Total bilirubin at the 2nd year (mg/dL) | 0.9 (0.6, 1.3) | 1.8 (1.0, 3.3) | 0.0003* |
| AST/ALT ratio at the 2nd year | 0.8 (0.6, 1.0) | 1.2 (0.8, 1.4) | 0.001* |
| AST at the 2nd year (U/L) | 47.5 (31, 84.3) | 63 (42.5, 102) | 0.06 |
| ALP at the 2nd year (U/L) | 252 (141, 377) | 274 (180, 574) | 0.16 |
| Triglyceride at the 2nd year (mg/dL) | 102 (68.3, 134) | 82.5 (51.5, 105.5) | 0.13 |
| Hemoglobin at the 2nd year (g/dL) | 14.0 (13.1, 15.3) | 13.2 (11.5, 14.4) | 0.01* |
| White cell count at the 2nd year | 6.5 (5.3, 8.4) | 4.8 (3.5, 6.9) | 0.003* |
| Platelet at the 2nd year (X 109/l) | 264 (212, 331) | 177 (98.5, 264) | 0.0003* |
| Rate of total bilirubin change per year (%) | 3.6 (−9.3, 25.0) | 16.7 (3.6, 70.0) | 0.003* |
| Rate of platelet count change per year (%) | −0.9 (−5.3, 6.6) | −5.6 (−16.9, −2.2) | 0.0003* |
| Duration from randomization to the first presence of varices (years) |
3.9 (3.0, 4.9) | 2.0 (1.9, 3.5) | 0.0004* |
Note:
P value < 0.05 and those variables with a P value < 0.2 by univariate analysis were included in Cox regression analysis.
In a Cox regression model, after adjusting for all significant variables from the univariate analysis (model 1), only treatment with UDCA, lower platelet count and higher total bilirubin at 2 years were significantly associated with the presence of new varices (Table 7). After removal of treatment with UDCA in model 2, the lower platelet count and higher total bilirubin at 2 years remained significantly associated with the presence of new varices. The median (IQR) platelet count and total bilirubin at the 2nd year of 25 patients with new varices were 177(× 109/l) (98.5-264) and 1.8 mg/dL (1.0-3.3), respectively. Using the ROC curves for the detection of new varices, we found that platelet count at the 2nd year of follow-up of 205 (× 109/l) was the best cut off value based on a sensitivity of 62.5%, specificity of 80.5%, PPV of 50%, and NPV of 87.3% with AUC of 0.75. The best cut off value of total bilirubin level at the 2nd year of follow-up for the detection of new varices was 1.7 mg/dL with a sensitivity of 56%, specificity of 87.7%, PPV of 58.3%, NPV of 86.6% and AUC of 0.74.
Table 7.
Cox regression model showing HR (95% CI) of predictors for the development of new varices in 124 PSC patients who had no varices at initial endoscopy.
| Multivariate analysis | B | SE | P value | OR | 95% CI (OR) |
|---|---|---|---|---|---|
| Model 1; | |||||
| - Total bilirubin at the 2nd year (mg/dL) |
0.45 | 0.12 | <0.001* | 1.6 | 1.2-2.0 |
| - Platelet at the 2nd year (X 109/l) | −0.01 | 0.003 | 0.001* | 0.989 | 0.983-0.995 |
| - Treatment with UDCA | 1.26 | 0.58 | 0.029* | 3.5 | 1.1-10.9 |
|
| |||||
| Model 2**; | |||||
| - Total bilirubin at the 2nd year (mg/dL) |
0.43 | 0.13 | 0.001* | 1.5 | 1.2-2.0 |
| - Platelet at the 2nd year (X 109/l) | −0.01 | 0.003 | 0.004* | 0.992 | 0.986-0.997 |
Note:
P value < 0.05, all variables with P values <0.2 by univariate analysis were included in Cox regression analysis (model 1).
Model 2; treatment with UDCA was removed.
A cumulative hazard curve of developing new varices in 124 patients at 2 years of follow-up showed that the cumulative hazard of developing new varices was 5.9%.
DISCUSSION
We demonstrate that a higher Mayo risk score and higher AST/ALT ratio were significantly associated with the presence of advanced liver fibrosis. Recently, the European Association for the study of the liver (EASL) clinical practice guidelines suggested that liver biopsy is not essential for the diagnosis of PSC(15). Thus, without the variables of the presence of liver fibrosis, the higher Mayo risk score and higher AST/ALT ratio at baseline were significantly associated with the presence of varices at initial endoscopy. We also found that treatment with UDCA, presence of lower platelet count and higher total bilirubin at the 2nd year of follow up were significantly associated with an increased risk of new varices in patients with PSC who had no varices at initial endoscopy. Our study also demonstrated that treatment with high dose UDCA was the strongest predictor for the presence of new varices and should not be used in the future in these patients. The paradoxical effect of high dose UDCA in our study on increasing risk of varices was unexpected.
Currently, there are no studies to determine the prognostic predictors for new varices in patients with PSC who have mild degrees of liver injury. Two-third of our patients had histologic stage 1-2 with an average (median; IQR) Mayo risk score of 0.296 (−0.273 to 0.931). We used a standard protocol to follow patients every 3 months with blood tests in our randomized control trial and found that total bilirubin and platelet count at the 2nd year of follow-up were useful predictors for an increased risk of new varices, regardless of treatment with UDCA.
From the report of the Baveno IV consensus, the hepatic vein pressure gradient (HVPG) is the most reliable predictor to detect varices (16); however, this method of hemodynamic monitoring is not available in many centers especially in developing countries. Screening all cirrhotic patients with endoscopy to detect the presence of varices may also result in a large number of unnecessary procedures and high costs (17). Non-invasive predictors of esophageal varices including Mayo risk score with value of at least 0.87 or a higher AST/ALT ratio of at least 1.12 may be used to select PSC patients for screening of varices. With clinical evaluation and blood tests during follow-up, the platelet count (<205,000/mm3) and total bilirubin level (>1.7 mg/dL) at the 2nd year of follow up may be used as alternative markers for patient-selection to undergo endoscopy. Bressler et al.(18) showed similar risk factors for esophageal varices at initial evaluation and suggested that patients with primary biliary cirrhosis (PBC) or PSC with a platelet count of less than 200,000/mm3, albumin level less than 40 g/l, and total bilirubin level higher than 20 micromol/l (or 1.17 mg/dL) should be screened for EV. However, the limitation of this study is the small number of patients with PSC (n=9). A recent multicenter longitudinal study investigated the relationship between varices and platelet count at the time of endoscopy (19). They found that the platelet count was not a good prognostic marker for gastroesophageal varices; however, HVPG was significantly correlated with platelet count at baseline, year 1 and year 5 (19). This study was limited by the exclusion of patients with PSC and/or PBC.
Our results showed that 6% of patients with PSC develop new varices at 2 years of follow up which was different from the result of previous study performed by Merli et al. showing that the incidence of EV was 5% at 1 year and 28% at 3 years (20). The different rate of new varices detection may be explained by the different study patient populations. Merli et al. included the cirrhotic patients caused by viral hepatitis B, viral hepatitis C and/or alcohol (20). Our current results also support the AASLD guideline of endoscopic surveillance in cirrhotic patients who had no varices at initial endoscopy with upper endoscopy performed every 2-3 years (21).
Our study is the first prospective study to report that a higher Mayo risk score and higher AST/ALT ratio at baseline are good predictors of varices at initial endoscopy with OR of 1.9 and 3.9, respectively, but not as good a predictor of developing new varices in patients with PSC who had no varices at initial endoscopy. Thus Mayo risk score and AST/ALT ratio at baseline seem to be more useful than liver biopsy for initial evaluation of varices in patients with PSC. A Mayo risk score without the need for a liver biopsy is a simple and noninvasive tool and it should be calculated for all patients with PSC at initial consultation to obtain 4-year survival estimates (10).
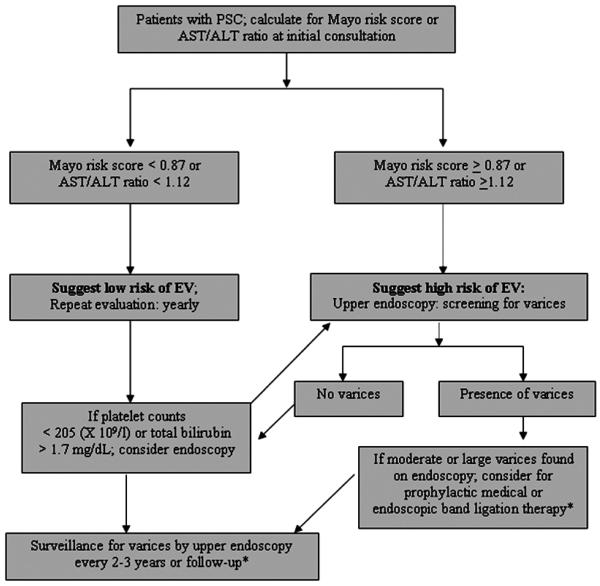
Twenty percent of our patients with new varices had either moderate or large varices on endoscopy. These patients should be considered for prophylactic medical or endoscopic band ligation therapy according to current guidelines (16, 21). Eighty percent of our patients with new varices had small varices on endoscopy. Currently, there is limited information to support the prophylactic treatment of patients with new small varices (22). There are no studies to date that have evaluated the progression of small varices in patients with PSC. A previous study from Italy which enrolled cirrhotic patients caused by alcohol or viral hepatitis showed that the rate of progression from small varices to larger varices was 12% at the 1st year and 25% at the 2nd year (20). To summarize our results, we propose an algorithm for screening and surveillance of varices in patients with PSC (Figure 1). Further study to identify the significant predictor of enlarged varices or the risk factors for bleeding from varices in patients with PSC should be performed.
Our study has some limitations. First, our study did not have complete follow-up at 5 years because 75% of the expected endpoints had been reached and the Data Safety and Monitoring Board reviewed the data and terminated the study due to futility. Second, only 33% of our assessed patients were enrolled due to several exclusion criteria. In this regard, the extrapolation to the general patients with PSC has to be done with caution. Lastly, we did not measure the maximum spleen bipolar diameter thus we can not calculate the platelet count/spleen diameter ratio which is another noninvasive diagnostic tool for esophageal varices (23, 24).
In conclusion, a higher Mayo risk score and higher AST/ALT ratio were significantly associated with the presence of advanced liver fibrosis. The higher Mayo risk score and higher AST/ALT ratio were significantly associated with the presence of varices at initial endoscopy. The presence of higher total bilirubin level at the 2nd year and lower platelet count at the 2nd year of follow-up were good predictors of the presence of new varices in patients with PSC who had no varices at initial endoscopy.
Supplementary Material
Acknowledgment
The project described was also supported by M01RR000065 awarded to Virginia Commonwealth University from the National Center for Research Resources
Funding support provided by NIDDK 56924 and Axcan Pharma
REFERENCES
- 1.Silveira MG, Lindor KD. Primary sclerosing cholangitis. Can J Gastroenterol. 2008;22:689–698. doi: 10.1155/2008/824168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bambha K, Kim WR, Talwalkar J, Torgerson H, Benson JT, Therneau TM, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003;125:1364–1369. doi: 10.1016/j.gastro.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Zein CO, Lindor KD, Angulo P. Prevalence and predictors of esophageal varices in patients with primary sclerosing cholangitis. Hepatology. 2004;39:204–210. doi: 10.1002/hep.20029. [DOI] [PubMed] [Google Scholar]
- 4.Chalasani N, Imperiale TF, Ismail A, Sood G, Carey M, Wilcox CM, et al. Predictors of large esophageal varices in patients with cirrhosis. Am J Gastroenterol. 1999;94:3285–3291. doi: 10.1111/j.1572-0241.1999.1539_a.x. [DOI] [PubMed] [Google Scholar]
- 5.Madhotra R, Mulcahy HE, Willner I, Reuben A. Prediction of esophageal varices in patients with cirrhosis. J Clin Gastroenterol. 2002;34:81–85. doi: 10.1097/00004836-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Olsson R, Hagerstrand I, Broome U, Danielsson A, Jarnerot G, Loof L, et al. Sampling variability of percutaneous liver biopsy in primary sclerosing cholangitis. J Clin Pathol. 1995;48:933–935. doi: 10.1136/jcp.48.10.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burak KW, Angulo P, Lindor KD. Is there a role for liver biopsy in primary sclerosing cholangitis? Am J Gastroenterol. 2003;98:1155–1158. doi: 10.1111/j.1572-0241.2003.07401.x. [DOI] [PubMed] [Google Scholar]
- 8.Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int. 2008;28:705–712. doi: 10.1111/j.1478-3231.2008.01691.x. [DOI] [PubMed] [Google Scholar]
- 9.van der Poorten D, Kwok A, Lam T, Ridley L, Jones DB, Ngu MC, et al. Twenty-year audit of percutaneous liver biopsy in a major Australian teaching hospital. Intern Med J. 2006;36:692–699. doi: 10.1111/j.1445-5994.2006.01216.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim WR, Therneau TM, Wiesner RH, Poterucha JJ, Benson JT, Malinchoc M, et al. A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc. 2000;75:688–694. doi: 10.4065/75.7.688. [DOI] [PubMed] [Google Scholar]
- 11.Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009 doi: 10.1002/hep.23082. Epub ahead of print:PMID:19585548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thuluvath PJ, Krishnan A. Primary prophylaxis of variceal bleeding. Gastrointest Endosc. 2003;58:558–567. doi: 10.1067/s0016-5107(03)01971-0. [DOI] [PubMed] [Google Scholar]
- 13.Harnois DM, Angulo P, Jorgensen RA, Larusso NF, Lindor KD. High-dose ursodeoxycholic acid as a therapy for patients with primary sclerosing cholangitis. Am J Gastroenterol. 2001;96:1558–1562. doi: 10.1111/j.1572-0241.2001.03777.x. [DOI] [PubMed] [Google Scholar]
- 14.Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336:691–695. doi: 10.1056/NEJM199703063361003. [DOI] [PubMed] [Google Scholar]
- 15.EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 16.de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2005;43:167–176. doi: 10.1016/j.jhep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 17.de Franchis R, Dell'Era A, Primignani M. Diagnosis and monitoring of portal hypertension. Dig Liver Dis. 2008;40:312–317. doi: 10.1016/j.dld.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Bressler B, Pinto R, El-Ashry D, Heathcote EJ. Which patients with primary biliary cirrhosis or primary sclerosing cholangitis should undergo endoscopic screening for oesophageal varices detection? Gut. 2005;54:407–410. doi: 10.1136/gut.2004.040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, et al. Platelet count is not a predictor of the presence or development of gastroesophageal varices in cirrhosis. Hepatology. 2008;47:153–159. doi: 10.1002/hep.21941. [DOI] [PubMed] [Google Scholar]
- 20.Merli M, Nicolini G, Angeloni S, Rinaldi V, De Santis A, Merkel C, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol. 2003;38:266–272. doi: 10.1016/s0168-8278(02)00420-8. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 22.Longacre AV, Garcia-Tsao G. A commonsense approach to esophageal varices. Clin Liver Dis. 2006;10:613–625. doi: 10.1016/j.cld.2006.08.016. x. [DOI] [PubMed] [Google Scholar]
- 23.Giannini EG, Zaman A, Kreil A, Floreani A, Dulbecco P, Testa E, et al. Platelet count/spleen diameter ratio for the noninvasive diagnosis of esophageal varices: results of a multicenter, prospective, validation study. Am J Gastroenterol. 2006;101:2511–2519. doi: 10.1111/j.1572-0241.2006.00874.x. [DOI] [PubMed] [Google Scholar]
- 24.Giannini E, Testa R. Noninvasive diagnosis of fibrosis: the truth is rarely pure and never simple. Hepatology. 2003;38:1312–1313. doi: 10.1053/jhep.2003.50500. author reply 1313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.