Abstract
Familial Alzheimer’s disease (FAD) is caused by mutations in amyloid precursor protein or presenilins (PS1, PS2). Many FAD-linked PS mutations affect intracellular calcium (Ca2+) homeostasis by mechanisms proximal to and independent of amyloid production, although the molecular details are controversial. Here, we demonstrate that several FAD-causing PS mutants enhance gating of the inositol trisphosphate receptor (InsP3R) Ca2+ release channel by a gain-of-function effect that mirrors the genetics of FAD and is independent of secretase activity. In contrast, wild type PS or PS mutants that cause frontotemporal dementia have no such effect. FAD PS alter InsP3R channel gating by modal switching. Recordings of endogenous InsP3R in lymphoblasts derived from individuals with FAD or cortical neurons of asymptomatic PS1-AD mice revealed they have higher occupancy in a high open probability burst mode compared to that of InsP3R in cells with wild-type PS, resulting in enhanced Ca2+ signaling. These results indicate that exaggerated Ca2+ signaling through InsP3R-PS interaction is a disease-specific and robust proximal mechanism in FAD.
INTRODUCTION
Alzheimer’s disease (AD) is a common form of dementia that involves slowly developing and ultimately fatal neurodegeneration. Most AD is sporadic and idiopathic and develops at ages over 60, but about 5% is inherited in an autosomal dominant manner due to mutations in amyloid precursor protein (APP) or presenilins (PS1, PS2) (1). Although familial Alzheimer’s disease (FAD) develops at ages as early as the late 30s, both familial and sporadic AD share hallmark features that include accumulation of β amyloid (Aβ) in extracellular plaques, intracellular neurofibrillary tangles comprised largely of hyper-phosphorylated tau, and cell atrophy and death in various brain regions (2–4). The consistent phenotypes suggest that both types of AD may share pathogenic origins. Nevertheless, the mechanisms by which these mutant proteins exert such devastating effects, and their roles and relationships in the two forms of AD, are still not clear. Insights into the molecular mechanisms and cellular functions of mutant proteins in FAD are likely to provide important clues into the etiology of AD pathogenesis and the identification of targets for therapeutic interventions.
Presenilins are transmembrane proteins that are synthesized on the endoplasmic reticulum (ER) and localized there (5). Together with nicastrin, APH-1 (anterior pharynx-defective 1), and PEN-2 (presenilin enhancer 2), PS forms a protein complex that is transported to the cell surface and to endosomes, where it functions as a γ-secretase that cleaves several type 1 transmembrane proteins, including APP (6, 7). γ-secretase cleavage of APP releases Aβ peptides, a major component of amyloid plaques in the brains of AD patients. Mutant PS are believed to affect APP processing by either enhancing the total production of Aβ or the relative proportion of the more amyloidogenic Aβ-42 form (8). In the amyloid hypothesis of AD, accumulation of amyloidogenic Aβ aggregates or oligomers is a proximal feature that causes neural toxicity leading to brain pathology (9, 10). However, FAD mutations in PS cause loss of secretase function, in contrast with the dominant gain-of-function indicated by the genetics of the disease (11). In addition to disrupting APP processing, many FAD-linked PS mutations affect intracellular calcium (Ca2+) homeostasis (12, 13). Although extracellular Aβ influences intracellular Ca2+ homeostasis in vitro (14, 15) and in vivo (16, 17), FAD-mutant PS also influences intracellular Ca2+ signaling by proximal, Aβ-independent mechanisms. Such Ca2+ signaling disruptions have manifested as attenuated capacitive Ca2+ entry (18–20), but most commonly as exaggerated Ca2+ liberation from the ER (18, 21–24), the major intracellular Ca2+ storage organelle. The molecular mechanisms underlying exaggerated ER Ca2+ release have been ascribed to enhanced loading of the ER lumen (23) due either to enhanced SERCA (sarco-endoplasmic reticulum Ca2+-ATPase) pump activity (25) or to disruption of a putative Ca2+ channel function of wild-type PS (26, 27). Alternately, exaggerated Ca2+ release has been accounted for by enhanced Ca2+ liberation from normal stores through inositol trisphosphate receptor (InsP3R) (21, 23) or ryanodine receptor (RyR) (22, 28, 29) Ca2+ release channels, both in vivo (22, 24, 28, 29) and in vitro (30–33), either as a consequence of enhanced channel abundance (28, 34–36) or, in the case of the InsP3R, enhanced activity in response to its ligand InsP3 (32, 37). Notably, enhanced agonist-induced InsP3R-mediated Ca2+ signals have been used diagnostically to identify individuals with FAD (31, 32). Biochemical interaction of the InsP3R with both wild-type (WT) and FAD-mutant PS1 and PS2 has been demonstrated (37). Single channel recordings of Sf9 insect cell InsP3R demonstrated that recombinant FAD-mutant PS1 and a FAD mutant-PS2 could enhance InsP3R Ca2+ release channel gating (37). These single channel studies were performed in the absence of Aβ or cellular pathology, suggesting that modulation of InsP3R gating is a fundamental mechanism that contributes to exaggerated Ca2+ signaling in FAD PS-expressing cells.
It is not known whether the effects of FAD PS on InsP3R gating represent a gain or loss of function. Moreover, although many (>100) PS mutations (especially in PS1) that cause FAD have been identified (38), only two FAD-mutant PS have been examined for their effects on InsP3R channel gating (37). In addition, some PS1 mutations result in frontotemporal dementia (FTD), a neurological disorder lacking Aβ accumulation (39, 40). If FAD PS-mediated alteration of InsP3R-mediated Ca2+ signaling is proximal in AD pathogenesis, then other FAD-mutant PS might be expected to have similar enhancing effects on InsP3R channel gating, whereas those associated with FTD might not. Previous studies of the effects of mutant PS on InsP3R investigated endogenous insect (Sf9 ovarian cells) and chicken (DT40 B lymphocytes) InsP3Rs (37), whereas AD, in which the pathological consequences are primarily in brain neurons, affects humans. Consequently, the relevance of these data in appropriate cell types with endogenous amounts of PS and InsP3R are unclear. Here, we studied InsP3R channel kinetics under the influence of several FAD- and FTD-mutant PS in four different systems, including transgenic AD mouse neurons, B-lymphoblasts derived from human FAD patient cells, and fibroblasts from PS 1 and 2 double knock-out cells. All FAD-linked PS mutations enhanced InsP3R single channel gating, leading to exaggerated intracellular Ca2+ signaling, whereas FTD-associated PS1 mutations did not affect InsP3R channel kinetics. Furthermore, the effects of FAD PS mutants were gain-of-function effects, consistent with the genetics of FAD. In contrast, the secretase activity of PS was not required. The results indicate that exaggerated Ca2+ signaling through InsP3R-PS interaction is a disease-specific and robust proximal mechanism in FAD.
RESULTS
Multiple FAD PS mutations modulate InsP3R channel gating by mode switching
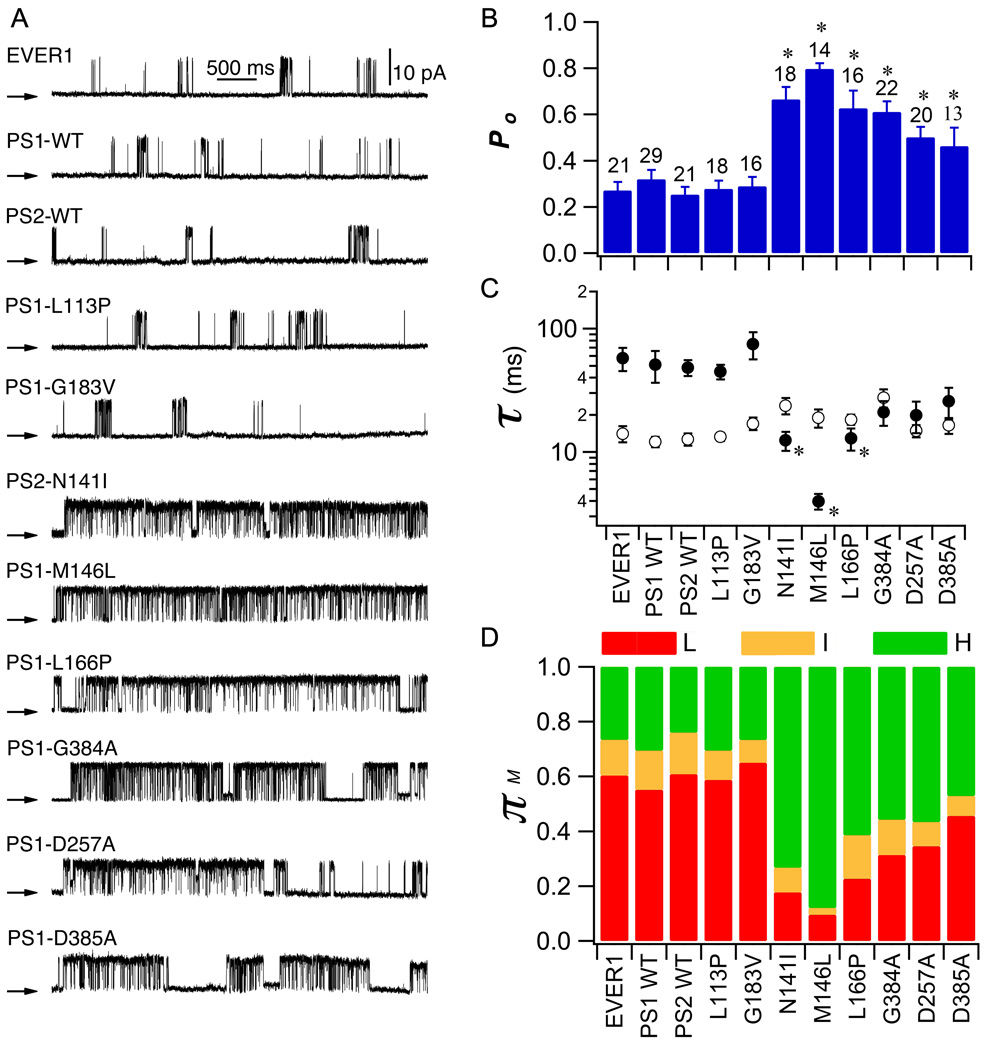
To determine whether enhanced InsP3R channel activity is a phenotype conserved in FAD PS-expressing cells, we recorded single InsP3R channel activities in the presence of one of eight different PS mutants (PS1-L113P (leucine at residue 113 substituted with proline), -M146L, -L166P, -G183V, -D257A, -G384A, -D385A and PS2-N141I). We performed single-channel patch-clamp electrophysiology of the outer membrane of isolated Sf9 cell nuclei (41) 48 hr after infecting cells with recombinant baculovirus (Fig. S1). Because enhancement of InsP3R activity is more apparent at sub-saturating InsP3 concentrations (37), we used 100 nM InsP3 and 1 µM Ca2+ to sub-optimally activate channel gating. We consistently detected InsP3R channels with open probability (Po) of 0.27 ± 0.04 in membrane patches from control EVER1-(an irrelevant ER transmembrane protein) infected nuclei (Fig. 1A and B). InsP3R channels recorded in membrane patches from PS1-WT- or PS2-WT-infected cells had Po similar to those from EVER1-infected control cells (Po = 0.32 ± 0.04 and 0.25 ± 0.03, respectively; p >0.05; Fig. 1A and B). In contrast, InsP3R channel Po was significantly enhanced by 250% in nuclei from cells infected with mutant PS1-M146L (Po = 0.81 ± 0.02; Fig. 1A and B) to a degree similar to that achieved with saturating ligand concentrations (37). Increased Po resulted from a marked reduction of channel mean closed-time (τc; Fig. 1C). FAD-mutant PS2 (N141I) also markedly enhanced InsP3R channel activity (Fig. 1A and B), with Po increased by 200% (0.66 ± 0.05; Fig. 1B), also mainly due to a significant reduction of τc (Fig. 1C). Similar results were obtained for two other FAD-causing PS1 mutants: InsP3R channel Po was increased 200% with PS1-L166P (Po = 0.63 ± 0.08) or PS1-G384A (Po = 0.61 ± 0.05; Fig. 1A and B). Thus, all four FAD PS mutants examined had similar effects on InsP3R channel activity. The γ-secretase-dead mutants PS1-D257A and PS1-D385A, which have mutations in intra-membrane sites involved in PS1 catalytic activity, also significantly enhanced InsP3R channel activity, although to a lesser extent than the FAD mutants (Po = 0.50 ± 0.05 and 0.46 ± 0.08, respectively; Fig. 1A and B). Thus, the secretase activity of PS is not required for its effects on InsP3R gating. Po of channels recorded from cells infected with FTD-associated mutant PS1-L113P and PS1-G183V were 0.28 ± 0.04 and 0.29 ± 0.04, respectively, not different from controls (Fig. 1A and B). Thus, several FAD-mutant PS have similar effects on InsP3R gating, and these effects are not recapitulated in PS mutants associated with a different neurological disease.
Fig. 1. Effect of recombinant PS on InsP3R single channel activity in Sf9 cells.
(A) Representative current recordings (+20 mV) in outer membrane patches of Sf9 cell nuclei infected with different recombinant PS baculoviruses. EVER1 served as an ER membrane protein infection control. Pipette solution contained 1µM free Ca2+ and 100 nM InsP3. Arrows indicate closed channel current level in this and all subsequent Figs. Summary of effects of PS on InsP3R channel Po (B), and mean open time τo (open circle) and mean closed time τc (filled circle) (C). (D) Summary of effects of PS on InsP3R modal gating. Bars: mean ± SEM. Asterisks: p < 0.05 by ANOVA compared with EVER1-infected cells.
To gain deeper insight into the mechanisms of InsP3R channel activation by FAD-mutant PS, we employed modal gating analysis. Previous studies demonstrated that ligand (InsP3, Ca2+) regulation of InsP3R gating is largely mediated by altering the propensity of the channel to gate in particular modes (42). Strongly activated channels gate in a high-Po H mode characterized by long bursting activities; an intermediate-Po I mode is characterized by fast channel openings and closings; and a low-Po L mode is characterized by long closed periods containing brief openings (42). In control nuclei isolated from EVER1-infected cells, the L gating mode was dominant, with the channel spending ~60% of its time in this mode and ~25% in the H mode (Fig. 1D). In nuclei from cells infected with either WT or FTD PS, similar modal gating distributions were observed (Fig. 1D). In contrast, the H mode was the dominant gating mode of InsP3R recorded from all of the FAD-causing mutant PS-expressing cells (Fig. 1D). Thus, FAD-mutant PS enhance InsP3R channel gating by mode switching, causing the channel to spend more time in the H mode at the expense primarily of the L mode (Fig. 1D; Fig. S2).
InsP3R single channel gating and InsP3-mediated Ca2+ signals are enhanced in human FAD B cells
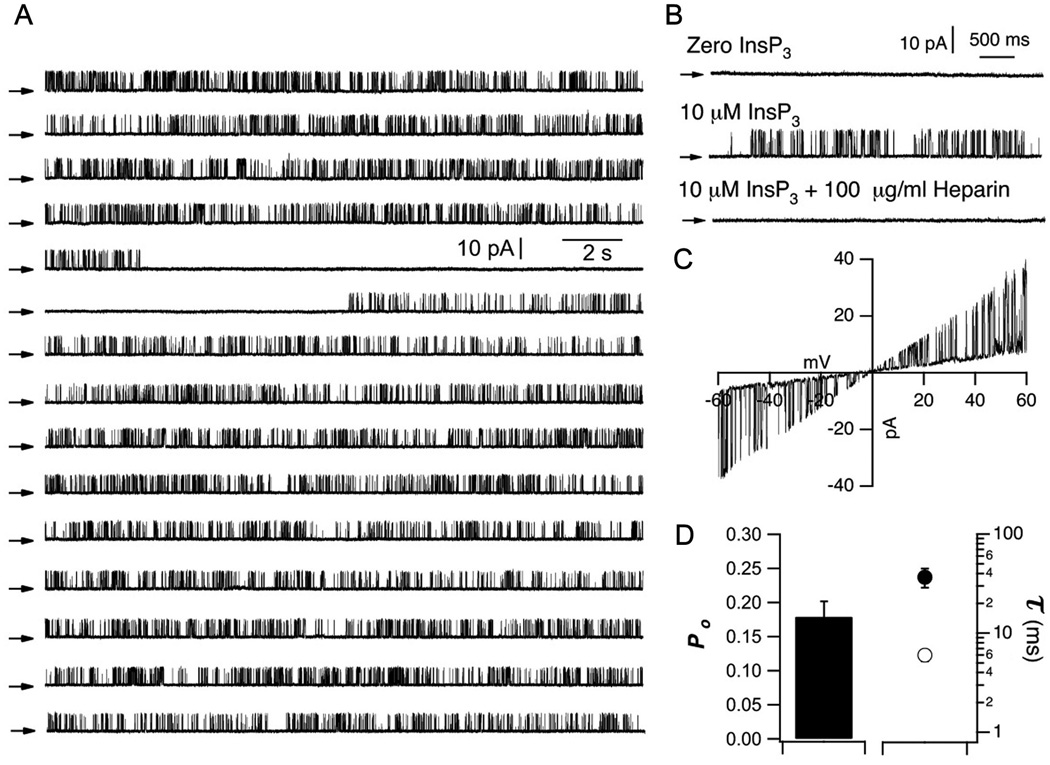
Enhancement of InsP3R channel activity by heterologous expression of mutant PS has been demonstrated in both Sf9 and DT40 cells [(37) and this study], systems that employ PS over-expressed in non-human cells. To determine the effects of endogenous PS in human cells, we studied InsP3R activity in normal and FAD human B cell lymphoblasts. Currents from endogenous human InsP3R single channels have never been previously recorded. Thus, we initially characterized endogenous InsP3R channels from human B lymphoblasts by nuclear membrane patch-clamp electrophysiology. In the absence of InsP3, no channel activity was apparent (n = 18; Fig. 2B), whereas with InsP3 (10 µM) in the pipette solution, we observed heparin-sensitive single channels with brief openings and long closings (n =15; Fig. 2A and B). These channels showed a linear I/V relationship with slope conductance ~475 pS (Fig. 2C), typical of mammalian InsP3R under these ionic conditions (43). InsP3R currents recorded from human B cells were long-lasting (Fig. 2A), with relatively low Po (0.18 ± 0.02, n=20; Fig. 2D).
Fig. 2. Characterization of endogenous InsP3R single channels in human B lymphoblasts.
(A) Continuous single InsP3R channel current trace (300 s) recorded from outer membrane of nucleus isolated from human B lymphoblaste at +20 mV with 10 µM InsP3 and 1 µM free Ca2+ in pipette solution. Arrows indicate closed channel current levels. (B) Representative current traces (+20 mV) in nuclei isolated from human B lymphoblasts. Channel activity required presence of InsP3 (n = 20) and was inhibited by heparin (n = 15). (C) I/V relationship obtained by ramping holding potential from −60 to +60 mV. (D) Summary of InsP3R channel Po, and mean open τo and closed τc durations.
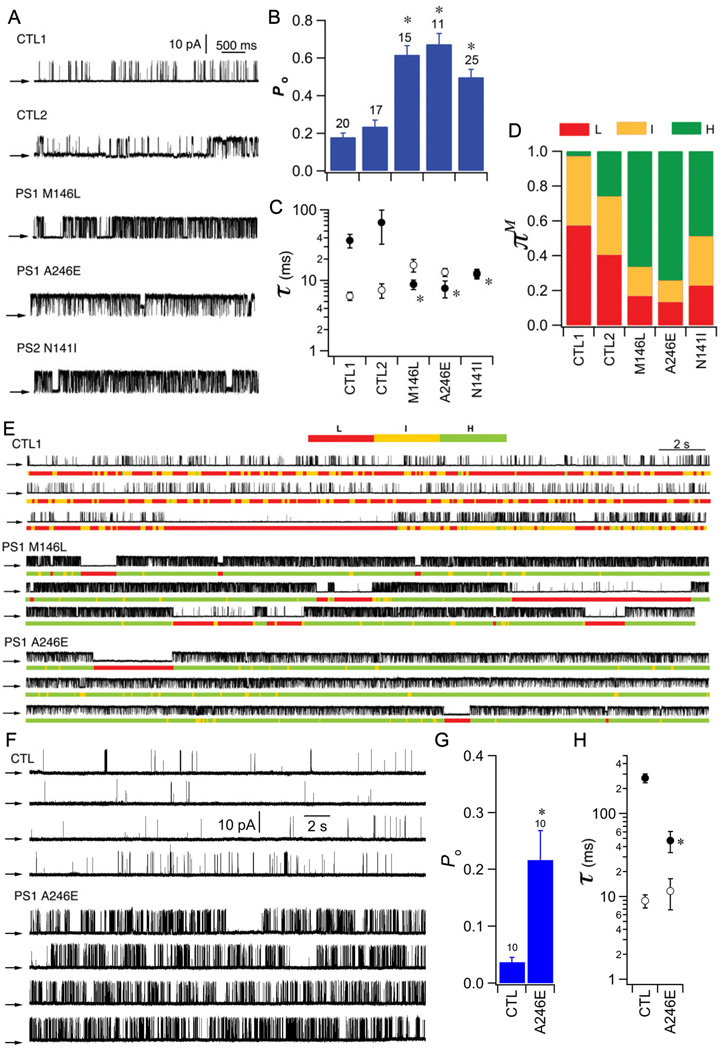
We compared InsP3R gating in B lymphoblasts derived from three individuals with FAD, harboring PS1-M146L, PS1-A246E, or PS2-N141I (FAD lymphocytes), with that in B-lymphoblasts from two different age-matched individuals without FAD or FAD-associated PS mutations (control lymphoblasts) (Table 1). InsP3R in control lymphoblasts from the two individuals without FAD had low channel Po (0.18 ± 0.02 and 0.23 ± 0.03, respectively; Fig. 3A,B) with channel activities characterized by brief openings and relatively long closings (Fig. 3A and C). InsP3R Po recorded from lymphoblasts from all three individuals with FAD were increased 200 to 300% when compared with those from control lymphoblasts (PS1-M146L: 0.62 ± 0.05; PS1-A246E: 0.67± 0.06; PS2-N141I: 0.50 ± 0.04; Fig. 3A and B), mainly due to a marked decrease in τc (Fig. 3C), with many channels bursting for extended periods (Fig. 3E). In control lymphoblasts, the L and I gating modes dominated channel kinetics, whereas InsP3R analyzed in FAD lymphoblasts spent 50 to 75% of the time in the high Po H mode (Fig. 3D and E). Analogous results were obtained with low (100 nM) InsP3. InsP3R Po was 0.04 ± 0.01 in control lymphoblasts from an individual without FAD, whereas Po was 0.22 ± 0.05 in PS1-A246E FAD lymphoblasts (Fig. 3F–G). These observations in human B-lymphoblasts with endogenous PS and InsP3R are similar to those made in Sf9 and DT40 cells. FAD-linked PS mutations therefore have a robust, common effect to enhance InsP3R single channel activity in insect, avian, and human cells.
Table 1.
Human FAD and control B-lymphoblast lines
| Cell Line | Genotype | Donor Age/Sex | AD present |
|---|---|---|---|
| AG07877 | PS1-M146L | 53 / M | Yes |
| AG06841 | PS1-A246E | 56 / M | Yes |
| AG09369 | PS2-N141I | 56 / M | Yes |
| AG09180 | Normal (CTL1) | 56 / M | No |
| AG08266 | Normal (CTL2) | 56 / M | No |
Fig. 3. Effect of FAD PS on InsP3R gating in human FAD B lymphoblasts.
(A) Representative InsP3R currents (+20 mV) in nuclei isolated from human FAD B lymphoblasts and control lymphoblasts from age-matched individuals without FAD activated with 10 µM InsP3 and 1 µM Ca2+ in pipette solution. Summary of channel Po (B), τo (open circles) and τc (filled circles) (C) and modal gating analysis (D). Asterisks: p < 0.05, ANOVA compared with CTL1. (E) Modal gating analyses. Each section shows continuous recording with gating mode assignment in color code below. In cells from normal individuals, low Po is associated with switching between L and I modes. In cells from all three individuals with FAD, enhanced gating is manifested by increased occupancy of H mode at expense of L mode. F-H. Single InsP3R channel current traces from human B cells activated by sub-optimal InsP3. (F) Representative currents (+20 mV) in isolated nuclei from human FAD lymphoblasts and age-matched control B lymphoblastss activated by sub-optimal 100 nM InsP3 and 1 µM Ca2+. Summary of InsP3R Po (G), and τo (open circle) and τc (filled circle) (H) from aged-matched control and FAD human B-lymphocblasts. Asterisks: p < 0.05 by student’s t-test.
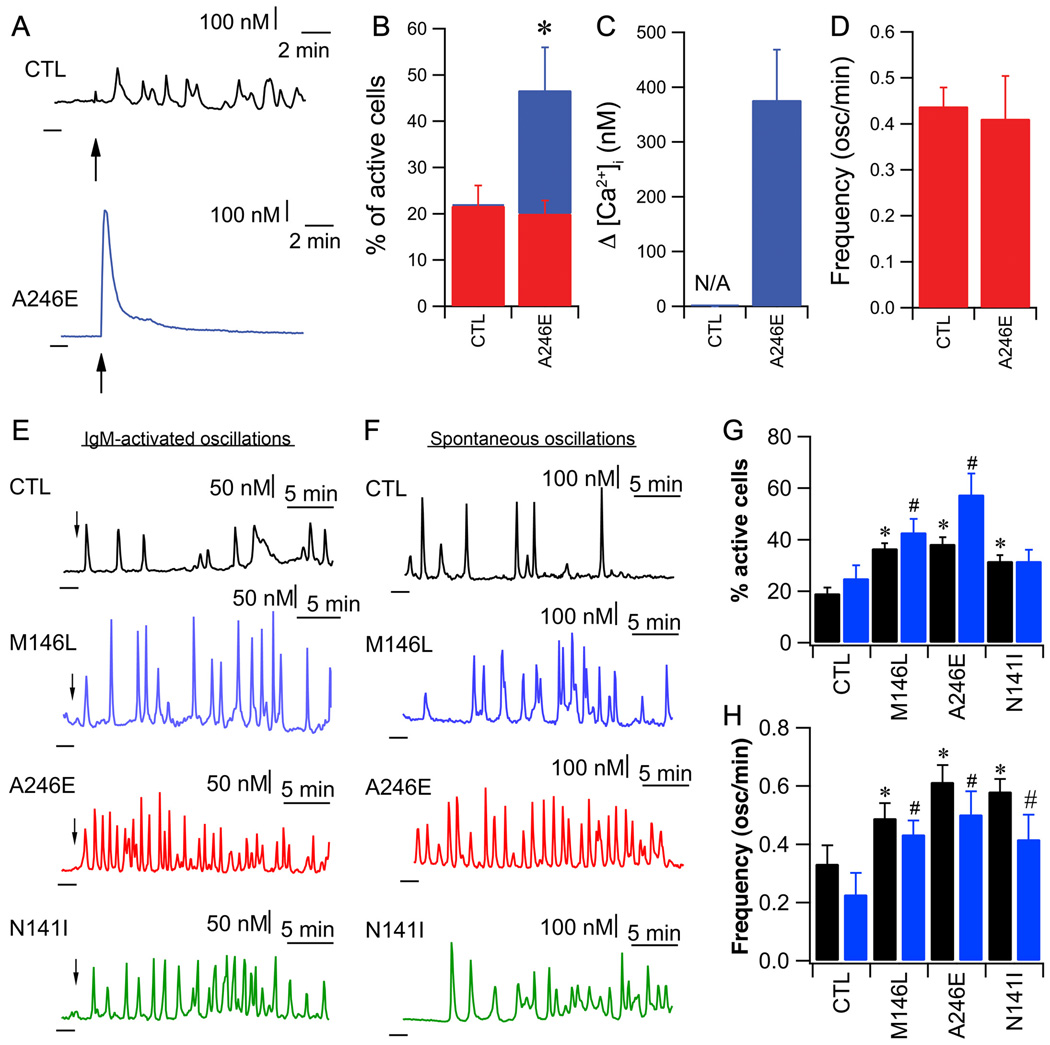
To determine whether these effects observed at the single-channel level are associated with altered [Ca2+]i signaling, we measured InsP3R-mediated Ca2+ signals in B lymphoblasts from the same individuals with FAD that were used for single-channel studies. InsP3R-mediated Ca2+ signals were elicited by cross-linking the B cell receptor (BCR) with IgM antibody. At high [IgM] (5µg/ml), 20% of cells responded with similar Ca2+ oscillations and spiking in both control and PS1-A246E FAD lymphoblasts (Fig. 4B and D), whereas a further 27% of the FAD lymphoblasts responded with exaggerated high-amplitude transient responses (Fig. 4A, B and C). With low-dose anti-IgM stimulation (50 ng/ml), Ca2+ oscillations/spiking were triggered in 19% ± 2% of control cells (Fig. 4E and G). Perfusion with xestospongin B, a membrane-permeable specific InsP3R inhibitor (44), reversibly inhibited them indicating that they were due to periodic Ca2+ release through the InsP3R (Fig. S3). In FAD lymphoblasts, both the percentage of responding cells and the oscillation and spiking frequency were increased (Fig. 4E, G and H). Perfusion with culture medium containing 10% FBS, which generates ongoing low InsP3 production (45), induced spontaneous Ca2+ oscillations/spiking in 25 ± 5% of control lymphoblasts (Fig. 4F and G). In contrast, the percentage of PS1 FAD lymphocytes displaying spontaneous Ca2+ oscillations was increased by 100% and the oscillation and spiking frequency doubled (Fig. 4F, G and H). The percentage of spontaneously oscillating PS2-N141I FAD cells was similar to that in control lymphoblasts, however, the oscillation frequency was increased (Fig. 4F, G and H). These responses are consistent with an enhanced sensitivity and activity of InsP3-mediated Ca2+ release in human FAD lymphoblasts, consistent with the enhanced InsP3R channel activity recorded in these cells.
Fig. 4. Exaggerated Ca2+ signaling in human FAD B lymphoblasts.
(A) Representative single cell Ca2+ responses to strong IgM stimulation (5µg/ml; arrow) in control human B lymphoblasts (CTL) or FAD lymphoblasts carrying PS1-A246E mutation. Dark lines below and to the left of each trace indicate zero Ca2+. (B) Responses to IgM stimulation. Percentages responding with Ca2+ oscillations (red) or large amplitude Ca2+ transients (blue). (C) Summary of peak amplitudes of high-amplitude transient Ca2+ responses triggered by 5 µg/ml anti-IgM. (D) Ca2+ oscillation frequency in response to anti-IgM. N = 3 experiments with 30 cells in each. Asterisk: p < 0.05, Student’s t-test. (E) Representative single cell Ca2+ responses to weak IgM stimulation (50 ng/ml; arrow) and (F) spontaneous oscillations during perfusion with serum-containing medium in lymphoblasts from unaffected (CTL) and FAD individuals. Dark lines: zero Ca2+ level. (G) Percentage of cells responding to IgM (black) or undergoing spontaneous Ca2+ oscillations in complete medium (blue). (H) Summaries of Ca2+ oscillation frequency in response to IgM (black) or spontaneous Ca2+ oscillations observed in complete medium (blue). Data in each group summarized from 4 experiments with 30 cells in each. Asterisks or #: p < 0.05 by ANOVA as compared with respective controls.
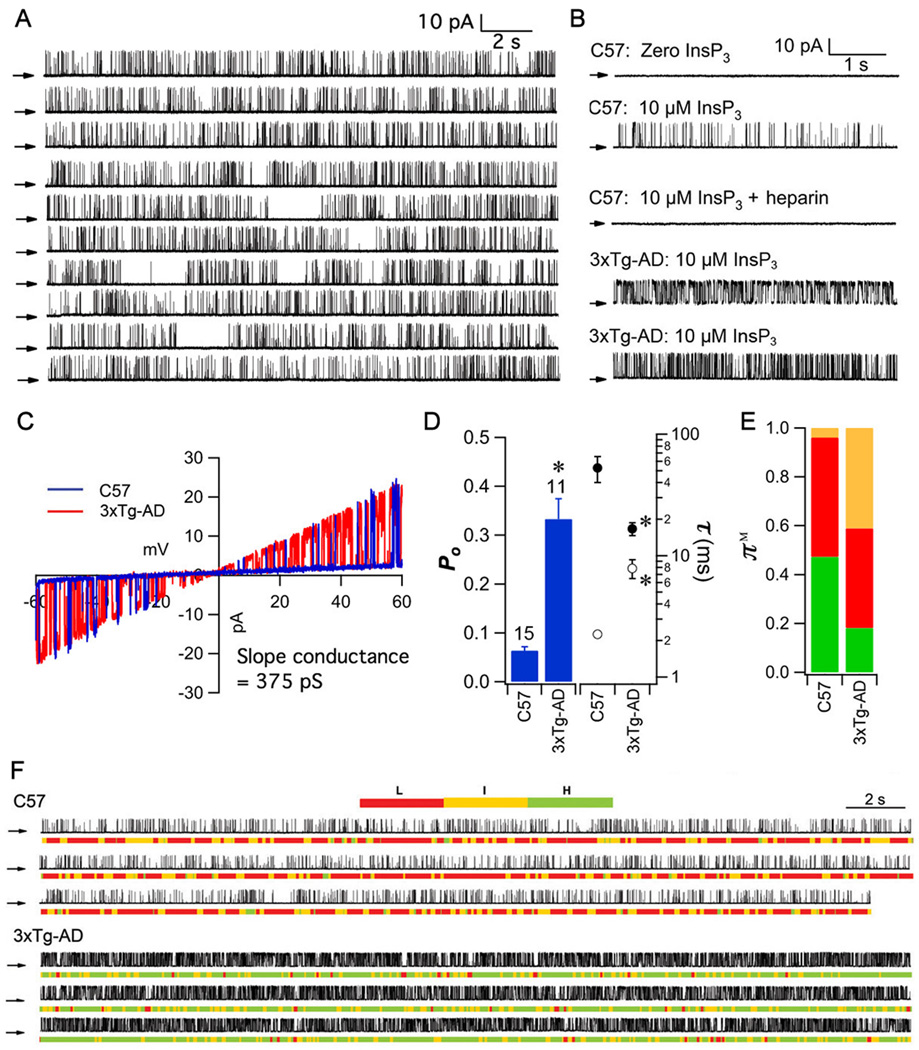
InsP3R channel gating is enhanced in FAD mouse cortical neurons
Ca2+ signaling disruption has been observed in fibroblast or lymphoblast lines derived from human FAD cells [here and (30, 32, 46)]. Our results above implicate mutant PS-enhanced InsP3R channel gating as the underlying mechanism. To determine if this molecular mechanism also operates in brain neurons, we isolated cortical neurons from embryonic day 14 to 16 (E14 to E16) WT C57BL/6 and 3×Tg-AD mice and recorded single InsP3R channel activities in nuclear envelopes from isolated nuclei. 3xTg-AD mice contain PS1-M146V knocked into the PS1 locus, and exhibit age-dependent amyloid plaques, neurofibrillary tangles, and cognitive decline starting at 3 to 6 months of age (3, 47). In nuclei isolated from control C57BL/6 mice, channel currents were not observed in the absence of InsP3 (Fig. 5B). With 10 µM InsP3, and 1 µM Ca2+, heparin-sensitive (Fig. 5B), channels with a linear slope conductance of ~375 pS (Fig. 5C) were recorded (Fig. 5A and B) with gating characterized by short openings (τo = 2.25 ± 0.11 ms) and relatively long closures (τc = 52.7 ± 12.7 ms) with Po = 0.06 ± 0.01 (Fig. 5D). Po was enhanced by 700% (0.43 ± 0.05; Fig. 5B and D) in nuclei isolated from 3xTg-AD mice. Increased Po was caused by markedly prolonged τo (10.22 ± 1.57 ms) together with shortened τc (14.61 ± 3.04 ms). The I and L modes dominated channel gating in control C57BL/6 neurons, whereas the H mode was the major gating mode in 3xTg-AD neurons (Fig. 5B,E, and F).
Fig. 5. InsP3R single channel activity in mouse primary embryonic cortical neurons.
(A) Continuous single InsP3R current trace (200 s) in outer membrane of nucleus isolated from embryonic cortical neuron (+40 mV with 10 µM InsP3 and 1 µM free Ca2+ in pipette solution). Arrows: closed channel current level. (B) Representative current traces (+40 mV) in nuclei from C57BL/6 (wild type) or 3xTg-AD mice (E14 to E16). Channel activities in both mouse lines required InsP3 and were inhibited by heparin. (C) InsP3-activated currents from C57BL/6 (blue) or 3xTg-AD (red) mice were linear with 375 pS slope conductance. (D) Summary of InsP3R channel Po, τo and τc in cortical neuron nuclei. Bars: mean ± SEM. Asterisks: p < 0.05 by unpaired Student’s t-test. (E) Summary of InsP3R modal gating analysis. Colors for gating modes same as Figs 1 and 3. (F) Modal gating analysis of InsP3R from cortical neurons. Each section is a continuous single channel current record with modal assignment indicated by color code. In cells from C57BL/6 mouse, channel gating is alternates between L and I modes, whereas in 3xTg-AD mouse, InsP3R gating alternates between H and I modes.
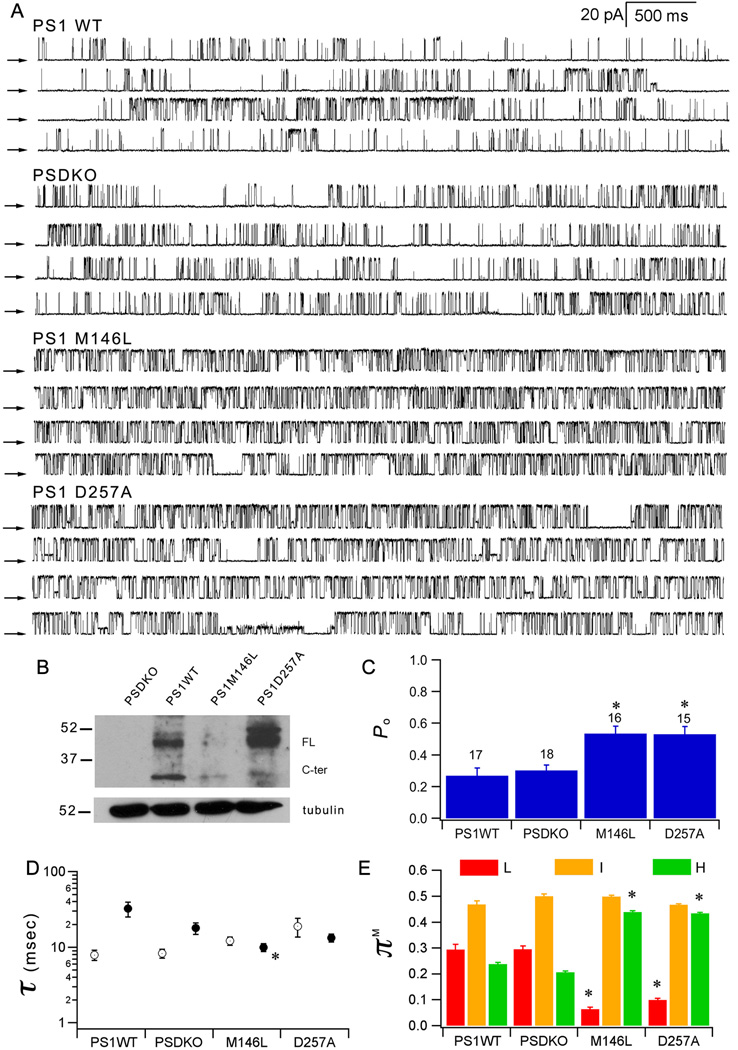
FAD PS enhancement of InsP3R channel gating is a gain-of-function effect
Our results reveal that FAD-mutant PS consistently enhances InsP3R channel gating. To explore the mechanisms involved, we recorded endogenous InsP3R channels in nuclei from embryonic fibroblasts (MEF) derived from PS double-knockout mice (48, 49). In the absence of PS, the endogenous MEF InsP3R Po was 0.30 ± 0.03 (Fig. 6). Stable expression of human PS1 by retroviral transduction was without effect on InsP3R Po (0.27 ± 0.05), whereas FAD mutant PS1-M146L approximately doubled channel gating activity (0.54 ± 0.05), by enhancing H-mode gating (Fig. 6). Similar results were obtained in independently-derived MEF clones (Fig. S4). These results indicate that the effects of FAD-mutant PS on InsP3R channel involve a gain of function. As shown above in Sf9 cells, this function is independent of PS secretase activity, because the secretase-dead PS1-D257A also enhanced channel activity (Fig. 6).
Fig. 6. FAD PS enhances InsP3R channel gating by gain-of-function effect.
(A) Continuous single InsP3R channel current traces recorded from outer membranes of nuclei isolated from PS deficient MEF (PSDKO) and PSDKO stably expressing human PS1-WT, FAD PS1-M146L or secretase-dead PS1-D257A (+40 mV; 10 µM InsP3 and 1 µM free Ca2+ in pipette solution). Arrows indicate closed channel current levels. (B) Western blot forPS1 in PS-deficient and stably transduced MEF cells. Summary of InsP3R channel Po (C), τo (open circle) and τc (filled circle) (D) and modal gating analysis (E). Asterisks: p < 0.05 by ANOVA compared with PSDKO.
DISCUSSION
In summary, the above results demonstrate a consistent and robust phenotype associated with the presence of mutant PS linked to FAD. In five different cell systems (four here and DT40 cells previously) from four species, FAD-causing mutant PS resulted in exaggerated responses of InsP3R Ca2+ release channels and exaggerated Ca2+ signals in response to agonist stimulation, as well as a small degree of constitutive Ca2+ signaling. The FAD-mutant PS phenotype involves gain-of-function effects, consistent with disease genetics, and is independent of the secretase function of PS. Moreover, the FAD-mutant PS phenotype is not observed in cells harboring either wild-type PS or PS mutants associated with a different disease, FTD. The FAD-mutant PS phenotype is manifested independently of any pathology associated with AD, and, in the mouse model, precedes such pathology. Moreover, it is apparent in physiologically-relevant cell types (cells derived from humans with FAD and AD mouse neurons) with all proteins present in endogenous amounts. We propose that exaggerated Ca2+ signaling through an InsP3R-PS interaction is a robust proximal gain-of-function molecular mechanism in FAD.
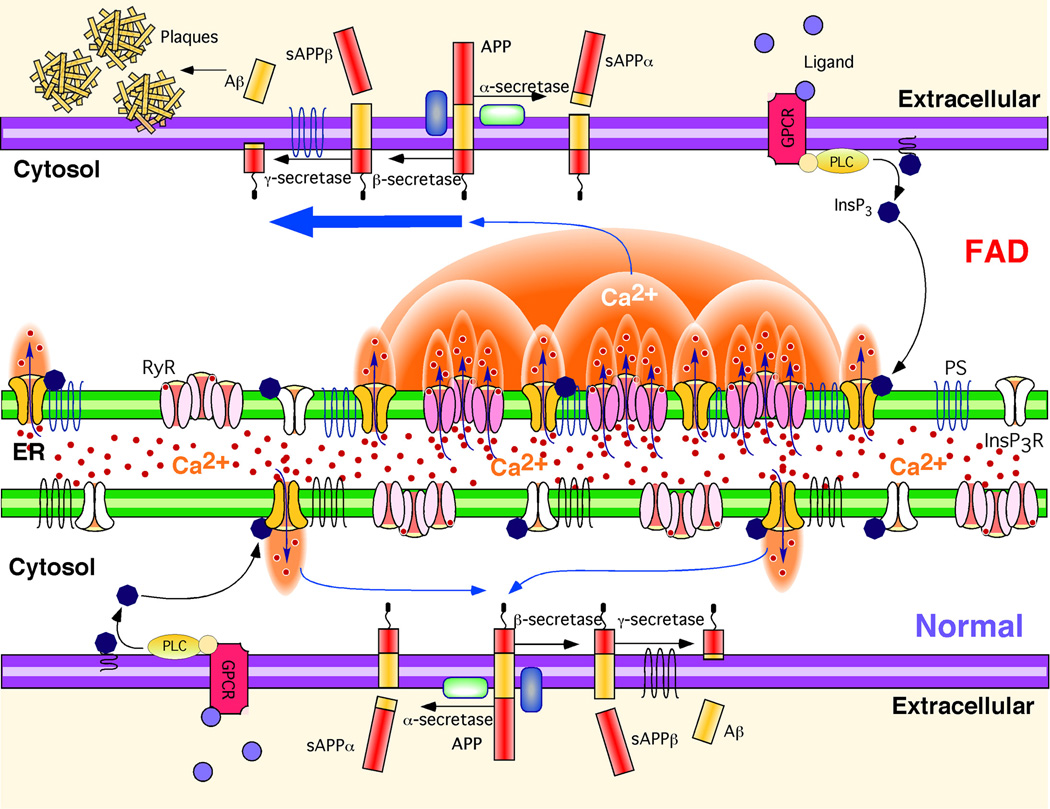
Our single channel analyses demonstrate that FAD-mutant PS enhances single channel activity of the InsP3R by affecting modal gating kinetics, the major mechanism by which InsP3 and Ca2+ regulate the channel (42). That FAD-mutant PS drives the channel into the H mode may have important physiological implications. The channel open time when it in the L gating mode (~10 ms) is short enough that it may not increase local [Ca2+] sufficiently to recruit additional InsP3R- or RyR-mediated Ca2+ release by Ca2+-induced Ca2+ release (CICR). In contrast, the much longer activity bursts of the channel in the H mode (>200 ms) will provide a sufficiently large flux of Ca2+ to enable a normally local Ca2+ signal to be amplified and propagated by CICR (50). Because InsP3R and RyR are clustered and spatially localized to different regions of cells to provide local [Ca2+]i signals as a critical element of physiological specificity, mode-shifting by mutant PS-induced FAD may result not only in exaggerated local Ca2+ signaling, but also a disruption of spatial specificity by enabling CICR to transmit the signals more globally (42, 50). Exaggerated and spatially disrupted Ca2+ signaling may in turn impinge on APP processing (16, 51–54), calpain activation (16, 54), and tau phosphorylation (55, 56), linking our findings here to the amyloid hypothesis of AD (Fig. 7).
Fig. 7. Hypothetical molecular mechanism of enhanced Aβ production due to Ca2+ disruption in FAD PS cells.
APP is processed by either α-secretase or β-secretase, the latter leading to Aβ generation after subsequent cleavage by γ-secretase. Stimulation of G-protein coupled receptors (GPCR) or other cell surface receptors by extracellular ligands activates phospholipase C (PLC), which cleaves phosphatidylinositol bisphosphate (PIP2) to produce InsP3. InsP3 binds to and activates the InsP3R to release Ca2+ from ER stores, increasing cytoplasmic Ca2+ concentration. In normal cells, these Ca2+ signals are tightly regulated in time, space, and amplitude. In FAD cells, mutant PS exerts stimulatory effects on InsP3R gating by modal switching to the H mode associated with prolonged channel openings. H mode gating generates exaggerated Ca2+ signaling by promoting additional release channel recruitment by CICR. Increased cytosolic Ca2+ concentration promotes β-secretase activity (52) and Aβ production (51, 54), which, together with mutant PS-enhanced production of amyloidogenic Aβ, results in plaque formation.
MATERIALS AND METHODS
Cell Culture
Spodoptera frugiperda cells (Sf9, BD Biosciences) were maintained as described (37, 41). Human PS baculovirus constructs (PS1-WT, PS1-L113P, PS1-M146L, PS1-L166P, PS1-G183V, PS1-D257A, PS1-G384A, PS1-D384A, PS2-WT and PS2-N141I) were subcloned into pFastBac1 and baculoviruses were generated using the Bac-to-Bac system (Invitrogen). Expression was confirmed by Western blotting with antibodies directed against PS1 or PS2 (anti-PS1 and anti-PS2, respectively) as described (37). B-lymphoblast lines derived from human FAD patients and normal individuals (Table I; Coriell Institute, Camden, NJ) were maintained at 37°C (95/5% air/CO2) in RPMI 1640 (Invitrogen) supplemented with 15% fetal bovine serum (Hyclone), 2 mM L-glutamine, 100 units/ml penicillin, and 100 µg/ml streptomycin. PS−/− (genetically deficient in PS1 and PS2), stable human PS1-WT, mutant PS1-M146L and PS1-D257A MEF cells were grown in DMEM supplemented with 10% fetal bovine serum (57, 58). To generate stable lines expressing comparable amounts of PS1 proteins, human PS1 cDNAs were introduced into pMX-IRES-EGFP retroviral vector, and PS retroviruses generated using Retro-X system (Clontech) were added to the parental PS−/− MEF cells, and GFP positive cells were sorted by FACS. PS expression was confirmed by Western blot.
Cortical neuron isolation
Primary cortical neurons were prepared from embryonic day 14 to 16 (E14 to E16) 3xTg-AD mice as described (37). Neurons from C57BL/6 mice (Charles River) served as controls. In brief, dams were killed with CO2, and embryos were removed by cesarean section. Brains from littermates were removed and placed into PBS. After the meninges were removed, cerebral cortices were dissected, minced, and digested with 0.25% trypsin in PBS at 37°C for 20 min. Dissociated cells were washed twice with DMEM supplemented with 10% FBS, triturated with a fire-polished Pasteur pipette and re-suspended in Neurobasal medium supplemented with 1x B27 (Invitrogen). All animal procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC).
Calcium imaging
Human B-lymphoblasts (Coriell Institute, Camden, NJ) were plated onto a CellTek-(BD Biosciences) coated glass-bottom perfusion chamber mounted on the stage of an inverted microscope (Eclipse TE2000; Nikon, Melville, NY) and incubated with fura-2 AM (2 µM; Invitrogen) for 30 min at room temperature in Hanks’ balanced salt solution (HBSS, Sigma, St. Louis, MO) containing 1% BSA. Cells were then continuously perfused with HBSS containing 1.8 mM CaCl2 and 0.8 mM MgCl2 (pH 7.4). Ca2+ signals were elicited by cross-linking the B cell receptor (BCR) with 50 ng/ml anti-human IgM antibody (SouthernBiotech, Birmingham, AL). In some experiments, cells were perfused with complete culture medium containing 10% FBS. Fura-2 was alternately excited at 340 and 380 nm, and the emitted fluorescence filtered at 510 nm was collected and recorded (37, 45) using a CCD-based imaging system running Ultraview software (PerkinElmer, Waltham, MA). Dye calibration was achieved by applying experimentally determined constants to the standard equation [Ca2+] = Kd·β·(R − R min)/(R max − R).
Electrophysiology
Preparation of isolated nuclei from cells was performed as described (37, 41, 45). In brief, cells were washed twice with PBS and suspended in nuclear isolation solution containing (in mM): 150 KCl, 250 sucrose, 1.5 β-mercapoethanol, 10 Tris-HCl, 0.05 phenylmethylsulphonyl fluoride and protease inhibitor cocktail (Complete, Roche Diagnosis, Indianapolis, IN), pH 7.3. Nuclei were isolated using a Dounce glass homogenizer and plated onto a 1-ml glass-bottomed dish containing standard bath solution (in mM): 140 KCl, 10 HEPES, 0.5 BAPTA, and 0.192 CaCl2 (free [Ca2+] = 90 nM). The pipette solution contained (in mM): 140 KCl, 10 HEPES, 0.5 dibromo-BAPTA, and 0.001 free Ca2+, pH 7.3. Free [Ca2+] in solutions was adjusted by Ca2+ chelators with appropriate affinities and confirmed by fluorometry as described (41). Data were recorded at room temperature and acquired using an Axopatch 200A amplifier (Axon Instruments), filtered at 1 kHz, and digitized at 5 kHz with an ITC-16 interface (Instrutech) and Pulse software (HEKA Electronik).
Data Analysis
Segment of current records exhibiting current levels for a single InsP3R channel were idealized using QuB software (University of Buffalo) with SKM algorithm (59, 60). Channel gating kinetics and modal gating behaviors were characterized as described (42). In brief, very short closing events (< 10 ms), presumably caused by ligand-independent transitions, were removed by burst analysis (61) after idealization with QuB. Modal gating assignment was then achieved by plotting and examining durations of channel burst (tb) and burst-terminating gaps (tg) as described (42). In Sf9 cells, we set Tb =100 ms and Tg = 200 ms for the detection of modal transitions. In both human B-lymphocytes and mouse cortical neurons, we set Tb = 50 ms and Tg = 100 ms for the detection of modal transitions. Data were summarized as the mean ± SEM, and the statistical significance of differences between means was assessed by using unpaired t tests or one-way ANOVA with Dunnett’s post hoc comparison test. Differences between means were accepted as statistically significant at the 95% level (p <0.05).
Supplementary Material
REFERENCES
- 1.Hutton M, Hardy J. The presenilins and Alzheimer’s disease. Hum. Mol. Genet. 1997;6:1639–1646. doi: 10.1093/hmg/6.10.1639. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J. A hundred years of Alzheimer’s disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 3.LaFerla FM, Oddo S. Alzheimer’s disease: Abeta, tau and synaptic dysfunction. Trends. Mol. Med. 2005;11:170–176. doi: 10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Annaert WG, Levesque L, Craessaerts K, Dierinck I, Snellings G, Westaway D, George-Hyslop PS, Cordell B, Fraser P, De Strooper B. Presenilin 1 controls gamma-secretase processing of amyloid precursor protein in pre-golgi compartments of hippocampal neurons. J. Cell. Biol. 1999;147:277–294. doi: 10.1083/jcb.147.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Wolfe MS, Selkoe DJ. Toward structural elucidation of the gamma-secretase complex. Structure. 2009;17:326–334. doi: 10.1016/j.str.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia W, Zhang J, Kholodenko D, Citron M, Podlisny MB, Teplow DB, Haass C, Seubert P, Koo EH, Selkoe DJ. Enhanced production and oligomerization of the 42-residue amyloid beta-protein by Chinese hamster ovary cells stably expressing mutant presenilins. J. Biol. Chem. 1997;272:7977–7982. doi: 10.1074/jbc.272.12.7977. [DOI] [PubMed] [Google Scholar]
- 9.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell. Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 10.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 11.Kelleher CT, Chiu R, Shin H, Bosdet IE, Krzywinski MI, Fjell CD, Wilkin J, Yin T, DiFazio SP, Ali J, Asano JK, Chan S, Cloutier A, Girn N, Leach S, Lee D, Mathewson CA, Olson T, O’Connor K, Prabhu AL, Smailus DE, Stott JM, Tsai M, Wye NH, Yang GS, Zhuang J, Holt RA, Putnam NH, Vrebalov J, Giovannoni JJ, Grimwood J, Schmutz J, Rokhsar D, Jones SJ, Marra MA, Tuskan GA, Bohlmann J, Ellis BE, Ritland K, Douglas CJ, Schein JE. A physical map of the highly heterozygous Populus genome: integration with the genome sequence and genetic map and analysis of haplotype variation. Plant J. 2007;50:1063–1078. doi: 10.1111/j.1365-313X.2007.03112.x. [DOI] [PubMed] [Google Scholar]
- 12.LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat. Rev. Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 13.Smith IF, Green KN, LaFerla FM. Calcium dysregulation in Alzheimer’s disease: recent advances gained from genetically modified animals. Cell Calcium. 2005;38:427–437. doi: 10.1016/j.ceca.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Huang HM, Ou HC, Hsueh SJ. Amyloid beta peptide enhanced bradykinin-mediated inositol (1,4,5)trisphosphate formation and cytosolic free calcium. Life Sci. 1998;63:195–203. doi: 10.1016/s0024-3205(98)00260-4. [DOI] [PubMed] [Google Scholar]
- 15.Mogensen HS, Beatty DM, Morris SJ, Jorgensen OS. Amyloid beta-peptide(25–35) changes [Ca2+] in hippocampal neurons. Neuroreport. 1998;9:1553–1558. doi: 10.1097/00001756-199805110-00057. [DOI] [PubMed] [Google Scholar]
- 16.Buxbaum JD, Ruefli AA, Parker CA, Cypess AM, Greengard P. Calcium regulates processing of the Alzheimer amyloid protein precursor in a protein kinase C-independent manner. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4489–4493. doi: 10.1073/pnas.91.10.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herms J, Schneider I, Dewachter I, Caluwaerts N, Kretzschmar H, Van Leuven F, Capacitive calcium entry is directly attenuated by mutant presenilin-1. independent of the expression of the amyloid precursor protein. J. Biol. Chem. 2003;278:2484–2489. doi: 10.1074/jbc.M206769200. [DOI] [PubMed] [Google Scholar]
- 19.Leissring MA, Akbari Y, Fanger CM, Cahalan MD, Mattson MP, LaFerla FM. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. J. Cell. Biol. 2000;149:793–798. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo AS, Cheng I, Chung S, Grenfell TZ, Lee H, Pack-Chung E, Handler M, Shen J, Xia W, Tesco G, Saunders AJ, Ding K, Frosch MP, Tanzi RE, Kim TW. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 2000;27:561–572. doi: 10.1016/s0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 21.Leissring MA, Paul BA, Parker I, Cotman CW, LaFerla FM. Alzheimer’s presenilin-1 mutation potentiates inositol 1,4,5-trisphosphate-mediated calcium signaling in Xenopus oocytes. J Neurochem. 1999;72:1061–1068. doi: 10.1046/j.1471-4159.1999.0721061.x. [DOI] [PubMed] [Google Scholar]
- 22.Smith IF, Hitt B, Green KN, Oddo S, LaFerla FM. Enhanced caffeine-induced Ca2+ release in the 3xTg-AD mouse model of Alzheimer’s disease. J Neurochem. 2005;94:1711–1718. doi: 10.1111/j.1471-4159.2005.03332.x. [DOI] [PubMed] [Google Scholar]
- 23.Stutzmann GE. Calcium dysregulation IP3 signaling and Alzheimer’s disease. Neuroscientist. 2005;11:110–115. doi: 10.1177/1073858404270899. [DOI] [PubMed] [Google Scholar]
- 24.Stutzmann GE, Caccamo A, LaFerla FM, Parker I. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer’s-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J Neurosci. 2004;24:508–513. doi: 10.1523/JNEUROSCI.4386-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green KN, Demuro A, Akbari Y, Hitt BD, Smith IF, Parker I, LaFerla FM. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. J. Cell. Biol. 2008;181:1107–1116. doi: 10.1083/jcb.200706171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson O, Tu H, Lei T, Bentahir M, de Strooper B, Bezprozvanny I. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J. Clin Invest. 2007;117:1230–1239. doi: 10.1172/JCI30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ERCa2+ leak channelsa function disrupted by familial Alzheimer’s disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakroborty S, Goussakov I, Miller MB, Stutzmann GE. Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice. J Neurosci. 2009;29:9458–9470. doi: 10.1523/JNEUROSCI.2047-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J Neurosci. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etcheberrigaray R, Hirashima N, Nee L, Prince J, Govoni S, Racchi M, Tanzi RE, Alkon DL. Calcium responses in fibroblasts from asymptomatic members of Alzheimer’s disease families. Neurobiol. Dis. 1998;5:37–45. doi: 10.1006/nbdi.1998.0176. [DOI] [PubMed] [Google Scholar]
- 31.Hirashima N, Etcheberrigaray R, Bergamaschi S, Racchi M, Battaini F, Binetti G, Govoni S, Alkon DL. Calcium responses in human fibroblasts: a diagnostic molecular profile for Alzheimer’s disease. Neurobiol. Aging. 1996;17:549–555. doi: 10.1016/0197-4580(96)00074-7. [DOI] [PubMed] [Google Scholar]
- 32.Ito E, Oka K, Etcheberrigaray R, Nelson TJ, McPhie DL, Tofel-Grehl B, Gibson GE, Alkon DL. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 1994;91:534–538. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leissring MA, LaFerla FM, Callamaras N, Parker I. Subcellular mechanisms of presenilin-mediated enhancement of calcium signaling. Neurobiol. Dis. 2001;8:469–478. doi: 10.1006/nbdi.2001.0382. [DOI] [PubMed] [Google Scholar]
- 34.Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J. Biol. Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- 35.Kasri NN, Kocks SL, Verbert L, Hebert SS, Callewaert G, Parys JB, Missiaen L, De Smedt H. Up-regulation of inositol 1,4,5-trisphosphate receptor type 1 is responsible for a decreased endoplasmic-reticulum Ca2+ content in presenilin double knock-out cells. Cell Calcium. 2006;40:41–51. doi: 10.1016/j.ceca.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Stutzmann GE, Smith I, Caccamo A, Oddo S, Parker I, Laferla F. Enhanced ryanodine-mediated calcium release in mutant PS1-expressing Alzheimer’s mouse models. Ann. N. Y. Acad. Sci. 2007;1097:265–277. doi: 10.1196/annals.1379.025. [DOI] [PubMed] [Google Scholar]
- 37.Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tandon A, Fraser P. The presenilins. Genome Biol. 2002;3:reviews3014. doi: 10.1186/gb-2002-3-11-reviews3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dermaut B, Kumar-Singh S, Engelborghs S, Theuns J, Rademakers R, Saerens J, Pickut BA, Peeters K, van den, Broeck M, Vennekens K, Claes S, Cruts M, Cras P, Martin JJ, Van Broeckhoven C, De Deyn PP. A novel presenilin 1 mutation associated with Pick’s disease but not beta-amyloid plaques. Ann. Neurol. 2004;55:617–626. doi: 10.1002/ana.20083. [DOI] [PubMed] [Google Scholar]
- 40.Raux G, Gantier R, Thomas-Anterion C, Boulliat J, Verpillat P, Hannequin D, Brice A, Frebourg T, Campion D. Dementia with prominent frontotemporal features associated with L113P presenilin 1 mutation. Neurology. 2000;55:1577–1578. doi: 10.1212/wnl.55.10.1577. [DOI] [PubMed] [Google Scholar]
- 41.Ionescu L, Cheung KH, Vais H, Mak DO, White C, Foskett JK. Graded recruitment and inactivation of single InsP3 receptor Ca2+-release channels: implications for quantal Ca2+ release. J. Physiol. 2006;573:645–662. doi: 10.1113/jphysiol.2006.109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ionescu L, White C, Cheung KH, Shuai J, Parker I, Pearson JE, Foskett JK, Mak DO. Mode switching is the major mechanism of ligand regulation of InsP3 receptor calcium release channels. J. Gen. Physiol. 2007;130:631–645. doi: 10.1085/jgp.200709859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li C, Wang X, Vais H, Thompson CB, Foskett JK, White C. Apoptosis regulation by Bcl-x(L) modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc. Natl. Acad. Sci. U.S.A. 2007;104:12565–12570. doi: 10.1073/pnas.0702489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaimovich E, Mattei C, Liberona JL, Cardenas C, Estrada M, Barbier J, Debitus C, Laurent D, Molgo J, Xestospongin B. a competitive inhibitor of IP3-mediated Ca2+ signalling in cultured rat myotubes isolated myonuclei and neuroblastoma (NG108−15) cells. FEBS Lett. 2005;579:2051–2057. doi: 10.1016/j.febslet.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 45.White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat. Cell. Biol. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grossmann A, Kukull WA, Jinneman JC, Bird TD, Villacres EC, Larson EB, Rabinovitch PS. Intracellular calcium response is reduced in CD4+ lymphocytes in Alzheimer’s disease and in older persons with Down’s syndrome. Neurobiol. Aging. 1993;14:177–185. doi: 10.1016/0197-4580(93)90094-r. [DOI] [PubMed] [Google Scholar]
- 47.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 48.Kang DE, Soriano S, Xia X, Eberhart CG, De Strooper B, Zheng H, Koo EH. Presenilin couples the paired phosphorylation of beta-catenin independent of axin: implications for beta-catenin activation in tumorigenesis. Cell. 2002;110:751–762. doi: 10.1016/s0092-8674(02)00970-4. [DOI] [PubMed] [Google Scholar]
- 49.Repetto E, Yoon IS, Zheng H, Kang DE. Presenilin 1 regulates epidermal growth factor receptor turnover and signaling in the endosomal-lysosomal pathway. J Biol Chem. 2007;282:31504–31516. doi: 10.1074/jbc.M704273200. [DOI] [PubMed] [Google Scholar]
- 50.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Green KN, LaFerla FM. Linking calcium to Abeta and Alzheimer’s disease. Neuron. 2008;59:190–194. doi: 10.1016/j.neuron.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 52.Hayley M, Perspicace S, Schulthess T, Seelig J. Calcium enhances the proteolytic activity of BACE1: An in vitro biophysical and biochemical characterization of the BACE1-calcium interaction. Biochim. Biophys. Acta. 2009;1788:1933–1938. doi: 10.1016/j.bbamem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 53.Pierrot N, Santos SF, Feyt C, Morel M, Brion JP, Octave JN. Calcium-mediated transient phosphorylation of tau and amyloid precursor protein followed by intraneuronal amyloid-beta accumulation. J. Biol. Chem. 2006;281:39907–39914. doi: 10.1074/jbc.M606015200. [DOI] [PubMed] [Google Scholar]
- 54.Querfurth HW, Selkoe DJ. Calcium ionophore increases amyloid beta peptide production by cultured cells. Biochemistry. 1994;33:4550–4561. doi: 10.1021/bi00181a016. [DOI] [PubMed] [Google Scholar]
- 55.Mattson MP. Antigenic changes similar to those seen in neurofibrillary tangles are elicited by glutamate and Ca2+ influx in cultured hippocampal neurons. Neuron. 1990;4:105–117. doi: 10.1016/0896-6273(90)90447-n. [DOI] [PubMed] [Google Scholar]
- 56.Mattson MP, Lovell MA, Ehmann WD, Markesbery WR. Comparison of the effects of elevated intracellular aluminum and calcium levels on neuronal survival and tau immunoreactivity. Brain Res. 1993;602:21–31. doi: 10.1016/0006-8993(93)90236-g. [DOI] [PubMed] [Google Scholar]
- 57.Kang DE, Soriano S, Frosch MP, Collins T, Naruse S, Sisodia SS, Leibowitz G, Levine F, Koo EH. Presenilin 1 facilitates the constitutive turnover of beta-catenin: differential activity of Alzheimer’s disease-linked PS1 mutants in the beta-catenin-signaling pathway. J. Neurosci. 1999;19:4229–4237. doi: 10.1523/JNEUROSCI.19-11-04229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang DE, Yoon IS, Repetto E, Busse T, Yermian N, Ie L, Koo EH. Presenilins mediate phosphatidylinositol 3-kinase/AKT and ERK activation via select signaling receptors. Selectivity of PS2 in platelet-derived growth factor signaling. J. Biol. Chem. 2005;280:31537–31547. doi: 10.1074/jbc.M500833200. [DOI] [PubMed] [Google Scholar]
- 59.Qin F, Auerbach A, Sachs F. Hidden Markov modeling for single channel kinetics with filtering and correlated noise. Biophys. J. 2000;79:1928–1944. doi: 10.1016/S0006-3495(00)76442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin F, Auerbach A, Sachs F. A direct optimization approach to hidden Markov modeling for single channel kinetics. Biophys. J. 2000;79:1915–1927. doi: 10.1016/S0006-3495(00)76441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Magleby KL, Pallotta BS. Burst kinetics of single calcium-activated potassium channels in cultured rat muscle. J. Physiol. 1983;344:605–623. doi: 10.1113/jphysiol.1983.sp014958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.We thank Drs. Rod Eckenhoff and Huafeng Wei for supplying mice, and Dustin Shilling for assistance with the culturing of the PS DKO MEF cells. Acknowledgement is made to the donors of ADR, a program of the American Health Assistance Foundation (A2008-137 to J.K.F.), the Alzheimer’s Association (IIRG-08-91662 to D.E.K) and to Core Research for Evolutional Science and Technology of JST, Japan (T.I.). K.-H.C. designed and performed the experiments, analyzed data and wrote the manuscript. L.M. developed recombinant baculoviruses, and performed infections, transfections and cell culture. D.-O.D.M. developed software for modal gating and single cell Ca2+ analyses, and assisted in the analyses. I.H. and T.I. developed recombinant baculoviral PS constructs. D.E.K. developed DKO MEF cells. J.K.F. designed and analyzed experiments and wrote the manuscript. None of the authors have competing interests.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.