Abstract
Envelopment of a herpesvirus particle is a complex process of which much is still to be learned. We previously identified the glycoprotein gpUL132 of human cytomegalovirus (HCMV) as an envelope component of the virion. In its carboxy-terminal portion, gpUL132 contains at least four motifs for sorting of transmembrane proteins to endosomes; among them are one dileucine-based signal and three tyrosine-based signals of the YXXØ and NPXY (where X stands for any amino acid, and Ø stands for any bulky hydrophobic amino acid) types. To investigate the role of each of these trafficking signals in intracellular localization and viral replication, we constructed a panel of expression plasmids and recombinant viruses in which the signals were rendered nonfunctional by mutagenesis. In transfected cells wild-type gpUL132 was mainly associated with the trans-Golgi network. Consecutive mutation of the trafficking signals resulted in increasing fractions of the protein localized at the cell surface, with gpUL132 mutated in all four trafficking motifs predominantly associated with the plasma membrane. Concomitant with increased surface expression, endocytosis of mutant gpUL132 was reduced, with a gpUL132 expressing all four motifs in mutated form being almost completely impaired in endocytosis. The replication of recombinant viruses harboring mutations in single trafficking motifs was comparable to replication of wild-type virus. In contrast, viruses containing mutations in three or four of the trafficking signals showed pronounced deficits in replication with a reduction of approximately 100-fold. Moreover, recombinant viruses expressing gpUL132 with three or four trafficking motifs mutated failed to incorporate the mutant protein into the virus particle. These results demonstrate a role of endocytosis of an HCMV envelope glycoprotein for incorporation into the virion and optimal virus replication.
Morphogenesis of herpesviruses is a complex multistep process which requires the assembly of an infectious viral particle containing >70 proteins. The proteins are organized into distinct regions in the particle, including the capsid containing viral DNA, the tegument, and the envelope of the virion, suggesting an ordered addition of viral proteins during assembly.
Most data in the literature favor a model in which the final virion is assembled by multiple budding and fusion events of the nascent particle. According to this model, genome-containing capsids acquire a first envelope by budding at the inner nuclear membrane in a step termed primary envelopment (34). By fusion of the primary envelope with the outer nuclear membrane, capsids are stripped from their envelope and translocated into the cytoplasm, where they accumulate tegument proteins through a poorly understood pathway (33). The final envelopment takes place when tegumented capsids bud into cytoplasmic membranes derived from compartments of the secretory pathway, including possibly the trans-Golgi network (TGN) and/or endosomal recycling compartment. Although incompletely defined, the corresponding cellular compartment in which envelopment takes place has been operationally defined as the assembly compartment (AC), and the process has been termed secondary envelopment (46).
For this process to yield infectious virions, all components of the mature viral envelope need to be present in the AC. Herpesviruses in general contain a complex envelope; in the case of human cytomegalovirus (HCMV) this includes at least 20 different proteins (58). It can be assumed that a high degree of spatial and temporal organization is required to localize all of these proteins to the correct compartment for incorporation into virions. An important question in the context of secondary envelopment is the intracellular transport pathway of membrane-associated glycoproteins that localize these viral proteins to the AC.
Herpesviral envelope glycoproteins can be transported to the plasma membrane of infected cells by a default pathway, and some of these proteins appear to exhibit functional activities once localized to the plasma membrane, such as complement binding or IgG Fc binding (18, 27, 51, 52). For a large number of envelope glycoproteins, however, it has been shown that they follow an internalization pathway after having reached the plasma membrane and accumulate in interior compartments of the cell, such as the TGN or vesicles derived from this cellular structure (1, 8, 9, 9, 17, 30, 42, 43). A well-studied member of this group of proteins is glycoprotein B (gB), which has been shown for a number of herpesviruses to be internalized from the plasma membrane and incorporated into the virion (4, 14, 21, 45, 57). However, the functional relevance of endocytosis for viral replication and/or pathogenesis is less clear. Endocytosis of herpesvirus glycoproteins has been implicated, for example, in cell-cell spread and evasion from the humoral immune response (16, 44).
Endocytosis of membrane proteins involves the interaction of the cytoplasmic tails of the respective proteins with intracellular adaptor proteins that mediate recruitment of the proteins to clathrin-coated pits for internalization (6). Two types of motifs have been shown to be of predominant importance: tyrosine-based YXXØ-type motifs (where Y stands for tyrosine, X stands for any amino acid, and Ø stands for any bulky hydrophobic amino acid) and LL (dileucine) motifs (6). The individual motifs are recognized with characteristic fine specificity by the adaptor protein (AP) complexes (AP-1, AP-2, AP-3, and AP-4) and by another family of adaptors designated GGAs (for Golgi-localized, gamma-ear containing, ARF binding) (48).
It is unknown whether all herpesviral envelope proteins containing potential motifs for retrieval from the plasma membrane will traffic through this pathway. In addition, whether internalization of glycoproteins affects the production of infectious viral particles is also largely unknown. For herpesviral glycoproteins that have been studied in detail, the results differ significantly between individual herpesviruses and specific viral glycoproteins. For example, endocytosis of gE of varicella-zoster virus (VZV) has been shown to be essential for viral replication, whereas it is irrelevant for replication of pseudorabies virus (PRV) (37, 55). For gB of several herpesviruses, endocytosis seems to be dispensable for virion formation since its inhibition has little effect on replication kinetics (3, 40). In the case of HCMV little information is available on endocytosis of envelope glycoproteins. Radsak and colleagues were the first to show that gB is retrieved from the infected cell surface prior to incorporation into the virion (45). These findings were confirmed by others (56). However, subsequent studies suggested that inhibition of endocytosis did not affect the production of infectious virus, indicating that endocytosis of gB is not required for production of infectious HCMV. Jarvis et al. reported that the expression of a dominant negative dynamin I protein that blocks clathrin-dependent endocytosis had no influence on total yield of HCMV during productive infection (23). Similarly, we have recently shown that endocytosis of gM, another HCMV envelope glycoprotein that contains an YXXØ motif in its carboxy-terminal domain, is not affected by deletion of the motif (25). Thus, available evidence suggests that endocytosis of envelope glycoproteins from the plasma membrane likely has a minor, if any, role in replication of HCMV in permissive cells in culture.
We have previously identified gpUL132, an envelope glycoprotein of HCMV which has no homologous counterparts in other herpesviruses and which contains multiple YXXØ and LL motifs for endocytosis in its cytoplasmic protein domain. In transfected cells the protein is exclusively localized within the TGN, and in infected cells it colocalizes with the AC (50). In this report we demonstrate that consecutive mutation of the endocytosis-associated motifs resulted in a corresponding increase in gpUL132 on the surface of transfected cells and nearly complete abrogation of endocytosis when all potential motifs were mutated. Recombinant viruses that expressed endocytosis-negative forms of gpUL132 showed similar replication deficits as a UL132 viral deletion mutant. In addition, forms of gpUL132 which failed to undergo endocytosis were not incorporated into virions. Our results indicated that for HCMV, endocytosis of gpUL132 represented an important pathway for incorporation of this envelope glycoprotein in virion assembly and virus replication.
MATERIALS AND METHODS
Cells and viruses.
Virus strains were propagated in primary human foreskin fibroblasts (HFF) grown in minimal essential medium supplemented with 5% fetal calf serum (FCS), glutamine (100 mg/liter), and gentamicin (350 mg/liter). Virions were isolated by glycerol-tartrate gradient centrifugation as described previously (53). HeLa cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% FCS, glutamine, and gentamicin. For immunofluorescence analysis, HeLa cells were grown in 24-well plates on 13-mm glass coverslips.
Preparation of eukaryotic expression constructs.
The construction of plasmid pcUL132 has been described before (50). To introduce single or multiple mutations within pcUL132, we used a combined chain reaction (CCR) which utilizes DNA amplification via PCR and ligation in a single reaction (5). A list of primers is provided in Table S1 in the supplemental material. Amplimers were inserted into pcDNA3.1 vector (Invitrogen) via BamHI and HindIII. To construct expression plasmids for UL132 containing a hemagglutinin ([HA] UL132HA) epitope, we first generated a plasmid containing the coding sequence of the UL132 authentic signal sequence (amino acids [aa] 1 to 27 of gpUL132). To this end, the signal sequence was first inserted into pcDNA3.1 via restriction sites HindIII and BamHI using the primers ul132-5-hind and ul132-sig-bam, giving rise to the plasmid pcUL132sig. The coding sequence for the HA epitope YPYDVPDYA was then inserted as a double-stranded oligonucleotide having BamHI and EcoRI single-stranded 5′ overhanging sequences into the BamHI and EcoRI sites of pcUL132sig, resulting in plasmid pcUL132sigHA. Finally, the coding sequence for gpUL132 and the mutant proteins was PCR amplified from the already existing pcUL132 wild-type (wt) and mutant plasmids and inserted into the EcoRI and NotI sites of pcUL132sigHA. The integrity of all constructed plasmids was confirmed by nucleotide sequencing. The GGA-1-GFP (where GFP is green fluorescent protein) plasmid was a kind gift of J. Bonifacino, NIH.
BAC mutagenesis and reconstitution of recombinant viruses.
Recombinant viruses on the genetic background of strain AD169 were constructed using bacterial artificial chromosome (BAC) HB5 (7). The recombinant viruses were generated by site-directed mutagenesis using a PCR-generated fragment electroporated into Escherichia coli strain DH10B harboring the HB5 BAC and expressing bacteriophage λ functions redαβγ from plasmid pBADαβγ (38). First, using conventional cloning procedures, the plasmid pcpoUL132HA was constructed. It contained the open reading frames (ORFs) UL131/Kan/UL132HA/IRL14 in the vector backbone of pcpo15-Link2 (11). A second pcpo15-based plasmid was also constructed containing the reading frames UL131/Kan/UL132m1-3HA/IRL14 (designated pcpoUL132m1-3HA). From these plasmids DNA fragments used for recombination were generated by PCR using the primers UL132M2-5 and UL132RV (where RV indicates recombinant virus). Following homologous recombination in E. coli, colonies carrying kanamycin and chloramphenicol resistance were selected. The resulting BACs were designated BAC-UL132HA and BAC-UL132m1-3HA, respectively. To construct BAC-UL132m3HA a recombination fragment was generated using primers UL132M2-5 and RV132mut3solo from template pcpoUL132HA; for BAC-UL132m4HA primers UL132M2-5 and RV132m1-4 and the template pcpUL132HA were used. The recombination fragment for BAC-UL132m1-4 was constructed from template pcpoUL132m1-3HA using the primers UL132M2-5 and RV132m1-4. For all BACs the correct insertion of the recombination cassette into the HCMV genome was verified by restriction enzyme digest and nucleotide sequencing of the flanking regions, including UL131 and IRL14. The BACs were used for transfection of fibroblasts and reconstitution of the respective infectious virus as described previously (50).
For replication analysis, HFF plated in six-well dishes were infected at a multiplicity of infection (MOI) of approximately 0.01. After adsorption of virus (4 h), the inoculum was removed and replaced by fresh medium. Supernatants were harvested at the time points indicated in the figures and stored at room temperature until use. Virus titers were determined by an indirect immunofluorescence assay using a monoclonal antibody (MAb) against the immediate-early protein IE1 of HCMV as described previously (2).
Construction and expression of chimeric TrkB/gpUL132 proteins.
A plasmid encoding the full-length rat TrkB sequence including a myc tag in the amino terminus was kindly provided by David Kaplan (Hospital for Sick Children, Toronto, Canada) and was used as the template for construction of the chimeric protein (31). Primers were designed to amplify codons 1 to 152 of TrkB and codons 107 to 270 of either wild-type UL132 or the mutant UL132m1-4, in which four predicted trafficking motifs were mutated. Compatible restriction sites were engineered into each product to permit ligation and construction of the fusion product of the two gene segments. The fusion genes were cloned into pcDNA4 (Invitrogen) and, after nucleotide sequencing to confirm the predicted sequence, were transiently expressed in 293 HEK cells to document expression of the expected protein. Expression in virus-infected HFF was carried out by a transfection/infection protocol previously described (26). Briefly, HFF were electroporated with the respective plasmid using Amaxa nucleofection (Lonza, Walkersville, MD) and subsequently infected with HCMV strain AD169 at an MOI of about 0.5. Virus-infected cells were subjected to antibody internalization assays as described below, and images were collected using an Olympus confocal microscope. In some cases larger numbers of cells were used to generate extracellular virus and virus-infected cells for analysis by immunoblotting. Extracellular virus was prepared by initially preclearing cell-free supernatant from transfected/infected cells, followed by pelleting the extracellular particles by high-speed centrifugation at 18,000 rpm for 70 min at 8°C.
Transient protein expression and image analysis.
HeLa cells grown on glass coverslips in 24-well plates were transfected with 0.8 μg of plasmid DNA using Lipofectamine (Invitrogen). Fibroblasts, also grown on glass coverslips in 24-well plates, were infected with the respective viruses at an MOI of 0.01. At the time points indicated in the figures. The coverslips were washed and fixed in 3% paraformaldehyde in phosphate-buffered saline (PBS). The fixed cells were permeabilized with Triton X-100 containing buffer and then blocked with PBS-1% bovine serum albumin (BSA). Primary antibodies, including a HA-specific MAb (Sigma-Aldrich) and sheep anti-TGN46 (Serotec), were then added. After samples were washed, antibody binding was detected with the appropriate secondary antibody conjugated with either fluorescein isothiocyanate (FITC) or tetramethyl rhodamine isothiocyanate (TRITC) (Dianova). Images were collected using a Zeiss Axioplan 2 fluorescence microscope fitted with a Visitron Systems charge-coupled device (CCD) camera (Puchheim, Germany) and processed using MetaView software and Adobe Photoshop.
Antibody internalization.
For antibody internalization, cells were washed for 5 min in PBS at room temperature, followed by an additional washing step with PBS at 4°C for 5 min. Then the cells were incubated with the first antibody for 60 min at 4°C in DMEM-2% fetal calf serum. For internalization, cells were first washed three times with ice-cold PBS-0.2% BSA and immediately fixed with 3% paraformaldehyde (0-min time point). To allow antibody internalization, cells were washed with DMEM-10% FCS at 37°C, followed by incubation at 37°C for the time intervals specified in the figures. Cells were then washed three times with PBS and fixed with 3% paraformaldehyde. To permeabilize cells, they were incubated for 4 min with PBS containing 0.1% Triton X-100. Cells were washed two times with PBS and incubated for 15 min in PBS containing 1% BSA, followed by the second antibody for 30 min.
Flow cytometry.
Single-cell suspensions were prepared from transfected or infected cells at the indicated time points and washed twice with PBS. The cell pellet was resuspended in 200 μl of buffer (5% FCS and 0.02% NaN3 in PBS) at 4°C and incubated for 10 min. To stain surface-exposed gpUL132, cells were incubated with an R-phycoerythrin (PE)-conjugated anti-HA antibody (Miltenyi Biotec) for 30 min at 4°C. Then the cells were fixed using Fix reagent (Invitrogen) and analyzed by flow cytometry using a FACSCalibur (Becton Dickinson). To stain for total gpUL132 protein, cells were treated with Fix and Perm reagent (Invitrogen) before the addition of the anti-HA antibody.
SDS-PAGE and immunoblotting.
Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% polyacrylamide gels under standard conditions. Proteins were transferred to nitrocellulose membranes, and membranes were blocked with PBS containing 0.1% Tween 20 and 5% powdered milk. Antibodies and sera were diluted in PBS containing 0.1% Tween 20 and 0.1% Tween 20-5% powdered milk, respectively. For detection of primary antibody binding, horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG antibody and an enhanced chemiluminescence detection system (Pharmacia Biotech) were used according to the manufacturer's instructions. Blots were stripped according to the manufacturer's protocol and reprobed with a monoclonal antibody specific for the major capsid protein of HCMV.
RESULTS
Mutation of sorting motifs in gpUL132 results in altered intracellular distribution.
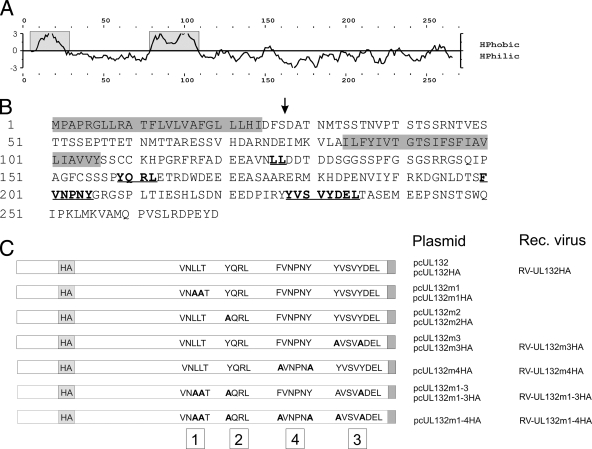
Within the carboxy-terminal domain of gpUL132, there are a number of amino acid motifs predicted to serve as signals for the intracellular sorting of transmembrane proteins (Fig. 1 B). These signals include a dileucine-based signal (LL127) and several tyrosine-based sorting signals (YQRL162, FVNPNY205, YVSV231, and YDEL235). All of these signals are potentially involved in retrograde transport of transmembrane proteins from the plasma membrane to the endosomal-lysosomal system (reviewed in reference 6). The redundancy of the sorting signals within the cytoplasmic tail of gpUL132 indicates that if all of these signals were functional, the intracellular traffic of gpUL132 would be tightly regulated.
FIG. 1.
Hydrophilicity profile, primary amino acid sequence, and summary of gpUL132 mutants that were used in this study. (A) Computer prediction of the hydrophilicity (HPhilic) of the gpUL132 polypeptide. The potential signal and membrane anchor sequences are indicated by boxes. HPhobic, hydrophobicity. (B) Primary amino acid sequence of gpUL132. The sorting signals which are relevant for this work are underlined and printed in bold. The potential signal and membrane anchor sequences are indicated by a shaded background. An arrow indicates the insertion site for the HA tag. (C) Cartoon of plasmids and recombinant viruses used in this study. The complete UL132 ORF is represented as an open bar. The individual sorting motifs and the respective mutant motifs are indicated and numbered from 1 to 4. The amino acid sequence YVSYDEL, which theoretically harbors the two sorting motifs YVSV231 and YDEL235, was treated as a single motif (motif 3). The positions of the amino-terminal HA tag and the carboxy-terminal myc tag (gray box) are indicated.
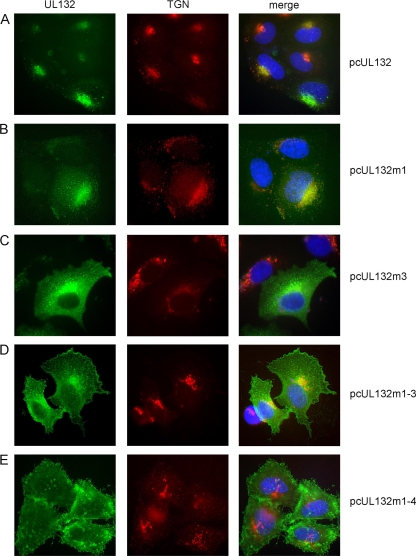
In order to analyze the contribution of the individual sorting signals to the intracellular distribution of gpUL132, we constructed a panel of expression plasmids that would give rise to proteins with mutations within the tyrosine-based sorting signals as well as the dileucine-based signal (Fig. 1C). The respective DNA fragments were inserted into the expression vector pcDNA3.1-myc/his allowing for the synthesis of recombinant gpUL132 proteins containing a myc epitope at the carboxyl terminus. The addition of the epitope tag did not influence the intracellular distribution of wt gpUL132 (data not shown). The plasmids were tested for gpUL132 expression and intracellular localization of the protein in transient expression assays using HeLa cells and indirect immunofluorescence for protein detection. As noted before, when expressed in the absence of other viral functions, wt gpUL132 colocalized almost exclusively with markers for the TGN (Fig. 2 A) (50). Mutation of the sorting motifs resulted in a gradual increase of the signal from gpUL132 that was initially almost completely colocalized with the TGN to areas outside the TGN (Fig. 2). The effect was subtle when the sorting motifs were mutated individually, i.e., mutations of the LL127 (UL132m1) motif or the YQRL162 (UL132m2) motif had little impact on the intracellular distribution of gpUL132. Only a slightly more diffuse signal compared to that of the wt protein was noted in the majority of the gpUL132-expressing cells (Fig. 2B and data not shown). In contrast, the combined deletion of the YVSV231 and YDEL235 (UL132m3) motifs resulted in a profound alteration in the intracellular distribution of gpUL132 (Fig. 2C). A more diffuse distribution of the UL132m3 mutant was noted, which was suggestive of surface expression. When the tyrosine-based motifs and the dileucine motif were mutated in combination (UL132m1-3) the boundary of the gpUL132-expressing cells became clearly visible, indicating extensive surface expression of the protein (Fig. 2D). In addition, little colocalization with the TGN was evident. When all four sorting signals were mutated (UL132m1-4), colocalization of gpUL132 with the TGN was completely abolished (Fig. 2E). Similar data were obtained following transfection of the plasmids into human fibroblasts, indicating that the observed effect was not cell type specific (data not shown).
FIG. 2.
Intracellular localization of wt gpUL132 and gpUL132 mutant proteins. HeLa cells were transfected with the indicated plasmids, and protein localization was assayed 48 h later by indirect immunofluorescence in permeabilized cells. gpUL132 was detected using as primary antibody an anti-myc antibody, and the TGN was detected by a polyclonal sheep serum against TGN46. Appropriate secondary antibodies were used to visualize binding of the primary antibody. The appearance of yellow in the merged pictures indicates colocalization of signals.
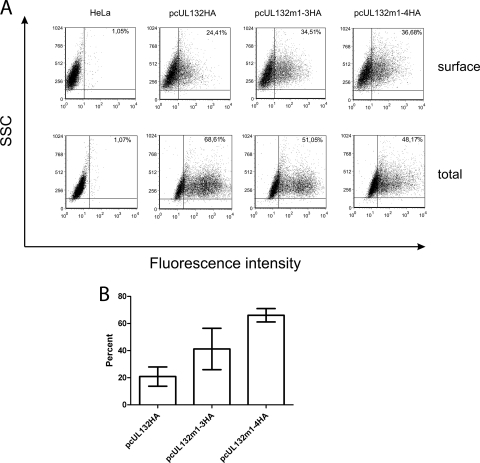
Flow cytometry was used to obtain more quantitative data of cell surface expression of gpUL132. To enable cell surface detection of the protein, a series of plasmids was constructed which allowed expression of a gpUL132 protein containing a hemagglutinin (HA) epitope tag at the amino terminus (gpUL132HA) (Fig. 1). Addition of the HA tag did not alter the intracellular distribution of the gpUL132 protein, as judged by indirect immunofluorescence analysis (data not shown). HeLa cells were transfected with the respective expression plasmids and stained with anti-HA antibody for gpUL132 exposed on the cells' surfaces and for total gpUL132 separately. In gpUL132-expressing cells, approximately 20% of the total gpUL132 protein was detected on the cell surface (Fig. 3). This percentage increased to 40% and 66% in cells expressing gpUL132m1-3HA and gpUL132m1-4HA, respectively. Thus, flow cytometry verified our previous results and further demonstrated intracellular relocalization of gpUL132 following deletion of the sorting motifs in the cytoplasmic tail.
FIG. 3.
Flow cytometry of wt gpUL132 and gpUL132 mutant protein after transient expression. HeLa cells were transfected with the indicated plasmids and subjected to flow cytometry analysis 48 h later. (A) Intact HeLa cells were stained with PE-conjugated anti-HA antibody and analyzed for cell surface expression of gpUL132 protein (top). Cells were analyzed in samples that were permeabilized before the addition of the anti-HA antibody (bottom). The percentage of cells staining for gpUL132 is indicated in the upper-right quadrant of each graph. SSC, side scatter. (B) Percentage of total gpUL132 detected on the surface of transfected cells. Values are the mean and range of three independent experiments.
gpUL132 undergoes endocytosis.
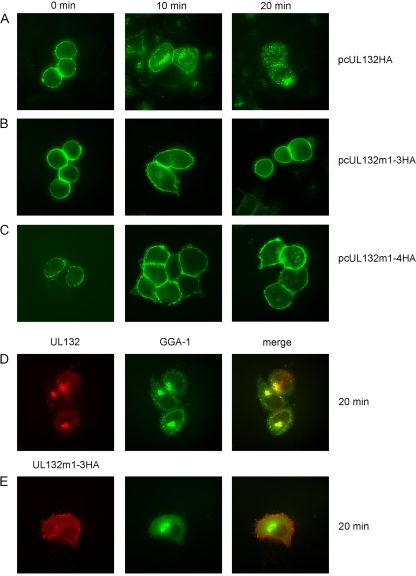
The possibility that gpUL132 is transported to the TGN by retrograde transport from the cell surface was analyzed next using an antibody-feeding protocol. HeLa cells were transfected with the respective plasmids, incubated 48 h after transfection with an anti-HA antibody at 4°C for 60 min, and then shifted to 37°C to allow internalization of the gpUL132-antibody complexes from the plasma membrane. In cells that were not shifted to 37°C, gpUL132HA and the mutant proteins were detected exclusively at the surfaces of cells (Fig. 4). Ten minutes after the shift to 37°C, wt gpUL132HA protein started to accumulate in numerous intracellular vesicles dispersed throughout the cell (Fig. 4A). Some of the vesicles accumulated in a perinuclear compartment. Surface localization was still detectable. At 20 min after the shift to 37°C most of the protein was no longer detectable at the surfaces of cells (Fig. 4A). In contrast to wt gpUL132HA, mutant proteins gpUL132m1-3HA and gpUL132m1-4HA remained at the cell surface during the 20-min observation period (Fig. 4B and C). The intracellular compartment to which gpUL132 was retrieved was analyzed by cotransfection of a plasmid expressing GGA-1-GFP, a cellular protein associated with the TGN (20). At 20 min after induction of endocytosis, gpUL132 showed considerable overlap with the GGA-1 signal, indicating that most of the protein underwent retrograde transport to the TGN (Fig. 4D). In contrast, mutant proteins gpUL132m1-3HA and gpUL132m1-4HA showed no colocalization with GGA-1 (Fig. 4E and data not shown). Overall, these data indicated that wt gpUL132 can reach the TGN via endocytosis from the plasma membrane and suggested that the trafficking motifs present within the carboxy-terminal part of the protein were responsible for the intracellular trafficking itinerary of gpUL132.
FIG. 4.
Endocytosis of wt gpUL132 and gpUL132 mutant proteins in transfected cells. (A to C) HeLa cells were transfected with the indicated plasmids and 48 h later incubated with anti-HA antibody at 4°C. To allow internalization of the gpUL132-IgG complex, cells were shifted to 37°C for the indicated times. Cells were fixed with paraformaldehyde, permeabilized, and incubated with FITC-conjugated anti-mouse antibody. (D and E) HeLa cells were cotransfected with gpUL132- and GGA-1-GFP-expressing plasmids as indicated. gpUL132 was labeled at the cell surface at 4°C with an anti-HA antibody and allowed to internalize for 20 min at 37°C. Following fixation and permeabilization of the cells, gpUL132 was detected using a Cy3-conjugated anti-HA antibody and GGA-1 by GFP fluorescence.
Replication of recombinant viruses expressing gpUL132 mutant proteins.
Since the combined mutation of the trafficking signals in gpUL132 resulted in a protein which was inefficiently retrieved from the cell surface, we could directly determine if endocytosis of gpUL132 was important for virus replication.
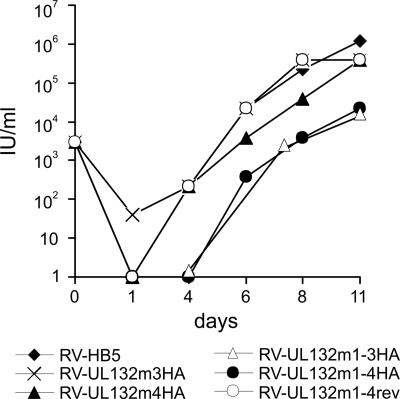
To test this possibility, a number of recombinant viruses in the genetic background of HCMV strain AD169 were constructed (7). In RV-UL132HA the ORF UL132 was replaced by the ORF UL132HA from the plasmid UL132HA, giving rise to a gpUL132 protein containing an HA epitope at the amino terminus and a myc epitope at the carboxyl terminus. This virus replicated with similar efficiency in human fibroblasts as the parent virus RV-HB5 originally described by Borst et al. (7), indicating that addition of the HA tag to gpUL132 had no influence on the replicative capacity of the virus (data not shown). RV-UL132m3HA and RV-UL132m4HA carried mutations in the YVSVYDEL235 and the FVNPNY205 motifs, respectively. RV-UL132m1-3HA and RV-UL132m1-4HA contained the UL132 reading frames from plasmids UL132m1-3HA and UL132m1-4HA harboring mutations in three and four trafficking signals, respectively (Fig. 1C). Recombinant viruses containing mutations in a single trafficking signal replicated with an efficiency similar to that of wt virus (Fig. 5). In contrast, production of infectious virus from cells infected with RV-UL132m1-3HA and RV-UL132m1-4HA was reduced by approximately 2 logs at day 11 postinfection (Fig. 5B) and was maintained at later time points (data not shown). The replication deficit was similar to that observed in RV-delUL132/AD, a UL132 deletion virus that has previously been shown to be impaired in replication (50). A revertant virus (RV-UL132m1-4rev), constructed from the RV-UL132m1-4HA BAC, replicated with an efficiency similar to that of the wt virus, indicating that the replication deficit was not the result of a second site mutation in RV-UL132m1-3HA or RV-UL132m1-4HA. From these findings, we concluded that the capacity of gpUL132 to be retrieved from the cell surface of virus-infected cells appeared to be required for the efficient assembly of infectious virus.
FIG. 5.
Replication of UL132 mutant recombinant viruses. Fibroblasts were seeded in six-well dishes and infected with the indicated recombinant viruses. At the indicated days postinfection, supernatants from the infected cultures were harvested, and infectious virus was titrated using indirect immunofluorescence with an antibody directed against IE1. IU, infectious units. The experiment was repeated three times, and a representative result is shown.
Endocytosis of gpUL132 mutant proteins in infected cells.
The replication characteristics of the recombinant viruses expressing mutant gpUL132 proteins indicated that a deficit in intracellular trafficking of gpUL132 results in reduced virus production. Whether in fact a defect in endocytosis was the underlying mechanism remained to be shown since results that were obtained using isolated expression of gpUL132 in transfected cells did not necessarily indicate that similar phenomena occur in HCMV-infected cells. In infected cells a plethora of virally encoded proteins or cellular proteins with virus-modified function and levels of expression could affect transport of gpUL132 and potentially override the effect that was observed in transfected HeLa cells. To analyze the intracellular distribution of the respective gpUL132 proteins, HFF were infected for 6 days with RV-UL132HA, RV-UL132m1-3HA, and RV-UL132m1-4HA, respectively, and the proteins were detected using an anti-HA antibody.
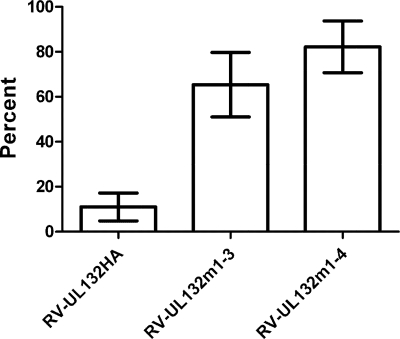
Using flow cytometry, we determined the ratio of total gpUL132 protein to surface-exposed protein in five independent experiments. In cells infected with RV-UL132HA approximately 11% of total gpUL132 was detectable at the cell surface (Fig. 6). In contrast, in cells infected with the mutant viruses RV-UL132m1-3HA and RV-UL132m1-4HA, an average of 65% and 82% of total gpUL132 was detected at the cell surface, respectively.
FIG. 6.
Flow cytometry of gpUL132 mutant proteins in infected cells. Fibroblasts were infected with the indicated recombinant viruses and subjected to flow cytometry 92 h later. Cells were analyzed for surface-exposed and total gpUL132, and the fraction of surface-exposed gpUL132 is shown. Data are the mean and range of five independent experiments.
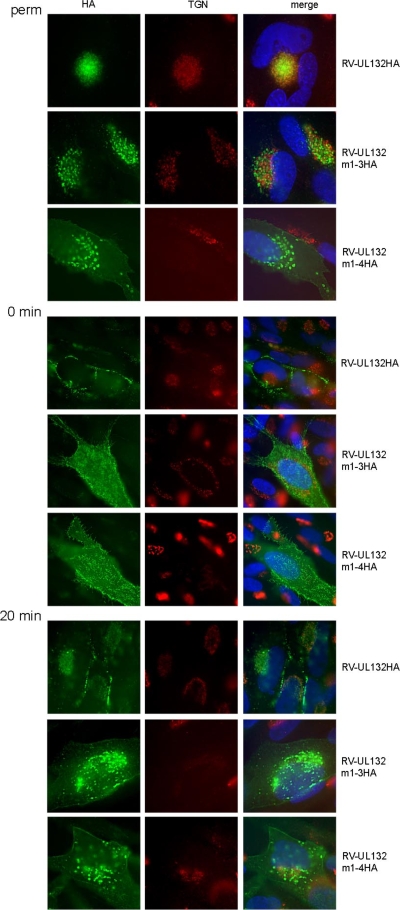
Next, we analyzed the intracellular distribution of gpUL132 by indirect immunofluorescence. When cells were fixed and permeabilized before the addition of antibody, wt gpUL132HA was detected in a fine-grained signal that was concentrated in an area also stained by an anti-TGN46 antibody (Fig. 7). However, the signals from both proteins were not completely overlapping. No surface staining was evident. In contrast, cells expressing the mutant gpUL132 proteins showed cell surface staining. In addition, the intracellular localization and appearance of the signals differed from those of wt gpUL132HA-expressing cells. The punctate signals were more widely distributed throughout the cytoplasm of the cell but still in an area close to the nucleus. Also, the signals were larger and had a doughnut shape, with larger vesicles in cells expressing gpUL132m1-4HA. Together with the change in intracellular gpUL132 distribution, the signal from the TGN was also altered, and in some of the cells that were infected with the gpUL132 mutant viruses, the TGN was hardly detectable, a phenomenon that is generally associated with HCMV infection in fibroblasts (22, 29).
FIG. 7.
Endocytosis of wt gpUL132 and gpUL132 mutant proteins in infected cells. Fibroblasts were infected with the indicated recombinant viruses for 120 h. Cells were fixed with paraformaldehyde and permeabilized before addition of the antibodies (perm). The intracellular localization of gpUL132 was detected with an anti-HA antibody. The TGN was stained using a polyclonal anti-TGN46 sheep serum. To analyze surface expression of gpUL132, cells were incubated with an anti-HA antibody at 4°C for 60 min, fixed with paraformaldehyde, and permeabilized (0 min). Following incubation with a sheep polyclonal anti-TGN46 polyclonal antibody, binding of the first antibodies was developed using appropriate secondary antibodies. Following incubation of cells at 4°C for 60 min with anti-HA antibody, gpUL132-antibody complexes were allowed to internalize for 20 min at 37°C (20 min). Thereafter, gpUL132 and TGN were stained as above.
Next, we visualized gpUL132 at the cell surface and after endocytosis (Fig. 7). Cells were cooled to 4°C, an anti-HA antibody was added to the culture medium for 60 min, and the cells were either immediately fixed or incubated at 37°C for 20 min to allow internalization of the anti-HA antibody-gpUL132 complexes from the plasma membrane. In wt gpUL132HA-expressing cells, we observed fine-grained signals at the surface which, similar to a string of pearls, were evident along the boundaries of the cells. In contrast, an exaggerated signal on the surface was observed in cells expressing gpUL132m1-3HA or gpUL132m1-4HA (Fig. 7). Twenty min after induction of endocytosis, gpUL132HA was still found at the surface in a fraction of cells, but in the majority of cells it accumulated in the cytoplasm in an area that was stained by the TGN46 antibody. The compartment where the protein accumulated was reminiscent of the staining found in permeabilized cells and likely represented the AC (Fig. 7). In cells infected with gpUL132m1-3HA or gpUL132m1-4HA, part of the protein was found to concentrate in structures similar to permeabilized cells. However, cell surface localization was also prominent. An isotype-matched control antibody did not stain the infected cells, indicating that the observed signals were independent of the viral or cellular expression of immunoglobulin Fc receptors (data not shown).
Incorporation of mutant gpUL132 protein in virus particles.
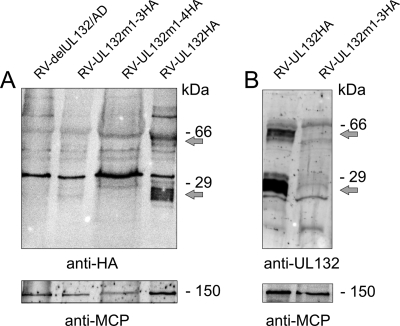
Finally, we analyzed the incorporation of the gpUL132 and mutant proteins into virus particles. Extracellular particles were purified 6 days after infection, and lysates were subjected to Western blotting using either an anti-HA antibody or a polyclonal anti-gpUL132 rabbit serum (Fig. 8). In viral particles purified from RV-UL132HA-infected cells the monoclonal anti-HA antibody stained two diffusely migrating proteins of estimated molecular masses of 22 to 28 kDa and 45 to 60 kDa, the mass of forms of gpUL132 previously documented in extracellular virus (Fig. 8A) (50). As expected, the sample from the UL132 deletion mutant virus (RV-delUL132/AD) did not show a specific reaction with the anti-HA antibody. Importantly, particles from both RV-UL132m1-3HA and RV-UL132m1-4HA did not contain detectable amounts of UL132-specific protein. A similar result was obtained using the polyclonal anti-UL132 rabbit serum (Fig. 8B). Since comparable amounts of particles were present in this analysis, as determined by reprobing the membranes with an antibody against the HCMV major capsid protein, this result indicated that gpUL132 mutant proteins which are defective in endocytosis were not incorporated into the virion.
FIG. 8.
Immunoblot analysis of recombinant viruses. Lysates of gradient-purified extracellular virus particles from the indicated recombinant virus strains were used for immunoblot analysis using either an anti-HA-specific antibody (A) or an anti-UL132 rabbit serum (B) for detection of gpUL132. The two viral forms of gpUL132 (50) are indicated by arrows. Following detection of gpUL132, the blots were stripped and developed with an antibody specific for the major capsid protein of HCMV (anti-MCP).
A chimeric protein expressing the cytoplasmic tail of gpUL132 is rapidly endocytosed and incorporated into extracellular virions in virus-infected cells.
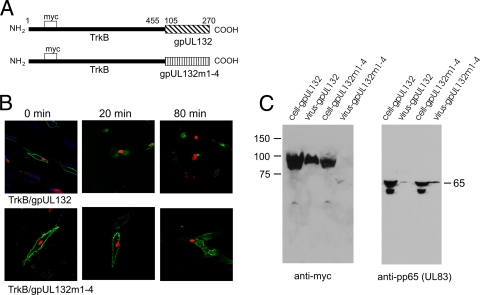
To eliminate the possibility of interactions between the extracellular and transmembrane domains of gpUL132 with other viral proteins leading to endocytosis and virion incorporation, we constructed a chimeric protein in which the extracellular and transmembrane domains of the receptor tyrosine kinase, TrkB, were fused to the cytoplasmic tail of wild-type gpUL132 or mutant gpUL132m1-4. TrkB is a type I glycoprotein that is the high-affinity receptor for brain-derived neurotrophic factor (BDNF) and undergoes tyrosine phosphorylation upon ligand binding (35, 49). We generated a chimeric protein from a TrkB construct encoding a myc tag in the amino terminus of TrkB, resulting in fusion between TrkB and the tails of gpUL132 and gpUL132m1-4 (Fig. 9A). Transient expression of plasmids encoding these constructs under the control of the HCMV IE1 promoter resulted in expression of the expected 100-kDa fusion protein detected by immunoblotting using anti-myc antibodies (data not shown). These plasmids were then transfected into HF cells using Amaxa nucleofection, and the transfected cells were infected 24 h later with HCMV. In some experiments, transfected cells/infected cells were grown on coverslips and processed in an antibody uptake/endocytosis assay. We noted expression of both the TrkB/gpUL132 wt and TrkB/gpUL132m1-4 chimeric proteins on the surfaces of transfected/infected cells at the initial time point, whereas at 20, 40, and 80 min the chimeric protein expressing the cytoplasmic tail of wild-type gpUL132 was quickly removed from the cell surface and accumulated in some cells in a perinuclear compartment (Fig. 9B). In contrast, the TrkB/gpUL132m1-4 construct remained easily detectable on the cell surface throughout the duration of the experiment (Fig. 9B). This result suggested that the phenotypes of gpUL132 and gpUL132m1-4 could be transferred to an unrelated protein by grafting of only the cytoplasmic tails of gpUL132 and of gpUL132m1-4 to the ectodomain of an unrelated protein.
FIG. 9.
Trafficking and virion incorporation of a TrkB/gpUL132 chimeric protein. (A) Cartoon illustrating the domains of the chimeric proteins. (B) Antibody internalization endocytosis assay of chimeric forms of TrkB molecules. HF cells, grown on 13-mm coverslips, were electroporated with the respective chimeric proteins as described in the text and infected with HCMV. On day 5 postinfection, cells were cooled to 4°C and incubated with an anti-myc MAb for 2 h. The cell cultures were then washed several times with warm medium, and individual coverslips were harvested at the indicated time points. After fixation in paraformaldehyde, the coverslips were reacted with an anti-IE1 MAb to identify infected cells and then developed with FITC-conjugated anti-mouse IgG1 (green) to detect internalized anti-myc antibodies and with TRITC-conjugated anti-mouse IgG2b (red) to detect anti-IE1 antibodies. (C) Virion incorporation of the TrkB/gpUL132 chimeric proteins. HF cells were electroporated with expression plasmids encoding the respective chimeric protein and then infected with HCMV 24 h later. Cells were harvested, and supernatant virus was collected by centrifugation. Viral proteins were solubilized and analyzed by immunoblotting using an anti-myc MAb to detect the chimeric protein and an anti-pp65 MAb to detect viral pp65.
To determine if the virion incorporation phenotype previously associated with the cytoplasmic tail of gpUL132 was also transferred to the chimeric protein, we concentrated the extracellular virions from the supernatant of transfected/infected cell cultures and compared the amount of the chimeric TrkB/gpUL132 proteins in extracellular virions and within the transfected/infected cells. We noted that TrkB/gpUL132 was efficiently incorporated into extracellular virions, whereas TrkB/gpUL132m1-4 could not be detected in extracellular virions (Fig. 9C). To ensure that similar levels of virions were analyzed in each virion preparation, we stripped the original membrane and reprobed with antibodies reactive with pp65 (UL83). Although we detected pp65 in both virion preparations as a single band, as has been previously published, there was considerably more protein in the virion preparation from cells that were transfected with the gpUL132m1-4 construct and infected with HCMV, indicating that the lack of the virion incorporation seen in these transfected/infected cells was not secondary to the analysis of limited amounts of extracellular virus (Fig. 9C). Several forms of pp65 were noted in the cells from both transfected/infected preparations, again consistent with the known degradation products of pp65 in virus-infected cells (Fig. 9C) (10). Together, these results clearly demonstrated that the cytoplasmic tail of gpUL132 contains trafficking signals that efficiently direct gpUL132 (or a chimeric protein) into the endocytic pathways, resulting in incorporation into progeny virions.
DISCUSSION
In a previous study gpUL132 was identified as a structural glycoprotein of HCMV. Deletion of the reading frame from laboratory-adapted strains or low-passage clinical isolates resulted in a replication deficit of the respective recombinant viruses (50). The function of gpUL132 is currently unknown. gpUL132 contains in its carboxy-terminal domain a number of different sequence motifs that are relevant for the intracellular transport of proteins. We have analyzed the function of the motifs that are potentially involved in endocytosis. To this end we have expressed mutant gpUL132 proteins either alone in transient transfections or in the context of a viral infection. The key role in the intracellular trafficking played by the signals in the carboxy-terminal domain of gpUL132 was further demonstrated by the results of the analysis of the intracellular trafficking exhibited by the chimeric protein consisting of the ectodomain of the receptor tyrosine kinase, TrkB, fused to the cytoplasmic tail of either the wild-type gpUL132 or the cytoplasmic tail of a mutant gpUL132 in which all predicted trafficking motifs were mutated (gpUL132m1-4). Utilizing these chimeric proteins, we demonstrated that mutation of the carboxy-terminal trafficking motifs leads to loss of endocytosis and incorporation into progeny virions compared to the chimeric TrkB protein expressing the wild-type gpUL132 tail. These data argued that the interactions between the cytoplasmic and/or transmembrane domain of gpUL132 with other viral proteins was unnecessary for endocytosis and localization to sites of virion assembly in the infected cell. At this time we cannot definitively rule out that in addition to trafficking signals, the carboxy-terminal tail of gpUL132 also expresses sequences that direct incorporation of this protein into the virion particle through interactions with other virion proteins; however, it is also clear that in the absence of the trafficking motifs expressed in the carboxy terminus of the tail of gpUL132, this protein cannot localize to sites of virus assembly. Finally, since we carried out our experiments in the presence of virus infection, we can also argue that if viral protein interactions critical for virion incorporation require the carboxy-terminal tail of gpUL132, then these interactions must take place at sites of virion assembly and not in proximity to the plasma membrane. Thus, our data demonstrated that the trafficking motifs in the tail of gpUL132 are important for endocytosis, intracellular localization of the protein, the kinetics of its intracellular transport, and, finally, the incorporation of gpUL132 into virions.
Among the trafficking signals that are found in gpUL132 are the more commonly used YXXØ and LL motifs. These motifs interact with the μ subunits of AP complexes which are mediators of clathrin-dependent endocytosis as well as intracellular sorting processes (6). In addition, gpUL132 harbors an FXNPXY (FVNPNY205) motif. FXNPXY signals have been shown to mediate rapid internalization of only a subset of type I membrane proteins and not other intracellular sorting events. The FXNPXY motif is likely not directly recognized by AP-2 but rather by proteins containing a domain known as PTB (phosphotyrosine-interacting domain) such as Dab2 (disabled-2) or the low-density lipoprotein receptor (LDLR) adaptor protein (32, 36). The FXNPXY signal in gpUL132 is unique among herpesvirus glycoproteins (M. Mach, unpublished observations). Interestingly, mutation of this unique motif in UL132 resulted in altered intracellular localization of gpUL132 but did not appreciably reduce virus yield, suggesting that its role in the trafficking of gpUL132 may be related to increasing the efficiency of endocytosis of this molecule, a function whose phenotype may not be appreciably altered in standard antibody internalization assays or virus yield assays.
Our findings in transient transfection assays indicated that the individual sorting signals have a differential influence on the intracellular distribution of gpUL132. Following isolated transient expression and analysis by indirect immunofluorescence, the wt gpUL132 protein was found to colocalize predominantly with markers from the TGN, indicating that it expresses all the necessary signals to be transported into this cellular compartment. Flow cytometry revealed a somewhat more complex picture and indicated that approximately 20% of the total wt gpUL132 protein in steady state was localized at the cell surface. The apparently discrepant distribution of gpUL132 on the surfaces of cells determined by indirect immunofluorescence or flow cytometry is explained by the large surface area of the transfected HeLa cells. In indirect immunofluorescence the fraction of gpUL132 that is associated with the cell surface was most probably not detected because the protein was distributed over the large surface area of an entire cell. Mutation of the LL127 (gpUL132m1) motif as well as the YQRL162 (gpUL132m2) motif had a rather subtle impact on intracellular distribution of the mutant protein, as determined by immunofluorescence. The respective mutant proteins continued to colocalize mainly with the TGN. In contrast, mutation of the YVSVYDEL235 (gpUL132m3) motif gave rise to a significantly different protein distribution, with drastically increased concentration of protein on the cell surface rather than in the TGN, indicating that this signal is more relevant in the intracellular sorting of gpUL132. Finally, combined mutation of LL, YXXØ, and FXNPXY (gpUL132m1-3 and gpUL132m1-4) motifs had the most striking effect, resulting in protein localization that was almost exclusively associated with the plasma membrane; TGN-colocalizing protein was no longer detectable. Again, flow cytometry provided a more quantitative estimate of the cell surface expression of these gpUL132 mutants and suggested that approximately 66% of the total gpUL132m1-4 protein was expressed on the cell surface. The differential usage of the various sorting signals is in accordance with results showing that recognition of the adaptor complexes is influenced by the nature of the X and Ø residues within the YXXØ sequence as well as by the context or spatial separation from the membrane (41). Differential effects of the individual motifs on protein trafficking have also been seen in other herpesvirus glycoproteins containing multiple YXXØ sorting motifs (14, 16, 55).
Mutation of trafficking signals not only resulted in different overall intracellular distribution of gpUL132 but also had an impact on retrieval kinetics from the plasma membrane. While transiently expressed gpUL132 wt protein was retrieved in large part from the TGN within 20 min, the gpUL132m1-3 and gpUL132m1-4 proteins remained at the cell surface during this time period. This result indicated that the mutations that were introduced in the gpUL132 protein likely affected the most relevant signals for retrograde transport. Thus, the dependence of gpUL132 on defined sorting motifs for intracellular transport resembled those described for other herpesvirus glycoproteins.
Interestingly, in infected cells we found a different distribution of gpUL132 mutant proteins compared to transiently expressed protein and also differences in the retrograde transport, emphasizing the importance of analyzing protein transport and localization in the context of the infected cell. In steady-state immunofluorescence in permeabilized cells, gpUL132 was found in the AC, as noted earlier, and in colocalization with markers from the TGN (50). No protein was detected at the cell surface. This finding was similar to that in transfected cells. Following infection the mutant proteins gpUL132m1-3 and gpUL132m1-4 also showed increased cell surface localization, but, in contrast to transiently expressed protein, a fraction of the protein was found within large intracellular vesicles which only partially overlapped with signals from the TGN marker protein TGN46. In addition, the AC was significantly more dispersed in cells infected with the RV-UL132m1-3 or RV-UL132m1-4 viruses than in wt-infected cells. There are at least two possible explanations for this finding. (i) The mutant gpUL132 protein is not efficiently transported to the cell surface in infected cells, and parts of the protein are retained in an aberrant intracellular compartment. (ii) The mutant protein is endocytosed from the plasma membrane but traffic to and incorporation into membranous structures in the AC are impaired secondary to mutations in these intracellular trafficking motifs. This would require that the infected cell provide a function for retrograde transport that is not available in transfected cells. Likely mechanisms could include complex formation of gpUL132 with additional HCMV-specific glycoproteins which undergo endocytosis. Linkage of different herpesvirus envelope glycoproteins in the plasma membrane, association with lipid rafts, and subsequent internalization have been demonstrated (15). Also, internalization of gI of PRV has been shown to be dependent on gE, a protein that is endocytosed by its own signal while gI is incapable of endocytosis due to the lack of the appropriate internalization signals (54). The fact that we observed endocytosis of mutant gpUL132 in infected cells and transport of the protein to structures that closely resemble those found in the steady-state analysis argues in favor of the latter possibility. In fact, we have preliminary evidence that the intracellular distribution of gB is altered in infected fibroblasts expressing gpUL132m1-4 protein (Mach, unpublished). Alternatively, it is possible that protein components of the plasma membrane of infected cells turn over more rapidly because of increased requirements for lipids during intracellular membrane formation required for assembly of virions.
Importantly, the gpUL132 mutant protein is transported to a compartment (cellular structure) which is not accessible for the developing virion during the late stages of virion morphogenesis. This is indicated by the fact that the mutant gpUL132 proteins are not incorporated into virions, coincident with a reduced replication capacity of the gpUL132 mutant viruses. The replication of RV-UL132m1-4HA was found to be equally impaired as that of a UL132 deletion virus. This effect could be a direct result of the lack of gpUL132 for virion morphogenesis. Alternatively and/or additionally, the aberrant retrograde transport of gpUL132 mutant proteins could include additional virus-encoded glycoproteins via complex formation and thus lead to a replication deficit. Although unlikely, it is theoretically possible that the loss of sites of interaction between the gpUL132 mutant protein and tegument proteins that are necessary for efficient secondary envelopment was the explanation for the defect in virus replication in this mutant virus (19, 24). The finding that the triple or quadruple gpUL132 mutant proteins are not incorporated into the virus particle is in contrast to findings with regard to other herpesvirus glycoproteins such as gE and Us9 of PRV or gB of HSV-1, for which endocytosis is not a prerequisite for incorporation into the virion (3, 9, 55).
Regardless of the underlying mechanism, our data show, to our knowledge for the first time, that endocytosis of an HCMV glycoprotein is important for optimal virus replication. In other herpesviruses, inhibition of endocytosis of glycoproteins has been reported to have different effects on replication of the specific members of this family of viruses. For example, complete inhibition of VZV replication has been observed for a virus encoding gE that cannot undergo endocytosis (37). Moderate reduction in virus replication for gB of HSV-1 and development of a small-plaque phenotype in cultured cells for gE of PRV have been associated with the respective envelope proteins with defects in endocytosis (3). Finally, it has been postulated that endocytosis of gB of PRV and HCMV is irrelevant for virus replication (23, 40). Thus, our results that demonstrate the importance of endocytosis of gpUL132 for optimal replication of HCMV are somewhat at odds with the previous findings on endocytosis of HCMV glycoproteins and replication efficiency. Early reports by Radsak showed that gB is incorporated into the virion following retrieval of the protein from the plasma membrane (45). These findings have been confirmed by others (56). Subsequent analyses, however, came to the conclusion that endocytosis of gB is irrelevant for virion formation and replication. Jarvis et al. analyzed virus replication in the presence of a dominant negative dynamin I protein and found no influence on viral replication (23). Dynamin I participates in clathrin-dependent endocytosis. There are, however, a number of clathrin-independent endocytosis mechanisms, and there is a growing body of evidence that the different endocytosis pathways are redundant and can compensate each other (13, 39, 47). On the other hand, the acidic cluster motif in the carboxy-terminal part of gB, which is an interactor sequence for PACS-1 (phosphofurin acidic cluster sorting protein 1), influencing the endosome to TGN transport, has been shown to be important for optimal viral replication, indicating that retrograde transport of gB may be important for optimal replication (12).
gB contains in its carboxy-terminal part a number of different motifs which could affect endocytosis (e.g., the predicted tyrosine-based motifs YQML848 and YRHL897). A systematic analysis of the importance of these motifs for endocytosis, incorporation into virions, and replication is lacking, and thus it is difficult at the moment to assess the role of endocytosis of gB for HCMV replication. Our data on gpUL132 as well as results on other herpesvirus glycoproteins indicate that mutation of single motifs potentially has little effect on intracellular transport of the protein and viral replication (55). The importance of PACS-1 binding sequences has also been seen in a recent study of HCMV gM. A viral mutant carrying a deletion in the acidic cluster motif which is present in the cytoplasmic tail of the protein exhibited a replication-impaired phenotype although this phenotype could not be described as robust (25, 28). Interestingly, an additional mutant having a mutation within an adjacent YXXØ sorting motif of gM also showed reduced replication capacity. However, mutation of the YXXØ motif did not result in increased cell surface expression of gM and had no significant impact on retrieval of the protein from the plasma membrane (25). Thus, for gM, the data suggested that the YXXØ motif within the carboxy-terminal tail may be of greater importance for intracellular trafficking of the protein to the AC than for surface expression and endocytosis. Alternatively, for the more abundant proteins like gB and gM, endocytosis might not be as critical as for gpUL132 since even when retrograde transport is impaired, sufficient amounts of protein for optimal replication still localize to the AC, either through residual endocytosis or via transport mechanisms not involving endocytosis. Another layer of complexity with respect to intracellular transport is the nature of the trafficking motif that is analyzed. It was reported, for example, that for gB of herpes simplex virus type 1 (HSV-1), mutation of the LL motif induces formation of giant syncytia, whereas mutation of a YXXØ motif reduced cell-cell fusion (3).
There is a possibility that endocytosis of gpUL132 is dependent on an antibody-dependent mechanism. For some herpesvirus glycoproteins, both antibody-dependent and -independent endocytosis has been described, and it was postulated that endocytosis of viral glycoproteins represents a mechanism to thwart efficient antibody-mediated lysis of infected cells (17, 57). Demonstration of antibody-independent endocytosis is normally performed by surface labeling of plasma membrane-associated proteins and detection of the labeled protein within intracellular sites after endocytosis has been allowed to proceed. We have used this approach to analyze antibody-independent endocytosis of gpUL132. However, while we could confirm endocytosis of gB, we were unable to sufficiently label gpUL132 on the surface of infected or transfected cells due to the short extracellular part of this glycoprotein and the low abundance in infected cells and virions (data not shown).
Based on its widespread occurrence, endocytosis of viral envelope glycoproteins, driven by a variety of different internalization signals, must have some functional relevance during the herpesvirus replication cycle, and the findings that the majority of herpesviral glycoproteins which undergo endocytosis are incorporated into the virion envelope support this hypothesis. However, thus far no unifying concept about the role of endocytosis for viral replication has emerged, and it will probably be difficult to develop, given the complexity of the various proteins and virus systems in question. For gpUL132 of HCMV, we can conclude that endocytosis of the protein is necessary for incorporation into the virion and optimal virus replication in fibroblasts.
Supplementary Material
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (MA 929/9) and the NIH (R01AI035602).
Footnotes
Published ahead of print on 5 May 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Alconada, A., U. Bauer, L. Baudoux, J. Piette, and B. Hoflack. 1998. Intracellular transport of the glycoproteins gE and gI of the varicella-zoster virus. gE accelerates the maturation of gI and determines its accumulation in the trans-Golgi network. J. Biol. Chem. 273:13430-13436. [DOI] [PubMed] [Google Scholar]
- 2.Andreoni, M., M. Faircloth, L. Vugler, and W. J. Britt. 1989. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J. Virol. Methods 23:157-167. [DOI] [PubMed] [Google Scholar]
- 3.Beitia Ortiz de Zarate, I., L. Cantero-Aguilar, M. Longo, C. Berlioz-Torrent, and F. Rozenberg. 2007. Contribution of endocytic motifs in the cytoplasmic tail of herpes simplex virus type 1 glycoprotein B to virus replication and cell-cell fusion. J. Virol. 81:13889-13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beitia Ortiz de Zarate, I., K. Kaelin, and F. Rozenberg. 2004. Effects of mutations in the cytoplasmic domain of herpes simplex virus type 1 glycoprotein B on intracellular transport and infectivity. J. Virol. 78:1540-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bi, W., and P. J. Stambrook. 1998. Site-directed mutagenesis by combined chain reaction. Anal. Biochem. 256:137-140. [DOI] [PubMed] [Google Scholar]
- 6.Bonifacino, J. S., and L. M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395-447. [DOI] [PubMed] [Google Scholar]
- 7.Borst, E. M., G. Hahn, U. H. Koszinowski, and M. Messerle. 1999. Cloning of the human cytomegalovirus (HCMV) genome as an infectious bacterial artificial chromosome in Escherichia coli: a new approach for construction of HCMV mutants. J. Virol. 73:8320-8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brideau, A. D., R. T. del, E. J. Wolffe, and L. W. Enquist. 1999. Intracellular trafficking and localization of the pseudorabies virus Us9 type II envelope protein to host and viral membranes. J. Virol. 73:4372-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brideau, A. D., L. W. Enquist, and R. S. Tirabassi. 2000. The role of virion membrane protein endocytosis in the herpesvirus life cycle. J. Clin. Virol. 17:69-82. [DOI] [PubMed] [Google Scholar]
- 10.Britt, W. J., and L. Vugler. 1987. Structural and immunological characterization of the intracellular forms of an abundant 68,000 Mr human cytomegalovirus protein. J. Gen. Virol. 68:1897-1907. [DOI] [PubMed] [Google Scholar]
- 11.Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9-14. [DOI] [PubMed] [Google Scholar]
- 12.Crump, C. M., C. H. Hung, L. Thomas, L. Wan, and G. Thomas. 2003. Role of PACS-1 in trafficking of human cytomegalovirus glycoprotein B and virus production. J. Virol. 77:11105-11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damm, E. M., L. Pelkmans, J. Kartenbeck, A. Mezzacasa, T. Kurzchalia, and A. Helenius. 2005. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. J. Cell Biol. 168:477-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan, Z., M. L. Grantham, M. S. Smith, E. S. Anderson, J. A. Cardelli, and M. I. Muggeridge. 2002. Truncation of herpes simplex virus type 2 glycoprotein B increases its cell surface expression and activity in cell-cell fusion, but these properties are unrelated. J. Virol. 76:9271-9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Favoreel, H. W., T. C. Mettenleiter, and H. J. Nauwynck. 2004. Copatching and lipid raft association of different viral glycoproteins expressed on the surfaces of pseudorabies virus-infected cells. J. Virol. 78:5279-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Favoreel, H. W., M. G. Van, H. J. Nauwynck, L. W. Enquist, and M. B. Pensaert. 2002. A tyrosine-based motif in the cytoplasmic tail of pseudorabies virus glycoprotein B is important for both antibody-induced internalization of viral glycoproteins and efficient cell-to-cell spread. J. Virol. 76:6845-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ficinska, J., M. G. Van, H. J. Nauwynck, K. Bienkowska-Szewczyk, and H. W. Favoreel. 2005. Pseudorabies virus glycoprotein gD contains a functional endocytosis motif that acts in concert with an endocytosis motif in gB to drive internalization of antibody-antigen complexes from the surface of infected monocytes. J. Virol. 79:7248-7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman, H. M., L. Wang, N. O. Fishman, J. D. Lambris, R. J. Eisenberg, G. H. Cohen, and J. Lubinski. 1996. Immune evasion properties of herpes simplex virus type 1 glycoprotein gC. J. Virol. 70:4253-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs, W., B. G. Klupp, H. Granzow, C. Hengartner, A. Brack, A. Mundt, L. W. Enquist, and T. C. Mettenleiter. 2002. Physical interaction between envelope glycoproteins E and M of pseudorabies virus and the major tegument protein UL49. J. Virol. 76:8208-8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh, P., and S. Kornfeld. 2004. The GGA proteins: key players in protein sorting at the trans-Golgi network. Eur. J. Cell Biol. 83:257-262. [DOI] [PubMed] [Google Scholar]
- 21.Heineman, T. C., and S. L. Hall. 2002. Role of the varicella-zoster virus gB cytoplasmic domain in gB transport and viral egress. J. Virol. 76:591-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Homman-Loudiyi, M., K. Hultenby, W. Britt, and C. Soderberg-Naucler. 2003. Envelopment of human cytomegalovirus occurs by budding into Golgi-derived vacuole compartments positive for gB, Rab 3, trans-Golgi network 46, and mannosidase II. J. Virol. 77:3191-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarvis, M. A., K. N. Fish, C. Soderberg-Naucler, D. N. Streblow, H. L. Meyers, G. Thomas, and J. A. Nelson. 2002. Retrieval of human cytomegalovirus glycoprotein B from cell surface is not required for virus envelopment in astrocytoma cells. J. Virol. 76:5147-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalejta, R. F. 2008. Tegument proteins of human cytomegalovirus. Microbiol. Mol. Biol. Rev. 72:249-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krzyzaniak, M., M. Mach, and W. J. Britt. 2007. The cytoplasmic tail of glycoprotein M (gpUL100) expresses trafficking signals required for human cytomegalovirus assembly and replication. J. Virol. 81:10316-10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krzyzaniak, M. A., M. Mach, and W. J. Britt. 2009. HCMV-encoded glycoprotein M (UL100) interacts with Rab11 effector protein FIP4. Traffic 10:1439-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenac, T., M. Budt, J. Arapovic, M. Hasan, A. Zimmermann, H. Simic, A. Krmpotic, M. Messerle, Z. Ruzsics, U. H. Koszinowski, H. Hengel, and S. Jonjic. 2006. The herpesviral Fc receptor fcr-1 down-regulates the NKG2D ligands MULT-1 and H60. J. Exp. Med. 203:1843-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mach, M., B. Kropff, M. Kryzaniak, and W. Britt. 2005. Complex formation by glycoproteins M and N of human cytomegalovirus: structural and functional aspects. J. Virol. 79:2160-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mach, M., K. Osinski, B. Kropff, U. Schloetzer-Schrehardt, M. Krzyzaniak, and W. Britt. 2007. The carboxy-terminal domain of glycoprotein N of human cytomegalovirus is required for virion morphogenesis. J. Virol. 81:5212-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maresova, L., T. J. Pasieka, E. Homan, E. Gerday, and C. Grose. 2005. Incorporation of three endocytosed varicella-zoster virus glycoproteins, gE, gH, and gB, into the virion envelope. J. Virol. 79:997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsh, H. N., C. I. Dubreuil, C. Quevedo, A. Lee, M. Majdan, G. S. Walsh, S. Hausdorff, F. A. Said, O. Zoueva, M. Kozlowski, K. Siminovitch, B. G. Neel, F. D. Miller, and D. R. Kaplan. 2003. SHP-1 negatively regulates neuronal survival by functioning as a TrkA phosphatase. J. Cell Biol. 163:999-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maurer, M. E., and J. A. Cooper. 2006. The adaptor protein Dab2 sorts LDL receptors into coated pits independently of AP-2 and ARH. J. Cell Sci. 119:4235-4246. [DOI] [PubMed] [Google Scholar]
- 33.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mettenleiter, T. C., B. G. Klupp, and H. Granzow. 2006. Herpesvirus assembly: a tale of two membranes. Curr. Opin. Microbiol. 9:423-429. [DOI] [PubMed] [Google Scholar]
- 35.Middlemas, D. S., R. A. Lindberg, and T. Hunter. 1991. trkB, a neural receptor protein-tyrosine kinase: evidence for a full-length and two truncated receptors. Mol. Cell. Biol. 11:143-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra, S. K., P. A. Keyel, M. J. Hawryluk, N. R. Agostinelli, S. C. Watkins, and L. M. Traub. 2002. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 21:4915-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moffat, J., C. Mo, J. J. Cheng, M. Sommer, L. Zerboni, S. Stamatis, and A. M. Arvin. 2004. Functions of the C-terminal domain of varicella-zoster virus glycoprotein E in viral replication in vitro and skin and T-cell tropism in vivo. J. Virol. 78:12406-12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muyrers, J. P., Y. Zhang, G. Testa, and A. F. Stewart. 1999. Rapid modification of bacterial artificial chromosomes by ET-recombination. Nucleic Acids Res. 27:1555-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naslavsky, N., R. Weigert, and J. G. Donaldson. 2004. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol. Biol. Cell 15:3542-3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nixdorf, R., B. G. Klupp, A. Karger, and T. C. Mettenleiter. 2000. Effects of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity. J. Virol. 74:7137-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohno, H., R. C. Aguilar, D. Yeh, D. Taura, T. Saito, and J. S. Bonifacino. 1998. The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J. Biol. Chem. 273:25915-25921. [DOI] [PubMed] [Google Scholar]
- 42.Olson, J. K., and C. Grose. 1997. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J. Virol. 71:4042-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasieka, T. J., L. Maresova, and C. Grose. 2003. A functional YNKI motif in the short cytoplasmic tail of varicella-zoster virus glycoprotein gH mediates clathrin-dependent and antibody-independent endocytosis. J. Virol. 77:4191-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasieka, T. J., L. Maresova, K. Shiraki, and C. Grose. 2004. Regulation of varicella-zoster virus-induced cell-to-cell fusion by the endocytosis-competent glycoproteins gH and gE. J. Virol. 78:2884-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radsak, K., M. Eickmann, T. Mockenhaupt, E. Bogner, H. Kern, A. Eis-Hubinger, and M. Reschke. 1996. Retrieval of human cytomegalovirus glycoprotein B from the infected cell surface for virus envelopment. Arch. Virol. 141:557-572. [DOI] [PubMed] [Google Scholar]
- 46.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandvig, K., M. L. Torgersen, H. A. Raa, and D. B. van. 2008. Clathrin-independent endocytosis: from nonexisting to an extreme degree of complexity. Histochem. Cell Biol. 129:267-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shiba, T., M. Kawasaki, H. Takatsu, T. Nogi, N. Matsugaki, N. Igarashi, M. Suzuki, R. Kato, K. Nakayama, and S. Wakatsuki. 2003. Molecular mechanism of membrane recruitment of GGA by ARF in lysosomal protein transport. Nat. Struct. Biol. 10:386-393. [DOI] [PubMed] [Google Scholar]
- 49.Soppet, D., E. Escandon, J. Maragos, D. S. Middlemas, S. W. Reid, J. Blair, L. E. Burton, B. R. Stanton, D. R. Kaplan, T. Hunter, K. Nikolics, and L. F. Parade. 1991. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell 65:895-903. [DOI] [PubMed] [Google Scholar]
- 50.Spaderna, S., B. Kropff, Y. Kodel, S. Shen, S. Coley, S. Lu, W. Britt, and M. Mach. 2005. Deletion of gpUL132, a structural component of human cytomegalovirus, results in impaired virus replication in fibroblasts. J. Virol. 79:11837-11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sprague, E. R., H. Reinhard, E. J. Cheung, A. H. Farley, R. D. Trujillo, H. Hengel, and P. J. Bjorkman. 2008. The human cytomegalovirus Fc receptor gp68 binds the Fc CH2-CH3 interface of immunoglobulin G. J. Virol. 82:3490-3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sprague, E. R., C. Wang, D. Baker, and P. J. Bjorkman. 2006. Crystal structure of the HSV-1 Fc receptor bound to Fc reveals a mechanism for antibody bipolar bridging. PLoS. Biol. 4:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talbot, P., and J. D. Almeida. 1977. Human cytomegalovirus: purification of enveloped virions and dense bodies. J. Gen. Virol. 36:345-349. [DOI] [PubMed] [Google Scholar]
- 54.Tirabassi, R. S., and L. W. Enquist. 1998. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J. Virol. 72:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tirabassi, R. S., and L. W. Enquist. 1999. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J. Virol. 73:2717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tugizov, S., E. Maidji, J. Xiao, and L. Pereira. 1999. An acidic cluster in the cytosolic domain of human cytomegalovirus glycoprotein B is a signal for endocytosis from the plasma membrane. J. Virol. 73:8677-8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Minnebruggen, G., H. W. Favoreel, and H. J. Nauwynck. 2004. Internalization of pseudorabies virus glycoprotein B is mediated by an interaction between the YQRL motif in its cytoplasmic domain and the clathrin-associated AP-2 adaptor complex. J. Virol. 78:8852-8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Varnum, S. M., D. N. Streblow, M. E. Monroe, P. Smith, K. J. Auberry, L. Pasa-Tolic, D. Wang, D. G. Camp, K. Rodland, S. Wiley, W. Britt, T. Shenk, R. D. Smith, and J. A. Nelson. 2004. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 78:10960-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.