Abstract
Bats are hosts to a variety of viruses capable of zoonotic transmissions. Because of increased contact between bats, humans, and other animal species, the possibility exists for further cross-species transmissions and ensuing disease outbreaks. We describe here full and partial viral genomes identified using metagenomics in the guano of bats from California and Texas. A total of 34% and 58% of 390,000 sequence reads from bat guano in California and Texas, respectively, were related to eukaryotic viruses, and the largest proportion of those infect insects, reflecting the diet of these insectivorous bats, including members of the viral families Dicistroviridae, Iflaviridae, Tetraviridae, and Nodaviridae and the subfamily Densovirinae. The second largest proportion of virus-related sequences infects plants and fungi, likely reflecting the diet of ingested insects, including members of the viral families Luteoviridae, Secoviridae, Tymoviridae, and Partitiviridae and the genus Sobemovirus. Bat guano viruses related to those infecting mammals comprised the third largest group, including members of the viral families Parvoviridae, Circoviridae, Picornaviridae, Adenoviridae, Poxviridae, Astroviridae, and Coronaviridae. No close relative of known human viral pathogens was identified in these bat populations. Phylogenetic analysis was used to clarify the relationship to known viral taxa of novel sequences detected in bat guano samples, showing that some guano viral sequences fall outside existing taxonomic groups. This initial characterization of the bat guano virome, the first metagenomic analysis of viruses in wild mammals using second-generation sequencing, therefore showed the presence of previously unidentified viral species, genera, and possibly families. Viral metagenomics is a useful tool for genetically characterizing viruses present in animals with the known capability of direct or indirect viral zoonosis to humans.
Bats belong to one of the most diverse, abundant, and widely distributed group of mammals. More than 1,100 bat species belong to the order of Chiroptera, representing approximately 20% of all mammalian species (54). Most bat species feed on insects and other arthropods, while others feed on fruit nectar, bird or mammal blood, and small vertebrates such as fish, frogs, mice, and birds (30). Of the 47 species of bats reported in the United States, most of them are insectivorous (http://www.batcon.org/).
Bats are considered the natural reservoir of a large variety of zoonotic viruses causing serious human diseases such as lyssaviruses, henipaviruses, severe acute respiratory syndrome coronavirus, and Ebola virus (6, 38, 46, 59, 63, 65). Characteristics of bats, including their genetic diversity, broad geological distribution, gregarious habits, high population density, migratory habits, and long life span (30, 58), likely endow them with the ability to host diverse viruses, some of which are also able to infect humans and other mammals (41, 63).
More than 80 virus species have been isolated or detected in bats using nucleic acid-based methods (6, 38, 59, 65). Viruses that have been recently discovered in bats include astroviruses, adeno-associated viruses (AAVs), adenoviruses, herpesviruses, and polyomavirus (8, 9, 13, 31, 32, 35, 37, 39, 40, 42, 61, 62, 68). For example, it was recently reported that a newly identified adenovirus isolated from bat guano was capable of infecting various vertebrate cell lines, including those of humans, monkeys, dogs, and pigs (35). With increasing human populations in previously wild areas, contact of bats with humans and with wild and domestic animals has increased, providing greater opportunities for cross-species transmissions of potentially pathogenic bat viruses. To better understand the range of viruses carried by bats, we undertook an initial characterization of the guano viromes of several common bat species in the United States.
The development of massively parallel sequencing technology makes is possible to reveal uncultured viral assemblages within biological or environmental samples (11, 28). To date, this approach has been used to characterize viruses in equine feces (7), human blood (5), tissue (14), human feces (3, 4, 15, 45, 60, 67), and human respiratory secretions (64), which in turn has facilitated the discovery of many novel viruses (18, 20, 25, 33, 47, 50). In the present study, we analyzed the viruses present in guano from several bat species in California and Texas, using sequence-independent PCR amplification, pyrosequencing, and sequence similarity searches.
MATERIALS AND METHODS
Collection of bat guano.
All of the bat species sampled are insectivorous. Plastic sheets were laid down on flat surfaces beneath bat roosts. Freshly produced bat guano was then collected 1 day later and stored at −80°C. Samples were collected from a bat roost near San Saba, TX, on two occasions that were 3 days apart during the summer of 2008. The roost (TM) was occupied mostly by Tadarida brasiliensis (Brazilian free-tailed bat). Three other species present in smaller numbers, Myotis velifer (Cave myotis), Nycticeus humeralis (evening bat), and Perimyotis subflavus (tricolored bat), are also known to share the roost and may have also been sampled.
Guano samples from northern California were collected from five different roosts at Point Reyes National Seashore. One roost (GF-4) was occupied by Antrozous pallidus (pallid bat), and the other four roosts (GF-3, -5, -6, and -7) were occupied by Myotis spp. and/or Tadarida brasiliensis (Table 1).
TABLE 1.
Summary of bat guano sample information
| Sample | Collection location | Collection time | No. of roosts | Major bat species | No. of random primers used at roost: |
Estimated no. of animals sampled | Library type | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TM | GF-3 | GF-4 | GF-5 | GF-6 | GF-7 | |||||||
| GF | Point Reyes, California | September 2009 | 5 (roosts GF-3 to -7) | Myotis spp. or/and Tadarida brasiliensis (roosts GF-3, -5, -6, and -7); Antrozous pallidus (roost GF4) | 3 | 12 | 4 | 4 | 6 | 384 | DNA and RNA | |
| TM | San Saba, Texas | 8 July 2008 | 1 | Tadarida brasiliensis | 4 at both time points | 96 | DNA and RNA | |||||
Sample preparation and viral nucleic acid extraction.
Bat guano was processed as previously described (60). Briefly, groups of 12 bat guano pellets from the same roosts were resuspended by vigorous vortexing in Hank's buffered saline solution (Gibco BRL) and cleared of debris by low-speed centrifugation (5 min at 11,000 × g). A total of 500 μl of guano supernatant was filtered through a 0.45-μm filter (Millipore) to remove bacterium-sized particles. The viral particles containing filtrate were digested with a mixture of DNases and RNase to remove unprotected nucleic acids (i.e., those not in viral capsids) (1). Viral nucleic acids were then extracted using the QIAamp viral RNA minikit (Qiagen).
DNA and RNA library construction and pyrosequencing.
Viral nucleic acid libraries were constructed by random PCR amplification as previously described (60). Both a RNA virus-only and DNA plus RNA virus sequence-independent amplifications were performed and then pooled prior to sequencing. For RNA virus-only amplification, an aliquot of the extracted viral nucleic acid collected from each pool of 12 guano pellets was treated with DNase (Ambion) to remove viral DNA. A total of 100 pmol of primer, consisting of an arbitrarily designed 20-base oligonucleotide followed by a randomized octamer sequence at the 3′ end, was then used in a reverse transcription (RT) reaction (Moloney murine leukemia virus reverse transcriptase; Promega). For the RNA plus DNA virus amplification, the DNase step prior to RT was excluded. A single round of DNA synthesis was then performed using Klenow fragment polymerase (New England Biolabs), followed by PCR amplification of double-stranded DNA using a primer consisting of only the 20-base fixed portion of the random primer.
A total of 37 distinct random primers (containing different 20-base fixed sequences) were applied to guano collected from the 6 bat roosts (Table 1). The number of primers assigned per roost was based on the number of guano pellets collected. Viral nucleic acids were therefore amplified from 37 pools of 12 guano pellets per pool, with each pellet presumed to be from a different animal. Guano obtained from up to 96 bats in the Texas roost (8 primers) and up to 348 bats in the 5 California roosts (29 primers) was analyzed (Table 1). To further improve viral nucleic acid sampling within each pool, the random PCR amplifications were performed in duplicate, starting with the Klenow-treated products, resulting in four PCRs per original pool (2 viral RNA-only inputs and 2 viral RNA plus DNA inputs). The DNA obtained from these four PCRs was mixed and purified, and the DNA concentration was measured. Equal amounts of DNA from each of the 37 different pools were then mixed together and run on a 2% agarose gel, and DNA fragments from the 500- to 1,000-bp region were excised and purified. The DNA was then sequenced on a single pyrosequencing gasket using GS FLX Titanium reagents (Roche).
A subset of random primer sequences used was previously published (60). The other random primers were designed by generating random sequences using Primo (http://www.changbioscience.com/primo/primor.html) that were then analyzed by BLASTn to remove those primers likely to bind to human and bacterial sequences.
Bioinformatics.
The pyrosequencing reads were grouped in 37 bins, according to their unique sequence tags (the 20 fixed bases of the random PCR primer). The fixed primer sequences plus eight additional downstream nucleotides (encoded by the 3′ NNNNNNNN part of the random primers), were then trimmed from each read. Trimmed reads within each sequence bin were then assembled by Sequencher software (Gene Codes), with a criterion of 95% identity or greater over at least 35 bp. Contigs were therefore assembled using sequences from at most 12 animals. When overlapping sequences in contigs contained mutations (due to pyrosequencing error or because multiple viral variants from different animals in the same pool were sequenced), the consensus sequence was used. The assembled sequence contigs and singlets greater than 100 bp were then compared to the GenBank nonredundant nucleotide and protein databases using BLASTn and BLASTx, respectively. Using BLAST searches, sequences were classified as likely originating from a eukaryotic virus, bacteria, phage, or eukaryote or deemed unclassifiable based on the taxonomic origin of the best-hit sequence. An E value of 0.001 was used as the cutoff value for significant hits.
Phylogenetic analysis.
Reference viral sequences from different viral families were obtained from GenBank. Amino acid sequence alignments were generated using ClustalW and implemented in MEGA 4.1 with the default settings (29). Aligned sequences were trimmed to match the genomic regions of the viral sequences obtained in our study and phylogenetic trees generated by MEGA4, using neighbor-joining with amino acid p distances and 1,000 bootstrap replicates. The GenBank accession numbers of the viral sequences used in the phylogenetic analyses are shown in the trees.
Nucleotide sequence accession numbers.
Trimmed and binned sequence reads and contigs of metagenomes from bat guano in California and Texas have been deposited in the GenBank sequence reads archive under accession number SRA012669. Sequences from the genomes described in more detail can be found under GenBank accession numbers HM228873 to HM228895 and HM234168 to HM234169.
RESULTS
Sequence data overview.
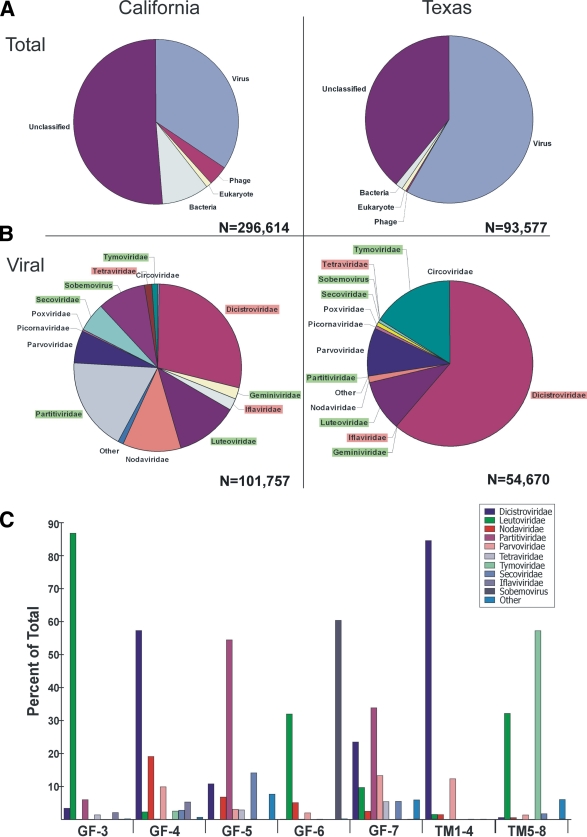
Approximately 390,000 raw sequences (average length, 296 bases) were generated from viral particle-enriched nucleic acids from bat guano. Sequence contigs were then formed and, together with singlets longer than 100 bases, were classified based on best BLAST scores (E value < 0.001) to taxonomically assigned sequences in the GenBank nonredundant database. Summaries of the classifications of viral nucleic acid in bat guano from California and Texas are shown in Fig. 1 A. Approximately 51% and 39% of all the sequence reads from California and Texas, respectively, had no significant similarity to any sequences in GenBank (E value > 0.001), similar to the percentages of unclassified sequences in a previous metagenomic study of human stool (60). The most abundant matches of viral sequences from bat guano in California and Texas were with eukaryotic viruses, with 34% and 58% of total reads, respectively. Sequences from both California and Texas bat guano yielded approximately 1% of eukaryote sequences, indicating the DNase and RNase treatment was largely effective in removing non-capsid-protected bat host nucleic acids.
FIG. 1.
Sequence classification for California and Texas bat guano-derived sequences based on BLASTx (E value < 0.001). (A) Percentages of sequences with similarity to those of eukaryotes, bacteria, phages, and eukaryotic virus in GenBank and to unclassifiable sequences. (B) Percentages of most abundant eukaryotic viral matches classified by viral families. Plant viruses are highlighted in green, insect viruses are highlighted in red, and mammalian viruses are not highlighted. (C) Eukaryotic viral families in California roost GF-3 to -7 and in one Texas roost at two collection time points (TM1-4 and TM5-8).
Phages in bat guano.
Based on prior studies, phages composed a significant fraction of human and equine fecal viral populations (3, 4, 7, 60). The levels of phage sequences in the feces of South Asian children with nonpolio acute flaccid paralysis and health contacts processed in the same manner were approximately 16% and 12% of total reads, respectively (60). In our study, the sequences with similarities to phages made up 4% and 0.1% of sequences in bat guano from California and Texas, respectively. Among the phages in bat guano samples from California, the majority belonged to the families Siphoviridae (67%) and Microviridae (28%), consistent with earlier viral metagenomic studies of which siphophages were the most abundant phages in human and equine feces (3, 4, 7). The most abundant sequence matches were to c2-like Lactococcus phages, T1-like enterobacterium phages, Chlamydia phage 3, and Spiroplasma phage 4 (data not shown).
Eukaryotic virus population in bat guano.
Many previously characterized and highly divergent eukaryotic viral sequences were detected in bat guano. The families of eukaryotic viruses that were found, based on their most significant BLASTx matches, are shown in Fig. 1B. Sequences of DNA viruses infecting eukaryotes made up a smaller fraction (approximately 10%) than eukaryotic RNA viral sequences in both California and Texas bat metagenomes. The DNA viruses were dominated by single-stranded DNA (ssDNA) viruses, including animal viruses from the families Parvoviridae and Circoviridae and plant viruses from the family Geminiviridae. Most of the proteins encoded by ssDNA eukaryotic virus-like sequences showed less than 60% amino acid identities to known viral protein, suggesting the presence of numerous novel viral species in bats. Sequences related to the newly discovered Cyclovirus genus in the family Circoviridae, commonly found in the tissues of hoofed farm animals and chickens as well as in human and wild chimpanzee feces, were also detected (33), showing that these viruses also exist in wild bats. Single-stranded RNA viruses belonged largely to the families Dicistroviridae, Nodaviridae, and Picornaviridae. Double-stranded RNA viral sequences in the family Partitiviridae (18%) were also detected in the bat guano from California.
Guano collected from bats in California had a more diverse viral composition than guano collected from bats in Texas, which may reflect the multiple roosts and bats species sampled in California. The most common eukaryotic viral families varied greatly between each of the five California roosts sampled (Fig. 1C). The guano viromes of the GF-3, -5, and -6 roosts were dominated by plant viruses, whereas the GF-4 roost, the only one with pallid bats, was richest in insect dicistroviruses, and the GF-7 roost had a more diversified virus profile. The viral compositions of the two guano samplings from the same Texas roost were highly distinct, with the earlier collection dominated by dicistroviruses and the later one by plant virus leutoviruses and tymoviruses (Fig. 1C, TM1-4 and TM5-8).
Insect viruses.
The largest fraction of the bat guano virome was related to insect viruses from the family Dicistroviridae, consisting of 29% of California viral sequences and 61% of Texas viral sequences, likely reflecting the insect-based diet of the bat species analyzed. Viral sequences related to viruses from Iflaviridae, Tetraviridae, Alphanodavirus, and Densovirinae were also detected. Most of the viruses were novel, sharing less than 60% amino acid (aa) similarity to known viral proteins, while some shared high (>90%) amino acid similarity with known insect viruses.
Viral sequences similar to those of Kashmir bee virus (12) and acute bee paralysis virus (19) were very abundant in bat guano from California and Texas. Sequences covered >70% of the complete genomes of these viruses (GenBank accession numbers HM228885 to HM228895). The translated full-length structural proteins shared 98% similarity with Kashmir bee virus and 97% aa similarity with acute bee paralysis virus, indicating that the viruses found in bat guano were variants of Kashmir bee virus and acute bee paralysis virus rather than new viral species. Because the sampled bats are nocturnal, it is unlikely that they feed on diurnal bees. Kashmir bee virus and acute bee paralysis virus may therefore also infect nocturnal bees or other insect hosts, or the bat species studied may have previously unknown dietary activities.
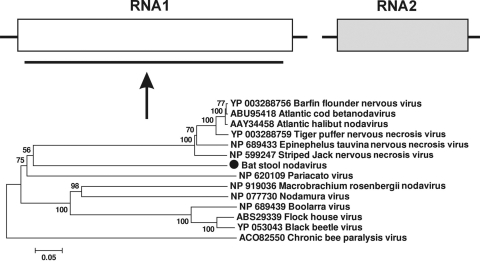
Viral sequences related to those of betanodaviruses known to infect fishes (36, 55) were also found in bat guano from California and Texas. We generated the almost full-length RNA1 segment (>90% coverage) of a nodavirus (bat guano-associated nodavirus GF-4), with a best match (E value of 3e−108) to Epinephelus tauvina nervous necrosis virus, with aa similarity of 34% (GenBank accession number HM228873). Phylogenetically, this nodavirus sequence in guano fell between the alphanodaviruses and betanodaviruses (Fig. 2). Given that none of the bat species sampled in our study are known to eat fish, this virus may represent a highly divergent insect nodavirus.
FIG. 2.
Phylogenetic analysis of bat guano-associated nodavirus GF-4 based on its 950-aa RNA-dependent RNA polymerase (RdRp) region. RNA1 of nodaviruses encodes the RdRp protein of ∼1,000 aa.
Plant viruses.
The second largest proportion of the bat guano viromes detected, with 46% of the viral sequences from Californian bats and 27% of those from Texan bats, was related to plant and fungal viral families, including Luteoviridae, Secoviridae, Tymoviridae, and Partitiviridae, and the Sobemovirus genus. Both previously characterized and newly identified plant viruses were detected. Plant viruses previously identified in human feces were mostly dominated by the tobamovirus pepper mild mottle virus (67).
Mammalian viruses in bat guano.
Sequences related to mammalian and bird viruses made up less than 10% of the viromes from bat guano in California and Texas. Viral sequences related to viruses from the families Parvoviridae, Circoviridae, Adenoviridae, Poxviridae, Picornaviridae, Astroviridae, and Coronaviridae were found in bat guano. Most of the sequence reads showed limited amino acid identity (<60%) with known viruses. Phylogenetic analyses were used to assess the relationship of novel viral sequences to known viruses.
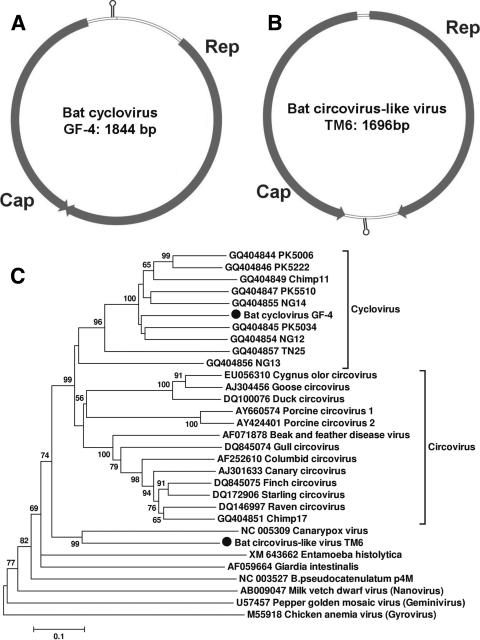
Bat cyclovirus and circovirus-like virus.
Cyclovirus, a new genus in the family Circoviridae, was recently described in stool samples from humans as well as in muscle tissue samples from hoofed farm animals and chickens from Pakistan and Nigeria (33). Stools from wild chimpanzees also contained cycloviruses (33). In California bat stool samples from roost GF-4, we identified a virus that is closely related to cycloviruses. The complete circular genome was 1,844 bp (GenBank accession number HM228874). The genome organization of bat cyclovirus GF-4 had characteristic features of cycloviruses, including two major inversely arranged open reading frames (ORFs) encoding the putative replication-associated protein (Rep; 281 aa) and capsid protein (Cap; 227 aa). A characteristic potential stem-loop structure with a conserved nonanucleotide motif (5′-TAATACTAT-3′) was also found in the 5′ intergenic region (between the start codons of the two major ORFs) (Fig. 3 A). The putative Rep proteins of the bat cyclovirus GF-4 had 45% to 68% aa similarity to cycloviruses found in human and chimpanzee feces and 39% to 43% similarity to the Rep proteins of porcine and avian circoviruses (data not shown).
FIG. 3.
(A) Genome organization of bat cyclovirus GF-4; (B) genome organization of bat circovirus-like virus TM6; (C) phylogenetic analysis of bat cyclovirus GF-4 and circovirus-like virus TM6 based on the complete amino acid sequence of the Rep protein (∼280 aa).
In Texas bat stool sample TM6, we found a small, circular DNA virus with a full-genome size of 1,696 bp (bat circovirus-like virus TM6) (GenBank accession number HM228876). The virus had two major ORFs arranged in opposite directions, with Rep at 264 aa and Cap at 226 aa, and two noncoding intergenic regions (Fig. 3B). The stem-loop structure also had the cyclovirus-conserved nonanucleotide motif (5′-TAATACTAT-3′) but was instead located at the 3′ intergenic region (between the stop codons of the two major ORFs).
A phylogenetic analysis of the complete Rep protein of bat cyclovirus GF-4 and bat circovirus-like virus TM6, including cycloviruses, circoviruses, chicken anemia virus (CAV), and non-Circoviridae Rep proteins from the plant Nanovirus milk vetch dwarf virus, Geminivirus pepper golden mosaic virus, canarypox virus, Bifidobacterium pseudocatenulatum plasmid pM4, Giardia intestinalis, and Entamoeba histolytica was performed (Fig. 3C). Examination of the phylogenetic tree showed that bat cyclovirus GF-4 grouped with known cycloviruses, forming a distinct species of cyclovirus. Bat circovirus-like virus TM6 fell outside the Circovirus and Cyclovirus clades, grouping with canarypox virus. While most closely related to the replicase sequence of canarypox virus, these two proteins showed only 40% aa similarity.
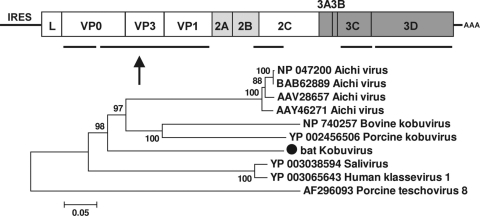
Bat kobuvirus.
Kobuvirus, a genus in the family Picornaviridae, currently contains three species: Aichi virus, bovine kobuvirus, and porcine kobuvirus. Kobuvirus-related viruses named salivirus and klassevirus have been recently described in human stool samples (20, 21, 34). Aichi virus and salivirus have been associated with human gastroenteritis, while bovine kobuvirus and porcine kobuvirus are associated with bovine and porcine diarrhea, respectively (26, 27, 48, 66). In one of the Texan sets of guano samples (TM7), we found approximately 400 reads which assembled into 5 contigs covering more than 60% of the viral genome of a virus closely related to kobuviruses (GenBank accession numbers HM228880 to HM228884). BLASTx searches showed that these contigs shared 39% to 59% aa similarity to kobuviruses. We tentatively named this virus bat kobuvirus. According to the International Committee on Taxonomy of Viruses (ICTV) (http://www.picornastudygroup.com/definitions/genus_definition.htm), the members of a picornavirus genus should share >40%, >40%, and >50% aa similarity in their P1, P2, and P3 regions, respectively. The largest contig (1,998 bp) covered about 70% of the P1 region and shared 46% aa similarity with the closest match, human Aichi virus. Bat kobuvirus therefore appears to be a new viral species within the genus Kobuvirus (Fig. 4). Phylogenetic analysis using the contig (1,335 bp) covering more than 80% of the 3-D region produces a similar tree topology (data not shown).
FIG. 4.
Phylogenetic analysis of bat kobuvirus based on its 660-aa partial P1 region. The P1 region of kobuviruses encodes structural proteins of ∼870 aa.
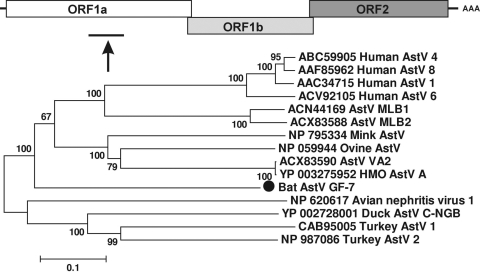
Bat astrovirus.
The family Astroviridae includes positive single-stranded RNA viruses, with genomes of 6.4 to 7.3 kb, encoding nonstructural proteins with ORF1a and ORF1b and structural protein with ORF2 (43). Astroviruses (AstV) have been identified in a variety of mammals and birds, including humans, cattle, pigs, sheep, mink, dogs, cats, mice, bats, chickens, and turkeys. In the California bat roost GF-7, we detected a highly divergent astrovirus-like sequence (677 bp) (GenBank accession number HM228876). The translated amino acid sequence most closely matched the serine protease region of the newly characterized human HMOAstV-A viral genome (34% similarity) (16, 23). Phylogenetic analysis based on this region yielded a tree topology that was congruent with those of analyses using other genome regions (8, 49, 68). The tree showed that the bat astrovirus GF-7 sequence fell in a basal position relative to other mamastroviruses (infecting mammals) between genera Mamastrovirus and Avastrovirus (infecting birds) (Fig. 5). Because the same genome region was not sequenced for recently reported bat astroviruses from China (8, 68), the relationship between bat astrovirus GF-7 and other bat astroviruses is currently unknown.
FIG. 5.
Phylogenetic analysis of bat astrovirus based on its 225-aa partial ORF1a region. ORF1a of astroviruses encodes nonstructural protein proteases of ∼900 aa.
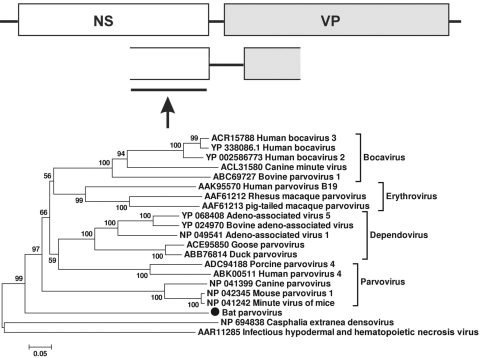
Bat parvovirus.
Classified within the Parvoviridae family Parvovirinae are a subfamily of linear, nonsegmented single-stranded DNA viruses largely infecting mammals but also some birds, with an average genome size of 4 to 6 kbp. Viruses in this subfamily possess two major ORFs, encoding the nonstructural protein (NS) and structural protein (VP) (22, 24, 57). In one of the guano samples from Texas bats (TM2), we generated a parvovirus-like sequence of 1,346 bases, including the C terminus of the nonstructural (NS) protein and the N terminus of the viral protein (VP) (GenBank accession number HM228877). Phylogenetic analysis based on this partial NS region demonstrated that this virus fell between sequences belonging to the subfamily Parvovirinae, infecting mammals/birds, and those belonging to the subfamily Densovirinae, infecting insects (Fig. 6).
FIG. 6.
Phylogenetic analysis of bat parvovirus based on its 210-aa partial nonstructural (NS) ORF. The NS ORF of parvoviruses encodes replication-associated protein REP of ∼700 aa.
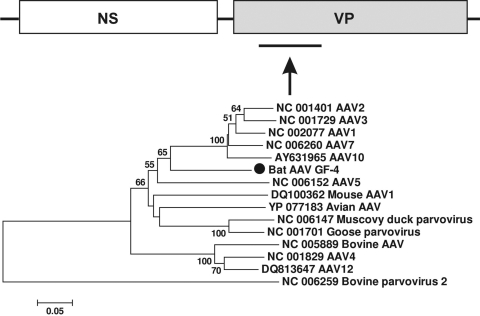
Bat adeno-associated virus and adenovirus.
Adeno-associated virus (AAV) belongs to the genus Dependovirus in the subfamily Parvovirinae. AAV usually requires coinfection with a helper adenovirus for its replication. AAV has been found in many vertebrate species, but no bat AAV has previously been reported (17, 44, 52, 53). Members of the family Adenoviridae are double-stranded DNA viruses, with relatively large genomes ranging from 26 to 45 kb. Adenovirus infection was identified in at least 40 vertebrate species, including mammals, birds, amphibians, reptiles, and fishes (35, 51, 52, 56). In the present study, we found both AAV and adenovirus sequences in guano collected from one of the California roosts (GF-4). The bat AAV sequence encoded a partial capsid protein VP1 (160 aa) (GenBank accession number HM228878), which exhibited 74% or less aa similarity over that region with known AAVs. Phylogenetically, bat AAV was related to known AAV as a deep-rooted lineage (Fig. 7). The bat adenovirus-related sequence was short (60 aa), showing 82% similarity over that region with that of polypeptide VIII of the capsid protein of tree shrew adenovirus (227 aa) (GenBank accession number HM234169). It also showed 80% aa similarity with the sequence of the recently characterized bat adenovirus strain TJM (polypeptide VIII; 222 aa) (35). The same genome region was not described for the other available bat adenovirus strains FBV1 and PPV1 (56).
FIG. 7.
Phylogenetic analysis of bat AAV based on its 160-aa partial capsid protein VP1 region. The VP1 protein of AAVs is ∼700 aa.
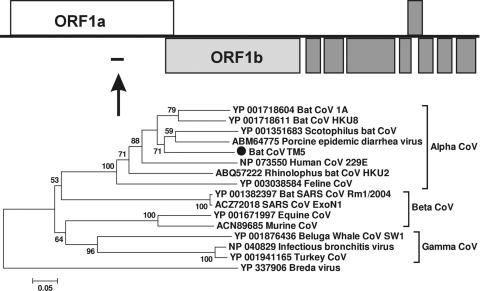
Bat coronavirus.
The family Coronaviridae includes positive single-stranded RNA viruses, with genomes of 16 to 31 kb. Bat coronaviruses have received much attention since the global outbreak caused by severe acute respiratory syndrome coronavirus (SARS-CoV), and numerous bat coronaviruses (CoVs) have been identified from different bat species (32). In our study, a CoV sequence (116 aa) (GenBank accession number HM234168) was detected in one of the guano samples from Texas (TM5), showing a best match to bat genus Scotophilus CoV 512 (73% aa identity). Phylogenetic analysis based on this partial replicase region confirmed that this virus is most closely related to Scotophilus bat CoV 512 (nonstructural proteins 8 and 9; 303 aa) but forms a distinct genetic lineage (Fig. 8).
FIG. 8.
Phylogenetic analysis of bat CoV based on its 110-aa partial replicase region. Nonstructural proteins 8 and 9 of CoV are ∼300 aa.
Another guano virus-like sequence had more ambiguous viral origins. A sequence of 200 aa in length showed a best BLASTx match to a simian hepatitis A virus in the family Picornaviridae (E value of 4e−17) (GenBank accession number HM228879) but phylogenetically fell between the families Picornaviridae and Dicistroviridae, reflecting the possible presence of a novel viral family (data not shown).
DISCUSSION
Our study examined the viral assemblages in the guano of bats from California and Texas. The viral metagenomic approach used here, involving the partial purification of viral nucleic acids, random PCR amplification, and pyrosequencing, detected viral sequences very closely related to known viruses as well as novel viruses.
The proportion of phage sequences was relatively low, compared with that of the viromes reported for human and equine feces (3, 4, 6, 60), consisting mostly of siphophages. The eukaryotic viruses included species from multiple DNA and RNA viral families. The majority of the eukaryotic viruses in bat guano from California and Texas were related to viruses infecting insects and plants. The presence of insect viruses in guano was not unexpected, considering the bat species from which we obtained guano are insectivorous. The high frequency of plant viral sequences might reflect the plant diet of the eaten insects.
Outbreaks of white-nose syndrome have been associated with infection with the Geomyces destructans fungus in the family Helotiaceae of the Ascomycota phylum (2). No outbreaks of the white-nose syndrome have been reported in Texas or California. Some of the Partitivirus-like sequences detected in guano from bats in California and Texas were related to those of viruses known to infect members of the phyla Ascomycota (which accounts for 75% of all fungal species) and Basidiomycota, indicating the likely presence of fungal viruses in the guts of bats.
The present study revealed numerous new mammalian viruses, including a highly divergent kobuvirus, astrovirus, parvovirus, AAV, adenovirus, and coronavirus. No close homologue of a known human viral pathogen was detected in our study. A large fraction of sequences was unclassifiable using BLAST methods. It is conceivable that viral sequences that are too divergent from those of known viruses to be recognized by BLAST methods were included in this large group of unclassifiable sequences.
Viral metagenomics, while providing sequence data on the most prevalent viruses present in a sample, is not currently as sensitive as PCR. For example, in a previous study, cycloviruses were found in approximately 9% of Nigerian stool samples (33), while these cycloviruses were not detected in the same cohort using viral metagenomics (data not shown). The viral survey reported here is therefore likely to underestimate the diversity of low-concentrations viruses in bat guano. Large differences in virome composition between guano samples taken only 3 days apart from the same bat roost in Texas were also observed. This result indicates that representative sampling of the enteric viruses in this population was not achieved and/or that the bat guano virome in this roost changed within a few days. Analyzing fecal pellets from a greater number of bats using deeper sequencing methods will result in improved sampling of the viral populations.
The viral metagenomic data obtained from the present study provide a preliminary view of the viromes in bat guano. Future study involving a wider sampling of bat species in different locations will doubtlessly increase our understanding of the diversity of viruses present in these mammals. Except for the recognition of viruses very closely related to known human pathogens, it is not possible to predict, based on genetic information alone, which bat viruses already are or may evolve into human pathogens, a rare occurrence also influenced by the extent of contact with bats. Further studies, such as in vitro replication using cell lines from different species in frequent contact with bats, may help define which viruses have zoonotic potential (10, 35, 63). The further characterization of the bat virome will therefore increase our understanding of mammalian virus diversity but may also be useful for the detection of potential zoonotic viruses. Identifying bat pathogens will also help preserve these mammals and their beneficial effects on the environment largely due to their voracious appetite for insects and role as pollinators.
Acknowledgments
We acknowledge NHLBI grant R01HL083254 and BSRI for sustained support to E.L.D. and support from Boston University's Center for Ecology and Conservation Biology to T.H.K.
The use of trade, product, or firm names is for descriptive purposes alone and does not imply endorsement by the U.S. Government.
Footnotes
Published ahead of print on 12 May 2010.
REFERENCES
- 1.Allander, T., S. U. Emerson, R. E. Engle, R. H. Purcell, and J. Bukh. 2001. A virus discovery method incorporating DNase treatment and its application to the identification of two bovine parvovirus species. Proc. Natl. Acad. Sci. U. S. A. 98:11609-11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blehert, D. S., A. C. Hicks, M. Behr, C. U. Meteyer, B. M. Berlowski-Zier, E. L. Buckles, J. T. Coleman, S. R. Darling, A. Gargas, R. Niver, J. C. Okoniewski, R. J. Rudd, and W. B. Stone. 2009. Bat white-nose syndrome: an emerging fungal pathogen? Science 323:227. [DOI] [PubMed] [Google Scholar]
- 3.Breitbart, M., M. Haynes, S. Kelley, F. Angly, R. A. Edwards, B. Felts, J. M. Mahaffy, J. Mueller, J. Nulton, S. Rayhawk, B. Rodriguez-Brito, P. Salamon, and F. Rohwer. 2008. Viral diversity and dynamics in an infant gut. Res. Microbiol. 159:367-373. [DOI] [PubMed] [Google Scholar]
- 4.Breitbart, M., I. Hewson, B. Felts, J. M. Mahaffy, J. Nulton, P. Salamon, and F. Rohwer. 2003. Metagenomic analyses of an uncultured viral community from human feces. J. Bacteriol. 185:6220-6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breitbart, M., and F. Rohwer. 2005. Method for discovering novel DNA viruses in blood using viral particle selection and shotgun sequencing. Biotechniques 39:729-736. [DOI] [PubMed] [Google Scholar]
- 6.Calisher, C. H., J. E. Childs, H. E. Field, K. V. Holmes, and T. Schountz. 2006. Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 19:531-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cann, A. J., S. E. Fandrich, and S. Heaphy. 2005. Analysis of the virus population present in equine faeces indicates the presence of hundreds of uncharacterized virus genomes. Virus Genes 30:151-156. [DOI] [PubMed] [Google Scholar]
- 8.Chu, D. K., L. L. Poon, Y. Guan, and J. S. Peiris. 2008. Novel astroviruses in insectivorous bats. J. Virol. 82:9107-9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chua, K. B., G. Crameri, A. Hyatt, M. Yu, M. R. Tompang, J. Rosli, J. McEachern, S. Crameri, V. Kumarasamy, B. T. Eaton, and L. F. Wang. 2007. A previously unknown reovirus of bat origin is associated with an acute respiratory disease in humans. Proc. Natl. Acad. Sci. U. S. A. 104:11424-11429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crameri, G., S. Todd, S. Grimley, J. A. McEachern, G. A. Marsh, C. Smith, M. Tachedjian, C. De Jong, E. R. Virtue, M. Yu, D. Bulach, J. P. Liu, W. P. Michalski, D. Middleton, H. E. Field, and L. F. Wang. 2009. Establishment, immortalisation and characterisation of pteropid bat cell lines. PLoS One 4:e8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delwart, E. L. 2007. Viral metagenomics. Rev. Med. Virol. 17:115-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Miranda, J. R., M. Drebot, S. Tyler, M. Shen, C. E. Cameron, D. B. Stoltz, and S. M. Camazine. 2004. Complete nucleotide sequence of Kashmir bee virus and comparison with acute bee paralysis virus. J. Gen. Virol. 85:2263-2270. [DOI] [PubMed] [Google Scholar]
- 13.Drexler, J. F., V. M. Corman, F. Gloza-Rausch, A. Seebens, A. Annan, A. Ipsen, T. Kruppa, M. A. Muller, E. K. Kalko, Y. Adu-Sarkodie, S. Oppong, and C. Drosten. 2009. Henipavirus RNA in African bats. PLoS One 4:e6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng, H., M. Shuda, Y. Chang, and P. S. Moore. 2008. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 319:1096-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkbeiner, S. R., A. F. Allred, P. I. Tarr, E. J. Klein, C. D. Kirkwood, and D. Wang. 2008. Metagenomic analysis of human diarrhea: viral detection and discovery. PLoS Pathog. 4:e1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkbeiner, S. R., L. R. Holtz, Y. Jiang, P. Rajendran, C. J. Franz, G. Zhao, G. Kang, and D. Wang. 2009. Human stool contains a previously unrecognized diversity of novel astroviruses. Virol. J. 6:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao, G., M. R. Alvira, S. Somanathan, Y. Lu, L. H. Vandenberghe, J. J. Rux, R. Calcedo, J. Sanmiguel, Z. Abbas, and J. M. Wilson. 2003. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc. Natl. Acad. Sci. U. S. A. 100:6081-6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaynor, A. M., M. D. Nissen, D. M. Whiley, I. M. Mackay, S. B. Lambert, G. Wu, D. C. Brennan, G. A. Storch, T. P. Sloots, and D. Wang. 2007. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 3:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Govan, V. A., N. Leat, M. Allsopp, and S. Davison. 2000. Analysis of the complete genome sequence of acute bee paralysis virus shows that it belongs to the novel group of insect-infecting RNA viruses. Virology 277:457-463. [DOI] [PubMed] [Google Scholar]
- 20.Greninger, A. L., C. Runckel, C. Y. Chiu, T. Haggerty, J. Parsonnet, D. Ganem, and J. L. DeRisi. 2009. The complete genome of klassevirus—a novel picornavirus in pediatric stool. Virol. J. 6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holtz, L. R., S. R. Finkbeiner, G. Zhao, C. D. Kirkwood, R. Girones, J. M. Pipas, and D. Wang. 2009. Klassevirus 1, a previously undescribed member of the family Picornaviridae, is globally widespread. Virol. J. 6:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones, M. S., A. Kapoor, V. V. Lukashov, P. Simmonds, F. Hecht, and E. Delwart. 2005. New DNA viruses identified in patients with acute viral infection syndrome. J. Virol. 79:8230-8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapoor, A., L. Li, J. Victoria, B. Oderinde, C. Mason, P. Pandey, S. Z. Zaidi, and E. Delwart. 2009. Multiple novel astrovirus species in human stool. J. Gen. Virol. 90:2965-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapoor, A., E. Slikas, P. Simmonds, T. Chieochansin, A. Naeem, S. Shaukat, M. M. Alam, S. Sharif, M. Angez, S. Zaidi, and E. Delwart. 2009. A newly identified bocavirus species in human stool. J. Infect. Dis. 199:196-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapoor, A., J. Victoria, P. Simmonds, E. Slikas, T. Chieochansin, A. Naeem, S. Shaukat, S. Sharif, M. M. Alam, M. Angez, C. Wang, R. W. Shafer, S. Zaidi, and E. Delwart. 2008. A highly prevalent and genetically diversified Picornaviridae genus in South Asian children. Proc. Natl. Acad. Sci. U. S. A. 105:20482-20487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khamrin, P., N. Maneekarn, A. Kongkaew, S. Kongkaew, S. Okitsu, and H. Ushijima. 2009. Porcine kobuvirus in piglets, Thailand. Emerg. Infect. Dis. 15:2075-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khamrin, P., N. Maneekarn, S. Peerakome, S. Okitsu, M. Mizuguchi, and H. Ushijima. 2008. Bovine kobuviruses from cattle with diarrhea. Emerg. Infect. Dis. 14:985-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristensen, D. M., A. R. Mushegian, V. V. Dolja, and E. V. Koonin. 2010. New dimensions of the virus world discovered through metagenomics. Trends Microbiol. 18:11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar, S., M. Nei, J. Dudley, and K. Tamura. 2008. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform. 9:299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunz, T. H., and M. B. Fenton. 2003. Bat Ecology. University of Chicago Press, Chicago, IL.
- 31.Kuzmin, I. V., M. Niezgoda, R. Franka, B. Agwanda, W. Markotter, J. C. Beagley, O. Y. Urazova, R. F. Breiman, and C. E. Rupprecht. 2008. Possible emergence of West Caucasian bat virus in Africa. Emerg. Infect. Dis. 14:1887-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau, S. K., K. S. Li, Y. Huang, C.-T. Shek, H. Tse, M. Wang, G. K. Choi, H. Xu, C. S. Lam, R. Guo, K.-H. Chan, B.-J. Zheng, P. C. Woo, and K.-Y. Yuen. 2010. Ecoepidemiology and complete genome comparison of different strains of severe acute respiratory syndrome-related Rhinolophus bat coronavirus in China reveal bats as a reservoir for acute, self-limiting infection that allows recombination events. J. Virol. 84:2808-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, L., A. Kapoor, B. Slikas, O. S. Bamidele, C. Wang, S. Shaukat, M. A. Masroor, M. L. Wilson, J. B. Ndjango, M. Peeters, N. D. Gross-Camp, M. N. Muller, B. H. Hahn, N. D. Wolfe, H. Triki, J. Bartkus, S. Z. Zaidi, and E. Delwart. 2010. Multiple diverse circoviruses infect farm animals and are commonly found in human and chimpanzee feces. J. Virol. 84:1674-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, L., J. Victoria, A. Kapoor, O. Blinkova, C. Wang, F. Babrzadeh, C. J. Mason, P. Pandey, H. Triki, O. Bahri, B. S. Oderinde, M. M. Baba, D. N. Bukbuk, J. M. Besser, J. M. Bartkus, and E. L. Delwart. 2009. A novel picornavirus associated with gastroenteritis. J. Virol. 83:12002-12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, Y., X. Ge, H. Zhang, P. Zhou, Y. Zhu, Y. Zhang, J. Yuan, L.-F. Wang, and Z. Shi. 2010. Host range, prevalence, and genetic diversity of adenoviruses in bats. J. Virol. 84:3889-3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, C., J. Zhang, F. Yi, J. Wang, X. Wang, H. Jiang, J. Xu, and Y. Hu. 2006. Isolation and RNA1 nucleotide sequence determination of a new insect nodavirus from Pieris rapae larvae in Wuhan city, China. Virus Res. 120:28-35. [DOI] [PubMed] [Google Scholar]
- 37.Luby, S. P., E. S. Gurley, and M. J. Hossain. 2009. Transmission of human infection with Nipah virus. Clin. Infect. Dis. 49:1743-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackenzie, J. S. 2005. Emerging zoonotic encephalitis viruses: lessons from Southeast Asia and Oceania. J. Neurovirol. 11:434-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maeda, K., E. Hondo, J. Terakawa, Y. Kiso, N. Nakaichi, D. Endoh, K. Sakai, S. Morikawa, and T. Mizutani. 2008. Isolation of novel adenovirus from fruit bat (Pteropus dasymallus yayeyamae). Emerg. Infect. Dis. 14:347-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKnight, C. A., A. G. Wise, R. K. Maes, C. Howe, A. Rector, M. Van Ranst, and M. Kiupel. 2006. Papillomavirus-associated basosquamous carcinoma in an Egyptian fruit bat (Rousettus aegyptiacus). J. Zoo Wildl. Med. 37:193-196. [DOI] [PubMed] [Google Scholar]
- 41.Messenger, S. L., C. E. Rupprecht, and J. S. Smith. 2003. Bats, emerging virus infections, and the rabies paradigm, p. 622-679. In T. H. Kunz and M. B. Fenton (ed.), Bat ecology. University of Chicago Press, Chicago, IL.
- 42.Misra, V., T. Dumonceaux, J. Dubois, C. Willis, S. Nadin-Davis, A. Severini, A. Wandeler, R. Lindsay, and H. Artsob. 2009. Detection of polyoma and corona viruses in bats of Canada. J. Gen. Virol. 90:2015-2022. [DOI] [PubMed] [Google Scholar]
- 43.Monroe, S., M. Carter, J. Hermann, D. Mitchell, and A. Sanchez-Fauquier. 2005. Astroviridae, p. 859-864. In C. Fauquet, M. Mayo, J. Maniloff, U. Desselberger, and L. Ball (ed.), Virus taxonomy: eighth report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, CA.
- 44.Mori, S., L. Wang, T. Takeuchi, and T. Kanda. 2004. Two novel adeno-associated viruses from cynomolgus monkey: pseudotyping characterization of capsid protein. Virology 330:375-383. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura, S., C. S. Yang, N. Sakon, M. Ueda, T. Tougan, A. Yamashita, N. Goto, K. Takahashi, T. Yasunaga, K. Ikuta, T. Mizutani, Y. Okamoto, M. Tagami, R. Morita, N. Maeda, J. Kawai, Y. Hayashizaki, Y. Nagai, T. Horii, T. Iida, and T. Nakaya. 2009. Direct metagenomic detection of viral pathogens in nasal and fecal specimens using an unbiased high-throughput sequencing approach. PLoS One 4:e4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Omatsu, T., S. Watanabe, H. Akashi, and Y. Yoshikawa. 2007. Biological characters of bats in relation to natural reservoir of emerging viruses. Comp. Immunol. Microbiol. Infect. Dis. 30:357-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quan, P. L., S. Junglen, A. Tashmukhamedova, S. Conlan, S. K. Hutchison, A. Kurth, H. Ellerbrok, M. Egholm, T. Briese, F. H. Leendertz, and W. I. Lipkin. 2010. Moussa virus: a new member of the Rhabdoviridae family isolated from Culex decens mosquitoes in Cote d'Ivoire. Virus Res. 147:17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reuter, G., A. Boldizsar, and P. Pankovics. 2009. Complete nucleotide and amino acid sequences and genetic organization of porcine kobuvirus, a member of a new species in the genus Kobuvirus, family Picornaviridae. Arch. Virol. 154:101-108. [DOI] [PubMed] [Google Scholar]
- 49.Rivera, R., H. H. Nollens, S. Venn-Watson, F. M. Gulland, and J. F. Wellehan, Jr. 2010. Characterization of phylogenetically diverse astroviruses of marine mammals. J. Gen. Virol. 91:166-173. [DOI] [PubMed] [Google Scholar]
- 50.Rosario, K., S. Duffy, and M. Breitbart. 2009. Diverse circovirus-like genome architectures revealed by environmental metagenomics. J. Gen. Virol. 90:2418-2424. [DOI] [PubMed] [Google Scholar]
- 51.Roy, S., L. H. Vandenberghe, S. Kryazhimskiy, R. Grant, R. Calcedo, X. Yuan, M. Keough, A. Sandhu, Q. Wang, C. A. Medina-Jaszek, J. B. Plotkin, and J. M. Wilson. 2009. Isolation and characterization of adenoviruses persistently shed from the gastrointestinal tract of non-human primates. PLoS Pathog. 5:e1000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmidt, M., H. Katano, I. Bossis, and J. A. Chiorini. 2004. Cloning and characterization of a bovine adeno-associated virus. J. Virol. 78:6509-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidt, M., A. Voutetakis, S. Afione, C. Zheng, D. Mandikian, and J. A. Chiorini. 2008. Adeno-associated virus type 12 (AAV12): a novel AAV serotype with sialic acid-and heparan sulfate proteoglycan-independent transduction activity. J. Virol. 82:1399-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simmons, N. B. 2005. Chiropttera, p. 312-529. In D. E. Wilson and D. M. Reeder (ed.), Mammal species of the world: a taxonomic reference. Smithsonian Institution Press, Washington, DC.
- 55.Skliris, G. P., J. V. Krondiris, D. C. Sideris, A. P. Shinn, W. G. Starkey, and R. H. Richards. 2001. Phylogenetic and antigenic characterization of new fish nodavirus isolates from Europe and Asia. Virus Res. 75:59-67. [DOI] [PubMed] [Google Scholar]
- 56.Sonntag, M., K. Muhldorfer, S. Speck, G. Wibbelt, and A. Kurth. 2009. New adenovirus in bats, Germany. Emerg. Infect. Dis. 15:2052-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tattersall, P., M. Bergoin, M. Bloom, K. Brown, R. Linden, and P. Tijssen. 2005. Paroviridae, p. 353-369. In C. Fauquet, M. Mayo, J. Maniloff, U. Desselberger, and L. Ball (ed.), Virus taxonomy: eighth report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, CA.
- 58.Teeling, E. C., M. S. Springer, O. Madsen, P. Bates, S. J. O'Brien, and W. J. Murphy. 2005. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 307:580-584. [DOI] [PubMed] [Google Scholar]
- 59.van der Poel, W. H., P. H. Lina, and J. A. Kramps. 2006. Public health awareness of emerging zoonotic viruses of bats: a European perspective. Vector Borne Zoonotic Dis. 6:315-324. [DOI] [PubMed] [Google Scholar]
- 60.Victoria, J. G., A. Kapoor, L. Li, O. Blinkova, B. Slikas, C. Wang, A. Naeem, S. Zaidi, and E. Delwart. 2009. Metagenomic analyses of viruses in stool samples from children with acute flaccid paralysis. J. Virol. 83:4642-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang, L. F., E. Hansson, M. Yu, K. B. Chua, N. Mathe, G. Crameri, B. K. Rima, J. Moreno-Lopez, and B. T. Eaton. 2007. Full-length genome sequence and genetic relationship of two paramyxoviruses isolated from bat and pigs in the Americas. Arch. Virol. 152:1259-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watanabe, S., N. Ueda, K. Iha, J. S. Masangkay, H. Fujii, P. Alviola, T. Mizutani, K. Maeda, D. Yamane, A. Walid, K. Kato, S. Kyuwa, Y. Tohya, Y. Yoshikawa, and H. Akashi. 2009. Detection of a new bat gammaherpesvirus in the Philippines. Virus Genes 39:90-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wibbelt, G., S. Speck, and H. Field. 2009. Methods for assessing diseases in bats, p. 775-794. In T. H. Kunz and S. Parsons (ed.), Ecological and behavioral methods for the study of bats. Johns Hopkins University Press, Baltimore, MD.
- 64.Willner, D., M. Furlan, M. Haynes, R. Schmieder, F. E. Angly, J. Silva, S. Tammadoni, B. Nosrat, D. Conrad, and F. Rohwer. 2009. Metagenomic analysis of respiratory tract DNA viral communities in cystic fibrosis and non-cystic fibrosis individuals. PLoS One 4:e7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong, S., S. Lau, P. Woo, and K. Y. Yuen. 2007. Bats as a continuing source of emerging infections in humans. Rev. Med. Virol. 17:67-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamashita, T., M. Ito, Y. Kabashima, H. Tsuzuki, A. Fujiura, and K. Sakae. 2003. Isolation and characterization of a new species of kobuvirus associated with cattle. J. Gen. Virol. 84:3069-3077. [DOI] [PubMed] [Google Scholar]
- 67.Zhang, T., M. Breitbart, W. H. Lee, J. Q. Run, C. L. Wei, S. W. Soh, M. L. Hibberd, E. T. Liu, F. Rohwer, and Y. Ruan. 2006. RNA viral community in human feces: prevalence of plant pathogenic viruses. PLoS Biol. 4:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu, H. C., D. K. Chu, W. Liu, B. Q. Dong, S. Y. Zhang, J. X. Zhang, L. F. Li, D. Vijaykrishna, G. J. Smith, H. L. Chen, L. L. Poon, J. S. Peiris, and Y. Guan. 2009. Detection of diverse astroviruses from bats in China. J. Gen. Virol. 90:883-887. [DOI] [PubMed] [Google Scholar]