Abstract
Respiratory syncytial virus (RSV) is the etiological agent of acute respiratory diseases, such as bronchiolitis and pneumonia. The exacerbated production of proinflammatory cytokines and chemokines in the airways in response to RSV is an important pillar in the development of these pathologies. As such, a keen understanding of the mechanisms that modulate the inflammatory response during RSV infection is of pivotal importance to developing effective treatment. The NF-κB transcription factor is a major regulator of proinflammatory cytokine and chemokine genes. However, RSV-mediated activation of NF-κB is far from characterized. We recently demonstrated that aside from the well-characterized IκBα phosphorylation and degradation, the phosphorylation of p65 at Ser536 is an essential event regulating the RSV-mediated NF-κB-dependent promoter transactivation. In the present study, using small interfering RNA and pharmacological inhibitors, we now demonstrate that RSV sensing by the RIG-I cytoplasmic receptor triggers a signaling cascade involving the MAVS and TRAF6 adaptors that ultimately leads to p65ser536 phosphorylation by the IKKβ kinase. In a previous study, we highlighted a critical role of the NOX2-containing NADPH oxidase enzyme as an upstream regulator of both the IκBαSer32 and p65Ser536 in human airway epithelial cells. Here, we demonstrate that inhibition of NOX2 significantly decreases IKKβ activation. Taken together, our data identify a new RIG-I/MAVS/TRAF6/IKKβ/p65Ser536 pathway placed under the control of NOX2, thus characterizing a novel regulatory pathway involved in NF-κB-driven proinflammatory response in the context of RSV infection.
Respiratory syncytial virus (RSV) is a major lung tropic respiratory pathogen typically causing mild illnesses. However, in infants, immunocompromised patients, and the elderly, acute lower respiratory diseases such as pneumonia and bronchiolitis can occur, which are associated with substantial rates of morbidity and mortality (24). Because RSV is highly contagious, virtually all children have at least one episode of RSV infection by the age of 3 years, leading to the most frequent reason for hospitalization of infants in developed countries (67). In addition, RSV is thought to be associated with long-term complications, such as recurrent wheezing and asthma (28, 61). Despite the major impact of this pathogen on human health, vaccines or effective antiviral treatment aimed at controlling RSV infection are not yet available (67). One of the major barriers to the establishment of an efficient treatment is linked to the absence of a complete understanding of the molecular mechanisms involved in the development of RSV-induced airway pathologies. Experimental evidence suggests that excessive inflammation triggered by the host plays a major role in the development of the clinical manifestations (17). Particularly, proinflammatory cytokines and chemokines, including RANTES, macrophage inflammatory protein 1α (MIP-1α), MCP-1, interleukin-8 (IL-8), and IP-10, produced by airway epithelial cells (AEC) in large and small airways are considered major players in the RSV-induced respiratory pathogenesis (3, 17, 40, 43, 46, 50, 52, 72). The mechanisms involved in the regulation of cytokines and chemokines in AEC are not well defined but rely mainly on the tight regulation of the NF-κB transcription factor activity. Infection with RSV induces a persistent activation of NF-κB, which likely leads to excessive NF-κB-mediated inflammatory genes expression (5, 13). Hence, elucidating the mechanisms of NF-κB activation in AEC after RSV infection has strong potential to the development of anti-inflammatory therapies.
RSV is a member of the family of single-stranded RNA Paramyxoviridae viruses. Cytoplasmic sensing of viral nucleic acids of RNA viruses is mediated by the recently identified family of ubiquitous DEXD/H-box helicase receptors (reviewed in references 2 and 44). Initially, retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA-5) members of DEXD/H-box helicases were thought to discriminate among different ligands and RNA viruses. However, several studies suggest a possible redundant function, as reported for influenza (62) or measles (4) viruses. The signaling pathways downstream of RIG-I and MDA-5 are initiated via a shared mechanism involving a caspase recruitment domain (CARD)-mediated interaction with the outer mitochondrial membrane-associated adaptor MAVS, also known as CARDIF/IPS-1/VISA (32). Once activated, MAVS dimerizes (1, 63) to provide an interface for the direct binding of various interacting partners, including members of the tumor necrosis factor (TNF) receptor-associated factor (TRAF) family, TRAF2, TRAF3, and TRAF6 (51, 59, 68), to form a macromolecular signaling complex that allows activation of diverse downstream signaling pathways. The role of RIG-I in RSV-induced NF-κB activation in A549 cells has previously been documented (36), but the role of MDA-5 remains unsolved. Interestingly, in a mouse model, while RIG-I was confirmed to play an essential role in RSV recognition, as demonstrated through its role in interferon (IFN)-stimulated gene (ISG) induction, MDA-5 was found to have an auxiliary function (37). However, in HeLa cells, while RIG-I was found to be critical for RSV-induced IFN-β production, only a minimal role for MDA-5 was observed (55). Besides the unclear role of MDA-5, downstream adaptors allowing RSV-induced NF-κB activation have not yet been characterized.
The NF-κB family of transcription factors is comprised of five mammalian members—p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), cRel, and RelB—involved in inflammatory and immunomodulatory responses, cell proliferation, and cell survival (18, 66). In unstimulated cells, cytoplasmic NF-κB subunits, mainly p65/p50 dimer in epithelial cells, interact with the inhibitory IκB molecules. The IκB-NF-κB complexes shuttle between the nucleus and the cytoplasm, with an equilibrium in favor of the cytoplasm (27). The classical NF-κB activation cascade is initiated by the stimulus-induced phosphorylation of the IκBα inhibitor by the IKK complex (IKKα/β/γ) that leads to IκBα polyubiquitination and subsequent degradation by the proteasome, liberation of NF-κB dimers, nuclear accumulation, and transcription of NF-κB target genes (27). The mechanisms of NF-κB activation in AEC during RSV infection are not fully characterized, but the importance of this classical pathway is supported by in vitro and in vivo studies (5, 8, 13, 14, 16, 22, 23, 26, 29, 38). Recently, multiple regulatory stimulus-dependent phosphorylations of Rel subunits were identified as key modulators of their nuclear localization, DNA-binding affinity, coactivator/corepressor association, and transactivation capacity (reviewed in references 45 and 56). We and others have previously shown that besides the well-characterized IκBα phosphorylation, a hallmark of the classical pathway, RSV triggers phosphorylation of p65 at Ser536 in human AEC (14, 36). Individual mutation of the Ser536 phosphoacceptor site dramatically impaired NF-κB-dependent promoter transactivation by p65 during RSV infection (14).
Work over the past two decades has shed significant light on the cell type- and stimulus-specific regulation of NF-κB activation by intracellular production of reactive oxygen species (ROS) (19). ROS-dependent regulation of NF-κB in the context of RSV infection was supported by studies demonstrating that antioxidant treatment, including butylated hydroxyanisol or N-acetylcysteine, interfered with RSV-induced p65 DNA-binding and IL-8, RANTES, or MCP-1 gene expression in human AEC (8, 9, 39, 49). We previously highlighted the key role of the NOX2-containing superoxide generating NADPH oxidase enzyme as an essential upstream regulator of RSV-induced p65Ser536 phosphorylation, as well as IκBα phosphorylation in AEC, thus identifying for the first time a specific source of ROS responsible for the oxidant-dependent regulation of NF-κB observed in RSV (14).
The signaling pathway leading to p65Ser536 phosphorylation appears to be stimulus specific. While IKKα/β phosphorylate p65Ser536 in vivo in response to various stimuli, including lipopolysaccharide, Ang II, TNF-α, or lymphotoxin β receptor signaling (12, 31, 53, 54), the IKK-related kinases, TBK1 and IKKɛ, phosphorylate p65Ser536 in response to TNF-α, IL-1, or the hepatitis B viral X protein (7, 15, 33). Additionally, the RSK-1 kinase phosphorylates p65Ser536 after Ang II stimulation or in response to p53 (6, 71). However, which of these kinases is involved in RSV-induced p65Ser536 phosphorylation and its NOX2-dependent regulation remains to be determined. Considering p65Ser536 phosphorylation as a potential therapeutic target to limit RSV-induced NF-κB activation and the resulting proinflammatory response, we deciphered the signaling pathway triggering p65Ser536 phosphorylation during RSV infection. In the present study, we show that RIG-I, but not MDA-5, is essential for the initiation of the signaling cascade leading to RSV-induced p65Ser536 phosphorylation, via a process that is dependent on MAVS, TRAF6, and the IKKβ kinase. Supporting our previous report that p65Ser536 is controlled by NOX2, we now demonstrate that RSV-induced IKK activity is dependent on NOX2.
MATERIALS AND METHODS
Reagents and plasmids.
Dimethyl sulfoxide (DMSO) and bovine serum albumin (BSA) were purchased from Sigma-Aldrich. U0126 was purchased from Biomol International, and the IKK2-IV inhibitor was purchased from Calbiochem. Poly(I:C) was from Invivogen. The pRL-null Renilla, P2(2×)TK-pGL3 NF-κB luciferase reporter, and ISG56prom-pGL3 interferon regulatory factor 3 (IRF-3) luciferase reporter constructs were previously described (14, 21). The IκBα (amino acids [aa] 1 to 55)-pGex-KG construct encoding the recombinant glutathione S-transferase (GST)-IκBα (aa 1 to 55) and the pTrack-flag-IKKβK44A plasmid encoding the dominant-negative form of IKKβ were as described earlier (25). The pCEP4-CD40 encoding plasmid (described in reference 48) and the CD40L-pcDNA3 encoding constructs were kindly provided by W. Mourad (CRCHUM, Montreal, Quebec, Canada).
RNAi oligonucleotide sequences and transfection.
Small interfering RNA (siRNA) oligonucleotides were obtained from Dharmacon. Target sequences of the RNAi used against the different genes are described in Table 1. siRNA oligonucleotide transfection was performed using the Oligofectamine reagent (Invitrogen) as previously described (14).
TABLE 1.
siRNA oligonucleotide sequences used in this study
| Target gene | Oligonucleotide | Sequence (5′-3′) | Source or reference |
|---|---|---|---|
| CTRL | Target | NNCAUAGCGUCCUUGAUCACA | 14 |
| Sense oligonucleotide | CAUAGCGUCCUUGAUCACAUU | 14 | |
| Antisense oligonucleotide | UGUGAUCAAGGACGCUAUGUU | 14 | |
| NOX2 | Target | NNGAAGACAACUGGACAGGAA | 14 |
| Sense oligonucleotide | GAAGACAACUGGACAGGAAUU | 14 | |
| Antisense oligonucleotide | UUCCUGUCCAGUUGUCUUCUU | 14 | |
| RIG-I | Target | NNAACGAUUCCAUCACUAUCCAU | Custom designed |
| Sense oligonucleotide | AACGAUUCCAUCACUAUCCAUdTdT | Custom designed | |
| Antisense oligonucleotide | AUGGAUAGUGAUGGAAUCGUUdTdT | Custom designed | |
| MDA-5 | Target | NNGGUGAAGGAGCAGAUUCAG | 41 |
| Sense oligonucleotide | GGUGAAGGAGCAGAUUCAGdTdT | 41 | |
| Antisense oligonucleotide | CUGAAUCUGCUCCUUCACCdTdT | 41 | |
| MAVS | Target | NNCCCACAGGGUCAGUUGUAU | 41 |
| Sense oligonucleotide | CCCACAGGGUCAGUUGUAUdTdT | 41 | |
| Antisense oligonucleotide | AUACAACUGACCCUGUGGGdTdT | 41 | |
| TRAF2 | Target | NNAGGGCAUAUAUGAAGAAGGCA | 64 |
| Sense oligonucleotide | AGGGCAUAUAUGAAGAAGGCAdTdT | 64 | |
| Antisense oligonucleotide | UGCCUUCUUCAUAUAUGCCCUdTdT | 64 | |
| TRAF3 | Target | NNAGAGUCAGGUUCCGAUGAU | 35 |
| Sense oligonucleotide | AGAGUCAGGUUCCGAUGAUdTdT | 35 | |
| Antisense oligonucleotide | AUCAUCGGAACCUGACUCUdTdT | 35 | |
| TRAF6 | Target | NNCUGUGCUGCAUCAAUGGCA | 11 |
| Sense oligonucleotide | CUGUGCUGCAUCAAUGGCAdTdT | 11 | |
| Antisense oligonucleotide | UGCCAUUGAUGCAGCACAGdTdT | 11 | |
| IKKɛ | Target | NNGAAGCAUCCAGCAGAUUCA | 60 |
| Sense oligonucleotide | GAAGCAUCCAGCAGAUUCAdTdT | 60 | |
| Antisense oligonucleotide | UGAAUCUGCUGGAUGCUUCdTdT | 60 | |
| IKKα | Target | NNGCAGGCUCUUUCAGGGACA | 54 |
| Sense oligonucleotide | GCAGGCUCUUUCAGGGACAUU | 54 | |
| Antisense oligonucleotide | UGUCCCUGAAAGAGCCUGCUU | 54 | |
| IKKβ | Target | NNGGUGGAAGAGGUGGUGAGC | 54 |
| Sense oligonucleotide | GGUGGAAGAGGUGGUGAGCUU | 54 | |
| Antisense oligonucleotide | GCUCACCACCUCUUCCACCUU | 54 | |
| TBK1 | Target | NNGCGGCAGAGUUAGGUGAAA | 60 |
| Sense oligonucleotide | GCGGCAGAGUUAGGUGAAAdTdT | 60 | |
| Antisense oligonucleotide | UUUCACCUAACUCUGCCGCdTdT | 60 |
Cell culture and infections.
A549 cells (American Type Culture Collection) were grown in F-12 nutrient mixture (Ham) medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (HI-FBS; Gibco) and 1% l-glutamine (Gibco). The initial stock of RSV A2 strain was obtained from Advanced Biotechnologies, Inc. Amplification was performed in HEp-2 cells (American Type Culture Collection) at a multiplicity of infection (MOI) of 0.1 until a 50% cytopathic effect was observed. Virus was purified by ultracentrifugation on 30% sucrose cushion after overnight centrifugation at 8,000 × g. The virus titer was determined by methylcellulose plaque-forming assays as previously described (20). UV inactivation of RSV A2 was performed by exposing the purified virus stock, kept on ice in serum-free medium (SFM), to UV light (∼20-cm distance) for 20 min. Inactivation was confirmed by using the methylcellulose plaque-forming assay. Infection of subconfluent A549 was performed at an MOI of 3, unless otherwise stated, in culture medium containing 2% HI-FBS. Where indicated, inhibitors or the corresponding vehicle were added for 1 h in SFM before the addition of RSV and 2% HI-FBS.
Luciferase reporter assays.
A549 cells in 24-well plates were cotransfected with the pRL-null Renilla (internal control, 50 ng), the P2(2×)TK-pGL3NF-κB, or the ISG56prom-pGL3 luciferase reporter (100 ng) constructs and the indicated expression plasmid (up to 500 ng) using the TransIT-LT1 transfection reagent (Mirus). Where indicated, RSV infection was performed at 8 h posttransfection. Poly(I:C) transfection was performed at 16 h posttransfection by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Quantification of luciferase was performed 24 h posttransfection using a dual luciferase reporter assay kit (Promega) according to the manufacturer's instructions. Relative luciferase activities were calculated as the luciferase to Renilla ratio.
Immunoblot analysis.
Whole-cell extracts (WCE) were prepared on ice in Nonidet P-40 (Igepal; Sigma) lysis buffer (14), quantified by using a Bio-Rad protein assay (Bio-Rad, Hercules, CA), and resolved by SDS-PAGE electrophoresis, followed by immunoblot analysis. Proteins were immunodetected using anti-p65 (Santa Cruz), anti-p65phosphoSer536 (Cell Signaling), anti-IκBα (Cell Signaling), anti-IκBαphosphoSer32 (Cell Signaling), anti-IRF-3-phosphoSer396 (58), anti-IRF-3 (Active Motif), anti-IKKβ (Imgenex), anti-IKKα (Cell Signaling), anti-TBK1 (Imgenex), anti-IKKɛ (eBioscience), anti-MAVS (Alexis Biochemicals), anti-RIG-I (Alexis Biochemicals), anti-MDA-5 (Alexis Biochemicals), anti-TRAF2 (BD Pharmingen), anti-TRAF3 (Santa Cruz), anti-TRAF6 (Santa Cruz), anti-ERK1/2 Thr202/204 Thr185/187 phospho-specific (Cell Signaling), anti-ERK1/2 (Cell Signaling), anti-actin (Chemicon International), and anti-RSV (Chemicon International) diluted in phosphate-buffered saline (PBS) containing 0.5% Tween and either 5% nonfat dry milk or 5% BSA. The membranes were further incubated for 1 h with horseradish peroxidase (HRP)-conjugated goat anti-rabbit or goat anti-mouse IgG (Kirkegaard & Perry Laboratories) in blocking solution. For NOX2 detection, cells were scraped and sonicated in 125 mM Tris-HCl (pH 6.8)-10% glycerol-2% sodium dodecyl sulfate (SDS)-0.1 M dithiothreitol (DTT) buffer containing 10 μg of leupeptin/ml, 20 μg of aprotinin/ml, and 1 μM pepstatin. Samples were quantified by using an RC-DC protein quantification assay (Bio-Rad), resolved by SDS-PAGE, and analyzed by immunoblotting with anti-gp91phox-Cter (obtained from G. Brandolin, CEA-Grenoble, Grenoble, France). The membranes were further incubated for 1 h with HRP-protein A (Bio-Rad). Equal loading was ensured by using anti-tubulin (Santa-Cruz) antibodies, followed by HRP-conjugated goat anti-mouse IgG. Immunoreactive bands were visualized by enhanced chemiluminescence using the Western Lightning Chemiluminescence Reagent Plus (Perkin-Elmer Life Sciences).
In vitro kinase assays.
Recombinant GST-IκBα fusion proteins used as substrates were produced in Escherichia coli BL21(DE3)/pLysS bacteria transformed with the IκBα (aa 1 to 55)-pGex-KG expression plasmid after induction with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 3 h at 37°C. After induction, bacterial pellet was lysed in PBS with 1% Triton X-100 and sonicated. Bacterial lysate was cleared by centrifugation for 15 min at 10,000 rpm at 4°C. Purification was performed by pulldown with glutathione-Sepharose beads. In vitro kinase assays were conducted using 80 μg of WCE immunoprecipitated by 1 μg of IKKγ antibodies (Santa Cruz). Immunocomplexes were washed in IKK kinase buffer (20 mM HEPES, 150 mM NaCl, 20 mM MgCl2, 1 mM DTT, 0.1 mM sodium orthovanadate, 20 mM glycerophosphate, 10 mM p-nitrophenylphosphate) and used in the kinase reaction with 10 μCi of [γ-32P] ATP, 20 μM ATP, and 1.0 μg of GST-IκBα (aa 1 to 55) substrate at 30°C for 30 min in kinase buffer. After resolution by SDS-PAGE, the substrate was detected by Coomassie blue staining of the lower part of the gel, and radioactivity incorporation (32P) was quantified by using a Typhoon Trio apparatus (Amersham Biosciences). The upper part of the gel was transferred to nitrocellulose membrane and IKKβ was detected by using anti-IKKβ (Imgenex).
Statistical analyses.
Data are presented as the mean ± the standard error of the mean (SEM). The statistical significance for comparison of two means was assessed by an unpaired Student t test. Analyses were performed using the Prism 5 software (GraphPad). Statistical relevance was evaluated using the following P values: P < 0.05 (*), P < 0.01 (**), or P < 0.001 (***).
RESULTS
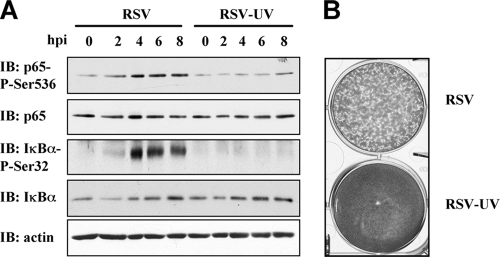
RSV-induced p65Ser536 phosphorylation is dependent on the recognition of replicative viral RNA by RIG-I, but not MDA-5.
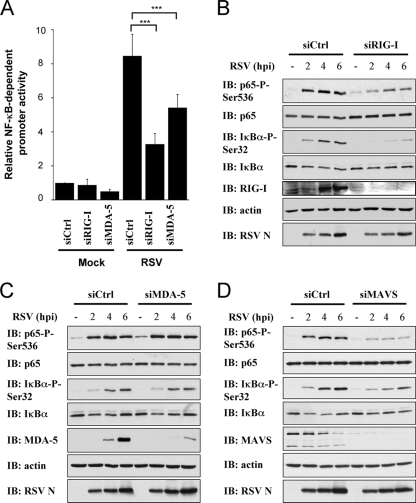
P65Ser536 phosphorylation during RSV infection of AEC plays an essential role in the regulation of p65 capacity to transactivate κB consensus-containing promoters (14). With the goal of identifying the molecular mechanisms controlling RSV-induced p65Ser536 phosphorylation, we first ascertained whether p65Ser536 phosphorylation was dependent on RSV replication. A549 cells were infected with RSV and replication-inactive UV-irradiated RSV at an MOI of 3 for 0 to 8 h postinfection (hpi). In accordance with previous data (14), RSV-induced p65Ser536 phosphorylation was detected with a similar kinetic pattern as IκBαSer32 phosphorylation, which is a hallmark of the classical pathway of NF-κB activation (Fig. 1 A). Viral replication is necessary to trigger both phosphorylations, since UV-irradiated RSV failed to trigger p65Ser536 and IκBαSer32 phosphorylations as detected by phospho-specific immunoblots. The efficiency of RSV replication disruption by UV treatment was assessed with a plaque-forming assay in Hep-2 monolayers (Fig. 1B). The role of RIG-I and MDA-5 in RSV-induced p65Ser536 phosphorylation was next investigated. First, to address the respective roles of RIG-I and MDA-5 in RSV-mediated NF-κB activation in human A549 cells, expression of RIG-I and MDA-5 was selectively abrogated by using transfection of small interfering RNA oligonucleotides (siRNA; Table 1). Interestingly, both RIG-I and MDA-5 downregulation significantly impaired the capacity of RSV to activate a NF-κB-promoter driven luciferase reporter. Stimulation of endogenous NF-κB activity by RSV was decreased by 61.3% in RIG-I-siRNA-transfected cells, and to a lesser extent by 36.2% in MDA-5-siRNA-transfected cells, compared to Ctrl-siRNA transfected cells (Fig. 2 A). Since RSV-induced NF-κB activation might result from different signaling pathways, the specific involvement of RIG-I and MDA-5 in p65Ser536 phosphorylation-dependent NF-κB activation was assessed by phospho-specific immunoblot analysis of WCE-derived from Ctrl-, RIG-I-, and MDA-5-specific siRNA (Table 1)-transfected A549 cells further infected with RSV for various times (0 to 6 h). In addition, to confirm the involvement of DEXD/H-box helicase receptors-dependent pathway, specific downregulation of MAVS by siRNA (Table 1) was performed. Although RSV potently stimulated p65Ser536 phosphorylation in Ctrl-siRNA-transfected cells, decreased expression of RIG-I or MAVS (Fig. 2B and D), but not of MDA-5 (Fig. 2C), reproducibly abolished RSV-induced p65Ser536 phosphorylation. Similar results were observed with regard to IκBαSer32 phosphorylation (Fig. 2B to D). It is noteworthy that MAVS levels decreased over time during RSV infection (Fig. 2D). This degradation was found to be proteasome dependent (data not shown) and thus most likely reflects the recently described proteasomal degradation of MAVS controlled by the PCBP2/AIP4 axis (70). This is the first demonstration of MAVS degradation in the context of RSV infection. Altogether, these results demonstrate that p65Ser536 phosphorylation during RSV-infection is triggered after recognition of replicative viral RNA exclusively by the RIG-I cytoplasmic sensor. Moreover, these results highlight a function of MDA-5 in a yet-uncharacterized IκBαSer32 phosphorylation- and p65Ser536 phosphorylation-independent NF-κB activation pathway.
FIG. 1.
Effective phosphorylation of p65ser536 is dependent on RSV replication. (A) A549 cells were left untreated or infected with RSV or UV-inactivated RSV at an MOI of 3 for the indicated times (hpi). WCE were resolved by SDS-PAGE and transferred onto nitrocellulose, and proteins were detected by immunoblotting (IB) with anti-IκBαSer32 phospho-specific (IκBα-P-Ser32), anti-IκBα, anti-p65Ser536 phospho-specific (p65-P-Ser536), and anti-p65 antibodies. Equal loading was verified by using anti-actin antibodies. Representative immunoblots of three different experiments are shown. (B) PFU assay of the Hep-2 monolayer infected with RSV or UV-inactivated RSV (RSV-UV).
FIG. 2.
RIG-I sensor, but not Mda-5, triggers signaling events controlling p65Ser536 phosphorylation. A549 were transfected with control (Ctrl) (A to D), RIG-I (A and B), Mda-5 (A and C), or MAVS (D) siRNA oligonucleotides (Table 1) as described in Materials and Methods. (A) siRNA-transfected A549 cells were further transfected with the P2(2×)TK-pGL3 NF-κB firefly luciferase and the pRL-null Renilla luciferase (internal control) reporter constructs and either mock or RSV infected for 16 h. The luciferase activities were measured and are expressed as the fold activation over the nonstimulated cells after normalization with Renilla luciferase activities. (***, P < 0.001; mean ± the SEM; n = 6.) The data are representative of three different experiments. (B) WCE derived from siRNA-transfected A549 cells infected with RSV at an MOI of 3 for various periods were resolved by SDS-PAGE, transferred onto nitrocellulose membrane, and immunoblotted (IB) with anti-IκBαSer32 phospho-specific (IκBα-P-Ser32), anti-IκBα, anti-p65Ser536 phospho-specific (p65-P-Ser536), anti-p65, anti-RSV (nucleocapsid protein [N] is shown), anti-RIG-I (A), anti-MDA-5 (B), anti-MAVS (C), and anti-actin antibodies. The data are representative of three independent experiments.
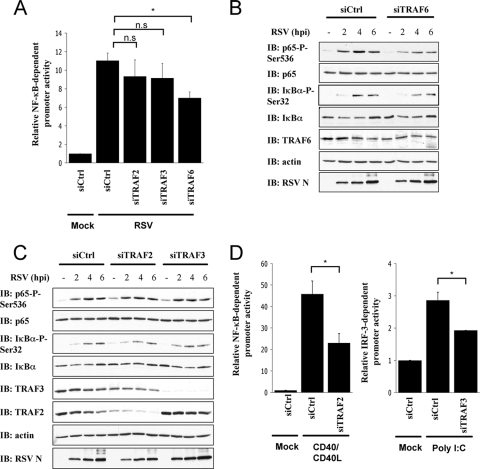
TRAF6 is required for RSV-induced NF-κB activation and p65Ser536 phosphorylation.
The signaling molecules acting downstream of MAVS regulating RSV-induced NF-κB activation have not yet been investigated. Importantly, whether the pathways leading to IκBαSer32 and p65Ser536 phosphorylations rely on the interaction with the same TRAF molecules, or if they diverge at this level as reported for the activation of the NF-κB and IRF-3 in the context of other infections (41), remained to be determined. Since TRAF2, TRAF3, and TRAF6 were demonstrated to act downstream of MAVS, their potential function in RSV-induced NF-κB activation was first investigated. TRAF2, TRAF3, and TRAF6 expressions were individually downregulated in A549 through transfection of specific siRNA (Table 1). RSV stimulated a NF-κB-driven luciferase promoter to a similar level in siCtrl-, siTRAF2-, and siTRAF3-transfected A549, while the stimulation was significantly decreased in siTRAF6-transfected cells (Fig. 3 A). Kinetic analysis of RSV infection (0 to 6 h) in the absence or presence of TRAF6-specific siRNA revealed that TRAF6 inhibition interfered with p65Ser536 phosphorylation (Fig. 3B). Similarly, IκBαSer32 phosphorylation was decreased when TRAF6 was downregulated (Fig. 3B). On the other hand, neither inhibition of TRAF2 nor TRAF3 expression affected p65Ser536 or IκBαSer32 phosphorylation (Fig. 3C). The capacity of siTRAF2 and siTRAF3 to inhibit CD40/CD40L-mediated activation of NF-κB- and poly(I:C)-mediated activation of IRF-3 monitored by luciferase reporter assays was used as a positive control of their efficacy (Fig. 3D). Taken together, these results demonstrate for the first time that TRAF6, but not TRAF2 or TRAF3, is required for RSV-mediated NF-κB activation. Additionally, these data reveal that TRAF6 controls the signaling events that ultimately lead to p65Ser536 and IκBαSer32 phosphorylations.
FIG. 3.
Downregulation of TRAF6, but not of TRAF2 or TRAF3, reduces RSV-induced p65Ser536 phosphorylation. A549 were transfected with control (Ctrl)-, TRAF2-, TRAF3-, or TRAF6-specific siRNA oligonucleotides (Table 1). (A) siRNA-transfected A549 cells were treated and data analyzed as described in Fig. 2A. (*, P < 0.05); n.s., not significantly different; mean ± the SEM; n = 6). The data are representative of two independent experiments. (B and C) WCE obtained from siRNA-transfected cells were analyzed by immunoblotting (IB) with anti-IκBαSer32 phospho-specific (IκBα-P-Ser32), anti-IκBα, anti-p65Ser536 phospho-specific (p65-P-Ser536), anti-p65, anti-RSV (nucleocapsid protein [N] is shown), anti-TRAF6 (B), anti-TRAF2 (C), anti-TRAF3 (C), and anti-actin antibodies. Immunoblots are representative of three independent experiments. (D) siRNA-transfected A549 cells were further cotransfected with CD40 and CD40L expressing constructs and the P2(2×)TK-pGL3 NF-κB firefly luciferase reporter constructs or with the ISG56prom-pGL3 IRF-3 firefly reporter construct, followed by poly(I:C). The pRL-null Renilla luciferase reporter construct was used as an internal control. The data were analyzed as described in Fig. 2A. (*, P < 0.05; mean ± the SEM; n = 3.)
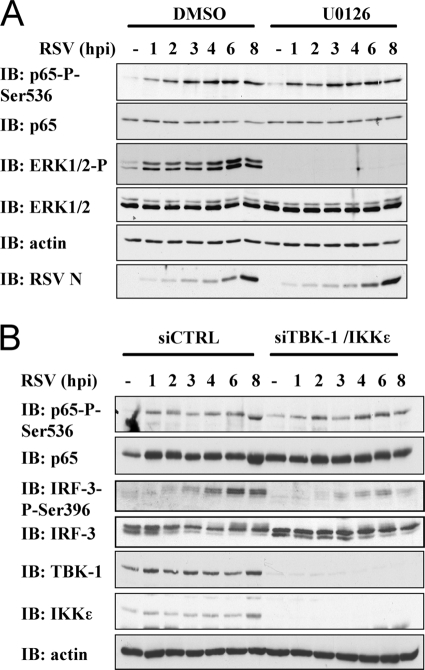
RSV-induced P65Ser536 phosphorylation is dependent on the IKKβ kinase.
Based on their involvement in p65Ser536 phosphorylation in different contexts, the IKK kinase subunits α and β, the IKK-related kinases TBK1 and IKKɛ, and the RSK-1 kinase are potential candidates that might be responsible for RSV-induced p65Ser536 phosphorylation. To discriminate between these kinases, pharmacological inhibition and/or siRNA-mediated downregulation strategies were used. First, inhibition of the MEK/ERK/RSK-1 signaling cascade was achieved through pretreatment of A549 cells with the U0126 inhibitor at 20 μM or the corresponding vehicle. Although the RSV-induced phosphorylation of ERK1/2, used as a positive control, was completely abolished by U0126 treatment, p65Ser536 phosphorylation was not altered compared to vehicle-treated cells (Fig. 4 A). Alternatively, the involvement of the IKK-related kinases, TBK1 and IKKɛ, was evaluated through cotransfection of siRNA efficiently downregulating their expression. Whereas RSV-induced IRF-3 phosphorylation, used as a positive control, was significantly decreased, RSV-induced p65Ser536 phosphorylation was similar to the level observed in siCtrl-transfected cells (Fig. 4B).
FIG. 4.
RSV-induced p65Ser536 phosphorylation is independent of the RSK-1, TBK1, and IKKɛ kinases. A549 were pretreated with DMSO (vehicle) or the U0126 inhibitor (20 μM) (A) or transfected with control (Ctrl)- or a combination of TBK-1- and IKKɛ-specific siRNA (Table 1) (B) before being left untreated or infected with RSV at an MOI of 3 for the indicated times (hpi). WCE were resolved by SDS-PAGE, and immunoblotted (IB) with anti-p65Ser536 phospho-specific (p65-P-Ser536), anti-p65, anti-RSV (nucleocapsid protein [N] is shown), and anti-actin antibodies. In panel A, the efficiency of U0126 was assessed by using anti-ERK1/2 phospho-specific and anti-ERK1/2 antibodies. In panel B, the efficiency of siRNA was ensured by using anti-TBK-1, anti-ΙΚΚɛ, anti-IRF-3-phosphoSer396, and anti-IRF-3 antibodies. The data are representative of three independent experiments.
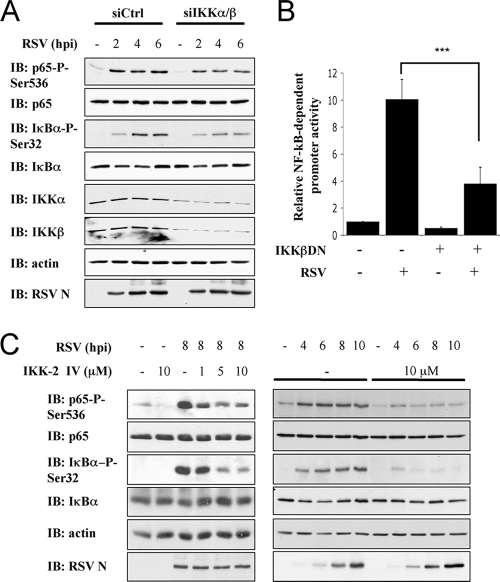
To investigate the potential involvement of IKKα/β, A549 were next cotransfected with siRNA targeting IKKα/β and further infected with RSV (0 to 6 h). As shown in Fig. 5 A, downregulation of IKKα/β expression resulted in a substantial reduction in RSV-induced p65Ser536 phosphorylation. As expected, inhibition of IKKα/β also interfered with RSV-induced IκBαSer32 phosphorylation. Ectopic expression of a dominant-negative mutant of IKKβ, IKKβK44A, significantly reduced RSV-induced activation of a NF-κB-driven luciferase promoter in A549 cells (Fig. 5B), thus confirming the previously documented role of the IKKβ kinase. The individual downregulation of IKKβ using an siRNA strategy was prevented since a significant induction of IKKα expression in IKKβ-siRNA-transfected cells was observed (data not shown), most likely through a compensation mechanism. Thus, to further characterize the potential specific role of IKKβ in p65Ser536 phosphorylation, we used the IKK-2 IV IKKβ-specific inhibitor. Treatment of A549 cells with IKK-2 IV inhibitor (0 to 10 μM) significantly decreased RSV-induced p65Ser536 phosphorylation in a dose-dependent manner (Fig. 5C). Kinetic analysis of RSV infection (0 to 10 hpi) demonstrated an inhibition of p65Ser536 phosphorylation, eliminating the possibility of a delayed kinetic in the presence of the IKK-2 IV inhibitor (Fig. 5C). As expected, similar results were obtained for RSV-induced IκBαSer32 phosphorylation (Fig. 5C). Taken together, these data demonstrate that the IKKβ kinase is required for the phosphorylation of p65Ser536 during RSV infection.
FIG. 5.
Phosphorylation of p65Ser536 during RSV infection is mediated by IKKβ. (A) A549 were transfected with control (Ctrl)- and a combination of IKKα- and IKKβ-specific siRNA (Table 1). siRNA-transfected A549 were further infected with RSV (MOI = 3) for the indicated times (hpi). WCE were resolved by SDS-PAGE and revealed by immunoblotting (IB) with anti-IκBαSer32 phospho-specific (IκBα-P-Ser32), anti-IκBα, anti-p65Ser536 phospho-specific (p65-P-Ser536), anti-p65, anti-RSV (nucleocapsid protein [N] is shown), anti-IKKα, anti-IKKβ, and anti-actin antibodies. The immunoblots are representative of three independent experiments. (B) A549 were cotransfected with the IKKβDN encoding plasmid and the P2(2×)TK-pGL3 NF-κB firefly luciferase and the pRL-null Renilla luciferase (internal control) reporter constructs and either mock or RSV infected. At 8 hpi, luciferase activities were measured and analyzed as described in Fig. 2A. (***, P < 0.001; mean ± the SEM; n = 9.) (C) A549 were pretreated with the IKKβ inhibitor, IKK-2 IV, at the indicated concentrations before being left untreated or infected with RSV at an MOI of 3 for various times (hpi). WCE were resolved by SDS-PAGE and analyzed by IB as in panel A. The immunoblots are representative of at least three experiments.
NOX2 regulates RSV-induced IKKβ activity to regulate p65Ser536 and IκBαSer32 phosphorylation.
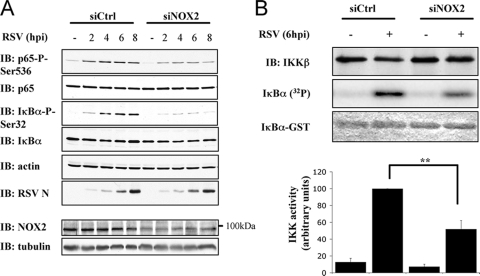
We recently showed that Paramyxoviridae viruses, including RSV, induce p65Ser536 and IκBαSer32 phosphorylations in AEC in a NOX2-containing superoxide-generating NADPH oxidase-dependent manner (14). To further characterize the role of NOX2 in the p65Ser536 phosphorylation pathway, A549 cells were transfected with Ctrl or NOX2 siRNA and infected with RSV for various times (0 to 8 hpi). As previously described, both p65Ser536 and IκBαSer32 phosphorylations were significantly diminished in siNOX2-transfected cells compared to siCtrl-transfected cells (Fig. 6 A). The activity of the IKK complex was quantified at 6 hpi by using a standard in vitro kinase assay procedure. As shown in Fig. 6B, interference with NOX2 expression significantly inhibited the capacity of RSV to stimulate IKK activity. In siNOX2-transfected cells, RSV-induced IKK activity was diminished by 52% compared to the activity measured in siCtrl-transfected cells. In summary, RSV-induced p65Ser536 phosphorylation is dependent on IKKβ activity, which is under the control of the NOX2-containing NADPH oxidase.
FIG. 6.
NOX2 controls IKK activity in RSV infection. (A) Control (Ctrl)- and NOX2-specific siRNA were transfected into A549. siRNA-transfected A549 were further infected with RSV at an MOI of 3 for the indicated times (hpi). WCE were resolved by SDS-PAGE and analyzed by immunoblotting (IB) as described in Fig. 5A. Efficiency of NOX2 downregulation was controlled by IB with anti-NOX2 antibodies. Equal loading was ensured using anti-tubulin antibodies. (B) WCE of siCtrl- and siNOX2-transfected A549 uninfected or infected with RSV for 6 hpi were subjected to an in vitro kinase assay as a substrate as described in Materials and Methods using immunoprecipitated IKK complex as a kinase and recombinant IκBα(1-55)-GST fusion protein as a substrate. The substrate was stained with Coomassie blue, and the IKK activity was measured by quantifying the incorporation of radioactivity (32P) normalized to the amount of immunoprecipitated IKKβ detected by immunoblotting (IB). The results of a representative experiment are presented. The quantifications are expressed as the mean ± the SEM of four experiments (**, P < 0.01).
DISCUSSION
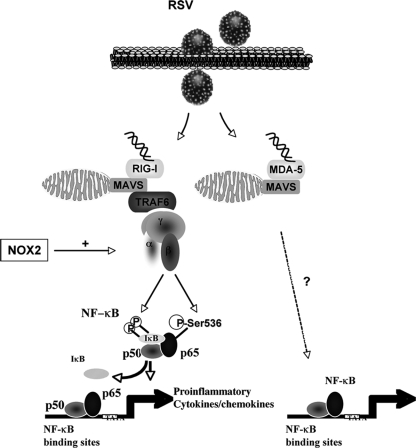
The importance of RSV as the causative agent for life-threatening acute lower respiratory tract diseases worldwide, taken together with the lack of an effective treatment or vaccine, makes the elucidation of the molecular mechanisms controlling RSV-associated diseases a necessity. The excessive inflammatory response, mediated by cytokines and chemokines, triggered by RSV infection of the epithelium is a major inducer of the pathogenesis and therefore constitutes a target of choice to improve the clinical symptoms. The NF-κB transcription factor is a key player in the transactivation of a network of proinflammatory genes, making it a potential therapeutic target to limit proinflammatory response. NF-κB regulation appears to be stimulus specific, and the molecular requirements for RSV-induced NF-κB activation in AEC are far from being resolved. A lot of groundwork has been completed, leading to the demonstration of the importance of RSV-triggered IKK activation, p65 nuclear accumulation, and DNA binding, which are characteristic of the classical pathway, in NF-κB activation (5, 8, 13, 16, 22, 23, 26, 29, 38). We and others have reported additional regulation by direct p65 phosphorylation at Ser276 and Ser536 (14, 36). We previously showed that phosphorylation at Ser536 is essential for RSV-induced p65-mediated promoter transactivation in AEC (14), but the mechanisms leading to p65Ser536 phosphorylation remained elusive. In the present study, we report the first analysis of the molecular requirement for RSV-induced p65Ser536 phosphorylation. We demonstrate that innate recognition of replicating RSV by both RIG-I and MDA-5 cytoplasmic sensors play a substantial role in RSV-induced NF-κB-mediated promoter transactivation in A549 cells. However, RSV-induced p65Ser536 phosphorylation, as well as the classical IκBαSer32 phosphorylation, is exclusively dependent on RIG-I, through a pathway involving MAVS, TRAF6, and the IKKβ kinase. Thus, a common signalosome regulates two essential steps in RSV-mediated NF-κB activation. Furthermore, we show that the IKKβ activity is dependent on the expression of the NOX2-containing NADPH oxidase that we previously (14) identified as an essential regulator of p65 and IκBα phosphorylations. A schematic diagram of a model of RSV-induced p65 activation is shown in Fig. 7.
FIG. 7.
Model of RSV-induced p65 activation. Upon sensing cytoplasmic RSV nucleic acid, the DEXD/H box helicase RIG-I signals to the MAVS adaptor located at the outer membrane of the mitochondria via CARD-domain interaction. MAVS dimerizes and interacts with TRAF6 to form the signalosome that activates the IKK kinase complex. Specifically, the IKKβ kinase subunit that is placed under the control of the NOX2-containing NADPH oxidase mediates the phosphorylation of IκBSer32 and p65Ser536. Phosphorylation of IκB is a hallmark of the classical pathway of NF-κB activation that induces its proteosomal degradation and the release of the p65/p50 dimer, which then migrates to the nucleus to modulate proinflammatory gene expression. Additional phosphorylation of p65 on Ser536 is essential for its capacity to transactivate genes (14). Finally, the DEXD/H box helicase MDA-5 appears to play a role in RSV-induced NF-κB-responsive promoter induction by a yet-undefined alternative pathway that is independent of IκBSer32 and p65Ser536 phosphorylations.
The specific contribution of RIG-I and MDA-5 in RSV-mediated activation of NF-κB was previously poorly delineated. Previous studies revealed a preferential role of RIG-I in RSV recognition in A549 and HeLa cells (36, 55). Interference with RIG-I in A549 was found to inhibit RSV-induced p65 nuclear accumulation and NF-κB DNA-binding (36). Our data now add to this picture, with the demonstration that IκBαSer32 phosphorylation, a hallmark of the classical activation pathway, is exclusively dependent on RIG-I and MAVS, strongly supporting the idea that the RIG-I/MAVS complex is upstream of the classical pathway of NF-κB activation. In a recent report, Liu et al. (36) excluded a role of MDA-5 in RSV sensing based on results showing that active RIG-I, but not MDA-5, binds RSV transcripts in a UV-cross-linking experiment. However, this result is in contrast with other studies reporting that in murine embryonic fibroblast (MEF) cells lacking MDA-5, RSV-induced ISG expression was relatively delayed (37), hence supporting an accessory physiological function of MDA-5 in amplifying RSV-induced innate immune response. Our results described in the present study reveal that MDA-5 plays a substantial role in NF-κB-driven promoter regulation in response to RSV infection, without acting through regulation of the classical pathway, as demonstrated by the lack of effect of its downregulation on RSV-induced IκBαSer32 or p65Ser536 phosphorylations. Alternative pathways of RSV-mediated NF-κB activation have recently been reported. In particular, RSV was shown to activate the noncanonical pathway involving NIK and IKKα kinases and the downstream processing of the p100 precursor into the p52 DNA-binding subunit that binds κB-consensus sites as a complex with the RelB subunit (10). Moreover, phosphorylation of p65 at Ser276, which regulates p65 transcriptional activity, was shown to be dependent on the MSK-1 kinase through a pathway independent of IκBα phosphorylation/degradation and distinct from p65Ser536 phosphorylation (30). Whether MDA-5 controls NF-κB activation through regulation of one of these pathways remains to be determined.
TRAF2 and TRAF6 E3 ubiquitin ligases are known key intermediates in NF-κB signaling pathways downstream of various membrane receptors, including CD40, TLRs, IL-1R, and T-cell receptor (65). Recently, MAVS was shown to bind to TRAF2, TRAF3, and TRAF6 (51, 59, 68). Our results demonstrate that only TRAF6 downregulation diminishes the capacity of RSV to trigger NF-κB activation through regulation of IκBαSer32 and p65Ser536 phosphorylations. These results are in accordance with previous reports demonstrating that the role of TRAF3 is restricted to MAVS-mediated activation of the IRF-3-mediated antiviral response, but not of NF-κB (51). TRAF6 was found to be essential for RIG-I-sensed RNA viruses, vesicular stomatitis virus, Newcastle disease virus, encephalomyocarditis virus, and Sendai virus (SeV) and induced NF-κB activation in MEF cells (34, 69). However, this finding is in contrast to a recent report by Mikkelsen et al. (42) that concluded that SeV-mediated IκBα phosphorylation was selectively dependent on TRAF2 and TAK1 but not on TRAF6. Similarly, characterization of MAVS requirement for TRAF2 or TRAF6 through deletion of TRAF6 and TRAF2 binding region in MAVS led to conflicting results regarding the capacity of the deletion mutant to trigger NF-κB activation by ectopic expression in HEK293 cells (59, 68). It remains unclear whether the observed discrepancies in the role of TRAF2/6 in mediating RIG-I-dependent NF-κB signaling in the context of different RNA virus infections is due to virus-specific mechanisms.
The direct phosphorylation of p65 subunit recently emerged as a novel stimulus-dependent regulatory mechanism controlling its nuclear localization, DNA-binding affinity, coactivator/corepressor association, or transactivation capacity (45, 57). Several kinases, including IKKα, β, IKKɛ, TBK1, and RSK-1, were found to be involved in the p65Ser536 phosphorylation pathway. Using a systematic analysis, we demonstrate that p65Ser536 phosphorylation in the context of RSV infection in A549 is dependent on IKKβ activity. In an in vivo study, Haeberle et al. (22) demonstrated that specific inhibition of IKKβ binding to the IKKγ regulatory subunit reduces inflammation induced by RSV in the lung of BALB/c mice. In the present study, we demonstrate that inhibition of IKKβ activity through another specific inhibitor, IKK-2 IV, reduces RSV-induced NF-κB activation by decreasing both ΙκΒαSer32 and p65Ser536 phosphorylations. This observation reinforces the previously documented concept that IKKβ activity represents a target of choice for the development of anti-inflammatory therapies for RSV-induced lower respiratory tract disease. Among upstream regulators, we previously provided direct proofs that the NOX2-containing NADPH oxidase specifically participates in the redox-sensitive regulation of RSV-induced ΙκΒαSer32 and p65Ser536 phosphorylations in human AEC (14). Our data now demonstrate a NOX2-dependent stimulation of IKK activity upon RSV infection. Oxidation of ΙΚΚβ at Cys179 negatively regulates NF-κB in response to anti-inflammatory stimuli (47). Thus, it is unlikely that IKKβ constitutes a direct target for redox modification during RSV infection. The upstream signalosome is an interesting candidate for NOX2-dependent regulation. Identification of the redox-target is currently under investigation. Our data shed light into the potential interest of targeting the RIG-I/MAVS/TRAF6 signalosome or the NOX2 NADPH oxidase to control early IKKβ activation as a means to reduce NF-κB-dependent cytokine genes expression and the subsequent inflammatory response during RSV infection.
Acknowledgments
We thank members of the laboratory for fruitful discussions and technical help. We are also grateful to G. Brandolin (CEA-Grenoble, Grenoble, France) for the NOX2 antibody and to W. Mourad (CRCHUM, Montreal, Quebec, Canada) for the CD40- and the CD40L-encoding constructs. We thank Christopher Rose and Loubna Jouan for critical reading of the manuscript.
This study was funded by operating grant MOP89807 from the Canadian Institutes of Health Research (CIHR) to N.G. and by funds from the Fonds de la Recherche en Santé du Québec (FRSQ). A.M. was the recipient of studentships from the FRSQ and CIHR. N.G. was the recipient of a Tier II Canada Research Chair.
Footnotes
Published ahead of print on 21 April 2010.
REFERENCES
- 1.Baril, M., M.-E. Racine, F. Penin, and D. Lamarre. 2009. MAVS dimer is a crucial signaling component of innate immunity and the target of hepatitis C virus NS3/4A protease. J. Virol. 83:1299-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barral, P. M., D. Sarkar, Z.-Z. Su, G. N. Barber, R. DeSalle, V. R. Racaniello, and P. B. Fisher. 2009. Functions of the cytoplasmic RNA sensors RIG-I and MDA-5: key regulators of innate immunity. Pharmacol. Ther. 124:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, S., W. Reed, F. W. Henderson, and T. L. Noah. 1997. RSV infection of human airway epithelial cells causes production of the chemokine RANTES. Am. J. Physiol. 272:L512-L520. [DOI] [PubMed] [Google Scholar]
- 4.Berghäll, H., J. Sirén, D. Sarkar, I. Julkunen, P. B. Fisher, R. Vainionpää, and S. Matikainen. 2006. The interferon-inducible RNA helicase, mda-5, is involved in measles virus-induced expression of antiviral cytokines. Microbes Infect. 8:2138-2144. [DOI] [PubMed] [Google Scholar]
- 5.Bitko, V., and S. Barik. 1998. Persistent activation of RelA by respiratory syncytial virus involves protein kinase C, underphosphorylated IκBβ, and sequestration of protein phosphatase 2A by the viral phosphoprotein. J. Virol. 72:5610-5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohuslav, J., L. F. Chen, H. Kwon, Y. Mu, and W. C. Greene. 2004. p53 induces NF-κB activation by an IκB kinase-independent mechanism involving phosphorylation of p65 by ribosomal S6 kinase 1. J. Biol. Chem. 279:26115-26125. [DOI] [PubMed] [Google Scholar]
- 7.Buss, H., A. Dorrie, M. L. Schmitz, E. Hoffmann, K. Resch, and M. Kracht. 2004. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-κB at serine 536 is mediated by multiple protein kinases including IκB kinase (IKK)-α, IKKβ, IKKɛ, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J. Biol. Chem. 279:55633-55643. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter, L. R., J. N. Moy, and K. A. Roebuck. 2002. Respiratory syncytial virus and TNF alpha induction of chemokine gene expression involves differential activation of Rel A and NF-κB1. BMC Infect. Dis. 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casola, A., N. Burger, T. Liu, M. Jamaluddin, A. R. Brasier, and R. P. Garofalo. 2001. Oxidant tone regulates RANTES gene expression in airway epithelial cells infected with respiratory syncytial virus. role in viral-induced interferon regulatory factor activation. J. Biol. Chem. 276:19715-19722. [DOI] [PubMed] [Google Scholar]
- 10.Choudhary, S., S. Boldogh, R. Garofalo, M. Jamaluddin, and A. R. Brasier. 2005. Respiratory syncytial virus influences NF-κB-dependent gene expression through a novel pathway involving MAP3K14/NIK expression and nuclear complex formation with NF-κB2. J. Virol. 79:8948-8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies, C. C., T. W. Mak, L. S. Young, and A. G. Eliopoulos. 2005. TRAF6 is required for TRAF2-dependent CD40 signal transduction in nonhemopoietic cells. Mol. Cell. Biol. 25:9806-9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douillette, A., A. Bibeau-Poirier, S. P. Gravel, J. F. Clement, V. Chenard, P. Moreau, and M. J. Servant. 2006. The proinflammatory actions of angiotensin II are dependent on p65 phosphorylation by the IκB kinase complex. J. Biol. Chem. 281:13275-13284. [DOI] [PubMed] [Google Scholar]
- 13.Fiedler, M. A., and K. Wernke-Dollries. 1999. Incomplete regulation of NF-κB by IκBα during respiratory syncytial virus infection in A549 cells. J. Virol. 73:4502-4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fink, K., A. Duval, A. Martel, A. Soucy-Faulkner, and N. Grandvaux. 2008. Dual role of NOX2 in respiratory syncytial virus- and Sendai virus-induced activation of NF-κB in airway epithelial cells. J. Immunol. 180:6911-6922. [DOI] [PubMed] [Google Scholar]
- 15.Fujita, F., Y. Taniguchi, T. Kato, Y. Narita, A. Furuya, T. Ogawa, H. Sakurai, T. Joh, M. Itoh, M. Delhase, M. Karin, and M. Nakanishi. 2003. Identification of NAP1, a regulatory subunit of IκB kinase-related kinases that potentiates NF-κB signaling. Mol. Cell. Biol. 23:7780-7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garofalo, R., M. Sabry, M. Jamaluddin, R. K. Yu, A. Casola, P. L. Ogra, and A. R. Brasier. 1996. Transcriptional activation of the interleukin-8 gene by respiratory syncytial virus infection in alveolar epithelial cells: nuclear translocation of the RelA transcription factor as a mechanism producing airway mucosal inflammation. J. Virol. 70:8773-8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garofalo, R. P., and H. Haeberle. 2000. Epithelial regulation of innate immunity to respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 23:581-585. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-κB puzzle. Cell 109(Suppl.):S81-S96. [DOI] [PubMed] [Google Scholar]
- 19.Gloire, G., S. Legrand-Poels, and J. Piette. 2006. NF-κB activation by reactive oxygen species: fifteen years later. Biochem. Pharmacol. 72:1493-1505. [DOI] [PubMed] [Google Scholar]
- 20.Grandvaux, N., F. Gaboriau, J. Harris, B. R. tenOever, R. Lin, and J. Hiscott. 2005. Regulation of arginase II by interferon regulatory factor 3 and the involvement of polyamines in the antiviral response. FEBS J. 272:3120-3131. [DOI] [PubMed] [Google Scholar]
- 21.Grandvaux, N., M. J. Servant, B. tenOever, G. C. Sen, S. Balachandran, G. N. Barber, R. Lin, and J. Hiscott. 2002. Transcriptional profiling of interferon regulatory factor 3 target genes: direct involvement in the regulation of interferon-stimulated genes. J. Virol. 76:5532-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haeberle, H. A., A. Casola, Z. Gatalica, S. Petronella, H. J. Dieterich, P. B. Ernst, A. R. Brasier, and R. P. Garofalo. 2004. IκB kinase is a critical regulator of chemokine expression and lung inflammation in respiratory syncytial virus infection. J. Virol. 78:2232-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haeberle, H. A., R. Takizawa, A. Casola, A. R. Brasier, H. J. Dieterich, N. Van Rooijen, Z. Gatalica, and R. P. Garofalo. 2002. Respiratory syncytial virus-induced activation of nuclear factor-κB in the lung involves alveolar macrophages and Toll-like receptor 4-dependent pathways. J. Infect. Dis. 186:1199-1206. [DOI] [PubMed] [Google Scholar]
- 24.Hall, C. B. 1998. Respiratory syncytial virus. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 25.Harris, J., S. Oliere, S. Sharma, Q. Sun, R. Lin, J. Hiscott, and N. Grandvaux. 2006. Nuclear accumulation of cRel following C-terminal phosphorylation by TBK1/IKKepsilon. J. Immunol. 177:2527-2535. [DOI] [PubMed] [Google Scholar]
- 26.Harris, J., and D. Werling. 2003. Binding and entry of respiratory syncytial virus into host cells and initiation of the innate immune response. Cell Microbiol. 5:671-680. [DOI] [PubMed] [Google Scholar]
- 27.Hayden, M. S., and S. Ghosh. 2008. Shared principles in NF-κB signaling. Cell 132:344-362. [DOI] [PubMed] [Google Scholar]
- 28.Jafri, H. S., S. Chavez-Bueno, A. Mejias, A. M. Gomez, A. M. Rios, S. S. Nassi, M. Yusuf, P. Kapur, R. D. Hardy, J. Hatfield, B. B. Rogers, K. Krisher, and O. Ramilo. 2004. Respiratory syncytial virus induces pneumonia, cytokine response, airway obstruction, and chronic inflammatory infiltrates associated with long-term airway hyper-responsiveness in mice. J. Infect. Dis. 189:1856-1865. [DOI] [PubMed] [Google Scholar]
- 29.Jamaluddin, M., A. Casola, R. P. Garofalo, Y. Han, T. Elliott, P. L. Ogra, and A. R. Brasier. 1998. The major component of IκBα proteolysis occurs independently of the proteasome pathway in respiratory syncytial virus-infected pulmonary epithelial cells. J. Virol. 72:4849-4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jamaluddin, M., B. Tian, I. Boldogh, R. P. Garofalo, and A. R. Brasier. 2009. Respiratory syncytial virus infection induces a reactive oxygen species-MSK1-phospho-Ser-276 RelA pathway required for cytokine expression. J. Virol. 83:10605-10615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang, X., N. Takahashi, N. Matsui, T. Tetsuka, and T. Okamoto. 2003. The NF-κB activation in lymphotoxin beta receptor signaling depends on the phosphorylation of p65 at serine 536. J. Biol. Chem. 278:919-926. [DOI] [PubMed] [Google Scholar]
- 32.Kawai, T., and S. Akira. 2008. Toll-like receptor and RIG-I-like receptor signaling. Ann. N. Y. Acad. Sci. 1143:1-20. [DOI] [PubMed] [Google Scholar]
- 33.Kim, H. R., S. H. Lee, and G. Jung. 2009. The hepatitis B viral X protein activates NF-κB signaling pathway through the upregulation of TBK1, FEBS Lett. [DOI] [PubMed]
- 34.Konno, H., T. Yamamoto, K. Yamazaki, J. Gohda, T. Akiyama, K. Semba, H. Goto, A. Kato, T. Yujiri, T. Imai, Y. Kawaguchi, B. Su, O. Takeuchi, S. Akira, Y. Tsunetsugu-Yokota, and J.-I. Inoue. 2009. TRAF6 establishes innate immune responses by activating NF-κB and IRF7 upon sensing cytosolic viral RNA and DNA. PLoS One 4:e5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao, G., M. Zhang, E. W. Harhaj, and S. C. Sun. 2004. Regulation of the NF-κB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J. Biol. Chem. 279:26243-26250. [DOI] [PubMed] [Google Scholar]
- 36.Liu, P., M. Jamaluddin, K. Li, R. P. Garofalo, A. Casola, and A. R. Brasier. 2007. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J. Virol. 81:1401-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loo, Y. M., J. Fornek, N. Crochet, G. Bajwa, O. Perwitasari, L. Martinez-Sobrido, S. Akira, M. A. Gill, A. Garcia-Sastre, M. G. Katze, and M. Gale, Jr. 2008. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 82:335-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mastronarde, J. G., B. He, M. M. Monick, N. Mukaida, K. Matsushima, and G. W. Hunninghake. 1996. Induction of interleukin (IL)-8 gene expression by respiratory syncytial virus involves activation of nuclear factor (NF)-κB and NF-IL-6. J. Infect. Dis. 174:262-267. [DOI] [PubMed] [Google Scholar]
- 39.Mastronarde, J. G., M. M. Monick, and G. W. Hunninghake. 1995. Oxidant tone regulates IL-8 production in epithelium infected with respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 13:237-244. [DOI] [PubMed] [Google Scholar]
- 40.Mellow, T. E., P. C. Murphy, J. L. Carson, T. L. Noah, L. Zhang, and R. J. Pickles. 2004. The effect of respiratory synctial virus on chemokine release by differentiated airway epithelium. Exp. Lung Res. 30:43-57. [DOI] [PubMed] [Google Scholar]
- 41.Michallet, M. C., E. Meylan, M. A. Ermolaeva, J. Vazquez, M. Rebsamen, J. Curran, H. Poeck, M. Bscheider, G. Hartmann, M. Konig, U. Kalinke, M. Pasparakis, and J. Tschopp. 2008. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity 28:651-661. [DOI] [PubMed] [Google Scholar]
- 42.Mikkelsen, S. S., S. B. Jensen, S. Chiliveru, J. Melchjorsen, I. Julkunen, M. Gaestel, J. S. C. Arthur, R. A. Flavell, S. Ghosh, and S. R. Paludan. 2009. RIG-I-mediated activation of p38 MAPK is essential for viral induction of interferon and activation of dendritic cells: dependence on TRAF2 and TAK1. J. Biol. Chem. 284:10774-10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller, A. L., T. L. Bowlin, and N. W. Lukacs. 2004. Respiratory syncytial virus-induced chemokine production: linking viral replication to chemokine production in vitro and in vivo. J. Infect. Dis. 189:1419-1430. [DOI] [PubMed] [Google Scholar]
- 44.Nakhaei, P., J. Hiscott, and R. Lin. 18 December 2009, posting date. STING-ing the antiviral pathway. J. Mol. Cell Biol. doi: 10.1093/jmcb/mjp048. [DOI] [PubMed]
- 45.Neumann, M., and M. Naumann. 2007. Beyond IκBs: alternative regulation of NF-κB activity. FASEB J. 21:2642-2654. [DOI] [PubMed] [Google Scholar]
- 46.Olszewska-Pazdrak, B., A. Casola, T. Saito, R. Alam, S. E. Crowe, F. Mei, P. L. Ogra, and R. P. Garofalo. 1998. Cell-specific expression of RANTES, MCP-1, and MIP-1α by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J. Virol. 72:4756-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pandey, M. K., S. K. Sandur, B. Sung, G. Sethi, A. B. Kunnumakkara, and B. B. Aggarwal. 2007. Butein, a tetrahydroxychalcone, inhibits nuclear factor (NF)-κB and NF-κB-regulated gene expression through direct inhibition of IκBα kinase β on cysteine 179 residue. J. Biol. Chem. 282:17340-17350. [DOI] [PubMed] [Google Scholar]
- 48.Reyes-Moreno, C., J. Girouard, R. Lapointe, A. Darveau, and W. Mourad. 2004. CD40/CD40 homodimers are required for CD40-induced phosphatidylinositol 3-kinase-dependent expression of B7.2 by human B lymphocytes. J. Biol. Chem. 279:7799-7806. [DOI] [PubMed] [Google Scholar]
- 49.Roebuck, K. A., L. R. Carpenter, V. Lakshminarayanan, S. M. Page, J. N. Moy, and L. L. Thomas. 1999. Stimulus-specific regulation of chemokine expression involves differential activation of the redox-responsive transcription factors AP-1 and NF-κB. J. Leukoc. Biol. 65:291-298. [DOI] [PubMed] [Google Scholar]
- 50.Sabroe, I., C. M. Lloyd, M. K. Whyte, S. K. Dower, T. J. Williams, and J. E. Pease. 2002. Chemokines, innate and adaptive immunity, and respiratory disease. Eur. Respir. J. 19:350-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saha, S. K., E. M. Pietras, J. Q. He, J. R. Kang, S.-Y. Liu, G. Oganesyan, A. Shahangian, B. Zarnegar, T. L. Shiba, Y. Wang, and G. Cheng. 2006. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J. 25:3257-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saito, T., R. W. Deskin, A. Casola, H. Haeberle, B. Olszewska, P. B. Ernst, R. Alam, P. L. Ogra, and R. Garofalo. 1997. Respiratory syncytial virus induces selective production of the chemokine RANTES by upper airway epithelial cells. J. Infect. Dis. 175:497-504. [DOI] [PubMed] [Google Scholar]
- 53.Sakurai, H., H. Chiba, H. Miyoshi, T. Sugita, and W. Toriumi. 1999. IκB kinases phosphorylate NF-κB p65 subunit on serine 536 in the transactivation domain. J. Biol. Chem. 274:30353-30356. [DOI] [PubMed] [Google Scholar]
- 54.Sakurai, H., S. Suzuki, N. Kawasaki, H. Nakano, T. Okazaki, A. Chino, T. Doi, and I. Saiki. 2003. Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-κB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J. Biol. Chem. 278:36916-36923. [DOI] [PubMed] [Google Scholar]
- 55.Sasai, M., M. Shingai, K. Funami, M. Yoneyama, T. Fujita, M. Matsumoto, and T. Seya. 2006. NAK-associated protein 1 participates in both the TLR3 and the cytoplasmic pathways in type I IFN induction. J. Immunol. 177:8676-8683. [DOI] [PubMed] [Google Scholar]
- 56.Schmitz, M. L., S. Bacher, and M. Kracht. 2001. I κB-independent control of NF-κB activity by modulatory phosphorylations. Trends Biochem. Sci. 26:186-190. [DOI] [PubMed] [Google Scholar]
- 57.Schmitz, M. L., I. Mattioli, H. Buss, and M. Kracht. 2004. NF-κB: a multifaceted transcription factor regulated at several levels. Chembiochem 5:1348-1358. [DOI] [PubMed] [Google Scholar]
- 58.Servant, M. J., N. Grandvaux, B. R. tenOever, D. Duguay, R. Lin, and J. Hiscott. 2003. Identification of the minimal phosphoacceptor site required for in vivo activation of interferon regulatory factor 3 in response to virus and double-stranded RNA. J. Biol. Chem. 278:9441-9447. [DOI] [PubMed] [Google Scholar]
- 59.Seth, R. B., L. Sun, C.-K. Ea, and Z. J. Chen. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell 122:669-682. [DOI] [PubMed] [Google Scholar]
- 60.Sharma, S., B. R. tenOever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148-1151. [DOI] [PubMed] [Google Scholar]
- 61.Sigurs, N., R. Bjarnason, F. Sigurbergsson, and B. Kjellman. 2000. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care Med. 161:1501-1507. [DOI] [PubMed] [Google Scholar]
- 62.Sirén, J., T. Imaizumi, D. Sarkar, T. Pietilä, D. L. Noah, R. Lin, J. Hiscott, R. M. Krug, P. B. Fisher, I. Julkunen, and S. Matikainen. 2006. Retinoic acid inducible gene-I and mda-5 are involved in influenza A virus-induced expression of antiviral cytokines. Microbes Infect. 8:2013-2020. [DOI] [PubMed] [Google Scholar]
- 63.Tang, E. D., and C.-Y. Wang. 2009. MAVS self-association mediates antiviral innate immune signaling. J. Virol. 83:3420-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trauzold, A., C. Roder, B. Sipos, K. Karsten, A. Arlt, P. Jiang, J. I. Martin-Subero, D. Siegmund, S. Muerkoster, L. Pagerols-Raluy, R. Siebert, H. Wajant, and H. Kalthoff. 2005. CD95 and TRAF2 promote invasiveness of pancreatic cancer cells. FASEB J. 19:620-622. [DOI] [PubMed] [Google Scholar]
- 65.Vallabhapurapu, S., and M. Karin. 2009. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 27:693-733. [DOI] [PubMed] [Google Scholar]
- 66.Vallabhapurapu, S., A. Matsuzawa, W. Zhang, P.-H. Tseng, J. J. Keats, H. Wang, D. A. A. Vignali, P. L. Bergsagel, and M. Karin. 2008. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-κB signaling. Nat. Immunol. 9:1364-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Welliver, R. C. 2004. Respiratory syncytial virus infection: therapy and prevention. Paediatr. Respir. Rev. 5(Suppl. A):S127-S133. [DOI] [PubMed] [Google Scholar]
- 68.Xu, L.-G., Y.-Y. Wang, K.-J. Han, L.-Y. Li, Z. Zhai, and H.-B. Shu. 2005. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol. Cell 19:727-740. [DOI] [PubMed] [Google Scholar]
- 69.Yoshida, R., G. Takaesu, H. Yoshida, F. Okamoto, T. Yoshioka, Y. Choi, S. Akira, T. Kawai, A. Yoshimura, and T. Kobayashi. 2008. TRAF6 and MEKK1 play a pivotal role in the RIG-I-like helicase antiviral pathway. J. Biol. Chem. 283:36211-36220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.You, F., H. Sun, X. Zhou, W. Sun, S. Liang, Z. Zhai, and Z. Jiang. 2009. PCBP2 mediates degradation of the adaptor MAVS via the HECT ubiquitin ligase AIP4. Nat. Immunol. 10:1300-1308. [DOI] [PubMed] [Google Scholar]
- 71.Zhang, L., Y. Ma, J. Zhang, J. Cheng, and J. Du. 2005. A new cellular signaling mechanism for angiotensin II activation of NF-κB: an IκB-independent, RSK-mediated phosphorylation of p65. Arterioscler. Thromb. Vasc. Biol. 25:1148-1153. [DOI] [PubMed] [Google Scholar]
- 72.Zhang, Y., B. A. Luxon, A. Casola, R. P. Garofalo, M. Jamaluddin, and A. R. Brasier. 2001. Expression of respiratory syncytial virus-induced chemokine gene networks in lower airway epithelial cells revealed by cDNA microarrays. J. Virol. 75:9044-9058. [DOI] [PMC free article] [PubMed] [Google Scholar]