Abstract
Human T-lymphotropic virus type 1 (HTLV-1) envelope (Env) glycoprotein mediates binding of the virus to its receptor on the surface of target cells and subsequent fusion of virus and cell membranes. To better understand the mechanisms that control HTLV-1 Env trafficking and activity, we have examined two protein-protein interaction motifs in the cytoplasmic domain of Env. One is the sequence YSLI, which matches the consensus YXXΦ motifs that are known to interact with various adaptor protein complexes; the other is the sequence ESSL at the C terminus of Env, which matches the consensus PDZ-binding motif. We show here that mutations that destroy the YXXΦ motif increased Env expression on the cell surface and increased cell-cell fusion activity. In contrast, mutation of the PDZ-binding motif greatly diminished Env expression in cells, which could be restored to wild-type levels either by mutating the YXXΦ motif or by silencing AP2 and AP3, suggesting that interactions with PDZ proteins oppose an Env degradation pathway mediated by AP2 and AP3. Silencing of the PDZ protein hDlg1 did not affect Env expression, suggesting that hDlg1 is not a binding partner for Env. Substitution of the YSLI sequence in HTLV-1 Env with YXXΦ elements from other cell or virus membrane-spanning proteins resulted in alterations in Env accumulation in cells, incorporation into virions, and virion infectivity. Env variants containing YXXΦ motifs that are predicted to have high-affinity interaction with AP2 accumulated to lower steady-state levels. Interestingly, mutations that destroy the YXXΦ motif resulted in viruses that were not infectious by cell-free or cell-associated routes of infection. Unlike YXXΦ, the function of the PDZ-binding motif manifests itself only in the producer cells; AP2 silencing restored the incorporation of PDZ-deficient Env into virus-like particles (VLPs) and the infectivity of these VLPs to wild-type levels.
Human T-lymphotropic virus type 1 (HTLV-1) envelope (Env), like most retroviral envelopes, is synthesized as a precursor protein in the endoplasmic reticulum, forms trimers, and is cleaved by a cellular furin-like protease as it transits through the trans-Golgi network on its way to the plasma membrane (7, 21, 31). Cleavage of the HTLV-1 Env precursor generates a 46-kDa surface subunit (SU, gp46) and a 21-kDa transmembrane protein (TM, gp21) (8, 43). SU contains the receptor-binding domain and is linked by a disulfide bond to TM, which anchors Env to the membrane and mediates fusion of virus and cell membranes after receptor engagement (11, 28, 40, 51). TM consists of extracellular, membrane-spanning, and cytoplasmic domains (31); the last contains motifs that direct Env trafficking, membrane targeting, and virion incorporation. HTLV-1 is poorly transmitted as cell-free virus, and there is good evidence supporting a model in which virions are transmitted in a polarized fashion between lymphocytes that are in close contact (22, 30). Unlike murine leukemia virus (MLV) and Mason-Pfizer monkey virus (MPMV) Envs, in which the cytoplasmic domain (CD) is cleaved by the virus-encoded protease to activate fusogenic activity (3, 6, 19, 42), the HTLV-1 Env cytoplasmic domain is not cleaved and HTLV-1 Env exists on the cell surface in a highly fusogenic state. In many respects, HTLV-1 Env resembles versions of MLV or MPMV Envs that lack C-terminal amino acids, e.g., with elevated cell-cell fusion activity and low virion infectivity. It is not exactly clear how HTLV-1 Env is controlled such that virus infection can proceed without cell-cell fusion, but it is probable that Env trafficking plays an important role. The cytoplasmic domain of HTLV-1 Env is relatively short and contains two important trafficking motifs: a YXXΦ motif (YSLI), which is involved in membrane protein trafficking and basolateral sorting in polarized epithelial cells (10), and a PDZ-binding motif (ESSL), which can interact with numerous PDZ proteins but is not found in other retroviral Envs (2).
The tyrosine-based sorting motif (YXXΦ, where Y is tyrosine, X is any amino acid, and Φ is a bulky hydrophobic amino acid) determines the trafficking and turnover of many membrane-spanning proteins in the cell (5, 39) and is present in most retroviral Env proteins (7). The YXXΦ motif interacts with the μ subunit of the heterotetrameric adaptor protein complexes AP1, AP2, AP3, and AP4. Each adaptor complex is involved in a specific trafficking pathway: AP1 and AP4 deliver cargo from the trans-Golgi network to the plasma membrane (13, 33, 48), AP2 directs the endocytosis of proteins from the cell surface, and AP3 is involved in lysosomal sorting (5, 12, 24, 35). Each type of μ subunit interacts with a distinct but overlapping type of tyrosine-based motif; the tyrosine and the Φ residues are most critical, but affinity is determined in large part by the variable amino acids at positions +1 and +2 relative to tyrosine and also by surrounding amino acids (5, 37). Furthermore, interactions between AP2 and the YXXΦ motif may be regulated by phosphorylation of μ2 (38, 47), by localized changes in phosphoinositide concentration, or by interactions between AP2 and docking factors (47). Although most retroviral Env proteins contain YXXΦ-sorting motifs, the sequences of the motifs and their roles in Env trafficking and function appear to vary widely among different retroviruses. For example, mutation of the YXXΦ motif in MLV Env interferes with basolateral targeting of Env and diminishes viral pathogenesis in vivo but has little effect on Env accumulation at the plasma membrane (9, 16, 23, 25, 29). Mutations in the YXXΦ motif in MPMV Env are similar to those in MLV Evn and also were reported to affect Env incorporation into virions (45). Mutation of the YXXΦ motif in HTLV-1 Env was previously shown to decrease Env endocytosis, increase cell-cell fusion, increase Env incorporation into virions, abolish basolateral targeting, and decrease virus infectivity (1, 10).
The most abundant protein-protein interaction domains in mammalian cells are the PDZ domains; more than 400 PDZ proteins are encoded in the human genome. PDZ domains are modular, recognize short C-terminal peptide motifs, and are often found in multiple copies or in combination with other protein interaction domains (36, 46, 50). PDZ proteins have the ability to form supramolecular scaffolds that coordinate signaling, synapse formation, cell polarity, and trafficking of interacting proteins (26, 44, 53). With respect to the last, it is important to note that PDZ proteins can delay the internalization of G protein-coupled receptors, ion channels, and membrane transporters (17, 41, 49, 52). Among retroviral Env proteins, only HTLV and simian T-lymphotropic virus (STLV) Envs contain putative PDZ-binding motifs. A yeast two-hybrid screen using the HTLV-1 Env cytoplasmic domain (CD) as bait identified the PDZ protein hDlg (human homolog of disc large protein) as a potential binding partner (2). In vitro pulldown experiments showed that a glutathione S-transferase (GST)-EnvCD fusion protein interacted with several PDZ proteins from cell lysates, one of which was hDlg. In one study, mutation of the PDZ-binding motif in HTLV-1 Env inhibited cell-cell fusion (2); in another study, hDlg small interfering RNA (siRNA) silencing caused a modest reduction in syncytium formation (54). Neither study examined how the PDZ-binding motif controls Env expression, membrane targeting, trafficking, or virus infectivity. Thus, it is still unclear which PDZ proteins interact with HTLV-1 Env in vivo and how those interactions affect Env trafficking and activity.
In this paper, functional interactions between the YXXΦ motif and the PDZ-binding motif in the cytoplasmic domain of HTLV-1 Env were investigated by mutagenesis of Env and by siRNA silencing of potential cellular interacting proteins. The YXXΦ motif in HTLV-1 Env appears to interact primarily with AP2 and AP3, which regulate Env endocytosis and lysosomal degradation, respectively. Mutations that ablated the YXXΦ motif increased Env accumulation on the cell surface. The PDZ-binding motif at the C terminus of Env appears to delay Env turnover. Mutation of the PDZ-binding element diminished Env accumulation in cells to very low levels, indicating that loss of the PDZ-binding motif accelerates Env degradation. Expression of Env with a mutated PDZ-binding motif could be restored to normal levels by also mutating the YXXΦ motif or by silencing AP2 or AP3. The ability of the PDZ-binding motif to alter the activity of the YXXΦ motif depends on the particular sequence of the latter. The attenuating effect of the PDZ-binding motif on Env endocytosis could be overcome by substitution of the YSLI motif in HTLV-1 Env with YXXΦ elements from other cell or virus proteins that are predicted to have higher affinities for AP2 than the YSLI motif of HTLV-1 Env.
MATERIALS AND METHODS
Cell culture.
Human kidney 293T cells and human cervical carcinoma HeLa cell lines HeLa-P4 (expressing CD4), HeLa-TZM (containing an HIV-1 long terminal repeat [LTR]-Luc reporter), and HeLa-Tat (expressing HIV-1 Tat) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and l-glutamine. Human Jurkat E6-1 cells (obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) and Raji/CD4 cells (provided by Vineet N. KewalRamani, NCI) were grown in RPMI 1640 medium containing 10% fetal bovine serum and l-glutamine.
Plasmids.
The HTLV-1 packaging plasmid pCMVHT1M-ΔEnv expresses all HTLV-1 gene products except envelope; the proviral 5′ LTR and 5′ untranslated region (5′ UTR) are replaced with a cytomegalovirus (CMV) immediate-early promoter. The HTLV-1 reporter plasmid pCMVRU5-GFPluc, which is used in cell-free infections, was derived from pHTC-GFPluc (14, 20) by joining the CMV promoter to the RU5 region of the 5′ LTR at the TATA box. For single-cycle cell-to-cell infections, we created the reporter plasmid pCRU5-HT1-inLuc; the reporter cassette within pCMVRU5-GFPluc was replaced with a cassette containing the CMV promoter, a luc gene (with a γ-globin intron), and a thymidine kinase (TK) poly(A) signal. The CMV-inLuc-TK poly(A) cassette is oriented in the opposite direction relative to viral vector transcription, but the intron is oriented in the sense direction, so that Luc is expressed only after virus replication. The HTLV-1 Env expression plasmid pCMV-HT1Env was constructed by PCR amplification of the env gene carried by the provirus clone pCS-HTLV such that the splice donor sequence at the 5′ end of the gene was destroyed without altering the Env amino acid sequence; the PCR product was inserted (replacing the 4070 env gene) in the vector pAmpho (Clontech). Deletions and site-directed mutations in the C terminus of HTLV-1 Env were made by overlapping PCR methods or by QuikChange mutagenesis (Stratagene). In pCMV-HT1Env-ΔPDZ, the proline codon immediately upstream of the ESSL PDZ-binding ligand was converted to a stop codon. An epitope-tagged version of the HTLV-1 Env expression plasmid was constructed by PCR methods such that a 12-amino-acid sequence containing the Myc epitope was inserted between the tyrosine motif (YSLI) and the PDZ-binding motif (ESSL) in the C terminus of Env. The Myc epitope-tagged plasmids are designated pCMV-HT1Env*; the resulting amino acid sequence of the C terminus is YSLINPEQKLISEEDLNPESSL, where the added amino acids are underlined.
Transfections and immunoblotting.
293T cells (1.8 × 106 cells per 60-mm dish) and HeLa-P4 cells (1.5 × 106 cells per 60-mm dish) were transfected with pCMVHTM-ΔEnv (0.8 μg) and HTLV-1 Env plasmid (0.2 μg) using TransIT-293 (Mirus) and FuGene 6 (Roche), respectively, according to the manufacturers' protocols. Virus-like particles (VLPs) were concentrated by ultracentrifugation, and viral pellets were dissolved in NuPAGE LDS sample buffer with reducing reagent (Invitrogen) and heated for 7 min at 90°C. Cells were lysed in TNT buffer (1% Triton X-100, 10 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1 mM EDTA) for 1 h on ice, and samples were prepared as described for VLPs. Cell and VLP extracts were fractioned by SDS-PAGE and transferred to Immobilon membranes. Equal loading of proteins was monitored by staining the membranes with Ponceau S solution (Sigma). Blots were probed with anti-HTLV1-gp46Env and anti-HTLV1-p19MA from ZeptoMetrix Corporation; anti-Myc and anti-VDAC (loading control) from Cell Signaling Technology; anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH) (loading control) from Santa Cruz Biotechnology; and anti-AP50 (AP2), anti-p47A (AP3), and anti-Dlg1 from BD Transduction Laboratories. Membranes were blocked with 5% nonfat milk in phosphate-buffered saline (PBS) with 0.02% Tween (Sigma) (PBST), probed with primary antibodies, washed with PBST, and developed with horseradish peroxidase (HRP)-conjugated secondary antibodies (Cell Signaling). Blots were washed again, and immunoreactive bands were detected with Chemi Glow reagent on an AlphaImager (Alpha Innotec).
Infection assays.
Single-cycle replication assays to quantify cell-free or cell-to-cell infection with HTLV-1 vectors have been described previously (15). Briefly, cell-free infections were performed by transfecting 293T cells with packaging plasmid (pCMVHTM-ΔEnv), reporter plasmid (pCMVRU5-GFPluc), and HTLV-1 Env expression plasmid. Forty-eight hours after transfection, the supernatant was filtered and added to HeLa-P4 cells, and luciferase activity was measured 48 h later using Promega luciferase reagent and a Lumat LB9501 luminometer (Berthold). Cell-to-cell infections were performed by transfecting Jurkat cells (106 cells/well in a 12-well plate) with pCMVHTM-ΔEnv packaging vector, pCRU5-HT1-inLuc reporter vector, and HTLV-1 Env expression plasmid using TransIT-Jurkat (Mirus) transfection reagent. On the following day, cells were washed with PBS and mixed with equal numbers of Raji/CD4 cells. Luciferase activity was measured 48 h later using Promega Brightglo luciferase reagent and a Lumat LB9501 luminometer (Berthold) following the manufacturers' directions (32).
Cell-cell fusion assay.
293T cells (5 × 104 cells/well in a 6-well plate) were transfected by treating cells with pCMVHT1-Env DNA and TransIT-293 transfection reagent (Mirus) for 5 h; cells were washed, and medium was changed. On the following day, HeLa-TZM cells (1 × 105) and HeLa-Tat cells (1 × 105) were added to the transfected 293T cells in a total volume of 4 ml. Luciferase activity was measured 48 h after cell mixing.
Fluorescence-activated cell sorter (FACS) analysis of Env surface expression.
293T cells (106 cells per 60-mm dish) were transfected by treating cells with pCMVHT1-Env DNA (0.2 μg), pCMV-GFP (green fluorescent protein) DNA (1 μg), and Lipofectamine 2000 (Invitrogen). On the following day, cells were collected and probed with anti-HTLV1-gp46Env in PBS containing 1% bovine serum albumin (BSA) on ice. Goat anti-mouse IgG1-phycoerythrin (PE) (BD) was used as the secondary antibody. Samples were analyzed with a FACSCalibur (BD) instrument.
siRNA silencing.
siRNAs (Qiagen) were made for silencing the μ subunits of AP2 and AP3 using the target sequences GUGGAUGCCUUUCGGGUCA and GGAGAACAGUUCUUGCGGC, respectively (24). The random-sequence siRNA was used as a control. HeLa-P4 cells were transfected with 20 nM siRNA using Lipofectamine 2000, followed 24 h later by transfection with pCMVHTM-ΔEnv (0.8 μg) and HTLV-1 Env expression plasmid (0.2 μg). Env expression in cells and Env incorporation into VLPs were analyzed by immunoblotting 48 h later. Silencing of hDlg1 was accomplished with predesigned On-Target SMARTpool siRNA (Dharmacon RNAi Technology).
shRNA silencing.
Small hairpin RNA (shRNA) (Openbiosystem) directed against the μ subunit of AP2 was used to silence AP2 in 293T cells. shRNA was delivered to 293T cells through lentivirus infection according the manufacturer's protocol.
Confocal microscopy.
HeLa-P4 cells (1 × 105 cells/well) were seeded on coverslips in a 12-well plate the day before transfection. Cells were transfected with HTLV-1 Env plasmid (0.2 μg) using Fugene transfection reagent. After 24 h, cells were fixed with 4% paraformaldehyde, permeabilized, and incubated with HTLV-1-infected patient serum (Scripps Laboratories). Fluorescein isothiocyanate (FITC)-conjugated donkey anti-human antibody was used for staining. The Golgi network was detected with wheat germ agglutinin labeled with Alexa Fluor 350. Slides were maintained in ProLong Antifade reagent and analyzed with a Zeiss LSM 510 inverted laser scan microscope.
RESULTS
The cytoplasmic domain of HTLV-1 Env contains two peptide motifs that modulate Env expression and function.
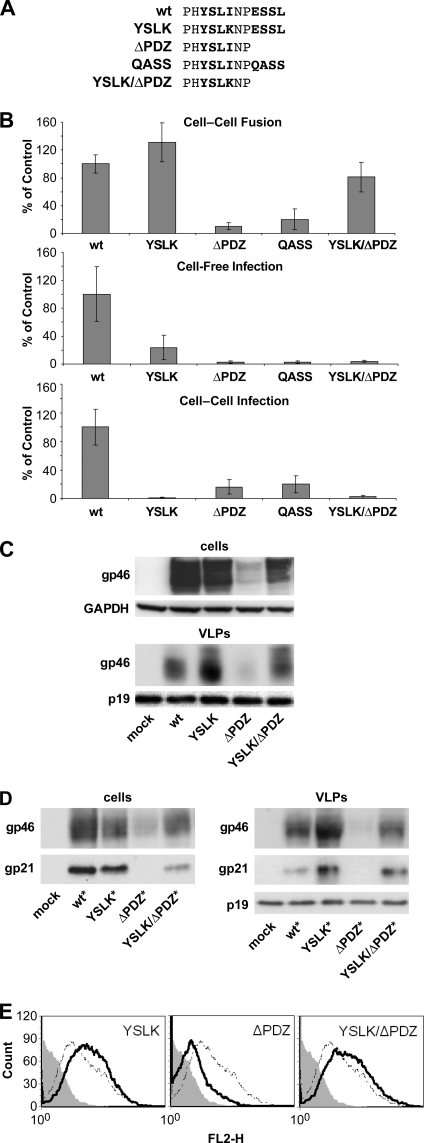
The cytoplasmic tail of HTLV-1 Env is reported to contain two peptide motifs that can mediate interactions with cellular proteins and regulate Env expression and activity. One motif is YSLI, which matches the YXXΦ consensus that is known to interact with various adapter proteins (10, 37). The other motif is the C-terminal ESSL element, which matches the position and consensus sequence for PDZ-binding motifs (36). We mutated or deleted these sequences, alone or in combination, as shown in Fig. 1A, and examined the resulting effects on Env activity and expression. The critical hydrophobic residue in the YSLI sequence was changed to YSLK (10). The ESSL motif either was deleted to result in ΔPDZ Env or was converted by site-directed mutagenesis to result in QASS. Both YXXΦ and PDZ-binding motifs were mutated in YSLK/ΔPDZ Env.
FIG. 1.
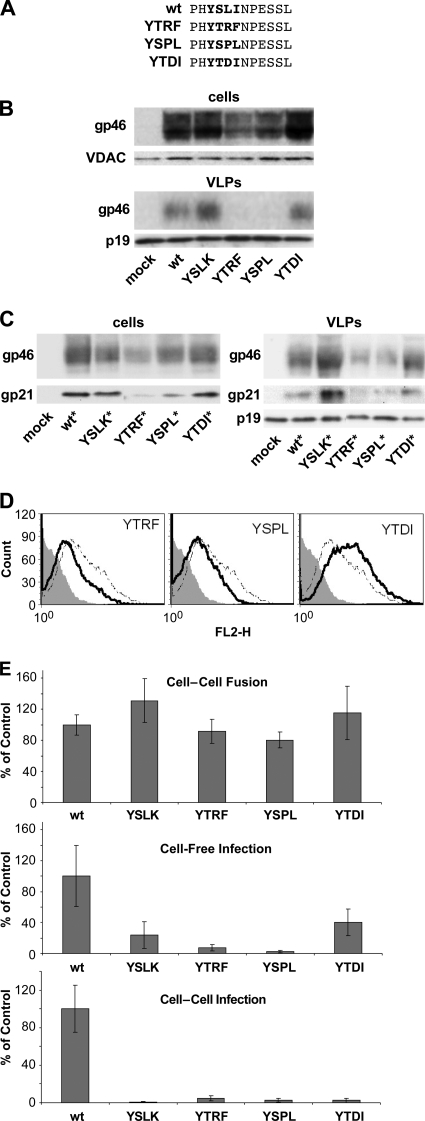
Two motifs in the C-terminal cytoplasmic domain of HTLV-1 Env modulate Env expression and functional activity. (A) Amino acid sequences of the C termini of WT and mutated HTLV-1 Envs. Amino acids comprising the tyrosine-based motif (YSLI) and the PDZ-binding motif (ESSL) are shown in bold. (B) Functional activities of WT and mutated versions of HTLV-1 Env were assessed in cell-cell fusion assays, cell-free infection assays, and cell-to-cell infection assays, as described in Materials and Methods. In cell-cell fusion assays, 293T cells were transfected with HTLV-1 Env plasmid and then cocultured with HeLa-Tat cells and HeLa-TZM cells; cell fusion resulted in luciferase expression. In cell-free infection assays, 293T cells were transfected with HTLV-1 pack aging (pCMVHT1M-ΔEnv) and reporter vectors and the indicated HTLV-1 Env expression plasmids; VLPs were then used to infect HeLa-P4 cells. In cell-to-cell infection assays, Jurkat cells were transfected with HTLV-1 vectors and then cocultured with Raji/CD4 cells. The results are presented as percentages relative to the value for the WT Env control and represent the means from at least three independent experiments; error bars show standard deviations. (C) 293T cells were transfected with pCMVHT1M-ΔEnv and either empty vector (mock) or the indicated HTLV-1 Env plasmids. Two days after transfection, cell lysates and VLP extracts were analyzed by Western blotting with anti-HTLV1 gp46 (SU) antibody. (D) 293T cells were transfected with pCMVHT1M-ΔEnv plus empty vector (mock) or the indicated myc-tagged HTLV-1 Env constructs. Two days later, cell lysates and VLP extracts were analyzed by immunoblotting. HTLV-1 Env SU (gp46) was detected with anti-HTLV-1 gp46 antibody, HTLV-1 TM (gp21) was detected with anti-myc antibody, and HTLV-1 MA was detected with anti-HTLV-1 p19 antibody. (E) 293T cells were transfected with HTLV-1 Env plasmids and a GFP-expressing plasmid; on the next day, cells were probed with anti-HTLV-1 gp46 (SU) for surface expression of Env. Samples were analyzed with a FACSCalibur instrument, and Env surface expression in GFP-positive cells was measured. These experiments were performed three times. Filled histogram, empty vector; dotted line, WT; solid line, Env with mutations. FL2-H, fluorescence in (585 ± 42 nm) channel.
First, we measured the abilities of wild-type (WT) and mutated Envs to mediate syncytium formation in a three-way cell fusion assay. Human 293T cells were transfected with an Env expression plasmid and cocultured with a mixture of two different HeLa cell lines: HeLa-Tat, which expresses HIV-1 Tat, and HeLa-TZM, which contains an HIV-1 LTR-controlled luciferase gene. Cell-cell fusion permits Tat activation of luciferase expression. The YSLK Env was consistently more fusogenic than WT Env (P < 0.05 according to the Student t test) (Fig. 1B). In contrast, the truncation or substitution of the PDZ ligand (ΔPDZ or QASS) resulted in significantly diminished cell fusion activity. Interestingly, the YSLK mutation restored cell fusion activity to that of ΔPDZ Env in the YSLK/ΔPDZ Env double mutant. These results suggest that the YSLK and ΔPDZ mutations do not affect fusion activity per se but rather affect Env accumulation at the plasma membrane.
We next examined the abilities of the various HTLV-1 Envs to mediate infection in single-cycle infection assays with HTLV-1 vectors. In cell-free infection experiments, VLPs were generated in 293T cells cotransfected with HTLV-1 packaging vector, HTLV-1 reporter vector encoding GFPluc, and an Env expression plasmid. Transduction of luciferase activity by VLPs containing YSLK Env was about 5-fold lower than that by VLPs containing WT Env (Fig. 1B). The other mutated Envs (ΔPDZ, QASS, and YSLK/ΔPDZ) were essentially inactive in cell-free infections. We have recently developed a single-cycle cell-to-cell infection assay for HTLV-1 in which Jurkat T cells are cotransfected with HTLV-1 vectors and Env expression plasmid and then cocultured with Raji/CD4 cells. In this assay, YSLK Env and YSLK/ΔPDZ Env were inactive, whereas ΔPDZ Env and QASS Env retained 15% to 20% of WT Env activity.
To determine whether the phenotypes displayed in cell fusion and infectivity assays were correlated with Env expression or virion incorporation, we performed Western blotting on cell extracts and VLP lysates from 293T cells cotransfected with HTLV-1 packaging plasmid and various Env expression plasmids (Fig. 1C). Immunoblots were probed with anti-gp46 (SU) antibody. The YSLK Env accumulated in cells at slightly lower levels than WT Env because YSLK was more efficiently incorporated into VLPs. The ΔPDZ Env did not accumulate in cells and was barely detectable in VLPs. However, the double mutation in YSLK/ΔPDZ Env restored Env expression in cells and incorporation into VLPs. These results mirror what was observed in the cell fusion assays.
We next examined the possibility that mutations in the cytoplasmic part of Env might destabilize interactions between the SU and TM subunits, leading to SU shedding. We constructed Env expression plasmids that contain an 11-amino-acid myc epitope tag between the YSLI and ESSL motifs. We could not add the epitope tag to the C terminus of Env, as this would block the PDZ ligand. The myc-tagged Env expression plasmid (WT* Env) produced the same level of Env as the untagged WT Env plasmid, and the myc-tagged protein had the same biological properties as WT Env (data not shown). The various Env mutations were engineered into the myc-tagged Env expression plasmid and examined by immunoblotting cell and VLP extracts as described above (Fig. 1D). Anti-myc antibody was used to detect gp21 (TM), and anti-gp46 antibody was used to detect SU. As shown in Fig. 1D, the ratios of SU to TM were the same for all mutated Envs. For example, both Env subunits were expressed from YSLK* Env at levels comparable to that for WT* Env, but neither subunit was detected in cells transfected with ΔPDZ* Env. Defects in virus infectivity of mutated Env proteins could not be attributed to changes in Env incorporation into VLPs or to SU shedding.
Immunoblot analysis allows comparison of only the overall amounts of Env in the cells. To determine how the mutations in the cytoplasmic part affect the expression of Env on the cell surface, we performed surface staining of gp46 followed by FACS analysis. Mutation in the tyrosine-based motif YSLK caused accumulation of Env on the cell surface, and the level of expression was higher than that for the WT (Fig. 1E).
The level of cell surface expression of ΔPDZ Env was lower than that for the WT, but the introduction of the mutation in the tyrosine-based motif (YSLK/ΔPDZ) restored the amount of the truncated Env on the cell surface.
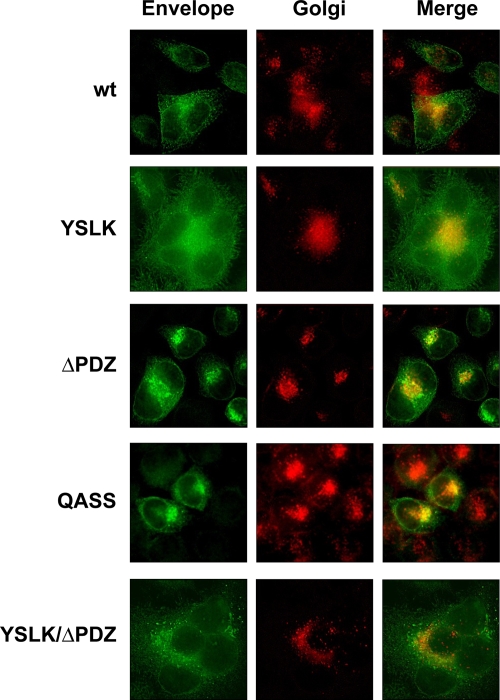
Immunofluorescent staining and confocal microscopy of HeLa cells transfected with the various Env plasmids confirmed that mutations of the PDZ-binding motif affected distribution of Env in cells (Fig. 2). Both ΔPDZ and QASS Env proteins colocalized mainly with Golgi network markers, whereas the WT, YSLK, and YSLK/ΔPDZ Envs were distributed throughout the cell, heavily staining in the endoplasmic reticulum and Golgi network and extensively decorating the plasma membrane.
FIG. 2.
Intracellular distribution of HTLV-1 envelope in HeLa-P4 cells. HeLa-P4 cells were transfected with the indicated HTLV-1 Env expression plasmids. Cells were permeabilized and stained with sera from HTLV-1-infected patients to detect Env (green) or with wheat germ agglutinin for the Golgi network (red). Distribution of Env with a truncated or substituted PDZ-binding ligand (ΔPDZ and QASS) in cells differed from that of other Env constructs and localized mainly in the Golgi network. Env with combined mutations (YSLK/ΔPDZ) was localized similarly to WT and YSLK Env. These experiments were performed three times.
The adaptor protein complexes AP2 and AP3 regulate HTLV-1 Env trafficking.
The above-described experiments indicate that mutation of the YXXΦ and PDZ-binding motifs affects Env accumulation and function, likely by modulating protein trafficking. Furthermore, the data suggest that the two motifs in Env may cooperate functionally. The YXXΦ motifs in membrane proteins interact with adaptor protein complexes; AP1 is responsible mainly for delivery of proteins from the Golgi network to the cell surface, AP2 is involved primarily in protein internalization from the plasma membrane, and AP3 directs cargo from early endosomes to lysosomes for degradation. Because YSLK Env accumulated at the plasma membrane, we could rule out AP1 as a determinant of Env trafficking.
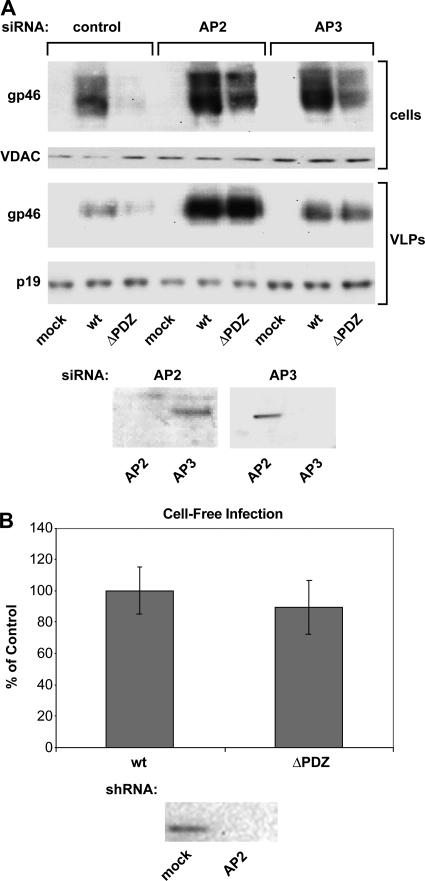
To determine the roles of AP2 and AP3 in Env expression, we abrogated AP2 and AP3 function by silencing the expression of subunits μ2 and μ3, respectively, with siRNA in HeLa-P4 cells. Env accumulation in cells and Env incorporation into VLPs were examined by immunoblotting (Fig. 3A). siRNA directed against AP2, but not AP3, specifically silenced AP2 expression (Fig. 3A, bottom). Likewise, siRNA against AP3 specifically silenced AP3, but not AP2, expression. Blocking AP2 production in cells transfected with the WT Env expression plasmid resulted in a significant increase in the amount of Env in cells and in VLPs (Fig. 3A). The effect of AP2 silencing on Env accumulation was even more pronounced in cells expressing ΔPDZ Env. Moreover, AP2 silencing caused increased cell surface expression of ΔPDZ Env (data not shown). This result is very similar to the effect that we observed by mutating the YXXΦ motif in Env.
FIG. 3.
siRNA silencing of AP2 and AP3 μ subunits caused increased accumulation of WT and ΔPDZ Env in cells and VLPs. (A) HeLa-P4 cells were transfected with siRNAs directed against the μ subunit of either AP2 or AP3 and then transfected with pCMVHT1M-ΔEnv plus the indicated HTLV-1 Env expression plasmids. Cell lysates were examined by immunoblotting with antibodies to the μ chains of AP2 and AP3 (bottom), which showed the efficiency of siRNA targeting and transfection. Anti-HTLV-1 gp46 antibody was used to probe for Env expression, and anti-HTLV-1 p19 antibody revealed that equal amounts of VLP extracts were loaded onto the gel. This experiment was performed three times. (B) shRNA delivered by lentiviral vector constructs and directed against the μ subunit of AP2 was used to silence AP2 in 293T cells, and cells were then transfected with pCMVHT1M-ΔEnv plus the indicated HTLV-1 Env expression plasmids. Cell-free infection was performed as described in Materials and Methods. Cell lysates were examined by immunoblotting with antibodies to μ chains of AP2 (bottom). Error bars show standard deviations.
AP3 mediates sorting of membrane cargo from the endosome to the lysosome and from the Golgi network to the lysosome. We observed that silencing of AP3 synthesis also increased the accumulation of WT and ΔPDZ Env proteins in cells and VLPs, although to a lesser extent than did AP2 silencing (Fig. 3A). It is likely that Env is degraded after internalization from the plasma membrane, but we cannot exclude the possibility that a certain amount of Env is directed from the Golgi network to lysosomes for degradation. Together with the AP2 silencing experiments, the results indicate that ΔPDZ Env is able to reach the plasma membrane but that it is rapidly internalized in an AP2-dependent manner. The interaction between the PDZ ligand in WT Env and a cellular PDZ protein possibly slows the process of endocytosis or may alter the downstream sorting events.
Using retroviral vectors to deliver shRNA, we were able to silence AP2 in 293T cells. As in HeLa-P4 cells, cell silencing of AP2 resulted in higher ΔPDZ Env incorporation into virions than was found with untreated cells (data not shown). Next, we determined whether truncation of the PDZ-binding motif affects infectivity of VLPs in the cell-free infectivity assay (Fig. 3B). Surprisingly, unlike results for the tyrosine-based motif, VLPs pseudotyped with Env lacking the PDZ motif were infectious (up to 89% of the WT level) in the cell-free infectivity assay. Based on these results, we conclude that, unlike that of the YXXΦ mutant, the phenotype of the PDZ-binding motif mutant is confined to the producer cell.
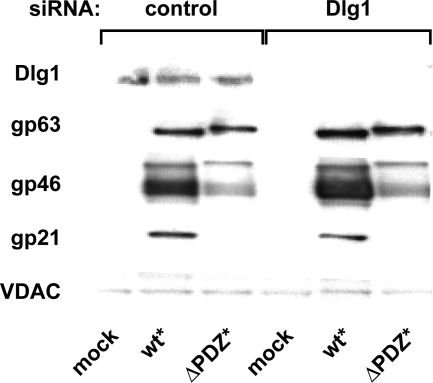
siRNA silencing of Dlg1 does not affect Env expression.
The PDZ ligand in HTLV-1 Env likely interacts with a cellular protein containing a PDZ domain. In a yeast two-hybrid screen with a “bait” containing the HTLV-1 Env PDZ ligand, the human homolog of Dlg1 was identified (2). Yeast two-hybrid screens with PDZ ligands derived from various other proteins have also identified Dlg1 as a binding partner, suggesting that Dlg1 interacts with many proteins or that it is simply more abundant than other PDZ proteins. We expected that if Dlg1 were the PDZ protein that interacted with the PDZ ligand in Env, abrogating Dlg1 expression would produce a phenotype similar to that of ΔPDZ Env. We therefore silenced Dlg1 expression with siRNA to test whether Dlg1 modulates HTLV-1 Env trafficking. Transfection of HeLa cells with Dlg1 siRNA reduced Dlg1 protein expression to undetectable levels on Western blots (Fig. 4). However, Dlg1 silencing did not alter Env accumulation in cells, indicating that Dlg1 is unlikely to be the critical binding partner for the PDZ ligand in HTLV-1 Env. The identity of the cellular protein that interacts with the PDZ ligand in Env remains to be established.
FIG. 4.
Dlg1 is not a binding partner for the PDZ-binding ligand of HTLV-1 Env. HeLa-P4 cells were transfected with pooled siRNA directed against Dlg1 and later transfected with the indicated HTLV-1 myc-tagged Env plasmids. Immunoblot analysis of cell lysates with antibody to Dlg1 confirmed the efficiency and specificity of Dlg1 silencing. Probing the blots with anti-myc antibody revealed bands corresponding to the Env precursor (gp63) and TM (gp21); anti-gp46 antibody detected HTLV-1 Env SU; and antibody to VDAC was used as a loading control. Silencing of Dlg1 did not cause significant changes in expression of WT* and ΔPDZ* Env. This experiment was repeated three times.
HTLV-1 Env function is dependent on a specific subset of YXXΦ motifs.
The μ subunits of AP complexes, μ1, μ2, μ3, and μ4, interact with distinct but overlapping sets of YXXΦ signals. The specificity and affinity of the interaction are determined in large part by amino acids at the X positions adjacent to the invariant tyrosine. The tyrosine-based signal in HTLV-1 Env is unusual in having a hydrophobic residue at the Y +2 position (YSLI), which would predict a poor interaction with AP2. For interaction with the μ2 subunit of AP2, arginine or proline is favored at the Y +2 position, and leucine and aspartate are disfavored (37). To determine how other YXXΦ signals affect HTLV-1 Env expression and activity, we replaced the native motif with the YXXΦ signals from the transferrin receptor (YTRF) (27), HIV-1 Env (YSPL) (4, 7), and vesicular stomatitis virus (VSV) G protein (YTDI) (7, 34) (Fig. 5A).
FIG. 5.
Substitution of the HTLV-1 tyrosine-based motif with different YXXΦ signals affected functions of Env. (A) Amino acid sequences of the C-terminal cytoplasmic domains of WT and variant Env proteins. The tyrosine-based motif is shown in bold. The YSLI (WT) element was replaced with the tyrosine-based motif from the transferrin receptor (YTRF), HIV-1 envelope (YSPL), or VSV-G protein (YTDI). (B) 293T cells were transfected with pCMVHT1M-ΔEnv plus the indicated Env expression plasmids. Two days later, cell and VLP lysates were examined by immunoblotting with antibody to HTLV-1 gp46 (SU). (C) 293T cells were transfected with pCMVHT1M-ΔEnv plus the indicated myc-tagged HTLV-1 Env expression plasmids. Two days later, cell and VLP lysates were examined by immunoblotting with antibody to HTLV-1 gp46 (SU); anti-myc, to detect gp21 (TM); or anti-HTLV-1 p19 (MA). (D) 293T cells were transfected with HTLV-1 Env plasmids; on the next day, cells were probed with anti-HTLV1 gp46 (SU) for surface expression of Env. Filled histogram, empty vector; dotted line, WT; solid line, Env with mutations. Samples were analyzed with a FACSCalibur instrument, and Env surface expression in GFP-positive cells was measured. These experiments were performed three times. (E) HTLV-1 Envs with WT or variant tyrosine-based motifs were tested in cell-cell fusion assays and infectivity assays as described for Fig. 1B. The results for the indicated Env variants are presented as percentages compared with the WT level. These experiments were performed at least three times; error bars show standard deviations.
The various YXXΦ signals were inserted into both untagged and myc-tagged versions of HTLV-1 Env, so that we could examine both gp46 (SU) and gp21 (TM) accumulation in cells and incorporation into VLPs by immunoblotting (Fig. 5B and C). Cells transfected with Env expression plasmids encoding YTRF or YSPL signals produced significantly lower levels of Env proteins and had less Env in VLPs than did WT Env. Moreover, the amounts of Env with these substitutions expressed on the cell surface were lower than that for the WT (Fig. 5D). These results are consistent with the more efficient AP2-controlled internalization of Env proteins that contain YTRF or YSPL signals, which display a higher-affinity interaction with subunit μ2 than does the YSLI motif in the WT Env. Levels of cellular accumulation and VLP incorporation of gp46 and gp21 were similar for WT Env and for YTDI Env accumulated on the cell surface, as one would expect for a tyrosine-based motif that also interacts poorly with AP2 (Fig. 5D).
In cell-cell fusion assays, the fusion activities of YTRF and YSPL Env proteins were comparable to those of WT Env, YSLK Env, and YTDI Env (Fig. 5E, top). Although YTRF Env and YSPL Env proteins were present at lower levels than the other Env constructs, levels of active protein sufficient to mediate cell-cell fusion were produced on the cell surface. In cell-free infection assays, only the YTDI Env retained significant activity, which was about 40% that of WT Env (Fig. 5E, middle). YTRF Env and YSPL Env retained less than 10% of WT Env activity. In cell-to-cell infection experiments, substitution of the YSLI motif in Env with any of the other signals (YTRF, YSPL, or YTDI) abolished infectivity (Fig. 5E, bottom). These results indicate not only that the YXXΦ motif is necessary for HTLV-1 Env function but that the particular subclass of signaling motif is important for proper control of Env trafficking and activity.
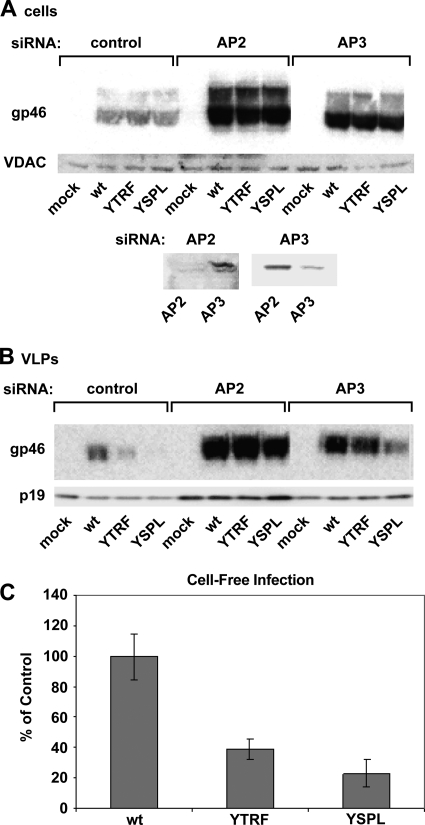
We next examined whether the biological differences among the Envs containing variations in the YXXΦ motif could be attributed to differences in their interactions with AP2 or AP3. Expression of AP2 or AP3 was inhibited using siRNA directed at subunit μ2 or μ3, respectively (Fig. 6A, bottom). siRNA-mediated silencing of AP2 resulted in a significant increase in cellular accumulation of WT, YTRF, and YSPL Envs (Fig. 6A). AP2 silencing caused an even greater increase in the incorporation of all Envs into VLPs (Fig. 6B), indicating that AP2-mediated internalization from the cell surface is a critical determinant of Env accumulation. AP3 silencing also resulted in increased levels of Env in cells and in VLPs, although not as great as with AP2 silencing. Increased Env levels in cells in which AP3 was silenced could reflect a delayed Env degradation, as AP3 might direct internalized proteins to lysosomes.
FIG. 6.
siRNA silencing of AP2 and AP3 increased the cellular accumulation and VLP incorporation of Env with variant tyrosine-based motifs. (A and B) HeLa-P4 cells were transfected with siRNA to the μ subunit of AP2 or AP3. Immunoblot analysis of cell lysates with antibodies to the μ chains of AP2 and AP3 shows the efficiency and specificity of siRNA silencing (A, bottom). After transfection with siRNA, cells were again transfected with pCMVHT1M-ΔEnv and the indicated Env expression plasmids. Blots were probed with anti-HTLV-1 gp46 (SU); anti-VDAC, as a loading control for cell lysates; or anti-HTLV-1 p19 (MA), to normalize VLP loading. This experiment was performed three times. (C) shRNA directed against the μ subunit of AP2 was used to silence AP2 in 293T cells (as described for Fig. 3B), and cells were then transfected with pCMVHT1M-ΔEnv plus the indicated HTLV-1 Env expression plasmids. Cell-free infection was performed as described in Materials and Methods. Error bars show standard deviations.
shRNA-mediated silencing in 293T cells restored incorporation of YTRF and YSPL into VLPs (data not shown), but this incorporation caused only slight increases in cell-free infectivity (38% and 23%, respectively) (Fig. 6C). These results point to the importance of YSLI for infectivity of HTLV1; YSLI cannot be substituted with just any functional tyrosine-based motif, confirming the importance of the sequence of the tyrosine-based motif.
DISCUSSION
We have confirmed and extended previous studies of the HTLV-1 Env cytoplasmic domain, which showed that mutations of critical amino acids in the YSLI element (e.g., ASLI or YSLK) resulted in elevated levels of cell surface expression of Env, increased Env incorporation into virions, and increased cell-cell fusion (10). Surprisingly, these changes were accompanied by almost complete loss of virus infectivity, either by cell-free or cell-to-cell routes of transmission. The YXXΦ motif interacts with the μ subunits of adaptor complexes, and we reasoned that interactions with AP2 and AP3 were the most relevant with respect to Env trafficking, as they mediate membrane protein internalization from the cell surface and lysosomal sorting, respectively. Silencing AP2 with siRNA directed against μ2 resulted in changes in Env expression and virion incorporation that were similar to the effects caused by mutation of the YSLI sequence; silencing AP3 produced changes that were similar to but less dramatic than those produced by AP2 silencing. Thus, the YSLI motif in HTLV-1 Env mediates interactions with AP2 and AP3 that play major roles in the control of Env trafficking and activity. In addition, Env expression was profoundly reduced by mutations in the putative PDZ-binding motif at the C terminus of Env, apparently due to accelerated endocytosis and degradation of Env. Expression of Env with a mutated PDZ-binding motif could be restored to wild-type levels by mutations that destroyed the YXXΦ motif or by siRNA-mediated silencing of AP2 and AP3. The data are consistent with a model in which the PDZ-binding motif interferes with AP2- and AP3-mediated turnover of Env. Furthermore, the ability of the PDZ-binding motif to alter the function of the YXXΦ motif depends on the sequence of the latter; YXXΦ motifs that are predicted to interact weakly with AP2 (such as YSLI) were modulated by PDZ protein binding, whereas elements that are predicted to interact with high affinity (such as YTRF or YSPL) were not.
To understand the mechanism by which the PDZ-binding motif modulates adaptor protein-mediated trafficking of HTLV-1 Env, it is important to know which PDZ protein(s) is involved. It was previously suggested that hDlg is the binding partner, based on yeast two-hybrid screens, in vitro pulldown assays, and colocalization of Env and hDlg in cells (2, 54). However, considering the number of PDZ proteins in cells and the limitations of the assays, we believe that these results could simply reflect the fact that hDlg is very abundantly expressed in cells. Previous studies did not investigate the effects of PDZ-binding motif mutations on Env expression and trafficking. It is expected that silencing of the PDZ protein that binds to HTLV-1 Env would result in effects on Env expression similar to those produced by mutating the PDZ-binding motif. By siRNA methods, we were able to reduce hDlg protein expression to levels that could not be detected by immunoblotting, but this had no effect on Env expression. These data suggest that there is a PDZ protein other than hDlg that binds to HTLV-1 Env and interferes with Env trafficking. Alternatively, silencing could be leaky, and a sufficient, although undetectable, amount of hDlg could be expressed to produce the delay in Env turnover. We are currently screening additional siRNAs to identify other PDZ proteins that may interact specifically with HTLV-1 Env. We realize, however, that there may be multiple PDZ proteins that can interact with HTLV-1 Env.
Because the PDZ proteins that interact with HTLV-1 are not known, we can only speculate on possible mechanisms by which PDZ protein binding to Env alters AP2- and AP3-mediated Env trafficking and propose experiments to test them. There are only two amino acids separating the YXXΦ and PDZ-binding motifs, so it is possible that PDZ protein and μ-subunit binding could compete by steric interference. We believe, however, that this is unlikely because insertion of an additional 10 amino acids (myc epitope) between the two motifs did not alter their behavior. An alternative possibility is that docking of Env to a PDZ protein scaffold would position Env in an environment where μ2 and μ3 affinity for the YSLI motif is diminished. It was previously established that phosphorylation of μ2 by the serine/threonine kinase AAK1 is required for high-affinity binding to the YXXΦ motif and for receptor-mediated endocytosis of transferrin (18). Association of Env with a supramolecular complex via PDZ protein interactions could delay Env endocytosis by excluding AAK1 from the area or by recruiting a phosphatase to maintain μ2 in an unphosphorylated state. The binding affinity of μ2 for YSLI is also likely to be regulated by local phosphoinositide concentrations, which could also be influenced by localization of Env to a PDZ scaffold (47). Finally, there are precedents for the control of targeting, trafficking, and recycling of ion channels, membrane transporters, and cell surface receptors as a result of their interactions with PDZ protein scaffolds that may help guide our understanding of HTLV-1 Env metabolism (17, 52).
HTLV-1 infection in vivo is believed to occur almost entirely between lymphocytes that are engaged in direct contact. It is logical to expect that HTLV-1 Env has acquired specific adaptations for this mode of transmission. Unlike MLV and MPMV Envs, where C-terminal cleavage of the TM R peptide by the viral protease in the virion activates fusogenic activity, HTLV-1 TM does not undergo cleavage and is present on the cell surface in a highly fusogenic state, resembling mutated versions of MLV and MPMV Env that lack C-terminal amino acids. In other words, HTLV-1 Env arrives at the cell surface ready to go, so it is critical to regulate the amount of Env on the cell surface and the length of time it resides there. Although all retroviral Env cytoplasmic domains have YXXΦ motifs for interaction with adaptor proteins, only the HTLV/STLV Envs contain a PDZ-binding motif. In addition to controlling Env trafficking, the opposing effects of adaptor complexes and PDZ proteins may direct the localization of Env to specific regions of the plasma membrane. For example, targeting Env to a specific microdomain by interaction with a PDZ scaffold could act to stabilize Env at that location; in regions lacking the interacting PDZ protein, Env would rapidly be internalized and degraded. One of the more puzzling results reported here and elsewhere is that inhibition of Env internalization by mutating the YXXΦ motif or substitution of HTLV1 YSLK with different tyrosine-based motifs reduced virus infectivity. Therefore, it is possible that the YXXΦ motif is also involved in postentry events.
Acknowledgments
We dedicate this work to the memory of David Derse.
We thank Jennifer Brown and Allen Kane (NCI, NIH) for graphics and Anne Arthur and Michael Tadle for editing the manuscript.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Published ahead of print on 12 May 2010.
REFERENCES
- 1.Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 73:1350-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blot, V., L. Delamarre, F. Perugi, D. Pham, S. Benichou, R. Benarous, T. Hanada, A. H. Chishti, M. C. Dokhelar, and C. Pique. 2004. Human Dlg protein binds to the envelope glycoproteins of human T-cell leukemia virus type 1 and regulates envelope mediated cell-cell fusion in T lymphocytes. J. Cell Sci. 117:3983-3993. [DOI] [PubMed] [Google Scholar]
- 3.Bobkova, M., J. Stitz, M. Engelstadter, K. Cichutek, and C. J. Buchholz. 2002. Identification of R-peptides in envelope proteins of C-type retroviruses. J. Gen. Virol. 83:2241-2246. [DOI] [PubMed] [Google Scholar]
- 4.Boge, M., S. Wyss, J. S. Bonifacino, and M. Thali. 1998. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 273:15773-15778. [DOI] [PubMed] [Google Scholar]
- 5.Bonifacino, J. S., and L. M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395-447. [DOI] [PubMed] [Google Scholar]
- 6.Brody, B. A., S. S. Rhee, M. A. Sommerfelt, and E. Hunter. 1992. A viral protease-mediated cleavage of the transmembrane glycoprotein of Mason-Pfizer monkey virus can be suppressed by mutations within the matrix protein. Proc. Natl. Acad. Sci. U. S. A. 89:3443-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byland, R., and M. Marsh. 2005. Trafficking of viral membrane proteins. Curr. Top. Microbiol. Immunol. 285:219-254. [DOI] [PubMed] [Google Scholar]
- 8.Copeland, T. D., W. P. Tsai, Y. D. Kim, and S. Oroszlan. 1986. Envelope proteins of human T cell leukemia virus type I: characterization by antisera to synthetic peptides and identification of a natural epitope. J. Immunol. 137:2945-2951. [PubMed] [Google Scholar]
- 9.Danis, C., J. Deschambeault, S. Do Carmo, E. A. Cohen, E. Rassart, and G. Lemay. 2004. The tyrosine-based YXXΦ targeting motif of murine leukemia virus envelope glycoprotein affects pathogenesis. Virology 324:173-183. [DOI] [PubMed] [Google Scholar]
- 10.Delamarre, L., C. Pique, A. R. Rosenberg, V. Blot, M. P. Grange, I. Le Blanc, and M. C. Dokhelar. 1999. The Y-S-L-I tyrosine-based motif in the cytoplasmic domain of the human T-cell leukemia virus type 1 envelope is essential for cell-to-cell transmission. J. Virol. 73:9659-9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delamarre, L., A. R. Rosenberg, C. Pique, D. Pham, I. Callebaut, and M. C. Dokhelar. 1996. The HTLV-I envelope glycoproteins: structure and functions. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13(Suppl. 1):S85-S91. [DOI] [PubMed] [Google Scholar]
- 12.Dell'Angelica, E. C., J. Klumperman, W. Stoorvogel, and J. S. Bonifacino. 1998. Association of the AP-3 adaptor complex with clathrin. Science 280:431-434. [DOI] [PubMed] [Google Scholar]
- 13.Dell'Angelica, E. C., C. Mullins, and J. S. Bonifacino. 1999. AP-4, a novel protein complex related to clathrin adaptors. J. Biol. Chem. 274:7278-7285. [DOI] [PubMed] [Google Scholar]
- 14.Derse, D., S. A. Hill, P. A. Lloyd, H. Chung, and B. A. Morse. 2001. Examining human T-lymphotropic virus type 1 infection and replication by cell-free infection with recombinant virus vectors. J. Virol. 75:8461-8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derse, D., S. A. Hill, G. Princler, P. Lloyd, and G. Heidecker. 2007. Resistance of human T cell leukemia virus type 1 to APOBEC3G restriction is mediated by elements in nucleocapsid. Proc. Natl. Acad. Sci. U. S. A. 104:2915-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grange, M. P., V. Blot, L. Delamarre, I. Bouchaert, A. Rocca, A. Dautry-Varsat, and M. C. Dokhelar. 2000. Identification of two intracellular mechanisms leading to reduced expression of oncoretrovirus envelope glycoproteins at the cell surface. J. Virol. 74:11734-11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haucke, V. 2006. Cargo takes control of endocytosis. Cell 127:35-37. [DOI] [PubMed] [Google Scholar]
- 18.Henderson, D. M., and S. D. Conner. 2007. A novel AAK1 splice variant functions at multiple steps of the endocytic pathway. Mol. Biol. Cell 18:2698-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson, L. E., R. Sowder, T. D. Copeland, G. Smythers, and S. Oroszlan. 1984. Quantitative separation of murine leukemia virus proteins by reversed-phase high-pressure liquid chromatography reveals newly described gag and env cleavage products. J. Virol. 52:492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill, S. A., P. A. Lloyd, S. McDonald, J. Wykoff, and D. Derse. 2003. Susceptibility of human T cell leukemia virus type I to nucleoside reverse transcriptase inhibitors. J. Infect. Dis. 188:424-427. [DOI] [PubMed] [Google Scholar]
- 21.Hunter, E., and R. Swanstrom. 1990. Retrovirus envelope glycoproteins. Curr. Top. Microbiol. Immunol. 157:187-253. [DOI] [PubMed] [Google Scholar]
- 22.Igakura, T., J. C. Stinchcombe, P. K. Goon, G. P. Taylor, J. N. Weber, G. M. Griffiths, Y. Tanaka, M. Osame, and C. R. Bangham. 2003. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299:1713-1716. [DOI] [PubMed] [Google Scholar]
- 23.Januszeski, M. M., P. M. Cannon, D. Chen, Y. Rozenberg, and W. F. Anderson. 1997. Functional analysis of the cytoplasmic tail of Moloney murine leukemia virus envelope protein. J. Virol. 71:3613-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janvier, K., and J. S. Bonifacino. 2005. Role of the endocytic machinery in the sorting of lysosome-associated membrane proteins. Mol. Biol. Cell 16:4231-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin, J., N. M. Sherer, G. Heidecker, D. Derse, and W. Mothes. 2009. Assembly of the murine leukemia virus is directed towards sites of cell-cell contact. PLoS Biol. 7:e1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim, E., and M. Sheng. 2004. PDZ domain proteins of synapses. Nat. Rev. Neurosci. 5:771-781. [DOI] [PubMed] [Google Scholar]
- 27.Kurten, R. C. 2003. Sorting motifs in receptor trafficking. Adv. Drug Deliv. Rev. 55:1405-1419. [DOI] [PubMed] [Google Scholar]
- 28.Li, K., S. Zhang, M. Kronqvist, M. Wallin, M. Ekstrom, D. Derse, and H. Garoff. 2008. Intersubunit disulfide isomerization controls membrane fusion of human T-cell leukemia virus Env. J. Virol. 82:7135-7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lodge, R., L. Delamarre, J. P. Lalonde, J. Alvarado, D. A. Sanders, M. C. Dokhelar, E. A. Cohen, and G. Lemay. 1997. Two distinct oncornaviruses harbor an intracytoplasmic tyrosine-based basolateral targeting signal in their viral envelope glycoprotein. J. Virol. 71:5696-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majorovits, E., M. Nejmeddine, Y. Tanaka, G. P. Taylor, S. D. Fuller, and C. R. Bangham. 2008. Human T-lymphotropic virus-1 visualized at the virological synapse by electron tomography. PLoS One 3:e2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manel, N., J. L. Battini, N. Taylor, and M. Sitbon. 2005. HTLV-1 tropism and envelope receptor. Oncogene 24:6016-6025. [DOI] [PubMed] [Google Scholar]
- 32.Mazurov, D., A. Ilinskaya, G. Heidecker, P. Lloyd, and D. Derse. 2010. Quantitative comparison of HTLV-1 and HIV-1 cell-to-cell infection with new replication dependent vectors. PLoS Pathog. 6:e1000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakatsu, F., and H. Ohno. 2003. Adaptor protein complexes as the key regulators of protein sorting in the post-Golgi network. Cell Struct. Funct. 28:419-429. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura, N., H. Plutner, K. Hahn, and W. E. Balch. 2002. The delta subunit of AP-3 is required for efficient transport of VSV-G from the trans-Golgi network to the cell surface. Proc. Natl. Acad. Sci. U. S. A. 99:6755-6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norris, F. A., E. Ungewickell, and P. W. Majerus. 1995. Inositol hexakisphosphate binds to clathrin assembly protein 3 (AP-3/AP180) and inhibits clathrin cage assembly in vitro. J. Biol. Chem. 270:214-217. [DOI] [PubMed] [Google Scholar]
- 36.Nourry, C., S. G. Grant, and J. P. Borg. 2003. PDZ domain proteins: plug and play! Sci. STKE 2003:RE7. [DOI] [PubMed]
- 37.Ohno, H., R. C. Aguilar, D. Yeh, D. Taura, T. Saito, and J. S. Bonifacino. 1998. The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J. Biol. Chem. 273:25915-25921. [DOI] [PubMed] [Google Scholar]
- 38.Olusanya, O., P. D. Andrews, J. R. Swedlow, and E. Smythe. 2001. Phosphorylation of threonine 156 of the mu2 subunit of the AP2 complex is essential for endocytosis in vitro and in vivo. Curr. Biol. 11:896-900. [DOI] [PubMed] [Google Scholar]
- 39.Pandey, K. N. 2009. Functional roles of short sequence motifs in the endocytosis of membrane receptors. Front. Biosci. 14:5339-5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pique, C., C. Lagaudriere-Gesbert, L. Delamarre, A. R. Rosenberg, H. Conjeaud, and M. C. Dokhelar. 2000. Interaction of CD82 tetraspanin proteins with HTLV-1 envelope glycoproteins inhibits cell-to-cell fusion and virus transmission. Virology 276:455-465. [DOI] [PubMed] [Google Scholar]
- 41.Puthenveedu, M. A., and M. von Zastrow. 2006. Cargo regulates clathrin-coated pit dynamics. Cell 127:113-124. [DOI] [PubMed] [Google Scholar]
- 42.Rein, A., J. Mirro, J. G. Haynes, S. M. Ernst, and K. Nagashima. 1994. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15E-p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J. Virol. 68:1773-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seki, M., H. Sashiyama, M. Hayami, and H. Shida. 1990. Intracellular processing and immunogenicity of the envelope proteins of human T-cell leukemia virus type I that are expressed from recombinant vaccinia viruses. Virus Genes 3:235-249. [DOI] [PubMed] [Google Scholar]
- 44.Sheng, M., and C. Sala. 2001. PDZ domains and the organization of supramolecular complexes. Annu. Rev. Neurosci. 24:1-29. [DOI] [PubMed] [Google Scholar]
- 45.Song, C., S. R. Dubay, and E. Hunter. 2003. A tyrosine motif in the cytoplasmic domain of Mason-Pfizer monkey virus is essential for the incorporation of glycoprotein into virions. J. Virol. 77:5192-5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tonikian, R., Y. Zhang, S. L. Sazinsky, B. Currell, J. H. Yeh, B. Reva, H. A. Held, B. A. Appleton, M. Evangelista, Y. Wu, X. Xin, A. C. Chan, S. Seshagiri, L. A. Lasky, C. Sander, C. Boone, G. D. Bader, and S. S. Sidhu. 2008. A specificity map for the PDZ domain family. PLoS Biol. 6:e239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Traub, L. M. 2009. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat. Rev. Mol. Cell Biol. 10:583-596. [DOI] [PubMed] [Google Scholar]
- 48.Traub, L. M., S. Kornfeld, and E. Ungewickell. 1995. Different domains of the AP-1 adaptor complex are required for Golgi membrane binding and clathrin recruitment. J. Biol. Chem. 270:4933-4942. [DOI] [PubMed] [Google Scholar]
- 49.Trejo, J. 2005. Internal PDZ ligands: novel endocytic recycling motifs for G protein-coupled receptors. Mol. Pharmacol. 67:1388-1390. [DOI] [PubMed] [Google Scholar]
- 50.van Ham, M., and W. Hendriks. 2003. PDZ domains—glue and guide. Mol. Biol. Rep. 30:69-82. [DOI] [PubMed] [Google Scholar]
- 51.Wallin, M., M. Ekstrom, and H. Garoff. 2004. Isomerization of the intersubunit disulphide-bond in Env controls retrovirus fusion. EMBO J. 23:54-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolfe, B. L., and J. Trejo. 2007. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic 8:462-470. [DOI] [PubMed] [Google Scholar]
- 53.Xavier, R., and B. Seed. 2005. PDZ domains and the politics of polarity in lymphocytes. Immunity 22:655-656. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida, S., M. Higuchi, T. Shoji, M. Yoshita, K. Ishioka, M. Takahashi, M. Oie, Y. Tanaka, M. Uchiyama, and M. Fujii. 2008. Knockdown of synapse-associated protein Dlg1 reduces syncytium formation induced by human T-cell leukemia virus type 1. Virus Genes 37:9-15. [DOI] [PubMed] [Google Scholar]