Abstract
Photoreceptors in metazoans can be grouped into two classes, with their photoreceptive membrane derived either from cilia or microvilli. Both classes use some form of the visual pigment protein opsin, which together with 11-cis retinaldehyde absorbs light and activates a G-protein cascade, resulting in the opening or closing of ion channels. Considerable attention has recently been given to the molecular evolution of the opsins and other photoreceptor proteins; much is also known about transduction in the various photoreceptor types. Here we combine this knowledge in an attempt to understand why certain photoreceptors might have conferred particular selective advantages during evolution. We suggest that microvillar photoreceptors became predominant in most invertebrate species because of their single-photon sensitivity, high temporal resolution, and large dynamic range, and that rods and a duplex retina provided primitive chordates and vertebrates with similar sensitivity and dynamic range, but with a smaller expenditure of ATP.
Introduction
Photoreceptors need large amounts of membrane for sensory and transduction proteins, and like other sensory cells they form this membrane from microvilli or from a modified cilium [1]. In microvillar photoreceptors, microvilli formed by evaginations of the plasma membrane are grouped together in structures called rhabdomeres, which contain the photopigment and other proteins essential for transduction. In ciliary photoreceptors, invaginations or membrane vesicles form just above the basal body of a cilium. Eakin [2] proposed that microvillar (or rhabdomeric) photoreceptors and ciliary photoreceptors constitute two broad classes which, with only a few exceptions, are separable and expressed in different animal groups. We now know that this is oversimplified: genetic studies show that both receptor types probably emerged from a single precursor [3,4], were present very early in the evolution of metazoans (Figure 1) [5] and have been described in nearly every phylum. This anatomical division seems nevertheless fundamental to photoreceptor function, as the opsin visual pigments (Figure 2A,B) used by the photoreceptors also divide into clear classes (Figure 2C). The ciliary use opsins from the c-opsin and Go-opsin subfamilies, and the microvillar always express r-opsins [5,6–8]. This separation of photoreceptor types and families of opsins is very old, likely present before the emergence of the bilateria [8].
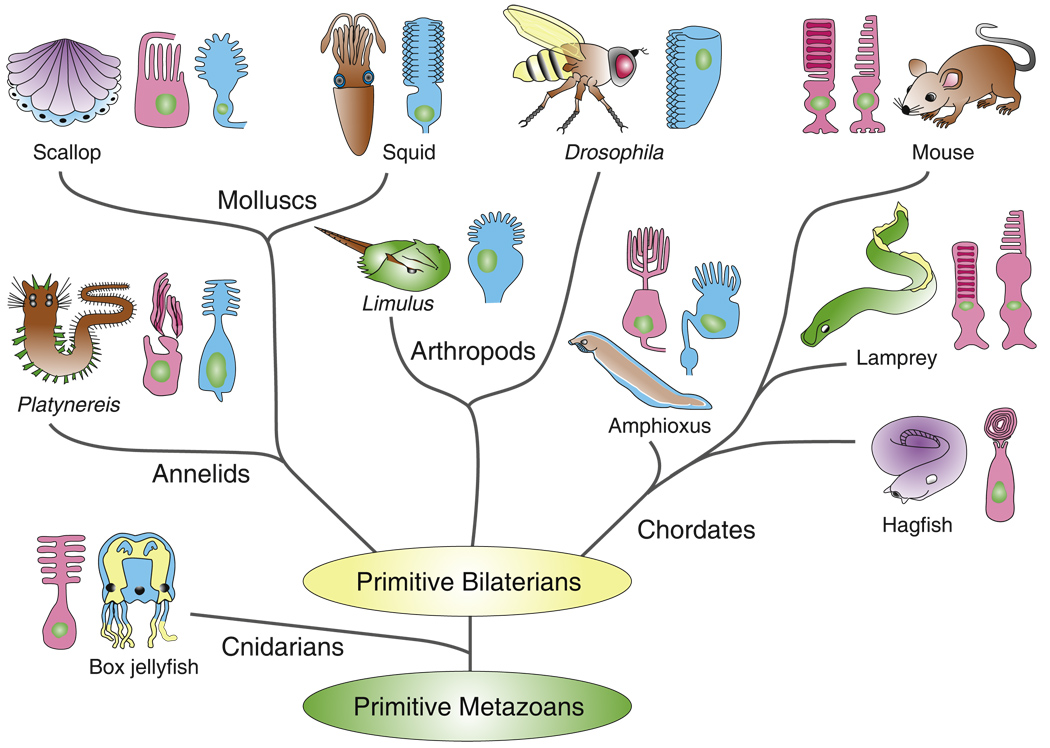
Figure 1. Phylogenetic tree of metazoans showing only animal groups or species discussed in this review, with photoreceptor types in principal eyes illustrated as ciliary (red) or microvillar (blue).
No attempt has been made to specify relationships among principal groups of bilaterians, since these remain controversial. Cnidarian embryos may have microvillar photoreceptors (see text), and it is likely that both photore-ceptor types were present very early in the evolution of metazoans. Mammals and other vertebrates have ciliary photoreceptors (rods and cones) and do not have photoreceptors with microvilli, but they may use a transduction cascade similar to the one used by microvillar photoreceptors in the intrinsically light-sensitive ganglion cells [16]. Phylogenetic tree is based upon [54,94,99]. Drawings of photoreceptors are from [3,5,56,61,95,100–103].
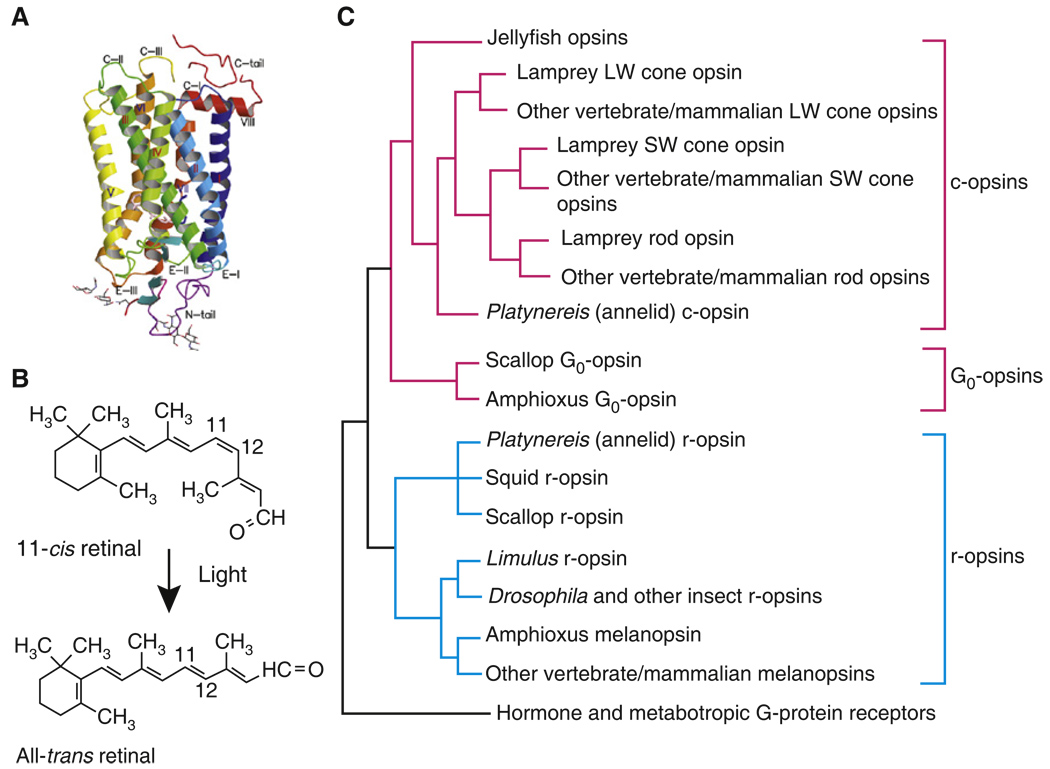
Figure 2. Opsins: a large family of closely related G-protein receptors that mediate phototransduction in all known metazoans.
(A) Crystal structure of bovine rhodopsin with seven transmembrane helical domains labeled with Roman numerals. The carboxyl terminus (above) faces the cytoplasm, the amino terminus (below) the extracellular space (or inside of disk of rod). Linking regions are labeled as C-I, C-II, etc. for cytoplasm and E-I, E-II, etc. for extracellular space (or inside of disk). (B) Lysine in the seventh transmembrane domain of opsin forms a covalent bond with the aldehyde of the chromophore retinal. Light produces a photoisomerization of 11-cis retinal to all-trans retinal and changes the conformation of the rest of the opsin protein, facilitating the binding of G-protein and triggering the phototransduction cascade (Figure 4). Most metazoans use retinal, but 3-dehydroretinal is found in some fresh-water vertebrates, and 3-hydroxyretinal in many insects. (C) Phylogenetic tree of opsins discussed in article; ciliary photoreceptors (red lines) have c-opsins and G0 opsins, and microvillar (blue lines) have r-opsins. Proteins in these three subfamilies show differences in amino acid sequence that are thought to be responsible for their different properties and interactions with different G proteins. The three different forms of photopigment (and others not shown) diverged very early probably among primitive metazoans. We show only the major branches of opsin families; considerable diversity exists within these families, for example between the different pigments for Drosophila or for SW and LW mammalian cone pigments, and more complete phylogenetic trees showing some of this diversity can be found in references [5–8,55], which provided the data for our figure. LW cones absorb light at long wavelengths (green to red); SW cones, in the blue and UV. Note that cone pigments evolved before rod. Structure in (A) reprinted with permission from [104].)
The most primitive extant metazoans with eyes are the cnidarians (corals, sea anemones, jellyfish, and hydroids). All photoreceptors of adult jellyfish that have been studied are ciliary with opsins distantly related to the c-opsins of vertebrates [8]. Cnidarian photoreceptors seem to share with vertebrate cones a low sensitivity to light and are adapted primarily for diurnal vision [9]. Only limited data are available on the G-proteins and effector enzymes in different cnidarian species [10,11], but these observations indicate that jellyfish employ cyclic nucleotide second messengers like every ciliary photoreceptor investigated thus far [3,12]. Microvillar photoreceptors may be present in some cnidarians, for example in jellyfish larvae which are free-swimming and eventually settle on the ocean substrate [13], where light intensities are generally lower. But for adult forms living near the ocean surface, only ciliary photoreceptors have so far been observed.
At some point prior to the cnidarian-bilaterian split, the r-opsin family and associated microvillar photoreceptors emerged (Figures 1 and 2C). These adopted a distinct transduction cascade based on phospholipase C (PLC) and Ca2+ signaling. Both ciliary and microvillar classes are still found in most phyla, sometimes side by side in the same eye [14], but often with one kind subserving spatial vision in the image-forming eyes and the other adopting an accessory role [3]. In the vast majority of protostomes, including arthropods, molluscs and annelids [3,12,15], the microvillar photoreceptors are more common in the principal eyes. On the other hand, in chordates including vertebrates, ciliary photoreceptors with their c-opsins and cGMP-based cascade came to dominate image acquisition in the principal eyes, but r-opsins and alternative transduction cascades were retained, for example in melanopsin-containing retinal ganglion cells which help to control pupillary diameter [16].
Why did microvillar photoreceptors come to dominate the majority of present-day invertebrates? Conversely, why was the ciliary solution preferred in the vertebrate lineage? To address these questions we compare the transduction cascades in the different photoreceptors and consider how they might be adapted for vision under different conditions.
We will argue that, in most invertebrate taxa, microvillar photoreceptors were preferred to invertebrate ciliary photoreceptors like those in jellyfish because of their defining characteristic: the microvilli, along with specific features of the PLC signaling cascade. These features enable a micro-villar photoreceptor to outperform the ciliary photoreceptors of invertebrates by supporting vision in very dim light with large responses to single photons. Microvillar photoreceptors can nevertheless adapt even to very bright illumination and the same cells can support vision in full daylight with the fastest photoreceptor responses known. The versatility of the microvillar photoreceptor allowed emerging bilaterian species to occupy mid and deep water as well as the ocean surface, and ultimately most terrestrial and aerial habitats. By comparison, the ciliary photoreceptors of invertebrates appear to be able to detect light over a restricted range of intensities with a reduced sensitivity and cannot function well in both dim and bright light.
Why then did ciliary photoreceptors come to dominate the chordates? We believe that this was due primarily to the invention of the high sensitivity rod, which supplemented the low sensitivity cone-like photoreceptors of invertebrates and allowed the principal eyes of chordates to cover the full intensity range, from starlight to daylight, by deploying two kinds of ciliary photoreceptors (rods and cones). Such duplex retinae have a distinct advantage over retinae with microvillar photoreceptors: duplex retinae are cheaper. The morphology and phototransduction cascade of the rods and cones enable them to count photons more efficiently in terms of the space they occupy, the materials and energy they use, and the accuracy with which they register photon hits. For this reason, the majority of vertebrates adopted a duplex retina with slow, high sensitivity rods for efficient scotopic vision in dim light, and lower sensitivity cones for fast and accurate photopic vision in bright light.
Transduction in Microvillar Receptors
We begin with primitive bilateria and the protostomes (Figure 1). Why were microvillar photoreceptors preferred in most invertebrate species to the ciliary photoreceptors that were already present, for example in jellyfish? The secret lies in the way the microvillar photoreceptors transduce the absorption of photons into an electrical response, and in particular with the microvilli themselves, which arise from the apical cell membrane and are both the defining characteristic of microvillar photoreceptors and a major contributor to their success. Typically 60 nm in diameter, microvilli range in length from 0.5 to 10 µm and are usually tightly packed into light absorbing structures, the rhabdomeres, which differ considerably in shape, size and position relative to the dioptric apparatus [17]. Within the microvilli, opsin is packed at a density of about 4000 µm−2, corresponding to about 1000 copies in a typical microvillus [18].
Microvilli presumably evolved in the first instance to provide a large area of light-absorbing membrane, but each microvillus also contains the core components of the transduction cascade (Figure 3) [12]. These include the heterotrimeric Gq protein, phospholipase C (PLCβ4), and in Drosophila, two classes of light-sensitive ‘transient receptor potential’ channels, TRP and TRPL [18–20], which admit both Na+ and Ca2+ ions to produce a depolarizing response to light (Figure 3). TRP homologues have also been identified in photoreceptors from the horseshoe crab Limulus [21] and squid [22] and are likely to be a common, and possibly universal feature of rhabdomeric photoreceptors.
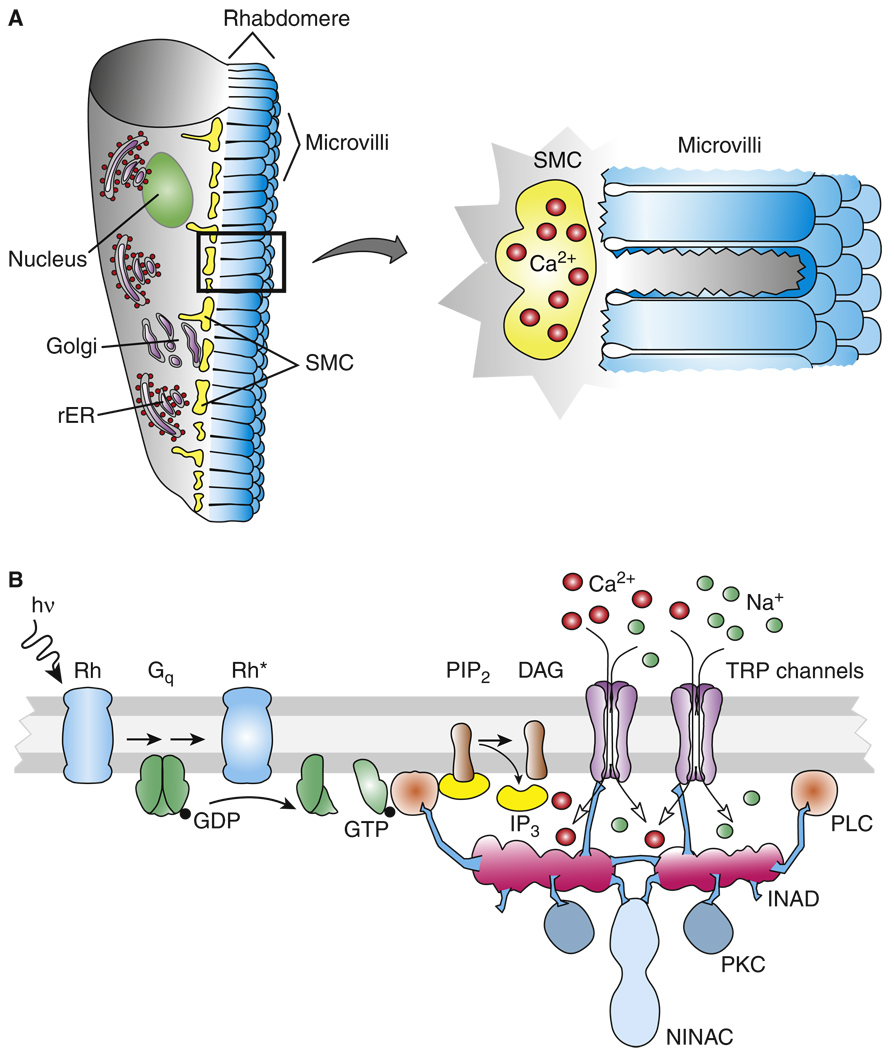
Figure 3. Transduction scheme of microvillar photoreceptor.
(A) Microvillar photoreceptor (left) and detail of microvilli with adjacent submicrovillar cisternae (SMCs) containing Ca2+ (right). (B) Major proteins and mechanisms in microvillar trans-duction. Schema is based on results from fly. In other species, IP3-induced Ca2+ release from the SMCs (not shown in diagram) is known to make an important contribution to microvillar transduction. Abbreviations: hν, light; Rh*, activated form of the photopigment rhodopsin; Gq, G protein containing αq subunit; GDP, guanosine diphosphate; GTP, guanosine triphosphate; PLC, phospholipase C; PIP2, phosphatidylinositol 4,5-bisphosphate; IP3, inositol 1,4,5-triphosphate; DAG, diacylgly-cerol; PKC, protein kinase C; rER, rough endoplasmic reticulum; NINAC, class III myosin; and INAD, a protein containing PDZ binding domains responsible for forming the signaling complex in a fly microvillus.
Because the initial stages of transduction are contained with the microvillus, it probably functions as a semi-autonomous unit, using its G proteins and PLC to produce a discrete response to the absorption of a photon by one of its rhodopsins. Within a typical volume of only 2 × 10−18 L, just one ion or molecule in a microvillus represents a concentration close to 1 µM; because distances of diffusion are short, a high concentration of reactants can be reached in a few milliseconds. Containment in the microvillus is, therefore, an important factor enabling both high gain and rapid response kinetics [23,24].
Flies take compartmentalization two stages further (Figure 3B). First, the activated channels are restricted to the microvillus where the photon is absorbed [24–26]. Second, several components of the cascade, including PLC, TRP and a protein kinase C (PKC) required for response inactivation, are assembled into a signaling complex by the INAD scaffolding protein, potentially further improving speed and reliability (reviewed in [19,27]). A single fly microvillus contains about 100 copies of each protein, but only 25 TRP channels since those are tetrameric [18,24]. Both opsin and the INAD complex are believed to be essentially immobile on the timescale of transduction, so that the G-protein is the only diffusible protein.
The exact mechanism of channel activation downstream of the cleavage of PIP2 to IP3 and DAG by PLC (Figure 3) is not completely resolved. In flies the channels are probably activated in the first instance by a membrane-delimited messenger — either DAG, a downstream metabolite, and/or the reduction in PIP2 (reviewed in [18,20]). In most other species studied [23], excitation is associated with, and possibly mediated by, the release of Ca2+ from IP3-sensitive ER stores called submicrovillar cisternae, localized to within 10–100 nm of the microvilli bases (Figure 3) [28]. Although the IP3-sensitive Ca2+ stores apparently play no role in fly, there is still a large increase in microvillar Ca2+ concentration mediated by the light-gated TRP channels, which are highly permeable to Ca2+ [29] in addition to Na+ [30].
Microvillar Photoreceptors Respond to Single Photons
Why is the mechanism of transduction in microvillar photoreceptors superior to that in the ciliary photoreceptors of cnidarians and other invertebrates? One reason is that microvillar phototransduction is much more sensitive. Of the comparatively few recordings made from invertebrate ciliary photoreceptors, none have been shown capable of detecting single photons. By contrast, microvillar photoreceptors in arthropods, molluscs and annelids can all produce large and often rapid responses to a single photon of light. These quantum bumps have amplitudes and durations ranging from 1 mV and 30 ms in large diurnal flies to 20 mV and 500 ms in nocturnal spiders [31], and they occur against a background of a low rate of spontaneous events and other sources of dark noise [32]. This high sensitivity would have given emerging bilaterians with microvillar photoreceptors an immediate advantage at dawn or dusk or in deeper water.
Several sources of amplification contribute to the high gain of quantum bumps. First there is a rapid generation of many activated G proteins and PLC molecules. In a fly microvillus, the absorption of a single photon by rhodopsin activates 5–10 G proteins and about 500 PIP2 molecules within approximately 50 ms [18,33]. There is then a critical additional amplification stage, mediated by a light-induced increase in cytosolic Ca2+. These Ca2+ signals, which exploit the huge Ca2+ concentration gradients across the plasma membrane or the vesicular membrane of intracellular Ca2+ stores, are an intrinsic feature of PLC signaling [34]. Many eukaryotic cells produce transient local increases in Ca2+, known in the cell-signaling field as Ca2+ ‘puffs’ and ‘sparks’. We suggest that the incorporation of this volatile Ca2+ signaling machinery into the phototransduction cascade was critical in enabling early protostomes with microvillar receptors to detect single photons.
How is the Ca2+-dependent amplification achieved in microvillar photoreceptors? In flies, during the latent period before the production of the bump, the concentration of a membrane-delimited lipid messenger builds up as successive PLC molecules are activated. At some point in time, which — because of the small number of molecules involved — varies stochastically from trial to trial, sufficient messenger is generated to overcome a threshold for the first TRP channel to open. The Ca2+ entering through just one TRP channel rapidly raises Ca2+ concentration throughout the microvillus, and acts synergistically on the remaining TRP channels to allow what were previously subthreshold concentrations of lipid messenger to open them [26]. This positive feedback produces an explosive activation of most of the channels in the microvillus, raising Ca2+ to near millimolar levels. Ca2+-dependent negative feedback then temporarily inactivates the channels to curtail the bump. The tiny dimensions of the microvillus ensure that once the channels close, Ca2+ is rapidly cleared from the microvillus by diffusion and Na/Ca exchange, to restore high sensitivity [18,24].
In IP3-dependent photoreceptors, such as those of Limulus and Lima, a broadly similar chain of events appears to occur, but much of the Ca2+ comes from IP3-induced release from intracellular stores known as the submicrovillar cisternae (Figure 3) [23]. The IP3 generated within the lumen of a single microvillus produces a highly localized release of Ca2+ from an underlying cisterna, raising the Ca2+ concentration in that microvillus and its immediate neighbors and facilitating the opening of the light-dependent channels by mechanisms that are still not fully resolved [21,23,35,36]. Whether the Ca2+ comes from inside or outside the cell, it facilitates a rapid and explosive event that is responsible for the quick rise and fall, as well as the large amplitude, of the quantum bump.
This Ca2+-dependent positive feedback produces high gain with fast kinetics, but has one disadvantage. Because of the highly nonlinear nature of this process, microvillar quantum bumps are variable in amplitude (Figure 5B), and are generated with a finite but characteristically variable latency (Figure 5C) of about 15–100 ms in Drosophila [26] and 70–300 ms in Limulus [37]. This spread of bump latencies over time, together with the time course of the bump itself, determines the temporal resolution of the cell as a whole.
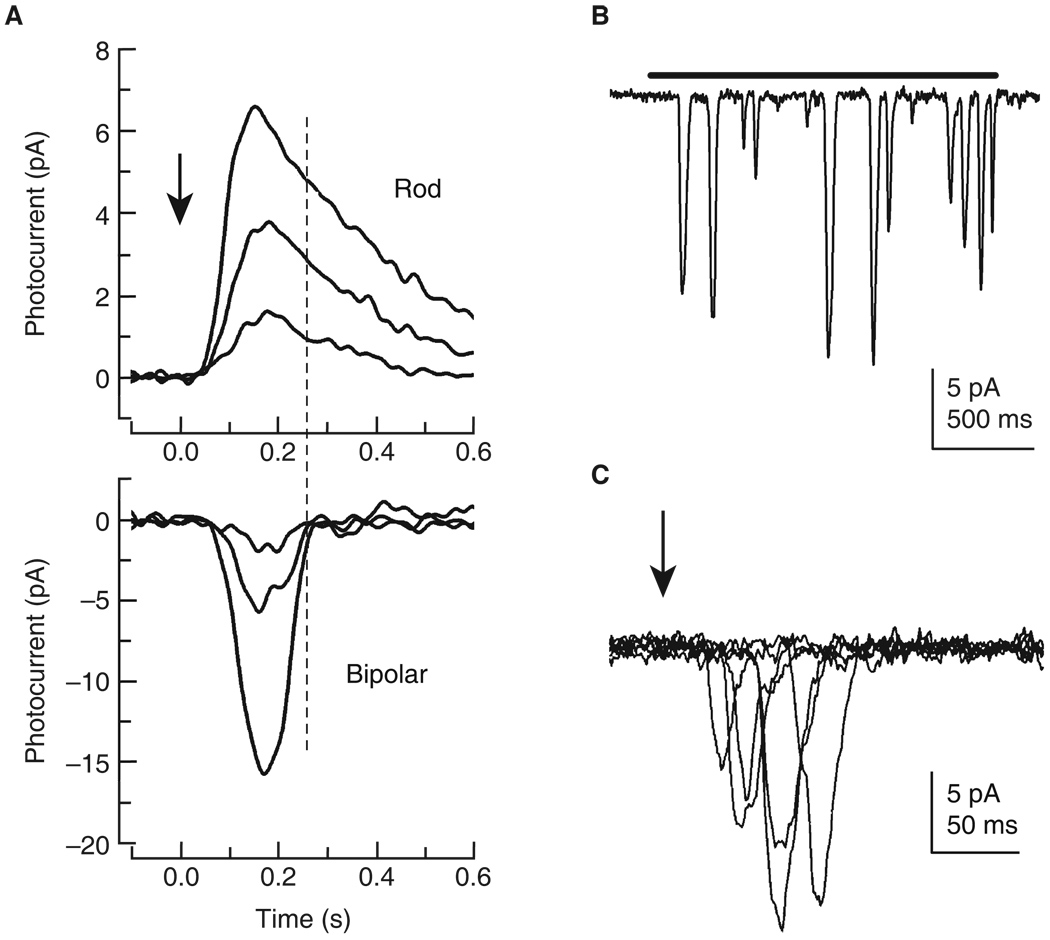
Figure 5. Light responses of rods and fly photoreceptors.
(A) Comparison of current waveform of light responses to 10 ms light flashes (arrow) of rod (above) and rod bipolar cell (below) from mouse. Light intensities were (for rod) 6.2, 12, and 25 photons/µm2; and for bipolar cell 0.6, 1.2, and 2.5 photons/µm2. For both rod and bipolar cell, amplitude increases with increasing light intensity but with little change in kinetics of waveform. The dashed line shows that bipolar cells mostly sum the initial part of the rod response, where variability is smallest [77,78]. Recordings generously provided by A. Sampath (see Figures 2 and 3 of [83]). (B) Quantum-bumps from Drosophila photoreceptor. A two second-long dim light flash (bar: about four effective photons s−1), elicits a train of discrete single photon responses, variable in amplitude but on average about 10 pA. The small (2 pA) events are caused by spontaneous G-protein activation. (C) Superimposed responses from a Drosophila photoreceptor to six 1 ms dim flashes (arrow) each containing only one effective photon. The single photon responses arise abruptly following a finite and variable latency and vary in amplitude. The summed response is consequently much noisier than is the bipolar cell response.
Microvillar Photoreceptors Can Also Function in Bright Light
The microvillar organization not only promotes photodetection with high gain in dim light, it also helps these photoreceptors reduce their gain by orders of magnitude to allow them to function in very bright illumination. The influx or release of Ca2+ in response to the trickle of photons in dim light is rapidly dissipated, but as illumination increases, the contributions of hundreds or thousands of individual microvilli, or local IP3-induced release sites, sum to raise the steady-state Ca2+ concentration throughout the cell. These global steady-state values, which in flies range from 150 nM in the dark to 10 µM in fully light-adapted cells [30], regulate the late-stage gain of phototransduction during light adaptation [38], greatly reducing the size, duration and latency of the quantum bump [37,39]. These changes in sensitivity enable microvillar photoreceptors to operate in full daylight, sometimes with exceptional temporal resolution. Houseflies can detect flicker up to 300 Hz [40], double the highest recorded vertebrate value of 140 Hz for the pigeon [41].
The dynamic range of the cell is also determined by the number of ‘transduction units’ that are available to handle absorbed photons, and the speed with which each unit can process a photon hit. In the fly these units probably correspond to single microvilli; a Drosophila photoreceptor with about 4 × 104 microvilli, each capable of processing at least 10 photons per second, can transduce at least 4 × 105 photons per second, roughly equivalent to the number absorbed in bright sunlight [39]. Because the blowfly photoreceptor has more microvilli and shorter quantum bumps, it can handle 5 × 106 per second [42].
To function in light this bright, flies must have a continuous supply of rhodopsin, and this is ensured by a process called pigment photoregeneration. This does not occur in any c-opsin of which we are aware, including the pigments of jellyfish and other invertebrates, but is characteristic of the r-opsins. The r-opsins are bistable, with one form containing 11-cis retinal (rhodopsin) and another containing all-trans retinal (metarhodopsin; Figure 2B). One photon converts rhodopsin to metarhodopsin, and a second reconverts the metarhodopsin back to rhodopsin, so that even in the brightest light these two forms reach a photoequilibrium determined by the spectral content of illumination and the absorption spectra of rhodopsin and metarhodopsin (reviewed in [43]). Photoregeneration enables an r-opsin to support vision in both dim and bright light. The chromophore can be tightly bound to the opsin, to reduce rates of spontaneous activation in the low light levels required for night vision, and be replenished at high rates in bright light [44]. Although there is a slow turnover of visual pigment by endocytosis and de novo synthesis (see, for example, [45]), photo-regeneration ensures that substantial rhodopsin remains available no matter what the conditions of illumination.
In summary, four characteristic features of the r-opsin transduction cascade give microvillar photoreceptors distinct advantages over the ciliary photoreceptors of jellyfish and other invertebrates. These are: first, sensitivity to single photons; second, the ability to light adapt over a huge dynamic range; third, high temporal resolution; and fourth, pigment photoregeneration. These advantages can be traced to three factors: first, ultracompartmentalization provided by concentrating the elements of the cascade in individual microvilli; second, an additional amplification stage mediated by Ca2+ release or influx, which is an intrinsic feature of microvillar PLC signaling; and third, a photorecon-vertible metarhodopsin, which is a feature of the r-opsin subfamily. We suggest that this combination of factors played a major role in the widespread adoption of microvillar photoreceptors in the principal eyes of most invertebrate species in the protostome lineage, including not only diurnal species and animals living near the ocean surface, but also nocturnal and deep-water species like giant squid, which have the largest eyes of any living creature.
Evolution of Chordate Photoreceptors
Despite the adoption of microvillar photoreceptors by many invertebrates, ciliary photoreceptors were retained in most taxa (for example, [46,47]), where they may have functioned much as in cnidarians, that is as diurnal cone-like detectors in bright light. In some invertebrates (for example Platynereis), ciliary photoreceptors are used alongside the microvillar but in separate organs and may play a role in specialized functions such as the entrainment of circadian rhythms [5]. In the scallop and file clam, both ciliary and microvillar photoreceptors are present in different layers of the same eyes and serve different functions. The microvillar responses are depolarizations resembling those of Drosophila in sensitivity and waveform [14,48]. The ciliary photoreceptors, on the other hand, produce hyperpolarizations with an opsin from the Go subfamily (Figure 2C), which may activate a guanylyl cyclase [49], increasing cGMP and opening K+ channels [50–52]. The responses of the ciliary photoreceptors in scallops and clams are much less sensitive than those of microvillar photoreceptors [14,51] and appear to serve the useful function of detecting decrements in illumination to mediate shadow responses [53].
Both microvillar and ciliary photoreceptors were also present in primitive chordates. Recent evidence indicates that the genome of the ancestral chordate resembled more closely that of amphioxus than that of any other presently living organism [54,55]. Amphioxus has four kinds of eyes [56]. There are frontal eyes, perhaps homologous to the principal eyes of vertebrates, and lamellar bodies, which fragment after metamorphosis; both contain ciliary photoreceptors. The Joseph cells and dorsal ocelli, on the other hand, are both microvillar. The microvillar photoreceptors appear to use an r-opsin closely related to the vertebrate light-sensitive ganglion cell pigment melanopsin [57], which couples to a Gq. Recordings have recently been made from both the Joseph cells and photoreceptors of dorsal ocelli, and they have many of the properties of microvillar photoreceptors in other species, including release of Ca2+ from internal stores and activation of a depolarizing inward current [58]. There are, as yet, no recordings from ciliary photoreceptors in amphioxus.
After amphioxus, the oldest living chordate relatives of the vertebrates are the urochordates (tunicates), the eyes of which have received comparatively little attention, but which seem as a group to show both ciliary and microvillar mechanisms [59], and the agnathans, hagfish and lampreys [54]. The eyes of hagfish lie beneath an unpigmented patch of skin and are small and simple, containing no lens (at least in adults) and a single kind of ciliary photoreceptor [60–62]. Because hagfish live under rocks or burrow in soft mud or sand [63], seldom in water less than 25 m deep and sometimes at depths greater than 1000 m [64], these photoreceptors are likely adapted to a dim-light environment and may be rather more sensitive than the ciliary photoreceptors of cnidarians and scallops; they may in fact be rods. The saccules of membrane presumably containing the photopigment appear to be completely enclosed in plasma membrane [60–62], much as in a rod. At present, however, there is no physiology or molecular biology available to support this identification.
The principal eyes of lampreys also have ciliary photoreceptors, and here considerably more is known. Lampreys express several kinds of c-opsin pigments [65,66] contained in two morphologically distinct groups of receptors called ‘short’ and ‘long’ [67], which appear to correspond to rods and cones. At least one of the c-opsins is orthologous to the rod Rh1 opsin of jawed vertebrates [7] and is therefore related to the pigment in our own rods, which in us and most probably also in lamprey mediates vision in dim light. Extracellular recordings suggest that some lamprey photoreceptors are much more sensitive than the ciliary photoreceptors of cnidarians or scallop [68], and the ‘short’ and ‘long’ photoreceptors of lamprey retina selectively express different forms of transducin [69] and the inhibitory PDE6 g subunit [70] with some of the properties of the mammalian rod and cone forms of these proteins. It seems quite likely, therefore, that rod c-opsin and functional rod photoreceptors are present in agnathans and evolved before the split between the jawed and jawless vertebrates [7].
We propose that, at some point during the Cambrian radiation, free-swimming primitive chordates had eyes with both microvillar and ciliary photoreceptors like those of amphioxus. Because cone pigments are older than rod pigments (Figure 2C) [6], and there is no evidence for a high-sensitivity, rod-like ciliary photoreceptor before the agnathans, we think it likely that the ciliary photoreceptors in primitive chordates were initially cone-like and, as in other invertebrate ciliary photoreceptors, much less sensitive than microvillar photoreceptors. We propose that the genome of these primitive chordates underwent gene duplications of c-opsin and the principal transduction proteins, including the visual pigments, transducin and the cyclic nucleotidegated channels, so that, in addition to cone-like ciliary photoreceptors, the primitive chordates developed rod photoreceptors with many of the properties of the rods of vertebrates [7].
The emergence of the duplex retina would have provided these organisms with both high-sensitivity detection in low light and low-sensitivity detection in bright light, albeit with a slower temporal resolution than fly microvillar photoreceptors, but covering at least as broad a range of light intensities. And, as we shall see, the adoption of a duplex retina would have permitted primitive chordates to detect light with a considerably smaller over-all expenditure of energy. Microvillar eyes in primitive chordates would then no longer have been needed and must have gradually disappeared, with r-opsin transduction retained in vertebrates only in the melanopsin-containing neurons of the inner retina.
Phototransduction in Rods
To see how the invention of rods and a duplex retina provided a competitive alternative to the microvillar solution, we refer first to the structure of the rod (Figure 4). As in the fly, the photopigment and enzymes of transduction are organized around units of membrane, but instead of microvilli, vertebrate photoreceptors have flattened membrane lamellae. More protein can be assembled into lamellae than into microvilli; the density of opsin in a rod is of the order of 3–5 × 104 µm−2 [71], nearly ten times higher than in the microvillar membrane of Drosophila, and this increases the efficiency of absorption of incident light. Moreover, the cell body and nucleus can be positioned below the lamellae rather than to one side, increasing the effective cross-sectional area of photoreceptive membrane over the retina as a whole and focusing light onto the outer segment [72].
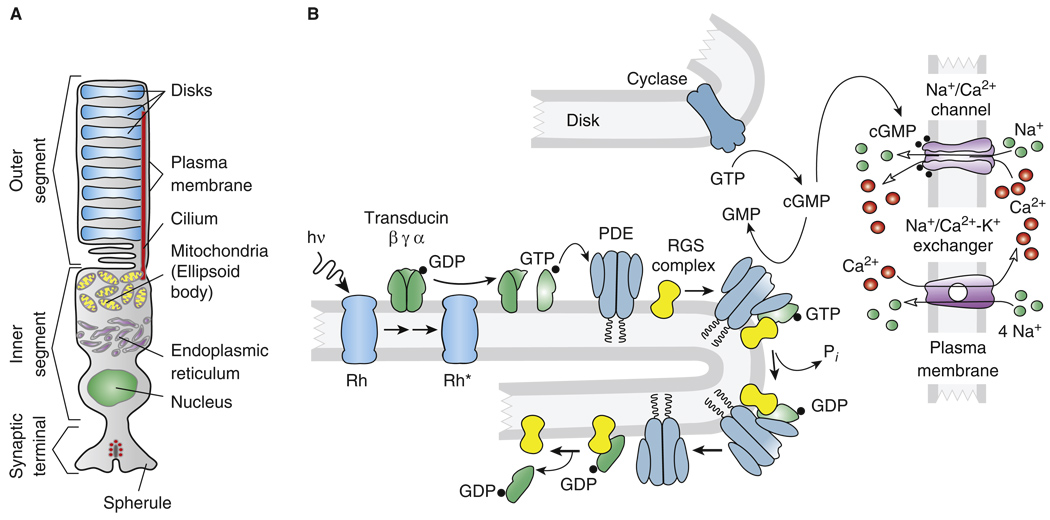
Figure 4. The vertebrate rod.
(A) Schematic anatomy of representative vertebrate rod. (B) Major proteins and mechanisms in vertebrate rod transduction. Abbreviations: hν, light; Rh*, activated form of the photopigment rhodopsin; GTP, guanosine triphosphate; GDP, guanosine diphosphate; cGMP, guanosine 3′,5′-cyclic monophosphate; GMP, guanosine monophosphate; PDE, guanosine nucleotide phosphodiesterase; RK, rhodopsin kinase; RGS complex, group of three proteins including RGS9 which accelerate the hydrolysis of GTP by the alpha subunit of transducin; and Pi, inorganic phosphate.
At some point in the evolution of the rod, the membrane lamellae containing the photopigment and enzymes of transduction became detached from the plasma membrane to form disks. Although it is still unclear what specific advantages the disks provide, we know that many of the proteins of the cascade [73] are embedded or peripherally attached to the disk (Figure 4B), where opsin and most of the rest of the transduction enzymes appear to diffuse relatively freely within the plane of the membrane. The disks act to some extent like larger versions of microvilli, forming independent units of transduction whose signals sum at least at dim intensities in a linear fashion to produce the photoreceptor response [74].
The initial events of transduction in rods are similar to those occurring in microvillar receptors (Figure 4B): photoisomerization of visual pigment to metarhodopsin II or Rh* produces transient binding of the G protein transducin (T), catalyzing the formation of about 20 TαGTPs per Rh* [12]. TαGTP activates the effector enzyme phosphodiesterase 6 (PDE6) by binding to its γ inhibitory subunits and displacing them from the active site of the PDE6 catalytic α and β subunits. The PDE6 then hydrolyzes cGMP, reducing the outer segment cGMP concentration and closing cation-permeable channels. The gain of transduction from Rh* to the decrease in cGMP is sufficiently large that a single photon closes of the order of 3–5% of the channels open in darkness. These channels are present at high concentration in the plasma membrane but have a low mean probability of opening and a rather low effective channel conductance in physiological solution [74]. This has the consequence that the total current in darkness is small, typically of the order of 25 pA in mammals and about twice this in amphibians. Because light closes channels instead of opening them, the total current never exceeds a few tens of pA. In microvillar photoreceptors such as Drosophila, on the other hand, light opens channels and activates currents which can be as large as several nA.
The molecular species of channel proteins and of many of the enzymes of transduction are different in rods and cones, and some of the proteins are present at substantially different concentrations in the two kinds of photoreceptors [75,76]. In ways still not fully understood, these differences must have been responsible for the development of the slower response kinetics and greater sensitivity of the rod. The pigments of rods and cones are also different, with rod pigments as a rule having a greater stability and lower frequency of spontaneous isomerization; as a result, they produce less background noise in darkness [12]. This stability must have played a role in increasing rod sensitivity, and it may also have prevented rods from operating in bright light by slowing the rate at which chromophore can be released from opsin for regeneration [44]. The evolution of these components may have gradually altered the properties of a subtype of ciliary photoreceptor in the retinae of emerging chordates, with each successive step increasing sensitivity and eventually producing a fully functional rod capable of detecting single photons but saturating in bright light.
The single-photon response of a rod is about 1 pA or 1 mV in amplitude and an order of magnitude smaller and slower than in many microvillar photoreceptors. However, and perhaps critically, the rod single-photon responses are much less variable in latency and amplitude [77,78]. Several factors contribute to reliability: firstly, the light-sensitive channels simply track cGMP concentration, which begins to decline as soon as the first PDE is activated. By contrast, microvillar photoreceptors use a thresholding mechanism to produce quantum bumps, and that introduces a highly variable latency. Secondly, amplification depends upon the number of Tα-GTPs and PDE6s activated, which in turn depends on the lifetime of Rh*. If this were determined by a first-order reaction, a high degree of stochastic variability would be expected. Variability is much lower, perhaps in part because Rh* turnoff requires multiple phosphorylations of rhodopsin by rhodopsin kinase and the binding of arrestin [79,80]. Because variability is low, responses to multiple photons absorbed in different disks sum linearly to produce a waveform nearly identical to that of the single-quantum response (Figure 5A). This is quite different from the situation in fly (Figure 5C).
The low variability of the single-photon response is important because it allows the rod pathway to improve its sensitivity by eliminating background noise (or dark noise) [81]. In mammals, for example, transmission from rods to bipolar cells is nonlinear, boosting the amplitude of the singlephoton response and allowing only signals that exceed a criterion amplitude to pass through the synapse. As a consequence, bipolar cells reject much of the noise produced by the transduction cascade and can improve the signal-to-noise ratio of dim-light vision by more than 300-fold over that expected from a linear combination of rod signals [82]. The bipolar cell registers primarily the initial waveform of the rod light response (Figure 5A), where variability in waveform is smallest [83]. Thus, when singlephoton signals from rod bipolar cells are neurally pooled to increase sensitivity at low light levels, much of the internal noise generated by phototransduction is eliminated. There is no evidence for a similar synaptic mechanism in flies, even among signals that are neurally pooled for high sensitivity in dim light [84].
Many arthropods are more highly specialized for nocturnal vision than are flies and have sensitivities comparable to nocturnal vertebrates [85]; but wherever they are recorded, microvillar quantum bumps vary so much in amplitude, latency and duration (Figure 5B,C) that this severe form of transducer noise will be difficult to eliminate. How nocturnal arthropods process quantum bumps is presently unknown, but any mechanism of temporal or spatial summation [17,86] could potentially limit visual performance in brighter illumination. Vertebrates can use rod vision with low acuity and poor temporal resolution but high sensitivity in dim light, then switch to cones and cone circuits with higher acuity and greater temporal resolution in bright light. This is an important advantage of a duplex retina.
Duplex Retinas Are More Energy Efficient
Vertebrate photoreceptors and duplex retinas are also more energy-efficient than their microvillar counterparts, and this may have provided a crucial selective advantage [87]. Drosophila photoreceptors and mouse rods consume nearly the same amount of ATP in darkness, and most of this is used by the Na+/K+ ATPase to pump ions [87,88]. Fluxes are high because both kinds of photoreceptors keep channels open in darkness to bias the membrane potential to more positive voltages, where their synapses respond to small changes in membrane potential [89,90]. As light intensity increases, the energy consumption of rods decreases as the cGMP channels close and the rods hyperpolarize [87], whereas in the depolarizing photoreceptors of fly, ATP consumption increases as both light-sensitive Na+ and voltage-sensitive K+ channels open [91]. Light increases the amount of ATP used for the biochemistry of transduction in both kinds of photoreceptors, but this source of ATP consumption is smaller in rods and limited by a variety of cellular mechanisms [87].
In many vertebrates, including mammals, bright light closes all of the channels in rods and decreases energy consumption by as much as five-fold; with large numbers of rods for night vision, this can considerably reduce energy consumption by the retina as a whole [87]. It is an open question whether the energy saving would have been quite this large in primitive chordates when rods first evolved. In jawless vertebrates, electrophysiological recording has been made only from whole lamprey retina (rather than from single rods and cones) and seems to show that rods continue to respond even in bright illumination [68]. These results are difficult to interpret but may indicate that lamprey rods represent an intermediate stage, perhaps resembling the rods of nocturnal geckos [92] which saturate in somewhat brighter illumination than the rods of mammals. Even without complete saturation, energy consumption in rods would still decrease with increasing light, since channels close, and a variety of additional mechanisms limit energy expenditure in bright illumination [87].
The hyperpolarization and closing of rod channels, along with their small effective channel conductance and low absolute value of total outer segment current, is likely to have contributed to reduced energy consumption in emerging duplex retinas, and this may also have been true for cones, though to a lesser extent. Cone photoreceptor channels do not completely close even in the brightest light [93], and cones require more energy for transduction [87].We estimate that in mammalian cones, the consumption of ATP instead of decreasing as in rods may increase in light by as much as a factor of two. This is nevertheless a smaller increase than that produced by light in many microvillar photoreceptors [88]. It is probably also smaller than that of the ciliary photoreceptors of molluscs, which use cyclic nucleotide-gated (CNG) K+ channels with currents that are much larger than in cones and increase with illumination [51]. In this respect, it would be important to know when ciliary photoreceptors first adopted CNG non-selective cation channels like those in rods and cones, because this development may have been an important step in the evolution of the ciliary retina. It is nevertheless striking how much less expensive rods are even than cones. The particular advantage of a duplex retina is that the high efficiency rods can be used for dim light vision with as great a sensitivity as microvillar photoreceptors, and then, when the light intensity increases, the rods can effectively be turned off, saturating either partially or completely, leaving the cones to function in bright light.
Evolution of Vertebrate Photoreceptors
We believe that the major step in the evolution of the vertebrate eye was the emergence in primitive chordates of rod photoreceptors in addition to cones to produce a duplex retina (see also [7]). We think it unlikely that the evolution of the vertebrate retina proceeded first by the emergence of a fully developed vertebrate-like eye with only cone photoreceptors before the appearance of the rod, as has been recently proposed [94]. The ciliary photoreceptors of invertebrates are sufficiently similar to vertebrate cones in their sensitivity and other response properties that a further development of a ciliary eye without rods would have been unlikely to confer any selective advantage in animals that also possessed the inherently more sensitive microvillar photoreceptors. Rods enabled ciliary photoreceptors to function in dim light with high efficiency. The low variability in latency and amplitude of the rod response could have improved the signal-to-noise ratio of summed signals from many receptors even before the emergence of the bipolar cell. Furthermore, saturation of the receptor response, even if only partial, would have reduced energy consumption in a duplex retina.
The mechanism of formation of 11-cis retinal, which for c-opsins and vertebrates does not occur by photoregeneration but requires an elaborate biochemical pathway, seems at first glance inferior to the scheme used by r-opsins and insects and is unlikely to have conferred any advantage to cones. For rods on the other hand, this mechanism, which consumes relatively little energy by comparison to the other processes in the rod [87], has the advantage that in the dark essentially 100% of the rhodopsin can be reconstituted [95,96]. This may however be less important than it would seem, since microvillar eyes (at least in some species) also have the capability of completely regenerating their visual pigment in darkness [45,97].
Summary and Conclusions
Primitive metazoans and bilaterians appear to have utilized two basic types of photoreceptor: a ciliary receptor of low sensitivity for diurnal vision and shadow detection similar to the one in cnidarians and some molluscs; and a microvillar photoreceptor of much greater sensitivity that could adapt to bright light and function in both dim and bright illumination. The principal eyes of emerging invertebrates used one type of receptor or the other, or sometimes both; but there are no known examples in protostomes of ciliary receptors that can signal individual photons, or of retinas with two kinds of ciliary receptors like the rods and cones of vertebrates. Although microvillar-based retinae often contain multiple photoreceptor classes, there is no need for the duplex retina division into high and low sensitivity types because one and the same photoreceptor can cover the full gamut from single photons to bright daylight. In the image-forming eyes of most invertebrate taxa, the microvillar photoreceptors were preferred because of their greater sensitivity and versatility, but ciliary receptors continued to be used by some species, including primitive chordates.
At some point in the evolution of vertebrates, rod photoreceptors emerged with a morphology and an array of transduction proteins different from those of the less sensitive cones. For reasons we still do not fully understand, the particular configuration of outer segment disks and distinctive forms of pigments, enzymes, and channels gave rods sufficient gain to signal reliably a single photon of light. The sensitivity of rod vision was improved further by the appearance of the bipolar cell, whose synapses filter out much of the background noise of rod vision. The invention of the rod permitted the emergence of a duplex retina, which combined the high sensitivity of rods with the ability of cones to adapt to bright illumination, thus providing an attractive alternative to microvillar-based retinae. Since the total currents of rods and cones are much smaller than those of invertebrate photoreceptors, and since light closes channels in vertebrates but opens them in insects, the ciliary photoreceptors of vertebrates require less energy for ion pumping. Within the context of a duplex retina, the fact that rods saturate completely in bright light becomes a distinct advantage, greatly reducing their energy utilization. Probably because of its lower cost, a duplex retina with ciliary receptors was preferred by the image-forming eyes of emerging chordates and is now found in all vertebrates without exception. The r-opsins with their microvillar signal cascades are retained only in the melanopsin-containing cells of the inner retina.
None of our conclusions is likely to be definitive because so many gaps remain in our knowledge of lower forms. We urgently need more electrical recordings, particularly from jellyfish and annelids, as well as from the ciliary photoreceptors of amphioxus and rods and cones of jawless vertebrates. We also need to know how photoreceptors are used in particular species and what pathways they serve, since this can also play a role in their selection [98]. In spite of the provisional nature of our arguments, we hope we have raised issues of importance, which future research may be able to address.
Acknowledgements
We are grateful to Margery Fain for drafting the figures and to Simon Conway Morris, Jeremy Niven, Alapakkam Sampath, and the reviewers of Current Biology for their helpful comments on earlier versions of the manuscript. Work was supported by NIH R01 EY01844 to G.L.F. and grants from the BBSRC, MRC, and Wellcome Trust to R.H.
References
- 1.Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr. Biol. 2009;19:R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eakin RM. Evolution of photoreceptors. Cold Spr. Harb. Symp. Quant. Biol. 1965;30:363–370. doi: 10.1101/sqb.1965.030.01.036. [DOI] [PubMed] [Google Scholar]
- 3.Arendt D. Evolution of eyes and photoreceptor cell types. Int. J. Dev. Biol. 2003;47:563–571. [PubMed] [Google Scholar]
- 4.Gehring WJ. New perspectives on eye development and the evolution of eyes and photoreceptors. J. Hered. 2005;96:171–184. doi: 10.1093/jhered/esi027. [DOI] [PubMed] [Google Scholar]
- 5.Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science. 2004;306:869–871. doi: 10.1126/science.1099955. [DOI] [PubMed] [Google Scholar]
- 6.Terakita A. The opsins. Genome Biol. 2005;6:213. doi: 10.1186/gb-2005-6-3-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pisani D, Mohun SM, Harris SR, McInerney JO, Wilkinson M. Molecular evidence for dim-light vision in the last common ancestor of the vertebrates. Curr. Biol. 2006;16:R318–R319. doi: 10.1016/j.cub.2006.03.090. author reply R320. [DOI] [PubMed] [Google Scholar]
- 8.Suga H, Schmid V, Gehring WJ. Evolution and functional diversity of jellyfish opsins. Curr. Biol. 2008;18:51–55. doi: 10.1016/j.cub.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 9.Garm A, Coates MM, Gad R, Seymour J, Nilsson DE. The lens eyes of the box jellyfish Tripedalia cystophora and Chiropsalmus sp. are slow and color-blind. J. Comp. Physiol. A. 2007;193:547–557. doi: 10.1007/s00359-007-0211-4. [DOI] [PubMed] [Google Scholar]
- 10.Kozmik Z, Ruzickova J, Jonasova K, Matsumoto Y, Vopalensky P, Kozmikova I, Strnad H, Kawamura S, Piatigorsky J, Paces V, et al. Assembly of the cnidarian camera-type eye from vertebrate-like components. Proc. Natl. Acad. Sci. USA. 2008;105:8989–8993. doi: 10.1073/pnas.0800388105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koyanagi M, Takano K, Tsukamoto H, Ohtsu K, Tokunaga F, Terakita A. Jellyfish vision starts with cAMP signaling mediated by opsin-G(s) cascade. Proc. Natl. Acad. Sci. USA. 2008;105:15576–15580. doi: 10.1073/pnas.0806215105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yau K-W, Hardie RC. Phototransduction motifs and variations. Cell. 2009;139:246–264. doi: 10.1016/j.cell.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nordstrom K, Wallen R, Seymour J, Nilsson D. A simple visual system without neurons in jellyfish larvae. Proc. Biol. Sci. 2003;270:2349–2354. doi: 10.1098/rspb.2003.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McReynolds JS, Gorman AL. Photoreceptor potentials of opposite polarity in the eye of the scallop, Pecten irradians. J. Gen. Physiol. 1970;56:376–391. doi: 10.1085/jgp.56.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernald RD. The evolution of eyes. Brain Behav. Evol. 1997;50:253–259. doi: 10.1159/000113339. [DOI] [PubMed] [Google Scholar]
- 16.Hankins MW, Peirson SN, Foster RG. Melanopsin: an exciting photopigment. Trends Neurosci. 2008;31:27–36. doi: 10.1016/j.tins.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson DE, Kelber A. A functional analysis of compound eye evolution. Arthropod Struct. Dev. 2007;36:373–385. doi: 10.1016/j.asd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Hardie RC, Postma M. Phototransduction in microvillar photoreceptors of Drosophila and other invertebrates. In: Basbaum K, Shepher, Westheimer, editors. The Senses - a Comprehensive Reference Vision Volume 1 (eds. Albright, Masland) Oxford: Academic Press; 2008. pp. 77–130. [Google Scholar]
- 19.Wang T, Montell C. Phototransduction and retinal degeneration in Drosophila. Pflugers Arch. 2007;454:821–847. doi: 10.1007/s00424-007-0251-1. [DOI] [PubMed] [Google Scholar]
- 20.Katz B, Minke B. Drosophila photoreceptors and signaling mechanisms. Front Cell Neurosci. 2009;3:2. doi: 10.3389/neuro.03.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bandyopadhyay BC, Payne R. Variants of TRP ion channel mRNA present in horseshoe crab ventral eye and brain. J. Neurochem. 2004;91:825–835. doi: 10.1111/j.1471-4159.2004.02773.x. [DOI] [PubMed] [Google Scholar]
- 22.Monk PD, Carne A, Liu SH, Ford JW, Keen JN, Findlay JBC. Isolation, cloning, and characterisation of a trp homologue from squid (Loligo forbesi) photoreceptor membranes. J. Neurochem. 1996;67:2227–2235. doi: 10.1046/j.1471-4159.1996.67062227.x. [DOI] [PubMed] [Google Scholar]
- 23.Nasi E, Gomez MdP, Payne R. In: Handbook of Biological Physics. Stavenga DG, de Grip WJ, Pugh EN, editors. Elsevier; 2000. pp. 389–448. [Google Scholar]
- 24.Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 2001;413:186–193. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- 25.Hamdorf K. Comparative Physiology and Evolution of Vision in Invertebrates. Volume VII/6A. Berlin: Springer-Verlag; 1979. The physiology of invertebrate visual pigments; pp. 145–224. [Google Scholar]
- 26.Henderson SR, Reuss H, Hardie RC. Single photon responses in Drosophila photoreceptors and their regulation by Ca2+ J. Physiol. 2000;524:179–194. doi: 10.1111/j.1469-7793.2000.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber A. Scaffolding proteins organize multimolecular protein complexes for sensory signal transduction. Eur. J. Neurosci. 2001;14:769–776. doi: 10.1046/j.0953-816x.2001.01704.x. [DOI] [PubMed] [Google Scholar]
- 28.Walz B, Baumann O. Structure and cellular physiology of Ca2+ stores in invertebrate photoreceptors. Cell Calcium. 1995;18:342–351. doi: 10.1016/0143-4160(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 29.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 30.Oberwinkler J, Stavenga DG. Calcium transients in the rhabdomeres of dark- and light-adapted fly photoreceptor cells. J. Neurosci. 2000;20:1701–1709. doi: 10.1523/JNEUROSCI.20-05-01701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pirhofer-Walzl K, Warrant E, Barth FG. Adaptations for vision in dim light: impulse responses and bumps in nocturnal spider photoreceptor cells (Cupiennius salei Keys) J. Comp. Physiol. A. 2007;193:1081–1087. doi: 10.1007/s00359-007-0263-5. [DOI] [PubMed] [Google Scholar]
- 32.Laughlin SB, Lillywhite PG. Intrinsic noise in locust photoreceptors. J. Physiol. 1982;332:25–45. doi: 10.1113/jphysiol.1982.sp014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardie RC, Martin F, Cochrane GW, Juusola M, Georgiev P, Raghu P. Molecular basis of amplification in Drosophila phototransduction. Roles for G protein, phospholipase C, and diacylglycerol kinase. Neuron. 2002;36:689–701. doi: 10.1016/s0896-6273(02)01048-6. [DOI] [PubMed] [Google Scholar]
- 34.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell. Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 35.del Pilar Gomez M, Nasi E. A direct signaling role for phosphatidylinositol 4,5-bisphosphate (PIP2) in the visual excitation process of microvillar receptors. J. Biol. Chem. 2005;280:16784–16789. doi: 10.1074/jbc.M414538200. [DOI] [PubMed] [Google Scholar]
- 36.Garger AV, Richard EA, Lisman JE. The excitation cascade of Limulus ventral photoreceptors: guanylate cyclase as the link between InsP3-mediated Ca2+ release and the opening of cGMP-gated channels. BMC Neurosci. 2004;5:7. doi: 10.1186/1471-2202-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong F, Knight BW, Dodge FA. Dispersion of latencies in photoreceptors of Limulus and the adapting-bump model. J. Gen. Physiol. 1980;76:517–537. doi: 10.1085/jgp.76.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu Y, Oberwinkler J, Postma M, Hardie RC. Mechanisms of light adaptation in Drosophila photoreceptors. Curr. Biol. 2005;15:1228–1234. doi: 10.1016/j.cub.2005.05.058. [DOI] [PubMed] [Google Scholar]
- 39.Juusola M, Hardie RC. Light adaptation in Drosophila photoreceptors: I. Response dynamics and signaling efficiency at 25 degrees C. J. Gen. Physiol. 2001;117:3–25. doi: 10.1085/jgp.117.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tatler B, O’Carroll DC, Laughlin SB. Temperature and the temporal resolving power of fly photoreceptors. J. Comp. Physiol. A. 2000;186:399–407. doi: 10.1007/s003590050439. [DOI] [PubMed] [Google Scholar]
- 41.Emmerton J. Vision. In: Abs M, editor. Physiology and Behaviour of the Pigeon. New York: Academic; 1983. pp. 245–266. [Google Scholar]
- 42.Howard J, Blakeslee B, Laughlin SB. The intracellular pupil mechanism and the maintenance of photoreceptor signal:noise ratios in the blowfly Lucilia cuprina. Proc. Roy. Soc. Lond. B. 1987;231:415–435. doi: 10.1098/rspb.1987.0053. [DOI] [PubMed] [Google Scholar]
- 43.Stavenga DG. Insect retinal pigments: Spectral characteristics and physiological functions. Progress Retinal Eye Res. 1996;15:231–259. [Google Scholar]
- 44.Osorio D, Nilsson DE. Visual pigments: trading noise for fast recovery. Curr. Biol. 2004;14:R1051–R1053. doi: 10.1016/j.cub.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 45.Blest AD. The rapid synthesis and destruction of photoreceptor membrane by a dinopid spider: a daily cycle. Proc. Roy. Soc. Lond. B. 1978;200:463–483. [Google Scholar]
- 46.Nilsson DE. Eyes as optical alarm systems in fan worms and ark clams. Phil. Trans. Roy. Soc. Lond. B. 1994;346:195–212. [Google Scholar]
- 47.von Salvini-Plawen L. Photoreception and the polyphyletic evolution of photoreceptors (with special reference to Mollusca) Am. Malacol. Bull. 2008;26:83–100. [Google Scholar]
- 48.Nasi E. Electrophysiological properties of isolated photoreceptors from the eye of Lima scabra. J. Gen. Physiol. 1991;97:17–34. doi: 10.1085/jgp.97.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomez MP, Nasi E. Light transduction in invertebrate hyperpolarizing photoreceptors: possible involvement of a Go-regulated guanylate cyclase. J. Neurosci. 2000;20:5254–5263. doi: 10.1523/JNEUROSCI.20-14-05254.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gorman AL, McReynolds JS. Ionic effects on the membrane potential of hyperpolarizing photoreceptors in scallop retina. J. Physiol. 1978;275:345–355. doi: 10.1113/jphysiol.1978.sp012193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez MP, Nasi E. The light-sensitive conductance of hyper-polarizing invertebrate photoreceptors: a patch-clamp study. J. Gen. Physiol. 1994;103:939–956. doi: 10.1085/jgp.103.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gomez dPM, Nasi E. Activation of light-dependent K+ channels in ciliary invertebrate photoreceptors involves cGMP but not the IP3/Ca2+ cascade. Neuron. 1995;15:607–618. doi: 10.1016/0896-6273(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 53.Hartline HK. The discharge of impulses in the optic nerve of Pecten in response to illumination of the eye. J. Cell. Comp. Physiol. 1938;11:465–478. [Google Scholar]
- 54.Putnam NH, Butts T, Ferrier DE, Furlong RF, Hellsten U, Kawashima T, Robinson-Rechavi M, Shoguchi E, Terry A, Yu JK, et al. The amphioxus genome and the evolution of the chordate karyotype. Nature. 2008;453:1064–1071. doi: 10.1038/nature06967. [DOI] [PubMed] [Google Scholar]
- 55.Holland LZ, Albalat R, Azumi K, Benito-Gutierrez E, Blow MJ, Bronner-Fraser M, Brunet F, Butts T, Candiani S, Dishaw LJ, et al. The amphioxus genome illuminates vertebrate origins and cephalochordate biology. Genome Res. 2008;18:1100–1111. doi: 10.1101/gr.073676.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lacalli TC. Sensory systems in amphioxus: a window on the ancestral chordate condition. Brain Behav. Evol. 2004;64:148–162. doi: 10.1159/000079744. [DOI] [PubMed] [Google Scholar]
- 57.Koyanagi M, Kubokawa K, Tsukamoto H, Shichida Y, Terakita A. Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr. Biol. 2005;15:1065–1069. doi: 10.1016/j.cub.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 58.Gomez dPM, Angueyra JM, Nasi E. Light-transduction in melanopsin-expressing photoreceptors of Amphioxus. Proc. Natl. Acad. Sci. USA. 2009;106:9081–9086. doi: 10.1073/pnas.0900708106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gorman AL, McReynolds JS, Barnes SN. Photoreceptors in primitive chordates: fine structure, hyperpolarizing receptor potentials, and evolution. Science. 1971;172:1052–1054. doi: 10.1126/science.172.3987.1052. [DOI] [PubMed] [Google Scholar]
- 60.Holmberg K. The hagfish retina: fine structure of retinal cells in Myxine glutinosa, L., with special reference to receptor and epithelial cells. Z. Zellforsch. Mikrosk. Anat. 1970;111:519–538. doi: 10.1007/BF00330929. [DOI] [PubMed] [Google Scholar]
- 61.Holmberg K. The hagfish retina: electron microscopic study comparing receptor and epithelial cells in the Pacific hagfish, Polistotrema stouti, with those in the Atlantic hagfish, Myxine glutinosa. Z. Zellforsch. Mikrosk. Anat. 1971;121:249–269. doi: 10.1007/BF00340676. [DOI] [PubMed] [Google Scholar]
- 62.Locket NA, Jørgensen JM. The eyes of hagfishes. In: Jørgensen JM, Lomholt JP, Weber RE, Malte H, editors. The Biology of Hagfishes. London: Chapman and Hall; 1998. pp. 539–556. [Google Scholar]
- 63.Strahan R. The behavior of Myxine and other Myxinoids. In: Brodal A, Fänge R, editors. The Biology of Myxine. Oslo: Universitetsforlaget; 1963. pp. 22–32. [Google Scholar]
- 64.Adam H, Strahan R. Notes on the habitat, aquarium maintenance, and experimental use of hagfishes. In: Brodal A, Fänge R, editors. The Biology of Myxine. Oslo: Universitetsforlaget; 1963. pp. 33–41. [Google Scholar]
- 65.Collin SP, Knight MA, Davies WL, Potter IC, Hunt DM, Trezise AE. Ancient colour vision: multiple opsin genes in the ancestral vertebrates. Curr. Biol. 2003;13:R864–R865. doi: 10.1016/j.cub.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 66.Davies WL, Collin SP, Hunt DM. Adaptive gene loss reflects differences in the visual ecology of basal vertebrates. Mol. Biol. Evol. 2009;26:1803–1809. doi: 10.1093/molbev/msp089. [DOI] [PubMed] [Google Scholar]
- 67.Holmberg K. The cyclostome retina. In: Crescitelli F, editor. The Visual System in Vertebrates, Handbook of Sensory Physiology vol. VII/5. Berlin: Springer; 1977. pp. 47–66. [Google Scholar]
- 68.Govardovskii VI, Lychakov DV. Visual cells and visual pigments of the lamprey, Lampetra fluviatilis. J. Comp. Physiol. A. 1984;154:279–286. [Google Scholar]
- 69.Muradov H, Kerov V, Boyd KK, Artemyev NO. Unique transducins expressed in long and short photoreceptors of lamprey Petromyzon marinus. Vision Res. 2008;48:2302–2308. doi: 10.1016/j.visres.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muradov H, Boyd KK, Kerov V, Artemyev NO. PDE6 in lamprey Petromyzon marinus: implications for the evolution of the visual effector in vertebrates. Biochemistry. 2007;46:9992–10000. doi: 10.1021/bi700535s. [DOI] [PubMed] [Google Scholar]
- 71.Liang Y, Fotiadis D, Filipek S, Saperstein DA, Palczewski K, Engel A. Organization of the G protein-coupled receptors rhodopsin and opsin in native membranes. J. Biol. Chem. 2003;278:21655–21662. doi: 10.1074/jbc.M302536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Solovei I, Kreysing M, Lanctot C, Kosem S, Peichl L, Cremer T, Guck J, Joffe B. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell. 2009;137:356–368. doi: 10.1016/j.cell.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 73.Wensel TG. Signal transducing membrane complexes of photoreceptor outer segments. Vision Res. 2008;48:2052–2061. doi: 10.1016/j.visres.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luo D-G, Kefalov V, Yau K-W. Phototransduction in rods and cones. In: Masland RH, Albright T, editors. The Senses. Vol. 1: Vision I, I. Vision. Amsterdam: Elsevier; 2008. pp. 269–301. [Google Scholar]
- 75.Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog. Retinal Eye Res. 2001;20:49–94. doi: 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]
- 76.Kawamura S, Tachibanaki S. Rod and cone photoreceptors: molecular basis of the difference in their physiology. Comp. Biochem. Physiol. A. 2008;150:369–377. doi: 10.1016/j.cbpa.2008.04.600. [DOI] [PubMed] [Google Scholar]
- 77.Rieke F, Baylor DA. Origin of reproducibility in the responses of retinal rods to single photons. Biophys. J. 1998;75:1836–1857. doi: 10.1016/S0006-3495(98)77625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Field GD, Rieke F. Mechanisms regulating variability of the single photon responses of mammalian rod photoreceptors. Neuron. 2002;35:733–747. doi: 10.1016/s0896-6273(02)00822-x. [DOI] [PubMed] [Google Scholar]
- 79.Hamer RD, Nicholas SC, Tranchina D, Liebman PA, Lamb TD. Multiple steps of phosphorylation of activated rhodopsin can account for the reproducibility of vertebrate rod single-photon responses. J. Gen. Physiol. 2003;122:419–444. doi: 10.1085/jgp.200308832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Doan T, Mendez A, Detwiler PB, Chen J, Rieke F. Multiple phosphorylation sites confer reproducibility of the rod’s single-photon responses. Science. 2006;313:530–533. doi: 10.1126/science.1126612. [DOI] [PubMed] [Google Scholar]
- 81.Okawa H, Sampath AP. Optimization of single-photon response transmission at the rod-to-rod bipolar synapse. Physiology. 2007;22:279–286. doi: 10.1152/physiol.00007.2007. [DOI] [PubMed] [Google Scholar]
- 82.Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron. 2002;34:773–785. doi: 10.1016/s0896-6273(02)00700-6. [DOI] [PubMed] [Google Scholar]
- 83.Sampath AP, Strissel KJ, Elias R, Arshavsky VY, McGinnis JF, Chen J, Kawamura S, Rieke F, Hurley JB. Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron. 2005;46:413–420. doi: 10.1016/j.neuron.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 84.Dubs A, Laughlin SB, Srinivasan MV. Single photon signals in fly photoreceptors and first order interneurones at behavioral threshold. J. Physiol. 1981;317:317–334. doi: 10.1113/jphysiol.1981.sp013827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Warrant EJ. Nocturnal bees. Curr. Bio. 2007;17:R991–R992. doi: 10.1016/j.cub.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 86.Warrant EJ. Seeing in the dark: vision and visual behaviour in nocturnal bees and wasps. J. Exp. Biol. 2008;211:1737–1746. doi: 10.1242/jeb.015396. [DOI] [PubMed] [Google Scholar]
- 87.Okawa H, Sampath AP, Laughlin SB, Fain GL. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr. Biol. 2008;18:1917–1921. doi: 10.1016/j.cub.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niven JE, Anderson JC, Laughlin SB. Fly photoreceptors demonstrate energy-information trade-offs in neural coding. PLoS Biol. 2007;5:e116. doi: 10.1371/journal.pbio.0050116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fain GL, Granda AM, Maxwell JM. Voltage signal of photoreceptors at visual threshold. Nature. 1977;265:181–183. doi: 10.1038/265181a0. [DOI] [PubMed] [Google Scholar]
- 90.Laughlin SB. Matching coding, circuits, cells, and molecules to signals: general principles of retinal design in the fly’s eye. Prog. Retinal Eye Res. 1994;13:165–196. [Google Scholar]
- 91.Weckström M, Laughlin SB. Visual ecology and voltage-gated ion channels in insect photoreceptors. Trends Neurosci. 1995;18:17–21. doi: 10.1016/0166-2236(95)93945-t. [DOI] [PubMed] [Google Scholar]
- 92.Kleinschmidt J, Dowling JE. Intracellular recordings from gecko photoreceptors during light and dark adaptation. J. Gen. Physiol. 1975;66:617–648. doi: 10.1085/jgp.66.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burkhardt DA. Light adaptation and photopigment bleaching in cone photoreceptors in situ in the retina of the turtle. J. Neurosci. 1994;14:1091–1105. doi: 10.1523/JNEUROSCI.14-03-01091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lamb TD, Collin SP, Pugh EN., Jr Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat. Rev. Neurosci. 2007;8:960–976. doi: 10.1038/nrn2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fain GL. Sensory Transduction. Sunderland, MA: Sinauer, Inc.; 2003. [Google Scholar]
- 96.Lamb TD. Evolution of vertebrate retinal photoreception. Phil. Trans. Roy. Soc. Lond. B. 2009;364:2911–2924. doi: 10.1098/rstb.2009.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vanhoutte KJ, Stavenga DG. Visual pigment spectra of the comma butterfly, Polygonia c-album, derived from in vivo epi-illumination microspectrophotometry. J. Comp. Physiol. A. 2005;191:461–473. doi: 10.1007/s00359-005-0608-x. [DOI] [PubMed] [Google Scholar]
- 98.Nilsson DE. The evolution of eyes and visually guided behaviour. Phil. Trans. Roy. Soc. Lond. B Biol Sci. 2009;364:2833–2847. doi: 10.1098/rstb.2009.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Conway Morris S. The Cambrian “explosion”: slow-fuse or megatonnage? Proc. Natl. Acad. Sci. USA. 2000;97:4426–4429. doi: 10.1073/pnas.97.9.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stierwald M, Yanze N, Bamert RP, Kammermeier L, Schmid V. The Sine oculis/Six class family of homeobox genes in jellyfish with and without eyes: development and eye regeneration. Dev. Biol. 2004;274:70–81. doi: 10.1016/j.ydbio.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 101.Dorlochter M, Stieve H. The Limulus ventral photoreceptor: Light response and the role of calcium in a classic preparation. Prog. Neurobiol. 1997;53:451–515. doi: 10.1016/s0301-0082(97)00046-4. [DOI] [PubMed] [Google Scholar]
- 102.Saibil H, Hewat E. Ordered transmembrane and extracellular structure in squid photoreceptor microvilli. J. Cell Biol. 1987;105:19–28. doi: 10.1083/jcb.105.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kleerekoper H. The sense organs. In: Hardisty HW, Potter IC, editors. The Biology of Lampreysm. vol. II. London and New York: Academic; 1972. pp. 373–404. [Google Scholar]
- 104.Okada T, Ernst OP, Palczewski K, Hofmann KP. Activation of rhodopsin: new insights from structural and biochemical studies. Trends Biochem. 2001;26:318–324. doi: 10.1016/s0968-0004(01)01799-6. [DOI] [PubMed] [Google Scholar]