Abstract
Biochemical and mechanistic aspects into how various hypometabolic states are initiated in mammals are poorly understood. Here, we show how a state of hypometabolism is initiated by 5′-AMP uptake by erythrocytes. Wild type, ecto-5′-nucleotidase-deficient, and adenosine receptor-deficient mice undergo 5′-AMP-induced hypometabolism in a similar fashion. Injection of 5′-AMP leads to two distinct declining phases of oxygen consumption (VO2). The phase I response displays a rapid and steep decline in VO2 that is independent of body temperature (Tb) and ambient temperature (Ta). It is followed by a phase II decline that is linked to Tb and moderated by Ta. Altering the dosages of 5′-AMP from 0.25- to 2-fold does not change the phase I response. For mice, a Ta of 15 °C is effective for induction of DH with the appropriate dose of 5′-AMP. Erythrocyte uptake of 5′-AMP leads to utilization of ATP to synthesize ADP. This is accompanied by increased glucose but decreased lactate levels, suggesting that glycolysis has slowed. Reduction in glycolysis is known to stimulate erythrocytes to increase intracellular levels of 2,3-bisphosphoglycerate, a potent allosteric inhibitor of hemoglobin's affinity for oxygen. Our studies showed that both 2,3-bisphosphoglycerate and deoxyhemoglobin levels rose following 5′-AMP administration and is in parallel with the phase I decline in VO2. In summary, our investigations reveal that 5′-AMP mediated hypometabolism is probably triggered by reduced oxygen transport by erythrocytes initiated by uptake of 5′-AMP.
Keywords: Erythrocyte; Glycolysis; Metabolism; Nucleoside Nucleotide Metabolism; Oxygen Transport; 2,3-Bisphophosglycerate; CD73; Adenosine Monophosphate; Adenosine Receptors; Hypometabolism
Introduction
Mammals in deep hypometabolism (DH)5 with severe hypothermia and stupor are only observed naturally during hibernation (1). During hibernation, an animal's overall metabolic rate, based on oxygen consumption, is a small fraction of its euthermic needs. The biochemical event that triggers this hypometabolism is an enigma. It is thought that non-hibernating mammals cannot undertake a similar hypometabolic process because deep hypothermia is often associated with ventricular fibrillation and cardiac arrest.
However, organs from non-hibernators can withstand a considerable ischemic period in a hypometabolic state. For example, during human organ transplantation, donated organs are transported in a cold ischemic state in the complete absence of blood circulation for many h (2), yet when implanted into a recipient, the restoration of blood flow and rewarming revives full organ function. This phenomenon also illustrates the important role of oxygen transportation by erythrocytes in metabolic activity. In addition, observations that exsanguination and prolonged hypoxia can result in hypothermia further demonstrate the importance of oxygen transportation and availability as factors that can modulate the metabolic rate in mammals (3, 4).
We have demonstrated that the metabolite 5′-AMP can induce mice to undergo transient hypometabolism with Tb as low as 26 °C, similar to a torpor-like state (5). The Tb of animals in torpor is defined to be at or moderately below 31 °C (6). Here, we demonstrate that 5′-AMP under the appropriate Ta can also be used to induce a reversible DH in which VO2 levels are less than 10% of normal euthermic demand. Our studies reveal that the 5′-AMP-mediated DH is accompanied by two distinct phases of VO2 decline. Immediately following 5′-AMP administration, the phase I decline in VO2 is steep, rapid, and independent of both Tb and Ta. This is followed by a gradual phase II decline that is moderated by Ta and Tb. Although our investigations show that erythrocytes take up 5′-AMP and use ATP to convert it to ADP, the majority of the 5′-AMP is rapidly catabolized. The uptake of 5′-AMP coincides with a rise in 2,3-bisphosphoglycerate (2,3-BPG) levels produced by the erythrocytes. The rise in 2,3-BPG is consistent with the observed phase I response because 2,3-BPG is a potent allosteric inhibitor of hemoglobin's affinity for oxygen. Spectral analysis of blood obtained from mice shortly after 5′-AMP administration revealed increased levels of deoxyhemoglobin.
EXPERIMENTAL PROCEDURES
Animals
These studies primarily used female mice (C57Bl/6), aged between 8 and 16 weeks. Mice were housed in a standard animal facility under a 12-h/12-h light/dark cycle with Ta between 22 and 24 °C. Male Sprague-Dawley rats weighing between 450 and 550 g were housed in similar facilities. All mouse studies were carried out under institutionally approved animal protocols HSC-AWC-06-078 (University of Texas Health Science Center) and NWC-06-039 (Nanjing University of Science and Technology). Rat experiments were undertaken with institutionally approved animal protocol HSC-AWC-07-046 (University of Texas Health Science Center). Dog experiments were undertaken with institutionally approved animal protocol NWC-06-039 (Nanjing University of Science and Technology). Care and use of animals for this study were in compliance with relevant animal welfare guidelines approved by the Animal Welfare Committee at the University of Texas Health Science Center.
DH Methods
Each mouse was injected intraperitoneally with the appropriate dosages of freshly prepared 5′-AMP (Sigma catalogue no. A1752-25G) dissolved in phosphate-buffered saline. For routine induction of DH, mice received 0.5 mg/g 5′-AMP and were maintained at 15 °C Ta throughout the entire procedure. Physical interaction with the animals was kept to a minimum once 5′-AMP had been administered. For rapid cooling, mice were given 0.125–0.5 mg/g of 5′-AMP and were kept at 4 °C for about 1 h before being transferred to a Ta of 15 °C. Core body temperature was measured by telemetry, using a G2 E-mitter (Columbus Instruments and Mini-Mitter Respironics). Oxygen consumption and carbon dioxide production rates were measured in an environmentally controlled comprehensive laboratory animal monitoring system (Columbus Instruments). Respiration rate was determined by counting the number of inhalations and exhalations over 10-s intervals. Heart rate and body temperature were measured with a mouse THM100 temperature and ECG monitoring system (Indus Instruments). Body temperature was manually monitored by a digital thermometer (Traceable, Fisher; catalogue no. 15-077-8) placed into the rectum.
Erythrocyte Uptake of [14C]Adenosine or 5′-[14C]AMP
Erythrocytes were isolated by differential centrifugation (1000 × g) from heparinized mouse blood, washed in a 2× volume of ice-cold PBS (four times), followed by ice cold Hanks' buffered salt solution (two times) with the final volume equal to the original blood volume. 1 μl of [14C]adenosine (43 mCi/mmol and 100 μCi/ml) or 5′- [14C]AMP (50 mCi/mmol and 100 μCi/ml) was mixed with 50 μl of erythrocytes and centrifuged either immediately or after incubation at 37 °C for 60 min. The supernatant was removed, and the erythrocyte pellet was washed with 1 ml of Hanks' buffered salt solution and repelleted. The pellet was treated with 50 μl of 10% trichloroacetic acid, and the supernatant was separated from insoluble products by centrifugation. Erythrocyte uptake of [14C]adenosine or 5′-[14C]AMP was determined by analyzing 10 μl of the supernatant in 10 ml of scintillant using a LKG 1209 counter. Products containing 14C were identified by TLC (Silica Gel 60 F254, EMD Chemicals, Inc.) in solvent containing butanol/methanol/water/ammonium hydroxide (60:20:19:1). All 14C-labeled nucleotides were obtained from GE Healthcare. The 14C-labeled nucleosides were obtained from MP Biomedicals. Levels of 2,3-BPG in erythrocytes were measured using a kit (Roche Applied Science). Hemoglobin content was determined by an automated hematology analyzer (Sysmex XT-2000i).
Metabolomics Profiling
Serum samples from four groups of mice (n = 7) were collected during euthermia, DH, arousal, and early recovery by centrifugation at 1000 × g for 10–15 min. All serum samples were frozen immediately upon collection and kept frozen until extraction. The frozen samples were shipped to Metabolon, Inc. for metabolomics profiling on their standard platform described previously (7). Briefly, the low molecular weight fractions of methanol extract were analyzed in parallel by gas chromatography and liquid chromatography in combination with mass spectrometry. Non-targeted metabolomics analyses were performed for both liquid chromatography-mass spectrometry and gas chromatography-mass spectrometry outputs, where each detected compound was identified via searching its spectral files through metabolomics libraries of about 1500 compounds at Metabolon. The data for each compound were median-centered and inter-quartile range-scaled. The company's technology platform strictly allows relative quantitation of peak areas for each metabolite. The analysis is based on the ratio changes when experimental animal groups are compared with the control animal group. All t tests are two-sided, applying a threshold of p < 0.05 for significance.
Glucose and Lactate Measurements
Glucose and lactate levels in erythrocytes were measured using assay kits (BioVision, Inc.). The erythrocytes were separated from serum by centrifugation, washed once in phosphate-buffered saline, and immediately frozen. Erythrocyte lysate was deproteinized by perchloric acid using a kit (BioVision, Inc). Supernatant from this extraction was used for glucose and lactate measurement. Protein levels of erythrocyte lysate were measured by a Bradford protein assay kit (Bio-Rad).
Spectral Analysis of Blood
Blood was obtained by cardiac puncture. The syringe containing the blood was frozen immediately in liquid nitrogen. To limit oxygen exposure, the thawed erythrocyte lysate was injected under a layer of paraffin oil placed in a 2-mm glass cuvette. Spectral analysis was carried out using a multiwavelength spectrophotometer HP8453 (Hewlett-Packard).
HPLC Fractionation of Adenine Nucleotides
Blood was rapidly removed from sacrificed mice and snap frozen in liquid nitrogen. Nucleotides were extracted from frozen samples using 0.4 n perchloric acid as described previously (8). Extracts were separated and quantified using reverse-phase HPLC (Waters, Millipore Corp.) on a Partissphere-bonded C18 cartridge column at a flow rate of 1.5 ml/min. The mobile phase was 0.02 m NH4H2PO4, pH 5.1, with a superimposed methanol gradient with the following time course: 0% for 0–4 min, 0–8% for 4–6 min, 8–20% for 6–8 min, and 20% for 8–18 min. All nucleotide and purine derivative standards were purchased from Sigma. Enzymatic confirmation of the adenylate peaks was carried out with snake venom nucleotidase and bovine ATPase treatment.
RESULTS
5′-AMP Induction of DH in Mammals
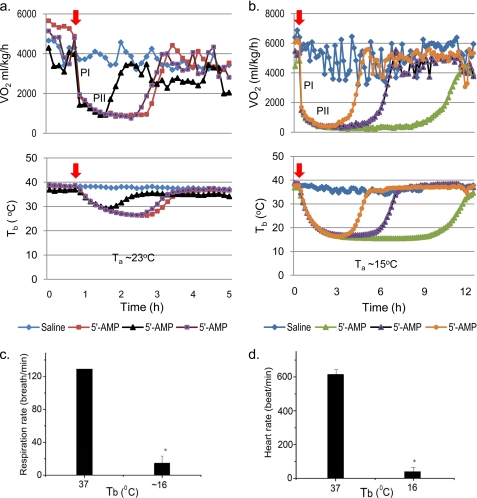
A key question raised from our earlier studies was whether 5′-AMP can be used to induce a DH state and what are the safe physiological boundaries. To investigate these issues, the effect of 5′-AMP on VO2, Tb, and the importance of Ta were assessed. Mice were injected with saline or 5′-AMP (0.5 mg/g), and then placed in either Ta of 23 °C (normal husbandry temperature) or Ta of 15 °C while their VO2 and Tb were simultaneously measured (Fig. 1, a and b). Mice that were given saline displayed VO2 levels ranging between 4000 and 6500 ml/kg/h regardless of whether Ta was 15 or 23 °C. The Tb of these mice measured by telemetry remained at about 37 °C. In contrast, about 10 min after 5′-AMP administration, the phase I VO2 had dropped steeply to below 2000 ml/kg/h for both groups of mice at Ta of 23 or 15 °C. Notably, Tb of the mice dropped only slightly over the same period. The phase II VO2 decline that followed corresponded with the major decline in Tb. For mice kept at a Ta of 23 °C, the phase II decline reached a nadir of about 1000 ml/kg/h and a Tb of 26–28 °C (Fig. 1a; also see supplemental Fig. 1). After about 2 h in the hypometabolic state, euthermic Tb and VO2 levels were restored. For mice kept at a Ta of 15 °C, the phase I response was similar to that of animals kept at 23 °C; however, their phase II decline was longer and deeper, reaching a nadir at about 300 ml/kg/h and a Tb of about 16 °C (Fig. 1b; also see supplemental Fig. 1). Normal respiration rates of mice were reduced from ∼120 to ∼10 breaths/min (Fig. 1c). In addition, heart rates were similarly reduced from ∼600 to about 50 beats/min (Fig. 1d). When laid on their sides or backs, mice appeared to be in a state of deep stupor with outstretched limbs but were responsive to tactile stimuli. Mice kept at a Ta of 23 °C did not display such behavior. Our observations indicate that mice enter DH when their Tb drops below 17 °C. Mice in DH occasionally display subconscious behaviors, such as hind limb scratching, flipping and rolling on their backs, and urination (see supplemental Video 1). Maintained at a Ta of 15 °C, arousal from DH occurs spontaneously for mice (Figs. 1b and 2 (c and d)). Once arousal was initiated, a parallel increase in VO2 and Tb was observed (Figs. 1b and 2 (c and d)). As Tb rose above 22 °C, a visible shivering period ensued, followed by the return of normal mouse behaviors, such as self-grooming. Short and long term assessment of the impact of DH on mouse cognitive function by Morris water maze studies revealed no significant difference in the escape latency of the animals after undergoing DH treatment (data not shown). Both male and female mice showed equal ability to enter DH. Rats and dogs given 5′-AMP and cooled to Tb of 18–20 °C (rats) and 20–22 °C (dogs) displayed a similar ability to undergo DH (see supplemental Fig. 2, a and b). Together, these observations demonstrate that 5′-AMP can be used to initiate reversible DH of mammals.
FIGURE 1.
Relationship between VO2, Tb, and Ta following 5′-AMP injection. a and b, simultaneous measurement of Tb and VO2 of mice given saline or 5′-AMP (0.5 mg/g, indicated by arrow) in individual metabolic chambers at Ta of 23 and 15 °C. Each mouse used in the study is represented by the same color trace for VO2 and Tb. Sampling time for both Tb and VO2 was about 8 min. Note that the phase I (PI) decline of VO2 is steep and rapid after administration of 5′-AMP. In contrast, the phase II (PII) VO2 decline is gradual and coincides with declining Tb. The colors reflect VO2 and Tb of independent measurement of different animals given either saline or 5′-AMP. c, respiration rate of mice (n = 4) with Tb ∼16 °C compared with 37 °C. d, heart rate of mice (n = 3) with Tb 16 °C compared with 37 °C. *, p < 0.001, paired t test. Error bars, S.E.M.
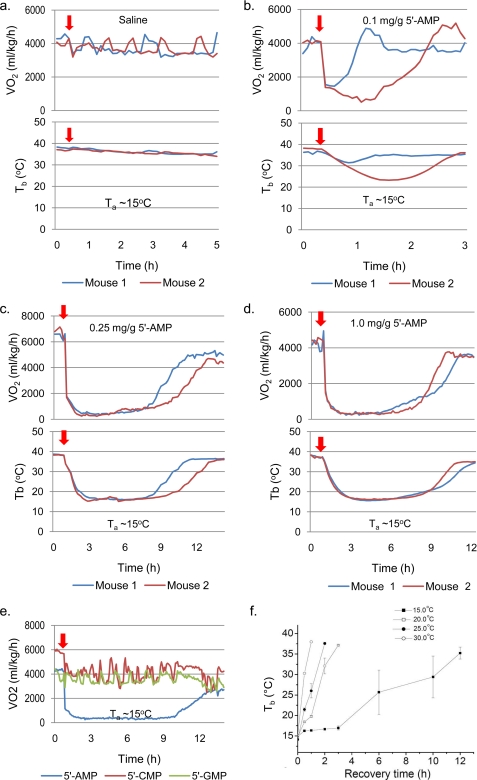
FIGURE 2.
DH as a function of 5′-AMP dosage. a–d, simultaneous measurement of Tb and VO2 of mice given different dosages of 5′-AMP and maintained at Ta of 15 °C. The different colors in the graph reflect VO2 and Tb of different animals used in the study. e, comparison of VO2 levels in mice given a similar amount (0.5 mg/g) of either 5′-AMP, 5′-CMP, or 5′-GMP. f, arousal from DH and the rise in Tb of mice (n = 4) maintained at various Ta values.
5′-AMP Dosages Initiate but Do Not Control the Length of DH
Next, we examined the concentration dependence of 5′-AMP in mediating the process of DH in mice. Five groups of mice were injected with saline or 5′-AMP doses ranging from 0.1 to 1.0 mg/g and then kept at a Ta of 15 °C. Our findings demonstrate that the concentration of 5′-AMP is important for entering into DH (Figs. 1b and 2 (a–d)). Lower dosages of 5′-AMP did not produce DH but instead a transient hypometabolic state with a moderate decrease in Tb (Fig. 2b). Surprisingly, increasing the dosage of 5′-AMP from 0.1 to 1 mg/g did not alter the phase I VO2 decline (Figs. 1b and 2 (b–d)). Comparing the Tb and VO2 profiles of mice revealed an aborted phase II when mice failed to enter DH (Fig. 2b). However, once DH was attained, there was considerable overlap in spontaneous arousal time between the different dosages of 5′-AMP administered (Figs. 1b and 2 (c and d)).
We further investigated whether other nucleoside monophosphates, such as cytidine monophosphate (5′-CMP) or guanosine monophosphate (5′-GMP), could produce a similar hypometabolic response in mice. Unlike the 5′-AMP-injected animals, mice given 5′-CMP or 5′-GMP (0.5 mg/g) displayed normal VO2 and activity throughout the study (Fig. 2e).
The effect of Ta on the arousal rate from DH was investigated. Mice in DH were kept at different Ta, and the time required for Tb to return to 37 °C was measured (Fig. 2f). An increase in Ta led to a faster recovery rate. These observations indicated that the appropriate Ta plays a major role in maintaining DH. However, differential arousal times at a constant Ta indicate the involvement of an endogenous control that remains unclear. Suppression of arousal by additional injections of 5′-AMP or a further decrease in Ta to less than 13 °C resulted in increased fatalities. Together, these studies indicate that the phase I VO2 response to 5′-AMP was not dependent on the Ta or the Tb of the animal. However, the phase II VO2 decline is associated with declining Tb of the animal and is moderated by Ta. Once the animal enters DH under a constant Ta, the arousal time is spontaneous and has no apparent relationship to the dose of 5′-AMP administered.
Dephosphorylation of 5′-AMP to Adenosine Is Not Essential for DH
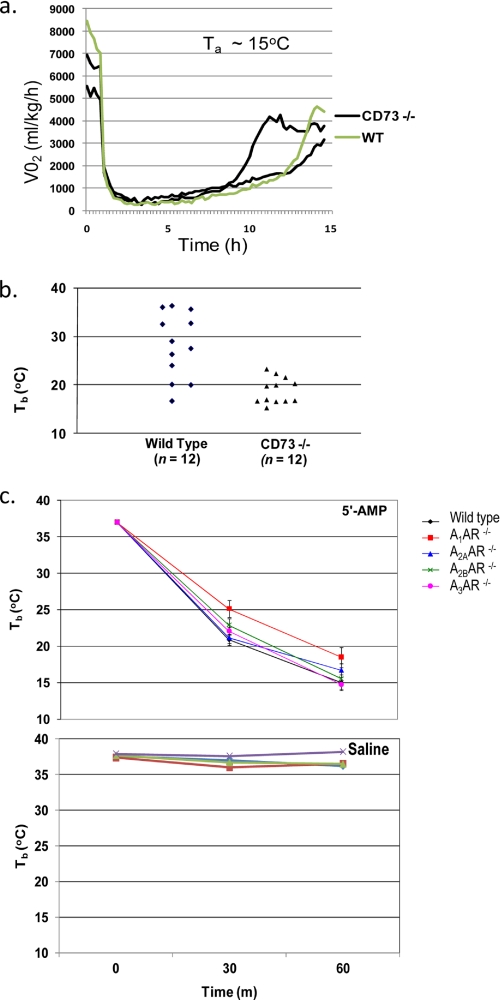
Circulating 5′-AMP is dephosphorylated to adenosine by the membrane-bound extracellular enzyme ecto-5′-nucleotidase/CD73 (9). Therefore, CD73−/− mice were used to investigate whether blocking 5′-AMP dephosphorylation affects the animal's hypometabolic responses to 5′-AMP. No differential VO2 response was observed between ecto-5′-nucleotidase/CD73-deficient and wild type mice given 5′-AMP (Fig. 3a). Next we investigated whether ecto-5′nucleotidase/CD73-deficient mice have a differential response to lower dosages of 5′-AMP. A cooling regime of Ta at 4 °C for 1 h was used to determine the lowest effective dosage to reduce Tb into the DH range. Using this cooling regime, a 5′-AMP dose of 0.125 mg/g was able to induce DH in the majority of ecto-5′-nucleotidase/CD73-deficient mice but was mostly unsuccessful in wild type animals (Fig. 3b). These observations suggest that the presence of ecto-5′-nucleotidase/CD73 in wild type mice reduces the effectiveness of lower doses of 5′-AMP to induce DH.
FIGURE 3.
Loss of adenosine receptors or ecto-5′-nucleotidase deficiency does not block DH by 5′-AMP. a, the VO2 profiles of ectonucleotidase/CD73-deficient (n = 2) and wild type mice following 5′-AMP (0.5 mg/g) administration. b, individual Tb of ectonucleotidase/CD73-deficient and wild type mice (n = 12) after 1 h at 4 °C Ta with injection of 0.125 mg/g 5′-AMP. c, top, Tb time course of mice (n = 4) with the adenosine receptor genotype A1−/−, A2a−/−, A2b−/−, or A3−/− or wild type were given 0.5 mg/g 5′-AMP after 1 h at 4 °C Ta. Bottom, Tb of mice of the same genotype given saline after 1 h at 4 °C Ta. Error bars, S.E.M.
The role played by adenosine receptors in DH was investigated using mice genetically engineered for adenosine receptor deficiency (10–13). Mice deficient in A1, A2A, A2B, or A3 adenosine receptors were compared with wild type animals for their response to 5′-AMP or saline. A similar cooling regime as above was used. Based on Tb, all of the different adenosine receptor-deficient and wild type mice showed equal ability to enter DH (Fig. 3c). Mice of the same genotype given saline did not change their Tb under a similar cooling regime.
Together, these findings demonstrate that neither the dephosphorylation of 5′-AMP into adenosine nor the presence of adenosine receptor-mediated processes is essential for 5′-AMP mediated hypometabolism.
DH Is Associated with Elevated Levels of 2,3-Bisphosphoglycerate
To gain biochemical insight into the fate and effects of 5′-AMP, we undertook a metabolomics analysis of serum metabolites from mice during the states of euthermia (preinjection), DH, at arousal and at early recovery. Liquid and gas chromatography and mass spectrometry analysis of the serum revealed that the level of 5′-AMP at DH (about 3 h postinjection) and arousal was less than 1.5-fold that observed in euthermic mice, indicating that the majority of the administered 5′-AMP had been catabolized. This conclusion is supported by the large increase in purine catabolites, including inosine, xanthine, xanthosine, hypoxanthine, urate, and allantoin during DH (Table 1). At early recovery, the majority of these purine catabolites had returned to levels observed in the preinjected animals (euthermia) except allantoin, an end product of purine catabolism in mice. A 2-fold increase in ADP over that of the euthermic state was observed during DH. The levels of adenosine during DH and arousal were lower during euthermia, suggesting that 5′-AMP was primarily catabolized through the AMP deaminase pathway.
TABLE 1.
Relative levels of purine intermediates in serum that showed significant alteration during euthermia, deep hypometabolism, arousal, and early recovery
For each group, the relative quantization value of each metabolite is the average obtained from seven mice. For each of the metabolites, the average euthermia control value is arbitrarily set at 1. The -fold change in DH, arousal, and early recovery is relative to the value obtained for euthermia. The probability that the changes were due to random factors is quantified by the p values, and the false positive rate is quantified by q values of t tests that are shown in italic type. (Note that for the data points with missing values, no acceptable measurement for that compound was obtained for the group.)
| Altered purine metabolites (n = 7) | DH/Euthermia |
Arousal/Euthermia |
Early recovery/Euthermia |
||||||
|---|---|---|---|---|---|---|---|---|---|
| -Fold change | p value | q value | -Fold change | p value | q value | -Fold change | p value | q value | |
| Xanthine | 8.83 | 0.000 | 0.001 | 4.97 | 0.012 | 0.007 | 1.13 | 0.356 | 0.127 |
| Xanthosine | 11.8 | 0.000 | 0.000 | 2.82 | 0.004 | 0.003 | 1.03 | 0.793 | 0.227 |
| Hypoxanthine | 8.69 | 0.004 | 0.005 | 3.07 | 0.033 | 0.017 | |||
| Inosine | 4.91 | 0.026 | 0.021 | 1.30 | 0.351 | 0.115 | |||
| Adenosine | 0.66 | 0.004 | 0.005 | 0.41 | 0.033 | 0.017 | 0.61 | 0.523 | 0.166 |
| 5′-AMP | 1.21 | 0.026 | 0.021 | 1.44 | 0.351 | 0.115 | 0.92 | 0.587 | 0.178 |
| ADP | 1.79 | 0.021 | 0.019 | 2.02 | 0.004 | 0.003 | 0.95 | 0.807 | 0.228 |
| Urate | 3.3 | 0.001 | 0.001 | 2.70 | 0.002 | 0.002 | 1.57 | 0.144 | 0.064 |
| Allantoin | 15.3 | 0.000 | 0.000 | 18.16 | 0.000 | 0.000 | 13.44 | 0.000 | 0.000 |
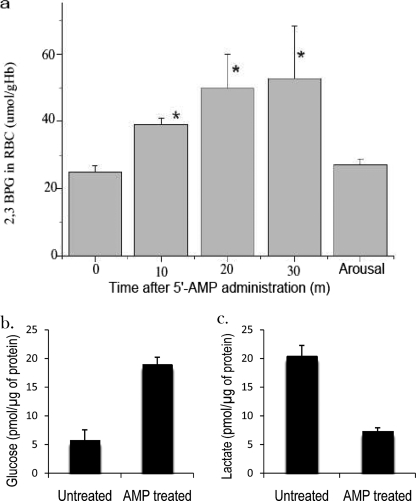
Metabolomics studies further revealed a higher level of 2,3-BPG in DH than euthermic state (data not shown). The enzyme that catalyzes 2,3-BPG biosynthesis from 1,3-BPG, bisphosphoglycerate mutase, is found only in erythrocytes and placenta (14). The observed increase in 2,3-BPG implicates erythrocytes as the source of 2,3-BPG and a likely cellular target of 5′-AMP. A time course assay of 2,3-BPG in erythrocytes revealed a rapid increase shortly after 5′-AMP administration to mice (Fig. 4a).
FIGURE 4.
Increased production of 2,3-bisphosphoglycerate induced by 5′-AMP uptake by erythrocytes. a, levels of 2,3-bisphosphoglycerate in erythrocytes/red blood cells (RBC) obtained from mice after administration of 5′-AMP (*, data shown as mean ± S.E.M. (error bars), p < 0.05, n = 6). b and c, glucose and lactate levels in erythrocytes isolated from 5′-AMP and untreated mice (data shown as mean ± S.E.M. p < 0.01, n = 4).
The large increase in 2,3-BPG levels could be an indicator of reduced glycolysis in the erythrocytes. Therefore, the levels of glucose and lactate in the erythrocytes obtained from 5′-AMP-treated and untreated mice were measured. Our analysis revealed that the level of glucose in the erythrocytes of 5′-AMP treated mice was over 3 times higher and the level of lactate was about 2 times lower than those from untreated animals (Fig. 4, b and c). Together, these findings suggest that the increase in 2,3-BPG levels was probably linked to reduce glucose utilization in the erythrocytes of 5′-AMP-treated mice.
Uptake of 5′-AMP by Erythrocytes and Elevated Levels of Deoxyhemoglobin
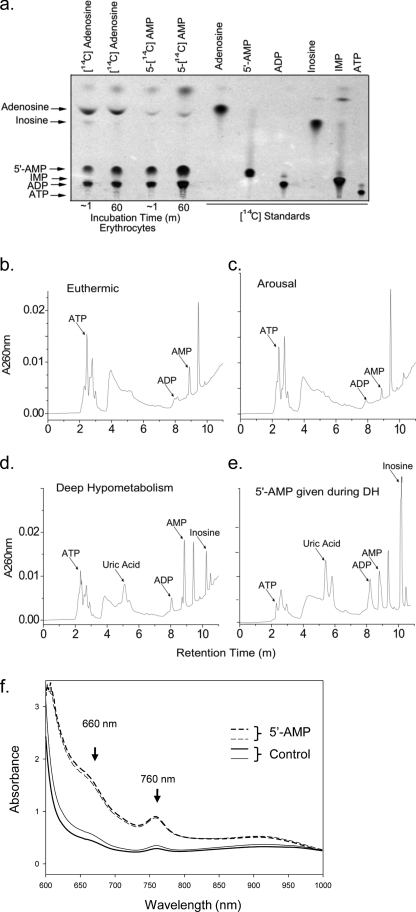
To verify whether 5′-AMP was directly taken up by erythrocytes, we incubated freshly isolated wild type mouse erythrocytes with radiolabeled [14C]adenosine or 5′-[14C]AMP. Using TLC, the catabolic and salvage products from [14C]adenosine or 5′- [14C]AMP were identified shortly after their addition to erythrocytes in vitro. Uptake of adenosine into erythrocytes occurred very rapidly as observed previously (15). TLC analysis showed that uptake of [14C]adenosine was primarily salvaged to form 5′- [14C]AMP and [14C]ADP (Fig. 5a). This would be consistent with previous observations that adenosine in erythrocytes is salvaged by adenosine kinase into 5′-AMP and then via adenylate kinase into ADP (16–18). Erythrocytes also displayed rapid uptake of 5′-[14C]AMP that drives the formation of [14C]ADP (Fig. 5a). No increase in [14C]ATP was observed, which is consistent with the adenylate equilibrium (ATP + 5′-AMP ↔ 2ADP) favoring the forward direction toward ADP driven by the influx of 5′-AMP. To expand on these observations, metabolite extracts were made from whole blood of mice during the states of euthermia and DH and at arousal. HPLC analysis revealed that extracts obtained from DH displayed a significant increase in purine catabolites, including inosine and uric acid (Fig. 5, b–e). The uptake of 5′-AMP into erythrocytes resulted in a significant rise in intracellular ADP and a corresponding drop in ATP levels. This change in the adenylate ratio is supported by observations that an additional injection of 5′-AMP during DH further increased ADP at the expense of ATP levels while also causing a large increase in inosine (Fig. 5e).
FIGURE 5.
Uptake of 5′-AMP by erythrocytes and increased deoxyhemoglobin levels. a, uptake of radiolabeled [14C]adenosine or 5′-[14C]AMP by isolated erythrocytes in vitro. Autoradiograph showing the TLC analysis of erythrocyte extracts compared with known radiolabeled purine standards. Shown are representative HPLC chromatograms of purine products extracted from blood obtained from mice in euthermia (b), in spontaneous arousal (c), and during DH (d) and an animal in DH given a second injection of 0.5 mg/g 5′-AMP and sacrificed 2 h later (e). HPLC of purine standards was used to determine the identity of the various peaks. f, absorbance spectrum of whole blood from mice (n = 2) treated with 5′-AMP (dashed lines) or untreated (solid lines). Blood was taken 10 min after intraperitoneal administration of 5′-AMP (0.5 mg/g).
Because 2,3-BPG allosterically inhibits hemoglobin affinity for oxygen, an increase in the levels of deoxyhemoglobin is predicted. Blood high in deoxyhemoglobin has a signature absorbance peak at 760 nm (19). In addition, oxyhemoglobin has a weaker absorbance than deoxyhemoglobin at 660 nm (20). Spectral analysis of blood from the 5′-AMP-treated mice displayed a signature peak absorbance at 760 nm. It also had a higher absorbance at 660 nm than blood obtained from untreated animals (Fig. 5f). These studies demonstrate that 5′-AMP uptake by the erythrocytes triggers the biochemical response that leads to an increase of 2,3-BPG, which in turn decreases hemoglobin affinity for oxygen.
DISCUSSION
Mice deficient in ectonucleotidase/CD73 or adenosine receptors enter DH with an efficiency similar to that of wild type animals. These two complementary but independent mouse genetic experiments demonstrate that dephosphorylation of 5′-AMP into adenosine was apparently not essential for DH. Our investigations revealed that unlike 5′-AMP-injected mice, the 5′-CMP- and 5′-GMP-injected mice did not show a decrease in VO2 levels. Because adenosine and adenylate nucleotides can be converted to 5′-AMP in vivo, administration of adenosine or adenylate nucleotides induces a transient decrease of VO2 in mice. Due to adenosine's low aqueous solubility and half-life of less than 10 s in plasma, DH of mice under similar conditions was not observed (see supplemental Fig. 3a). Unlike the rapid drop observed for 5′-AMP following its administration, other adenylate nucleotides, including cAMP, displayed a more gradual decline in VO2 (supplemental Fig. 3, b and c; data not shown). We surmised that the slower response is consistent with the breakdown of cAMP to 5′-AMP by phosphodiesterases; however, the concentration of 5′-AMP generated from cAMP did not reach the levels required for DH to take hold. A slower VO2 response was also observed for ADP and ATP given at similar concentration (data not shown).
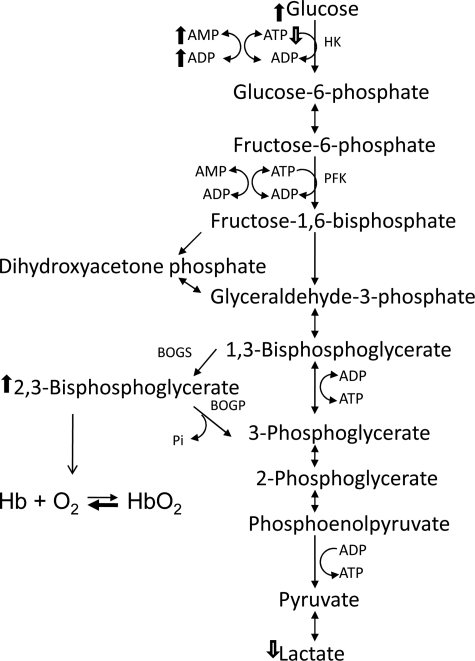
Previous studies have shown that 5′-[11C]AMP cannot cross the blood brain barrier (16). Rather, the radiotracer was found primarily in red blood cells and in two highly perfused organs, lung and heart. In the blood, the uptake of 5′-[11C]AMP was observed to alter the ADP/ATP ratio, as predicted by the adenylate equilibrium (16). Our studies demonstrate that 5′-[14C]AMP was taken up rapidly by erythrocytes from wild type and CD73−/− mice. TLC analysis revealed that the uptake of 5′-AMP into erythrocytes drives the production of ADP. In addition, our HPLC analysis revealed that uptake of 5′-AMP by erythrocytes alters the relative levels of ADP and ATP, which is consistent with the adenylate equilibrium. We propose that the influx of 5′-AMP into erythrocytes drives the adenylate equilibrium (ATP + 5′-AMP ↔ 2ADP) to increase ADP production, utilizing intracellular ATP (Fig. 6). The first step of glycolysis carried out by hexokinase (glucose to glucose 6-phosphate) requires ATP to proceed (21). Our studies demonstrate that glucose is highly elevated, but lactate is decreased during DH, which is consistent with a reduced level of glycolysis. Metabolic modeling studies have demonstrated that the production of 2,3-BPG from 1,3-BPG is enhanced when glycolysis slows as a response to stabilize ATP levels in the erythrocytes (22). A small change in ATP levels can have a large effect on 2,3-BPG production in erythrocytes (22).
FIGURE 6.
A proposed model for 5′-AMP-mediated hypometabolism via erythrocytes. The model indicates altered levels of glycolytic intermediates and adenylates observed. In the erythrocytes, uptake of 5′-AMP drives the production of ADP at the expense of ATP. In turn, glycolytic steps dependent on ATP at hexokinase (HK) were slowed, resulting in the buildup of glucose and decreased production of lactate. The reduction in glycolytic rate will drive the production of 2,3-BPG from 1,3-BPG by the enzyme bisphophoglycerate mutase to stabilize intracellular ATP levels. The increase in 2,3-BPG allosterically reduces the affinity of hemoglobin (Hb) for oxygen, which could explain the rapid and steep decline in VO2 illustrated by phase I and increased deoxyhemoglobin levels.
In erythrocytes, 2,3-BPG regulates the affinity of hemoglobin for oxygen in an allosteric manner (21). The affinity of 2,3-BPG for deoxygenated hemoglobin is very high, but it binds only weakly to oxygenated hemoglobin (21, 22). Thus, a rise in 2,3-BPG in the erythrocyte following 5′-AMP administration would lower its ability to transport oxygen. The phase I VO2 profile could reflect a reduced rate of oxygen transport by the erythrocytes rather than a sudden shutdown of the animal's metabolic rate. This is supported by the observation that the amplitude of the phase I decline in VO2 was very similar over a wide dosage range of 5′-AMP, an observation consistent with allosteric regulation. In addition, blood from mice given 5′-AMP has much higher deoxyhemoglobin levels than blood from untreated animals. We propose that an allosteric suppression of hemoglobin's affinity for oxygen by 2,3-BPG results in delivery of less oxygen to organs, and this in turn triggers a physiological adaption to decrease overall metabolic activity in vivo. When the body's heat loss to the environment is greater than that generated endogenously, the Tb will decline toward the Ta. As Tb drops, the overall demand for oxygen also decreases, thereby creating an interdependent loop between Tb and VO2, which is consistent with the observed phase II VO2 and Tb profiles. If maintained at the appropriate Ta, the animal's Tb will attain an equilibrium between the energy released by reduced metabolic activity and the heat loss to the environment, thereby allowing the animal to maintain DH. Gradual rewarming of the animal, leading to increased VO2, triggers arousal from DH. However, the regulation of arousal remains complex because mice arouse at vastly different times even when Ta remains constant.
Metabolic inhibitors, such as 2-deoxyglucose and hydrogen sulfide (H2S), are able to induce torpor and “suspended animation” of small mammals (23, 24). How these metabolic inhibitors achieve reduced Tb is currently unclear. However, the effects of these inhibitors on erythrocyte functions are well established. Inhaled H2S gas is metabolized by erythrocytes into reactants (HbO2 + 2H2S → 2S + Hb + H2O) that would deplete oxyhemoglobin levels (25). Erythrocytes lack mitochondria and are unable to carry out β-oxidation. Therefore, inhibitors of β-oxidation, such as mercaptoacetate, do not disrupt erythrocyte ATP production and therefore do not induce torpor of hamsters (23). However, inhibition of hexokinase, the first enzymatic step of glycolysis, by 2-deoxygluocose would severely impact the ability of erythrocytes to produce ATP. In addition, hypothermia of mammals can be induced by exsanguination, a process in which a large volume of blood is removed from the body (3, 26). Furthermore, hypothermia is a documented response to prolonged hypoxia (4). Together, these findings suggest that erythrocytes could be a common target for the various agents known to induce hypometabolism in mammals. In summary, we have demonstrated a method using a natural metabolite, 5′-AMP, together with the appropriate Ta, to initiate, maintain, and terminate DH of diverse species of mammals.
Supplementary Material
Acknowledgments
We thank Drs. J. Lever and R. Kellems for helpful comments and reading of the manuscript, Dr. L. Thompson for providing the CD73−/− mice, Dr. J. Chen for the A2 AR−/− mice, and Dr. V. Berka and Dr. A. L. Tsai for much assistance in blood spectral analysis.
This work was supported, in whole or in part, by a National Institutes of Health Director Pioneer award (to C. C. L.) (NIH/NDPA: 5DP1 OD000895).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3 and Movie 1.
Supported in part by a NASA/Texas Space Grant Consortium Fellowship.
Supported in part by Ministry of Education of P. R. China.
- DH
- deep hypometabolism
- BPG
- bisphosphoglycerate
- Ta
- ambient temperature
- Tb
- body temperature
- HPLC
- high pressure liquid chromatography
- VO2
- oxygen consumption.
REFERENCES
- 1.Heldmaier G., Ortmann S., Elvert R. (2004) Respir. Physiol. Neurobiol. 141, 317–329 [DOI] [PubMed] [Google Scholar]
- 2.Simpkins C. E., Montgomery R. A., Hawxby A. M., Locke J. E., Gentry S. E., Warren D. S., Segev D. L. (2007) Am. J. Transplant. 7, 99–107 [DOI] [PubMed] [Google Scholar]
- 3.Ikeda S. (1965) Tohoku J. Exp. Med. 87, 185–198 [DOI] [PubMed] [Google Scholar]
- 4.Wood S. C. (1991) Annu. Rev. Physiol. 53, 71–85 [DOI] [PubMed] [Google Scholar]
- 5.Zhang J., Kaasik K., Blackburn M. R., Lee C. C. (2006) Nature 439, 340–343 [DOI] [PubMed] [Google Scholar]
- 6.Overton J. M., Williams T. D. (2004) Physiol. Behav. 81, 749–754 [DOI] [PubMed] [Google Scholar]
- 7.Lawton K. A., Berger A., Mitchell M., Milgram K. E., Evans A. M., Guo L., Hanson R. W., Kalhan S. C., Ryals J. A., Milburn M. V. (2008) Pharmacogenomics 9, 383–397 [DOI] [PubMed] [Google Scholar]
- 8.Knudsen T. B., Winters R. S., Otey S. K., Blackburn M. R., Airhart M. J., Church J. K., Skalko R. G. (1992) Teratology 45, 91–103 [DOI] [PubMed] [Google Scholar]
- 9.Thompson L. F., Eltzschig H. K., Ibla J. C., Van De Wiele C. J., Resta R., Morote-Garcia J. C., Colgan S. P. (2004) J. Exp. Med. 200, 1395–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J. F., Huang Z., Ma J., Zhu J., Moratalla R., Standaert D., Moskowitz M. A., Fink J. S., Schwarzschild M. A. (1999) J. Neurosci. 19, 9192–9200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salvatore C. A., Tilley S. L., Latour A. M., Fletcher D. S., Koller B. H., Jacobson M. A. (2000) J. Biol. Chem. 275, 4429–4434 [DOI] [PubMed] [Google Scholar]
- 12.Sun D., Samuelson L. C., Yang T., Huang Y., Paliege A., Saunders T., Briggs J., Schnermann J. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 9983–9988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csóka B., Németh Z. H., Virág L., Gergely P., Leibovich S. J., Pacher P., Sun C. X., Blackburn M. R., Vizi E. S., Deitch E. A., Haskó G. (2007) Blood 110, 2685–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pritlove D. C., Gu M., Boyd C. A., Randeva H. S., Vatish M. (2006) Placenta 27, 924–927 [DOI] [PubMed] [Google Scholar]
- 15.Noji T., Karasawa A., Kusaka H. (2004) Eur. J. Pharmacol. 495, 1–16 [DOI] [PubMed] [Google Scholar]
- 16.Mathews W. B., Nakamoto Y., Abraham E. H, Scheffel U., Hilton J., Ravert H. T., Tatsumi M., Rauseo P. A., Traughber B. J., Salikhova A. Y., Dannals R. F., Wahl R. L. (2005) Mol. Imaging Biol. 7, 203–208 [DOI] [PubMed] [Google Scholar]
- 17.Tsuboi K. K., Chervenka C. H. (1975) J. Biol. Chem. 250, 132–140 [PubMed] [Google Scholar]
- 18.Hawkins C. F., Bagnara A. S. (1987) Biochemistry 26, 1982–1987 [DOI] [PubMed] [Google Scholar]
- 19.Chance B., Leigh J. S., Miyake H., Smith D. S., Nioka S., Greenfeld R., Finander M., Kaufmann K., Levy W., Young M. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 4971–4975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shepherd A. P., Sutherland J. C., Wilson A. F. (1975) J. Appl. Physiol. 39, 152–155 [DOI] [PubMed] [Google Scholar]
- 21.Lehninger A. (1982) Principles of Biochemistry, 2nd Ed., W. H. Freeman, New York [Google Scholar]
- 22.Mulquiney P. J., Kuchel P. W. (1999) Biochem. J. 342, 597–604 [PMC free article] [PubMed] [Google Scholar]
- 23.Dark J., Miller D. R., Zucker I. (1994) Am. J. Physiol. 267, R496–R501 [DOI] [PubMed] [Google Scholar]
- 24.Blackstone E., Morrison M., Roth M. B. (2005) Science 308, 518. [DOI] [PubMed] [Google Scholar]
- 25.Evans C. L. (1967) Q. J. Exp. Physiol. Cogn. Med. Sci. 52, 231–248 [DOI] [PubMed] [Google Scholar]
- 26.Rhee P., Talon E., Eifert S., Anderson D., Stanton K., Koustova E., Ling G., Burris D., Kaufmann C., Mongan P., Rich N. M., Taylor M., Sun L. (2000) J. Trauma 48, 439–447; discussion 447–450 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.