Abstract
The serine protease granzyme B (GrB) is the most potent proapoptotic cytotoxin of the granule exocytosis pathway of cytotoxic lymphocytes. GrB is synthesized as a zymogen (proGrB) and activated in cytotoxic granules by the lysosomal cysteine protease cathepsin C (CatC) which removes the N-terminal dipeptide Gly-Glu. It has been shown recently that mice lacking CatC nonetheless express significant residual GrB activity, indicating the presence of additional proGrB convertases. Here, we describe an assay to assess activation of proGrB and show that the amino-peptidase cathepsin H (CatH) has proGrB convertase activity in vitro, whereas dipeptidylpeptidase II does not. We generated mice lacking both CatC and CatH expression (CatCH−/−) and found that their lymphocytes have reduced convertase activity compared with those from CatC-deficient mice. Despite this, cytotoxic lymphocytes from CatCH−/− mice retain cytotoxic activity and some residual GrB activity. We conclude that CatH can act as an additional proGrB convertase and that other protease/s (apart from dipeptidylpeptidase II) must also possess convertase activity. This indicates a great deal of functional redundancy in GrB maturation, which would prevent pathogen-mediated immune suppression by via convertase inhibition.
Keywords: Cellular Immune Response, Death Protease, Gene Knock-out, Lymphocyte, Protease, Protein Processing, Cathepsin, Cytotoxic Lymphocyte, Granzyme B
Introduction
Cytotoxic lymphocytes (CLs)6 include natural killer and CD8-positive T cells and are responsible for eliminating virus-infected and cancerous cells. CLs induce cell death through two distinct pathways, by signaling through death receptors or activating the granule exocytosis pathway. In the latter, specialized secretory lysosomes (“granules”) release their contents into the synapse formed between the killer and the target cell (1, 2). These deadly contents include perforin, a pore-forming protein capable of lysing cells at high concentration and granzymes, a family of serine proteases. The cell death signaling pathways of granzymes A (GrA) and B (GrB) are the best studied; however, in vitro proapoptotic function has also been attributed to most of the other granzymes. GrB is a potent cytotoxin that induces apoptosis in vitro when applied to cells at nanomolar concentrations with purified perforin (3). Perforin is critical for granzyme-mediated cell death, as it facilitates entry of proteases into the target cell cytosol, where they can access and cleave substrates to bring about target cell death (4).
GrB is synthesized and activated through a mechanism common to other serine proteases that reside in lysosomes (5). Following entry into the secretory pathway, its signal peptide is removed in the endoplasmic reticulum, leaving GrB as a zymogen (proGrB), with an inhibitory dipeptide at the newly formed N terminus (6, 7). Upon reaching the Golgi, proGrB follows the mannose-6-phosphate pathway into the secretory lysosomes, where its processing is completed by the cysteine amino-dipeptidase cathepsin C (CatC) (8, 9). Although CatC is certainly sufficient to activate proGrB, its necessity has been questioned by two lines of evidence. First, activated lymphocytes derived from humans with congenital deficiency of CatC (Papillon-Lefevre syndrome) contain active GrB and kill target cells with similar efficiency to healthy controls (10). Papillon-Lefevre syndrome patients present principally with manifestations of neutrophil dysfunction, such as severe gingivitis, because the neutrophil serine proteases cathepsin G and elastase depend completely on CatC for processing (10, 11). Secondly, lymphocytes from CatC−/− mice possess active GrB and kill target cells almost as efficiently as wild-type mice (12). This evidence led us to postulate that additional proteases must be capable of activating proGrB. We therefore developed an assay for GrB convertase activity and tested the candidate aminopeptidases dipeptidylpeptidase II (DPPII) and cathepsin H (CatH). We found that CatH, but not DPPII, has GrB convertase activity; however, lymphocytes deficient in both CatC and CatH are still capable of generating active GrB, indicating that additional GrB convertases exist in intact CLs.
EXPERIMENTAL PROCEDURES
Enzymes and Reagents
All reagents were obtained from either Merck or Sigma unless otherwise specified. Bovine CatC was purchased from Sigma (catalog no. 9032-68-2). Human CatH was purchased from Merck (catalog no. 60748-73-4). Human DPPII was purchased from Biomol (catalog no. SE564-0010).
Cathepsin Active Site Titrations
To obtain accurate specific activities, CatC and CatH were titrated with the irreversible active site inhibitors Ala-4-(I)Phe-DMK (a gift from Merck Frosst Canada (13)) and E-64 (Sigma), respectively. The residual activity was assessed on the substrate Gly-Phe-7-amino-4-methylcoumarin (CatC) or l-Arg-7-amido-4-methylcoumarin (CatH), purchased from MP Biomedicals and Sigma, respectively.
Production of proGrB
Recombinant human ProGrB was produced in Pichia pastoris and purified from culture supernatant as described previously for GrB (14), except that the construct was designed to include the natural two-residue prodomain (Gly-Glu) downstream of the enterokinase (EK) cleavage site. Removal of the His tag by EK was verified by submitting 2 μg of proGrB for amino acid sequencing via Edman degradation (Monash Proteomics Facility) as described previously (15).
ProGrB Convertase Assay
20 pmol of proGrB was incubated with various amounts of protease in 10 μl of buffer containing 50 mm MES, 150 mm NaCl, and 5 mm dithiothreitol, pH 5.5. The reaction mix was then added to 90 μl of buffer containing 200 mm Tris, 150 mm NaCl, pH 7.4, to optimize the pH for GrB activity. GrB activity was subsequently assayed using the quenched fluorescence substrate, aminobenzoyl-IEPDSSMESK- dinitrophenyl as described previously, which is specific for human granzyme B (15, 16).
Convertase Assay Performed with Lymphocyte Lysates
Splenocytes were washed in saline and then lysed at 1 × 108 cells/ml in buffer containing 1% IGEPAL, 100 mm MES, pH 5.5. Allostimulated splenocytes were washed in saline and then lysed at 4 × 107 cells/ml in buffer containing 0.1% IGEPAL, 250 mm NaCl, 2.5 mm EDTA, and 25 mm HEPES, pH 7.2. 10 μl of lysate was incubated overnight with 10 μl of buffer containing 20 pmol of proGrB, 50 mm MES, 150 mm NaCl, and 5 mm dithiothreitol, pH 5.5. This reaction was then assayed for GrB activity as above.
Mice
C57BL/6 (H-2b) and BALB/c (H-2d) mice were purchased from the Walter and Eliza Hall Institute of Medical Research. The GrA and GrB cluster-deficient mice (17) were kindly provided by Markus Simon (Max-Planck-Institut fur Immunbiologie, Freiburg, Germany) and bred and maintained at the Peter MacCallum Cancer Institute. As the GrB cluster-deficient mice were generated using 129 embryonic stem cells, the double knock-out mice were backcrossed for eight generations onto a C57BL/6 background. A PCR-screening protocol (17) was used for genotyping. Christine Pham (Washington University School of Medicine, St.Louis, MO) kindly provided the CatC−/− (designated previously as DPP1) mice (18), which had been backcrossed onto the C57BL/6 background for 11 generations. A PCR protocol provided by C. Pham was used to verify the disruption of the wild-type CatC gene. CatH−/− mice were generated by replacing exon 5 through intron 9 of the CatH gene with a targeting construct comprising internal ribosome entry site, a lacZ reporter, and a G418 resistance cassette (neor) using homologous recombination in HM1-mouse embryonic stem cells. CatH-deficient mice do not show a gross morphological phenotype and no impairment of reproductive capacity or breeding behavior or nursing. A full account of generation and phenotyping of CatH−/− mice will be published elsewhere.7 The CatC/CatH double knock-out mice were bred to homozygosity at the Peter MacCallum Cancer Institute and used at 6–8 weeks of age. The development, fertility (litter size and sex), and life span of the CatC/H−/− mice were normal. Examination of the major organs, including the lymphoid organs, was normal. Fluorescence-activated cell sorter analysis was performed to verify that the splenic subpopulations were unaltered (data not shown). Approval for all mouse experiments was through the Peter MacCallum Cancer Centre animal ethics committee.
Cell Culture and Reagents
The mouse mastocytoma cell line P815 (H-2d), was maintained in Dulbecco's modified Eagle's medium with high glucose (4.5 g/liter, JRH Biosciences) supplemented with 10% heat-inactivated fetal bovine serum (JRH Biosciences), 2 mm glutamine (Invitrogen), 0.1 mm 2-mercaptoethanol, 100 units/ml penicillin and 100 μg/ml streptomycin (Invitrogen). Allogeneic (H-2b anti H-2d) mouse cytotoxic T lymphocytes (CTL) were generated by one-way mixed lymphocyte reaction (MLR) as described previously (12) and maintained in RPMI medium (Invitrogen) supplemented as above, with the addition of 100 μm nonessential amino acids, 1 mm sodium pyruvate (Invitrogen), and 50 international units/ml recombinant interleukin-2. For most assays, CTLs were used after 7 days, but for some assays, CTL, which had been restimulated for a further 3 days, were used. The effector cells generated from each strain were >80% CD8+ T cells and displayed equivalent levels of activation markers (CD25, CD69). Western blot analysis demonstrated similar levels of perforin and GrB protein in each of the effector cell populations (data not shown). Standard 51Cr and 125I-DNA release assays (19) were used to compare the membrane damage and the DNA fragmentation induced in P815 target cells by the CTL.
Endogenous Granzyme Activity Assays
GrA (tryptase) and GrB (aspase) esterolytic activity presence in whole cell lysates of primary and secondarily stimulated CTLs were measured as described previously (20) by the hydrolysis of synthetic peptide thiobenzylester substrates: Boc-Ala-Ala-Asp-thiobenzyl (kindly provided by Dr Jim Powers, Georgia Institute of Technology, Atlanta, Georgia) for GrB and Boc-l-lysine-S-benzyl (Sigma) for GrA.
RESULTS
Production of Recombinant proGrB
Potential candidates for proGrB activation would almost certainly need to be present in lysosomes and have amino-peptidase activity. Other than CatC, two proteases that fit these criteria are DPPII and CatH. DPPII is a serine dipeptidase with a strong preference for Pro at the P1 residue, making it unlikely to activate proGrB, as the dipeptide typically cleaved from proGrB is Gly-Glu (21, 22). CatH is the only other known cathepsin with aminopeptidase activity and, as a monopeptidase, would have to sequentially remove the two amino acids of the proGrB pro-dipeptide. Further processing would be precluded, as the exposed N-terminal Ile then forms a salt bridge with the Asp in the active site of the protease, resulting in a rapid conformational change (5).
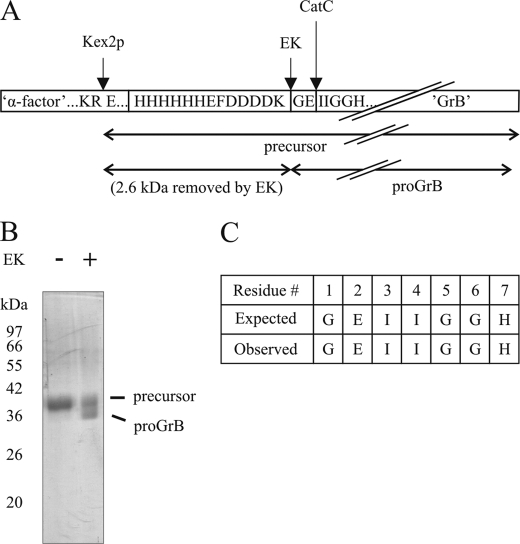
To test candidate proteases for proGrB convertase activity, we produced recombinant proGrB in Pichia pastoris in a manner similar to that described previously for GrB (14). The precursor protein comprises an α-factor polypeptide (removed by Kex2p during biosynthesis), a His6 tag, and EK cleavage site, followed by the native prodomain of human GrB (Gly-Glu) and the mature GrB sequence (Fig. 1A). The Kex2p-processed precursor protein was purified by cation-exchange and nickel affinity chromatography and treated with EK to remove the additional N-terminal domains, leaving only proGrB. As shown in Fig. 1B, treatment of the precursor protein with EK slightly reduced its size, consistent with removal of the His tag and EK site. (The panel shows an incomplete EK digestion to illustrate this). A sample of completely digested proGrB was N-terminally sequenced, confirming that processing by EK was complete and correct (Fig. 1C).
FIGURE 1.
Production of recombinant human proGrB. A, diagram of the precursor protein produced in P. pastoris. Processing points (Kex2p, removal of α-factor polypeptide; EK, removal of His tag; CatC, removal of dipeptide prodomain) are indicated by arrows. B, a sample of the proGrB precursor treated with EK. Coomassie stained 12.5% SDS-PAGE gel shows partial EK processing of proGrB precursor to illustrate the decrease in size following removal of the His tag and EK site. C, N-terminal amino acid sequence of recombinant EK-treated proGrB.
A Convertase Assay for proGrB
A convertase assay for proGrB activation incorporated an initial activation step at pH 5.5 to mimic the lysosomal conditions required for CatC, followed by adjustment of the pH to 7.4 for optimal GrB activity. Under these conditions, CatC was able to activate proGrB in a dose-dependent manner (Fig. 2A). Overnight (16 h) treatment of proGrB with CatC showed that the GrB generated remained active and stable under prolonged incubation (Fig. 2B).
FIGURE 2.
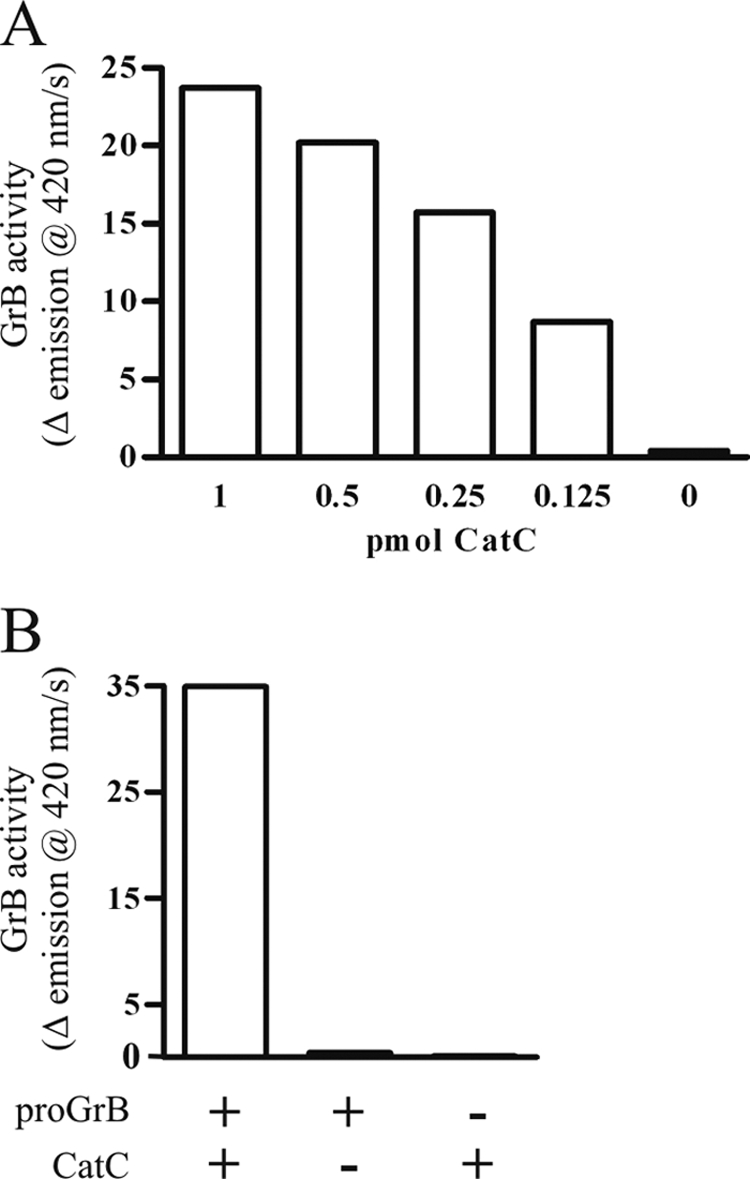
ProGrB convertase assay. The activation of proGrB following treatment with CatC was assessed at 37 °C using a quenched fluorescence substrate, aminobenzoyl-IEPDSSMESK-dinitrophenyl. 2-Fold serial dilutions of CatC (20–2.5 pmol) were used to activate 20 pmol of proGrB. A, following a 1-h incubation at pH 5.5, the pH was adjusted to pH 7.4, substrate was added, and the resultant GrB activity was assayed. B, the stability and maximal activation of 20 pmol proGrB was assessed following treatment with 20 pmol CatC overnight (16 h) at pH 5.5. Data shown are representative of three experiments.
Testing Candidate proGrB Convertases
CatH and DPPII were tested for their ability to activate proGrB. As predicted, DPPII did not produce any increase in GrB activity over untreated proGrB despite prolonged incubation and a favorable stoichiometric ratio (Fig. 3B). By contrast, CatH produced a dose-dependent activation of proGrB (Fig. 3A). Overnight treatment of proGrB with CatH produced ∼50% of the GrB activity produced by CatC (Fig. 3B). CatC, CatH, and DPPII did not hydrolyze the GrB substrate in the absence of proGrB (data not shown). To the best of our knowledge, these experiments are the first demonstrating a role for CatH in activating any protease, and the first demonstration of a proGrB convertase other than CatC. To demonstrate that CatH activates proGrB by removing the same two residues as CatC, a sample of CatH-treated proGrB was N-terminally sequenced. This showed that the new N terminus is the same as that observed for CatC-treated proGrB, Ile-Ile-Gly-Gly (data not shown). CatH likely achieves this by sequentially removing the two residues of the pro-dipeptide.
FIGURE 3.
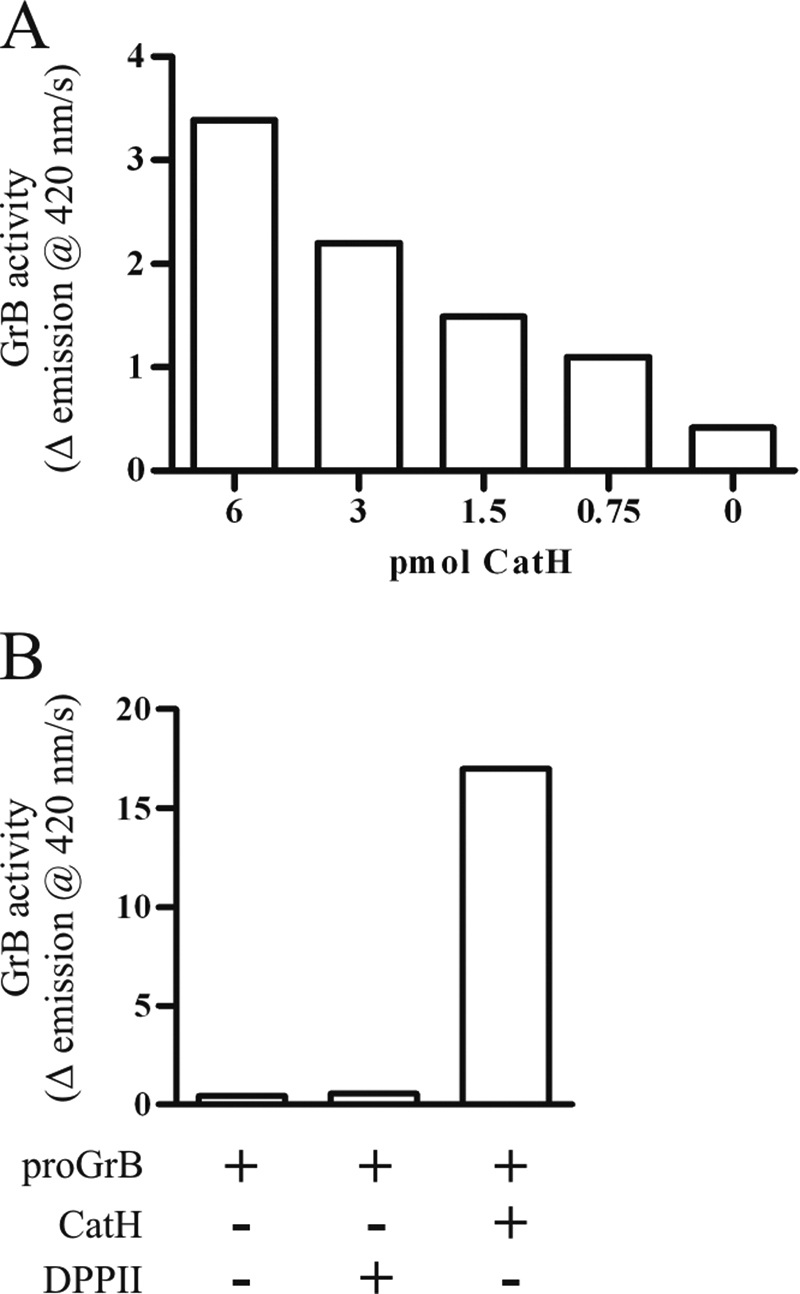
CatH, but not DPPII, activates proGrB. The activation of proGrB by treatment with CatH or DPPII was assessed in the convertase assay. A, GrB activity was assessed after 1 h of incubation with convertase. B, maximal activity was assessed following overnight (16 h) incubation of 20 pmol proGrB with 20 pmol CatH or DPPII. Data shown are representative of at least two experiments.
Activation of proGrB with Mouse Spleen Lysates
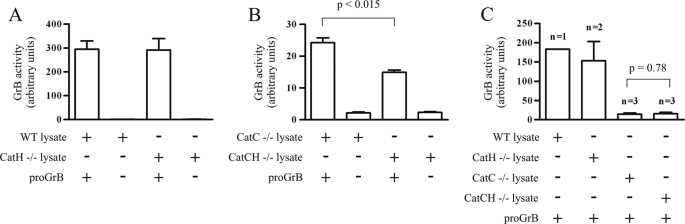
To investigate the involvement of CatH in activation of proGrB in vivo, CatC−/− mice were bred with CatH−/− mice to produce mice deficient in both proteases (CatCH−/−). Lysates were made from the spleens of wild-type BL/6, CatC−/−, CatH−/−, and CatCH−/− mice and tested for proGrB convertase activity. As the substrate aminobenzoyl-IEPDSSMESK-dinitrophenyl is specific for human GrB, it was possible to use mouse cell lysates as a source of activating proteases in the convertase assay without endogenous mouse GrB confounding the assay (Fig. 4, A and B). As expected, wild-type and CatH−/− cell lysates both produced a high level of substrate turnover from activation of proGrB, as they both contain CatC (Fig. 4A). Also, as expected from previous studies, CatC−/− cell lysate exhibited some convertase activity. By contrast, there was significantly less convertase activity in CatCH−/− lysates (Fig. 4B), strongly supporting a role for CatH in proGrB activation in vivo.
FIGURE 4.
CatH has proGrB convertase activity in vivo. ProGrB (20 pmol) was incubated overnight at pH 5.5 with lysates from 1 × 106 unstimulated splenocytes (A and B) or from 4 × 105 allogeneic CTL (C) derived from wild-type BL/6 (WT), CatH−/−, CatC−/−, or CatCH−/− mice. The resultant GrB activity was assessed using the quenched fluorescence substrate. Each assay was performed using lysates from three mice of each strain (with the exception of the CTL, where one wild-type BL/6 and two CatH−/− lysates were used), and the mean ± S.E. was calculated. Student's t tests were performed, and p values indicate significant differences in activity generated by the CatC−/− and CatCH−/− lysates.
Lysates produced from activated lymphocytes derived from the different mouse lines were also used in the proGrB convertase assay. Again, wild-type and CatH−/− lysates generated the greatest amount of GrB activity; however, in this case, CatC−/− and CatCH −/− cells produced equivalent convertase activity (Fig. 4C). This suggests that additional activating protease(s) are up-regulated in these cells on stimulation and that CatH is not important for activating proGrB in this context.
Allostimulated CatCH−/− Splenocytes Retain Cytotoxic Capacity
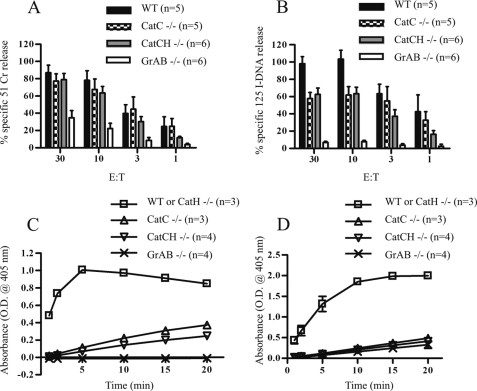
To test the functional consequences on cytotoxic activity of losing both CatC and CatH, in vitro one-way MLRs were used to generate alloreactive (H-2b anti-H-2d) CTLs. Cells from primary 7-day MLRs were compared for their capacity to induce 51Cr release assay (a marker of plasma membrane disruption) and 125I-DNA release (a measure of DNA fragmentation) at varying effector to target ratios (Fig. 5, A and B, respectively). As reported previously, CatC−/− CTLs induced similar 51Cr release from P815 target cells as BL/6 CTLs. Consistent with a reduction in the levels of active GrB produced by these cells, 125I-DNA release was reduced compared with wild-type BL/6 but remained markedly higher than in CTLs generated from mice, in which both the GrA and GrB genes were disrupted. These observations are consistent with the observed phenotype of GrB−/− mice, which demonstrate reduced target cell DNA fragmentation in short term assays (23). At all but the lowest effector to target ratio used, the CatCH −/− CTLs produced levels of 51Cr and 125I-DNA release similar to those of CatC−/− CTLs. To determine whether the cytotoxic activity of CatCH−/− CTLs could be explained by residual GrB activity, lysates of CTLs generated in the MLR were tested for aspase activity. This confirmed that CatCH−/− CTL contained GrB activity equivalent to that seen in CatC−/− CTLs (Fig. 5C). An assay for BLTase (“tryptase”) activity showed that, like the CatC−/− CTLs, CatCH −/− CTLs do not contain active GrA (Fig. 5D). This confirmed that the activation of proGrA is totally dependent on CatC (12). Taken together, our results show that whereas both CatC and CatH have proGrB convertase activity, at least one additional protease must be capable of processing the GrB pro-form in their absence.
FIGURE 5.
Effector lymphocytes from both CatC−/− and CatCH−/− mice retain sufficient GrB activity to induce DNA fragmentation in target cells. Membrane damage and DNA fragmentation induced in P815 target cells were assessed using 51Cr (A) or 125I-DNA (B) release. CTLs from three separate primary MLR (at days 6 and 7) were used at the effector:target (E:T) ratio indicated. Aspase (GrB) and tryptase (GrA) activities were measured in substrate cleavage assays using specific peptide substrates, Boc-Ala-Ala-Asp-S-benzyl for GrB (C) or Boc-L-lysine-S-benzyl for GrA (D). Lysates were prepared from 7-day primary MLR. Each individual assay was performed using 30 μg protein, and the assays were repeated using lysates from multiple MLR as indicated. The total number of assays performed are indicated (n), and each assay was done in triplicate. The data were pooled, and the mean ± S.E. is shown.
DISCUSSION
We show here that there are multiple pathways to granzyme B activation, which include cathepsin H. Granzymes and perforin play crucial roles in protecting mammals against viruses and other intracellular pathogens, as demonstrated by the increased susceptibility of perforin- or granzyme-deficient mice and perforin-deficient humans to overwhelming virus infection. For example, perforin-deficient mice are exquisitely sensitive to the mousepox virus ectromelia, due to their inability to mount an effective CTL response (17). GrAB−/− mice are also highly sensitive to ectromelia, although these mice are able to generate functional anti-ectromelia CTLs (18). The defect in the defense of GrAB−/− mice against ectromelia therefore remains unclear.
In other infections, granzymes interfere directly in viral replication and apoptosis of the infected cell. This is perhaps best exemplified by recent studies in human adenovirus V infection, in which GrB and granzyme H (GrH) were shown to cooperatively limit viral replication and assembly and to hasten the death of infected cells. The capsid protein L4–100K produced in infected cells acts as a competitive inhibitor of GrB. However, GrH efficiently cleaves L4–100K to relieve the block on GrB-mediated apoptosis (24). In addition, GrH cleaves proteins pivotal in viral DNA synthesis such as DNA-binding protein, thereby limiting viral replication.
Recently it has also been demonstrated that GrB has extracellular matrix remodelling ability, suggesting that it may contribute to lymphocyte migration or hemostasis during inflammation (25, 26). Together, these graphic demonstrations of the importance of GrB in immune function provide a clear evolutionary rationale for redundancy in the mechanisms that underpin GrB activation.
Acknowledgments
We thank Dion Kaiserman for advice and assistance with protein production and analysis, Leanne McNiff for mouse breeding, José Villadangos and Christine Pham for providing the cathepsin H- and the cathepsin C-deficient mice, and Merck Frosst Canada for the cathepsin C inhibitor.
C. Peters and T. Reinheckel, manuscript in preparation.
- CL
- cytotoxic lymphocyte
- CatC
- cathepsin C
- CatH
- cathepsin H
- GrA
- granzyme A
- GrB
- granzyme B
- proGrB
- pro-granzyme B
- MES
- 4-morpholineethanesulfonic acid
- GrH
- granzyme H
- DPPII
- dipeptidylpeptidase II
- EK
- enterokinase
- CTL
- cytotoxic T lymphocytes
- MLR
- mixed lymphocyte reaction.
REFERENCES
- 1.Burkhardt J. K., Hester S., Lapham C. K., Argon Y. (1990) J. Cell Biol. 111, 2327–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yannelli J. R., Sullivan J. A., Mandell G. L., Engelhard V. H. (1986) J. Immunol. 136, 377–382 [PubMed] [Google Scholar]
- 3.Kaiserman D., Bird C. H., Sun J., Matthews A., Ung K., Whisstock J. C., Thompson P. E., Trapani J. A., Bird P. I. (2006) J. Cell Biol. 175, 619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi L., Kraut R. P., Aebersold R., Greenberg A. H. (1992) J. Exp. Med. 175, 553–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvesen G., Enghild J. J. (1990) Biochemistry 29, 5304–5308 [DOI] [PubMed] [Google Scholar]
- 6.Caputo A., Garner R. S., Winkler U., Hudig D., Bleackley R. C. (1993) J. Biol. Chem. 268, 17672–17675 [PubMed] [Google Scholar]
- 7.Lobe C. G., Finlay B. B., Paranchych W., Paetkau V. H., Bleackley R. C. (1986) Science 232, 858–861 [DOI] [PubMed] [Google Scholar]
- 8.Griffiths G. M., Isaaz S. (1993) J. Cell Biol. 120, 885–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smyth M. J., McGuire M. J., Thia K. Y. (1995) J. Immunol. 154, 6299–6305 [PubMed] [Google Scholar]
- 10.Pham C. T., Ivanovich J. L., Raptis S. Z., Zehnbauer B., Ley T. J. (2004) J. Immunol. 173, 7277–7281 [DOI] [PubMed] [Google Scholar]
- 11.de Haar S. F., Jansen D. C., Schoenmaker T., De Vree H., Everts V., Beertsen W. (2004) Hum. Mutat. 23, 524. [DOI] [PubMed] [Google Scholar]
- 12.Sutton V. R., Waterhouse N. J., Browne K. A., Sedelies K., Ciccone A., Anthony D., Koskinen A., Mullbacher A., Trapani J. A. (2007) J. Cell Biol. 176, 425–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Méthot N., Rubin J., Guay D., Beaulieu C., Ethier D., Reddy T. J., Riendeau D., Percival M. D. (2007) J. Biol. Chem. 282, 20836–20846 [DOI] [PubMed] [Google Scholar]
- 14.Sun J., Bird C. H., Buzza M. S., McKee K. E., Whisstock J. C., Bird P. I. (1999) Biochem. Biophys. Res. Commun. 261, 251–255 [DOI] [PubMed] [Google Scholar]
- 15.Sun J., Whisstock J. C., Harriott P., Walker B., Novak A., Thompson P. E., Smith A. I., Bird P. I. (2001) J. Biol. Chem. 276, 15177–15184 [DOI] [PubMed] [Google Scholar]
- 16.Prakash M. D., Bird C. H., Bird P. I. (2009) Immunol. Cell Biol. 87, 249–254 [DOI] [PubMed] [Google Scholar]
- 17.Simon M. M., Hausmann M., Tran T., Ebnet K., Tschopp J., ThaHla R., Müllbacher A. (1997) J. Exp. Med. 186, 1781–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pham C. T., Ley T. J. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 8627–8632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton V. R., Vaux D. L., Trapani J. A. (1997) J. Immunol. 158, 5783–5790 [PubMed] [Google Scholar]
- 20.Davis J. E., Sutton V. R., Browne K. A., Trapani J. A. (2003) J. Immunol. Methods 276, 59–68 [DOI] [PubMed] [Google Scholar]
- 21.Leiting B., Pryor K. D., Wu J. K., Marsilio F., Patel R. A., Craik C. S., Ellman J. A., Cummings R. T., Thornberry N. A. (2003) Biochem. J. 371, 525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maes M. B., Lambeir A. M., Gilany K., Senten K., Van der Veken P., Leiting B., Augustyns K., Scharpé S., De Meester I. (2005) Biochem. J. 386, 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heusel J. W., Wesselschmidt R. L., Shresta S., Russell J. H., Ley T. J. (1994) Cell 76, 977–987 [DOI] [PubMed] [Google Scholar]
- 24.Andrade F., Fellows E., Jenne D. E., Rosen A., Young C. S. (2007) EMBO J. 26, 2148–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buzza M. S., Dyson J. M., Choi H., Gardiner E. E., Andrews R. K., Kaiserman D., Mitchell C. A., Berndt M. C., Dong J. F., Bird P. I. (2008) J. Biol. Chem. 283, 22498–22504 [DOI] [PubMed] [Google Scholar]
- 26.Buzza M. S., Zamurs L., Sun J., Bird C. H., Smith A. I., Trapani J. A., Froelich C. J., Nice E. C., Bird P. I. (2005) J. Biol. Chem. 280, 23549–23558 [DOI] [PubMed] [Google Scholar]