Abstract
N-Methyl-d-aspartate (NMDA) receptors are expressed at excitatory synapses throughout the brain and are essential for neuronal development and synaptic plasticity. Functional NMDA receptors are tetramers, typically composed of NR1 and NR2 subunits (NR2A–D). NR2A and NR2B are expressed in the forebrain and are thought to assemble as diheteromers (NR1/NR2A, NR1/NR2B) and triheteromers (NR1/NR2A/NR2B). NR2A and NR2B contain cytosolic domains that regulate distinct postendocytic sorting events, with NR2A sorting predominantly into the degradation pathway, and NR2B preferentially trafficking through the recycling pathway. However, the interplay between these two subunits remains an open question. We have now developed a novel approach based on the dimeric feature of the α- and β-chains of the human major histocompatibility complex class II molecule. We created chimeras of α- and β-chains with the NR2A and NR2B C termini and evaluated endocytosis of dimers. Like chimeric proteins containing only a single NR2A or NR2B C-terminal domain, major histocompatibility complex class II-NR2A homodimers sort predominantly to late endosomes, whereas NR2B homodimers traffic to recycling endosomes. Interestingly, NR2A/NR2B heterodimers traffic preferentially through the recycling pathway, and NR2B is dominant in regulating dimer trafficking in both heterologous cells and neurons. In addition, the recycling of NR2B-containing NMDARs in wild-type neurons is not significantly different from NR2A−/− neurons. These data support a dominant role for NR2B in regulating the trafficking of triheteromeric NMDARs in vivo. Furthermore, our molecular approach allows for the direct and selective evaluation of dimeric assemblies and can be used to define dominant trafficking domains in other multisubunit protein complexes.
Keywords: Glutamate Receptors Ionotropic (AMPA, NMDA); MHC; Receptor Endocytosis; Sorting; Trafficking
Introduction
In the nervous system, trafficking of glutamate receptors to excitatory synapses is critical in regulating synaptic communication between neurons. In addition, changes in synaptic strength underlie many forms of synaptic plasticity, which are regulated, at least in part, by the dynamic expression of glutamate receptors on the postsynaptic membrane (1–3). Therefore, there has been intense interest in defining the molecular determinants within glutamate receptors that regulate their trafficking and synaptic expression. However, the immense heterogeneity of glutamate receptors complicates the study of endogenous glutamate receptor trafficking. For example, there are three subtypes of ionotropic glutamate receptors (NMDA,2 AMPA, and kainate), and each receptor subtype is composed of multiple subunits.
NMDA receptors (NMDARs) are tetramers composed of NR1, NR2 (NR2A-D), and NR3 (NR3A-B) subunits. NR1 is a single gene product expressed throughout the brain. In contrast, the four NR2 subunits are differentially distributed in the central nervous system and confer unique functional properties on endogenous NMDARs (4). In hippocampus and cortex, NMDARs are typically NR1/NR2B heteromers early in development, but the subunit composition is developmentally regulated with NR2A expression increasing over time (5, 6). In mature hippocampal and cortical neurons, both NR2A and NR2B are expressed, and NMDARs consist of a combination of NR1/NR2B, NR1/NR2A, and NR1/NR2A/NR2B heteromers (7).
The NR2 subunits contain divergent sequences that regulate unique protein-protein interactions and distinct receptor trafficking properties (8, 9). For example, the NR2A and NR2B intracellular C-terminal domains contain trafficking motifs that regulate NMDAR endocytosis and intracellular trafficking (10–13). However, the postendocytic itineraries of these NMDA receptor subunits are distinct. Studies in heterologous cells and neurons have shown that NR2A-containing receptors undergo endocytosis and lysosomal sorting for receptor degradation, whereas NR2B-containing receptors undergo endocytosis and preferentially recycle to the plasma membrane (14, 15). The subunit-specific postendocytic sorting is conserved when the NR2 C termini are appended to the simple, single transmembrane-spanning reporter protein Tac (interleukin 2 receptor), demonstrating that the NR2 intracellular domains contain the relevant sorting information and can function independently. However, it has not been possible to identify the postendocytic sorting itinerary of NMDARs containing a combination of both NR2A and NR2B subunits.
In the present study, we developed a novel strategy using the α-subunit and β-subunit of the major histocompatibility complex class II molecule (MHC II) as a reporter protein to study the contributions of individual NR2 subunit cytosolic domains in the intracellular trafficking of assembled dimers. By engineering chimeras between the α- and β-chains of MHC II with the NR2A and NR2B C-terminal domains, we find that the NR2B C terminus plays a dominant role in trafficking of NR2A/NR2B heterodimers in both heterologous cells and neurons. In support of this finding, we find that NR2B recycling does not differ significantly in NR2A knock-out mice (NR2A−/−), consistent with NR2B playing a central role in the trafficking of NR2A/NR2B-containing NMDARs in vivo.
EXPERIMENTAL PROCEDURES
DNA Constructs
The following cDNA constructs were obtained as gifts: green fluorescent protein (GFP)-Rab9 from Dr. Juan Bonifacino (NICHD, National Institutes of Health, Bethesda, MD), GFP-Rab11 from Dr. James Goldenring (Vanderbilt University, Nashville, TN), GFP-NR2B from Dr. Stefano Vicini (Georgetown University, Washington, D.C.). Tailless HLA-DRα (amino acids 1–239) and HLA-DRβ (amino acids 1–250) lacking the entire cytosolic domain were modified using PCR from the HLA-DRα and HLA-DRβ cDNAs that were previously described (16). Both NR2A (amino acids 1304–1464) and NR2B (amino acids 1315–1482) C termini were amplified by PCR and were subcloned into the truncated MHC II α- and β-chains in pcDNA3.1 using EcoRI restriction enzyme sites. The sequences of all chimeric constructs were confirmed by automated sequence analysis.
Antibodies
Mouse monoclonal antibodies that recognize the HLA-DRβ-chain alone (XD5.A11) (17) or only when dimerized with α-chain (L243) were used as tissue culture supernatants and have been described previously (18). Monoclonal rabbit anti-GFP antibody was obtained from Invitrogen (Eugene, OR). Unconjugated rabbit IgG and unconjugated mouse IgG antibody were obtained from Sigma.
Immunostaining for Surface Protein Expression in HeLa Cells
HeLa cells grown on glass coverslips were transfected with the cDNAs indicated (4 μg of DNA/well in a 6-well dish) using the Calcium Phosphate Transfection kit (Clontech) and analyzed 36 h later. Transfected cells were washed once with ice-cold PBS and labeled with anti-αβ-dimer antibody (L243) or anti-β-chain antibody (XD5.A11) on ice for 1 h. After three washes in ice-cold PBS, the cells were fixed in 4% paraformaldehyde in PBS for 15 min at room temperature. The cells were permeabilized in 0.25% Triton X-100 in PBS, blocked in 10% normal goat serum in PBS, and labeled with Alexa Fluor 568-conjugated anti-mouse IgG2A secondary antibody for 30 min at room temperature. After multiple washes in PBS, the coverslips were mounted (Prolong Antifade kit; Invitrogen), and cells were visualized using a 63 × objective on a Zeiss LSM510 confocal microscope (Carl Zeiss, Thornwood, NY). A series of optical sections was collected at intervals of 0.34 μm.
Immunofluorescent Internalization Assay in HeLa Cells
Transfected HeLa cells were incubated with anti-αβ-dimer antibody (L243) on ice for 1 h before cells were returned to 37 °C for the indicated amounts of time to allow internalization. The cells were washed and fixed in 4% paraformaldehyde in PBS for 15 min at room temperature before being permeabilized in 0.25% Triton X-100 in PBS for 5 min. After blocking in 10% normal goat serum, the cells were labeled with secondary antibodies, washed multiple times, and mounted on glass slides.
Recycling Assay in HeLa Cells
Transfected HeLa cells were incubated with anti-αβ-dimer (L243) antibody on ice for 1 h before incubation at 37 °C for 30 min to allow internalization. The cells were washed multiple times and labeled with excess unconjugated secondary antibody on ice for 1 h to “block” all surface receptors. Cells were incubated at 37 °C for 30 min to allow recycling or maintained on ice to prevent protein trafficking as a control. The cells were washed three times in PBS, fixed, and blocked before being incubated with Alexa Fluor 568-conjugated anti-mouse IgG2A secondary antibody for 30 min to label the surface receptors (red). Cells were permeabilized and blocked before being incubated with Alexa Fluor 488-conjugated anti-mouse IgG2A secondary antibody for 30 min to label internalized receptors that had not recycled back to the plasma membrane (green). The level of recycling was plotted as the ratio of the average fluorescence intensities of recycled receptors (red) versus the total of surface and internalized receptors (red+green) fluorescence intensities.
Recycling Assay in Cultured Primary Neurons
Transfected neurons were incubated with anti-GFP antibody at room temperature for 10 min before cells were returned to 37 °C for 30 min to allow internalization. The cells were gently washed three times and labeled with excess unconjugated secondary antibody for 20 min at room temperature to block all remaining surface receptors. Supplemental Fig. S1 shows that after the 20-min incubation with unconjugated secondary antibody, labeling of any surface-remaining receptors was blocked. Cells were incubated at 37 °C for 30 min to allow recycling or incubated at room temperature as a control. The cells were washed 3 times in PBS, fixed in 4% PFA with sucrose, blocked in 10% normal goat serum, and incubated with Alexa 568 secondary antibody for 30 min to label the surface receptors (red). Cells were then permeabilized, blocked, and incubated with Alexa Fluor 647-conjugated secondary antibody for 30 min to label internalized receptors that had not recycled back to the plasma membrane (green). The amount of recycled protein was plotted as the ratio of the average fluorescence intensities of recycled receptors (red) versus the total of surface and internalized receptors (red+green) fluorescence intensities.
RESULTS AND DISCUSSION
Use of MHC II α- and β-Chains as Reporter Molecules to Study Protein Dimerization and Trafficking
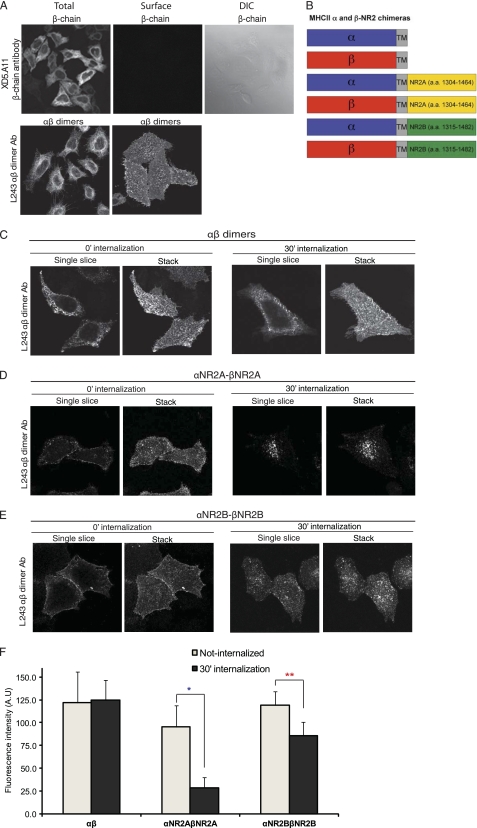
Tac has been used as a plasma membrane reporter molecule and has been instrumental in defining trafficking and sorting motifs encoded in various proteins (11, 13, 14, 19, 20). However, these studies have been limited by the fact that Tac is a monomer, thereby restricting analysis to monomeric receptors or unassembled proteins. Therefore, we have now developed a novel approach using MHC II proteins to study protein trafficking. MHC II is composed of α- and β-chains that must oligomerize to reach the plasma membrane. Furthermore, there are antibodies that specifically recognize an extracellular epitope on the fully assembled αβ-heterodimer (18). We generated several truncated, cytoplasmic tailless, MHC II α- and β-chains, and we found that when the MHC II β-chain was expressed alone, it was not expressed on the cell surface, whereas coexpression with the MHC II α-chain led to dimerization and the αβ-complexes were robustly expressed on the cell surface (Fig. 1A, surface/αβ-dimer). To evaluate endocytosis, HeLa cells expressing αβ-dimers were labeled on ice for 1 h with the MHC II αβ-heterodimer-specific antibody (L243) and returned to conditioned media for 30 min to allow internalization. We observed robust steady-state surface expression of tailless αβ-dimers (stack images) with negligible endocytosis (single-slice images) at both 0 and 30 min of internalization (Fig. 1C). Statistical analysis in Fig. 1F also indicates same level of αβ-dimer expression before and after internalization, statistically confirming that αβ-dimers do not degrade and are stably expressed on the cell surface.
FIGURE 1.
Tailless MHC II-αβ-dimers are stably expressed on the plasma membrane, whereas MHC II-αβ-NR2 chimeras undergo endocytosis. A, MHC II β-monomers are not expressed on the cell surface unless coexpressed and dimerized with MHC II α-chains. HeLa cells were transiently transfected with MHC II β-chain alone or with MHC II α-chain. For surface staining, live cells were labeled with anti-human MHC II β-chain antibody (XD5.A11) or anti-MHC II αβ-dimer (L243) antibody as described under “Experimental Procedures.” In contrast, total protein expression was visualized by labeling fixed cells with the β-chain antibody. B, schematic diagrams of the MHC II αβ-NMDA receptor (NR2 subunit) chimeras are shown. The distal C terminus of NR2A (amino acids 1304–1464) or NR2B (amino acids 1315–1482) was appended to the cytosolic domain of the MHC II α- and β-chains. C–E, αβ-dimers are robustly expressed on the cell surface at steady state at 30 min (C), whereas NR2A (D) and NR2B (E) homodimers undergo internalization. Cells expressing αβ, αNR2A-βNR2A, or αNR2B-βNR2B dimers were labeled on ice for 30 min before returning to conditioned media at 37 °C to allow internalization for 30 min. Cells were fixed, permeabilized, and labeled with anti-mouse Alexa Fluor 568-conjugated secondary antibody. Images were collected using a 63 × objective at intervals of 0.34 μm. F, αβ, αNR2A-βNR2A, and αNR2B-βNR2B dimer expression was quantified before and after 30 min of internalization in HeLa cells using National Institutes of Health ImageJ software (n = 11–28, n = 3). Data represent means ± S.E. (error bars) of the fluorescence intensities of αβ, αNR2A-βNR2A, and αNR2B-βNR2B dimers. Student's t test was performed; *, p < 0.001 relative to αNR2A-βNR2A expression in cells before internalization; **, p < 0.01 relative to αNR2B-βNR2B expression in cells before internalization.
αβ-NR2A or NR2B Homodimers Undergo Internalization
We generated chimeras of the truncated MHC II α- or β-chains and the distal NR2A or NR2B C-terminal domains (Fig. 1B). To analyze whether homomeric dimers of the NR2A or NR2B C termini drive endocytosis of surface-expressed MHC II, we expressed αNR2A-βNR2A or αNR2B-βNR2B in HeLa cells. Robust endocytosis was observed for both NR2A and NR2B homodimers. After 30 min of internalization, αNR2A-βNR2A homodimers exhibited a pronounced reduction in surface expression and distinct endosomal distribution mainly in the perinuclear region (Fig. 1D). In contrast, αNR2B-βNR2B homodimers showed pronounced residual surface expression, and they were also present in endosomes (Fig. 1E). Quantification of αNR2A-βNR2A and αNR2B-βNR2B homodimer expression before and after internalization in Fig. 1F reveals ∼75% reduction of NR2A homodimer expression after 30 min of internalization, whereas NR2B homodimer expression is only reduced ∼25% after internalization, confirming the distinct intracellular sorting pathways of NR2A and NR2B homodimers. These data are consistent with NR2A predominantly trafficking through the degradation pathway whereas NR2B preferentially sorts through recycling endosomes and traffics back to the cell surface. These data demonstrate that the C-terminal domains of both NR2A and NR2B drive endocytosis of homodimers and suggest that NR2A- and NR2B-containing homodimers undergo distinct postendocytic sorting events.
NR2A Homodimers Traffic to Late Endosomes, whereas NR2B Homodimers Preferentially Recycle to the Plasma Membrane
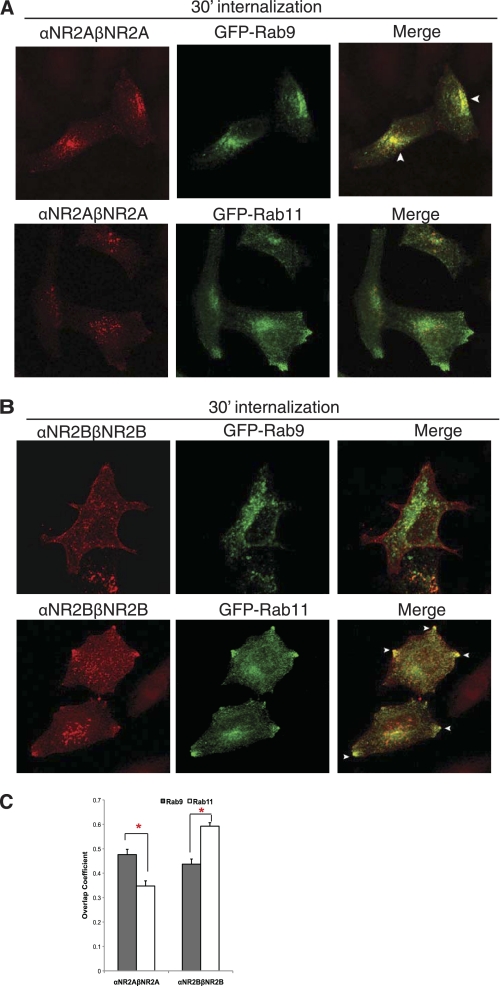
We next examined the postendocytic fate of homomeric αNR2A-βNR2A and αNR2B-βNR2B dimers using immunofluorescence microscopy. After 30 min of endocytosis, αNR2A-βNR2A was colocalized extensively with GFP-Rab9 in perinuclear late endosomes (Fig. 2, A and C), whereas αNR2B-βNR2B homodimers were colocalized with GFP-Rab11 in recycling endosomes at or near the cell membrane (Fig. 2, B and C). These results show that homomeric NR2A dimers preferentially traffic to late endosomes, whereas homomeric NR2B dimers preferentially traffic to recycling endosomes. This is consistent with previous studies showing that NR2B-containing NMDARs preferentially recycle, whereas NR2A-containing NMDARs do not (14, 15).
FIGURE 2.
αNR2A-βNR2A dimers traffic to late endosomes following endocytosis, whereas αNR2B-βNR2B dimers preferentially traffic through recycling endosomes. A, homomeric αNR2A-βNR2A dimers colocalize with the late endosomal marker GFP-Rab9 at 30 min of internalization. B, homomeric αNR2B-βNR2B dimers colocalize with the recycling endosomal marker GFP-Rab11. Cells expressing αNR2A-βNR2A or αNR2B-βNR2B together with GFP-Rab9 or GFP-Rab11 were labeled with MHC II αβ-dimer (L243) antibody on ice for 45 min and returned to conditioned media for 30 min at 37 °C. The cells were fixed and permeabilized, and the immunoreactivity was visualized with Alexa Fluor 568-conjugated (red) secondary antibody. Images were collected using a 63 × objective at intervals of 0.34 μm. Projection images are shown. Arrows indicate colocalization. C, statistical analysis of the amount of colocalization between αNR2A-βNR2A and αNR2B-βNR2B dimers with GFP-Rab9 or GFP-Rab11 after 30 min of internalization. Colocalization analysis was measured with the software NIH ImageJ Overlap coefficient plug-in. Values represent the mean ± S.E. (error bars) of overlap coefficient (n = 15–20, n = 4). Student's t test was performed; *, p < 0.01.
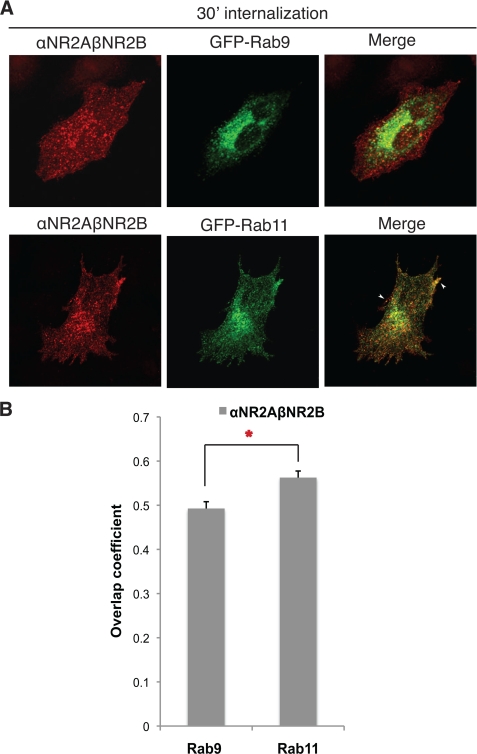
αNR2A-βNR2B Heterodimers Preferentially Sort to Recycling Endosomes and Recycle to the Plasma Membrane
It has not been possible to evaluate the trafficking itinerary of diheteromeric and triheteromeric NMDARs due to the lack of specific tools to monitor particular subpopulations of receptors. To determine the relative contribution of NR2B and NR2A in NMDAR trafficking, we next evaluated the postendocytic sorting itinerary of heteromeric αNR2A-βNR2B dimers. After 30 min of endocytosis, αNR2A-βNR2B heterodimers colocalized well with GFP-Rab11 and less well with GFP-Rab9 (Fig. 3, A, upper panel, and B). Interestingly, αNR2A-βNR2B dimers colocalized particularly well with GFP-Rab11 on the cell surface along the distal edges of the cell in a distribution that is typical of recycling endosomes (Fig. 3, A, lower panel, and B). The patterns of αNR2A-βNR2B heterodimer colocalization with GFP-tagged Rab9 and Rab11 are essentially indistinguishable from that of the αNR2B-βNR2B homodimers. It should be noted that the overlap coefficient of αNR2B-βNR2B homodimers (Fig. 2C) and αNR2A-βNR2B heterodimers (Fig. 3B) with Rab11 are not significantly different from each other (0.59 versus 0.56). Thus, the postendocytic recycling itinerary of NR2B is dominant over the lysosomal sorting of NR2A in heteromeric complexes.
FIGURE 3.
Heterodimers of αNR2A-βNR2B preferentially traffic through recycling endosomes. A, heteromeric αNR2A-βNR2B dimers preferentially sort to recycling endosomes. Cells expressing αNR2A-βNR2B and GFP-Rab9 or GFP-Rab11 were labeled with MHC II αβ-dimer (L243) antibody on ice for 45 min and returned to conditioned media for 30 min at 37 °C. The cells were fixed and permeabilized, and the immunoreactivity was visualized with Alexa Fluor 568-conjugated (red) secondary antibody. Images were collected using a 63 × objective at intervals of 0.34 μm. Projection images are shown. Arrows indicate colocalization. B, statistical analysis of the amount of colocalization between αNR2A-βNR2B dimers with GFP-Rab9 or GFP-Rab11. Colocalization analysis was measured with the software NIH ImageJ Overlap coefficient plug-in. Values represent the mean ± S.E. (error bars) of overlap coefficient (n = 17–25, n = 4). Student's t test was performed; *, p < 0.01.
To examine heterodimer recycling directly, we performed an antibody-based recycling assay. We expressed the MHC II-NMDAR chimeras in HeLa cells and monitored the amount of protein that was recycled over 30 min. We observed almost no recycling of homomeric αNR2A-βNR2A dimers (Fig. 4, A and E). By contrast, internalized homomeric αNR2B-βNR2B dimers, heteromeric αNR2A-βNR2B dimers, and heteromeric αNR2B-βNR2A dimers showed significant recycling back to the cell surface (Fig. 4, B–E). These results are consistent with our colocalization analyses demonstrating that NR2B-containing homodimers and heterodimers preferentially sort to recycling endosomes and reveal NR2B-dependent recycling of NR2 heteromers.
FIGURE 4.
Homomeric αNR2B-βNR2B dimers and heteromeric αNR2A-βNR2B or αNR2B-βNR2A dimers recycle to the plasma membrane. A, αNR2A-βNR2A dimers do not recycle to the plasma membrane efficiently. B–D, homomeric αNR2B-βNR2B dimers (B), as well as heteromeric αNR2A-βNR2B (C) and αNR2B-βNR2A (D) dimers undergo robust recycling. HeLa cells expressing αNR2A-βNR2A, αNR2B-βNR2B, αNR2A-βNR2B, or αNR2B-βNR2A were labeled with MHC II-αβ-dimer (L243) antibody on ice for 1 h and returned to conditioned media for 30 min at 37 °C. Remaining surface receptors were blocked with unconjugated secondary antibody. Cells were again incubated at 37 °C for 20 min, fixed, and blocked, and recycled receptors were labeled with Alexa Fluor 568-conjugated (red) secondary antibody. Cells were permeabilized, and internalized receptors were labeled with Alexa Fluor 488-conjugated secondary antibody. Images were collected at intervals of 0.34 μm, and projection images are shown. E, dimer recycling in HeLa cells was quantified with Metamorph software (n = 20, n = 4). Data represent means ± S.E. (error bars) of the recycled/internalized fluorescence intensities ratios. Student's t test was performed; *, p < 0.02 relative to 4 °C (CTL); **, p < 0.02 relative to recycled cells expressing αNR2A-βNR2A.
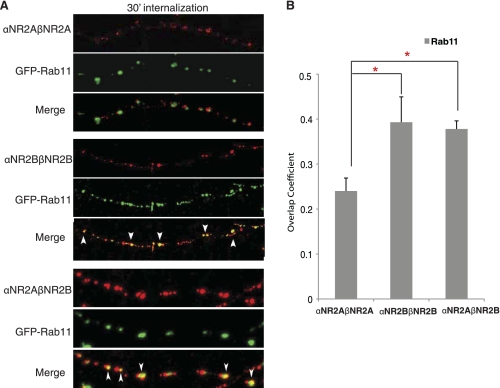
Differential Postendocytic Sorting of αNR2A-βNR2A, αNR2B-βNR2B, and αNR2A-βNR2B Dimers in Hippocampal Neurons
To determine whether these same rules govern the sorting of NR2A/NR2B homo- and heterodimers in neurons, we examined the postendocytic trafficking of αβ-NR2A/NR2B dimers in hippocampal neurons. We transfected dissociated hippocampal neurons with MHC II αNR2A-βNR2A, αNR2B-βNR2B, or αNR2A- βNR2B together with the recycling endosomal marker GFP-Rab11 and quantified colocalization between these proteins. After 30 min of internalization, αNR2B-βNR2B showed almost double the amount of colocalization with GFP-Rab11 as αNR2A-βNR2A (Fig. 5B), consistent with our findings in HeLa cells that NR2B homodimers prefer the recycling pathway compared with NR2A. Most importantly, trafficking of αNR2B-βNR2B homodimers and αNR2A-βNR2B heterodimers was indistinguishable in neurons, with each construct showing a high degree of overlap with the recycling endosomal marker GFP-Rab11 (Fig. 5, A and B). These data demonstrate that both homomeric αNR2B-βNR2B and heteromeric αNR2A-βNR2B dimers preferentially sorted to recycling endosomes in neurons and that the distal C terminus of NR2B is a dominant regulator of receptor trafficking in heterologous cells and neurons.
FIGURE 5.
Heteromeric αNR2A-βNR2B dimers preferentially traffic through recycling endosomes in hippocampal neurons. A, hippocampal neurons were transiently transfected at 9/10 days in vitro with αNR2A-βNR2A, αNR2B-βNR2B, or αNR2A-βNR2B together with GFP-Rab11. Five days after transfection, cells were labeled with anti-αβ-dimer antibody (L243) for 15 min at room temperature, antibody was removed, and neurons were returned to conditioned media and incubated at 37 °C for 30 min to allow internalization. Cells were fixed, permeabilized, and immunoreactivity was visualized with Alexa Fluor 568-conjugated (red) secondary antibody. Arrows indicate colocalization. B, statistical analysis of the amount of colocalization between various αβ-dimers and the endosomal marker GFP-Rab11 is shown. A series of optical sections was collected at 0.34 μm using a Zeiss LSM 510 confocal microscope. Colocalization analysis was measured with the software ImageJ Overlap coefficient plug-in. Values represent mean ± S.E. (error bars) of overlap coefficient (n = 14–20, n = 3–4). Student's t test was performed; *, p < 0.01.
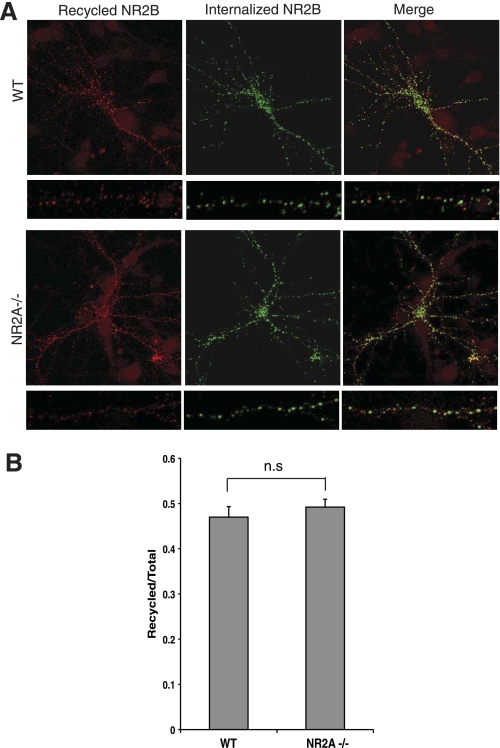
Recycling of NR2B Is Unaffected in NR2A−/− Hippocampal Neurons
The data presented thus far strongly suggest that in vivo heteromers containing NR2B will preferentially recycle at synapses. To demonstrate conclusively that the NR2B sorting motif is dominant for NMDAR trafficking, we analyzed NMDAR recycling in NR2A-deficient neurons. We expressed full-length NR2B containing an N-terminal GFP epitope tag in hippocampal neurons derived either from wild-type (WT) or NR2A knock-out mice (NR2A−/−) and examined NMDAR recycling. Quantitative analysis revealed that the amount of NR2B recycled back onto the cell surface in NR2A−/− neurons was not significantly different from that seen in WT neurons (Fig. 6). These data demonstrate that the trafficking of NR2B was not affected by NR2A expression and are consistent with our findings that NR2B contains dominant sorting signals that regulate postendocytic NMDAR trafficking.
FIGURE 6.
Recycling of internalized NR2B is not affected in NR2A−/− hippocampal neurons. A, levels of recycled NR2B-containing NMDA receptors were not significantly different in WT versus NR2A−/− neurons. Cultured hippocampal neurons (derived from WT or NR2A−/− mice) were transfected with GFP-NR2B at 11 days in vitro. After 3 days, neurons were incubated with anti-GFP antibody and incubated in conditioned media at 37 °C. Remaining GFP antibody-bound receptors on the surface were blocked by unconjugated secondary antibody. Neurons were incubated in conditioned media at 37 °C to allow recycling. Cells were fixed, and recycled receptors were labeled with Alexa Fluor 568-conjugated secondary antibody (red). Internalized receptors were visualized by labeling with Alexa Fluor 647-conjugated secondary antibody (green) after permeabilization. B, quantification of NR2B recycling in A is shown. Fluorescence intensities were measured using Metamorph software (n = 43–46 cells; n = 3 independent experiments). The ratios of recycled receptors (red) to total (red + green) of GFP-NR2B in WT and NR2A−/− neurons are presented. The error bars represent mean ± S.E.; n.s., nonspecific; p > 0.4, Student's t test.
NMDAR endocytosis is developmentally regulated in neurons such that NMDARs undergo robust endocytosis and rapidly recycle back to the postsynaptic membrane early in development (13, 15). At this stage of development, endogenous NMDARs contain NR2B, and the dynamic recycling of endogenous NMDARs early in development fits very well with the presence of dominant endocytic and recycling motifs in NR2B present in NMDAR heteromers. By contrast, later in development endogenous NMDARs become more stable on the plasma membrane. This stabilization corresponds with the increased expression of NR2A. Our data show that the NR2A cytosolic domain contains endocytic motifs, but the fate of internalized NR2A is degradation in lysosomes. It should be pointed out that the different extent of NMDAR endocytosis and recycling observed during development is not solely due to differences in NR2A/NR2B expression, as other factors such as expression of NMDAR-binding proteins or NR1-dependent regulation of NMDARs affect NMDAR trafficking and surface expression. Nevertheless, our novel use of the heterodimeric MHC II proteins as a reporter has clearly shown that the NR2B cytosolic domain can act as a dominant recycling signal and likely contributes to the developmental differences observed in NMDAR trafficking. Furthermore, we anticipate this approach will be a valuable tool to dissect out domains and motifs that are important in the trafficking of multimeric protein complexes.
Supplementary Material
Acknowledgments
We thank Drs. Juan Bonifacino, James Goldenring, and Stefano Vicini for GFP-Rab9, GFP-Rab11, and GFP-NR2B cDNAs, respectively. We thank the NINDS Light Imaging Facility, National Institutes of Health, in particular the faculty manager Dr. Carolyn Smith, for advice and expertise in the collection and analysis of confocal images. We acknowledge J. Nagle and D. Kauffman (NINDS Sequencing Facility) for automated DNA sequence analysis.
This work was supported by the Intramural Research Program of NINDS and NCI, National Institutes of Health.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- NMDA
- N-methyl-d-aspartate
- NMDAR
- NMDA receptor
- MHC II
- major histocompatibility complex class II
- GFP
- green fluorescent protein
- PBS
- phosphate-buffered saline
- WT
- wild type.
REFERENCES
- 1.Malinow R., Malenka R. C. (2002) Annu. Rev. Neurosci. 25, 103–126 [DOI] [PubMed] [Google Scholar]
- 2.Wenthold R. J., Prybylowski K., Standley S., Sans N., Petralia R. S. (2003) Annu. Rev. Pharmacol. Toxicol. 43, 335–358 [DOI] [PubMed] [Google Scholar]
- 3.Bredt D. S., Nicoll R. A. (2003) Neuron 40, 361–379 [DOI] [PubMed] [Google Scholar]
- 4.Cull-Candy S. G., Leszkiewicz D. N. (2004) Sci. STKE 2004, re16. [DOI] [PubMed] [Google Scholar]
- 5.Monyer H., Burnashev N., Laurie D. J., Sakmann B., Seeburg P. H. (1994) Neuron 12, 529–540 [DOI] [PubMed] [Google Scholar]
- 6.Sans N., Petralia R. S., Wang Y. X., Blahos J., 2nd, Hell J. W., Wenthold R. J. (2000) J. Neurosci. 20, 1260–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheng M., Cummings J., Roldan L. A., Jan Y. N., Jan L. Y. (1994) Nature 368, 144–147 [DOI] [PubMed] [Google Scholar]
- 8.Lau C. G., Zukin R. S. (2007) Nat. Rev. 8, 413–426 [DOI] [PubMed] [Google Scholar]
- 9.Chen B. S., Roche K. W. (2007) Neuropharmacology 53, 362–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavezzari G., McCallum J., Lee R., Roche K. W. (2003) Neuropharmacology 45, 729–737 [DOI] [PubMed] [Google Scholar]
- 11.Scott D. B., Michailidis I., Mu Y., Logothetis D., Ehlers M. D. (2004) J. Neurosci. 24, 7096–7109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barria A., Malinow R. (2005) Neuron 48, 289–301 [DOI] [PubMed] [Google Scholar]
- 13.Roche K. W., Standley S., McCallum J., Dune Ly C., Ehlers M. D., Wenthold R. J. (2001) Nat. Neurosci. 4, 794–802 [DOI] [PubMed] [Google Scholar]
- 14.Lavezzari G., McCallum J., Dewey C. M., Roche K. W. (2004) J. Neurosci. 24, 6383–6391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Washbourne P., Liu X. B., Jones E. G., McAllister A. K. (2004) J. Neurosci. 24, 8253–8264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinet V., Vergelli M., Martin R., Bakke O., Long E. O. (1995) Nature 375, 603–606 [DOI] [PubMed] [Google Scholar]
- 17.Radka S. F., Machamer C. E., Cresswell P. (1984) Hum. Immunol. 10, 177–186 [DOI] [PubMed] [Google Scholar]
- 18.Lampson L. A., Levy R. (1980) J. Immunol. 125, 293–299 [PubMed] [Google Scholar]
- 19.Hawkins L. M., Prybylowski K., Chang K., Moussan C., Stephenson F. A., Wenthold R. J. (2004) J. Biol. Chem. 279, 28903–28910 [DOI] [PubMed] [Google Scholar]
- 20.Standley S., Roche K. W., McCallum J., Sans N., Wenthold R. J. (2000) Neuron 28, 887–898 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.