Abstract
Ca2+/calmodulin (CaM)-dependent protein kinase II (CaMKII) is a major mediator of physiological glutamate signaling involved in higher brain functions. Here, we show CaMKII involvement in pathological glutamate signaling relevant in stroke. The novel inhibitor tatCN21 was neuroprotective even when added hours after glutamate insults. By contrast, the “traditional” inhibitor KN93 attenuated excitotoxicity only when present during the insult. Both inhibitors efficiently blocked Ca2+/CaM-stimulated CaMKII activity, CaMKII interaction with NR2B and aggregation of CaMKII holoenzymes. However, only tatCN21 but not KN93 blocked the Ca2+-independent “autonomous” activity generated by Thr-286 autophosphorylation, the hallmark feature of CaMKII regulation. Mutational analysis further validated autonomous CaMKII activity as the drug target crucial for post-insult neuroprotection. Overexpression of CaMKII wild type but not the autonomy-deficient T286A mutant significantly increased glutamate-induced neuronal death. Maybe most importantly, tatCN21 also significantly reduced infarct size in a mouse stroke model (middle cerebral arterial occlusion) when injected (1 mg/kg intravenously) 1 h after onset of arterial occlusion. Together, these data demonstrate that inhibition of autonomous CaMKII activity provides a promising therapeutic avenue for post-insult neuro-protection after stroke.
Keywords: Calcium, Calcium Calmodulin-dependent Protein Kinase (CaMK), Cell Death, Neuron, Neurotransmitters
Introduction
Glutamate is the most abundant excitatory neurotransmitter in the mammalian brain. However, excessive glutamate release causes Ca2+-dependent excitotoxic neuronal death in pathological situations such as focal cerebral ischemia (stroke) (for review see Refs. 1–4). Most glutamate receptors are involved in excitotoxicity, but especially important are the Ca2+-permeable ionotropic receptors such as the NMDA4-type glutamate receptor (NMDAR) (5–8). Extensive attempts to develop a stroke therapy by targeting glutamate receptors have resulted in disappointment (for review see Refs. 9, 10), suggesting that alternative strategies will be necessary. Currently, the only approved pharmacological treatment of stroke patients is hemolytic therapy with tissue plasminogen activator. However, less than 2% of patients actually receive tissue plasminogen activator. Although tissue plasminogen activator is effective in stroke caused by blood clots, it is actually contraindicated in hemorrhagic stroke, and diagnostic evaluation pushes most patients beyond the therapeutically effective time window (11–13).
The Ca2+/calmodulin (Ca2+/CaM)-dependent protein kinase II (CaMKII) is a major physiological downstream target of glutamate-induced Ca2+ signaling (for review see Refs. 14–17) and was examined in this study for involvement in pathological excitotoxic glutamate signaling. CaMKII is highly expressed in brain where it participates in NMDAR-dependent long term potentiation and learning and memory (14–17). CaMKII forms multimeric holoenzymes, and each kinase subunit is activated separately by Ca2+/CaM. An inter-subunit autophosphorylation at Thr-286 renders the kinase “autonomous” that is active even after Ca2+/CaM dissociates. This autonomy is thought to be a form of molecular memory and is indeed important in learning and memory (14–18). The role of CaMKII in pathological glutamate signaling is unclear, as previous reports have indicated that CaMKII may either promote (19–22) or attenuate (23–26) neuronal cell death. A possible reason for the apparent contradictions was that excitotoxic glutamate affects not only CaMKII activity but also its protein interactions and subcellular localization, such as binding to the NMDAR and translocation to postsynaptic sites (27–30), and self-aggregation and extrasynaptic clustering (31–34). These glutamate effects on CaMKII may modulate neuron survival following excitotoxic insult in different ways. Another possible reason for the apparent contradictions in previous studies was the use of inhibitors now known to also target non-CaMKII proteins. KN93, the most commonly used CaMKII inhibitor, also inhibits CaMKI and CaMKIV (35), as well as voltage-gated Ca2+ and K+ channels (36, 37). CaMKII inhibitors derived from the autoinhibitory region, such as AC3-I and AIP, also inhibit other protein kinases such as myosin light chain kinase (MLCK), PKA, and PKD (38–40), and some studies indicated a low potency of CaMKII inhibition (IC50 ∼30 μm) (41). Highly specific inhibitors are now available with CN peptides such tatCN21 (42), which are derived from the natural CaMKII inhibitory protein CaM-KIIN (43).
Here, we demonstrated a role for CaMKII in glutamate excitotoxicity by several lines of evidence. Remarkably, the CaMKII inhibitor tatCN21 was neuroprotective also when applied hours after a glutamate insult in vitro or in a mouse stroke model. Biochemical and mutational analysis identified specifically the “autonomous” form of CaMKII activity as the relevant drug target for post-insult neuroprotection.
EXPERIMENTAL PROCEDURES
Materials
CaMKIIα and CaM were isolated and purified as described previously (29, 30, 42). Reagents were obtained from Sigma, except for the following: inhibitor peptides and controls (Biomatix, Wilmington, DE, and Global Peptides, Fort Collins, CO); neuron culture supplies, ethidium homodimer 2 (EtDH2), Hoechst 33258, and Lipofectamine 2000 (Invitrogen); KN93, KN92, and STO-609 (Calbiochem); d-APV (Tocris Bioscience, St. Louis, MO); paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA); lactate dehydrogenase assay (LDH) kit (Roche Applied Science); antibodies against MAP2 (Pharmingen); total CaMKIIα (CBα2; Invitrogen); and phospho-Thr-286 (PhosphoSolutions, Aurora, CO). The sequences of tatCN21 and the reverse sequence control tatRev were described previously (42); the additional control peptide tatCtrl was a fusion of the tat sequence to a scrambled CN21 sequence (VKEPRIDGKPVRLRGQKSDRI).
Cell Culture and Transfection
Medium density primary disassociated hippocampal or cortical neuron cultures were prepared from newborn Sprague-Dawley rats, plated onto poly-d-lysine-coated 24-well dishes (≈40,000–50,000/well), and maintained at 37 °C, 5% CO2 in Neurobasal A media with B-27 supplement, 50 units/ml penicillin/streptomycin, 2 mm Glutamax. Glial growth was inhibited with 70 μm 5-fluoro-2′-deoxyuridine and 140 μm uridine. Neurons were transfected by electroporation (AMAXA Biosystems, Basel, Switzerland) prior to plating or at 7 or 10 days in vitro (DIV) with Lipofectamine 2000, as described previously (29, 30, 42). Cells were cultured with 100 μm d-APV following transfection with CaMKII constructs. APV was washed from cells prior to inducing cell death.
Neuron Death Assays
7 DIV hippocampal neurons (unless indicated otherwise) were generally insulted with 400 μm glutamate (or 300 μm NMDA) for 5 min (which leads to ∼80% neuronal death; see Fig. 1B). 200 μm glutamate was used when induction of submaximal death was desired (such as in overexpression experiments); 100 μm glutamate was used in older neurons, which are more sensitive. Thus, all insults were done in the generally used glutamate range of 50–500 μm (44–46). Following glutamate treatment, medium was exchanged, and neurons were returned to 37 °C and 5% CO2 for 20–24 h. Cell death was then assessed using an LDH assay kit or by staining with 1 μm EtDH2 (10 min). Cells were fixed in 4% paraformaldehyde and stained with 10 ng/μl Hoechst 33258 for 30 min.
FIGURE 1.
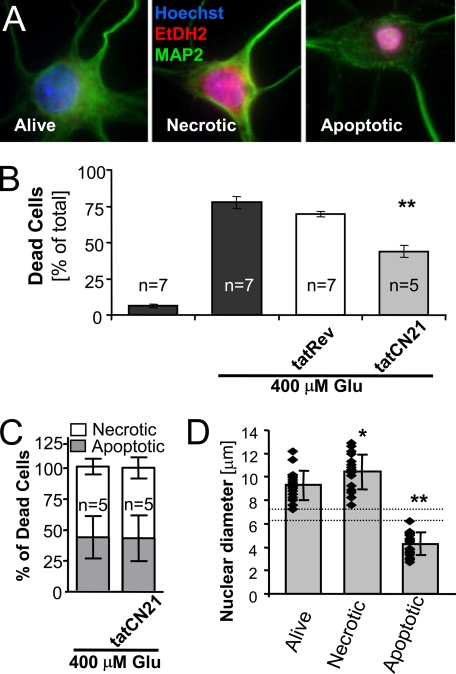
CaMKII inhibition reduced excitotoxic neuron death assessed by cell staining. A, EtDH2 stained necrotic and late stage apoptotic nuclei but was excluded from the nuclei of healthy cells. Hoechst counter-stained all nuclei; anti-MAP2 immunocytochemistry and morphology identified neurons. B, live/dead cell counting revealed that 400 μm glutamate induced death in ∼75% of neurons, which was significantly reduced by 5 μm tatCN21 (**, p < 0.001 in Dunnett's t test ANOVA post hoc analysis) but not by the control peptide tatRev. C, although tatCN21 reduced total cell death after glutamate treatment (see B), it did not change the ratio of apoptotic to necrotic cells. D, neurons subjectively classified as apoptotic versus necrotic by morphology (see A) have distinct, nonoverlapping nuclear diameters (** indicates p < 0.001 in Dunnett's t test ANOVA post hoc analysis), as expected based on nuclear condensation during apoptosis. Compared with healthy neurons, nuclear diameter of necrotic neurons was slightly but significantly increased (* indicates p < 0.05 in analysis as above), consistent with mild nuclear swelling. Individual data points (n = 18) and the mean are indicated. Error bars show standard deviation.
Neurons were incubated with 5 μm tatCN21 (or control peptide), 10 μm KN93, 10 μm KN92 (Calbiochem), 100 μm d-APV, or 5 μm STO-609 either 20 min prior to glutamate treatment or different times after glutamate insult, as indicated. Results were normalized by setting untreated conditions to zero and glutamate treated to one, unless indicated otherwise. Significance was determined as indicated, generally using a one-way ANOVA followed by either Dunnett's (to compare multiple treatments to control) or Newman-Keule's multiple comparison test (to compare among multiple treatments).
CaMKII Activity Assays
CaMKIIα (20 nm subunit concentration) activity was assessed in 50 mm PIPES, pH 7.2, 0.1 mg/ml bovine serum albumin, 10 mm MgCl2, 100 μm [γ-32P]ATP (∼1 Ci/mmol), 50 μm AC2 substrate, and 1 mm, 1 μm Ca2+/CaM (stimulated) or 0.5 mm EGTA (autonomous) for 1 min at 30 °C, essentially as described previously (42). For measurement of autonomy, CaMKII (100 nm) was pre-phosphorylated as in the stimulated conditions, but for 5 min on ice and without AC2 and radioactivity. CaMKII autophosphorylation at Thr-286 was verified by Western blot as described previously (29, 42, 47).
CaMKII Binding to NR2B-c
To assess the effect of inhibitors on CaMKII binding to the NR2B subunit, GST-NR2B-c was immobilized on an anti-glutathione S-transferase-coated microtiter plate, as described previously (29, 30, 42). 100 nm CaMKIIα, 0.5 mm Ca2+, and 1.5 μm CaM was added together with the indicated concentrations of inhibitor. After washing, the bound protein was disassociated from the plates by boiling in SDS loading buffer. Eluted CaMKII was detected by Western blot as described previously (29, 30, 42).
CaMKII Self-association Assays
For standard assays, purified 0.5 μm CaMKIIα was combined with 25 μm PIPES, pH 6.5, 20 mm KCl, 10 mm MgCl2, 0.1 mg/ml bovine serum albumin, 0.1% Tween 20, 0.5 mm dithiothreitol, 0.5 mm CaCl2, 2.0 μm CaM, and 10 μm ATP. Where indicated, 1 μm tatCN21 or 2 μm KN93 was included. The mixtures were prepared on ice and then incubated for 5 min at room temperature prior to centrifugation at 4 °C for 30 min (16,000 × g). CaMKII in the supernatant and pellet was detected by Western blot as described previously (29, 30, 42).
Mouse MCAO Stroke Model
Focal ischemia was induced in male C57BL/6J mice by occluding the anterior communicating and middle cerebral arteries for 1 h while measuring physiological parameters (heart rate and arterial blood pressure, pH levels, PaCO2, and PaO2), as described previously (48–50). 24 h later, infarct size was assessed by cutting 1-mm-thick coronal sections and staining them with 2% triphenyltetrazolium chloride, which stains live tissue red and leaves dead tissue white. Sections were imaged using an Olympus microscope and photographed with a Hitachi CCD camera, and infarct volume was calculated based on area and slice thickness.
RESULTS
tatCN21 Protects Neurons from Excitotoxic Death
The peptide tatCN21 is a new, highly selective and cell-penetrating inhibitor of CaMKII and thus is an excellent tool to clarify if CaMKII stimulation promotes or reduces excitotoxic death. Therefore, the effect of tatCN21 (5 μm, ∼50-fold IC50) on glutamate-induced neuron death was measured in hippocampal neurons cultured for 7 DIV. Neurons were insulted with glutamate for 5 min, in the presence or absence of tatCN21. 20–24 h later, nuclei of dead neurons were stained with EtHD2, a dye that is excluded from live cells. Nuclei of all cells were counter-stained using Hoechst 33258 or 4′,6-diamidino-2-phenylindole, and neurons were identified by morphology and/or anti-MAP2 immunocytochemistry. Necrotic and apoptotic neurons were distinguished by nuclear condensation (Fig. 1A); additionally, apoptotic neurons showed reduced and irregular MAP2 staining (Fig. 1A), consistent with previous observations (51–54). This approach revealed that tatCN21 significantly reduced glutamate-induced neuronal death, indicating that CaMKII stimulation promotes excitotoxicity (Fig. 1B). Although reducing overall death, tatCN21 did not affect the ratio of neurons with necrotic versus apoptotic appearance after a glutamate insult, suggesting that CaMKII promotes neuron death both with apoptotic and necrotic morphology (Fig. 1C). The decision to classify a neuron as necrotic or apoptotic was made subjectively; however, post hoc measurements of their nuclear diameters highlights that there is a clear objective distinction (Fig. 1D).
Further Pharmacological Evidence That CaMKII but Not CaMKI or CaMKIV Promotes NMDAR-mediated Excitotoxicity
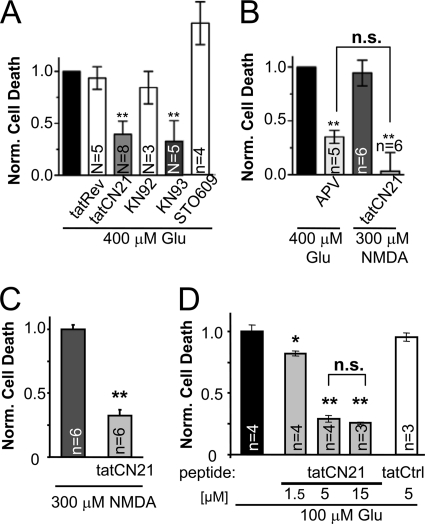
The tatCN21 peptide, but not the reverse sequence control peptide tatRev, showed the same neuroprotective effect in another assay of cell death, which measures release of LDH from dead and dying cells (Fig. 2A). This LDH assay allows rapid quantification of death in large populations of cells. Thus, we decided to also test other inhibitors of CaMK activity as follows: KN93, an inhibitor of CaMKI, CaMKII, and CaMKIV; KN92, the inactive control substance; and STO-609, an inhibitor of CaMKK, a CaM-dependent upstream kinase required for full activation of CaMKI and CaMKIV but not CaMKII. Neuroprotection was seen for KN93 but not KN92 or STO-609 (Fig. 2A), further supporting the conclusion that CaMKII but not CaMKI or CaMKIV promotes excitotoxic death.
FIGURE 2.
CaMKII inhibition reduced excitotoxic neuron death assessed by LDH release. A, CaMKII inhibition by 5 μm tatCN21 or 10 μm KN93 reduced glutamate-induced death also when measured by LDH assay (** indicates p < 0.001 in Dunnett's t test ANOVA post hoc analysis). The controls tatRev and KN92 had no effect, whereas the CaMKI and IV inhibitor STO-609 (5 μm) increased cell death (p < 0.05 in analysis as above). B, glutamate-induced neuron death was significantly reduced by the NMDAR inhibitor d-APV (100 μm), and NMDA-induced neuron death was significantly inhibited by tatCN21 (5 μm) (** indicates p < 0.001 compared with glutamate in Newman-Keuls multiple comparison test after ANOVA; n.s. indicates that there was no difference between these two conditions). C, tatCN21 inhibited NMDA-induced death in an additional independent experiment (p < 0.001 in Student's t test), similar to what was observed for glutamate-induced death in A. D, CaMKII inhibition by 5 and 15 μm tatCN21 showed significant neuroprotection (**, p < 0.001) that was indistinguishable between the two concentrations (n.s.), whereas 1.5 μm tatCN21 also significant neuroprotection but to a much lesser extent (*, p < 0.01; all in Newman-Keuls multiple comparison test after ANOVA). Thus, 5 μm tatCN21 is sufficient to elicit maximal neuroprotection levels. In all panels, results shown are mean ± S.E.; N indicates the number of independent experiments; n indicates the number of coverslips.
Overstimulation of most glutamate receptors can lead to cell death, but the NMDAR appears to be the most sensitive death-mediating receptor in excitotoxicity (1–5). Indeed, the NMDAR inhibitor APV significantly reduced glutamate-induced neuron death in our assay, as expected (Fig. 2B). Additionally, direct receptor overstimulation by the agonist NMDA induced a similar level of cell death as the glutamate insult. More importantly, tatCN21 was neuroprotective also after NMDA treatment, in two independent experiments (Fig. 2, B and C).
The tatCN21 concentration used in the excitotoxicity experiments (5 μm) is ∼100-fold IC50 of CaMKII inhibition and is thus a reasonable pharmacological dose. Indeed, the maximal neuroprotective effect of tatCN21 was reached at 5 μm, as neuroprotection was not further increased by a 3-fold higher concentration (Fig. 2D).
Overexpression of CaMKIIα Increases Neuron Death
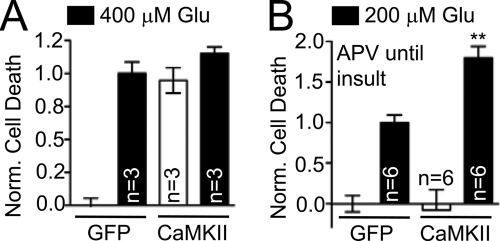
The involvement of CaMKII in neuronal cell death, which was strongly indicated by the pharmacological data, was further tested by a molecular biology approach. AMAXA electroporation yielded a transfection rate of >30% in 7 DIV hippocampal neurons, allowing assessment of the cell death by LDH assays. Overexpression of CaMKIIα significantly increased basal level of neuronal cell death (without glutamate insult), to a degree that could not be significantly further increased by an insult with 400 μm glutamate (Fig. 3A) (suggesting that prolonged glutamate released from dying transfected neurons caused death also of the untransfected neurons in the dish, to a similar extent as the 5-min glutamate bath application). Incubation of the CaMKII-expressing cultures with APV immediately after transfection reduced neuronal cell death back to near basal levels (Fig. 3B), indicating that the CaMKII-mediated basal death required spontaneous activation of NMDAR in the cultures. When neurons that were cultured in presence of APV were then insulted with 200 μm glutamate (after removal of the APV), excitotoxic cell death was significantly higher in the CaMKII-expressing cultures than in green fluorescent protein expressing controls (Fig. 3B). Taken together, the pharmacological and molecular data strongly support that CaMKII activation promotes neuronal cell death in excitotoxicity.
FIGURE 3.
Overexpression of mGFP-CaMKIIα increased basal and glutamate-induced neuron death. A, overexpression of mGFP-CaMKIIα in 7 DIV hippocampal neurons substantially increased basal cell death (p < 0.001), but 400 μm glutamate did not cause a further increase (p > 0.05 in Newman-Keuls multiple comparison test after ANOVA). B, in mGFP-CaMKIIα overexpressing neurons, basal cell death was reduced to normal levels by culturing cells with the NMDAR inhibitor d-APV. However, glutamate-induced cell death was significantly increased (d-APV was washed from the cells prior to glutamate treatment) (**, p < 0.001 in Newman-Keuls multiple comparison test after ANOVA). Results shown are mean ± S.E. in all panels.
tatCN21 but Not KN93 Protects against Excitotoxicity When Added Post-insult
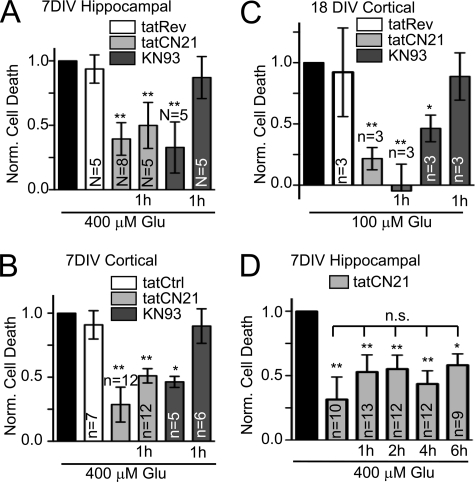
Remarkably, tatCN21 protected from excitotoxic neuronal cell death even when added 1 h after the insult (Fig. 4, A–C), which is within a clinically relevant window of therapeutic opportunity. The same post-insult neuroprotection by tatCN21 was seen in 7 DIV hippocampal neurons (Fig. 4A), 7 DIV cortical neurons (Fig. 4B), and 18 DIV cortical neurons (Fig. 4C). By contrast, the traditional CaMK inhibitor KN93 was neuroprotective only when present during the insult but not when added 1 h later (Fig. 4, A–C). This further supports involvement of CaMKII and shows that some but not all CaMKII inhibitors are protective in a clinically relevant time frame.
FIGURE 4.
Post-insult neuroprotection by tatCN21 but not KN93. A, 7 DIV hippocampal neurons. B, 7 DIV cortical neurons; C, 18 DIV cortical neurons, tatCN21 (5 μm) significantly reduced glutamate-induced cell death when added during the insult and when added 1 hour after insult. By contrast, KN93 (10 μm) only reduced cell death when added during the insult. (**, p < 0.01; *, p < 0.05; compared with glutamate treatment without inhibitors, in Dunnett's t test ANOVA post hoc analysis.) D, tatCN21 significantly reduced glutamate-induced neuron death even when added up to 6 h after insult (**, p < 0.01; *, p < 0.05). There was no statistically significant difference between the different times of tatCN21 treatment (n.s. (not significant) p > 0.05; all in Newman-Keuls multiple comparison test after ANOVA). In all panels, results shown are mean ± S.E., and N (number of independent experiments) or n (number of independent wells, from at least two independent experiments, except for C) are indicated.
To investigate the time window of post-insult neuroprotective potential, tatCN21 was also added at later time points (Fig. 4D). tatCN21 showed significant neuroprotection even when added up to 6 h after the glutamate insult (Fig. 4D).
tatCN21 but Not KN93 Inhibits Autonomous CaMKII
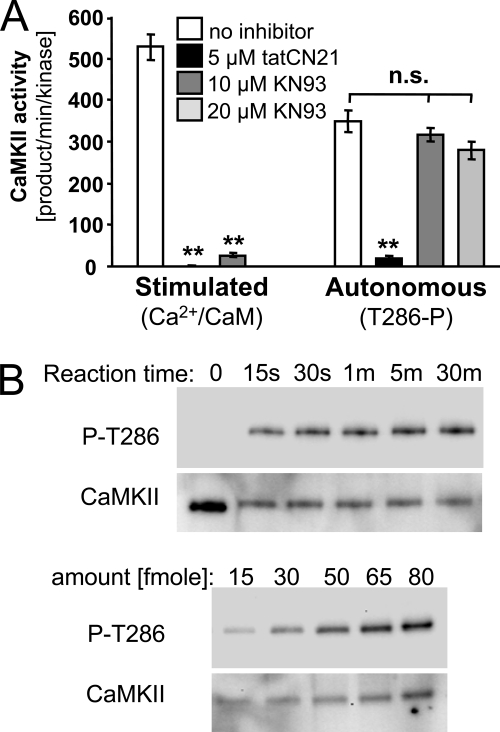
Why does one CaMKII inhibitor (tatCN21) result in post-insult neuroprotection whereas another one (KN93) does not? To answer this question, we compared the effects of the two inhibitors on several changes in CaMKII activity and protein interactions that can be induced be Ca2+ signals generated by excitotoxic levels of glutamate. Both tatCN21 and KN93 efficiently inhibited Ca2+/CaM-stimulated activity of CaMKII (Fig. 5A). However, only tatCN21 but not KN93 blocked Ca2+-independent autonomous CaMKII activity (Fig. 5A). For generating autonomous activity, CaMKII Thr-286 autophosphorylation was first stimulated by Ca2+/CaM (and verified by Western blot; Fig. 5B); autonomous activity was then measured after chelating Ca2+ with EGTA. Thus, when present during a glutamate insult, KN93 would prevent CaMKII stimulation and generation of autonomous activity. However, once autonomous CaMKII activity is generated, it can be inhibited only by tatCN21 but not KN93, providing an explanation for the post-insult protection seen only for tatCN21 but not KN93.
FIGURE 5.
tatCN21 but not KN93 inhibits autonomous CaMKII activity. A, tatCN21 (5 μm) but not KN93 (10 μm or 20 μm) inhibited Ca2+-independent autonomous CaMKII activity (after 5 min Thr-286 autophosphorylation on ice) in a cell-free assay using purified CaMKIIα and measuring phosphorylation of AC2 peptide substrate for 1 min at 30 °C. By contrast, both inhibitors efficiently inhibited Ca2+/CaM-stimulated CaMKII activity (**, p < 0.001 Dunnett's t test ANOVA post hoc analysis; n.s. (not significant) indicates no statistical difference, with p > 0.07 in ANOVA). Error bars indicate S.E. B, effective Thr-286 autophosphorylation on ice was verified by Western blot analysis with a phospho-Thr-286-specific antibody (P-T286) after different reactions times, indicating maximal phosphorylation even after 30 s (upper panels). Total CaMKII was stained as control. Loading different amounts of CaMKII after 10 min autophosphorylation showed that the detection was in the linear range (lower panels, but from same blot and exposures as the upper panels).
Both tatCN21 and KN93 Inhibit NR2B-binding and Aggregation of CaMKII
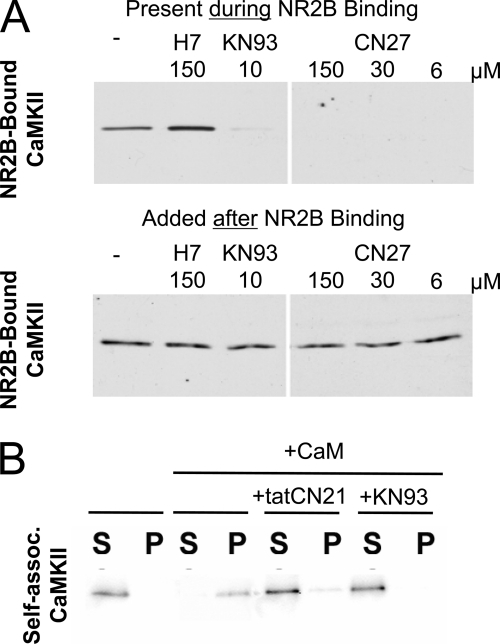
Physiological glutamate signals can induce translocation of CaMKII to postsynaptic sites, a process likely mediated by regulated binding to the NMDAR subunit NR2B (27–30). In biochemical assays, KN93 as well as tatCN21 and other related CN peptides (such as CN27) prevented Ca2+/CaM-induced binding of purified CaMKIIα to the cytoplasmic C terminus of NR2B immobilized on 96-well plates (Fig. 6A) (42). By contrast, the general kinase inhibitor H7 did not inhibit this interaction (Fig. 6A). When CaMKII was allowed to bind to NR2B first, none of the inhibitors caused any measurable dissociation of the bound kinase (Fig. 6A). Thus, both CaMKII inhibitor classes had the same effect on interaction with NR2B in vitro, and none of them affected the interaction once it was formed.
FIGURE 6.
CN inhibitor peptides and KN93 inhibited CaMKII binding to NR2B and aggregation of holoenzymes. A, binding of purified CaMKIIα to immobilized GST-NR2Bc was inhibited by both KN93 and CN27, as has been previously shown for tatCN21. By contrast, the general kinase inhibitor H7 did inhibit binding, and none of the inhibitors reversed the CaMKII binding to NR2Bc when added after the binding had already been established. NR2Bc-bound CaMKII was detected by Western blot. B, tatCN21 and KN93 both prevented formation of sedimentable CaMKII aggregates that are induced in vitro by Ca2+/CaM at low pH. Shown are Western blot analysis of centrifugation supernatants (S) and pellets (P).
Excitotoxic glutamate and ischemic conditions can induce formation of extra-synaptic CaMKII clusters, a process likely mediated by the aggregation of multiple CaMKII holoenzymes (31–34). Indeed, mimicking ischemic conditions in a test tube (pH <6.8, low ATP, and addition of Ca2+/CaM) triggered precipitation of purified CaMKII holoenzymes into aggregates that can be pelleted by centrifugation (Fig. 6B). Addition of either KN93 or tatCN21 inhibited formation of these precipitates (Fig. 6B). This tatCN21 effect further supports the proposed model of inter-holoenzyme T-site/Thr-286 region interaction as underlying mechanism for holoenzyme aggregation (34, 55), as tatCN21 acts by T-site binding (42). Inhibitor effects on pre-formed aggregates were not tested, because such CaMKII aggregates are quickly reversible even without any inhibitor within cells (32, 33). Thus, taken together, post-insult neuroprotection by tatCN21 cannot be explained by effects on CaMKII localization mediated by NR2B binding or self-aggregation.
Significantly Increased Glutamate-induced Death by Overexpression of CaMKIIα Wild Type but Not the Autonomy-deficient T286A Mutant
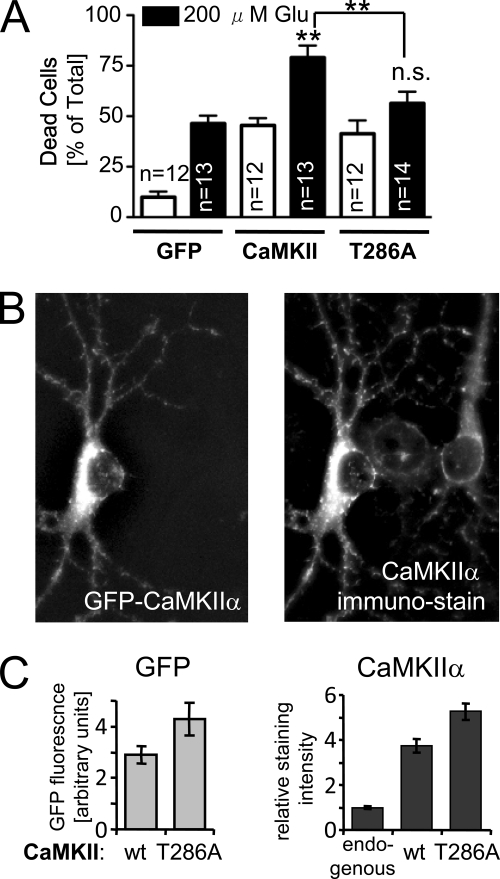
Comparing the biochemical effects of tatCN21 with KN93 strongly indicated autonomous CaMKII activity as the drug target important in post-insult neuroprotection. Thus, the effect of CaMKII autonomy on excitotoxicity was further elucidated using the autonomy-deficient CaMKII T286A mutant. Cell death of transfected 7 DIV hippocampal neurons was assessed by EtDH2 staining (compare Fig. 1A). Overexpression of CaMKII wild type and the T286A mutant enhanced basal neuronal cell death to a similar degree (Fig. 7A). However, a 5-min insult with 200 μm glutamate increased cell death significantly more in neurons expressing CaMKII wild type than in neurons expressing the T286A mutant (Fig. 7A). Thus, although excessive stimulated CaMKII activity appears sufficient to increase cell death induced by continuing basal activation, autonomous CaMKII activity is required for the massive increase in neuronal death after a single strong but brief insult.
FIGURE 7.
Mutational analysis further implicated a role for CaMKII autonomy in excitotoxic neuronal cell death. A, overexpression of mGFP-CaMKIIα wild type but not the autonomy-deficient T286A mutant significantly increased glutamate-induced neuron death (**, p < 0.001; n.s. (not significant) p > 0.05 in Newman-Keuls multiple comparison test after ANOVA, compared with GFP, if not indicated otherwise). Basal cell death was increased by both mGFP-CaMKIIα wild type and T286A mutant (based on analysis as above). Two-way ANOVA indicated significant differences for glutamate treatment, transfection condition (p < 0.001), and their interaction (p < 0.05), with Bonferroni post hoc analysis indicating that glutamate increased cell death for each transfection condition. Transfected cells were cultured with d-APV until the glutamate treatment; cell death was assessed by live/dead cell counting. The number of individual coverslips (n) analyzed in four independent experiments is indicated. Results are shown as mean ± S.E. B, mGFP-CaMKIIα expression in transfected neurons (identified by GFP fluorescence of mGFP-CaMKIIα; left panel) increases the level of total CaMKIIα over untransfected neurons (as assessed by immunostaining with the anti-CaMKIIα antibody CBα2; right panel). C, quantification of experiments as shown in B showed that mGFP-CaMKIIα wild type (wt) was expressed at slightly lower levels than the T286A mutant (p < 0.048, t test) and that total CaMKIIα immunostaining intensities were ∼4–5-fold higher in transfected neurons (wild type or T286A) than in untransfected neurons (endogenous).
Expression levels of CaMKIIα wild type and the T286A mutant were compared by quantifying green fluorescent protein fluorescence in the transfected neurons (Fig. 7, B and C). The T286A mutant was expressed at slightly higher levels than CaMKII wild type (Fig. 7C), excluding the possibility that the different effect on excitotoxic death was caused by inefficient expression of the T286A mutant. Immunostaining of total CaMKIIα indicated ∼4–5-fold overexpression over endogenous CaMKIIα (Fig. 7, B and C). Note that CaMKII expression starts already at birth, but is restricted to hippocampal neurons (56), and that α is the prominent CaMKII isoform already at 7 DIV, a time when its expression level reaches almost half of maximum (57).
tatCN21 Is Neuroprotective in a Mouse Model of Stroke
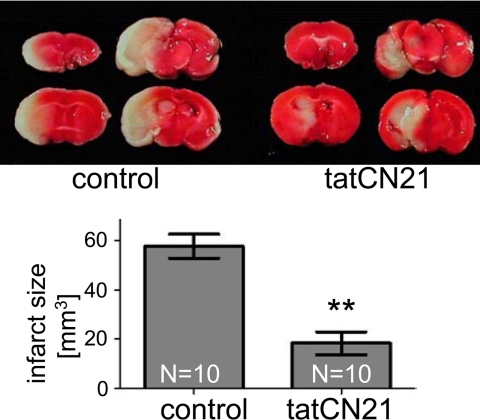
Maybe most importantly, tatCN21 was also neuroprotective in a mouse MCAO model of stroke. Injection of tatCN21 (1 mg/kg intravenously) at reperfusion, 1 h after onset of the occlusion, dramatically reduced the infarct size 24 h later, compared with saline control (Fig. 8). The occlusion did not result in any detectable differences in any physiological parameters (heart rate, arterial blood pressure, pH levels, PaCO2, and PaO2) between control and tatCN21-treated animals as a result (data not shown). Inactive tat fusion control peptides are well established to leave infarct size unaffected (for examples see 8, 58–60). Thus, CaMKII autonomy is indeed a promising drug target for stroke therapy.
FIGURE 8.
tatCN21 significantly reduced infarct size in a mouse stroke model, even when injected 1 h after onset of arterial occlusion. Infarct areas (in white in the upper panels) were determined by triphenyltetrazolium chloride staining, 24 h after MCAO. Injection of tatCN21 (1 mg/kg intravenously) at reperfusion, 1 h after the occlusion, significantly reduced the reconstructed infarct volume (**, p < 0.01 in t test, n = 10; lower panel). Results are shown as mean ± S.E.
DISCUSSION
CaMKII is well established as an important mediator of physiological glutamate signals (14–17), but its role in pathological excitotoxic glutamate signaling has been less clear. This study shows that CaMKII activity promotes excitotoxic cell death in hippocampal and cortical neurons, using a combination of several pharmacological inhibitors and CaMKII overexpression (Figs. 1–3). Most importantly, the Ca2+-independent autonomous CaMKII activity that is generated by Thr-286 autophosphorylation was identified as a drug target for post-insult neuroprotection (Figs. 4–8); tatCN21, a highly specific inhibitor of stimulated and autonomous CaMKII activity (42), protected neurons even when added hours after a glutamate insult (Fig. 4), whereas the “traditional” KN93 inhibited only stimulated but not autonomous CaMKII activity (Fig. 5) and was neuroprotective only when present during the insult (Fig. 4). Furthermore, overexpression of the autonomy-deficient T286A mutant increased glutamate excitotoxicity significantly less than overexpression of CaMKII wild type (Fig. 7). Finally, neuroprotection by tatCN21 was also demonstrated in an in vivo model of stroke (Fig. 8), demonstrating that CaMKII autonomy is indeed a promising drug target.
The role of CaMKII in neuronal cell death has been examined before but yielded conflicting conclusions (19–26). A variety of different approaches performed here now clearly support that CaMKII activation promotes excitotoxic death. Most importantly, however, this study shows for the first time that a CaMKII inhibitor can provide neuroprotection even when applied in a therapeutically relevant time window hours after the insult, not only in primary culture but also an animal model. In addition to acute conditions such stroke and global ischemia, excitotoxicity is thought to be a major cause in chronic neurodegenerative diseases (1–4). Chronic use of CaMKII inhibitors, which would be necessary in slow neurodegeneration, will likely be contraindicated due to the important role of CaMKII activity in learning and memory. However, for acute treatment of stroke or global ischemia (within 1 day or up to 1 week post-insult), temporary impairment of learning would be more than acceptable. In contrast to the current very limited treatment options (see Introduction), CaMKII inhibition is not expected to have contraindications in hemorrhagic stroke, making it a potential therapy for any stroke patient. Although development of a stroke therapy that targets CaMKII autonomy is still a far-away goal, even the current inhibitor tatCN21 may prove to be clinically useful, as tat fusion peptides and even proteins can cross the blood-brain barrier (8, 61). Indeed, tatCN21 was effective in the MCAO stroke model even after the clinically relevant route of systemic delivery by intravenous injection.
What are possible reasons why previous reports came to conflicting conclusions about the role of CaMKII in excitotoxicity? We had considered that the different effects of excessive glutamate on CaMKII activity (stimulated and autonomous) and targeting (to synapses and extra-synaptic clusters) may have different consequences in promoting or protecting from cell death. Then the different CaMKII inhibitors could differentially affect cell survival, depending on which subset of glutamate effects on the kinase they inhibit. However, instead, our results suggest autonomous activity as the most important glutamate-regulated CaMKII property promoting excitotoxic death; the other glutamate-induced CaMKII changes did not appear to significantly affect cell survival either way. Thus, the reason for apparent discrepancies among previous findings may rather lie in the limited selectivity of KN93, which inhibits additionally CaMKI, CaMKIV, and L-type Ca2+ channels (35–37), as well as AIP and AC3-I, which inhibit additionally MLCK, protein kinase C, and PKD (38–40). Indeed, our results indicate that the CaMKI and -IV inhibitor STO-609 enhanced excitotoxic death of hippocampal neurons. Such death-increasing effect of CaMKIV inhibition was also predicted by previous studies (62–64). Thus, KN93 could enhance excitotoxicity in specific situations or neuronal cell types where the toxic effect of CaMKI/IV inhibition outweighs the protective effect of CaMKII inhibition. However, although the limited drug selectivity may explain some contradictory reports, it does not explain the results of a study using knock-out mice, which had indicated a neuroprotective effect of CaMKII (23). It is possible that a complete lack of a protein has a profoundly different effect than inhibition of that same protein. Maybe more importantly, lack of CaMKIIα for a prolonged period of time (during all of development of the knock-out mice) may cause maladaptive compensatory effects indirectly leading to higher glutamate sensitivity. Indeed, CaMKIIα knock-out mice are epileptic (65), consistent with greater excitability.
What are the potential downstream effectors through which autonomous CaMKII activity may contribute to excitotoxic neuronal death? These could include CaMKII-induced potentiation of glutamate receptors (14–17) and L-type Ca2+ channels (66). Additionally, CaMKII has recently been shown to directly interact with connexin hemichannels (67), which are important for maintaining neuronal homeostasis and for neuron-glia communication and have been implicated in glutamate-induced cell death (68–70). However, the actual mechanism(s) linking CaMKII to neuronal death may be not yet identified. A neuroprotective effect of CaMKII inhibition could not have been directly predicted from the literature. In fact, many downstream effects of CaMKII phosphorylation are actually involved in promoting cell survival, including inhibition of neuronal nitric-oxide synthase (8, 71) and activation of extracellular signal-regulated kinase (ERK) (72) and cAMP-response element-binding protein (73).
In summary, we now have several lines of evidence demonstrating the role of CaMKII in glutamate excitotoxicity. Importantly, autonomous CaMKII activity was singled out as a promising drug target for effective post-insult neuroprotection, not only in culture but also in vivo.
Acknowledgments
We thank Drs. Mark Dell'Acqua, Kim Heidenreich, and Manisha Patel (University of Colorado Denver) for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants P30NS048154 (University of Colorado, Denver, center grant), P20RR017677-08, and R01NS052644 (to K. U. B.). This work was also supported by Veterans Affairs Merit Review (to M. S. K.) and State of Colorado TTO BDEGP. The University of Colorado is currently seeking patent protection for tatCN21 and its uses.
Supported by National Institutes of Health Training Grant F31NS061584.
Supported by National Institutes of Health Training Grant T32GM007635.
- NMDAR
- NMDA receptor
- CaMKII
- Ca2+/calmodulin-dependent protein kinase II
- NMDA
- N-methyl-d-aspartic acid
- MCAO
- middle cerebral arterial occlusion
- ANOVA
- analysis of variance
- PIPES
- 1,4-piperazinediethanesulfonic acid
- LDH
- lactate dehydrogenase.
REFERENCES
- 1.Doyle K. P., Simon R. P., Stenzel-Poore M. P. (2008) Neuropharmacology 55, 310–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hara M. R., Snyder S. H. (2007) Annu. Rev. Pharmacol. Toxicol. 47, 117–141 [DOI] [PubMed] [Google Scholar]
- 3.Aarts M. M., Tymianski M. (2004) Curr. Mol. Med. 4, 137–147 [DOI] [PubMed] [Google Scholar]
- 4.Bramlett H. M., Dietrich W. D. (2004) J. Cereb. Blood Flow Metab. 24, 133–150 [DOI] [PubMed] [Google Scholar]
- 5.Choi D. W., Koh J. Y., Peters S. (1988) J. Neurosci. 8, 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frandsen A., Drejer J., Schousboe A. (1989) J. Neurochem. 53, 297–299 [DOI] [PubMed] [Google Scholar]
- 7.Noh K. M., Yokota H., Mashiko T., Castillo P. E., Zukin R. S., Bennett M. V. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 12230–12235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aarts M., Liu Y., Liu L., Besshoh S., Arundine M., Gurd J. W., Wang Y. T., Salter M. W., Tymianski M. (2002) Science 298, 846–850 [DOI] [PubMed] [Google Scholar]
- 9.Ginsberg M. D. (2008) Neuropharmacology 55, 363–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villmann C., Becker C. M. (2007) Neuroscientist 13, 594–615 [DOI] [PubMed] [Google Scholar]
- 11.Weinberger J. M. (2006) J. Neurol. Sci. 249, 101–109 [DOI] [PubMed] [Google Scholar]
- 12.Kaur J., Zhao Z., Klein G. M., Lo E. H., Buchan A. M. (2004) J. Cereb. Blood Flow Metab. 24, 945–963 [DOI] [PubMed] [Google Scholar]
- 13.Wang X., Tsuji K., Lee S. R., Ning M., Furie K. L., Buchan A. M., Lo E. H. (2004) Stroke 35, 2726–2730 [DOI] [PubMed] [Google Scholar]
- 14.Wayman G. A., Lee Y. S., Tokumitsu H., Silva A. J., Silva A., Soderling T. R. (2008) Neuron 59, 914–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lisman J., Schulman H., Cline H. (2002) Nat. Rev. Neurosci. 3, 175–190 [DOI] [PubMed] [Google Scholar]
- 16.Hudmon A., Schulman H. (2002) Annu. Rev. Biochem. 71, 473–510 [DOI] [PubMed] [Google Scholar]
- 17.Colbran R. J., Brown A. M. (2004) Curr. Opin. Neurobiol. 14, 318–327 [DOI] [PubMed] [Google Scholar]
- 18.Giese K. P., Fedorov N. B., Filipkowski R. K., Silva A. J. (1998) Science 279, 870–873 [DOI] [PubMed] [Google Scholar]
- 19.Hajimohammadreza I., Probert A. W., Coughenour L. L., Borosky S. A., Marcoux F. W., Boxer P. A., Wang K. K. (1995) J. Neurosci. 15, 4093–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laabich A., Cooper N. G. (2000) Brain Res. Mol. Brain Res. 85, 32–40 [DOI] [PubMed] [Google Scholar]
- 21.Takano H., Fukushi H., Morishima Y., Shirasaki Y. (2003) Brain Res. 962, 41–47 [DOI] [PubMed] [Google Scholar]
- 22.Fan W., Agarwal N., Cooper N. G. (2006) Brain Res. 1067, 48–57 [DOI] [PubMed] [Google Scholar]
- 23.Waxham M. N., Grotta J. C., Silva A. J., Strong R., Aronowski J. (1996) J. Cereb. Blood Flow Metab. 16, 1–6 [DOI] [PubMed] [Google Scholar]
- 24.Mabuchi T., Kitagawa K., Kuwabara K., Takasawa K., Ohtsuki T., Xia Z., Storm D., Yanagihara T., Hori M., Matsumoto M. (2001) J. Neurosci. 21, 9204–9213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linseman D. A., Bartley C. M., Le S. S., Laessig T. A., Bouchard R. J., Meintzer M. K., Li M., Heidenreich K. A. (2003) J. Biol. Chem. 278, 41472–41481 [DOI] [PubMed] [Google Scholar]
- 26.Bok J., Wang Q., Huang J., Green S. H. (2007) Mol. Cell. Neurosci. 36, 13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strack S., McNeill R. B., Colbran R. J. (2000) J. Biol. Chem. 275, 23798–23806 [DOI] [PubMed] [Google Scholar]
- 28.Gardoni F., Caputi A., Cimino M., Pastorino L., Cattabeni F., Di Luca M. (1998) J. Neurochem. 71, 1733–1741 [DOI] [PubMed] [Google Scholar]
- 29.Bayer K. U., De Koninck P., Leonard A. S., Hell J. W., Schulman H. (2001) Nature 411, 801–805 [DOI] [PubMed] [Google Scholar]
- 30.Bayer K. U., LeBel E., McDonald G. L., O'Leary H., Schulman H., De Koninck P. (2006) J. Neurosci. 26, 1164–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudmon A., Aronowski J., Kolb S. J., Waxham M. N. (1996) J. Biol. Chem. 271, 8800–8808 [DOI] [PubMed] [Google Scholar]
- 32.Hudmon A., Lebel E., Roy H., Sik A., Schulman H., Waxham M. N., De Koninck P. (2005) J. Neurosci. 25, 6971–6983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao-Cheng J. H., Vinade L., Smith C., Winters C. A., Ward R., Brightman M. W., Reese T. S., Dosemeci A. (2001) Neuroscience 106, 69–78 [DOI] [PubMed] [Google Scholar]
- 34.Vest R. S., O'Leary H., Bayer K. U. (2009) FEBS Lett. 583, 3577–3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enslen H., Sun P., Brickey D., Soderling S. H., Klamo E., Soderling T. R. (1994) J. Biol. Chem. 269, 15520–15527 [PubMed] [Google Scholar]
- 36.Ledoux J., Chartier D., Leblanc N. (1999) J. Pharmacol. Exp. Ther. 290, 1165–1174 [PubMed] [Google Scholar]
- 37.Li G., Hidaka H., Wollheim C. B. (1992) Mol. Pharmacol. 42, 489–498 [PubMed] [Google Scholar]
- 38.Hvalby O., Hemmings H. C., Jr., Paulsen O., Czernik A. J., Nairn A. C., Godfraind J. M., Jensen V., Raastad M., Storm J. F., Andersen P., et al. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 4761–4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith M. K., Colbran R. J., Soderling T. R. (1990) J. Biol. Chem. 265, 1837–1840 [PubMed] [Google Scholar]
- 40.Backs J., Backs T., Neef S., Kreusser M. M., Lehmann L. H., Patrick D. M., Grueter C. E., Qi X., Richardson J. A., Hill J. A., Katus H. A., Bassel-Duby R., Maier L. S., Olson E. N. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 2342–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen H. X., Otmakhov N., Strack S., Colbran R. J., Lisman J. E. (2001) J. Neurophysiol. 85, 1368–1376 [DOI] [PubMed] [Google Scholar]
- 42.Vest R. S., Davies K. D., O'Leary H., Port J. D., Bayer K. U. (2007) Mol. Biol. Cell 18, 5024–5033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang B. H., Mukherji S., Soderling T. R. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 10890–10895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi D. W. (1987) J. Neurosci. 7, 369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi D. W., Maulucci-Gedde M., Kriegstein A. R. (1987) J. Neurosci. 7, 357–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michaels R. L., Rothman S. M. (1990) J. Neurosci. 10, 283–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bayer K. U., De Koninck P., Schulman H. (2002) EMBO. J. 21, 3590–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu J., Novgorodov S. A., Chudakova D., Zhu H., Bielawska A., Bielawski J., Obeid L. M., Kindy M. S., Gudz T. I. (2007) J. Biol. Chem. 282, 25940–25949 [DOI] [PubMed] [Google Scholar]
- 49.Yu J., Zhu H., Ko D., Kindy M. S. (2008) Brain Res. 1238, 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Govindaswami M., Brown S. A., Yu J., Zhu H., Bishop P. D., Kindy M. S., Oeltgen P. R. (2008) Acad. Emerg. Med. 15, 250–257 [DOI] [PubMed] [Google Scholar]
- 51.Felipo V., Grau E., Miñana M. D., Grisolía S. (1993) J. Neurochem. 60, 1626–1630 [DOI] [PubMed] [Google Scholar]
- 52.Li G. L., Farooque M., Lewen A., Lennmyr F., Holtz A., Olsson Y. (2000) APMIS 108, 98–106 [DOI] [PubMed] [Google Scholar]
- 53.Buddle M., Eberhardt E., Ciminello L. H., Levin T., Wing R., DiPasquale K., Raley-Susman K. M. (2003) Brain Res. 978, 38–50 [DOI] [PubMed] [Google Scholar]
- 54.Antonelli T., Tomasini M. C., Fournier J., Mazza R., Tanganelli S., Pirondi S., Fuxe K., Luca F. (2008) Cereb. Cortex 18, 1748–1757 [DOI] [PubMed] [Google Scholar]
- 55.Hudmon A., Kim S. A., Kolb S. J., Stoops J. K., Waxham M. N. (2001) J. Neurochem. 76, 1364–1375 [DOI] [PubMed] [Google Scholar]
- 56.Bayer K. U., Löhler J., Schulman H., Harbers K. (1999) Brain Res. Mol. Brain Res. 70, 147–154 [DOI] [PubMed] [Google Scholar]
- 57.Fink C. C., Bayer K. U., Myers J. W., Ferrell J. E., Jr., Schulman H., Meyer T. (2003) Neuron 39, 283–297 [DOI] [PubMed] [Google Scholar]
- 58.Borsello T., Clarke P. G., Hirt L., Vercelli A., Repici M., Schorderet D. F., Bogousslavsky J., Bonny C. (2003) Nat. Med. 9, 1180–1186 [DOI] [PubMed] [Google Scholar]
- 59.Sun H. S., Doucette T. A., Liu Y., Fang Y., Teves L., Aarts M., Ryan C. L., Bernard P. B., Lau A., Forder J. P., Salter M. W., Wang Y. T., Tasker R. A., Tymianski M. (2008) Stroke 39, 2544–2553 [DOI] [PubMed] [Google Scholar]
- 60.Yu C. Z., Li C., Pei D. S., Zong Y. Y., Shi Q., Wen X. R., Guan Q. H., Hang D., Hou X. Y., Zhang G. Y. (2009) Neurochem. Res. 34, 2008–2021 [DOI] [PubMed] [Google Scholar]
- 61.Schwarze S. R., Ho A., Vocero-Akbani A., Dowdy S. F. (1999) Science 285, 1569–1572 [DOI] [PubMed] [Google Scholar]
- 62.Chun H., Hao W., Honghai Z., Ning L., Yasong W., Chen D. (2009) Brain Res. 1257, 75–88 [DOI] [PubMed] [Google Scholar]
- 63.Marshall J., Dolan B. M., Garcia E. P., Sathe S., Tang X., Mao Z., Blair L. A. (2003) Neuron 39, 625–639 [DOI] [PubMed] [Google Scholar]
- 64.Tremper-Wells B., Mathur A., Beaman-Hall C. M., Vallano M. L. (2002) J. Neurochem. 81, 314–324 [DOI] [PubMed] [Google Scholar]
- 65.Butler L. S., Silva A. J., Abeliovich A., Watanabe Y., Tonegawa S., McNamara J. O. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 6852–6855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grueter C. E., Abiria S. A., Dzhura I., Wu Y., Ham A. J., Mohler P. J., Anderson M. E., Colbran R. J. (2006) Mol. Cell 23, 641–650 [DOI] [PubMed] [Google Scholar]
- 67.Alev C., Urschel S., Sonntag S., Zoidl G., Fort A. G., Höher T., Matsubara M., Willecke K., Spray D. C., Dermietzel R. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 20964–20969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oguro K., Jover T., Tanaka H., Lin Y., Kojima T., Oguro N., Grooms S. Y., Bennett M. V., Zukin R. S. (2001) J. Neurosci. 21, 7534–7542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bargiotas P., Monyer H., Schwaninger M. (2009) Curr. Mol. Med. 9, 186–194 [DOI] [PubMed] [Google Scholar]
- 70.Contreras J. E., Sánchez H. A., Véliz L. P., Bukauskas F. F., Bennett M. V., Sáez J. C. (2004) Brain Res. Brain Res. Rev. 47, 290–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rameau G. A., Chiu L. Y., Ziff E. B. (2004) J. Biol. Chem. 279, 14307–14314 [DOI] [PubMed] [Google Scholar]
- 72.Illario M., Cavallo A. L., Bayer K. U., Di Matola T., Fenzi G., Rossi G., Vitale M. (2003) J. Biol. Chem. 278, 45101–45108 [DOI] [PubMed] [Google Scholar]
- 73.Wheeler D. G., Barrett C. F., Groth R. D., Safa P., Tsien R. W. (2008) J. Cell Biol. 183, 849–863 [DOI] [PMC free article] [PubMed] [Google Scholar]