Abstract
Ceruloplasmin is a multicopper oxidase required for correct iron homeostasis.Previously, we have identified a ceruloplasmin mutant associated with the iron overload disease aceruloplasminemia, which was unable to acquire copper from the mammalian pump ATP7B but could be produced in an enzymatically active form in yeast. Here, we report the expression of recombinant ceruloplasmin in the yeast Pichia pastoris and the study of the role of five surface-exposed loops in copper incorporation by comparing the efficiencies of mammalian ATP7B and yeast Ccc2p. The possibility to “mix and match” mammalian and yeast multicopper oxidases and copper ATPases can provide clues on the molecular features underlying the process of copper loading in multicopper oxidases.
Keywords: Copper, Golgi, Iron, Neurodegeneration, Protein Metal Ion Interaction, Protein Sorting, Aceruloplasminemia, Ceruloplasmin, Ferroportin
Introduction
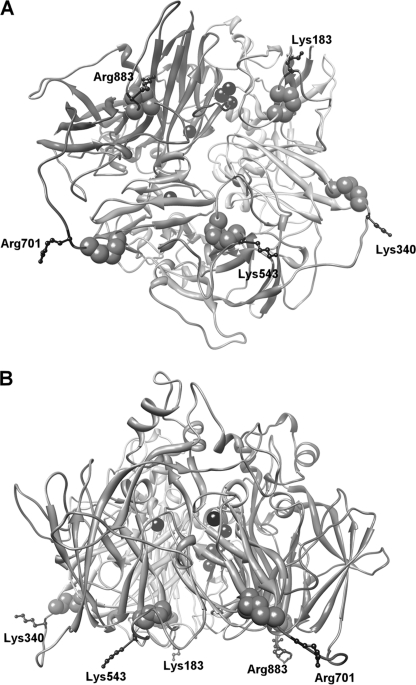
Ceruloplasmin (Cp)3 is a complex protein present in vertebrates, which belongs to the family of the multicopper oxidases. These enzymes are able to couple the single electron oxidation of substrates to complete reduction of dioxygen to water. Multicopper oxidases possess multiple copper-binding sites with different structural and functional properties; type 1 blue copper is the primary electron acceptor from the substrate, and a trinuclear cluster formed by type 2 and binuclear type 3 copper constitutes the oxygen binding and reduction site (1). Cp is a multidomain protein made up of six plastocyanin-like domains, the interface between domains 6 and 1 hosting the catalytically essential trinuclear copper cluster and domains 2, 4, and 6 harboring a type 1 copper site each. Cp is predominantly secreted by hepatocytes, where the P-type ATPase ATP7B incorporates copper into apo-Cp during transit in the trans-Golgi network (2). A GPI-anchored form of Cp (Cp-GPI) has also been identified, mainly in the brain, where it resides on the plasma membrane of astrocytes (3). Synthesis of this isoform is via alternative splicing, causing the replacement of the five C-terminal amino acids of the secreted protein by 30 alternative residues that lead to the addition of the GPI anchor (4). The ferroxidase activity of Cp is required for proper iron homeostasis; lack of oxidase-active Cp leads to internalization and degradation of ferroportin (Fpn), the only known mammalian iron exporter (5). Furthermore, genetic defects of the Cp gene cause aceruloplasminemia, a rare autosomal iron overload disease with clinical manifestations, including retinal degeneration, diabetes mellitus, and neurological symptoms, which include ataxia, involuntary movements, and dementia (6). Missense Cp mutants associated with aceruloplasminemia are beginning to be characterized and can be broadly classified in different groups according to their ability to stabilize Fpn on the plasma membrane of cells silenced for endogenous Cp-GPI. Nonfunctional mutants are inactive due to retention in the endoplasmic reticulum or secretion as apo-Cp lacking copper, and partially or fully functional mutants are enzymatically active. Mutant Cp R701W is atypical in that it is found in an unusually young patient in heterozygous form (7). We have previously reported that Cp R701W is unable to be loaded with copper by ATP7B, but it can acquire the prosthetic metal from the yeast copper ATPase Ccc2p. Moreover, this mutant is dominant over wild type Cp and induces fragmentation of the Golgi complex with re-localization of ATP7B (8). Arg701 is located in a large solvent-exposed loop connecting domains 4 and 5, and corresponding loops connect the other domains of Cp. Despite a low degree of sequence homology, all these loops start with a CX(R/K) motif, with the cysteine residue stabilizing the loop by forming a disulfide bridge (Fig. 1 and supplemental Fig. S1). We have recently shown that mutation of Cp basic residues Lys340 or Arg883 into tryptophan on two of these loops causes rapid degradation of Fpn. However, K340W and R883W mutants were found to be not dominant over wild type Cp, at variance with the homologous R701W mutant. On the other hand, all three mutants (R701W, K340W, and R883W) are enzymatically active when produced in yeast (8). These findings suggest that Cp loops could play a critical role in copper incorporation and that the process of copper loading in yeast versus mammalian cells is less structurally demanding.
FIGURE 1.
Three-dimensional structure of human ceruloplasmin. Bottom view (A) and side view (B) of the protein show the six-domain structure with the copper atoms indicated by black spheres. The cysteine residues and the basic residues of the five loops containing the CX(R/K) motif are shown in space fill (light gray) and ball-and-stick representation (dark gray), respectively. The figure was produced with Chimera (18).
Heterologous expression of multicopper oxidases is generally quite challenging; a survey of the literature shows that bacteria are useful only for expression of prokaryotic multicopper oxidase. Eukaryotic enzymes have been expressed with varying luck in yeast, mammalian, or insect cells. Human Cp has been produced in the methylotrophic yeast Pichia pastoris under control of the inducible AOX1 promoter (9). However, not only yields were well below 1 mg/liter but the whole procedure was quite complex and took several days to be completed. In this work, we have set up a different system based on an engineered P. pastoris strain harboring an inactivated gene for the endogenous ferroxidase Fet3p, and we have used the strong constitutive glyceraldehyde-3-phosphate dehydrogenase promoter to drive expression of recombinant Cp. The rationale for using a fet3Δ strain was that of avoiding contamination of recombinant Cp by the endogenous yeast ferroxidase.
Expression of Cp mutants in this system has allowed us to expand our investigation on a peculiar dominant negative Cp mutant associated with aceruloplasminemia, i.e. R701W. Moreover, the role of all surface-exposed loops in the process of copper incorporation has been analyzed by comparing yeast ATPase Ccc2p with the mammalian homologous ATP7B for their ability to deliver copper to Cp. The possibility of “mixing and matching” yeast and mammalian ferroxidases and copper ATPases can provide clues on the molecular mechanism of copper incorporation into complex proteins such as the multicopper oxidases.
EXPERIMENTAL PROCEDURES
Yeast Strains and Media
P. pastoris strain JC300 (ade1, arg4, his4) was a generous gift of J. Cregg (10). Yeast cells were grown at 30 °C in YPD or in minimal dextrose (MD) medium with the appropriate auxotrophic supplements. The bioavailable iron content of the medium was varied by addition of the membrane-impermeable iron chelator bathophenanthroline disulfonate (BPS, 80–160 μm for YPD or 20 μm for MD).
Constructs
The coding sequence for human Cp, both GPI-linked and secreted isoforms, was cloned SacI-XhoI in the integrative pIB2 expression vector (11) under control of the constitutive glyceraldehyde-3-phosphate dehydrogenase promoter. A FLAG epitope tag was introduced by PCR in the coding sequence of Cp to replace Arg481, and Cp mutants were constructed as described (8). The mutant Cp was moved from the mammalian expression vector pCMV Tag4B in pIB2 and sequence-verified. The coding sequence for P. pastoris Fet3p with a C-terminal FLAG epitope tag was cloned EcoRI-XhoI in pCMV Tag4B. The coding sequence for P. pastoris Ccc2p was cloned BamHI-XhoI in pCMV Tag4B to introduce a C-terminal FLAG tag.
Construction of fet3Δ Strain
One-step gene disruption was employed to inactivate the FET3 gene. Plasmid pBSFet3, which contains the P. pastoris Fet3p coding sequence and 3′-untranslated region, was first digested with EcoRI and SpeI, filled-in with Klenow enzyme, and religated to remove the BamHI site in the polylinker. pBSFet3 was then digested with BamHI to remove 335 bp of Fet3p coding sequence, including ligands for type 1 copper and the trinuclear cluster; the P. pastoris ADE1 gene was amplified from pBLAde (10) and cloned BamHI in pBSFet3. To create the fet3Δ strain, the fet3::ADE1 cassette was obtained by digestion with BsmI and BglII and electroporated in P. pastoris JC300. Electrocompetent JC300 cells were prepared by the method of Wu and Letchworth (12). White Ade+ colonies were plated on MD with or without 20 μm BPS, and after 48 h at 30 °C, they were scored for lack of growth. Colonies with impaired growth in BPS were further screened by PCR on genomic DNA, and lack of Fet3p oxidase activity was used to confirm inactivation of the FET3 gene. Genomic DNA was extracted by glass bead lysis, and PCR was carried out with the following primers: forward 5′-actggtgccgagccaattcc-3′ and reverse 5′-gacctcgagctatcagttcaataactc-3′ with the following conditions: 95 °C for 5 min followed by 25 cycles at 95 °C for 1 min, 58 °C for 1 min, 72 °C for 2 min 30 s, and a final extension at 72 °C for 7 min. The frequency of correct gene replacement was estimated to be less than 2% (two colonies out of about 150 screened). The fet3Δ strain grew poorly in BPS but was otherwise normal.
Expression, Purification, and Characterization of Recombinant Cp
The expression plasmids with the Cp cDNA were linearized with SalI and electroporated in the fet3Δ strain. The presence of Cp was confirmed by PCR on genomic DNA of selected His+ colonies.
Expression of secreted Cp was performed in MD, buffered with 50 mm potassium phosphate, pH 6, and supplemented with 5 μg/ml arginine, 100 μm CuSO4, and 50 μm Fe(NH4)2(SO4)2. Culture supernatants were concentrated 40-fold with Amicon Ultra 15 devices and processed for immunodetection. Denaturing and nondenaturing SDS-PAGE, staining for oxidase activity with o-dianisidine and Western blot, were performed as described (8). Recombinant Cp was partially purified by anion exchange chromatography on DEAE-Sephacel and quantified by indirect ELISA. Total protein was determined by the Bradford assay (Bio-Rad). Oxidase activity was assayed with o-dianisidine (13). More details can be found in the supplemental material.
Mammalian cell culture, silencing, transfection, and staining for immunofluorescence microscopy were performed as described previously (8). The presence of the GFP moiety fused to Fpn allowed direct visualization of the iron exporter by epifluorescence. The polyclonal anti-ATP7B antibody was from Novus Biologicals. Small interfering RNA oligonucleotide pool matching selected regions of rat ATP7B was obtained from Qiagen. Cells were visualized using an inverted DMI 6000 confocal scanner microscope TCS SP5 (Leica Microsystems CMS GmbH) with a ×63 oil immersion objective. Images were acquired using Leica Application Suite Advanced Fluorescence software.
RESULTS
Expression of Recombinant Cp in a P. pastoris fet3Δ Strain
A fet3Δ strain of the methylotrophic yeast P. pastoris was constructed as a host for heterologous expression of human Cp. The FET3 gene was deleted to avoid contamination of recombinant Cp with the endogenous yeast ferroxidase. This would naturally happen when expressing membrane-bound Cp-GPI, but it could also occur with secreted Cp. In fact, although Fet3p is firmly bound to the plasma membrane, it has been reported that a fraction of Fet3p can be released into the medium by spontaneous limited proteolysis (14), and this was shown to happen also in the strain expressing recombinant Cp (9). The FLAG-tagged Cp-GPI isoform produced in yeast could be easily purified (supplemental Fig. S2A), but it was unexpectedly found to be enzymatically inactive. Immunofluorescence microscopy of yeast cells expressing Cp-GPI suggested that the recombinant protein was not fully processed, as most of Cp-GPI stained on intracellular membranes rather than on plasma membrane (supplemental Fig. S2B). Substitution of the native signal peptide sequence of Cp with that of Fet3p did not improve the situation (data not shown).
The soluble isoform of human Cp was therefore expressed in P. pastoris fet3Δ and was shown to be correctly secreted by Western blot analysis of concentrated culture supernatants, suggesting that the protein was in this case properly processed. However, affinity purification failed, likely due to shielding of the epitope tag by extra oligosaccharide chains. Partial purification by anion exchange chromatography yielded a 40–60% pure protein (supplemental Fig. S3A), with a yield of about 150 μg/liter of culture. The recombinant protein was enzymatically active, as demonstrated by nondenaturing SDS-PAGE and staining of the gel with o-dianisidine (supplemental Fig. S3B) Although yield and purity were not impressive, the recombinant protein was obviously devoid of any Fet3p contamination, which made it suitable for the determination of oxidase activity reported below.
Characterization of Cp Missense Mutants Localized on Interdomain Loops
The possibility of obtaining recombinant soluble Cp not contaminated with Fet3p allowed us to exploit the fet3Δ P. pastoris system to express a series of Cp missense mutants structurally related to a peculiar dominant negative mutant involved in aceruloplasminemia and to assess both their oxidase and Fpn-related functional activities.
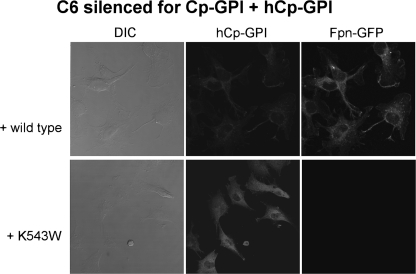
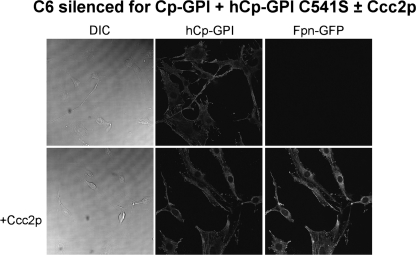
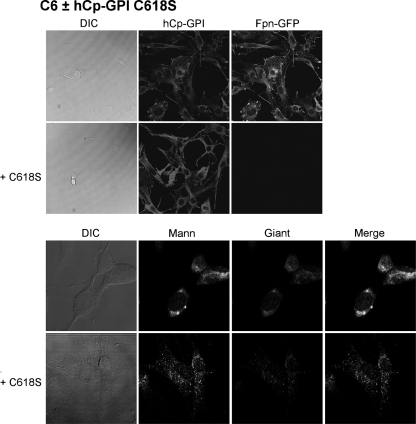
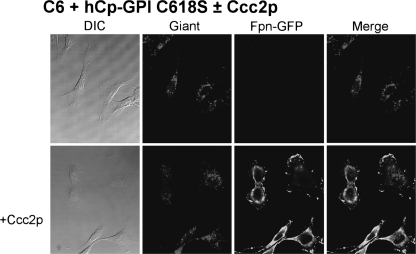
A wide set of mutants was analyzed, which targeted specific basic residues located in surface loops of Cp. As shown in Fig. 1 and in supplemental Fig. S1, there are five loops that connect the six domains in the Cp structure. The loop going from residue 699 to 710 contains a critical arginine at position 701. Mutation R701W was found in a heterozygous young patient affected by aceruloplasminemia, and our previous work revealed that this mutant has a dominant negative effect over wild type Cp and that it induces fragmentation/dispersal of the Golgi apparatus (8). Moreover, the R701W mutant is not loaded with copper by ATP7B, but it can acquire the metal from yeast Ccc2p. Basic residues corresponding to Arg701 within the CX(R/K) motif of every other loop were replaced by tryptophan to mimic the R701W mutation. The Arg/Lys → Trp Cp mutants were expressed in yeast, and their ability to maintain Fpn-GFP on the plasma membrane was assessed by transfection in mammalian cells silenced for endogenous Cp-GPI. We have already reported preliminary results for mutants K340W and R883W (8). All Arg/Lys → Trp mutants (K183W, K340W, K543W, and R883W) essentially behaved like the R701W mutant, in that they were nonfunctional in mammalian cells (representatively shown for K543W in Fig. 2), but showed significant oxidase activity when expressed and partially purified in yeast (supplemental Fig. 3B and Table 1). These results indicate that mutation of the basic residue of all loops dramatically interferes with proper copper loading of Cp in mammalian cells but not in yeast. However, at variance with the R701W mutant, the other four Arg/Lys → Trp mutants did not show the dominant negative effect over wild type Cp and did not affect Golgi morphology (data not shown), suggesting a peculiar role for the Arg701-containing loop. We explored the role of the disulfide bridges preceding the basic residues by substituting each cysteine residue with serine. Cys → Ser mutants C181S, C338S, C541S, and C881S were unable to rescue Fpn-GFP (i.e. to prevent its degradation) unless yeast Ccc2p was co-expressed in C6 cells, as representatively shown in Fig. 3 for C541S. Interestingly, P. pastoris Ccc2p appeared to be more efficient than its homolog from Saccharomyces cerevisiae, with cell-surface Fpn-GFP present in practically all cells, as compared with about 30% of the cells obtained by co-transfections with S. cerevisiae Ccc2p (8). This is not due to different transfection efficiencies and/or expression levels of the ATPases, as demonstrated by Western blot analysis (supplemental Fig. 4). The Cys → Ser mutants were not dominant over wild type Cp, and Golgi morphology was normal as well (data not shown). On the other hand, disruption of the disulfide bridge of the Arg701 loop was much more devastating because not only mutation of Cys699 (the cysteine on the loop) but also of Cys618 (the corresponding internal cysteine) led to Cys → Ser mutants that exhibited the same phenotype of R701W, being nonfunctional and dominant over wild type Cp (Fig. 4, upper panel) and able to induce fragmentation of the Golgi apparatus (Fig. 4, lower panel). Specific Golgi markers were used to show that vesicles formed in the presence of mutant C618S originated from the Golgi apparatus. As shown in Fig. 4 (lower panel), in untransfected cells mannosidase II co-localized with the Golgi matrix protein Giantin, yielding the expected perinuclear localization. When cells were transfected with hCp-GPI C618S, both Golgi markers appeared to be redistributed from the typical ribbon pattern to a vesicular haze extensively dispersed throughout the cytosol. Identical results were obtained with hCp-GPI C699S. Co-expression with Ccc2p rescued Fpn-GFP, even though the Golgi apparatus was dispersed (Fig. 5).
FIGURE 2.
Cp mutant K543W is unable to rescue Fpn-GFP in C6 silenced for endogenous ceruloplasmin. Rat glioma C6 cells were silenced for Cp-GPI. After 24, they were transfected with Fpn-GFP and human Cp-GPI K543W. Cells were analyzed after 24 h by epifluorescence (Fpn-GFP) and immunostaining (hCp). DIC, differential interference contrast.
TABLE 1.
Enzymatic and functional activity of human Cp expressed in P. pastoris
| Ceruloplasmin | Oxidase activitya | Specific activity | Functional complementationb |
|---|---|---|---|
| Wild type | 0.078 | 100 | +++ |
| R701W | 0.053 | 68 | −D |
| K183W | 0.071 | 91 | − |
| K340W | 0.075 | 96 | − |
| K543W | 0.077 | 99 | − |
| R883W | 0.052 | 67 | − |
| C699S | 0.062 | 79 | −D |
| C618S | 0.034 | 44 | −D |
a ΔA540/h/μg Cp at 37 °C pH 6 (blank, sample boiled for 5 min).
b Functional complementation in C6 cells silenced for endogenous Cp-GPI and co-transfected with Fpn-GFP and the corresponding Cp mutant. D, dominant over wild type Cp.
FIGURE 3.
Analysis of complementation by Cp-GPI C541S of endogenous ceruloplasmin silencing in C6 cells. Rat glioma C6 cells were silenced for Cp-GPI. After 24 h, they were transfected with Fpn-GFP and human Cp-GPI C541S. Yeast ATPase Ccc2p ability to deliver copper to the mutant Cp was assessed by further co-transfection with recombinant P. pastoris Ccc2p-FLAG construct. Cells were analyzed after 24 h by epifluorescence (Fpn-GFP) or immunofluorescence (hCp). DIC, differential interference contrast.
FIGURE 4.
Human Cp-GPI C618S is dominant over wild type CP and induces dispersal of the Golgi apparatus. Upper panel, rat glioma C6 cells were transfected with Fpn-GFP and, when indicated, with hCp-GPI C618S. After 24 h, they were analyzed by epifluorescence (Fpn-GFP) or immunofluorescence (hCp). Lower panel, higher magnification images of C6 cells nontransfected or transfected with Cp-GPI C618S and immunostained for Golgi markers (mannosidase, Mann; Giantin, Giant). DIC, differential interference contrast.
FIGURE 5.
Yeast Ccc2p can reverse the effect of Cp-GPI C618S. Rat glioma C6 cells were transfected with Fpn-GFP and with hCp-GPI C618S. After 24 h, they were analyzed by epifluorescence (Fpn-GFP) or immunofluorescence (hCp). Co-transfection with P. pastoris Ccc2p rescues Fpn-GFP even though Golgi still appears fragmented. DIC, differential interference contrast.
Both C618S and C699S mutants were found to be catalytically active when expressed in yeast (supplemental Fig. 3B), with 44 and 79% activity, respectively, relative to wild type (Table 1), indicating that mutation of the cysteine residues did not induce gross folding defects. Thus, Cp mutations R701W, C699S, and C618S all interfere with Golgi morphology and copper loading selectively in mammalian cells. It should be noted that immunofluorescence analysis indicated that ATP7B levels were not changed by overexpression of any of the Cp-GPI Arg/Lys → Trp and Cys → Ser mutants (data not shown).
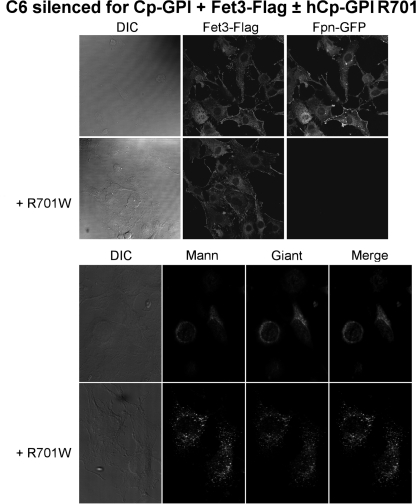
The mechanism of copper incorporation in multicopper oxidases is not yet fully understood. It has been established that copper loading takes place in the trans-Golgi both in mammalian and yeast cells and requires the copper-transporting ATPases ATP7B and Ccc2p, respectively. A highly specific structural interaction between the ATPase and the acceptor protein does not seem to be strictly necessary, because yeast has been widely used as a model system to analyze the function of ATP7B variants causing Wilson disease by evaluating Fet3p activity in a ccc2Δ strain (15, 16). To assess whether the activity of ATP7B was impaired in cells transfected with the Cp mutant R701W, we exploited the ability of ATP7B to deliver copper to Fet3p by testing it in mammalian cells in the absence and presence of Cp-GPI R701W. Fig. 6 shows that the yeast ferroxidase is functional in C6 cells silenced for Cp-GPI, as evidenced by the presence of Fpn-GFP on the membrane. On the other hand, when Cp-GPI R701W is co-expressed, Fet3p correctly reaches the plasma membrane, but it is inactive (Fig. 6). Moreover, lack of activity of Fet3p is accompanied by dispersion of the Golgi apparatus (Fig. 6). Thus, the expression of Cp-GPI R701W impairs the proper functioning of ATP7B, which becomes unable to discharge copper into the target ferroxidase, whichever it is. Consistent with our previous findings, Golgi fragmentation induced by the presence of Cp-GPI R701W does not affect protein sorting to the plasma membrane (Fig. 6). Similar results were obtained with the other two mutants with dominant negative properties, i.e. C618S and C699S (data not shown).
FIGURE 6.
Effect of Cp-GPI R701W on copper loading of yeast ferroxidase Fet3p by ATP7B. Upper panel, C6 cells silenced for Cp-GPI were transfected with Fpn-GFP, Fet3p-FLAG, and when indicated with hCp GPI-R701W. After 24 h, cells were examined by epifluorescence (Fpn-GFP) or immunofluorescence (Fet3-FLAG, upper panel). Lower panel, C6 cells silenced for Cp-GPI were transfected with Fet3p-FLAG and when indicated with hCp-GPI R701W. After 24 h, cells were examined by immunostaining for Golgi markers (mannosidase, Mann; and Giantin, Giant). DIC, differential interference contrast.
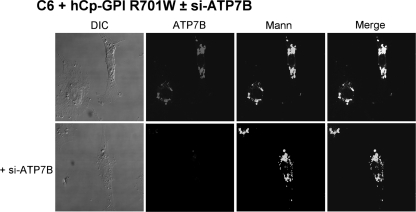
Golgi dispersal could be due to abnormal interaction of mutant Cp and ATP7B with consequent inactivation of the ATPase or to some other phenomenon triggered by mutant Cp but independent of ATP7B. To test the involvement of the copper ATPase in the induction of Golgi fragmentation, cells expressing Cp-GPI R701W were silenced for ATP7B, and Golgi status was analyzed. Results obtained indicate that the Golgi is dispersed in C6 cells silenced for ATP7B and transfected with Cp-GPI R701W (Fig. 7), suggesting that it is an intrinsic property of the mutant Cp that gives rise to the changes in Golgi morphology.
FIGURE 7.
Golgi is fragmented in C6 silenced for ATP7B and transfected with Cp-GPI R701W. Cells were silenced for ATP7B when indicated and then transfected with Cp-GPI R701W. After 24 h, they were examined by immunostaining for ATP7B and mannosidase (Mann). DIC, differential interference contrast.
DISCUSSION
The results shown here strongly indicate that the basic residues of the five loops connecting the six domains of Cp, and the disulfide bridges that stabilize the loops, are required for proper copper loading. The observation that the yeast ATPase Ccc2p is able to load copper in the Arg/Lys → Trp and Cys → Ser mutants is intriguing. The yeast and mammalian pumps share significant sequence homology. However, detailed structural information on the copper ATPases is limited to the metal- and ATP-binding domains (17), and little is known about the short loops connecting transmembrane regions on the luminal side of the membrane. Inspection of the sequence of the four predicted loops reveals that, apart from being quite short, they are not particularly conserved among ATP7B and P. pastoris and S. cerevisiae Ccc2p, and they have quite different charge and hydrophobicity. Thus, the different behavior of the two pumps could be due to differences in the structure of the ATPase on the luminal side of the Golgi apparatus.
A central observation is that disruption of the native structure of the external loops of Cp is not per se sufficient to prevent the ATPase-assisted copper loading. A possible explanation is that a conformational rearrangement of Cp is required for proper copper loading and that mutation of the basic residues on the loops, or disruption of the preceding disulfide bridges, impairs the conformational rearrangement by anomalously increasing the affinity between Cp and ATP7B (but not Ccc2p). For instance, “closure” of domains 5 and 6 onto domain 1 could be necessary for the delivery of copper to take place onto the trinuclear copper cluster (whose ligands are provided by both domains 6 and 1). Because the loops are located in a flat region at the bottom of the Cp structure (Fig. 1 and supplemental Fig. S1), it is tempting to speculate that this region is responsible for improper interaction with ATP7B when the native loop structure is destroyed. This model would be consistent with the topology of ATP7B, which places the metal-binding domains on the cytosolic side of the membrane and predicts exit of copper from the transmembrane channel into the lumen.
A much more complex scenario is set by mutations R701W, C699S, and C618S at the loop connecting domains 4 and 5 of Cp, which also induce fragmentation/dispersal of the Golgi. Here, not only is ATP7B no longer able to deliver copper to the mutant Cp but it cannot load the metal onto the wild type protein or even on a different ferroxidase like Fet3p, although Ccc2p is still working. Moreover, the presence of the mutant protein leads to the appearance of a plethora of vesicles clearly originating from the Golgi apparatus. Dispersal of the Golgi could lead to a lack of copper delivery either by unavailability of ATP7B or by true inactivation of the transporter, explaining the dominant phenotype of the mutant Cp. Exit of Cp from the endoplasmic reticulum and arrival to the Golgi are necessary to alter Golgi morphology, but the functionality of the Golgi apparatus is not grossly damaged, i.e. anterograde transport to the plasma membrane and endocytosis are still active.4 In turn, the altered morphology of the Golgi apparatus is responsible for the dysfunction of ATP7B, but not of Ccc2p, with the latter keeping the ability to pump copper inside the Golgi lumen even when the apparatus is dispersed. The hypothesis that Ccc2p can win on ATP7B in a dispersed Golgi simply because it is overexpressed cannot be ruled out. Clearly, overexpression guarantees that a significant fraction of Ccc2p is present in all scattered vesicles of a fragmented Golgi. However, it should be noted that a number of loop mutants that do not induce rearrangement of the Golgi apparatus are also assisted by Ccc2p, but not by ATP7B, in their copper loading, both in yeast (where Ccc2p is not overexpressed) and in mammalian cells. Moreover, we found that recombinant Ccc2p and ATP7B almost fully co-localize in C6 cells expressing Cp-GPI R701W (supplemental Fig. 5), suggesting that ATP7B impairment is unlikely due to spatial misplacing.
Why does yeast Ccc2p but not mammalian ATP7B deliver copper to all the Cp loop mutants (independent of the cell context and of whether the Golgi is dispersed or not)? An additional explanation could be that the two ATPases have different rates of copper transport, and if Ccc2p works faster than ATP7B, it could pump much more copper in the trans-Golgi lumen, leading to incorporation of the metal into Cp simply by providing a more copper-rich environment. A higher concentration of copper would be beneficial for all those Cp mutants with defects that lower the protein affinity for copper, either by interfering with the copper-binding sites or by interfering with folding in a state competent for copper incorporation. Direct measurements of copper transport by purified copper ATPases are very scarce, and comparison of Ccc2p and ATP7B catalytic efficiency is not straightforward, mainly because of differences in expression and/or reconstitution systems. Thus, the possibility that our results could be explained by a higher kinetic efficiency of P. pastoris Ccc2p cannot be discarded.
Future work will be aimed at understanding what is the molecular defect of Cp R701W that causes Golgi dispersal. Interestingly, we found that the double mutant C699S/R701W was nonfunctional, but not dominant, and Golgi morphology was normal (data not shown). This finding suggests that this region of Cp is probably involved in multiple functional interactions with different partners that are critical for correct maturation of the protein, but the fact that only replacement of Arg701 with tryptophan or disruption of the Cys618–Cys699 disulfide bridge causes the dominant phenotype remains puzzling.
Supplementary Material
Acknowledgments
We thank Drs. T. Persichini and M. Colasanti for useful discussions.
This work was supported by Telethon-Italy Grant GGP06173 (to G. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures” and Figs. 1–5.
N. Maio, F. Polticelli, T. Persichini, G. Rizzo, M. Colasanti, G. De Franceso, M. C. Bonaccorsi di Patti, and G. Musci, manuscript in preparation.
- Cp
- ceruloplasmin
- BPS
- bathophenanthroline disulfonic acid
- Fpn
- ferroportin
- GFP
- green fluorescent protein
- GPI
- glycosylphosphatidylinositol
- MD
- minimal dextrose.
REFERENCES
- 1.Solomon E. I., Sundaram U. M., Machonkin T. E. (1996) Chem. Rev. 96, 2563–2606 [DOI] [PubMed] [Google Scholar]
- 2.Lutsenko S., Barnes N. L., Bartee M. Y., Dmitriev O. Y. (2007) Physiol. Rev. 87, 1011–1046 [DOI] [PubMed] [Google Scholar]
- 3.Patel B. N., David S. (1997) J. Biol. Chem. 272, 20185–20190 [DOI] [PubMed] [Google Scholar]
- 4.Patel B. N., Dunn R. J., David S. (2000) J. Biol. Chem. 275, 4305–4310 [DOI] [PubMed] [Google Scholar]
- 5.De Domenico I., Ward D. M., di Patti M. C., Jeong S. Y., David S., Musci G., Kaplan J. (2007) EMBO J. 26, 2823–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyajima H. (2003) Neuropathology 23, 345–350 [DOI] [PubMed] [Google Scholar]
- 7.Kuhn J., Miyajima H., Takahashi Y., Kunath B., Hartmann-Klosterkoetter U., Cooper-Mahkorn D., Schaefer M., Bewermeyer H. (2005) J. Neurol. 252, 111–113 [DOI] [PubMed] [Google Scholar]
- 8.Bonaccorsi di Patti M. C., Maio N., Rizzo G., De Francesco G., Persichini T., Colasanti M., Polticelli F., Musci G. (2009) J. Biol. Chem. 284, 4545–4554 [DOI] [PubMed] [Google Scholar]
- 9.Bielli P., Bellenchi G. C., Calabrese L. (2001) J. Biol. Chem. 276, 2678–2685 [DOI] [PubMed] [Google Scholar]
- 10.Lin Cereghino G. P., Lin Cereghino J., Sunga A. J., Johnson M. A., Lim M., Gleeson M. A., Cregg J. M. (2001) Gene 263, 159–169 [DOI] [PubMed] [Google Scholar]
- 11.Sears I. B., O'Connor J., Rossanese O. W., Glick B. S. (1998) Yeast 14, 783–790 [DOI] [PubMed] [Google Scholar]
- 12.Wu S., Letchworth G. J. (2004) BioTechniques 36, 152–154 [DOI] [PubMed] [Google Scholar]
- 13.Schosinsky K. H., Lehmann H. P., Beeler M. F. (1975) Clin. Chem. 21, 757–759 [PubMed] [Google Scholar]
- 14.Bonaccorsi di Patti M. C., Bellenchi G. C., Bielli P., Calabrese L. (1999) Arch. Biochem. Biophys. 372, 295–299 [DOI] [PubMed] [Google Scholar]
- 15.Hsi G., Cullen L. M., Macintyre G., Chen M. M., Glerum D. M., Cox D. W. (2008) Hum. Mutat. 29, 491–501 [DOI] [PubMed] [Google Scholar]
- 16.Hung I. H., Suzuki M., Yamaguchi Y., Yuan D. S., Klausner R. D., Gitlin J. D. (1997) J. Biol. Chem. 272, 21461–21466 [DOI] [PubMed] [Google Scholar]
- 17.Lutsenko S., Gupta A., Burkhead J. L., Zuzel V. (2008) Arch. Biochem. Biophys. 476, 22–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E. (2004) J. Comput. Chem. 25, 1605–1612 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.